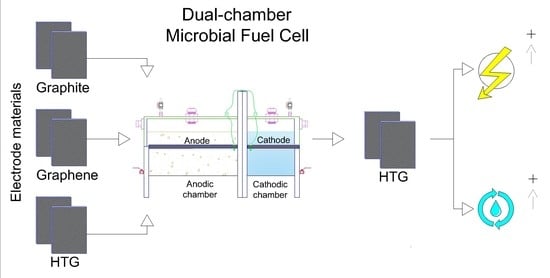
Assessment of Graphite, Graphene, and Hydrophilic-Treated Graphene Electrodes to Improve Power Generation and Wastewater Treatment in Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
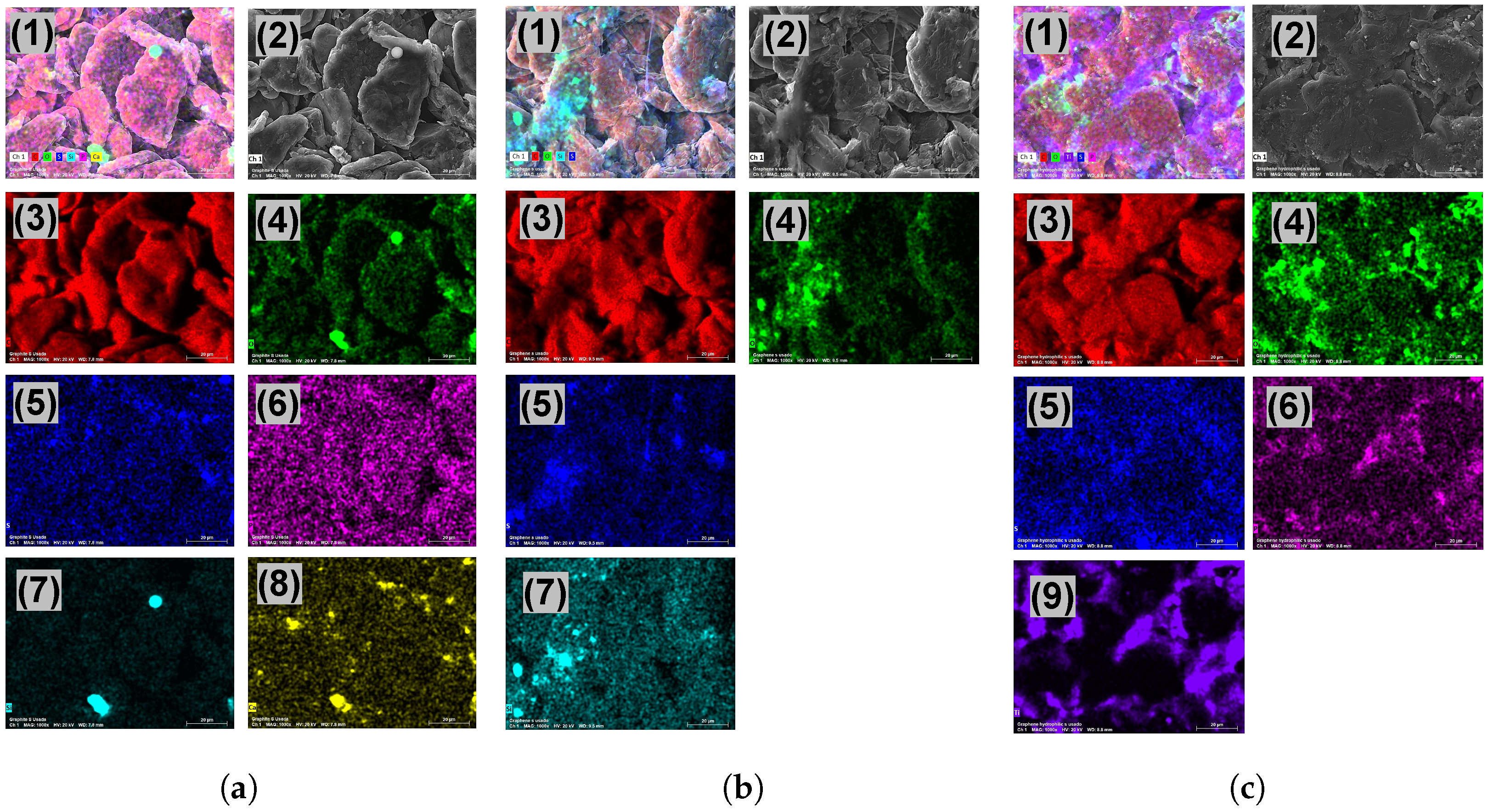
2.1. Characterization of Materials
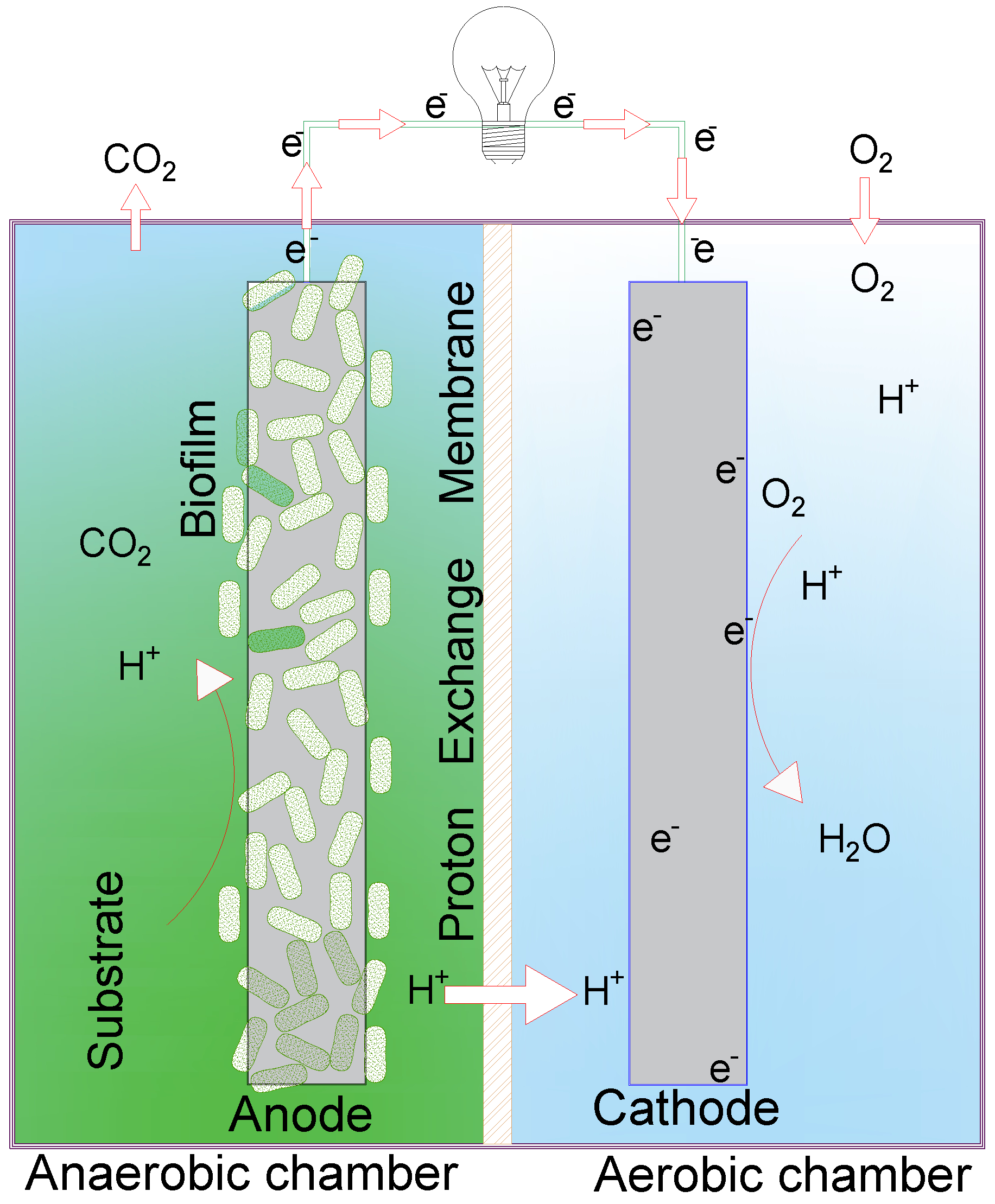
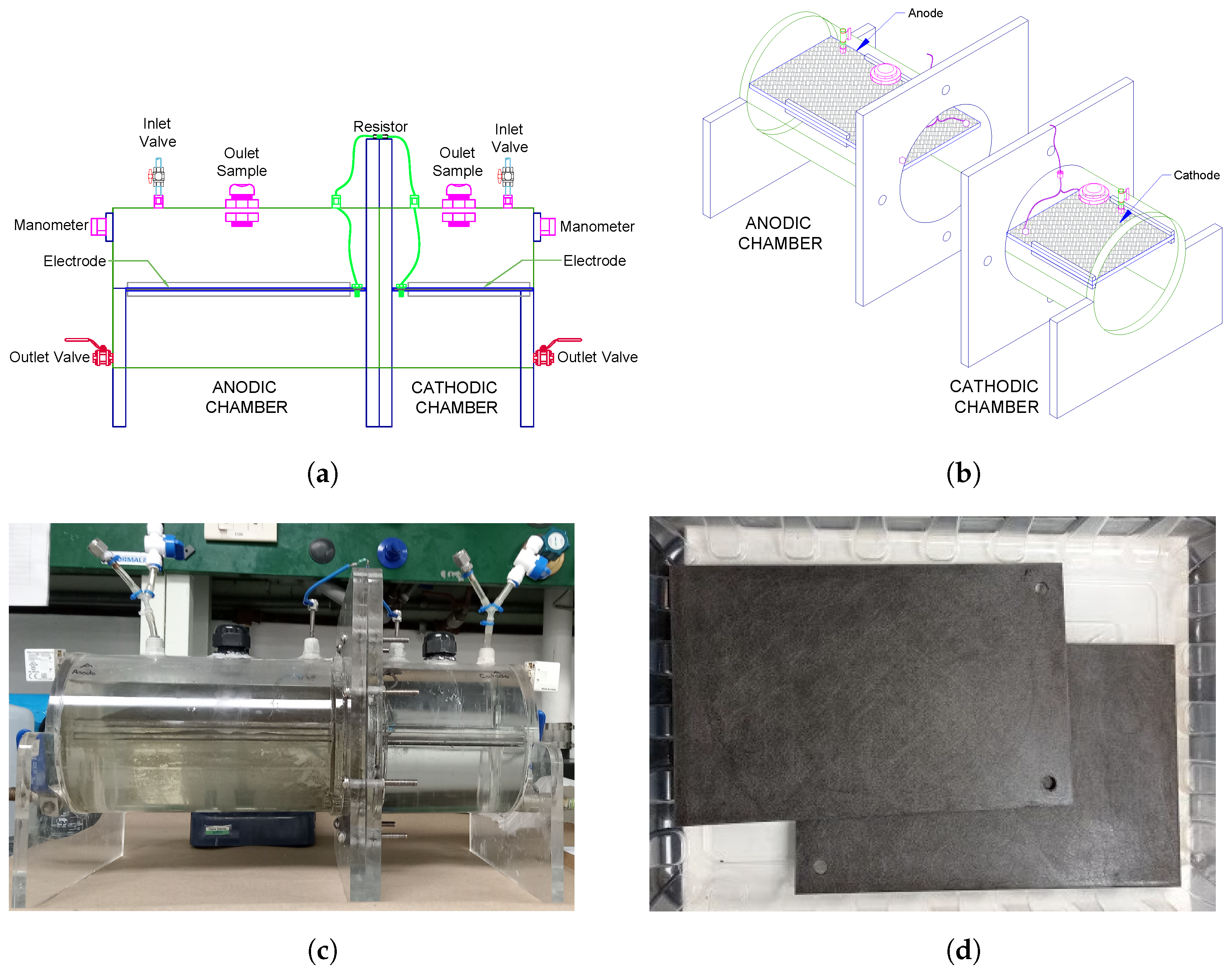
2.2. Experimental Device
2.3. Inoculum Preparation
2.4. Evaluation of MFC Performance
3. Results and Discussion
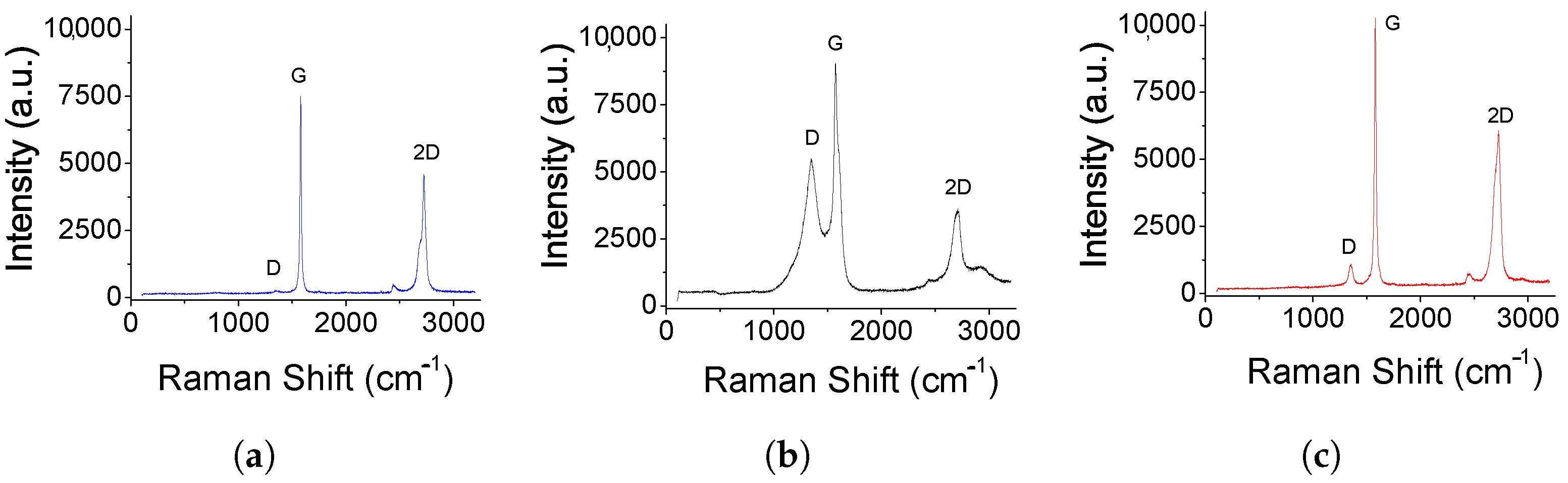
3.1. Characterization of the Electrode Materials
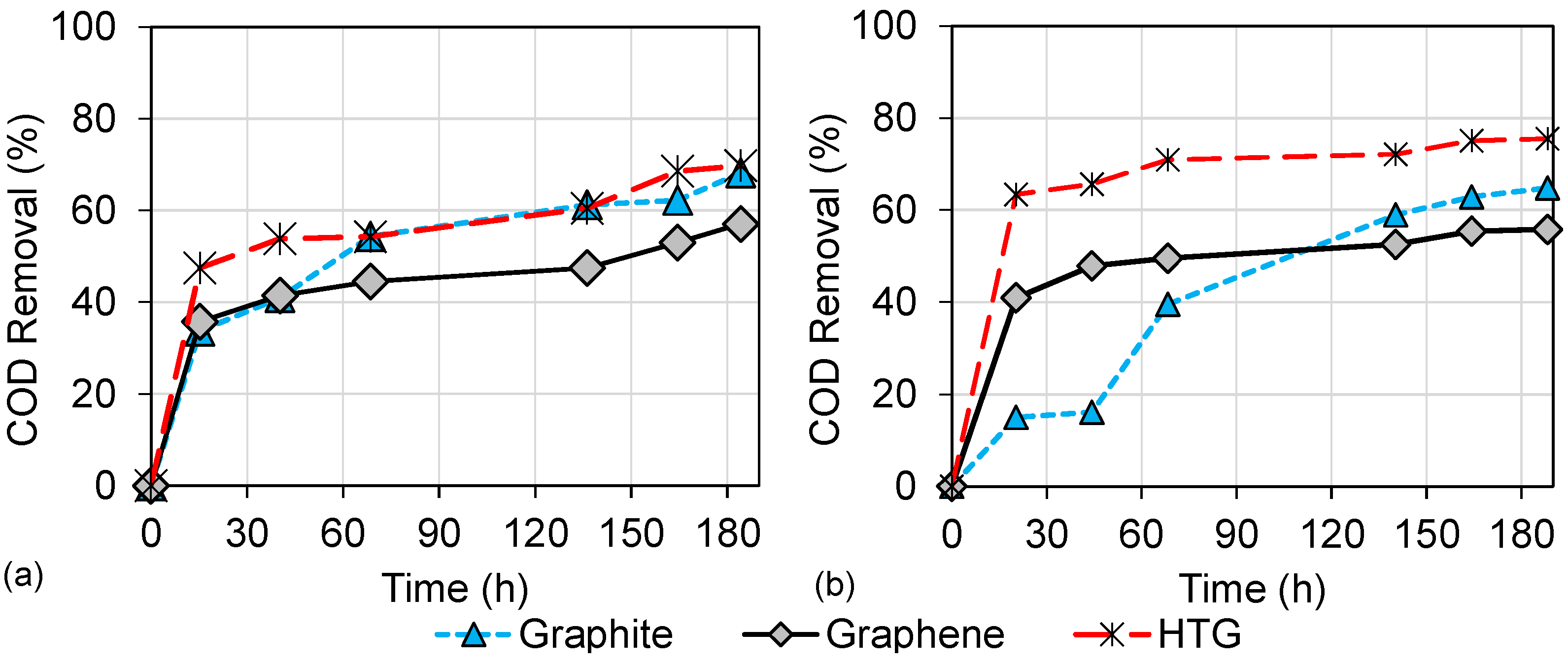
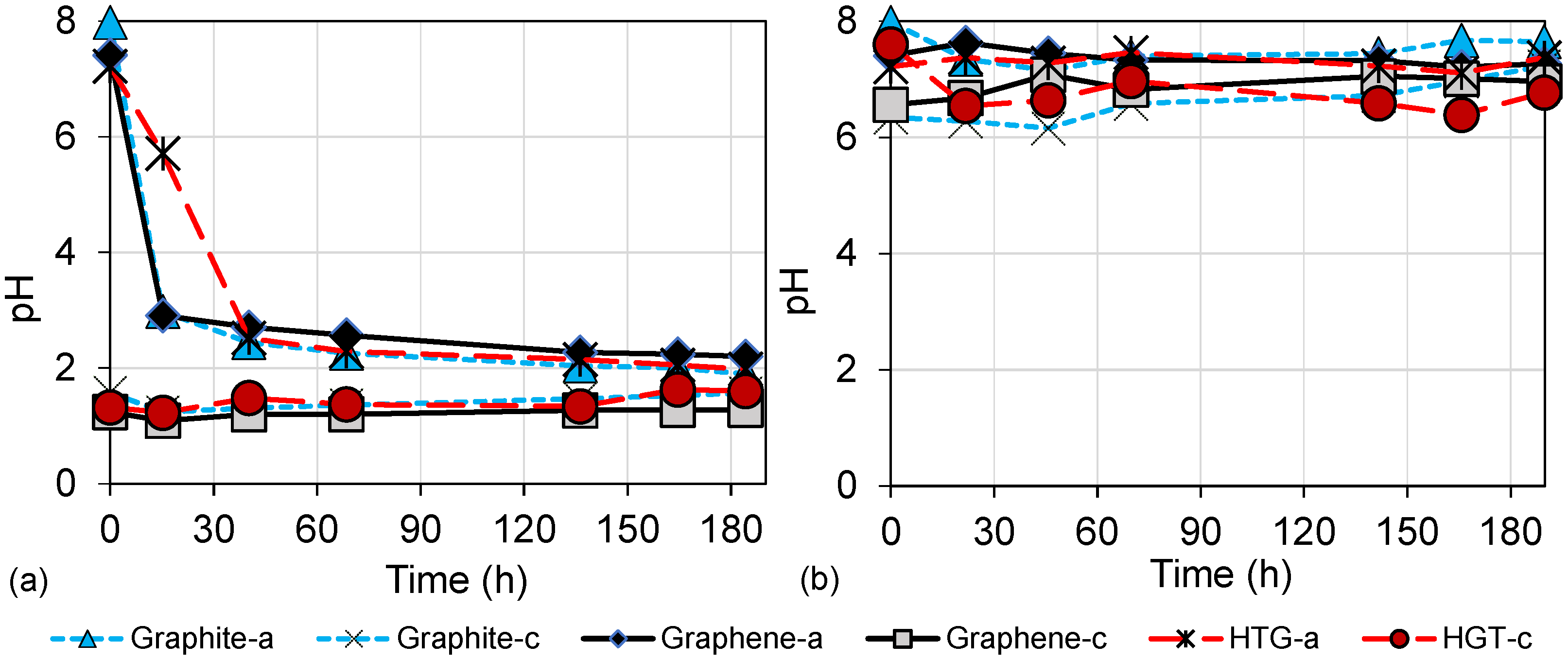
3.2. Wastewater Biodegradation
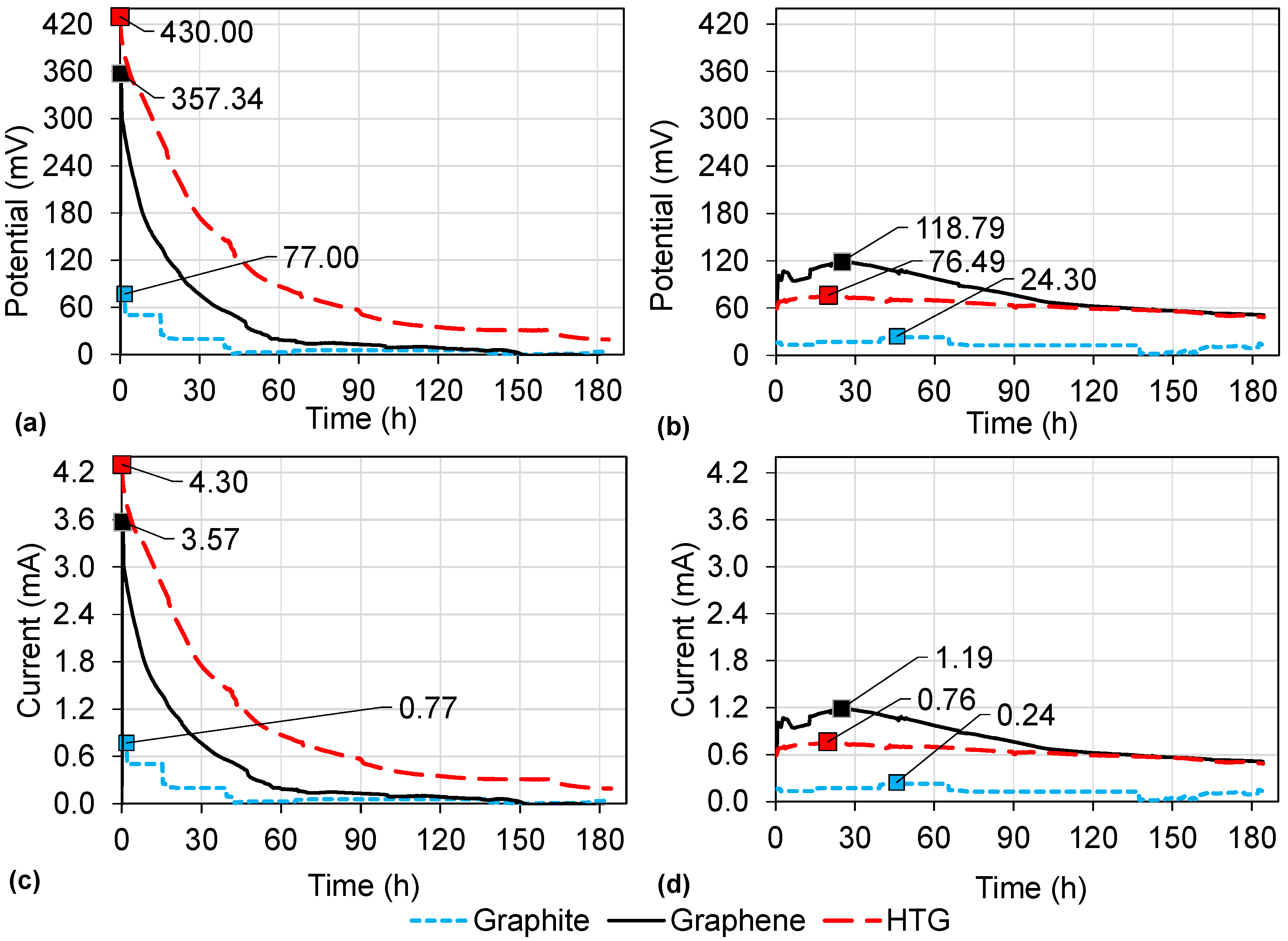
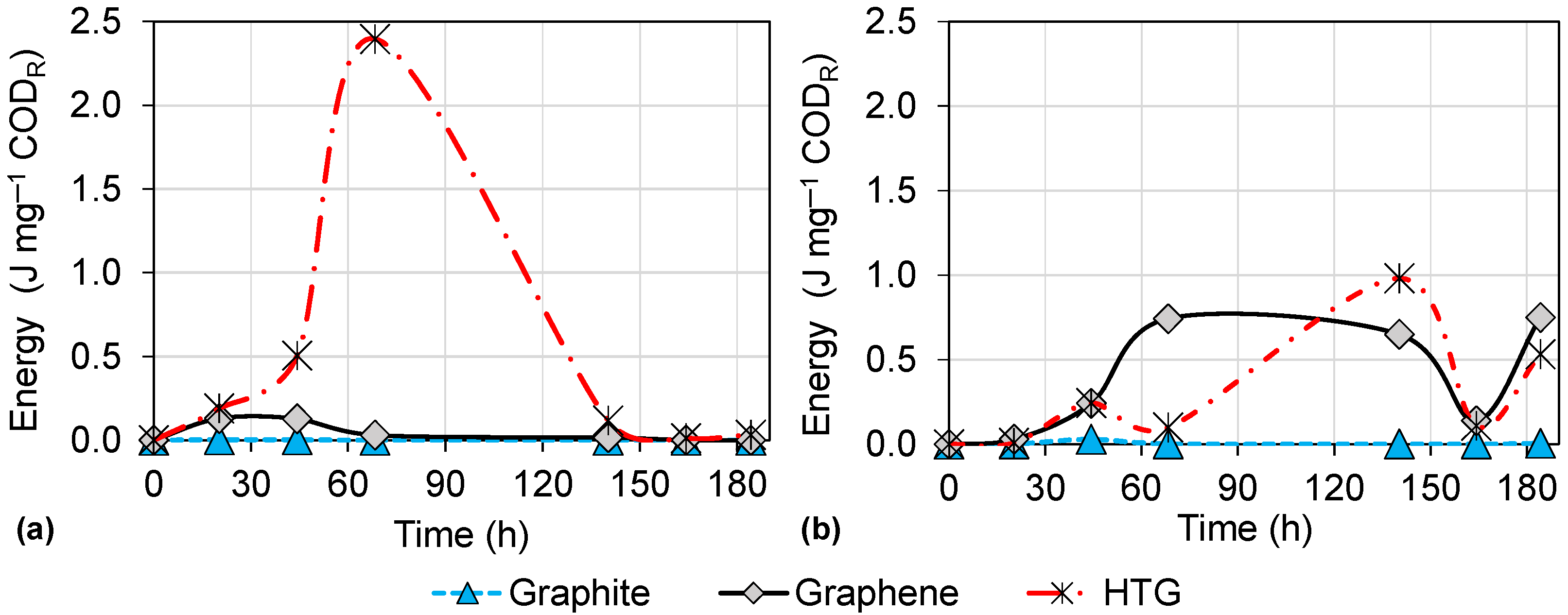
3.3. Energy Production in DC-MFCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HTG | Hydrophilic-treated graphene |
| DC-MFC | Dual chamber microbial fuel cell |
| HCl | Hydrochloric acid |
| COD | Chemical Oxygen Demand |
| CE | Coulombic efficiency |
| Ti | Titanium |
References
- Yang, Y.; Liu, T.; Zhu, X.; Zhang, F.; Ye, D.; Liao, Q.; Li, Y. Boosting Power Density of Microbial Fuel Cells with 3D Nitrogen-Doped Graphene Aerogel Electrode. Adv. Sci. 2016, 3, 1600097. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Min, B.; Logan, B.E. Continuous Electricity Generation from Domestic Wastewater and Organic Substrates in a Flat Plate Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef] [PubMed]
- Menicucci, J.; Beyenal, H.; Marsili, E.; Veluchamy; Demir, G.; Lewandowski, Z. Procedure for Determining Maximum Sustainable Power Generated by Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 1062–1068. [Google Scholar] [CrossRef]
- González del Campo, A.; Cañizares, P.; Lobato, J.; Rodrigo, M.; Fernandez Morales, F.J. Effects of External Resistance on Microbial Fuel Cell’s Performance; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Song, T.S.; Yan, Z.S.; Zhao, Z.W.; Jiang, H.L. Removal of organic matter in freshwater sediment by microbial fuel cells at various external resistances. J. Chem. Technol. Biotechnol. 2010, 85, 1489–1493. [Google Scholar] [CrossRef]
- Borja-Maldonado, F.; Zavala, M.Á.L. Contribution of configurations, electrode and membrane materials, electron transfer mechanisms, and cost of components on the current and future development of microbial fuel cells. Heliyon 2022, 8, e09849. [Google Scholar] [CrossRef] [PubMed]
- Pavoski, G.; Maraschin, T.; Fim, F.d.C.; Balzaretti, N.M.; Galland, G.B.; Moura, C.S.; de Souza Basso, N.R. Few Layer Reduced Graphene Oxide: Evaluation of the Best Experimental Conditions for Easy Production. Mater. Res. 2016, 20, 53–61. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Losic, D.; Saint, C.P.; Pant, D. Applications of graphene in microbial fuel cells: The gap between promise and reality. Renew. Sustain. Energy Rev. 2017, 72, 1389–1403. [Google Scholar] [CrossRef]
- Tang, J.; Yuan, Y.; Liu, T.; Zhou, S. High-capacity carbon-coated titanium dioxide core-shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J. Power Sources 2015, 274, 170–176. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Fan, Z.; Liu, J.; Zhang, H. Graphene-Based Electrodes. Adv. Mater. 2012, 24, 5979–6004. [Google Scholar] [CrossRef]
- Lawson, K.; Rossi, R.; Regan, J.M.; Logan, B.E. Impact of cathodic electron acceptor on microbial fuel cell internal resistance. Bioresour. Technol. 2020, 316, 123919. [Google Scholar] [CrossRef]
- Jadhav, D.; Ghadge, A.; Mondal, D.; Ghangrekar, M. Comparison of oxygen and hypochlorite as cathodic electron acceptor in microbial fuel cells. Bioresour. Technol. 2014, 154, 330–335. [Google Scholar] [CrossRef]
- Dai, H.; Yang, H.; Liu, X.; Zhao, Y.; Liang, Z. Performance of sodium bromate as cathodic electron acceptor in microbial fuel cell. Bioresour. Technol. 2016, 202, 220–225. [Google Scholar] [CrossRef] [PubMed]
- López Zavala, M.; Torres Delenne, P.; González Peña, O. Improvement of Wastewater Treatment Performance and Power Generation in Microbial Fuel Cells by Enhancing Hydrolysis and Acidogenesis, and by Reducing Internal Losses. Energies 2018, 11, 2309. [Google Scholar] [CrossRef]
- Kim, C.; Lee, C.R.; Song, Y.E.; Heo, J.; Choi, S.M.; Lim, D.H.; Cho, J.; Park, C.; Jang, M.; Kim, J.R. Hexavalent chromium as a cathodic electron acceptor in a bipolar membrane microbial fuel cell with the simultaneous treatment of electroplating wastewater. Chem. Eng. J. 2017, 328, 703–707. [Google Scholar] [CrossRef]
- Napoli, L.; Lavorante, M.; Franco, J.; Sanguinetti, A.; Fasoli, H. Effects on Nafion® 117 Membrane using Different Strong Acids in Various Concentrations. J. New Mater. Electrochem. Syst. 2013, 16, 151–156. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Kabiri, K.; Solati-Hashjin, M.; Moaddel, H. Novel nanocomposite proton exchange membranes based on Nafion® and AMPS-modified montmorillonite for fuel cell applications. J. Membr. Sci. 2010, 365, 286–293. [Google Scholar] [CrossRef]
- Rodrigo, M.; Cañizares, P.; Lobato, J.; Paz, R.; Sáez, C.; Linares, J. Production of electricity from the treatment of urban waste water using a microbial fuel cell. J. Power Sources 2007, 169, 198–204. [Google Scholar] [CrossRef]
- Eaton, A.; Clesceri, L.; Franson, M.; Association, American Public Health; Rice, E.; Greenberg, A.; Association, American Water Works; Federation, Water Environment. Standard Methods for the Examination of Water & Wastewater; Number v. 21 in Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Siburian, R.; Sihotang, H.; Lumban Raja, S.; Supeno, M.; Simanjuntak, C. New Route to Synthesize of Graphene Nano Sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef]
- Kumara, G.R.A.; Pitawala, H.M.G.T.A.; Karunarathne, B.; Mantilaka, M.M.M.G.P.G.; Rajapakse, R.M.G.; Huang, H.H.; De Silva, K.K.H.; Yoshimura, M. Development of a chemical-free floatation technology for the purification of vein graphite and characterization of the products. Sci. Rep. 2021, 11, 22713. [Google Scholar] [CrossRef]
- Zhao, C.e.; Wang, W.J.; Sun, D.; Wang, X.; Zhang, J.R.; Zhu, J.J. Nanostructured Graphene/TiO2 Hybrids as High-Performance Anodes for Microbial Fuel Cells. Chem. Eur. J. 2014, 20, 7091–7097. [Google Scholar] [CrossRef]
- Polverino, S.; Del Rio Castillo, A.E.; Brencich, A.; Marasco, L.; Bonaccorso, F.; Morbiducci, R. Few-Layers Graphene-Based Cement Mortars: Production Process and Mechanical Properties. Sustainability 2022, 14, 784. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A. Chemical Functionalization of Graphene Family Members. Phys. Sci. Rev. 2017, 2, 20160103. [Google Scholar] [CrossRef]
- Wan, X.; Lu, H.; Kang, J.; Li, S.; Yue, Y. Preparation of graphene-glass fiber-resin composites and its electromagnetic shielding performance. Compos. Interfaces 2018, 25, 883–900. [Google Scholar] [CrossRef]
- Tatarova, E.; Dias, A.; Henriques, J.; Abrashev, M.; Bundaleska, N.; Kovacevic, E.; Bundaleski, N.; Cvelbar, U.; Valcheva, E.; Arnaudov, B.; et al. Towards large-scale in free-standing graphene and N-graphene sheets. Sci. Rep. 2017, 7, 10175. [Google Scholar] [CrossRef]
- Rao, K.S.; Senthilnathan, J.; Liu, Y.F.; Yoshimura, M. Role of peroxide ions in formation of graphene nanosheets by electrochemical exfoliation of graphite. Sci. Rep. 2014, 4, 4237. [Google Scholar] [CrossRef]
- Cai, T.; Jiang, N.; Zhen, G.; Meng, L.; Song, J.; Chen, G.; Liu, Y.; Huang, M. Simultaneous energy harvest and nitrogen removal using a supercapacitor microbial fuel cell. Environ. Pollut. 2020, 266, 115154. [Google Scholar] [CrossRef] [PubMed]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef]
- Feng, H.; Liang, Y.; Guo, K.; Chen, W.; Shen, D.; Huang, L.; Zhou, Y.; Wang, M.; Long, Y. TiO2 Nanotube Arrays Modified Titanium: A Stable, Scalable, and Cost-Effective Bioanode for Microbial Fuel Cells. Environ. Sci. Technol. Lett. 2016, 3, 420–424. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.; Ajayi, F.F.; Park, W.; Chang, I.S.; Kim, I.S. Mass Transport through a Proton Exchange Membrane (Nafion) in Microbial Fuel Cells. Energy Fuels 2008, 22, 169–176. [Google Scholar] [CrossRef]
- Kalaiselvan, N.; Glivin, G.; Bakthavatsalam, A.; Mariappan, V.; Premalatha, M.; Raveendran, P.S.; Jayaraj, S.; Sekhar, S.J. A waste to energy technology for Enrichment of biomethane generation: A review on operating parameters, types of biodigesters, solar assisted heating systems, socio economic benefits and challenges. Chemosphere 2022, 293, 133486. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Nelson, B. Invited review: Anaerobic fermentation of dairy food wastewater. J. Dairy Sci. 2012, 95, 6188–6203. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Christel, S.; Roman, P.; Wong, Z.L.; Bijmans, M.F.; Dopson, M. Electricity generation from an inorganic sulfur compound containing mining wastewater by acidophilic microorganisms. Res. Microbiol. 2016, 167, 568–575. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH Measurement and Homeostasis in Bacteria and Archaea. Adv. Microb. Physiol. 2009, 55, 317. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, B.; Yanagawa, K.; Ha, N.T.; Goel, R.; Terashima, M.; Yasui, H. Effects of low pH conditions on decay of methanogenic biomass. Water Res. 2020, 179, 115883. [Google Scholar] [CrossRef]
- EG & G Technical Servoces, Inc. Fuel Cell Handbook, 7th ed.; National Technical Information Service, Virginia, U.S. Department of Commerce: Springfield, VA, USA, 2004; p. 427.
- Lew, K. Acids and Bases; Essential Chemistry; Chelsea House: New York, NY, USA, 2009. [Google Scholar]
- Ge, Z.; Zhang, F.; Grimaud, J.; Hurst, J.; He, Z. Long-term investigation of microbial fuel cells treating primary sludge or digested sludge. Bioresour. Technol. 2013, 136, 509–514. [Google Scholar] [CrossRef]
- Jang, J.K.; Pham, T.H.; Chang, I.S.; Kang, K.H.; Moon, H.; Cho, K.S.; Kim, B.H. Construction and operation of a novel mediator- and membrane-less microbial fuel cell. Process Biochem. 2004, 39, 1007–1012. [Google Scholar] [CrossRef]
- Jannelli, N.; Anna Nastro, R.; Cigolotti, V.; Minutillo, M.; Falcucci, G. Low pH, high salinity: Too much for microbial fuel cells? Appl. Energy 2017, 192, 543–550. [Google Scholar] [CrossRef]
- Dopson, M.; Ni, G.; Sleutels, T.H. Possibilities for extremophilic microorganisms in microbial electrochemical systems. FEMS Microbiol. Rev. 2016, 40, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Borole, A.P.; O’Neill, H.; Tsouris, C.; Cesar, S. A microbial fuel cell operating at low pH using the acidophile Acidiphilium cryptum. Biotechnol. Lett. 2008, 30, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, B.; Kim, J.; Lee, T.; Yu, J. Electricity generation and microbial community in microbial fuel cell using low-pH distillery wastewater at different external resistances. J. Biotechnol. 2014, 186, 175–180. [Google Scholar] [CrossRef] [PubMed]


| Sc | Material | Catholyte | Voltage | Current | Density Power | COD | |
|---|---|---|---|---|---|---|---|
| Anode | Cathode | mV | (mA) | mW·m | Removal (%) | ||
| 1 | Graphite | Graphite | HCl | 77.00 | 0.770 | 1.002 | 68.19 |
| 2 | Graphene | Graphene | HCl | 357.34 | 3.573 | 22.147 | 57.09 |
| 3 | HTG | HTG | HCl | 430.00 | 4.300 | 32.070 | 69.78 |
| 4 | Graphite | Graphite | DW | 24.30 | 0.243 | 0.102 | 64.81 |
| 5 | Graphene | Graphene | DW | 118.79 | 1.188 | 2.448 | 55.85 |
| 6 | HTG | HTG | DW | 74.90 | 0.749 | 0.973 | 75.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borja-Maldonado, F.; López Zavala, M.Á. Assessment of Graphite, Graphene, and Hydrophilic-Treated Graphene Electrodes to Improve Power Generation and Wastewater Treatment in Microbial Fuel Cells. Bioengineering 2023, 10, 378. https://doi.org/10.3390/bioengineering10030378
Borja-Maldonado F, López Zavala MÁ. Assessment of Graphite, Graphene, and Hydrophilic-Treated Graphene Electrodes to Improve Power Generation and Wastewater Treatment in Microbial Fuel Cells. Bioengineering. 2023; 10(3):378. https://doi.org/10.3390/bioengineering10030378
Chicago/Turabian StyleBorja-Maldonado, Fátima, and Miguel Ángel López Zavala. 2023. "Assessment of Graphite, Graphene, and Hydrophilic-Treated Graphene Electrodes to Improve Power Generation and Wastewater Treatment in Microbial Fuel Cells" Bioengineering 10, no. 3: 378. https://doi.org/10.3390/bioengineering10030378
APA StyleBorja-Maldonado, F., & López Zavala, M. Á. (2023). Assessment of Graphite, Graphene, and Hydrophilic-Treated Graphene Electrodes to Improve Power Generation and Wastewater Treatment in Microbial Fuel Cells. Bioengineering, 10(3), 378. https://doi.org/10.3390/bioengineering10030378