Host Immune Regulation in Implant-Associated Infection (IAI): What Does the Current Evidence Provide Us to Prevent or Treat IAI?
Abstract
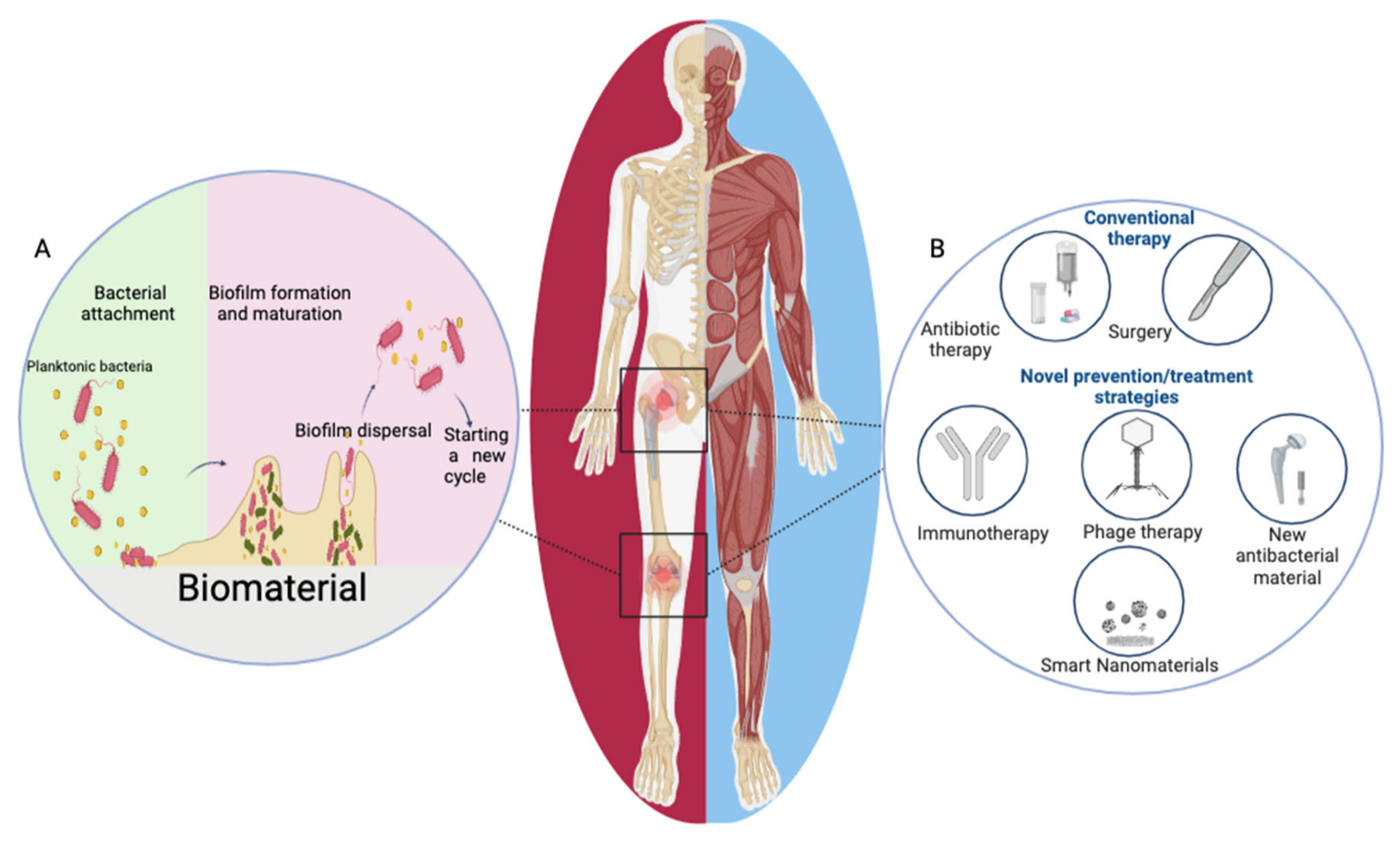
1. Introduction
2. Challenges in the Diagnosis and Treatment of IAI
3. The Role of Host Immunity in IAI
3.1. Impact of Prostheses on Host Immune Status
3.2. Biofilm Formation on Implants
3.3. Immune Evasion in Biofilm Infection
4. Role of Main Host Immune Cells in IAI
4.1. Neutrophils and Macrophages
4.2. Myeloid-Derived Suppressor Cells (MDSCs)
4.3. T Lymphocytes and Other Immune Cells
5. Prevention and Treatment of IAI
5.1. Antibacterial Materials and Immune-Evasive Coatings for Orthopedic Implants
5.2. Nanomaterials in IAI Prevention
5.3. Phage Therapy in IAI
5.4. Immune Modulation in IAI
5.4.1. Monoclonal Antibodies (mAbs)
5.4.2. Immune Checkpoint Molecules
5.4.3. Cytokine Modulation
6. Conclusions Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.-J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic Burden of Periprosthetic Joint Infection in the United States. J. Arthroplast. 2012, 27, 61–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Rosman, C.W.K.; van Dijl, J.M.; Sjollema, J. Interactions between the foreign body reaction and Staphylococcus aureus biomaterial-associated infection. Winning strategies in the derby on biomaterial implant surfaces. Crit. Rev. Microbiol 2021, 48, 624–640. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef]
- VanEpps, J.S.; Younger, J.G. Implantable Device Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Uçkay, I.; Pittet, D.; Vaudaux, P.; Sax, H.; Lew, D.; Waldvogel, F. Foreign body infections due to Staphylococcus epidermidis. Ann. Med. 2009, 41, 109–119. [Google Scholar] [CrossRef]
- Shoji, M.M.; Chen, A.F. Biofilms in Periprosthetic Joint Infections: A Review of Diagnostic Modalities, Current Treatments, and Future Directions. J. Knee Surg. 2020, 33, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Dostert, M.; Belanger, C.R.; Hancock, R.E.W. Design and Assessment of Anti-Biofilm Peptides: Steps Toward Clinical Application. J. Innate Immun. 2019, 11, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.C.; Son, M.-S.; Chang, E.T.; Zimmerli, W.; Parvizi, J. Are We Winning or Losing the Battle With Periprosthetic Joint Infection: Trends in Periprosthetic Joint Infection and Mortality Risk for the Medicare Population. J. Arthroplast. 2018, 33, 3238–3245. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.K.; Zeng, I.; Ravi, S.; Zhu, M.; Vince, K.G.; Young, S.W. Periprosthetic Joint Infection Is the Main Cause of Failure for Modern Knee Arthroplasty: An Analysis of 11,134 Knees. Clin. Orthop. Relat. Res. 2017, 475, 2194–2201. [Google Scholar] [CrossRef]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Parvizi, J. Diagnosing periprosthetic joint infection: Has the era of the biomarker arrived? Clin. Orthop. Relat. Res. 2014, 472, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, P.D.; Benedetto, E.D.D.; Salviato, D.; Beltrame, A.; Gisonni, R.; Cainero, V.; Causero, A. Acute periprosthetic knee infection: Is there still a role for DAIR? Acta Biomed. 2017, 88, 84–91. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Beswick, A.D.; Whitehouse, M.R.; Wylde, V.; Blom, A.W. Debridement, antibiotics and implant retention for periprosthetic joint infections: A systematic review and meta-analysis of treatment outcomes. J. Infect. 2018, 77, 479–488. [Google Scholar] [CrossRef]
- Qasim, S.N.; Swann, A.; Ashford, R. The DAIR (debridement, antibiotics and implant retention) procedure for infected total knee replacement—A literature review. SICOT J. 2017, 3, 2. [Google Scholar] [CrossRef]
- Zhu, M.F.; Kim, K.; Cavadino, A.; Coleman, B.; Munro, J.T.; Young, S.W. Success Rates of Debridement, Antibiotics, and Implant Retention in 230 Infected Total Knee Arthroplasties: Implications for Classification of Periprosthetic Joint Infection. J. Arthroplast. 2021, 36, 305–310.e1. [Google Scholar] [CrossRef]
- Pangaud, C.; Ollivier, M.; Argenson, J.-N. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. EFORT Open Rev. 2019, 4, 495–502. [Google Scholar] [CrossRef]
- Wang, Q.; Goswami, K.; Kuo, F.-C.; Xu, C.; Tan, T.L.; Parvizi, J. Two-Stage Exchange Arthroplasty for Periprosthetic Joint Infection: The Rate and Reason for the Attrition After the First Stage. J. Arthroplast. 2019, 34, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.J.; Vegari, D.; Ho, A.; Zmistowski, B.; Parvizi, J. Two-stage Exchange Arthroplasty for Infected Total Knee Arthroplasty: Predictors of Failure. Clin. Orthop. Relat. Res. 2011, 469, 3049. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Doloff, J.C.; Veiseh, O.; Vegas, A.J.; Tam, H.H.; Farah, S.; Ma, M.; Li, J.; Bader, A.; Chiu, A.; Sadraei, A.; et al. Colony Stimulating Factor-1 Receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat. Mater. 2017, 16, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Kenneth Ward, W. A review of the foreign-body response to subcutaneously-implanted devices: The role of macrophages and cytokines in biofouling and fibrosis. J. Diabetes Sci. Technol. 2008, 2, 768–777. [Google Scholar] [CrossRef]
- Amin Yavari, S.; Castenmiller, S.M.; van Strijp, J.A.G.; Croes, M. Combating Implant Infections: Shifting Focus from Bacteria to Host. Adv. Mater. 2020, 32, e2002962. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Kubes, P. Cellular and molecular choreography of neutrophil recruitment to sites of sterile inflammation. J. Mol. Med. 2011, 89, 1079–1088. [Google Scholar] [CrossRef]
- Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. Neutrophils in Biomaterial-Guided Tissue Regeneration: Matrix Reprogramming for Angiogenesis. Tissue Eng. Part. B Rev. 2021, 27, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Si, H.-B.; Yang, T.-M.; Zeng, Y.; Zhou, Z.-K.; Pei, F.-X.; Lu, Y.-R.; Cheng, J.-Q.; Shen, B. Correlations between inflammatory cytokines, muscle damage markers and acute postoperative pain following primary total knee arthroplasty. BMC Musculoskelet. Disord. 2017, 18, 265. [Google Scholar] [CrossRef] [PubMed]
- Langkilde, A.; Jakobsen, T.L.; Bandholm, T.Q.; Eugen-Olsen, J.; Blauenfeldt, T.; Petersen, J.; Andersen, O. Inflammation and post-operative recovery in patients undergoing total knee arthroplasty-secondary analysis of a randomized controlled trial. Osteoarthr. Cartil. 2017, 25, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.E.; Yamada, K.J.; Fallet, R.; Odvody, J.; Schwarz, D.M.; Lyden, E.R.; Anderson, M.J.; Alter, R.; Vidlak, D.; Hartman, C.W.; et al. Orthopaedic Surgery Elicits a Systemic Anti-Inflammatory Signature. J. Clin. Med. 2020, 9, 2123. [Google Scholar] [CrossRef]
- Jubel, J.M.; Randau, T.M.; Becker-Gotot, J.; Scheidt, S.; Wimmer, M.D.; Kohlhof, H.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. sCD28, sCD80, sCTLA-4, and sBTLA Are Promising Markers in Diagnostic and Therapeutic Approaches for Aseptic Loosening and Periprosthetic Joint Infection. Front. Immunol. 2021, 12, 687065. [Google Scholar] [CrossRef]
- Zimmerli, W.; Waldvogel, F.A.; Vaudaux, P.; Nydegger, U.E. Pathogenesis of foreign body infection: Description and characteristics of an animal model. J. Infect. Dis. 1982, 146, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Lew, P.D.; Waldvogel, F.A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J. Clin. Investig. 1984, 73, 1191–1200. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Bernard, L.; Vaudaux, P.; Merle, C.; Stern, R.; Huggler, E.; Lew, D.; Hoffmeyer, P. The inhibition of neutrophil antibacterial activity by ultra-high molecular weight polyethylene particles. Biomaterials 2005, 26, 5552–5557. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends. Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Triffault-Fillit, C.; Ferry, T.; Laurent, F.; Pradat, P.; Dupieux, C.; Conrad, A.; Becker, A.; Lustig, S.; Fessy, M.H.; Chidiac, C.; et al. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: A prospective cohort study. Clin. Microbiol. Infect. 2019, 25, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Corvec, S.; Portillo, M.E.; Pasticci, B.M.; Borens, O.; Trampuz, A. Epidemiology and New Developments in the Diagnosis of Prosthetic Joint Infection. Int. J. Artif. Organs. 2012, 35, 923–934. [Google Scholar] [CrossRef]
- Tornero, E.; García-Oltra, E.; García-Ramiro, S.; Martínez-Pastor, J.C.; Bosch, J.; Climent, C.; Morata, L.; Camacho, P.; Mensa, J.; Soriano, A. Prosthetic joint infections due to Staphylococcus aureus and coagulase-negative staphylococci. Int. J. Artif. Organs. 2012, 35, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral. Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Prabhakara, R.; Harro, J.M.; Leid, J.G.; Harris, M.; Shirtliff, M.E. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect. Immun. 2011, 79, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Shahrour, H.; Dandache, I.; Martínez-López, A.L.; González-Gaitano, G.; Chokr, A.; Martínez-de-Tejada, G. An antibiotic potentiator retains its activity after being immobilized on silicone and prevents growth of multidrug-resistant Pseudomonas aeruginosa biofilms. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111876. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Yamada, K.J.; Kielian, T. Biofilm-Leukocyte Cross-Talk: Impact on Immune Polarization and Immunometabolism. J. Innate Immun. 2019, 11, 280–288. [Google Scholar] [CrossRef]
- Heim, C.E.; Vidlak, D.; Scherr, T.D.; Kozel, J.A.; Holzapfel, M.; Muirhead, D.E.; Kielian, T. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J. Immunol. 2014, 192, 3778–3792. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef]
- Heim, C.E.; Bosch, M.E.; Yamada, K.J.; Aldrich, A.L.; Chaudhari, S.S.; Klinkebiel, D.; Gries, C.M.; Alqarzaee, A.A.; Li, Y.; Thomas, V.C.; et al. Lactate production by Staphylococcus aureus biofilm inhibits HDAC11 to reprogramme the host immune response during persistent infection. Nat. Microbiol. 2020, 5, 1271–1284. [Google Scholar] [CrossRef]
- Kristian, S.A.; Birkenstock, T.A.; Sauder, U.; Mack, D.; Götz, F.; Landmann, R. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J. Infect. Dis. 2008, 197, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Rada, B. Interactions between Neutrophils and Pseudomonas aeruginosa in Cystic Fibrosis. Pathogens 2017, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Le, K.Y.; Khan, B.A.; Nguyen, T.H.; Hunt, R.L.; Bae, J.S.; Kabat, J.; Zheng, Y.; Cheung, G.Y.C.; Li, M.; et al. Resistance to leukocytes ties benefits of quorum-sensing dysfunctionality to biofilm infection. Nat. Microbiol. 2019, 4, 1114–1119. [Google Scholar] [CrossRef]
- Post, V.; Wahl, P.; Uçkay, I.; Ochsner, P.; Zimmerli, W.; Corvec, S.; Loiez, C.; Richards, R.G.; Moriarty, T.F. Phenotypic and genotypic characterisation of Staphylococcus aureus causing musculoskeletal infections. Int. J. Med. Microbiol. 2014, 304, 565–576. [Google Scholar] [CrossRef]
- Campoccia, D.; Testoni, F.; Ravaioli, S.; Cangini, I.; Maso, A.; Speziale, P.; Montanaro, L.; Visai, L.; Arciola, C.R. Orthopedic implant infections: Incompetence of Staphylococcus epidermidis, Staphylococcus lugdunensis, and Enterococcus faecalis to invade osteoblasts. J. Biomed. Mater. Res. A 2016, 104, 788–801. [Google Scholar] [CrossRef]
- Jhunjhunwala, S. Neutrophils at the Biological-Material Interface. ACS Biomater. Sci. Eng. 2018, 4, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- De Vor, L.; Rooijakkers, S.H.M.; van Strijp, J.A.G. Staphylococci evade the innate immune response by disarming neutrophils and forming biofilms. FEBS Lett. 2020, 594, 2556–2569. [Google Scholar] [CrossRef] [PubMed]
- Günther, F.; Wabnitz, G.H.; Stroh, P.; Prior, B.; Obst, U.; Samstag, Y.; Wagner, C.; Hänsch, G.M. Host defence against Staphylococcus aureus biofilms infection: Phagocytosis of biofilms by polymorphonuclear neutrophils (PMN). Mol. Immunol. 2009, 46, 1805–1813. [Google Scholar] [CrossRef]
- Kovtun, A.; Bergdolt, S.; Wiegner, R.; Radermacher, P.; Huber-Lang, M.; Ignatius, A. The crucial role of neutrophil granulocytes in bone fracture healing. Eur. Cell. Mater. 2016, 32, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhunwala, S.; Aresta-DaSilva, S.; Tang, K.; Alvarez, D.; Webber, M.J.; Tang, B.C.; Lavin, D.M.; Veiseh, O.; Doloff, J.C.; Bose, S.; et al. Neutrophil Responses to Sterile Implant Materials. PLoS ONE 2015, 10, e0137550. [Google Scholar] [CrossRef]
- White, P.C.; Chicca, I.J.; Cooper, P.R.; Milward, M.R.; Chapple, I.L.C. Neutrophil Extracellular Traps in Periodontitis: A Web of Intrigue. J. Dent. Res. 2016, 95, 26–34. [Google Scholar] [CrossRef]
- Ríos-López, A.L.; González, G.M.; Hernández-Bello, R.; Sánchez-González, A. Avoiding the trap: Mechanisms developed by pathogens to escape neutrophil extracellular traps. Microbiol. Res. 2021, 243, 126644. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Musselman, R.M.; Olivares-Navarrete, R. Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater. Sci. 2020, 8, 2289–2299. [Google Scholar] [CrossRef]
- Wantha, S.; Alard, J.-E.; Megens, R.T.A.; van der Does, A.M.; Döring, Y.; Drechsler, M.; Pham, C.T.N.; Wang, M.-W.; Wang, J.-M.; Gallo, R.L.; et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ. Res. 2013, 112, 792–801. [Google Scholar] [CrossRef]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.D.; Krupka, T.; Anderson, J.M. iNOS-mediated generation of reactive oxygen and nitrogen species by biomaterial-adherent neutrophils. J. Biomed. Mater. Res. A 2007, 80, 381–390. [Google Scholar] [CrossRef]
- Benoit, M.; Desnues, B.; Mege, J.-L. Macrophage polarization in bacterial infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef]
- Campoccia, D.; Mirzaei, R.; Montanaro, L.; Arciola, C.R. Hijacking of immune defences by biofilms: A multifront strategy. Biofouling 2019, 35, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Mège, J.-L.; Mehraj, V.; Capo, C. Macrophage Polarization and Bacterial Infections. Curr. Opin. Infect Dis. 2011, 24, 230–234. [Google Scholar] [CrossRef]
- Hanke, M.L.; Heim, C.E.; Angle, A.; Sanderson, S.D.; Kielian, T. Targeting Macrophage Activation for the Prevention and Treatment of Staphylococcus Aureus Biofilm Infections. J. Immunol. 2013, 190, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Pidwill, G.R.; Gibson, J.F.; Cole, J.; Renshaw, S.A.; Foster, S.J. The Role of Macrophages in Staphylococcus Aureus Infection. Front. Immunol. 2021, 11, 3506. [Google Scholar] [CrossRef]
- Brann, K.R.; Fullerton, M.S.; Onyilagha, F.I.; Prince, A.A.; Kurten, R.C.; Rom, J.S.; Blevins, J.S.; Smeltzer, M.S.; Voth, D.E. Infection of Primary Human Alveolar Macrophages Alters Staphylococcus aureus Toxin Production and Activity. Infect. Immun. 2019, 87, e00167-19. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ. Res. 2016, 119, 414–417. [Google Scholar] [CrossRef]
- Batista-Gonzalez, A.; Vidal, R.; Criollo, A.; Carreño, L.J. New Insights on the Role of Lipid Metabolism in the Metabolic Reprogramming of Macrophages. Front. Immunol. 2019, 10, 2993. [Google Scholar] [CrossRef]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Tacke, R.; Sun, J.; Uchiyama, S.; Polovina, A.; Nguyen, D.G.; Nizet, V. Protection Against Lethal Multidrug-Resistant Bacterial Infections Using Macrophage Cell Therapy. Infect. Microbes Dis. 2019, 1, 61–69. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.E.; Vidlak, D.; Odvody, J.; Hartman, C.W.; Garvin, K.L.; Kielian, T. Human prosthetic joint infections are associated with myeloid-derived suppressor cells (MDSCs): Implications for infection persistence. J. Orthop. Res. 2018, 36, 1605–1613. [Google Scholar] [CrossRef]
- Young, M.R.; Newby, M.; Wepsic, H.T. Hematopoiesis and Suppressor Bone Marrow Cells in Mice Bearing Large Metastatic Lewis Lung Carcinoma Tumors. Cancer Res. 1987, 47, 100–105. [Google Scholar]
- Youn, J.-I.; Gabrilovich, D.I. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 2010, 40, 2969–2975. [Google Scholar] [CrossRef]
- Bergenfelz, C.; Leandersson, K. The Generation and Identity of Human Myeloid-Derived Suppressor Cells. Front. Oncol. 2020, 10, 109. [Google Scholar] [CrossRef]
- Medina, E.; Hartl, D. Myeloid-Derived Suppressor Cells in Infection: A General Overview. J. Innate Immun. 2018, 10, 407–413. [Google Scholar] [CrossRef]
- Peng, K.-T.; Hsieh, C.-C.; Huang, T.-Y.; Chen, P.-C.; Shih, H.-N.; Lee, M.S.; Chang, P.-J. Staphylococcus aureus biofilm elicits the expansion, activation and polarization of myeloid-derived suppressor cells in vivo and in vitro. PLoS ONE 2017, 12, e0183271. [Google Scholar] [CrossRef]
- Heim, C.E.; Vidlak, D.; Scherr, T.D.; Hartman, C.W.; Garvin, K.L.; Kielian, T. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J. Immunol. 2015, 194, 3861–3872. [Google Scholar] [CrossRef]
- Korn, M.F.; Stein, R.R.; Dolf, A.; Shakeri, F.; Buness, A.; Hilgers, C.; Masson, W.; Gravius, S.; Kohlhof, H.; Burger, C.; et al. High-Dimensional Analysis of Immune Cell Composition Predicts Periprosthetic Joint Infections and Dissects Its Pathophysiology. Biomedicines 2020, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Watters, C.; Fleming, D.; Bishop, D.; Rumbaugh, K.P. Host Responses to Biofilm. Prog. Mol. Biol. Transl. Sci. 2016, 142, 193–239. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.K.; Jensen, H.E.; Koch, J.; Bjarnsholt, T.; Eickhardt, S.; Shirtliff, M. Specific Antibodies to Staphylococcus aureus Biofilm Are Present in Serum from Pigs with Osteomyelitis. In Vivo 2015, 29, 555–560. [Google Scholar]
- Prabhakara, R.; Harro, J.M.; Leid, J.G.; Keegan, A.D.; Prior, M.L.; Shirtliff, M.E. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect. Immun. 2011, 79, 5010–5018. [Google Scholar] [CrossRef]
- Vantucci, C.E.; Ahn, H.; Fulton, T.; Schenker, M.L.; Pradhan, P.; Wood, L.B.; Guldberg, R.E.; Roy, K.; Willett, N.J. Development of systemic immune dysregulation in a rat trauma model of biomaterial-associated infection. Biomaterials 2020, 264, 120405. [Google Scholar] [CrossRef] [PubMed]
- Seebach, E.; Kubatzky, K.F. Chronic Implant-Related Bone Infections-Can Immune Modulation be a Therapeutic Strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, E.S.; Mellman, I. Cell biology of antigen processing in vitro and in vivo. Annu Rev. Immunol. 2005, 23, 975–1028. [Google Scholar] [CrossRef]
- Muthukrishnan, G.; Masters, E.A.; Daiss, J.L.; Schwarz, E.M. Mechanisms of Immune Evasion and Bone Tissue Colonization That Make Staphylococcus aureus the Primary Pathogen in Osteomyelitis. Curr. Osteoporos. Rep. 2019, 17, 395–404. [Google Scholar] [CrossRef]
- Goodyear, C.S.; Silverman, G.J. Death by a B cell superantigen: In vivo VH-targeted apoptotic supraclonal B cell deletion by a Staphylococcal Toxin. J. Exp. Med. 2003, 197, 1125–1139. [Google Scholar] [CrossRef]
- Ton-That, H.; Liu, G.; Mazmanian, S.K.; Faull, K.F.; Schneewind, O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. USA 1999, 96, 12424–12429. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-C.; Wu, T.-S.; Hsu, Y.-J.; Chang, C.-J.; Lin, C.-S.; Chia, J.-H.; Wu, T.-L.; Huang, T.-T.; Martel, J.; Ojcius, D.M.; et al. NK cells kill mycobacteria directly by releasing perforin and granulysin. J. Leukoc. Biol. 2014, 96, 1119–1129. [Google Scholar] [CrossRef]
- Endsley, J.J.; Torres, A.G.; Gonzales, C.M.; Kosykh, V.G.; Motin, V.L.; Peterson, J.W.; Estes, D.M.; Klimpel, G.R. Comparative antimicrobial activity of granulysin against bacterial biothreat agents. Open Microbiol. J. 2009, 3, 92–96. [Google Scholar] [CrossRef]
- Gonzales, C.M.; Williams, C.B.; Calderon, V.E.; Huante, M.B.; Moen, S.T.; Popov, V.L.; Baze, W.B.; Peterson, J.W.; Endsley, J.J. Antibacterial role for natural killer cells in host defense to Bacillus anthracis. Infect. Immun. 2012, 80, 234–242. [Google Scholar] [CrossRef]
- Theresine, M.; Patil, N.D.; Zimmer, J. Airway Natural Killer Cells and Bacteria in Health and Disease. Front. Immunol. 2020, 11, 585048. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The Multifaceted Biology of Plasmacytoid Dendritic Cells. Nat Rev Immunol 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.-H.L.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Armbruster, N.S.; Günter, M.; Biljecki, M.; Klenk, J.; Heumos, S.; Autenrieth, S.E. PSM Peptides from Community-Associated Methicillin-Resistant Staphylococcus aureus Impair the Adaptive Immune Response via Modulation of Dendritic Cell Subsets in vivo. Front. Immunol. 2019, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Balraadjsing, P.P.; de Jong, E.C.; Grijpma, D.W.; Tanck, M.W.; Zaat, S.A. Poly(trimethylene carbonate) and poly(D,L-lactic acid) modify human dendritic cell responses to staphylococci but do not affect Th1 and Th2 cell development. Eur. Cell Mater. 2018, 35, 103–116. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, S.; Qu, X.; Yue, B. Recent Advances in Research on Antibacterial Metals and Alloys as Implant Materials. Front. Cell. Infect. Microbiol. 2021, 11, 693939. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Zhu, C.; Zhang, Q.; Yu, J.; Wang, J.; Wang, Q.; Tang, J.; Zhou, H.; Shen, H. Advanced antibacterial activity of biocompatible tantalum nanofilm via enhanced local innate immunity. Acta Biomater. 2019, 89, 403–418. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Li, Y.; Gu, Y.-X.; Zhang, C.-N.; Lai, H.-C.; Shi, J.-Y. Ta-Coated Titanium Surface with Superior Bacteriostasis And Osseointegration. Int. J. Nanomed. 2019, 14, 8693–8706. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Song, J. Anti-periprosthetic infection strategies: From implant surface topographical engineering to smart drug-releasing coatings. ACS Appl. Mater. Interfaces 2021, 13, 20921–20937. [Google Scholar] [CrossRef]
- Chae, K.; Jang, W.Y.; Park, K.; Lee, J.; Kim, H.; Lee, K.; Lee, C.K.; Lee, Y.; Lee, S.H.; Seo, J. Antibacterial infection and immune-evasive coating for orthopedic implants. Sci. Adv. 2020, 6, eabb0025. [Google Scholar] [CrossRef]
- Mela, I.; Kaminski, C.F. Nano-vehicles give new lease of life to existing antimicrobials. Emerg. Top. Life Sci. 2020, 4, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Contera, S.; Bernardino de la Serna, J.; Tetley, T.D. Biotechnology, nanotechnology and medicine. Emerg. Top. Life Sci. 2020, 4, 551–554. [Google Scholar] [CrossRef]
- Kumar, M.; Curtis, A.; Hoskins, C. Application of Nanoparticle Technologies in the Combat against Anti-Microbial Resistance. Pharmaceutics 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.; Lawlor, A.; Whelan, A.; Regan, F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst 2008, 133, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Huo, S.; Tang, H.; Qu, X.; Yue, B. Smart Nanomaterials for Treatment of Biofilm in Orthopedic Implants. Front. Bioeng. Biotechnol. 2021, 9, 694635. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-J.; Hsu, S.-H.; Tsai, C.-L. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef]
- Nishanth, R.P.; Jyotsna, R.G.; Schlager, J.J.; Hussain, S.M.; Reddanna, P. Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: Role of ROS-NFκB signaling pathway. Nanotoxicology 2011, 5, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Genevière, J.; McCallin, S.; Huttner, A.; Pham, T.-T.; Suva, D. A systematic review of phage therapy applied to bone and joint infections: An analysis of success rates, treatment modalities and safety. EFORT Open Rev. 2021, 6, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Colak, M.; Yilmaz, B.C.; Ersoz, G.; Kutateladze, M.; Gozlugol, M. Bacteriophage therapy in implant-related infections: An experimental study. J. Bone Joint Surg. Am. 2013, 95, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Kelly, N.; Elliott, L.; Grant, A.; Wilkinson, M.; Hazratwala, K.; McEwen, P. Evaluation of Bacteriophage Anti-Biofilm Activity for Potential Control of Orthopedic Implant-Related Infections Caused by Staphylococcus aureus. Surg. Infect. (Larchmt.) 2019, 20, 16–24. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Xiao, C.; He, S.; Yao, H.; Bao, G. Efficacy of Phage Therapy in Controlling Rabbit Colibacillosis and Changes in Cecal Microbiota. Front. Microbiol. 2017, 8, 957. [Google Scholar] [CrossRef]
- Taha, M.; Abdelbary, H.; Ross, F.P.; Carli, A.V. New Innovations in the Treatment of PJI and Biofilms-Clinical and Preclinical Topics. Curr. Rev. Musculoskelet. Med. 2018, 11, 380–388. [Google Scholar] [CrossRef]
- De Vor, L.; van Dijk, B.; van Kessel, K.; Kavanaugh, J.S.; de Haas, C.; Aerts, P.C.; Viveen, M.C.; Boel, E.C.; Fluit, A.C.; Kwiecinski, J.M.; et al. Human monoclonal antibodies against Staphylococcus aureus surface antigens recognize in vitro and in vivo biofilm. eLife 2022, 11, e67301. [Google Scholar] [CrossRef]
- Raafat, D.; Otto, M.; Reppschläger, K.; Iqbal, J.; Holtfreter, S. Fighting Staphylococcus aureus biofilms with monoclonal antibodies. Trends Microbiol. 2019, 27, 303–322. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, M.; Yi, S.; Liu, S.; Li, B.; Yu, R.; Guo, Q.; Zhang, X.; Yu, C.; Li, J.; et al. Monoclonal Antibody Targeting Staphylococcus aureus Surface Protein A (SasA) Protect Against Staphylococcus aureus Sepsis and Peritonitis in Mice. PLoS ONE 2016, 11, e0149460. [Google Scholar] [CrossRef]
- Varshney, A.K.; Kuzmicheva, G.A.; Lin, J.; Sunley, K.M.; Bowling, R.A.; Kwan, T.-Y.; Mays, H.R.; Rambhadran, A.; Zhang, Y.; Martin, R.L.; et al. A natural human monoclonal antibody targeting Staphylococcus Protein A protects against Staphylococcus aureus bacteremia. PLoS ONE 2018, 13, e0190537. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, N.; Ishikawa, M.; Nishitani, K.; Beck, C.A.; Tsuchiya, H.; Mesfin, A.; Kates, S.L.; Daiss, J.L.; Xie, C.; Schwarz, E.M. Immunotherapy synergizes with debridement and antibiotic therapy in a murine 1-stage exchange model of MRSA implant-associated osteomyelitis. J. Orthop. Res. 2018, 36, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J.; Wherry, E.J. Combination Cancer Therapies with Immune Checkpoint Blockade: Convergence on Interferon Signaling. Cell 2016, 165, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Dyck, L.; Mills, K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Svabek, C.; Vazquez-Guillamet, C.; Sato, B.; Rasche, D.; Wilson, S.; Robbins, P.; Ulbrandt, N.; Suzich, J.; Green, J.; et al. Targeting the programmed cell death 1: Programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care 2014, 18, R3. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef]
- Andrews, L.P.; Yano, H.; Vignali, D.A.A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: Breakthroughs or backups. Nat. Immunol. 2019, 20, 1425–1434. [Google Scholar] [CrossRef]
- McCulloch, T.R.; Wells, T.J.; Souza-Fonseca-Guimaraes, F. Towards efficient immunotherapy for bacterial infection. Trends Microbiol. 2021, 30, 158–169. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Prince, N.; Penatzer, J.A.; Shackleford, T.L.; Stewart, E.K.; Dietz, M.J.; Boyd, J.W. Tissue-level cytokines in a rodent model of chronic implant-associated infection. J. Orthop Res. 2021, 39, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Prince, N.; Penatzer, J.A.; Dietz, M.J.; Boyd, J.W. Localized cytokine responses to total knee arthroplasty and total knee revision complications. J. Transl. Med. 2020, 18, 330. [Google Scholar] [CrossRef] [PubMed]
- Vaudaux, P.; Grau, G.E.; Huggler, E.; Schumacher-Perdreau, F.; Fiedler, F.; Waldvogel, F.A.; Lew, D.P. Contribution of tumor necrosis factor to host defense against staphylococci in a guinea pig model of foreign body infections. J. Infect. Dis. 1992, 166, 58–64. [Google Scholar] [CrossRef]
- Wang, Y.; Ashbaugh, A.G.; Dikeman, D.A.; Zhang, J.; Ackerman, N.E.; Kim, S.E.; Falgons, C.; Ortines, R.V.; Liu, H.; Joyce, D.P.; et al. Interleukin-1β and tumor necrosis factor are essential in controlling an experimental orthopedic implant-associated infection. J. Orthop. Res. 2020, 38, 1800–1809. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maimaiti, Z.; Li, Z.; Xu, C.; Fu, J.; Hao, L.-B.; Chen, J.-Y.; Chai, W. Host Immune Regulation in Implant-Associated Infection (IAI): What Does the Current Evidence Provide Us to Prevent or Treat IAI? Bioengineering 2023, 10, 356. https://doi.org/10.3390/bioengineering10030356
Maimaiti Z, Li Z, Xu C, Fu J, Hao L-B, Chen J-Y, Chai W. Host Immune Regulation in Implant-Associated Infection (IAI): What Does the Current Evidence Provide Us to Prevent or Treat IAI? Bioengineering. 2023; 10(3):356. https://doi.org/10.3390/bioengineering10030356
Chicago/Turabian StyleMaimaiti, Zulipikaer, Zhuo Li, Chi Xu, Jun Fu, Li-Bo Hao, Ji-Ying Chen, and Wei Chai. 2023. "Host Immune Regulation in Implant-Associated Infection (IAI): What Does the Current Evidence Provide Us to Prevent or Treat IAI?" Bioengineering 10, no. 3: 356. https://doi.org/10.3390/bioengineering10030356
APA StyleMaimaiti, Z., Li, Z., Xu, C., Fu, J., Hao, L.-B., Chen, J.-Y., & Chai, W. (2023). Host Immune Regulation in Implant-Associated Infection (IAI): What Does the Current Evidence Provide Us to Prevent or Treat IAI? Bioengineering, 10(3), 356. https://doi.org/10.3390/bioengineering10030356






