(Bio)printing in Personalized Medicine—Opportunities and Potential Benefits
Abstract
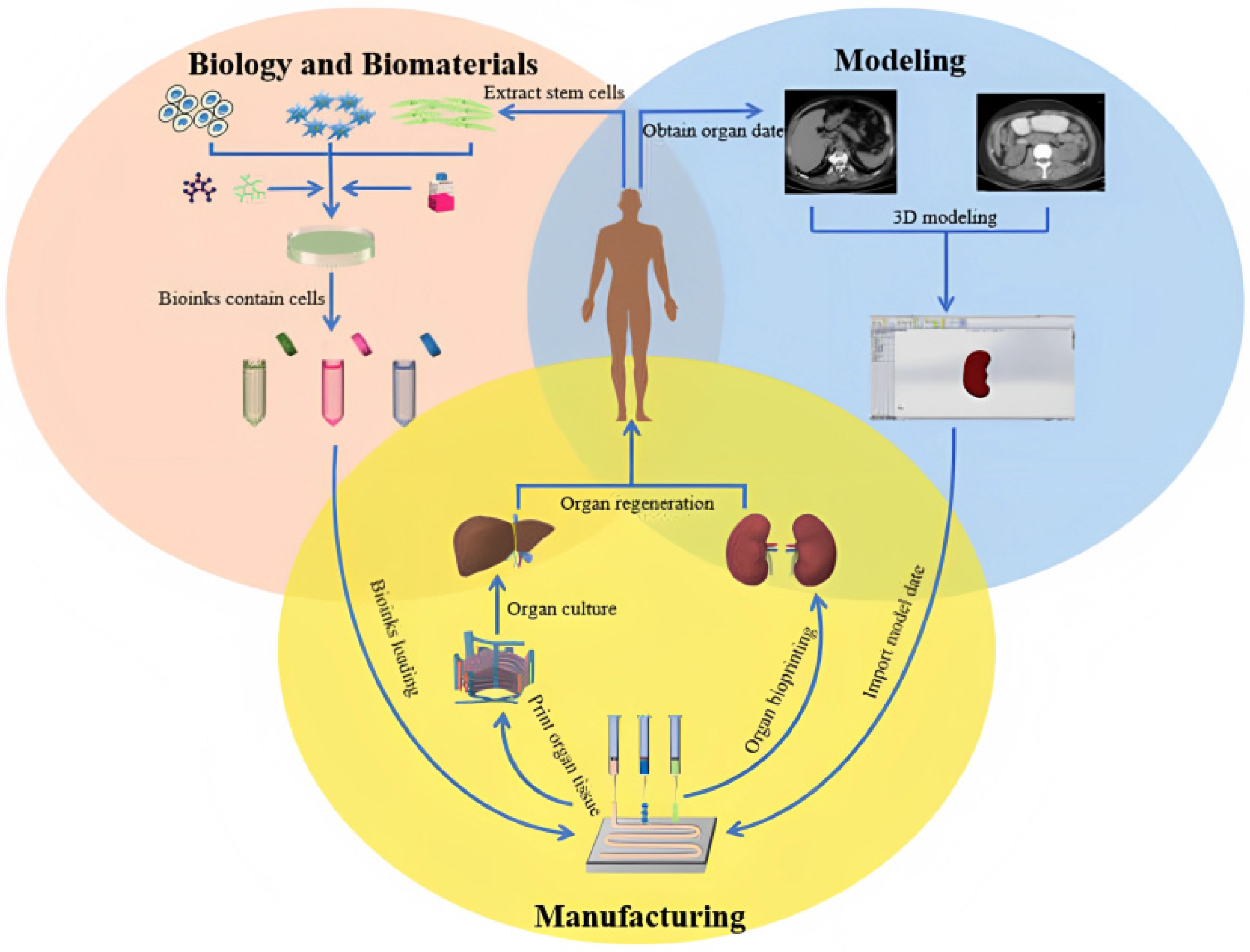
1. Introduction
2. Materials and Methods
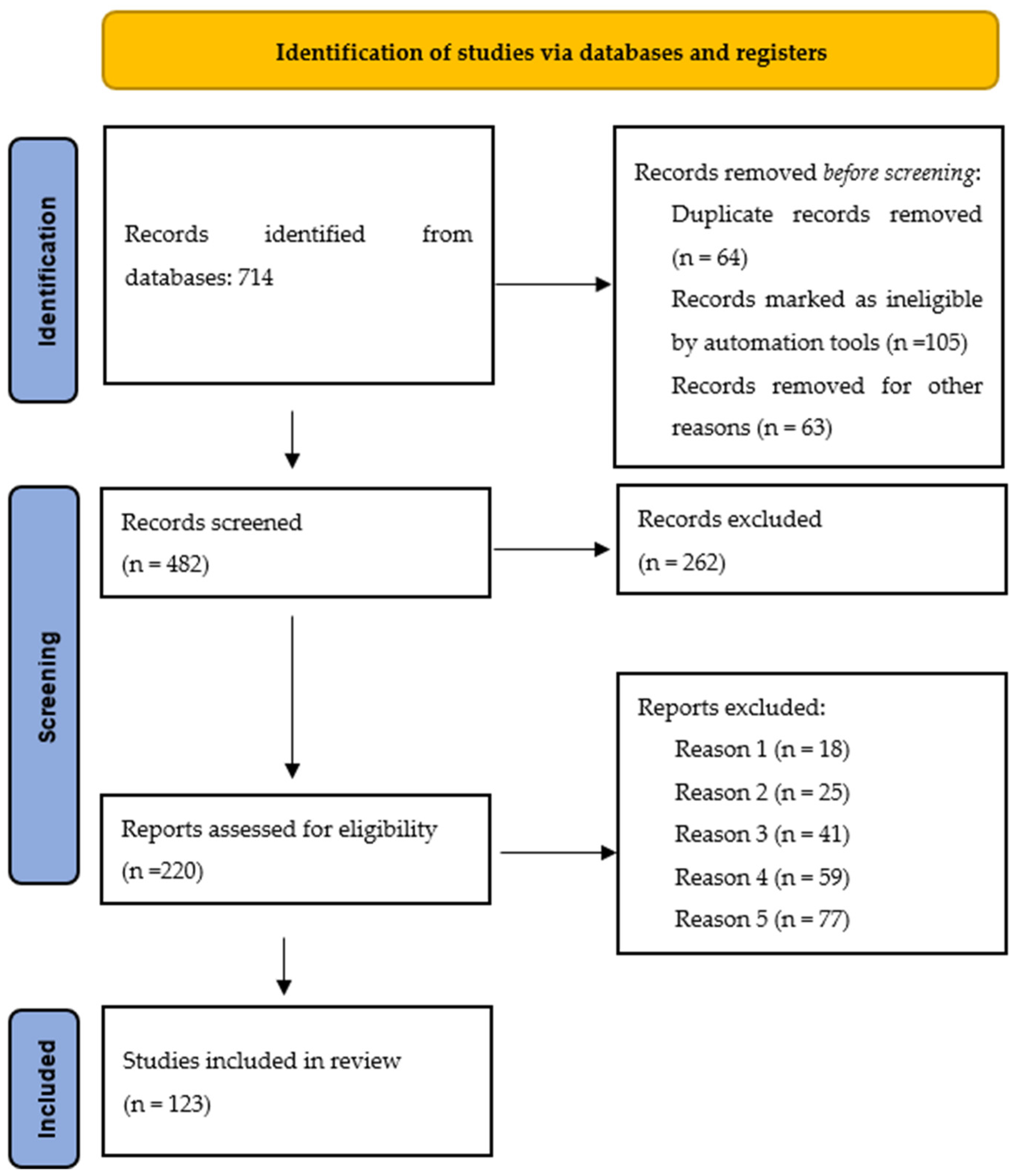
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Analysis
3. Results and Discussion
3.1. Benefits of Organ Transplantation
3.1.1. Transplant Organ Shortage
3.1.2. Overcoming Gender Differences in Transplantation
3.1.3. Probability of Reduction of Transplant Rejection
3.1.4. Removal of Congenital Defects of Various Tissues and Organs
3.2. Drug Research and Development
3.3. Surgery Planning and Medical Training for Young Doctors
4. Perspectives
5. Study Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Malkoc, V. Challenges and the future of 3D bioprinting. J. Biomed. Imaging Bioeng. 2018, 1, 62–63. [Google Scholar]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Kilova, K.; Milkov, D.; Mateva, N. Are personal digital devices applicable in healthcare? Health Policy Manag. 2019, 19, 324–325. [Google Scholar]
- Buonafede, F.; Felice, G.; Lamperti, F.; Piscitello, L. Additive manufacturing and global value chains: An empirical investigation at the country level. In Progress in International Business Research; Limited, E.P., Ed.; Emerald Publishing Limited: Bingley, UK, 2018; Volume 13, pp. 295–323. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Santos, T.G.; Miranda, R.M. Revisiting fundamental welding concepts to improve additive manufacturing: From theory to practice. Prog. Mater. Sci. 2020, 107, 107. [Google Scholar] [CrossRef]
- QUANTIFIER|English Meaning—Cambridge Dictionary. Available online: https://dictionary.cambridge.org/dictionary/english/bioprinting (accessed on 17 February 2023).
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Garreta, E.; Oria, R.; Tarantino, C.; Pla-Roca, M.; Prado, P.; Fernández-Avilés, F.; Campistol, J.M.; Samitier, J.; Montserrat, N. Tissue engineering by decellularization and 3D bioprinting. Mater. Today 2017, 20, 166–178. [Google Scholar] [CrossRef]
- Nasiri, S.; Khosravani, M.R. Progress and challenges in fabrication of wearable sensors for health monitoring. Sens. Actuators A Phys. 2020, 312, 112105. [Google Scholar] [CrossRef]
- Ahmad, I.; Hee, L.M.; Abdelrhman, A.M.; Imam, S.A.; Leong, M.S. Development, characterization, and power management of electromagnetic-type hybrid vibro-acoustic energy harvester for wireless sensor nodes. Sens. Actuators A Phys. 2023, 351, 114154. [Google Scholar] [CrossRef]
- Sukanya, V.S.; Nalinikanta, P.; Rath, S.N. Recent approaches in clinical applications of 3D printing in neonates and pediatrics. Eur. J. Pediatr. 2021, 180, 323–332. [Google Scholar] [CrossRef]
- Wagner, M.; Werther, T.; Unger, E.; Kasprian, G.; Dovjak, G.; Dorfer, C.; Schned, H.; Steinbauer, P.; Goeral, K.; Olischar, M.; et al. Development of a 3D printed patient-specific neonatal brain simulation model using multimodality imaging for perioperative management. Pediatr. Res. 2022, 91, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Elalouf, A. Immune response against the biomaterials used in 3D bioprinting of organs. Transpl. Immunol. 2021, 69, 101446. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Abraham, A.S.; Ibarra, C.; Agarwal, R.; Geibel, J.; Mulligan, D.C. 3D Bioprinting in Transplantation. In Technological Advances in Organ Transplantation; Springer: Cham, Switzerland, 2017; pp. 261–276. [Google Scholar] [CrossRef]
- Banerjee, D.; Singh, Y.P.; Datta, P.; Ozbolat, V.; O’Donnell, A.; Yeo, M.; Ozbolat, I.T. Strategies for 3D bioprinting of spheroids: A comprehensive review. Biomaterials 2022, 291, 121881. [Google Scholar] [CrossRef]
- Wu, Y.; Fuh, J.; Ozbolat, I.T. 3D Bioprinting in Tissue and Organ Regeneration; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–294. [Google Scholar] [CrossRef]
- Mayner, J.; Demeester, E.; Engler, A.J. Combining Genetic and Mechanical Factors to Model Disease. In Material-Based Mechanobiology; The Royal Society of Chemistry: London, UK, 2022; pp. 309–337. [Google Scholar] [CrossRef]
- Song, D.; Xu, Y.; Liu, S.; Wen, L.; Wang, X. Progress of 3D Bioprinting in Organ Manufacturing. Polymers 2021, 13, 3178. [Google Scholar] [CrossRef]
- Aldaadaa, A.; Owji, N.; Knowles, J. Three-dimensional Printing in Maxillofacial Surgery: Hype versus Reality. J. Tissue Eng. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Khatri, C.; Hanna, S.A.; Ashrafian, H.; Sarraf, K.M. Use of three-dimensional printing in preoperative planning in orthopaedic trauma surgery: A systematic review and meta-analysis. World J. Orthop. 2020, 11, 57–67. [Google Scholar] [CrossRef]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef]
- Chandrakar, B. (Bhumika) 3D Printing in Pharmaceutical and Medical Applications Recent Achievements and Challenges. Int. J. Health Sci. 2022, 6, 8109–8121. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Fern, J.L.C.; Kee, A.T.K.; Kou, J.; Jing, J.L.J.; Her, H.C.; Yong, H.S.; Ming, H.C.; Bhattamisra, S.K.; et al. 3D printing for oral drug delivery: A new tool to customize drug delivery. Drug Deliv. Transl. Res. 2020, 10, 986–1001. [Google Scholar] [CrossRef]
- Bhuskute, H.; Shende, P.; Prabhakar, B. 3D Printed Personalized Medicine for Cancer: Applications for Betterment of Diagnosis, Prognosis and Treatment. AAPS PharmSciTech 2022, 23, 8. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Vrana, N.E.; Gupta, S.; Mitra, K.; Rizvanov, A.A.; Solovyeva, V.V.; Antmen, E.; Salehi, M.; Ehterami, A.; Pourchet, L.; Barthes, J.; et al. From 3D printing to 3D bioprinting: The material properties of polymeric material and its derived bioink for achieving tissue specific architectures. Cell Tissue Bank. 2022, 23, 417–440. [Google Scholar] [CrossRef]
- Kumar, A.; Jacob, A. Techniques in scaffold fabrication process for tissue engineering applications: A review. J. Appl. Biol. Biotechnol. 2022, 10, 163–176. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef]
- Sánchez Rodríguez, D.A.; Ramos-Murillo, A.I.; Godoy-Silva, R.D. Tissue engineering, 3D-Bioprinting, morphogenesis modelling and simulation of biostructures: Relevance, underpinning biological principles and future trends. Bioprinting 2021, 24, e00171. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, L.; Ma, L.; Luo, Y.; Yang, H.; Cui, Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering 2019, 5, 777–794. [Google Scholar] [CrossRef]
- Mermin-Bunnell, A. Integrating Bioprinted Organs into Our Healthcare System. Intersect Stanf. J. Sci. Technol. Soc. 2020, 14, 1–16. [Google Scholar]
- Mapanao, A.K.; Voliani, V. Three-dimensional tumor models: Promoting breakthroughs in nanotheranostics translational research. Appl. Mater. Today 2020, 19, 100552. [Google Scholar] [CrossRef]
- Zhu, Y.; Kang, E.; Wilson, M.; Basso, T.; Chen, E.; Yu, Y.; Li, Y.-R. 3D Tumor Spheroid and Organoid to Model Tumor Microenvironment for Cancer Immunotherapy. Organoids 2022, 1, 149–167. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef]
- Prendergast, M.E.; Burdick, J.A. Recent Advances in Enabling Technologies in 3D Printing for Precision Medicine. Adv. Mater. 2020, 32, 1902516. [Google Scholar] [CrossRef]
- Mao, H.; Yang, L.; Zhu, H.; Wu, L.; Ji, P.; Yang, J.; Gu, Z. Recent advances and challenges in materials for 3D bioprinting. Prog. Nat. Sci. Mater. Int. 2020, 30, 618–634. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bastani, B. The present and future of transplant organ shortage: Some potential remedies. J. Nephrol. 2020, 33, 277–288. [Google Scholar] [CrossRef]
- Jiménez Oliver, K. Overview of Organ Donation. Mex. J. Med. Res. ICSA 2023, 11, 55–63. [Google Scholar] [CrossRef]
- Patil, R.Y.; Patil, Y.H.; Gupta, S.K.; Bannore, A. Organ Donation and Transplantation Challenges, Opportunities, and the Role of Blockchain. In Disruptive Developments in Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2022; pp. 325–339. [Google Scholar] [CrossRef]
- Mills, P.A.S.; Mills, D.K. Reduced supply in the organ donor market and how 3D printing can address this shortage: A critical inquiry into the collateral effects of driverless cars. Appl. Sci. 2020, 10, 6400. [Google Scholar] [CrossRef]
- Rana Khalid, I.; Darakhshanda, I.; Rafi, A.R. 3D Bioprinting: An attractive alternative to traditional organ transplantation. Arch. Biomed. Sci. Eng. 2019, 5, 007–018. [Google Scholar] [CrossRef]
- Momper, J.D.; Misel, M.L.; McKay, D.B. Sex differences in transplantation. Transplant. Rev. 2017, 31, 145–150. [Google Scholar] [CrossRef]
- Matej, V.; Karol, G.; Monika, B.; Juraj, M.; Ivana, D. Age and sex disparity in infectious complications after kidney transplantation. Bratisl. Med. J. 2022, 123, 463–469. [Google Scholar] [CrossRef]
- Vinson, A.J.; Zhang, X.; Dahhou, M.; Süsal, C.; Döhler, B.; Sapir-Pichhadze, R.; Cardinal, H.; Melk, A.; Wong, G.; Francis, A.; et al. Age-dependent Sex Differences in Graft Loss After Kidney Transplantation. Transplantation 2022, 106, 1473–1484. [Google Scholar] [CrossRef]
- Serrano, M.T.; Sabroso, S.; Esteban, L.M.; Berenguer, M.; Fondevila, C.; Lorente, S.; Cortés, L.; Sanchez-Antolin, G.; Nuño, J.; De la Rosa, G.; et al. Mortality and Causes of Death After Liver Transplantation: Analysis of Sex Differences in a Large Nationwide Cohort. Transpl. Int. 2022, 35, 10263. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, U.; Issachar, A.; Cohen-Naftaly, M.; Brown, M.; Nesher, E. Gender specific survival rates after deceased donor liver transplantation: A retrospective cohort. Ann. Med. Surg. 2022, 79, 103933. [Google Scholar] [CrossRef] [PubMed]
- Paisarntanawat, N. Alternatives to Organ Replacement. Int. J. Form. Sci. Curr. Future Res. Trends 2022, 13, 87–101. [Google Scholar]
- Tibbetts, J.H. The Future of BioprintingMultidisciplinary teams seek to create living human organs. BioScience 2021, 71, 564–570. [Google Scholar] [CrossRef]
- Yu, J.; Park, S.A.; Kim, W.D.; Ha, T.; Xin, Y.Z.; Lee, J.; Lee, D. Current Advances in 3D Bioprinting Technology and Its Applications for Tissue Engineering. Polymers 2020, 12, 2958. [Google Scholar] [CrossRef] [PubMed]
- Varkey, M.; Visscher, D.O.; Van Zuijlen, P.P.M.; Atala, A.; Yoo, J.J. Skin bioprinting: The future of burn wound reconstruction? Burn. Trauma 2019, 7, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Huang, J.F.; Lin, J.L.; Yang, D.J.; Xu, T.Z.; Chen, D.; Zan, X.; Wu, A.M. 3D bioprinting in orthopedics translational research. J. Biomater. Sci. Polym. Ed. 2019, 30, 1172–1187. [Google Scholar] [CrossRef]
- Yang, S.; Lin, H.; Luo, C. Meta-Analysis of 3D Printing Applications in Traumatic Fractures. Front. Surg. 2021, 8, 352. [Google Scholar] [CrossRef]
- Voelker, R. 3D-Printed Implant Is Approved to Replace Ankle Joint Bone. JAMA 2021, 325, 1246. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, S.; Ishtiaq, I.; Aslam, K.; Ali, U.; Mehak, S.; Khan, S.; Sajjad, S.; Babar, M. 3D bioprinting—A step towards heart tissue regeneration. J. Appl. Biotechnol. Bioeng. 2021, 8, 1–4. [Google Scholar] [CrossRef]
- Kamolz, L.P.; Griffith, M.; Finnerty, C.; Kasper, C. Skin Regeneration, Repair, and Reconstruction. Biomed Res. Int. 2015, 2015, 892031. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Frejo, L.; Grande, D.A. 3D-bioprinted tracheal reconstruction: An overview. Bioelectron. Med. 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Mahfouzi, S.H.; Safiabadi Tali, S.H.; Amoabediny, G. 3D bioprinting for lung and tracheal tissue engineering: Criteria, advances, challenges, and future directions. Bioprinting 2021, 21, e00124. [Google Scholar] [CrossRef]
- Stocco, E.; Porzionato, A.; De Rose, E.; Barbon, S.; De Caro, R.; Macchi, V. Meniscus regeneration by 3D printing technologies: Current advances and future perspectives. J. Tissue Eng. 2022, 13, 314211065860. [Google Scholar] [CrossRef]
- Wang, Z.; Agrawal, P.; Zhang, Y.S. Nanotechnologies and Nanomaterials in 3D (Bio)printing toward Bone Regeneration. Adv. NanoBiomed Res. 2021, 1, 2100035. [Google Scholar] [CrossRef]
- Mao, S.; Pang, Y.; Liu, T.; Shao, Y.; He, J.; Yang, H.; Mao, Y.; Sun, W. Bioprinting of in vitro tumor models for personalized cancer treatment: A review. Biofabrication 2020, 12, 042001. [Google Scholar] [CrossRef]
- Bom, S.; Martins, A.M.; Ribeiro, H.M.; Marto, J. Diving into 3D (bio)printing: A revolutionary tool to customize the production of drug and cell-based systems for skin delivery. Int. J. Pharm. 2021, 605, 120794. [Google Scholar] [CrossRef]
- Moldovan, N.I. Three-Dimensional Bioprinting of Anatomically Realistic Tissue Constructs for Disease Modeling and Drug Testing. Tissue Eng.—Part C Methods 2021, 27, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Jackson, S.J.; Thomas, G.J. Human tissue models in cancer research: Looking beyond the mouse. DMM Dis. Model. Mech. 2017, 10, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ho, C.C.; Zhang, C.; Wang, B. A Review on the 3D Printing of Functional Structures for Medical Phantoms and Regenerated Tissue and Organ Applications. Engineering 2017, 3, 653–662. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Yu, D.F.; Zhao, J.G.; Wu, Y.L.; Zheng, B. 3D Printout Models vs. 3D-Rendered Images: Which Is Better for Preoperative Planning? J. Surg. Educ. 2016, 73, 518–523. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv. Healthc. Mater. 2017, 6, 75. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2020, 48, 536–555. [Google Scholar] [CrossRef]
- Segaran, N.; Saini, G.; Mayer, J.L.; Naidu, S.; Patel, I.; Alzubaidi, S.; Oklu, R. Application of 3d printing in preoperative planning. J. Clin. Med. 2021, 10, 917. [Google Scholar] [CrossRef]
- Baba, M.; Matsumoto, K.; Yamasaki, N.; Shindo, H.; Yano, H.; Matsumoto, M.; Otsubo, R.; John Lawn, M.; Matsuo, N.; Yamamoto, I.; et al. Development of a Tailored Thyroid Gland Phantom for Fine-Needle Aspiration Cytology by Three-Dimensional Printing. J. Surg. Educ. 2017, 74, 1039–1046. [Google Scholar] [CrossRef]
- Wu, A.-M.; Wang, K.; Wang, J.-S.; Chen, C.-H.; Yang, X.-D.; Ni, W.-F.; Hu, Y.-Z. The addition of 3D printed models to enhance the teaching and learning of bone spatial anatomy and fractures for undergraduate students: A randomized controlled study. Ann. Transl. Med. 2018, 6, 403. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, S.W.H. Impact of the educational technology use in undergraduate pharmacy teaching and learning—A systematic review. Pharm. Educ. 2021, 21, 159–168. [Google Scholar] [CrossRef]
- Losco, C.D.; Grant, W.D.; Armson, A.; Meyer, A.J.; Walker, B.F. Effective methods of teaching and learning in anatomy as a basic science: A BEME systematic review: BEME guide no. 44. Med. Teach. 2017, 39, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Milano, E.G.; Capelli, C.; Wray, J.; Biffi, B.; Layton, S.; Lee, M.; Caputo, M.; Taylor, A.M.; Schievano, S.; Biglino, G. Current and future applications of 3D printing in congenital cardiology and cardiac surgery. Br. J. Radiol. 2019, 92, 1094. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, D.; Fahimipour, A.; Abasian, P.; Saber, S.S.; Seyedi, M.; Ghanavati, S.; Ahmad, A.; De Stephanis, A.A.; Taghavinezhaddilami, F.; Leonova, A.; et al. 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications. Acta Biomater. 2021, 122, 26–49. [Google Scholar] [CrossRef]
- Kröger, E.; Dekiff, M.; Dirksen, D. 3D printed simulation models based on real patient situations for hands-on practice. Eur. J. Dent. Educ. 2017, 21, e119–e125. [Google Scholar] [CrossRef]
- Abbasov, I.B. Three-Dimensional Bioprinting of Organs: Modern Trends. Crit. Rev. Biomed. Eng. 2022, 50, 19–34. [Google Scholar] [CrossRef] [PubMed]
- GODT—Organ Donation and Transplantation. Activities Executive Summary 2020 International. 2022. Available online: www.transplant-observatory.org (accessed on 6 December 2022).
- van Daal, M.; Muntinga, M.E.; Steffens, S.; Halsema, A.; Verdonk, P. Sex and Gender Bias in Kidney Transplantation: 3D Bioprinting as a Challenge to Personalized Medicine. Women’s Health Rep. 2020, 1, 218–223. [Google Scholar] [CrossRef]
- Bertassoni, L.E. Bioprinting of Complex Multicellular Organs with Advanced Functionality—Recent Progress and Challenges Ahead. Adv. Mater. 2022, 34, 3. [Google Scholar] [CrossRef]
- Kasturi, M.; Mathur, V.; Agarwal, P.; Srinivasan, V.; Vasanthan, K.S. 3D Printing for Tissue Regeneration. In Advances in 3D Printing [Working Title]; Intech Open: London, UK, 2022; p. 3. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, e1805510. [Google Scholar] [CrossRef]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef]
- Shin, A. The History, Current Status, Benefits, and Challenges of 3D Printed Organs. arXiv 2022, arXiv:2207.13212. [Google Scholar]
- Su, X.; Wang, T.; Guo, S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen. Ther. 2021, 16, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Fortunato, G.M.; Hann, S.Y.; Ayan, B.; Vajanthri, K.Y.; Presutti, D.; Cui, H.; Chan, A.H.P.; Costantini, M.; Onesto, V.; et al. Recent advances in bioprinting technologies for engineering cardiac tissue. Mater. Sci. Eng. C 2021, 124, 11205. [Google Scholar] [CrossRef]
- Dodziuk, H. Applications of 3D printing in healthcare. Kardiochirurgia Torakochirurgia Pol. 2016, 13, 283–293. [Google Scholar] [CrossRef]
- Agarwal, S.; Saha, S.; Balla, V.K.; Pal, A.; Barui, A.; Bodhak, S. Current Developments in 3D Bioprinting for Tissue and Organ Regeneration—A Review. Front. Mech. Eng. 2020, 6, 589171. [Google Scholar] [CrossRef]
- Liaw, C.Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Asare-Ose, A. 3D and Bio-Printing as an Efficient and Safe Alternative to 3D and Bio-Printing as an Efficient and Safe Alternative to Traditional Methods and Materials in Cardiovascular Diseases Traditional Methods and Materi. Available online: https://idun.augsburg.edu/cgi/viewcontent.cgi?article=2231&context=etd (accessed on 6 December 2022).
- Birla, R.K.; Williams, S.K. 3D bioprinting and its potential impact on cardiac failure treatment: An industry perspective. APL Bioeng. 2020, 4, 1. [Google Scholar] [CrossRef]
- Morris, S. Future of 3D printing: How 3D bioprinting technology can revolutionize healthcare? Birth Defects Res. 2018, 110, 1098–1101. [Google Scholar] [CrossRef]
- Bose, S.; Bandyopadhyay, A. Additive Manufacturing: Future of Manufacturing in a Flat World. Addit. Manuf. 2020, 2, 380–401. [Google Scholar] [CrossRef]
- Desanlis, A.; Albouy, M.; Rousselle, P.; Thépot, A.; Dos Santos, M.; Auxenfans, C.; Marquette, C. Validation of an implantable bioink using mechanical extraction of human skin cells: First steps to a 3D bioprinting treatment of deep second degree burn. J. Tissue Eng. Regen. Med. 2021, 15, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Watt, S.M.; Pleat, J.M. Stem cells, niches and scaffolds: Applications to burns and wound care. Adv. Drug Deliv. Rev. 2018, 123, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Jang, J.; Jo, H.J.; Kim, W.-H.; Kim, B.; Chun, H.-J.; Lim, D.; Han, D.-W. Advances and Innovations of 3D Bioprinting Skin. Biomolecules 2022, 13, 55. [Google Scholar] [CrossRef]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for Organ Regeneration. Adv. Healthc. Mater. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Peng, W.; Datta, P.; Ayan, B.; Ozbolat, V.; Sosnoski, D.; Ozbolat, I.T. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017, 57, 26–46. [Google Scholar] [CrossRef]
- Aquino, R.P.; Barile, S.; Grasso, A.; Saviano, M. Envisioning smart and sustainable healthcare: 3D Printing technologies for personalized medication. Futures 2018, 103, 35–50. [Google Scholar] [CrossRef]
- Koçak, E.; Yıldız, A.; Acartürk, F. Three dimensional bioprinting technology: Applications in pharmaceutical and biomedical area. Colloids Surf. B Biointerfaces 2021, 197, 111396. [Google Scholar] [CrossRef]
- Mastrangeli, M.; Millet, S.; van den Eijnden-Van Raaij, J. Organ-on-chip in development: Towards a roadmap for organs-on-chip. ALTEX 2019, 36, 650–668. [Google Scholar] [CrossRef]
- Biselli, E.; Agliari, E.; Barra, A.; Bertani, F.R.; Gerardino, A.; De Ninno, A.; Mencattini, A.; Di Giuseppe, D.; Mattei, F.; Schiavoni, G.; et al. Organs on chip approach: A tool to evaluate cancer-immune cells interactions. Sci. Rep. 2017, 7, 12737. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Melchels, F.P.W.; Ferreira, M.J.S.; Moxon, S.R.; Potjewyd, G.; Dargaville, T.R.; Kimber, S.J.; Domingos, M. Emulating Human Tissues and Organs: A Bioprinting Perspective Toward Personalized Medicine. Chem. Rev. 2020, 120, 11128–11174. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Zein, N.N.; Quintini, C.; Miller, C.M.; Tsoulfas, G. Three-dimensional (3D) printing and liver transplantation. 3D Print. Appl. Med. Surg. 2020, 1, 97–116. [Google Scholar] [CrossRef]
- Bozkurt, Y.; Karayel, E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J. Mater. Res. Technol. 2021, 14, 1430–1450. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, X.; Li, L.; Chen, Z.N.; Gao, G.; Yao, R.; Sun, W. 3D printing human induced pluripotent stem cells with novel hydroxypropyl chitin bioink: Scalable expansion and uniform aggregation. Biofabrication 2018, 10, 44101. [Google Scholar] [CrossRef]
- AbouHashem, Y.; Dayal, M.; Serafin, S.; Štrkalj, G. Students’ attitudes toward body image donation for 3D printing. Clin. Anat. 2017, 30, 1005–1006. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, D. A systematic review of clinical value of three-dimensional printing in renal disease. Quant. Imaging Med. Surg. 2018, 8, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Chan, A.; Ong, Y.S.; Chua, C.K. Deep learning for fabrication and maturation of 3D bioprinted tissues and organs. Virtual Phys. Prototyp. 2020, 15, 340–358. [Google Scholar] [CrossRef]
- Durfee, W.K.; Iaizzo, P.A. Medical Applications of 3D Printing. In Engineering in Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 527–543. ISBN 9780128130681. [Google Scholar] [CrossRef]
- Brazier, M.; Cave, E. Organ and tissue transplantation. In Medicine, Patients, and the Law; Penguin UK: London, UK, 2016; pp. 517–544. ISBN 978-1-7849-9136-4. [Google Scholar]
- Ford, S.; Minshall, T. Invited review article: Where and how 3D printing is used in teaching and education. Addit. Manuf. 2019, 25, 131–150. [Google Scholar] [CrossRef]
- Sattar, M.U.; Palaniappan, S.; Lokman, A.; Hassan, A.; Shah, N.; Riaz, Z. Effects of virtual reality training on medical students’ learning motivation and competency. Pak. J. Med. Sci. 2019, 35, 852–857. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D printed medicines: Which techniques and polymers are more successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed]
| Key Benefit/Topic | Area of Application/Significance | Authors |
|---|---|---|
| Transplant organ shortage | Organ transplantation | Bastani 2020 [41] |
| Organ and tissue donation | Oliver 2023 [42] | |
| Blockchain-specific approach for the prevention or monitoring of organ trafficking | Patil et al., 2023 [43] | |
| the challenges and opportunities in tackling the potential reduction in organ donations | Mills 2020 [44] | |
| 3D Bioprinting as an alternative to traditional organ transplantation | Iram et al., 2019 [45] | |
| Overcoming gender differences in transplantation | Sex differences in transplantation | Momper et al., 2017 [46] |
| Post-transplant infections | Vnucak et al., 2022 [47] | |
| Age-dependent sex differences after transplantation | Vinson et al., 2022 [48] | |
| Sex-based discrepancies between donors and recipients | Serrano et al., 2022 [49] | |
| Sex disparities in post-transplant survival | Gabbay et al., 2022 [50] | |
| Probability reduction of transplant rejection | Alternatives to Organ Replacement | Paisarntanawat 2022 [51] |
| Multidisciplinary teams seek to create living human organs | Tibbetts 2021 [52] | |
| Applications of 3D bioprinting technology for tissue engineering | Yu et al., 2020 [53] | |
| Skin bioprinting: the future of burn wound reconstruction | Varkey et al., 2019 [54] | |
| Orthopedics | Zheng et al., 2019 [55] | |
| Traumatic fractures | Yang et al., 2021 [56] | |
| Implants | Voelker 2021 [57] | |
| Heart tissue regeneration | Shahzadi et al., 2021 [58] | |
| Skin regeneration, repair, and reconstruction | Kamolz 2022 [59] | |
| Removal of congenital defects of various tissues and organs | Orthopedics | Zheng et al., 2019 [55] |
| Components of the human heart | Lee et al., 2019 [60] | |
| Tracheal reconstruction | Frejo and Grande 2019 [61] | |
| Bioprinting Skin | Kang et al., 2022 [61] | |
| Lung and tracheal tissue engineering | Mahfouzi et al., 2021 [62] | |
| Meniscus regeneration | Stocco et al., 2022 [63] | |
| Bone Regeneration | Wang et al., 2021 [64] | |
| Drug research and development | Personalized cancer treatment | Mao et al., 2020 [65] |
| Production of drug and cell-based systems | Bom et al., 2021 [66] | |
| Tissue constructs for disease modelling and drug testing | Moldovan 2021 [67] | |
| 3D bioprinting as alternatives to animal research | Van Norman 2019 [68] | |
| Cancer Research | Jackson and Thomas 2017 [69] | |
| Surgery Planning and Medical training for young doctors | Functional Structures for Medical Phantoms | Wang et al., 2017 [70] |
| Comparison between 3D printout models and 3D-rendered images | Zheng et al., 2018 [71] | |
| Healthy and diseased models | Gu et al., 2017 [72] | |
| Preoperative Surgical Planning | Tejo-Otero et al., 2022 [73] | |
| Preoperative Surgical Planning | Segaran et al., 2021 [74] | |
| Phantom for Fine-Needle Aspiration Cytology | Baba et al., 2017 [75] | |
| Teaching and learning of bone spatial anatomy | Wu et al., 2018 [76] | |
| Pharmacy education | Lee and Lee 2021 [77] | |
| Teaching and learning in anatomy | Losco et al., 2017 [78] | |
| Cardiac surgery | Milano et al., 2019 [79] | |
| Dentistry and maxillofacial surgery | Khorsandi et al., 2021 [80] | |
| 3D printed simulation models | Kröger et al., 2017 [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shopova, D.; Yaneva, A.; Bakova, D.; Mihaylova, A.; Kasnakova, P.; Hristozova, M.; Sbirkov, Y.; Sarafian, V.; Semerdzhieva, M. (Bio)printing in Personalized Medicine—Opportunities and Potential Benefits. Bioengineering 2023, 10, 287. https://doi.org/10.3390/bioengineering10030287
Shopova D, Yaneva A, Bakova D, Mihaylova A, Kasnakova P, Hristozova M, Sbirkov Y, Sarafian V, Semerdzhieva M. (Bio)printing in Personalized Medicine—Opportunities and Potential Benefits. Bioengineering. 2023; 10(3):287. https://doi.org/10.3390/bioengineering10030287
Chicago/Turabian StyleShopova, Dobromira, Antoniya Yaneva, Desislava Bakova, Anna Mihaylova, Petya Kasnakova, Maria Hristozova, Yordan Sbirkov, Victoria Sarafian, and Mariya Semerdzhieva. 2023. "(Bio)printing in Personalized Medicine—Opportunities and Potential Benefits" Bioengineering 10, no. 3: 287. https://doi.org/10.3390/bioengineering10030287
APA StyleShopova, D., Yaneva, A., Bakova, D., Mihaylova, A., Kasnakova, P., Hristozova, M., Sbirkov, Y., Sarafian, V., & Semerdzhieva, M. (2023). (Bio)printing in Personalized Medicine—Opportunities and Potential Benefits. Bioengineering, 10(3), 287. https://doi.org/10.3390/bioengineering10030287









