An In Vivo Rat Study of Bioresorbable Mg-2Zn-2Ga Alloy Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Mg-2 wt.% Zn-2 wt.% Ga Alloy Sample Preparation
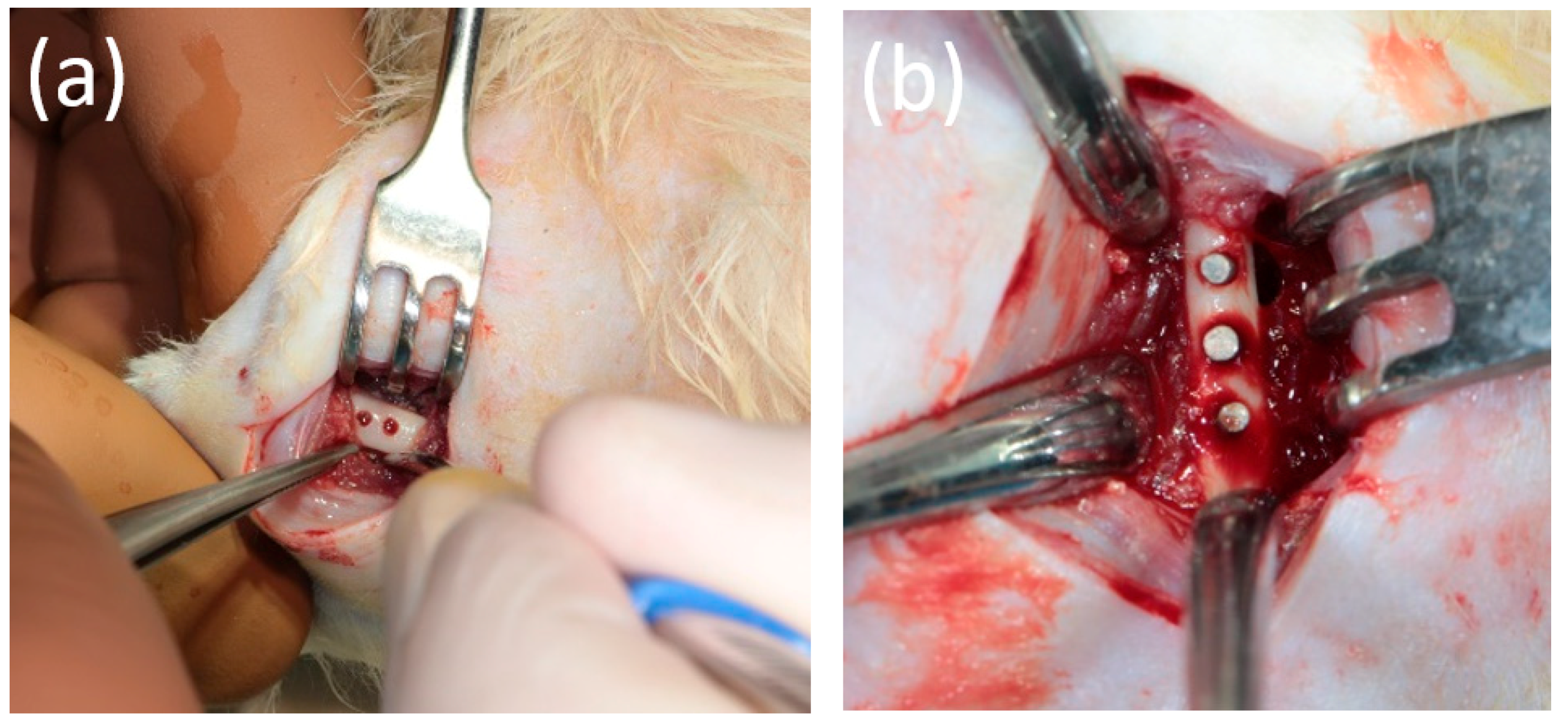
2.2. Animal Experiment
2.3. Ultrasound Examination
2.4. X-ray Examination
2.5. Microfocus Computed Tomography (micro-CT) Examination
2.6. SEM and EDX
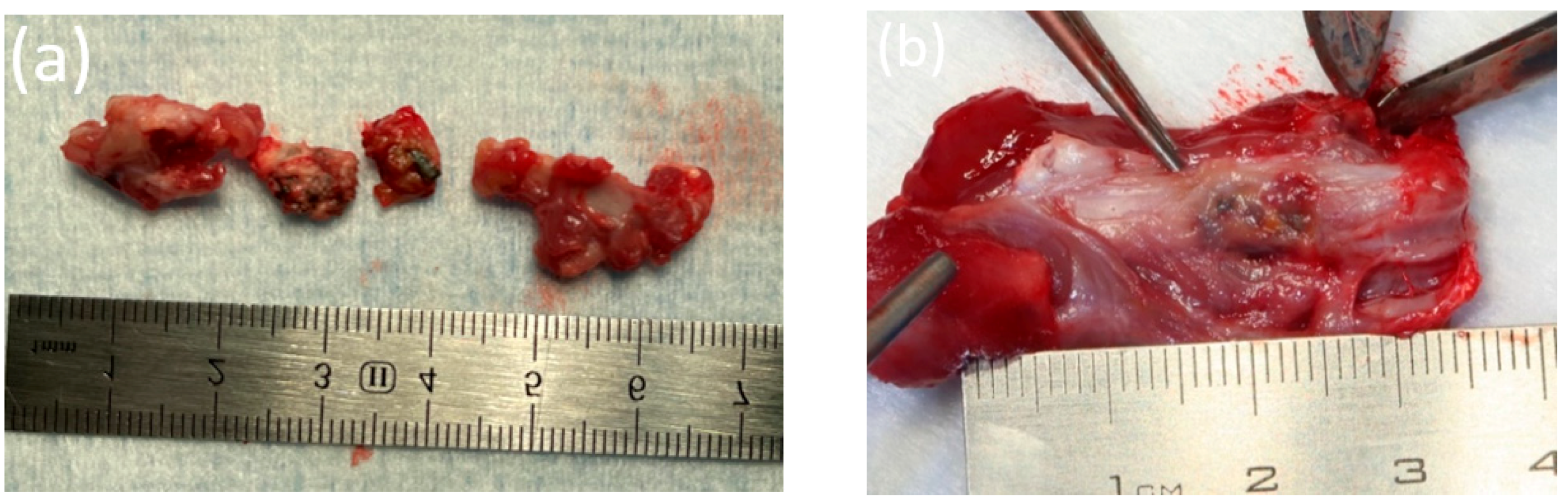
2.7. Histological Evaluation of Bone Fragments
3. Results
3.1. Results of Laboratory Studies
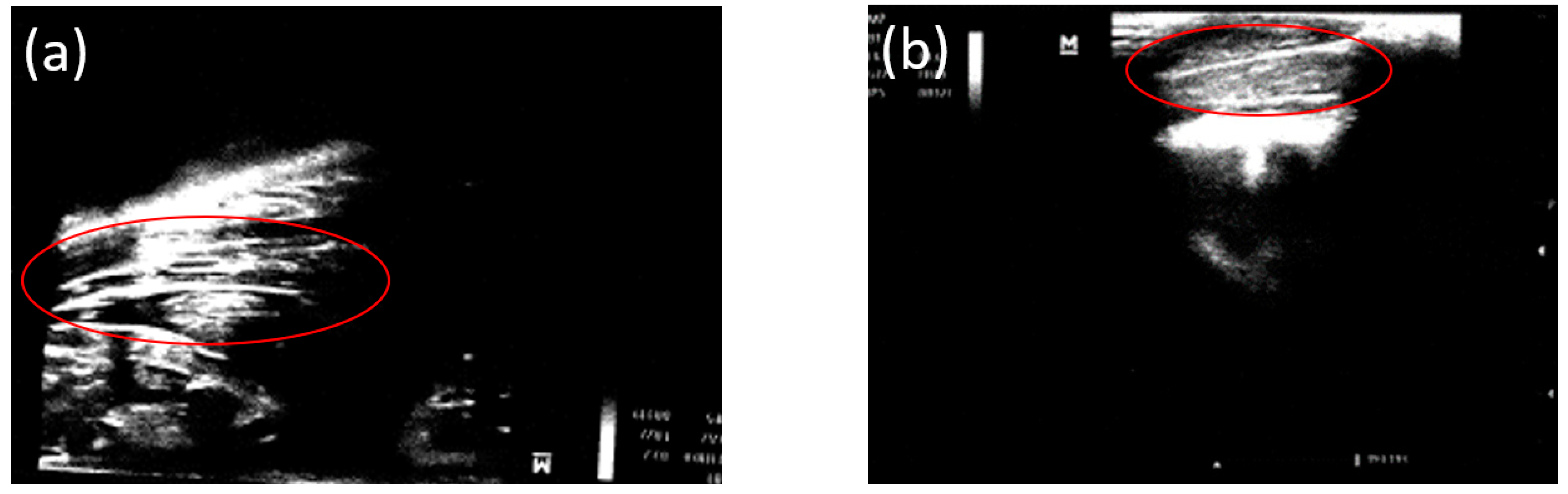
3.2. Ultrasound
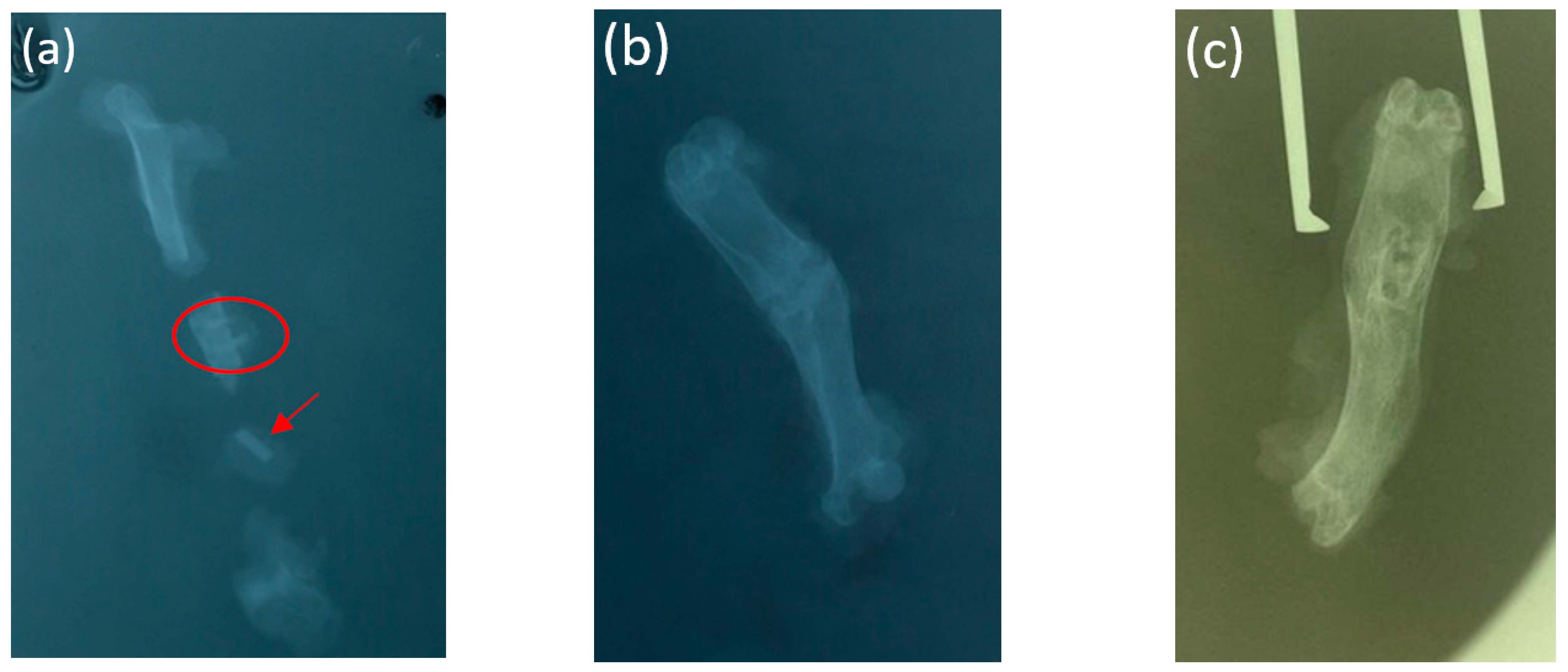
3.3. X-ray Examination
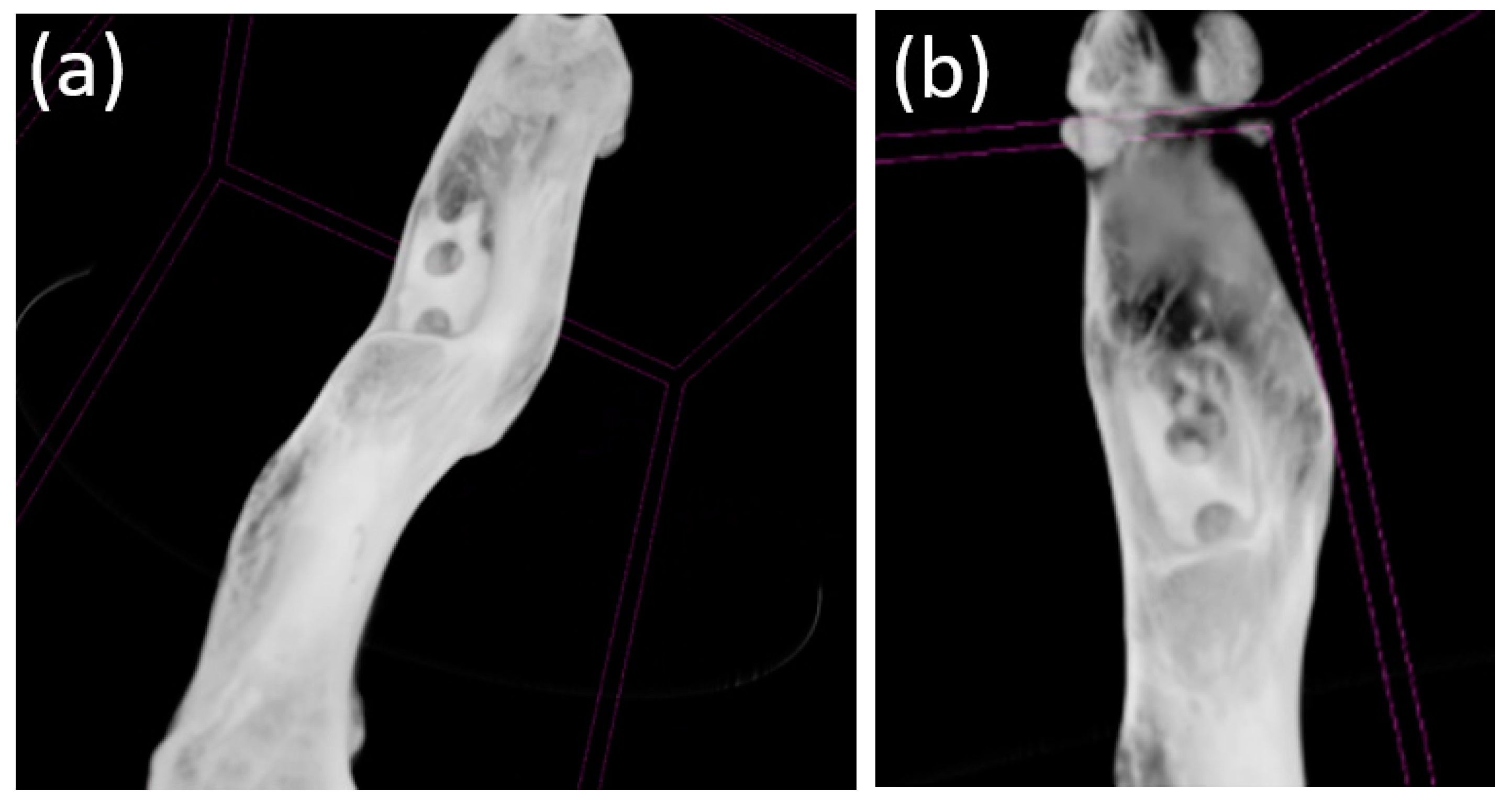
3.4. Cone Beam Computed Tomography
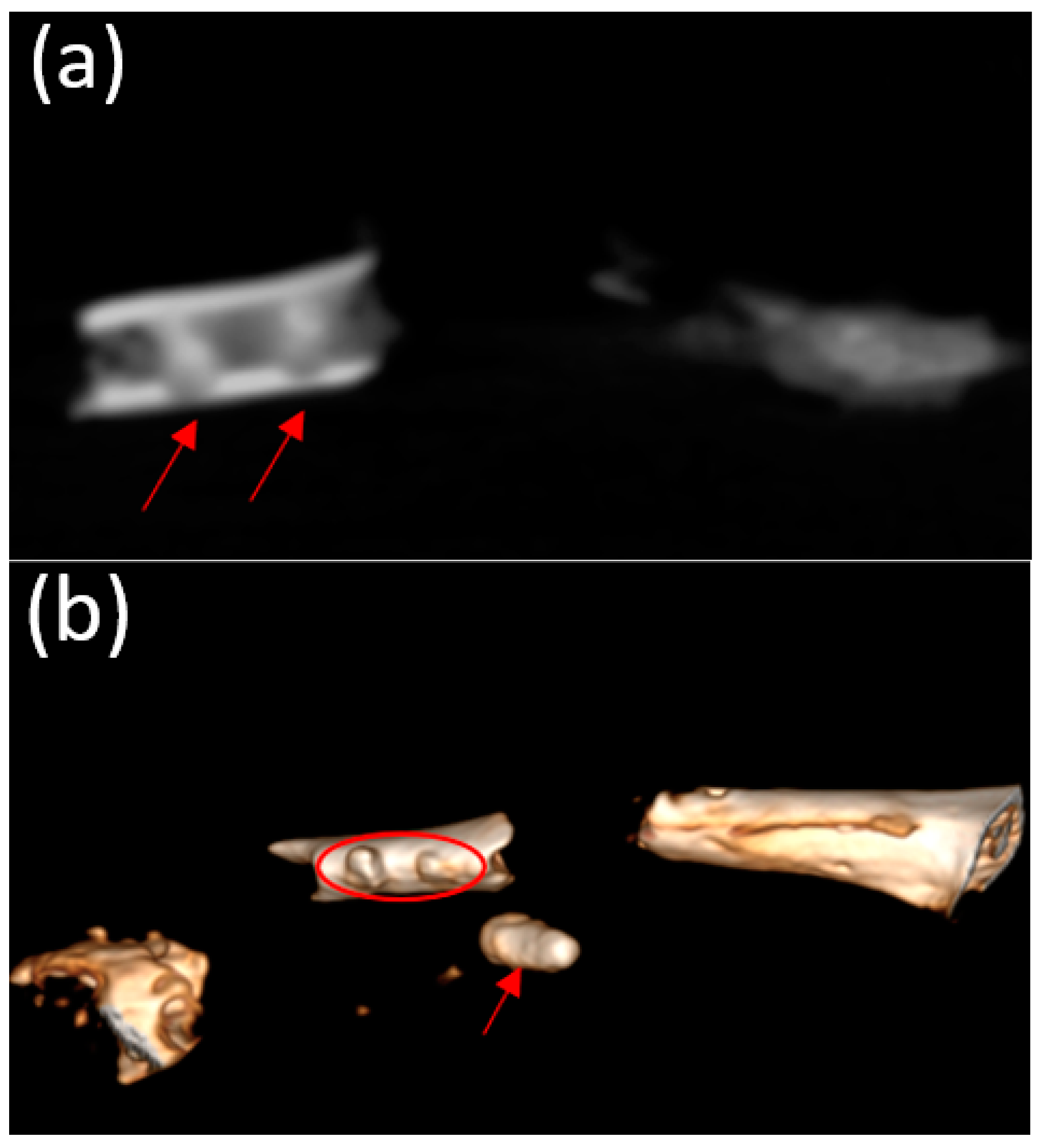
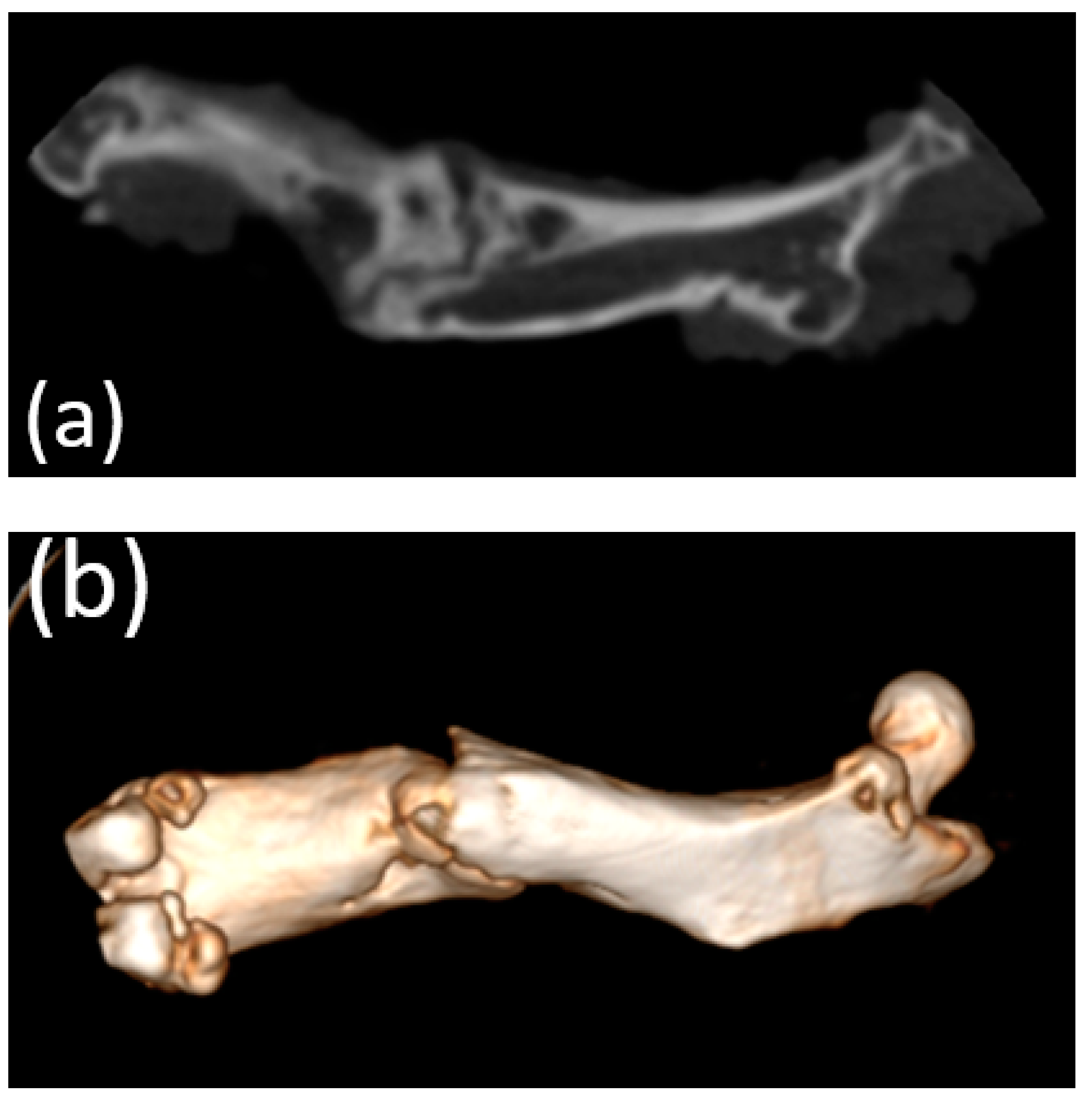
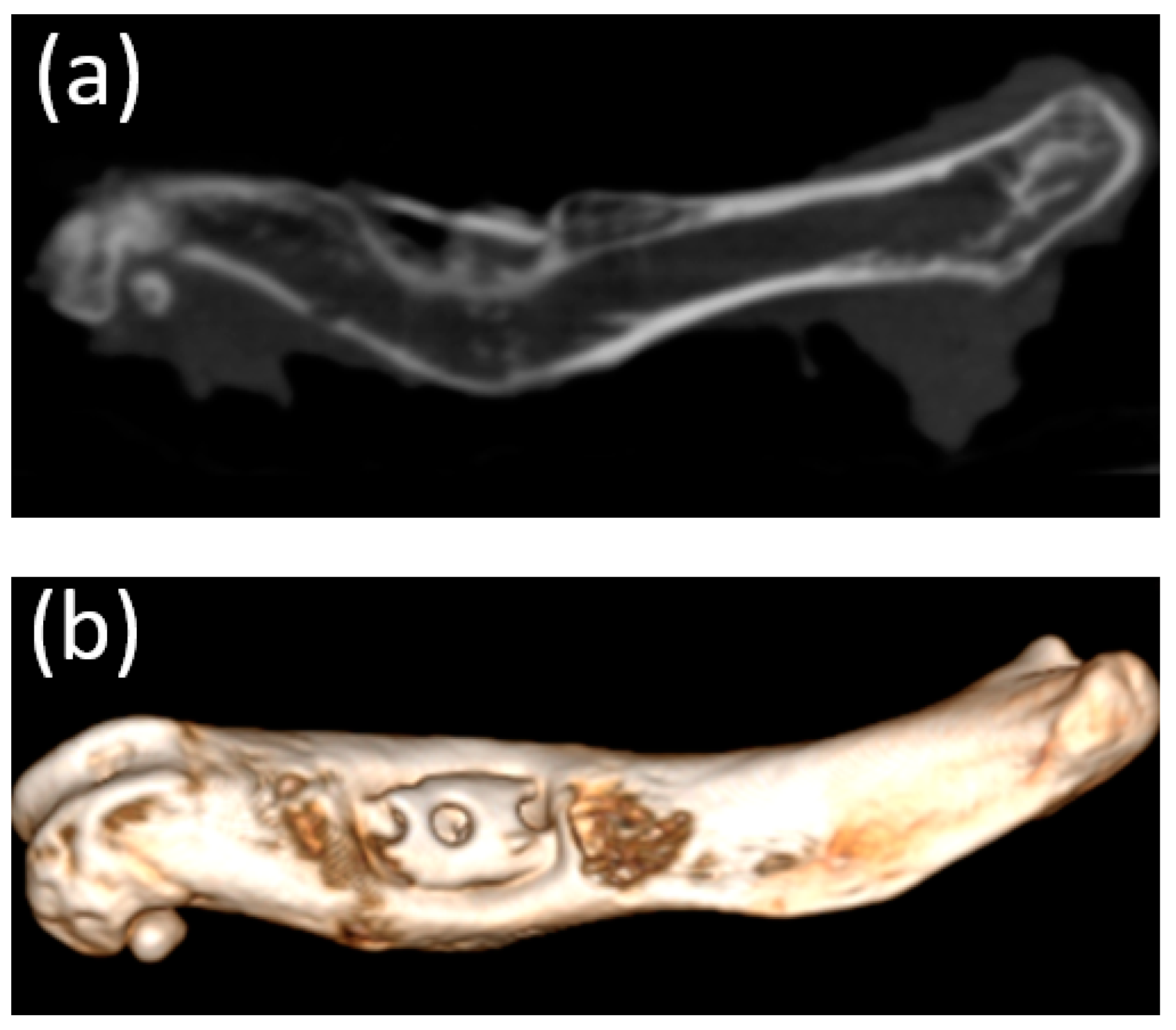
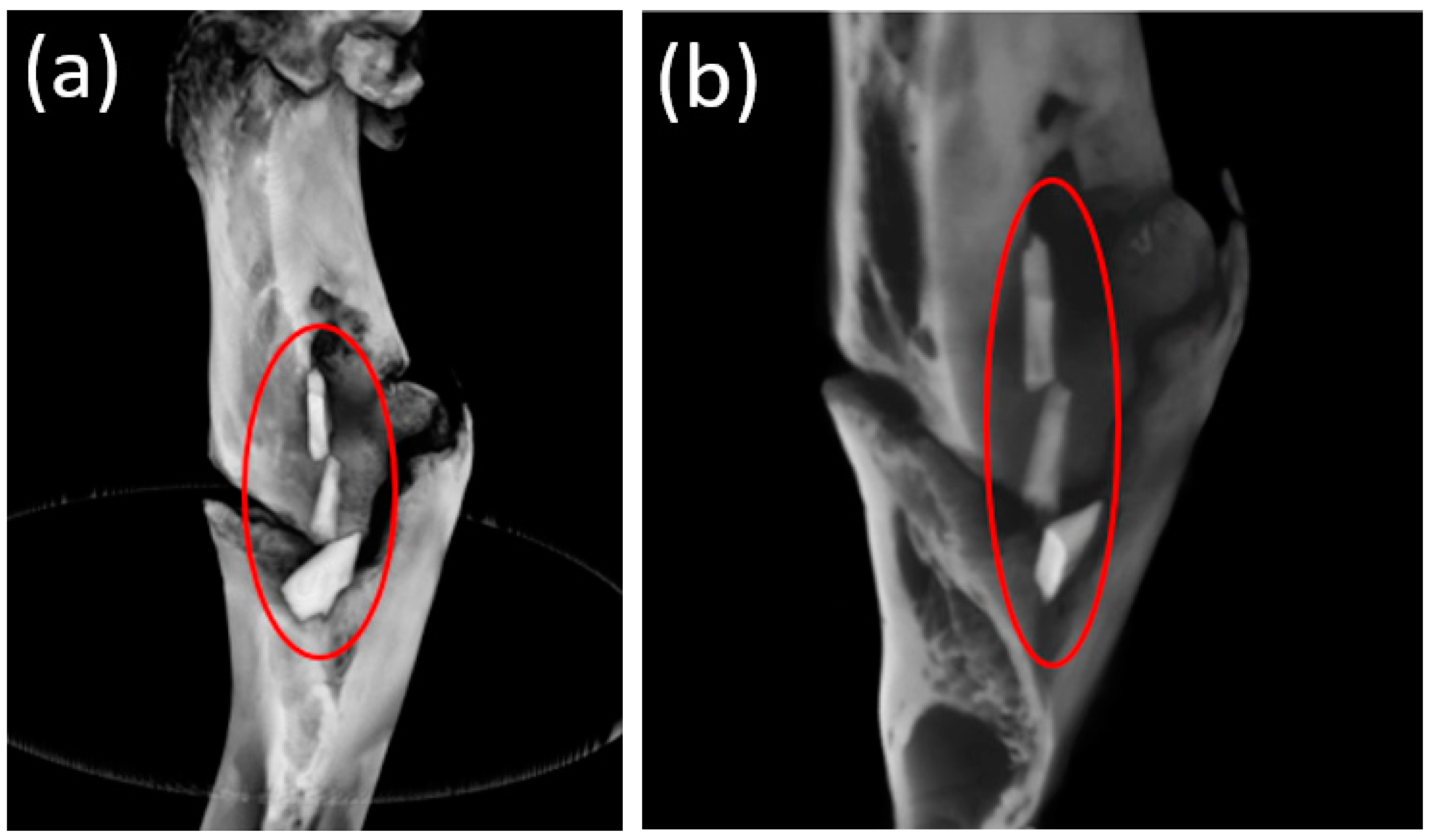
3.5. Micro-CT
3.6. Scanning Electron Microscopy Characterization
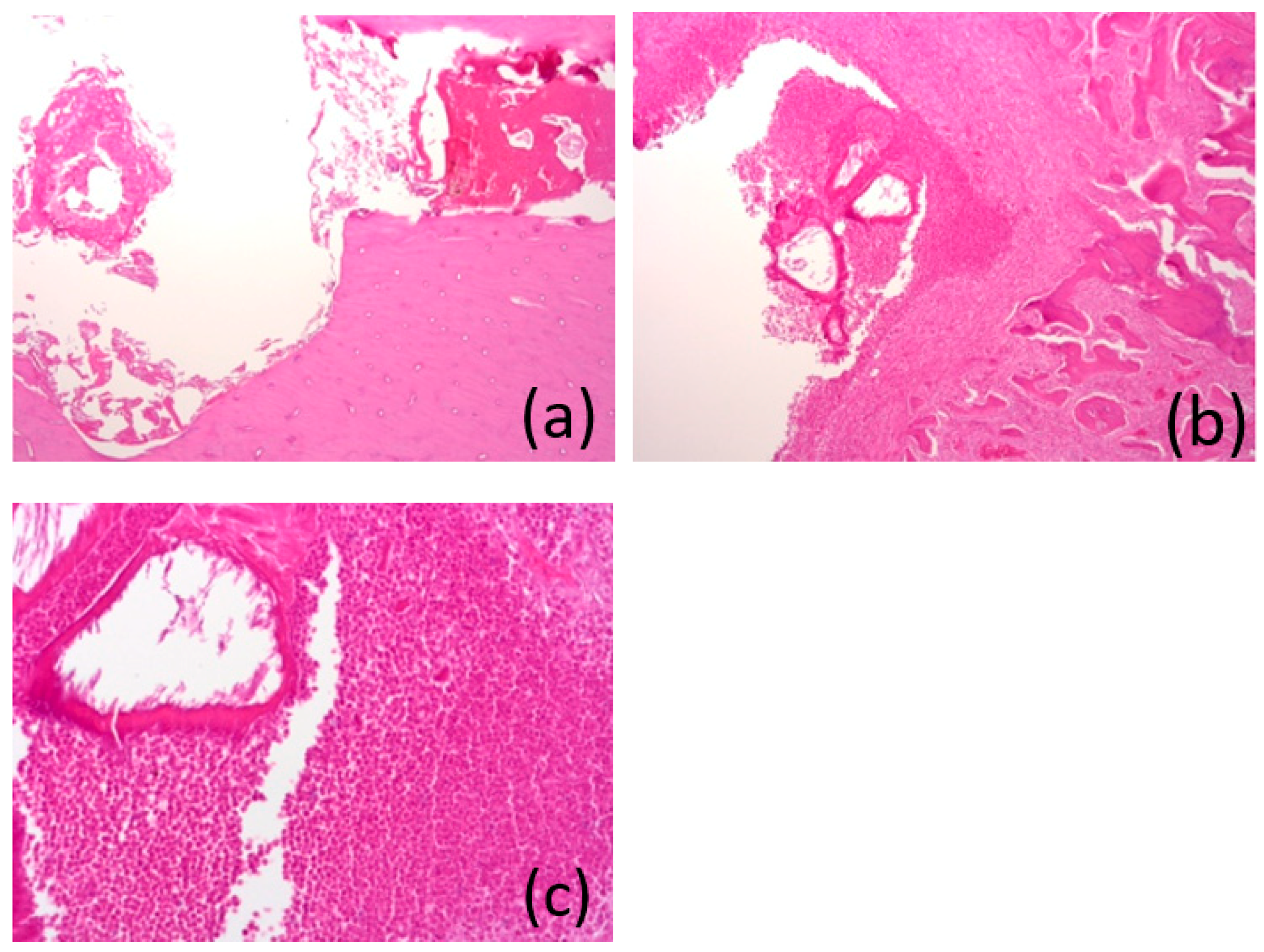
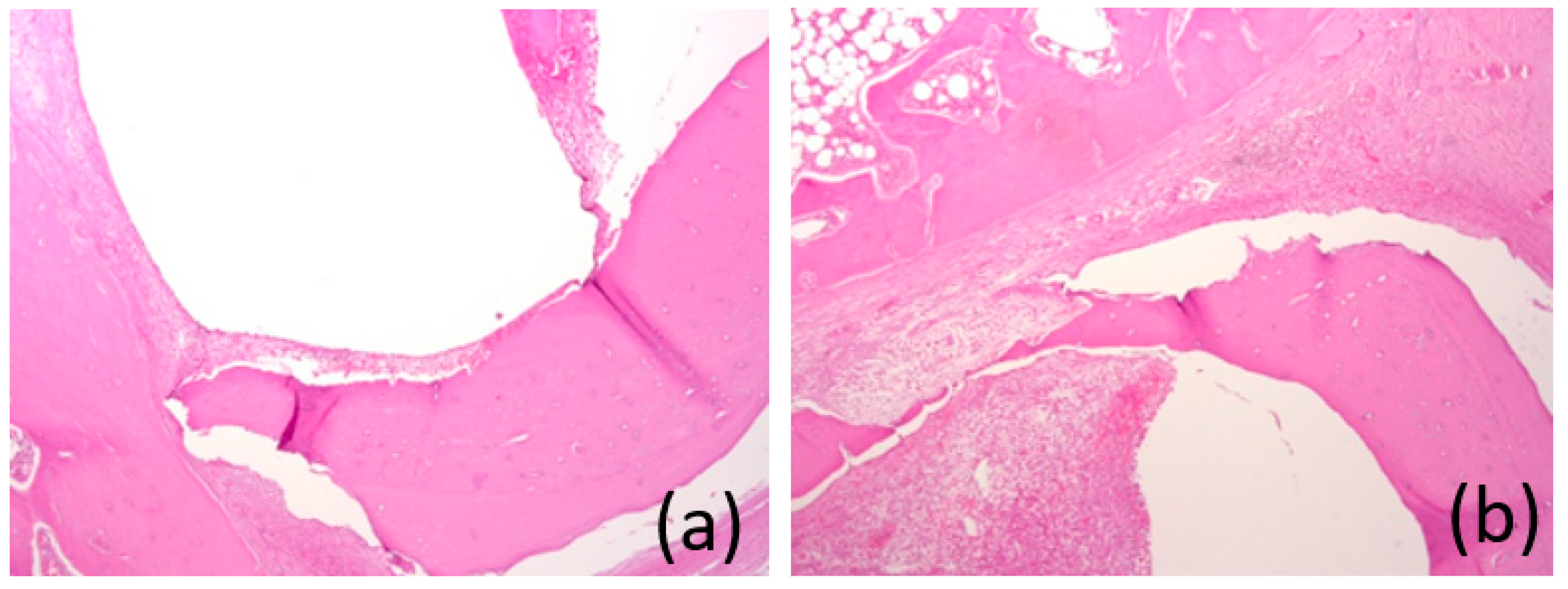
3.7. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoniac, I.; Miculescu, M.; Mănescu, V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef]
- Chagnon, M.; Guy, L.-G.; Jackson, N. Evaluation of Magnesium-Based Medical Devices in Preclinical Studies: Challenges and Points to Consider. Toxicol. Pathol. 2019, 47, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.M.A.; Baig, Z.; Ahmed, S.W.; Hussain, G.; Subramaniam, K.; Hastuty, S.; Rao, T.V.V.L.N. Biocompatibility and corrosion resistance of metallic biomaterials. Corros. Rev. 2020, 38, 381–402. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Y.; Chen, X.-H.; Yang, J.A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental Theory of Biodegradable Metals—Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Verma, R.P. Titanium based biomaterial for bone implants: A mini review. Mater. Today Proc. 2020, 26, 3148–3151. [Google Scholar] [CrossRef]
- Ding, W. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef]
- Niu, J.; Yuan, G.; Liao, Y.; Mao, L.; Zhang, J.; Wang, Y.; Huang, F.; Jiang, Y.; He, Y.; Ding, W. Enhanced biocorrosion resistance and biocompatibility of degradable Mg–Nd–Zn–Zr alloy by brushite coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4833–4841. [Google Scholar] [CrossRef]
- Lukyanova, E.; Anisimova, N.; Martynenkoa, N.; Kiselevsky, M.; Dobatkina, S.; Estrin, Y. Features of in vitro and in vivo behaviour of magnesium alloy WE43. Mater. Lett. 2018, 215, 308–311. [Google Scholar] [CrossRef]
- Wolff, M.; Luczak, M.; Schaper, J.G.; Wiese, B.; Dahms, M.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. In vitro biodegradation testing of Mg-alloy EZK400 and manufacturing of implant prototypes using PM (powder metallurgy) methods. Bioact. Mater. 2018, 3, 213–217. [Google Scholar] [CrossRef]
- Cooper, H.J.; Urban, R.M.; Wixson, R.L.; Meneghini, R.M.; Jacobs, J.J. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J. Bone Jt. Surg. Am. 2013, 95, 865–872. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Jiang, W.; Cipriano, A.F.; Tian, Q.; Zhang, C.; Lopez, M.; Sallee, A.; Lin, A.; Cortez Alcaraz, M.C.; Wu, Y.; Zheng, Y.; et al. In vitro evaluation of MgSr and MgCaSr alloys via direct culture with bone marrow derived mesenchymal stem cells. Acta Biomater. 2018, 72, 407–423. [Google Scholar] [CrossRef]
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. J. Biometals 2019, 23, 185–193. [Google Scholar] [CrossRef]
- Rahman, M.; Dutta, N.K.; Roy Choudhury, N. Magnesium Alloys with Tunable Interfaces as Bone Implant Materials. Front. Bioeng. Biotechnol. 2020, 8, 564. [Google Scholar] [CrossRef]
- Zhang, J.; Shang, Z.; Jiang, Y.; Zhang, K.; Li, X.; Ma, M.; Li, Y.; Ma, B. Biodegradable Metals for Bone Fracture Repair in Animal Models: A Systematic Review. Regen. Biomater. 2020, 8, rbaa047. [Google Scholar] [CrossRef]
- Kasinath, R.; Ernsberg, C.; Vass, S.; Ginn, S.N.; Qu, H.; Tong, W. Orthopedic Implant Having a Crystalline Gallium-Containing Hydroxyapatite Coating and Methods for Making the Same. U.S. Patent No. 11,141,505, 30 April 2020. [Google Scholar]
- Melnikov, P.; Teixeira, A.R.; Malzac, A.; Coelho, M.D.B. Gallium-containing hydroxyapatite for potential use in orthopedics. Mater. Chem. Phys. 2009, 117, 86–90. [Google Scholar] [CrossRef]
- Ma, Z.; Fu, Q. Therapeutic Effect of Organic Gallium on Ovariectomized Osteopenic Rats by Decreased Serum Minerals and Increased Bone Mineral Content. Biol. Trace Elem. Res. 2010, 133, 342–349. [Google Scholar] [CrossRef]
- Bernstein, L.R. Mechanisms of Therapeutic Activity for Gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar]
- Warrell, R.P., Jr.; Bockman, R.S.; Coonley, C.J.; Isaacs, M.; Staszewski, H. Gallium nitrate inhibits calcium resorption from bone and is effective treatment for cancer-related hypercalcemia. J. Clin. Investig. 1984, 73, 1487–1490. [Google Scholar] [CrossRef]
- Warrell, R.P., Jr.; Skelos, A.; Alcock, N.W.; Bockman, R.S. Gallium Nitrate for Acute Treatment of Cancer-related Hypercalcemia: Clinicopharmacological and Dose Response Analysis. Cancer Res. 1986, 46, 4208–4212. [Google Scholar]
- Warrell, R.P., Jr.; Israel, R.; Frisone, M.; Snyder, T.; Gaynor, J.J.; Bockman, R.S. Gallium Nitrate for Acute Treatment of Cancer-Related Hypercalcemia: A Randomized, Double-Blind Comparison to Calcitonin. Ann. Intern. Med. 1988, 108, 669–674. [Google Scholar] [CrossRef]
- Warrell, R.P., Jr.; Bosco, B.; Weinerman, S.; Levine, B.; Lane, J.; Bockman, R.S. Gallium Nitrate for Advanced Paget Disease of Bone: Effectiveness and Dose-Response Analysis. Ann. Intern. Med. 1990, 113, 847–851. [Google Scholar] [CrossRef]
- Matkovic, V.; Apseloff, G.; Shepard, D.R.; Gerber, N. Use of gallium to treat Paget’s disease of bone: A pilot study. Lancet 1990, 335, 72–75. [Google Scholar] [CrossRef]
- Niesvizky, R. Gallium nitrate in multiple myeloma: Prolonged survival in a cohort of patients with advanced-stage disease. Semin. Oncol. 2003, 30, 20–24. [Google Scholar] [CrossRef]
- Collery, P.; Keppler, B.; Madoulet, C.; Desoize, B. Gallium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 283–296. [Google Scholar] [CrossRef]
- Bazhenov, V.; Li, A.; Iliasov, A.; Bautin, V.; Plegunova, S.; Koltygin, A.; Komissarov, A.; Abakumov, M.; Redko, N.; Shin, K.S. Corrosion Behavior and Biocompatibility of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. Materials 2022, 13, 294. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium Biomaterials-Road to the Clinic. Bioengineering 2022, 9, 107. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; Haude, M.; Kuku, K.; Hideo-Kajita, A.; Ince, H.; Abizaid, A.; Tölg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; et al. In vivo serial invasive imaging of the second-generation drug-eluting absorbable metal scaffold (Magmaris—DREAMS 2G) in de novo coronary lesions: Insights from the BIOSOLVE-II first-in-man trial. Int. J. Cardiol. 2018, 255, 22–28. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Wang, W.; Huang, H.; Pei, J.; Qu, H.; Yuan, G.; Li, Y. The degradation and transport mechanism of a mg-Nd-Zn-Zr stent in rabbit common carotid artery: A 20-month study. Acta Biomater. 2018, 69, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.C.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-based biodegradable alloys: Degradation, application, and alloying elements. Interv. Med. Appl. Sci. 2017, 9, 27–38. [Google Scholar] [CrossRef]
- Zhao, N.; Zhu, D. Endothelial responses of magnesium and other alloying elements in magnesium-based stent materials. Metallomics 2015, 7, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, A.A.; Fernandes, P.R.A.; Peach, C.; Cooper, G.; Diver, C.; Bartolo, P.J. Metallic bone fixation implants: A novel design approach for reducing the stress shielding phenomenon. Virtual Phys. Prototyp. 2017, 12, 141–151. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Johnston, S.; Dargusch, M.; Atrens, A. Building towards a standardised approach to biocorrosion studies: A review of factors influencing Mg corrosion in vitro pertinent to in vivo corrosion. Sci. China Mater. 2018, 61, 475–500. [Google Scholar] [CrossRef]
- Sun, Y.; Helmholz, H.; Willumeit-Römer, R. Preclinical in Vivo Research of Magnesium-Based Implants for Fracture Treatment: A Systematic Review of Animal Model Selection and Study Design. J. Magnes. Alloy. 2021, 9, 351–361. [Google Scholar] [CrossRef]
- Nene, S.S.; Kashyap, B.P.; Prabhu, N.; Estrin, Y.; Al-Samman, T. Biocorrosion and biodegradation behavior of ultralight Mg–4Li–1Ca (LC41) alloy in simulated body fluid for degradable implant applications. J. Mater. Sci. 2015, 50, 3041–3050. [Google Scholar] [CrossRef]
- Drobyshev, A.; Komissarov, A.; Redko, N.; Gurganchova, Z.; Statnik, E.S.; Bazhenov, V.; Sadykova, I.; Miterev, A.; Romanenko, I.; Yanushevich, O. Bone Remodeling Interaction with Magnesium Alloy Implants Studied by SEM and EDX. Materials 2022, 15, 7529. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone Regeneration Strategies: Engineered Scaffolds, Bioactive Molecules and Stem Cells Current Stage and Future Perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials Evolution of Bone Plates for Internal Fixation of Bone Fractures: A Review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Buijs, G.J.; Stegenga, B.; Bos, R.R.M. Efficacy and Safety of Biodegradable Osteofixation Devices in Oral and Maxillofacial Surgery: A Systematic Review. J. Dent. Res. 2006, 85, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; van Bakelen, N.B.; Dijkstra, P.U.; Vissink, A.; Bos, R.R.M.; van Minnen, B. Efficacy and Morbidity of Biodegradable versus Titanium Osteosyntheses in Orthognathic Surgery: A Systematic Review with Meta-Analysis and Trial Sequential Analysis. Eur. J. Oral Sci. 2021, 129, e12800. [Google Scholar] [CrossRef]
- Gareb, B.; van Bakelen, N.; Buijs, G.; Jansma, J.; de Visscher, J.; Hoppenreijs, T.; Bergsma, J.; van Minnen, B.; Stegenga, B.; Bos, R. Comparison of the Long-Term Clinical Performance of a Biodegradable and a Titanium Fixation System in Maxillofacial Surgery: A Multicenter Randomized Controlled Trial. PLoS ONE 2017, 12, e0177152. [Google Scholar] [CrossRef]
- Yaremchuk, M.J.; Posnick, J.C. Resolving Controversies Related to Plate and Screw Fixation in the Growing Craniofacial Skeleton. J. Craniofac. Surg. 1995, 6, 525–538. [Google Scholar] [CrossRef]
- Viljanen, J.; Kinnunen, J.; Bondestam, S.; Majola, A.; Rokkanen, P.; Törmälä, P. Bone Changes after Experimental Osteotomies Fixed with Absorbable Self-Reinforced Poly-L-Lactide Screws or Metallic Screws Studied by Plain Radiographs, Quantitative Computed Tomography and Magnetic Resonance Imaging. Biomaterials 1995, 16, 1353–1358. [Google Scholar] [CrossRef]
- Destatis. Vollstationär Behandelte Patientinnen und Patienten in Krankenhäuser 2018; Destatis Statistisches Bundesamt: Wiesbaden, Germany, 2019. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/_inhalt.html (accessed on 9 October 2019).
- Prediger, B.; Mathes, T.; Probst, C.; Pieper, D. Elective Removal vs. Retaining of Hardware after Osteosynthesis in Asymptomatic Patients—A Scoping Review. Syst. Rev. 2020, 9, 225. [Google Scholar] [CrossRef]
- Minkowitz, R.B.; Bhadsavle, S.; Walsh, M.; Egol, K.A. Removal of Painful Orthopaedic Implants after Fracture Union. J. Bone Jt. Surg. 2007, 89, 1906–1912. [Google Scholar] [CrossRef]
- Müller, M.; Mückley, T.; Hofmann, G.O. Kosten Und Komplikationen Der Materialentfernung. Trauma Und Berufskrankh. 2007, 9, S297–S301. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J.V. A Review on Current Research Status of the Surface Modification of Zn-Based Biodegradable Metals. Bioact. Mater. 2022, 7, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Price, C.T.; Langford, J.R.; Liporace, F.A. Essential Nutrients for Bone Health and a Review of Their Availability in the Average North American Diet. Open Orthop. J. 2012, 6, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and Human Health: An Update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef]
- Aghion, E.; Levy, G.; Ovadia, S. In vivo behavior of biodegradable Mg-Nd-Y-Zr-Ca alloy. J. Mater. Sci. Mater. Med. 2012, 23, 805–812. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.-M. Bone-implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011, 7, 432–440. [Google Scholar] [CrossRef]
- Jing, X.; Ding, Q.; Wu, Q.; Su, W.; Yu, K.; Su, Y.; Ye, B.; Gao, Q.; Sun, T.; Guo, X. Magnesium-based materials in orthopaedics: Material properties and animal models. Biomater. Transl. 2021, 2, 197–213. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Kulyasova, O.B.; Khudododova, G.D.; Dyakonov, G.S.; Zheng, Y.; Valiev, R.Z. Effect of Microstructure Refinement on the Corrosion Behavior of the Bioresorbable Mg-1Zn-0.2Ca and Mg-1Ca Alloys. Materials 2022, 15, 6749. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, N.; Zhang, J.; Zhang, S.; Zhao, C.; Xia, J. Degradation of Mg-6Zn alloy stents does not influence the healing of the common bile duct in vivo. Exp. Ther. Med. 2017, 13, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
- Hänzi, A.C.; Gerber, I.; Schinhammer, M.; Löffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg-Y-Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Reifenrath, J.; Bormann, D.; Meyer-Lindenberg, A. Magnesium alloys as promising degradable implant materials in orthopaedic research. In Magnesium Alloys—Corrosion and Surface Treatments; Czerwinski, F., Ed.; Intech: Rijek, Croatia, 2011; pp. 93–108. [Google Scholar]
- Bernhardt, R.; Kuhlisch, E.; Schulz, M.C.; Eckelt, U.; Stadlinger, B. Comparison of Bone-Implant Contact and Bone-Implant Volume between 2D-Histological Sections and 3D-SRµCT Slices. Eur. Cells Mater. 2012, 23, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.B.; Hansson, H.A.; Albrektsson, T. Qualitative Interfacial Study between Bone and Tantalum, Niobium or Commercially Pure Titanium. Biomaterials 1990, 11, 277–280. [Google Scholar] [CrossRef]





| Diameter, mm | Length, mm | Resorption, % | Resorption Rate, mm/week | |
|---|---|---|---|---|
| Before installation | 1.70 × 1.70 × 1.70 | 5.0 | - | - |
| 1 month post-operation | 1.46 × 1.40 × 1.36 | 4.6 × 4 × 4 | 17% | 0.04 |
| 3 months post-operation | 0.7 × 1.0 × 0.8 | 3.9 × 3.7 × 3.4 | 49% | 0.09 |
| 6 months post-operation | Not rendered | Not rendered | 100% | 0.06 |
| Element Concentration, wt.% | |||
|---|---|---|---|
| Element Name | After 1 Month | After 3 Months | After 6 Months |
| Ca | 5.32 ± 0.01 | 3.93 ± 0.01 | 0.35 ± 0.01 |
| P | 13.46 ± 0.02 | 1.95 ± 0.01 | 0.23 ± 0.01 |
| Mg | 11.80 ± 0.02 | - | - |
| Ca/P | ~0.4 | ~2 | ~1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drobyshev, A.; Gurganchova, Z.; Redko, N.; Komissarov, A.; Bazhenov, V.; Statnik, E.S.; Sadykova, I.A.; Sviridov, E.; Salimon, A.I.; Korsunsky, A.M.; et al. An In Vivo Rat Study of Bioresorbable Mg-2Zn-2Ga Alloy Implants. Bioengineering 2023, 10, 273. https://doi.org/10.3390/bioengineering10020273
Drobyshev A, Gurganchova Z, Redko N, Komissarov A, Bazhenov V, Statnik ES, Sadykova IA, Sviridov E, Salimon AI, Korsunsky AM, et al. An In Vivo Rat Study of Bioresorbable Mg-2Zn-2Ga Alloy Implants. Bioengineering. 2023; 10(2):273. https://doi.org/10.3390/bioengineering10020273
Chicago/Turabian StyleDrobyshev, Alexey, Zaira Gurganchova, Nikolay Redko, Alexander Komissarov, Viacheslav Bazhenov, Eugene S. Statnik, Iuliia A. Sadykova, Eugeny Sviridov, Alexey I. Salimon, Alexander M. Korsunsky, and et al. 2023. "An In Vivo Rat Study of Bioresorbable Mg-2Zn-2Ga Alloy Implants" Bioengineering 10, no. 2: 273. https://doi.org/10.3390/bioengineering10020273
APA StyleDrobyshev, A., Gurganchova, Z., Redko, N., Komissarov, A., Bazhenov, V., Statnik, E. S., Sadykova, I. A., Sviridov, E., Salimon, A. I., Korsunsky, A. M., Zayratyants, O., Ushmarov, D., & Yanushevich, O. (2023). An In Vivo Rat Study of Bioresorbable Mg-2Zn-2Ga Alloy Implants. Bioengineering, 10(2), 273. https://doi.org/10.3390/bioengineering10020273










