Radiographic, Biomechanical and Histological Characterization of Femoral Fracture Healing in Aged CD-1 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
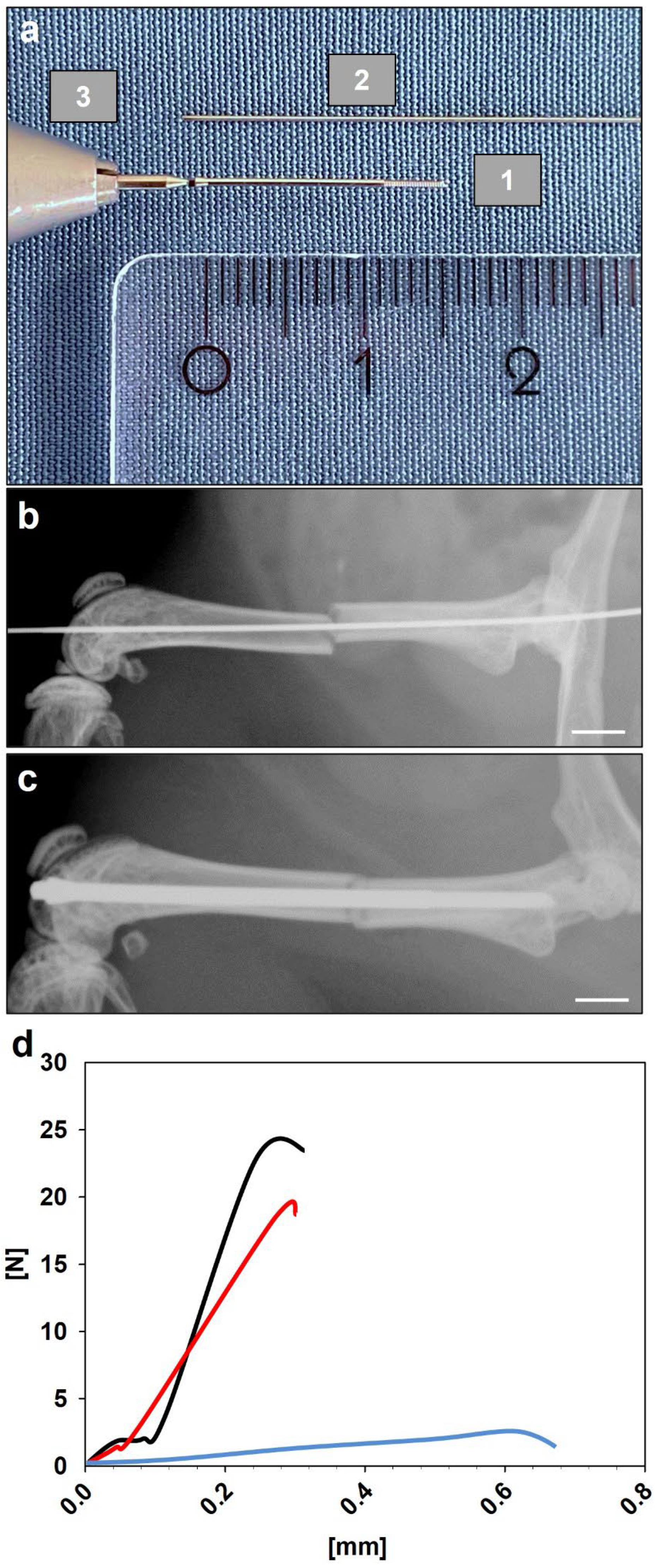
2.2. Surgical Procedure
2.3. Radiographic Analysis
2.4. Biomechanical Analysis
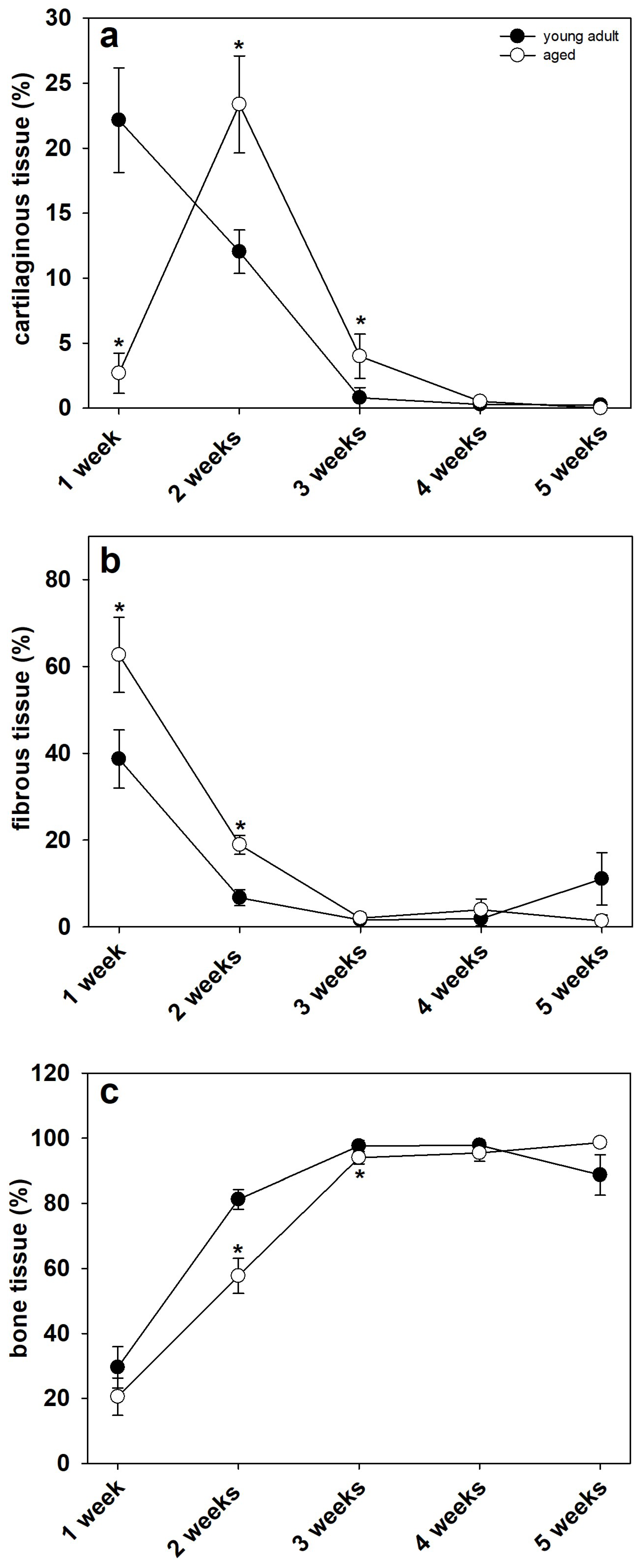
2.5. Histological Analysis
2.6. Statistics
3. Results
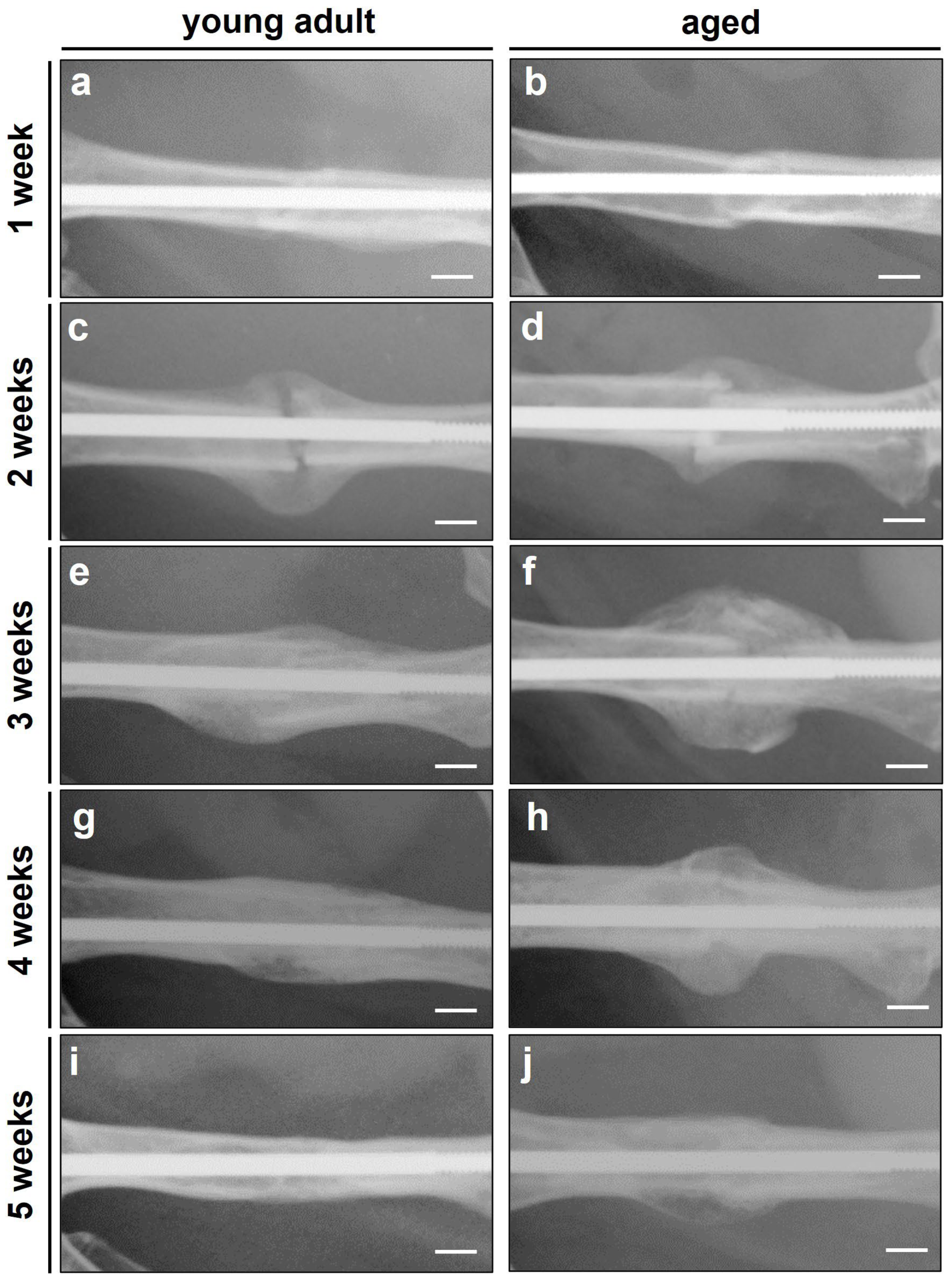
3.1. Radiographic Analysis
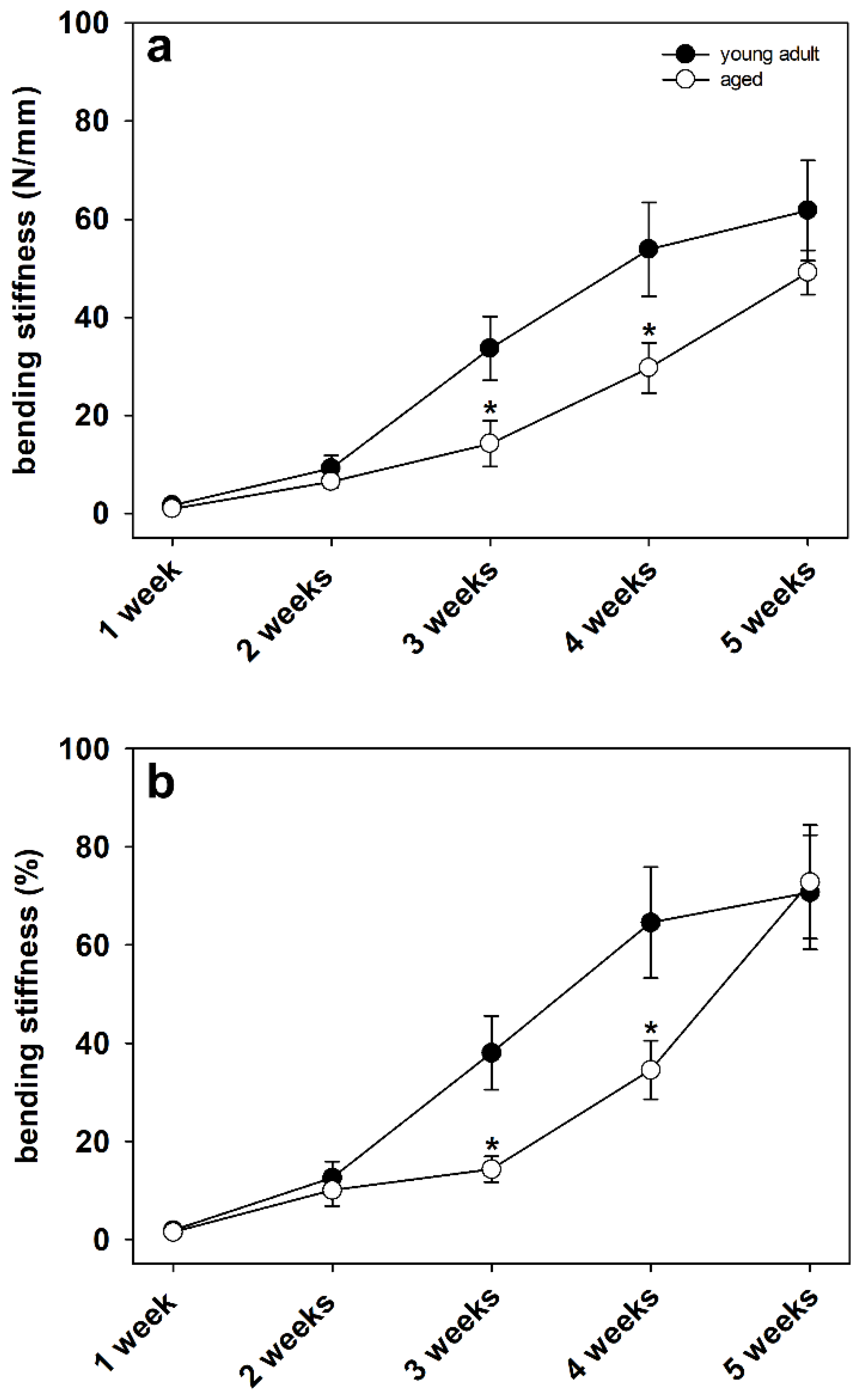
3.2. Biomechanical Analysis
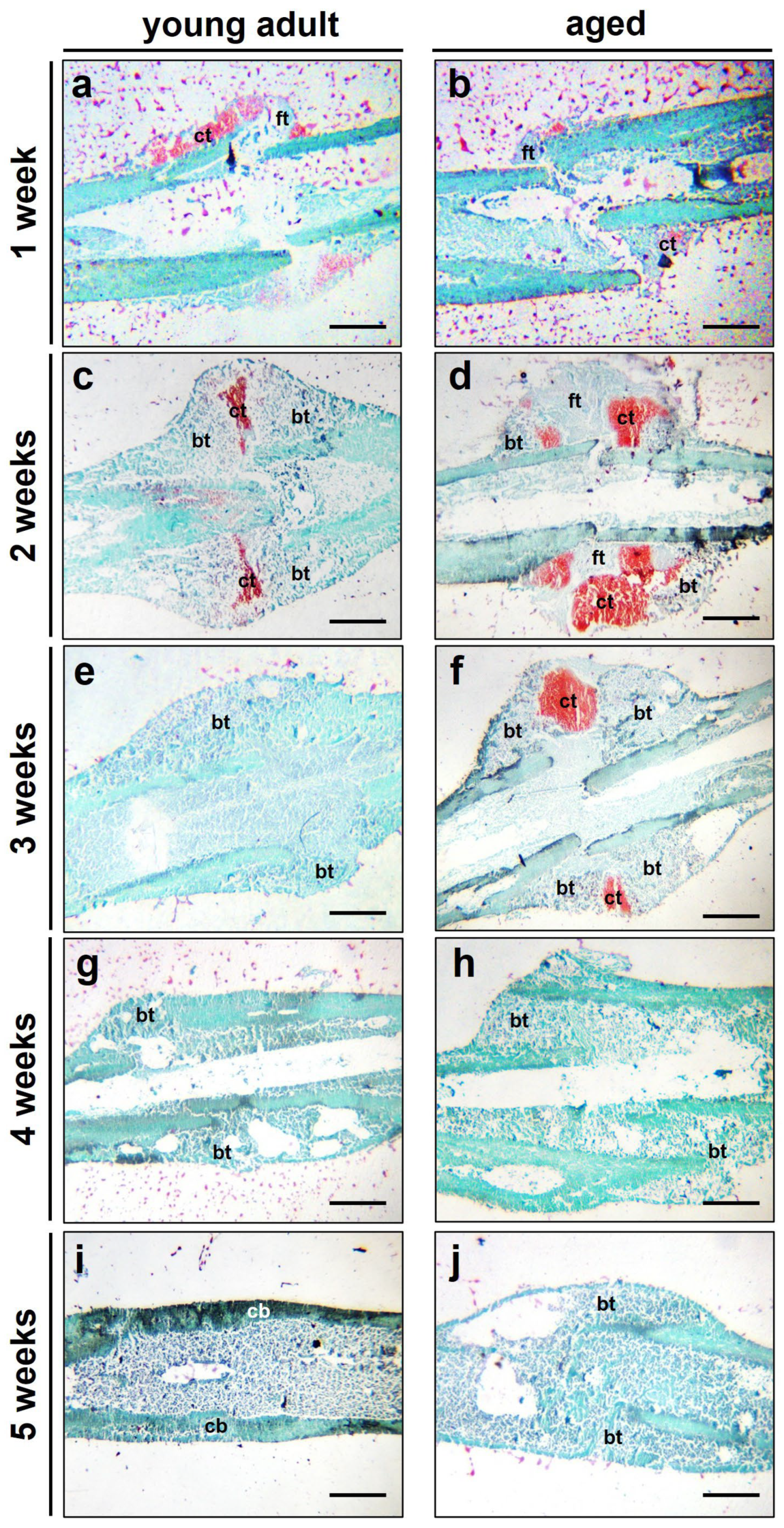
3.3. Histological Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rollmann, M.F.; Herath, S.C.; Braun, B.J.; Holstein, J.H.; Pohlemann, T.; Menger, M.D.; Histing, T. In-hospital mortality of pelvic ring fractures in older adults now and then: A pelvic registry study. Geriatr. Gerontol. Int. 2019, 19, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Maffulli, N. Hip fractures. An epidemiological review. Bull. Hosp. Jt. Dis. 1999, 58, 197–201. [Google Scholar] [PubMed]
- Cauley, J.A.; Thompson, D.E.; Ensrud, K.; Scott, J.C.; Black, D. Risk of mortality following clinical fractures. Osteoporos. Int. 2000, 11, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Miclau, T.; Chow, S.K.-H.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Histing, T.; Garcia, P.; Holstein, J.H.; Klein, M.; Matthys, R.; Nuetzi, R.; Steck, R.; Laschke, M.W.; Wehner, T.; Bindl, R.; et al. Small animal bone healing models: Standards, tips, and pitfalls results of a consensus meeting. Bone 2011, 49, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Menger, M.M.; Laschke, M.W.; Orth, M.; Pohlemann, T.; Menger, M.D.; Histing, T. Vascularization strategies in the prevention of non-union formation. Tissue Eng. Part B Rev. 2021, 27, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Histing, T.; Stenger, D.; Scheuer, C.; Metzger, W.; Garcia, P.; Holstein, J.H.; Klein, M.; Pohlemann, T.; Menger, M.D. Pantoprazole, a proton pump inhibitor, delays fracture healing in mice. Calcif. Tissue Int. 2012, 90, 507–514. [Google Scholar] [CrossRef]
- Herath, S.C.; Lion, T.; Klein, M.; Stenger, D.; Scheuer, C.; Holstein, J.H.; Morsdorf, P.; Rollmann, M.F.; Pohlemann, T.; Menger, M.D.; et al. Stimulation of angiogenesis by cilostazol accelerates fracture healing in mice. J. Orthop. Res. 2015, 33, 1880–1887. [Google Scholar] [CrossRef]
- Garcia, P.; Speidel, V.; Scheuer, C.; Laschke, M.W.; Holstein, J.H.; Histing, T.; Pohlemann, T.; Menger, M.D. Low dose erythropoietin stimulates bone healing in mice. J. Orthop. Res. 2011, 29, 165–172. [Google Scholar] [CrossRef]
- Holstein, J.H.; Orth, M.; Scheuer, C.; Tami, A.; Becker, S.C.; Garcia, P.; Histing, T.; Morsdorf, P.; Klein, M.; Pohlemann, T.; et al. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone 2011, 49, 1037–1045. [Google Scholar] [CrossRef]
- Orth, M.; Shenar, A.K.; Scheuer, C.; Braun, B.J.; Herath, S.C.; Holstein, J.H.; Histing, T.; Yu, X.; Murphy, W.L.; Pohlemann, T.; et al. VEGF-loaded mineral-coated microparticles improve bone repair and are associated with increased expression of epo and RUNX-2 in murine non-unions. J. Orthop. Res. 2019, 37, 821–831. [Google Scholar] [CrossRef]
- Menger, M.M.; Bremer, P.; Scheuer, C.; Rollmann, M.F.; Braun, B.J.; Herath, S.C.; Orth, M.; Spater, T.; Pohlemann, T.; Menger, M.D.; et al. Pantoprazole impairs fracture healing in aged mice. Sci. Rep. 2020, 10, 22376. [Google Scholar] [CrossRef]
- Orth, M.; Baudach, J.; Scheuer, C.; Osche, D.; Veith, N.T.; Braun, B.J.; Rollmann, M.F.; Herath, S.C.; Pohlemann, T.; Menger, M.D.; et al. Erythropoietin does not improve fracture healing in aged mice. Exp. Gerontol. 2019, 122, 1–9. [Google Scholar] [CrossRef]
- Holstein, J.H.; Matthys, R.; Histing, T.; Becker, S.C.; Fiedler, M.; Garcia, P.; Meier, C.; Pohlemann, T.; Menger, M.D. Development of a stable closed femoral fracture model in mice. J. Surg. Res. 2009, 153, 71–75. [Google Scholar] [CrossRef]
- Homburger, F.; Russfield, A.B.; Weisburger, J.H.; Lim, S.; Chak, S.; Weisburger, E.K. Aging Changes in CD-1 HaM/ICR Mice Reared Under Standard Laboratory Conditions. JNCI J. Natl. Cancer Inst. 1975, 55, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Schoen, M.; Rotter, R.; Schattner, S.; Mittlmeier, T.; Claes, L.; Vollmar, B.; Gradl, G. Introduction of a new interlocked intramedullary nailing device for stabilization of critically sized femoral defects in the rat: A combined biomechanical and animal experimental study. J. Orthop. Res. 2008, 26, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Tonna, E.A.; Cronkite, E.P. The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine. Clin. Orthop. Relat. 1963, 30, 218–233. [Google Scholar]
- Colnot, C.; Thompson, Z.; Miclau, T.; Werb, Z.; Helms, J.A. Altered fracture repair in the absence of MMP9. Development 2003, 130, 4123–4133. [Google Scholar] [CrossRef]
- Menger, M.M.; Laschke, M.W.; Nussler, A.K.; Menger, M.D.; Histing, T. The vascularization paradox of non-union formation. Angiogenesis 2022, 25, 279–290. [Google Scholar] [CrossRef]
- Fang, T.D.; Salim, A.; Xia, W.; Nacamuli, R.P.; Guccione, S.; Song, H.M.; Carano, R.A.; Filvaroff, E.H.; Bednarski, M.D.; Giaccia, A.J.; et al. Angiogenesis is required for Successful Bone Induction Buring Distraction Osteogenesis. J. Bone Miner. Res. 2005, 20, 1114–1124. [Google Scholar] [CrossRef]
- Murnughan, M.; Li, G.; Marsh, D.R. Nonsteroidal anti-inflammatory drug-induced fracture nonunion: An inhibition of angiogenesis? J. Bone Jt. Surg. Am. 2006, 88, 140–147. [Google Scholar]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef]
- Kon, T.; Cho, T.J.; Aizawa, T.; Yamazaki, M.; Nooh, N.; Graves, D.; Gerstenfeld, L.C.; Einhorn, T.A. Expression of osteoprotegerin, receptor activator of NF-κB Ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001, 16, 1004–1014. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 4–9. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Osta, B.; Benedetti, G.; Miossec, P. Classical and Paradoxical Effects of TNF-α on Bone Homeostasis. Front. Immunol. 2014, 13, 48. [Google Scholar] [CrossRef]
- Lopez, E.M.; Leclerc, K.; Ramsukh, M.; EL Parente, P.; Patel, K.; Aranda, C.J.; Josephson, A.M.; Remark, L.H.; Kirby, D.J.; Buchalter, D.B.; et al. Modulating the systemic and local adaptive immune response after fracture improves bone regeneration during aging. Bone 2022, 157, 116324. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Josephson, A.M.; Bradaschia-Correa, V.; Lee, S.; Leclerc, K.; Patel, K.S.; Lopez, E.M.; Litwa, H.P.; Neibart, S.S.; Kadiyala, M.; Wong, M.Z.; et al. Age related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 6995–7004. [Google Scholar] [CrossRef]
- Josephson, A.M.; Leclerc, K.; Remark, L.H.; Lopeź, E.M.; Leucht, P. Systemic NF-κB-mediated inflammation promotes an aging phenotype in skeletal stem/progenitor cells. Aging 2021, 13, 13421–13429. [Google Scholar] [CrossRef]
- Barrios, C.; Brostrom, L.A.; Stark, A.; Walheim, G. Healing complications after internal fixation of trochanteric hip fractures: The prognostic value of osteoporosis. J. Orthop. Trauma 1993, 7, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Namkung-Matthai, H.; Appleyard, R.; Jansen, J.; Hao Lin, J.; Maastricht, S.; Swain, M.; Mason, R.S.; Murrell, G.A.; Diwan, A.D.; Diamond, T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 2001, 28, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Li, W.; Xu, S.W.; Yang, D.S.; Wang, Y.; Lin, M.; Zhao, G.F. Osteoporosis influences the middle and late periods of fracture healing in a rat osteoporotic model. Chin. J. Traumatol. 2005, 8, 111–116. [Google Scholar] [PubMed]
- Langeland, N. Effects of oestradiol-17 beta benzoate treatment on fracture healing and bone collagen synthesis in female rats. Acta Endocrinol. 1975, 80, 603–612. [Google Scholar] [CrossRef]
- Melhus, G.; Solberg, L.B.; Dimmen, S.; Madsen, J.E.; Nordsletten, L.; Reinholt, F.P. Experimental osteoporosis induced by ovariectomy and vitamin D deficiency does not markedly affect fracture healing in rats. Acta Orthop. 2007, 78, 393–403. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The role of endogenous bone morphogenetic proteins in normal skeletal repair. Injury 2009, 40, S4–S7. [Google Scholar] [CrossRef]
- Histing, T.; Kuntz, S.; Stenger, D.; Scheuer, C.; Garcia, P.; Holstein, J.H.; Klein, M.; Pohlemann, T.; Menger, M.D. Delayed fracture healing in aged senescence-accelerated P6 mice. J. Investig. Surg. 2013, 26, 30–35. [Google Scholar] [CrossRef]
- Lopas, L.A.; Belkin, N.S.; Mutyaba, P.L.; Gray, C.F.; Hankenson, K.D.; Ahn, J. Fractures in Geriatric Mice Show Decreased Callus Expansion and Bone Volume. Clin. Orthop. Relat. Res. 2014, 472, 3523–3532. [Google Scholar] [CrossRef]
- Gibon, E.; Lu, L.; Goodman, S.B. Aging, inflammation, stem cells, and bone healing. Stem Cell Res. Ther. 2016, 7, 44. [Google Scholar] [CrossRef]
- Fayolle, C.; Labrune, M.; Berteau, J.-P. Raman spectroscopy investigation shows that mineral maturity is greater in CD-1 than in C57BL/6 mice distal femurs after sexual maturity. Connect. Tissue Res. 2020, 61, 409–419. [Google Scholar] [CrossRef]
- Ward, W.E.; Kim, S.; Chan, D.; Fonseca, D. Serum equol, bone mineral density and biomechanical bone strength differ among four mouse strains. J. Nutr. Biochem. 2005, 16, 743–749. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menger, M.M.; Manuschewski, R.; Ehnert, S.; Rollmann, M.F.; Maisenbacher, T.C.; Tobias, A.L.; Menger, M.D.; Laschke, M.W.; Histing, T. Radiographic, Biomechanical and Histological Characterization of Femoral Fracture Healing in Aged CD-1 Mice. Bioengineering 2023, 10, 275. https://doi.org/10.3390/bioengineering10020275
Menger MM, Manuschewski R, Ehnert S, Rollmann MF, Maisenbacher TC, Tobias AL, Menger MD, Laschke MW, Histing T. Radiographic, Biomechanical and Histological Characterization of Femoral Fracture Healing in Aged CD-1 Mice. Bioengineering. 2023; 10(2):275. https://doi.org/10.3390/bioengineering10020275
Chicago/Turabian StyleMenger, Maximilian M., Ruben Manuschewski, Sabrina Ehnert, Mika F. Rollmann, Tanja C. Maisenbacher, Anne L. Tobias, Michael D. Menger, Matthias W. Laschke, and Tina Histing. 2023. "Radiographic, Biomechanical and Histological Characterization of Femoral Fracture Healing in Aged CD-1 Mice" Bioengineering 10, no. 2: 275. https://doi.org/10.3390/bioengineering10020275
APA StyleMenger, M. M., Manuschewski, R., Ehnert, S., Rollmann, M. F., Maisenbacher, T. C., Tobias, A. L., Menger, M. D., Laschke, M. W., & Histing, T. (2023). Radiographic, Biomechanical and Histological Characterization of Femoral Fracture Healing in Aged CD-1 Mice. Bioengineering, 10(2), 275. https://doi.org/10.3390/bioengineering10020275





