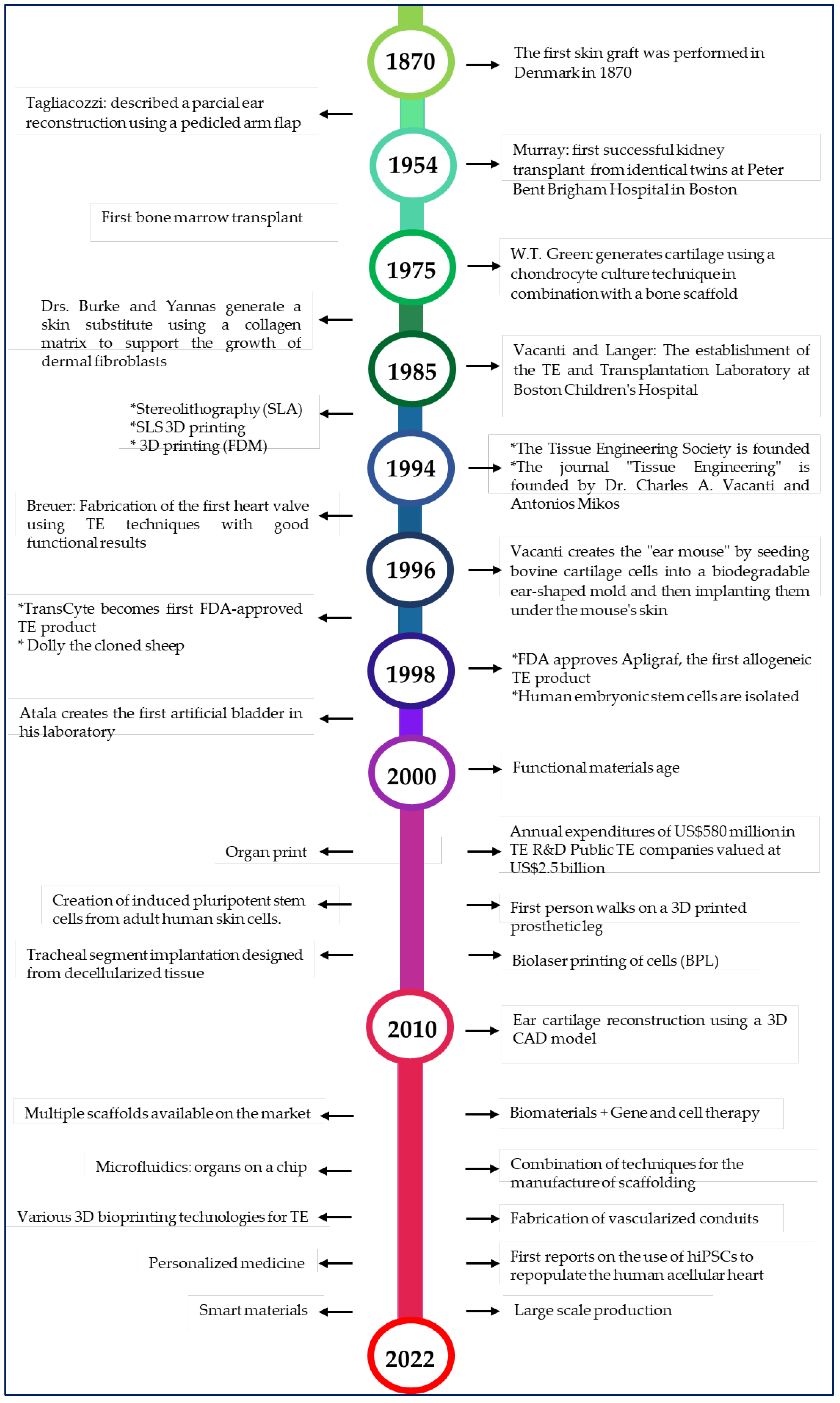
Polymeric Materials, Advances and Applications in Tissue Engineering: A Review
Abstract
1. Introduction

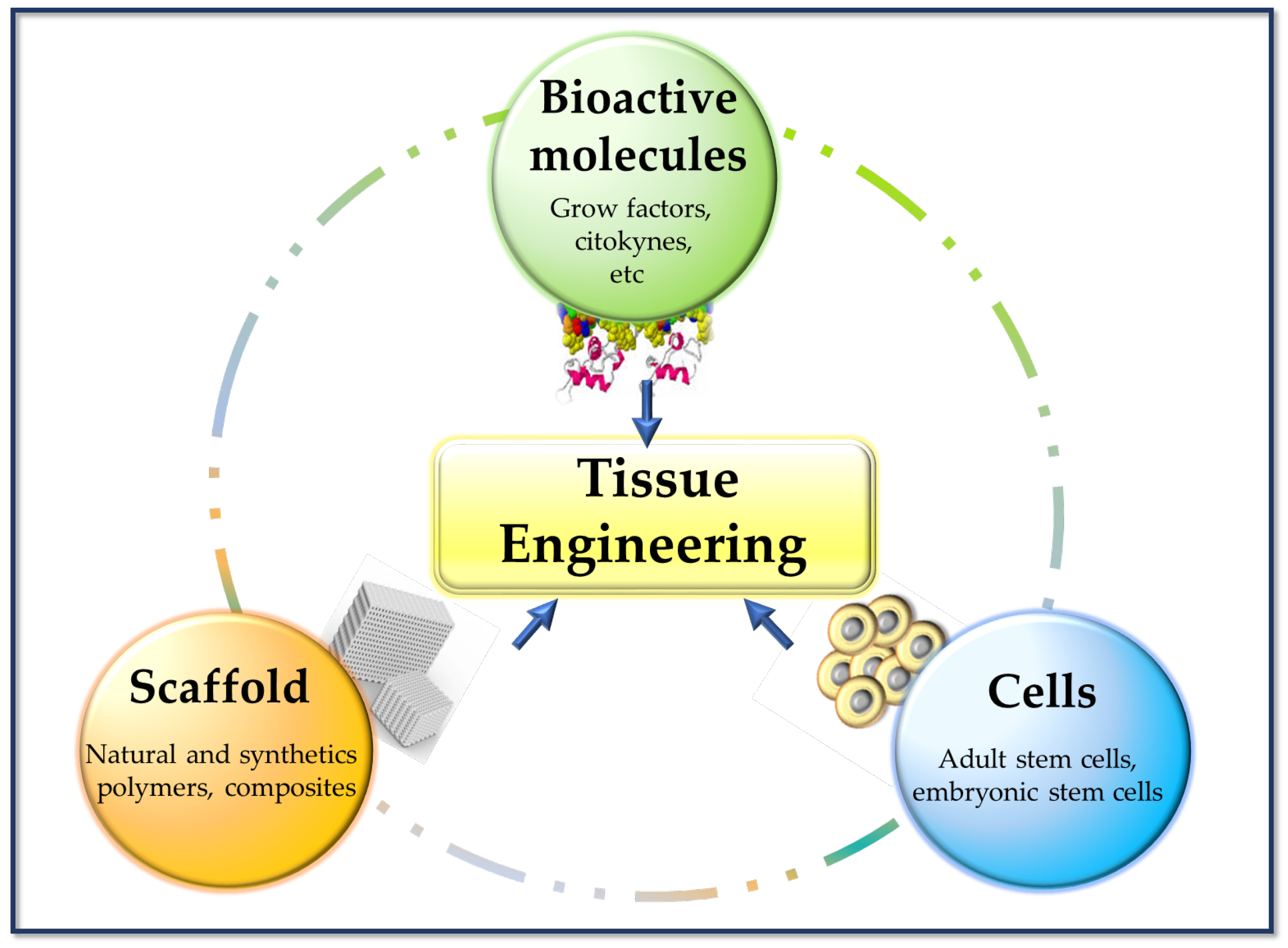
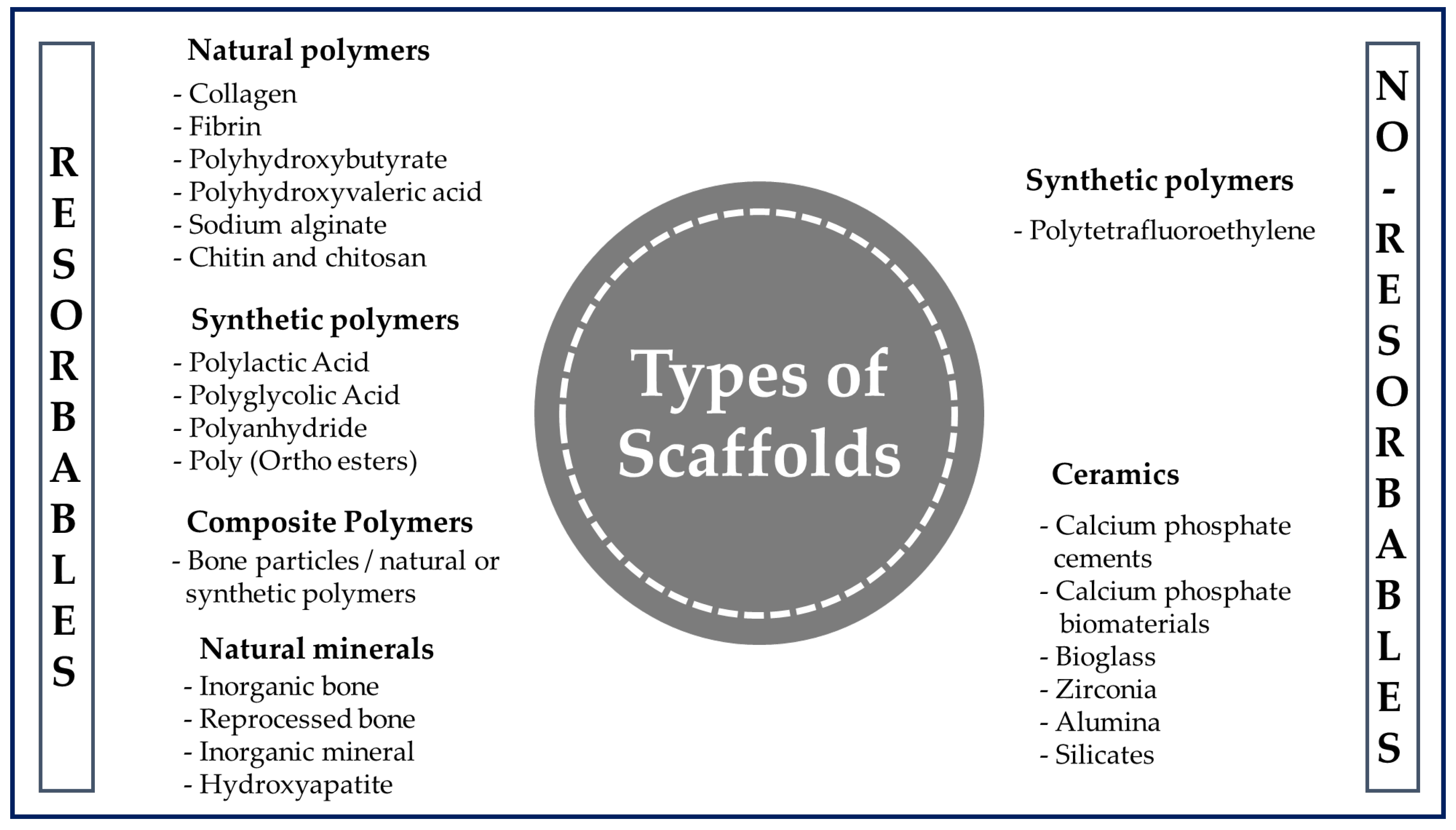
2. Biomaterials for Tissue Engineering Scaffolds Fabrication
| Feature | Description |
|---|---|
| Adequate intrinsic physical and mechanical properties | This is defined by the microarchitecture and surface microtextures (surface topography). An ideal microarchitecture should be highly porous, with defined and interconnected pore sizes and a high surface area to volume ratio to allow for better vascularization, mass transfer, and cell growth. |
| Biocompatibility | It must produce the desired effect, be safe, and cause the minimum degree of inflammation once implanted. |
| Bioactivity | The biomaterial-cell interaction favors cell adhesion and proliferation, facilitating contact between cells and their migration over a prolonged period. Therefore, scaffolds can include biological molecules on their surface to promote cell adhesion or can also serve as a delivery vehicle or reservoir for growth-stimulating substances such as growth factors to accelerate regeneration. |
| Mimic EMC | It must be capable of mimicking the native tissue, providing an environment of optimal protection and nutrition. |
| Bioabsorption | It must be bioabsorbed in a controlled and appropriate time so that the new tissue replaces the space initially occupied by the biomaterials. |
| Versatility | They must be adaptable to different manufacturing techniques. |
| Translational perspective | The scaffold must be reproducible, accessible, and scalable to enable its use in high-demand applications for large tissues. |
3. Polymers for Tissue Engineering
4. Strategies for Fabrication of Scaffolds in Tissue Engineering
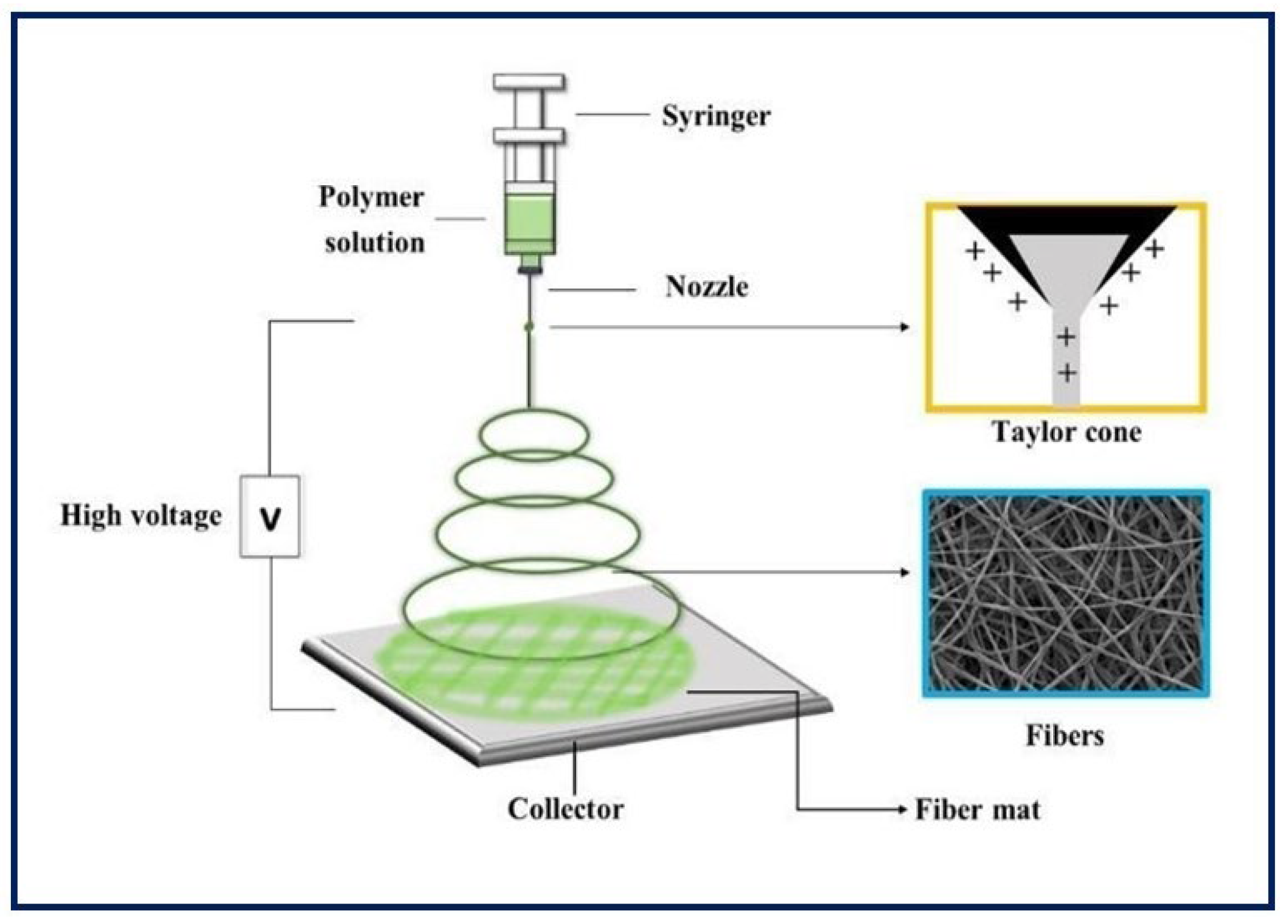
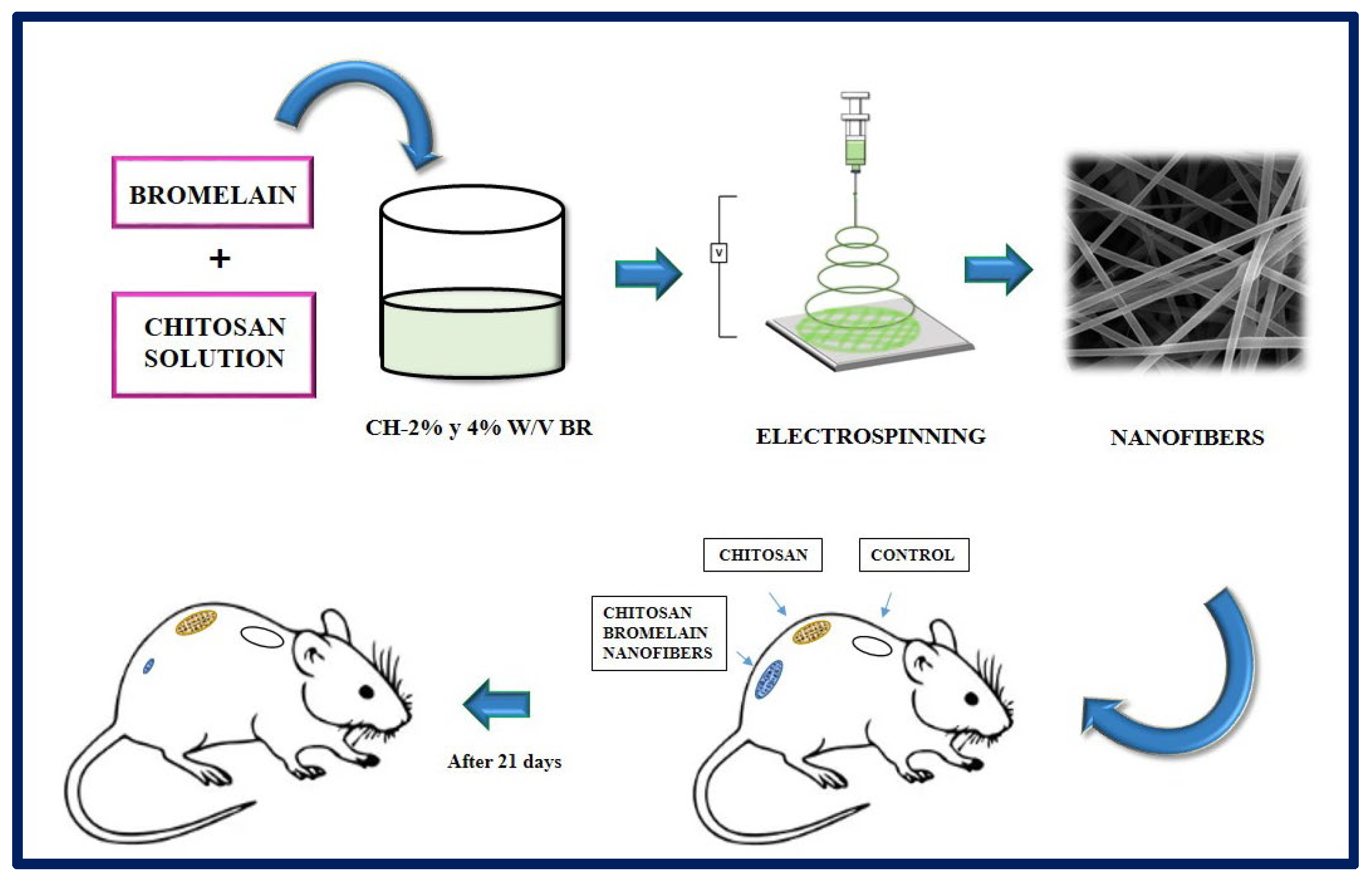
4.1. Electrospinning
4.2. Molecular Self-Assembly
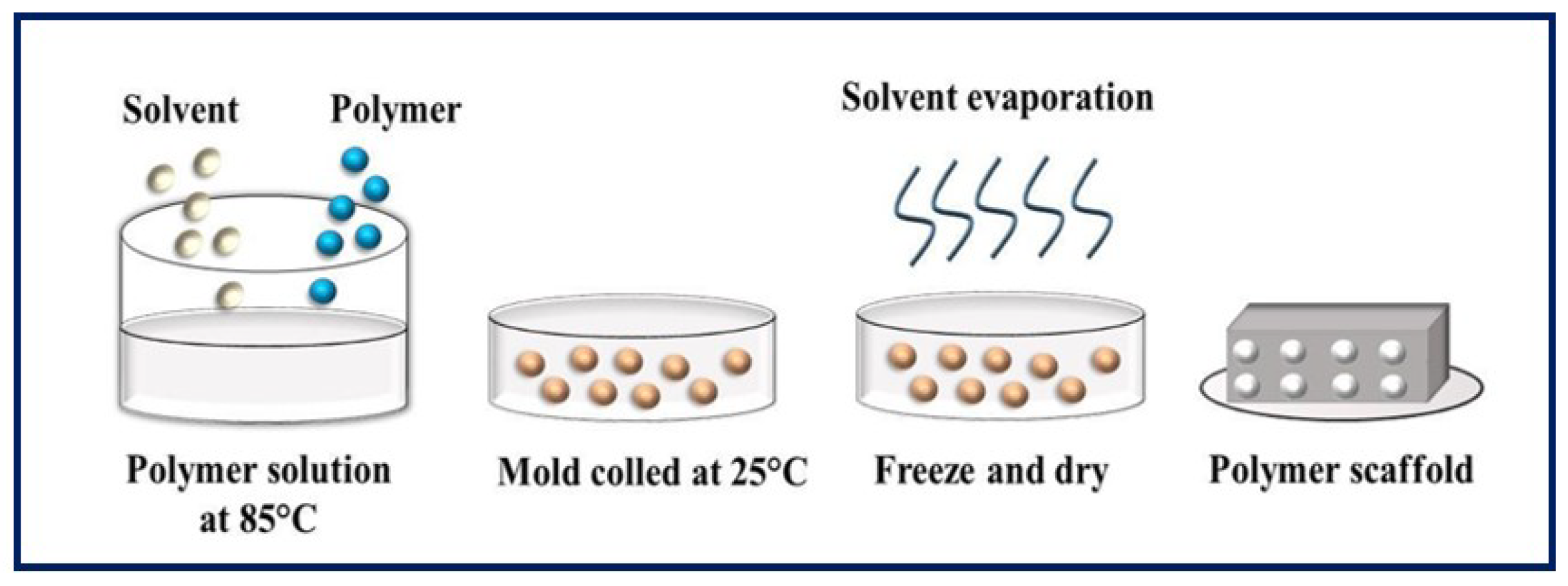
4.3. Phase Separation
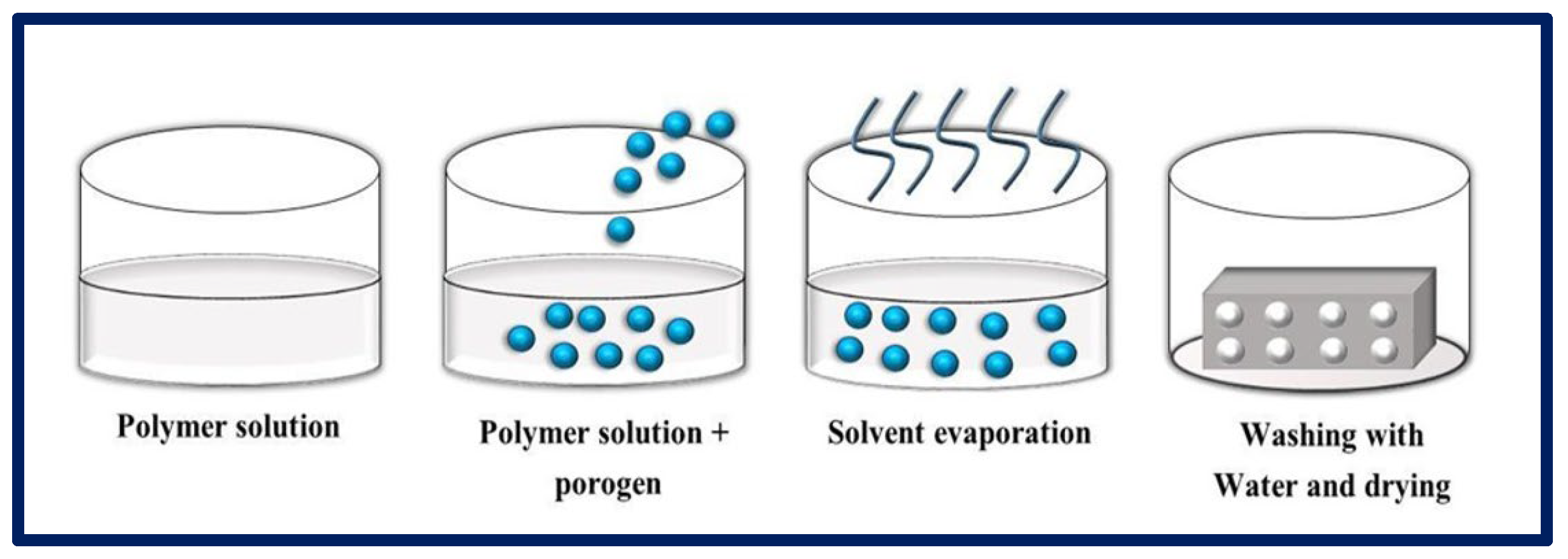
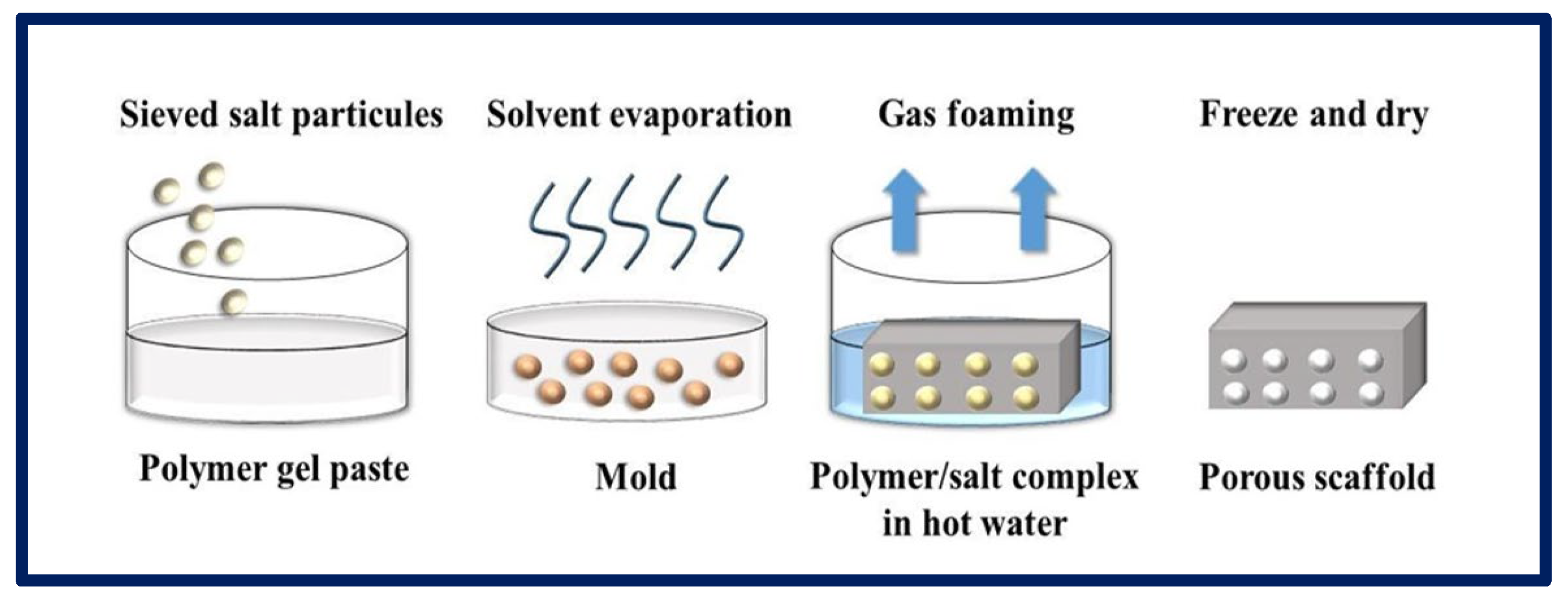
4.4. Particulate Leaching
4.5. Gas Foaming
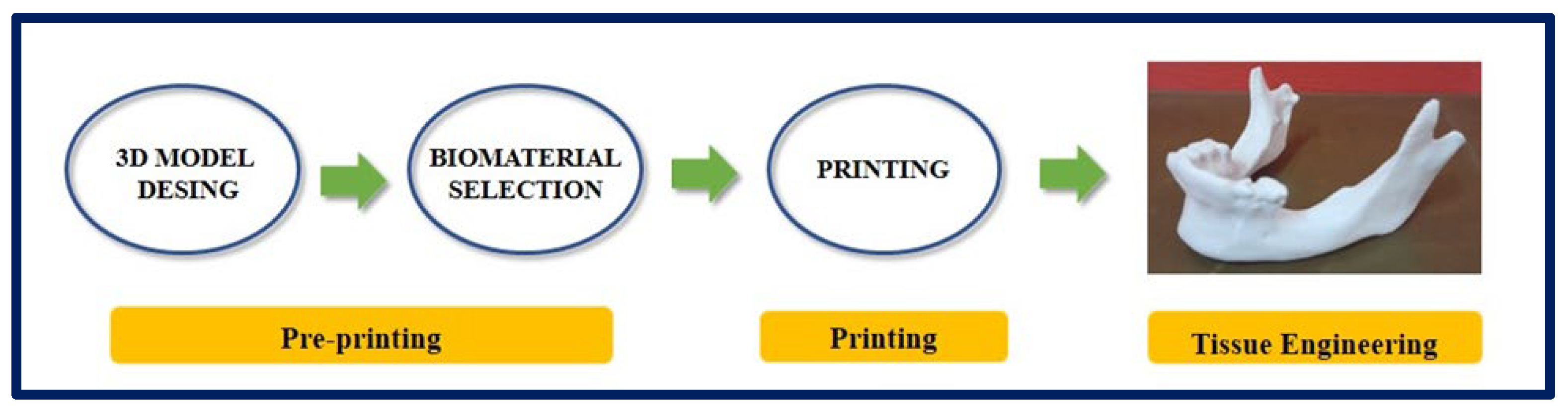
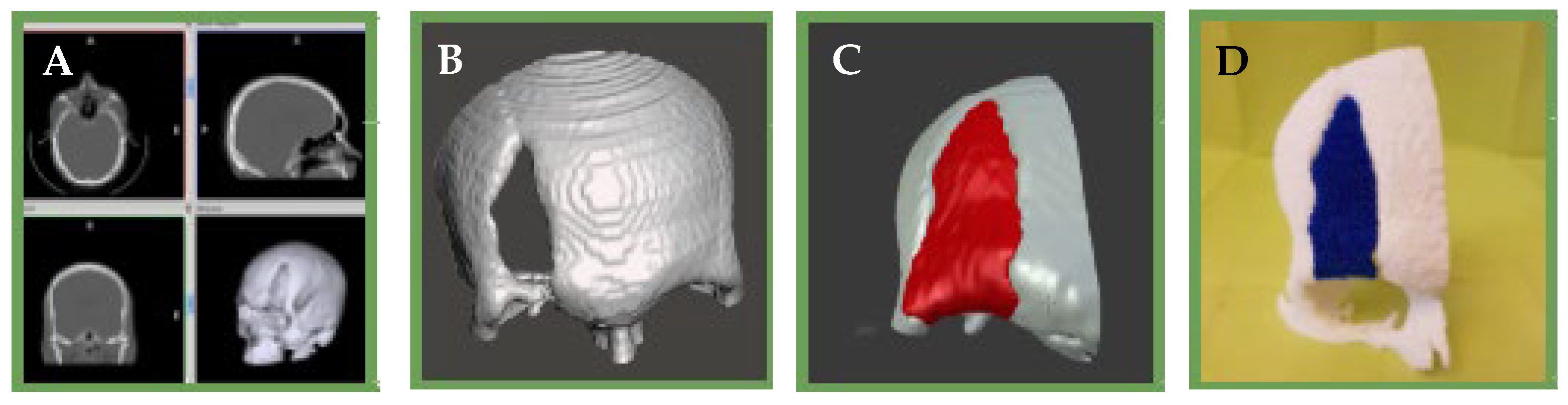
4.6. Rapid Prototyping
4.7. Decellularization
4.8. Cell Sheets
5. Smart Materials
6. Functionalization Strategies to Promote Bioactivity in Polymeric Supports
7. Cells for Tissue Engineering: Stem Cells
8. Stem Cell Classification
9. Cell Replacement Therapies Combined with Tissue Engineering Strategies: More Complex Than Is Believed
10. Applications of Polymers for Tissue Engineering in Experimental Models
10.1. Epithelial Tissue Engineering
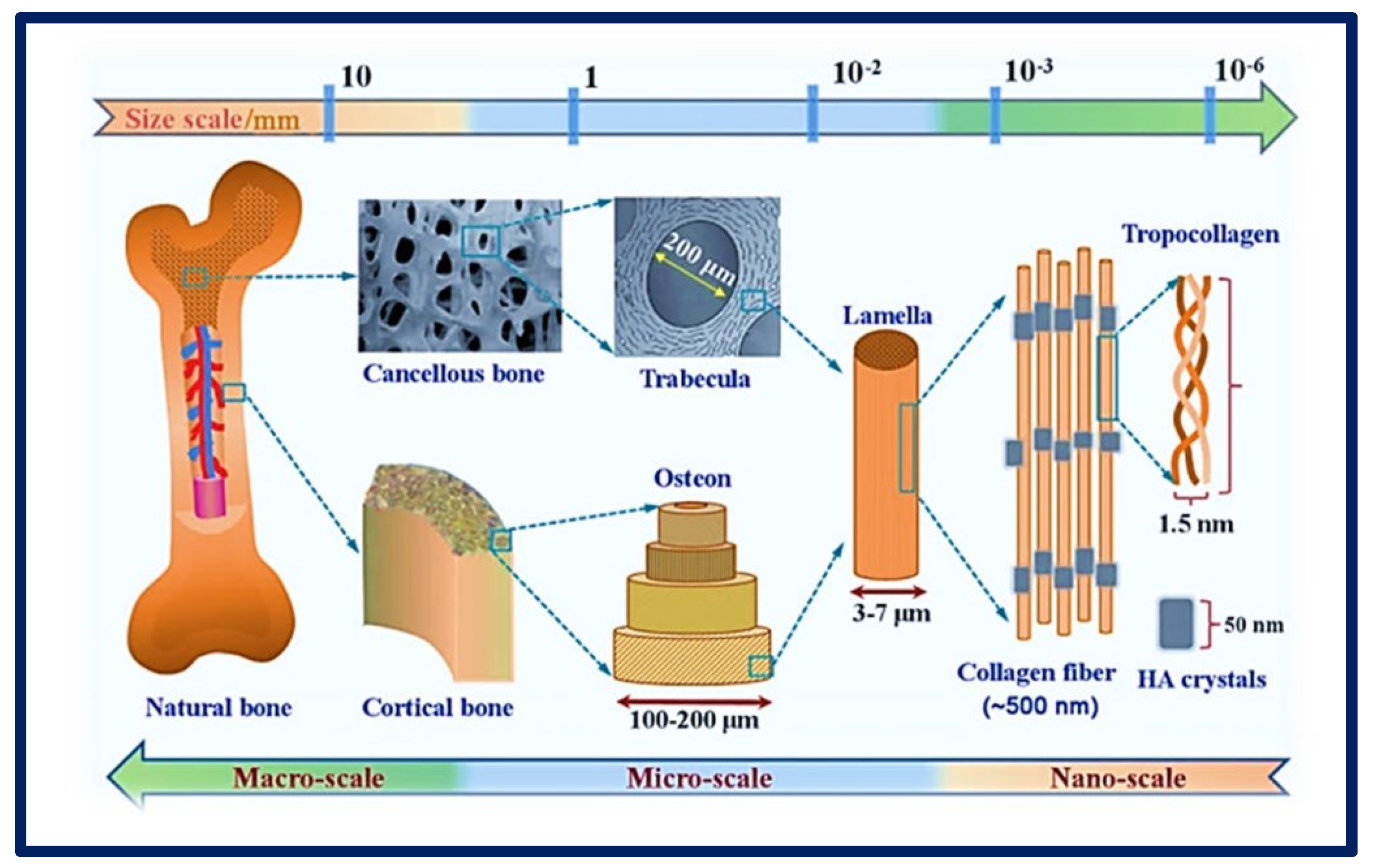
10.2. Bone Tissue Engineering
10.3. Urinary Tissue Engineering
10.4. Uterus Tissue Engineering
10.5. Vascular Tissue Engineering
10.6. Cardiac Tissue Engineering
10.7. Cartilage Tissue Engineering
10.8. Neural Tissue Engineering
10.9. Adipose Tissue Engineering
11. Tissue Engineering Polymers in Clinical Applications
12. Tissue Engineering Polymers in the Market
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Chang, J.; Zhu, Y.; Wu, C. 3D printing of bioinspired biomaterials for tissue regeneration. Adv. Health Mater. 2020, 27, e2000208. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G. Diseño y preparación de matrices poliméricas porosas para ingeniería de tejidos biológicos. Anales. Acad. Nac. CS Ex. Fis. Nat. 2017, 59, 115–130. [Google Scholar]
- Del Barrio Cortés, E.; Matutano Molina, C.; Rodríguez-Lorenzo, L.; Cubo-Mateo, N. Generation of Controlled Micrometric Fibers inside Printed Scaffolds Using Standard FDM 3D Printers. Polymers 2023, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, V.D.; Zeng, P.; Li, B.; Zhang, Y. In vitro cell culture in hollow microfibers with porous structures. Biomater. Sci. 2020, 8, 2175–2188. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric scaffolds for regenerative medicine. Polym. Rev. 2011, 51, 23–52. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef]
- Vacanti, J.P.; Vacanti, C.A. The history and scope of tissue engineering. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–8. [Google Scholar]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Bio-medical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; Al Ammari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrà, A.; Bhawani, S.A.; Ibrahim, M.N.M.; Khan, A.; Alorfi, H.S.; Asiri, A.M.; Hussein, M.A.; Khan, I.; Umar, K. Utilizing Biomass-Based Graphene Oxide–Polyaniline–Ag Electrodes in Microbial Fuel Cells to Boost Energy Generation and Heavy Metal Removal. Polymers 2022, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jung, J.-Y.; Shin, H.-W.; Park, J.-W.; Bang, J.; Huh, J. Loop and Bridge Conformations of ABA Triblock Comb Co-polymers: A Conformational Assessment for Molecular Composites. Polymers 2022, 14, 2301. [Google Scholar] [CrossRef] [PubMed]
- Hansapaiboon, S.; Bulatao, B.P.; Sorasitthiyanukarn, F.N.; Jantaratana, P.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Fabrication of Curcumin Diethyl γ-Aminobutyrate-Loaded Chitosan-Coated Magnetic Nanocarriers for Improvement of Cytotoxicity against Breast Cancer Cells. Polymers 2022, 14, 5563. [Google Scholar] [CrossRef]
- El-Dessouky, H.M.; McHugh, C. Multifunctional auxetic and honeycomb composites made of 3D woven carbon fibre preforms. Sci. Rep. 2022, 12, 22593. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.I.; Astudillo, S.; Mina Hernandez, J.H.; Saavedra, M.; Zapata, P.A.; Valencia-Llano, C.H.; Chaur, M.N.; Grande-Tovar, C.D. Synthesis, Characterization, and Optimization Studies of Polycaprolactone/Polylactic Acid/Titanium Dioxide Nanoparti-cle/Orange Essential Oil Membranes for Biomedical Applications. Polymers 2023, 15, 135. [Google Scholar] [CrossRef]
- Harris, J.P.; Burrell, J.C.; Struzyna, L.A.; Chen, H.I.; Serruya, M.D.; Wolf, J.A.; Duda, J.; Cullen, K. Emerging regenerative medicine and tissue engineering strategies for Parkinson’s disease. NPJ Park. Dis. 2020, 6, 4. [Google Scholar] [CrossRef]
- Diego Rodriguez, E.; Villanueva Peña, A.; Roca Edreira, A.; Martín García, B.; Meana Infiesta, A.; Gómez Llames, S. Estado actual de la ingeniería de tejidos en urología: Revisión de la literatura. Actas Urol. Esp. 2004, 28, 636–645. [Google Scholar] [CrossRef]
- Chaignaud, B.E.; Langer, R.; Vacanti, J.P. The history of tissue engineering using synthetic biodegradable polymer scaffolds and cells. In Synthetic Biodegradable Polymer Scaffolds; Atala, A., Mooney, D.J., Eds.; Birkhäuser Boston: Boston, MA, USA, 1996; pp. 1–14. [Google Scholar]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Kengla, C.; Lee, S.J.; Yoo, J.J. AtalaA. 3-D bioprinting technologies for tissue engineering applications. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–288. [Google Scholar]
- Woodfield, T.; Lim, K.; Morouço, P.; Levato, R.; Malda, J.; Melchels, F. Biofabrication in tissue engineering. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 236–266. [Google Scholar]
- Garreta, E.; Oria, R.; Tarantino, C.; Pla-Roca, M.; Prado, P.; Fernández-Avilés, F.; Campistol, J.M.; Samitier, J.; Montserrat, N. Tissue engineering by decellularization and 3D bioprinting. Mater. Today 2017, 20, 166–178. [Google Scholar] [CrossRef]
- Meyer, U. The history of tissue engineering and regenerative medicine in perspective. In Fundamentals of Tissue Engineering and Regenerative Medicine; Meyer, U., Handschel, J., Wiesmann, H.P., Meyer, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 5–12. [Google Scholar]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef]
- Vacanti, C.A. History of tissue engineering and a glimpse into its future. Tissue Eng. 2006, 12, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Yousefi, A.-M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.S.; Kandasubramanian, B. Progress in the advancement of porous biopolymer scaffold: Tissue engineering application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
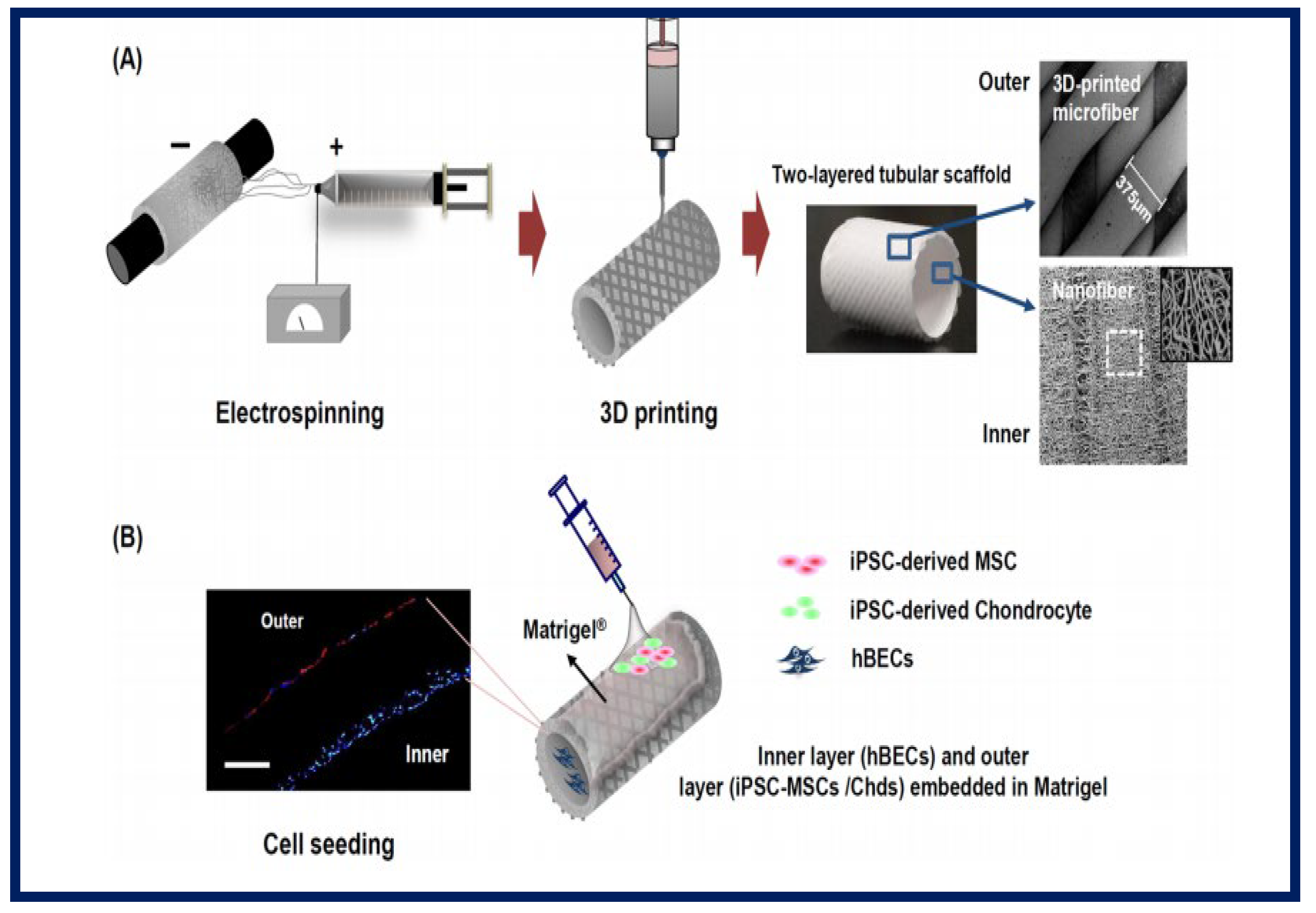
- Kim, I.G.; Park, S.A.; Lee, S.-H.; Choi, J.S.; Cho, H.; Lee, S.J.; Kwon, Y.-W.; Kwon, S.K. Transplantation of a 3D-printed tracheal graft combined with iPS cell-derived MSCs and chondrocytes. Sci. Rep. 2020, 10, 4326. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef]
- Chung, H.J.; Park, T.G. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 249–262. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef]
- Moreno, M.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Scaffolds for bone regeneration: State of the art. Curr. Pharm. Des. 2016, 22, 2726–2736. [Google Scholar] [CrossRef] [PubMed]
- Moreno Madrid, A.P.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Sabino, M.A.; Loaiza, M.; Dernowsek, J.; Rezende, R.; Da Silva, J.V.L. Técnicas para la fabricación de andamios poliméricos con aplicaciones en ingenierÍa de tejidos. Rev. LatinAm. Metal. Mat. 2017, 37, 1–27. [Google Scholar]
- Perniconi, B.; Costa, A.; Aulino, P.; Teodori, L.; Adamo, S.; Coletti, D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 2011, 32, 7870–7882. [Google Scholar] [CrossRef]
- Aguero Luztonó, L.; Zaldivar Silva, D.; Escobar Ivirico, J.L. Liberación de cefalexina a partir de hidrogeles de poli (acrilamida-co-ácido metacrílico). Biomecánica 2000, 18, 58–62. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Chan, J.P.; Battiston, K.G.; Santerre, J.P. Synthesis and characterization of electrospun nanofibrous tissue engineering scaffolds generated from in situ polymerization of ionomeric polyurethane composites. Acta Biomater. 2019, 96, 161–174. [Google Scholar] [CrossRef]
- Pierre, J.; Maria, K.; Solene-Emmanuelle, B.; Hany, N.; Valerie, V.; Mathieu, P.; Jerome, L.; Patricia, F.; Yong, C.; Philippe, M.; et al. Nanofibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy. Biomaterials 2016, 80, 157–168. [Google Scholar]
- Mata, A.; Kim, E.J.; Boehm, C.A.; Fleischman, A.J.; Muschler, G.F.; Roy, S. A three-dimensional scaffold with precise micro-architecture and surface micro-textures. Biomaterials 2009, 30, 4610–4617. [Google Scholar] [CrossRef]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer- and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Sun, H. Manufacture of Biomaterials. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 116–134. [Google Scholar]
- Yu, T.-T.; Cui, F.-Z.; Meng, Q.-Y.; Wang, J.; Wu, D.-C.; Zhang, J.; Kou, X.-X.; Yang, R.-L.; Liu, Y.; Zhang, Y.S.; et al. Influence of surface chemistry on adhesion and osteo/odontogenic differentiation of dental pulp stem cells. ACS Biomater. Sci. Eng. 2017, 3, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Boey, F.; Lao, L.L. Implanted cardiovascular polymers: Natural, synthetic and bio-inspired. Prog. Polym. Sci. 2008, 33, 853–874. [Google Scholar] [CrossRef]
- Hamid Akash, M.S.; Rehman, K.; Chen, S. Natural and synthetic polymers as drug carriers for delivery of therapeutic proteins. Polym. Rev. 2015, 55, 371–406. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. [Google Scholar]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni Vishakha, S.; Butte Kishor, D.; Rathod Sudha, S. Natural polymers—A comprehensive review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1597–1613. [Google Scholar]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2018, 64, 1–36. [Google Scholar] [CrossRef]
- Qian, Z.; Radke, D.; Jia, W.; Tahtinen, M.; Wang, G.; Zhao, F. Bioengineering scaffolds for regenerative engineering. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 444–461. [Google Scholar]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109768. [Google Scholar] [CrossRef]
- Gómez Perez, C.A. Desarrollo de Implantes Craneofaciales Utilizando Técnicas de Diseño y Manufactura Avanzadas. Master’s Thesis, Facultad de Ingeniería, Universidad Autonoma de San Luis Potosí, Cuernavaca, Mexico, 2015. [Google Scholar]
- Coluzza, I.; Pisignano, D.; Gentili, D.; Pontrelli, G.; Succi, S. Ultrathin Fibers from Electrospinning Experiments under Driven Fast-Oscillating Perturbations. Phys. Rev. Appl. 2014, 2, 054011. [Google Scholar] [CrossRef]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ma, Q.; Yu, W.; Li, D.; Dong, X.; Liu, G.; Yu, H. Comparison of different electrospinning technologies for the production of arrays with multifunctional properties: Fluorescence, conduction and magnetism. J. Phys. D Appl. Phys. 2020, 53, 155301. [Google Scholar] [CrossRef]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the fiber diameter during electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef]
- Dzenis, Y. Material science. Spinning continuous fibers for nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Ewaldz, E.; Brettmann, B. Molecular interactions in electrospinning: From polymer mixtures to supramolecular assemblies. ACS Appl. Polym. Mater. 2019, 1, 298–308. [Google Scholar] [CrossRef]
- Jana, S.; Cooper, A.; Ohuchi, F.; Zhang, M. Uniaxially aligned nanofibrous cylinders by electrospinning. ACS Appl. Mater. Interfaces 2012, 4, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yu, X.; Chai, Q.; Ayres, N.; Steckl, A.J. Stimuli-Responsive Self-Immolative Polymer Nanofiber Membranes Formed by Coaxial Electrospinning. ACS Appl. Mater. Interfaces 2017, 9, 11858–11865. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2019, 5, 61–81. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Chan, C.-H.; Tang, W.C. Alignment of multiple electrospun piezoelectric fiber bundles across serrated gaps at an incline: A method to generate textile strain sensors. Sci. Rep. 2017, 7, 15436. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Ezazi, N.Z.; Liu, D.; Santos, H.A. Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Tan, H.-L.; Kai, D.; Pasbakhsh, P.; Teow, S.-Y.; Lim, Y.-Y.; Pushpamalar, J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: A potential scaffold for tissue engineering. Colloids Surf. B Biointerfaces 2020, 188, 110713. [Google Scholar] [CrossRef] [PubMed]
- Prabu, G.T.V.; Dhurai, B. A Novel Profiled Multi-Pin Electrospinning System for Nanofiber Production and Encapsulation of Nanoparticles into Nanofibers. Sci. Rep. 2020, 10, 4302. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Aggeli, A.; Ingham, E.; McPherson, M.J. Production of self-assembling biomaterials for tissue engineering. Trends Biotechnol. 2009, 27, 423–433. [Google Scholar] [CrossRef]
- Hughes, E.A.B.; Chipara, M.; Hall, T.J.; Williams, R.L.; Grover, L.M. Chemobrionic structures in tissue engineering: Self-assembling calcium phosphate tubes as cellular scaffolds. Biomater. Sci. 2020, 8, 812–822. [Google Scholar] [CrossRef]
- Ebrahimi, M. Biomimetic principle for development of nanocomposite biomaterials in tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 287–306. [Google Scholar]
- Gong, C.; Sun, S.; Zhang, Y.; Sun, L.; Su, Z.; Wu, A.; Wei, G. Hierarchical nanomaterials via biomolecular self-assembly and bioinspiration for energy and environmental applications. Nanoscale 2019, 11, 4147–4182. [Google Scholar] [CrossRef]
- Panda, J.J.; Chauhan, V.S. Short peptide based self-assembled nanostructures: Implications in drug delivery and tissue engineering. Polym. Chem. 2014, 5, 4431–4449. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, C.; Akakuru, O.U.; Su, Z.; Wu, A.; Wei, G. The design and biomedical applications of self-assembled two-dimensional organic biomaterials. Chem. Soc. Rev. 2019, 48, 5564–5595. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Feng, M.; Chen, X.; Sun, J. Multidimensional (0D-3D) functional nanocarbon: Promising material to strengthen the photocatalytic activity of graphitic carbon nitride. Green Energy Environ. 2021, 6, 823–845. [Google Scholar] [CrossRef]
- Saunders, L.; Ma, P.X. Self-Healing Supramolecular Hydrogels for Tissue Engineering Applications. Macromol. Biosci. 2019, 19, e1800313. [Google Scholar] [CrossRef]
- Nakamatsu, J.; Torres, F.G.; Troncoso, O.P.; Min-Lin, Y.; Boccaccini, A.R. Processing and characterization of porous structures from chitosan and starch for tissue engineering scaffolds. Biomacromolecules 2006, 7, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.A.; Rao, P.S. Synthesis of polymeric nanomaterials for biomedical applications. In Nanomaterials in Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 27–63. [Google Scholar]
- Nam, Y.S.; Park, T.G. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J. Biomed. Mater. Res. 1999, 47, 8–17. [Google Scholar] [CrossRef]
- Rana, D.; Ratheesh, G.; Ramakrishna, S.; Ramalingam, M. Nanofiber composites in cartilage tissue engineering. In Nanofiber Composites for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 325–344. [Google Scholar]
- Rowlands, A.S.; Lim, S.A.; Martin, D.; Cooper-White, J.J. Polyurethane/poly (lactic-co-glycolic) acid composite scaffolds fabricated by thermally induced phase separation. Biomaterials 2007, 28, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.B.; Reddy, G.S.M.; Sivanjineyulu, V.; Jayaramudu, J.; Varaprasad, K.; Sadiku, E.R. Hydrophobic/hydrophilic nanostructured polymer blends. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Elsevier: Amsterdam, The Netherlands, 2016; pp. 385–411. [Google Scholar]
- Ghalia, M.A.; Dahman, Y. Advanced nanobiomaterials in tissue engineering. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 141–172. [Google Scholar]
- Salehi, M.; Farzamfar, S.; Bozorgzadeh, S.; Bastami, F. Fabrication of Poly(L-Lactic Acid)/Chitosan Scaffolds by Solid-Liquid Phase Separation Method for Nerve Tissue Engineering: An In Vitro Study on Human Neuroblasts. J. Craniofac. Surg. 2019, 30, 784–789. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; McNulty, J.; Salick, M.R.; Peng, X.-F.; Turng, L.-S. Approaches to Fabricating Multiple-Layered Vascular Scaffolds Using Hybrid Electrospinning and Thermally Induced Phase Separation Methods. Ind. Eng. Chem. Res. 2016, 55, 882–892. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.-Y.; Huh, M.; Yun, S.I. Porous Poly (3-hydroxybutyrate) Scaffolds Prepared by Non-Solvent-Induced Phase Separation for Tissue Engineering. Macromol. Res. 2020, 28, 835–843. [Google Scholar] [CrossRef]
- Yousefi, A.-M.; Liu, J.; Sheppard, R.; Koo, S.; Silverstein, J.; Zhang, J.; James, P.F. I-Optimal Design of Hierarchical 3D Scaffolds Produced by Combining Additive Manufacturing and Thermally Induced Phase Separation. ACS Appl. Bio Mater. 2019, 2, 685–696. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L. Fabrication of scaffolds in tissue engineering: A review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.C.; Suhaimin, I.; Kassim, S.; Zubir, S.; Abdullah, T. Effect of modified solvent casting/particulate leaching (SCPL) technique on the properties of bioactive glass reinforced polyurethane scaffold for biomedical applications. JPS 2019, 30, 115–126. [Google Scholar] [CrossRef]
- Costantini, M.; Barbetta, A. Gas foaming technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 127–149. [Google Scholar]
- Dugad, R.; Radhakrishna, G.; Gandhi, A. Recent advancements in manufacturing technologies of microcellular polymers: A review. J. Polym. Res. 2020, 27, 182. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, S.; Yin, S.; Jiang, F.; Zhou, M.; Yang, G.; Sun, N.; Zhang, W.; Jiang, X. In situ gas foaming based on magnesium particle degradation: A novel approach to fabricate injectable macroporous hydrogels. Biomaterials 2020, 232, 119727. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; An, J. Introduction to rapid prototyping of biomaterials. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar]
- Touri, M.; Kabirian, F.; Saadati, M.; Ramakrishna, S.; Mozafari, M. Additive manufacturing of biomaterials—The evolution of rapid prototyping. Adv. Eng. Mater. 2019, 21, 1800511. [Google Scholar] [CrossRef]
- Ahmed, N. Direct metal fabrication in rapid prototyping: A review. J. Manuf. Process. 2019, 42, 167–191. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Bertoldi, K.; Gabbrielli, R.; Velders, A.H.; Feijen, J.; Grijpma, D.W. Mathematically defined tissue engineering scaffold architectures prepared by stereolithography. Biomaterials 2010, 31, 6909–6916. [Google Scholar] [CrossRef]
- Mazzanti, V.; Malagutti, L.; Mollica, F. FDM 3D Printing of Polymers Containing Natural Fillers: A Review of their Mechanical Properties. Polymers 2019, 11, 1094. [Google Scholar] [CrossRef]
- Kundu, A.; Mccoy, L.; Azim, N.; Nguyen, H.; Rajaraman, S. Fabrication and Characterization of 3D Printed, 3D Microelectrode Arrays for Interfacing with a Peripheral Nerve-on-a-Chip. ACS Biomater. Sci. Eng. 2021, 7, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Kruth, J.P.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Robinson, D.K.R.; Lagnau, A.; Boon, W.P.C. Innovation pathways in additive manufacturing: Methods for tracing emerging and branching paths from rapid prototyping to alternative applications. Technol. Forecast. Soc. Chang. 2019, 146, 733–750. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Varkey, M.; Visscher, D.O.; van Zuijlen, P.P.M.; Atala, A.; Yoo, J.J. Skin bioprinting: The future of burn wound reconstruction. Burn. Trauma 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Ng, W.L.; An, J.; Chua, C.K.; Tan, L.P. Layer-by-layer ultraviolet assisted extrusion-based (UAE) bioprinting of hydrogel constructs with high aspect ratio for soft tissue engineering applications. PLoS ONE 2019, 14, e0216776. [Google Scholar] [CrossRef]
- Willemsen, K.; Nizak, R.; Noordmans, H.J.; Castelein, R.M.; Weinans, H.; Kruyt, M.C. Challenges in the design and regulatory approval of 3D-printed surgical implants: A two-case series. Lancet Digit. Health 2019, 1, e163–e171. [Google Scholar] [CrossRef]
- Ng, W.L.; Chua, C.K.; Shen, Y.-F. Print Me an Organ! Why We Are Not There Yet. Prog. Polym. Sci. 2019, 97, 101145. [Google Scholar] [CrossRef]
- Syed, O.; Walters, N.J.; Day, R.M.; Kim, H.-W.; Knowles, J.C. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 2014, 10, 5043–5054. [Google Scholar] [CrossRef]
- Sehic, E.; Brännström, M.; Hellström, M. Progress in Preclinical Research on Uterus Bioengineering That Utilizes Scaffolds Derived from Decellularized Uterine Tissue. Biomed. Mater. Devices 2022, 1–8. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, E.A.; Willacy, O.; Madsen, C.D.; Rafaeva, M.; Heumüller, S.E.; Bock, F.; Sengle, G.; Koch, M.; Imhof, T.; Zaucke, F.; et al. Decellularization and antibody staining of mouse tissues to map native extracellular matrix structures in 3D. Nat. Protoc. 2019, 14, 3395–3425. [Google Scholar] [CrossRef]
- Teodori, L.; Costa, A.; Marzio, R.; Perniconi, B.; Coletti, D.; Adamo, S.; Gupta, B.; Tárnok, A. Native extracellular matrix: A new scaffolding platform for repair of damaged muscle. Front. Physiol. 2014, 5, 218. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Isenberg, B.; Wong, J.Y. Building structure into engineered tissues. Mater. Today 2006, 9, 54–60. [Google Scholar] [CrossRef]
- Aulino, P.; Costa, A.; Chiaravalloti, E.; Perniconi, B.; Adamo, S.; Coletti, D.; Marrelli, M.; Tatullo, M.; Teodori, L. Muscle Extracellular Matrix Scaffold Is a Multipotent Environment. Int. J. Med. Sci. 2015, 12, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Bousnaki, M.; Beketova, A.; Kontonasaki, E. A Review of in Vivo and Clinical Studies Application of cell sheet and scaffold technology for periodontal ligament regeneration. Biomolecules 2022, 12, 435. [Google Scholar] [CrossRef]
- You, Q.; Lu, M.; Li, Z.; Zhou, Y. Cell Sheet Technology as an Engineering-Based Approach to Bone Regeneration. Int. J. Nanomed. 2022, 17, 6491–6511. [Google Scholar] [CrossRef]
- Sekine, H.; Okano, T. Tubular Cardiac Tissue Bioengineered from Multi-Layered Cell Sheets for Use in the Treatment of Heart Failure. Card. Tissue Eng. 2022, 2485, 227–242. [Google Scholar]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liao, Y.; Zhang, Y.; Shekh, M.I.; Zhang, J.; You, Z.; Du, B.; Lian, C.; Hel, Q. Novel nanofibrous membrane-supporting stem cell sheets for plasmid delivery and cell activation to accelerate wound healing. Bioeng. Transl. Med. 2022, 7, e10244. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Okano, T. Cell Sheet Engineering. Mater. Today 2004, 7, 42–47. [Google Scholar] [CrossRef]
- Alhashmi Alamer, F.; Almalki, G.A. Fabrication of Conductive Fabrics Based on SWCNTs, MWCNTs and Graphene and Their Applications: A Review. Polymers 2022, 14, 5376. [Google Scholar] [CrossRef]
- Vallejos, S.; Trigo-López, M.; Arnaiz, A.; Miguel, Á.; Muñoz, A.; Mendía, A.; García, J.M. From Classical to Advanced Use of Polymers in Food and Beverage Applications. Polymers 2022, 14, 4954. [Google Scholar] [CrossRef]
- Mountaki, S.A.; Kaliva, M.; Loukelis, K.; Chatzinikolaidou, M.; Vamvakaki, M. Responsive Polyesters with Alkene and Carboxylic Acid Side-Groups for Tissue Engineering Applications. Polymers 2021, 13, 1636. [Google Scholar] [CrossRef]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef]
- Chandy, T. Biocompatibility of materials and its relevance to drug delivery and tissue engineering. In Biointegration of Medical Implant Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 297–331. [Google Scholar]
- Hetemi, D.; Pinson, J. Surface functionalisation of polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef]
- Custódio, C.A.; del Campo, A.; Reis, R.L.; Mano, J.F. Smart instructive polymer substrates for tissue engineering. In Smart Polymers and Their Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 411–438. [Google Scholar]
- Chai, C.; Leong, K.W. Biomaterials approach to expand and direct differentiation of stem cells. Mol. Ther. 2007, 15, 467–480. [Google Scholar] [CrossRef]
- Bolger, M.; Groynom, R.; Bogie, K.; Lavik, E. Reporter scaffolds for clinically relevant cell transplantation studies. Ann. Biomed. Eng. 2020, 48, 1982–1990. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Allen, P.; Song, Y.H.; Wachs, R.A.; Du, Y.; Ellington, A.D.; Schmidt, C.E. Oligonucleotide-functionalized hydrogels for sustained release of small molecule (aptamer) therapeutics. Acta Biomater. 2020, 102, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, Y.; Chen, X.; Zeng, C.; Hu, Q.; Yin, W.; Li, W.; Xie, H.; Zhang, B.; Huang, X.; et al. Nanoparticle-modified chitosan-agarose-gelatin scaffold for sustained release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Zhao, R.; Wang, C.; Qiu, F.; Sun, B.; Ji, H.; Qiu, J.; Wang, C. Enhanced adhesion and proliferation of human umbilical vein endothelial cells on conductive PANI-PCL fiber scaffold by electrical stimulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 106–112. [Google Scholar] [CrossRef]
- Alanezi, A.A.; Neau, S.H.; D’mello, A.P. Development and application of a modified method to determine the encapsulation efficiency of proteins in polymer matrices. AAPS PharmSciTech 2020, 21, 248. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Li, X.-R.; Wei, W.-J.; Wei, Z.-Y.; Zhang, C.-R.; Wang, F.; Dawes, H.; Guo, S.-C. Polymeric coating on β-TCP scaffolds provides immobilization of small extracellular vesicles with surface-functionalization and ZEB1-Loading for bone defect repair in diabetes mellitus. Biomaterials 2022, 283, 121465. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Peng, K.; Fu, X.; Zhang, E.; Lv, F.; Liu, L.; Zhang, N.; Wang, Y.; Wang, S.; et al. Supramolecular nanofibers for encapsulation and in situ differentiation of neural stem cells. Adv. Healthc. Mater. 2020, 9, e1901295. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Pradeep, S. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Sirova, M.; Rossmann, P.; Thielecke, H.; Boterberg, V.; Rihova, B.; Schacht, E.; Dubruel, P. Surface modification of polyimide sheets for regenerative medicine applications. Biomacromolecules 2010, 11, 2731–2739. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Hong, Y.; Fu, Q.; He, Q.; Mechakra, A.; Zhu, Q.; Zhou, F.; Liang, R.; Li, C.; et al. Tissue-Adhesive Paint of Silk Microparticles for Articular Surface Cartilage Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 22467–22478. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Huang, W.; Wu, L.; An, Y.; Xuan, T.; He, H.; Ye, M.; Qi, L.; Wu, J. Bioactive ECM Mimic Hyaluronic Acid Dressing via Sustained Releasing of bFGF for Enhancing Skin Wound Healing. ACS Appl. Bio Mater. 2020, 3, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Kim, Y.S.; Xie, V.Y.; Smith, B.T.; Watson, E.; Lam, J.; Pearce, H.A.; Engel, P.S.; Mikos, A.G. Modular, tissue-specific, and biodegradable hydrogel cross-linkers for tissue engineering. Sci. Adv. 2019, 5, eaaw7396. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, S.; Fan, J.; Hwang, H.S.; Aghaloo, T.; Lee, M. Smoothened agonist sterosome immobilized hybrid scaffold for bone regeneration. Sci. Adv. 2020, 6, eaaz7822. [Google Scholar] [CrossRef] [PubMed]
- Adipurnama, I.; Yang, M.-C.; Ciach, T.; Butruk-Raszeja, B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: A review. Biomater. Sci. 2016, 5, 22–37. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Chang, J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today 2018, 24, 41–56. [Google Scholar] [CrossRef]
- Trucco, M. Regeneration of the pancreatic beta cell. J. Clin. Investig. 2005, 115, 5–12. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Moore, K.A.; Lemischka, I.R. Stem cells and their niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G. Stem cells in tissue engineering. Nature 2001, 414, 118–121. [Google Scholar] [CrossRef]
- Cai, W. Engineering in Translational Medicine, 2014th ed.; Springer: London, UK, 2013. [Google Scholar]
- Evans, N.D.; Gentleman, E.; Polak, J.M. Scaffolds for stem cells. Mater. Today 2006, 9, 26–33. [Google Scholar] [CrossRef]
- Deng, W. Induced pluripotent stem cells: Paths to new medicines. A catalyst for disease modelling, drug discovery and regenerative therapy. EMBO Rep. 2010, 11, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Colman, A.; Dreesen, O. Induced pluripotent stem cells and the stability of the differentiated state. EMBO Rep. 2009, 10, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Kimbrel, E.A.; Lanza, R. Next-generation stem cells—Ushering in a new era of cell-based therapies. Nat. Rev. Drug Discov. 2020, 19, 463–479. [Google Scholar] [CrossRef]
- Fernández Vallone, V.B.; Romaniuk, M.A.; Choi, H.; Labovsky, V.; Otaegui, J.; Chasseing, N.A. Mesenchymal stem cells and their use in therapy: What has been achieved. Differentiation 2013, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, M.S.; Ferlini, A. Urinary stem cells as tools to study genetic disease: Overview of the literature. J. Clin. Med. 2019, 8, 627. [Google Scholar] [CrossRef]
- Duffy, G.P.; Robinson, S.T.; O’Connor, R.; Wylie, R.; Mauerhofer, C.; Bellavia, G.; Straino, E.; Cianfarani, F.; Mendez, K.; Beatty, R.; et al. Implantable therapeutic reservoir systems for diverse clinical applications in large animal models. Adv. Health Mater. 2020, 9, e2000305. [Google Scholar] [CrossRef]
- Chhabra, R.; Peshattiwar, V.; Pant, T.; Deshpande, A.; Modi, D.; Sathaye, S.; Tibrewala, A.; Dyawanapelly, S.; Jain, R.D.; Dandekar, P. In Vivo Studies of 3D Starch–Gelatin Scaffolds for Full-Thickness Wound Healing. ACS Appl. Bio Mater. 2020, 3, 2920–2929. [Google Scholar] [CrossRef]
- Del Sol, A.; Thiesen, H.J.; Imitola, J.; Carazo Salas, R.E. Big-Data-Driven Stem Cell Science and Tissue Engineering: Vision and Unique Opportunities. Cell Stem Cell 2017, 20, 157–160. [Google Scholar] [CrossRef]
- Little, M.; Liu, G.-H.; Shenoy, K.V.; Vunjak-Novakovic, G.; Radisic, M. Engineering tissues and organs: The road to the clinic. Cell 2020, 81, 22–23. [Google Scholar]
- Abutaleb, N.O.; Truskey, G.A. Human iPSCs Stretch to Improve Tissue-Engineered Vascular Grafts. Cell Stem Cell 2020, 26, 136–137. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. Stem cells, regenerative medicine, and Prometheus. Lancet 2018, 391, 814. [Google Scholar] [CrossRef]
- Cossu, G.; Birchall, M.; Brown, T.; De Coppi, P.; Culme-Seymour, E.; Gibbon, S.; Hitchcock, J.; Mason, C.; Montgomery, J.; Morris, S.; et al. Lancet Commission: Stem cells and regenerative medicine. Lancet 2018, 391, 883–910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y.; Jiang, D.; Zhang, J.; Sheng, Z.; Xie, C.; Peng, Z.; et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 2020, 258, 120287. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Synthetic biodegradable polymers for tissue engineering and drug delivery. Curr. Opin. Solid State Mater. Sci. 1998, 3, 246–251. [Google Scholar] [CrossRef]
- Bayat, S.; Amiri, N.; Pishavar, E.; Kalalinia, F.; Movaffagh, J.; Hashemi, M. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019, 229, 57–66. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Sampath, S.; Muthusamy, S.; John, M.A. Importance of crosslinking strategies in designing smart biomaterials for bone tissue engineering: A systematic review. Mater. Sci. Eng. C 2018, 96, 941–954. [Google Scholar] [CrossRef]
- Samadian, H.; Khastar, H.; Ehterami, A.; Salehi, M. Bioengineered 3D nanocomposite based on gold nanoparticles and gelatin nanofibers for bone regeneration: In vitro and in vivo study. Sci. Rep. 2021, 11, 13877. [Google Scholar] [CrossRef]
- Abbas, T.O.; Ali, T.A.; Uddin, S. Urine as a Main Effector in Urological Tissue Engineering—A Double-Edged Sword. Cells 2020, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Bivalacqua, T.J. Regenerative medicine in urology: The future of urinary reconstruction. Trends Urol. Men’s Health 2020, 11, 9–12. [Google Scholar] [CrossRef]
- Adamowicz, J.; Pasternak, I.; Kloskowski, T.; Gniadek, M.; Van Breda, S.V.; Buhl, M.; Balcerczyk, D.; Gagat, M.; Grzanka, D.; Strupinski, W.; et al. Development of a conductive biocomposite combining graphene and amniotic membrane for replacement of the neuronal network of tissue-engineered urinary bladder. Sci. Rep. 2020, 10, 8524. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, R.S.; Williams, J.K.; Yoo, K.W.; Yoo, J.J.; Atala, A. A tissue-engineered uterus supports live births in rabbits. Nat. Biotechnol. 2020, 38, 1280–1287. [Google Scholar] [CrossRef]
- Takehara, H.; Sakaguchi, K.; Sekine, H.; Okano, T.; Shimizu, T. Microfluidic vascular-bed devices for vascularized 3D tissue engineering: Tissue engineering on a chip. Biomed. Microdevices 2019, 22, 9. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Feng, Y.; Yang, Y.; Wei, Z.; Zhao, W.; Zhao, C. A chitosan modified asymmetric small-diameter vascular graft with anti-thrombotic and anti-bacterial functions for vascular tissue engineering. J. Mater. Chem. B 2019, 8, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Miyagawa, S.; Toyofuku, T.; Fukushima, S.; Kawamura, T.; Kawamura, A.; Kashiyama, N.; Nakamura, Y.; Toda, K. Syngeneic Mesenchymal Stem Cells Reduce Immune Rejection After Induced Pluripotent Stem Cell-Derived Allogeneic Cardiomyocyte Transplantation. Sci. Rep. 2020, 10, 4593. [Google Scholar] [CrossRef]
- Fiamingo, A.; Montembault, A.; Boitard, S.-E.; Naemetalla, H.; Agbulut, O.; Delair, T.; Campana-Filho, S.P.; Menasché, P.; David, L. Chitosan Hydrogels for the Regeneration of Infarcted Myocardium: Preparation, Physicochemical Characterization, and Biological Evaluation. Biomacromolecules 2016, 17, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Domengé, O.; Ragot, H.; Deloux, R.; Crépet, A.; Revet, G.; Boitard, S.E.; Simon, A.; Mougenot, N.; David, L.; Delair, T.; et al. Efficacy of epicardial implantation of acellular chitosan hydrogels in ischemic and nonischemic heart failure: Impact of the acetylation degree of chitosan. Acta Biomater. 2021, 119, 125–139. [Google Scholar] [CrossRef]
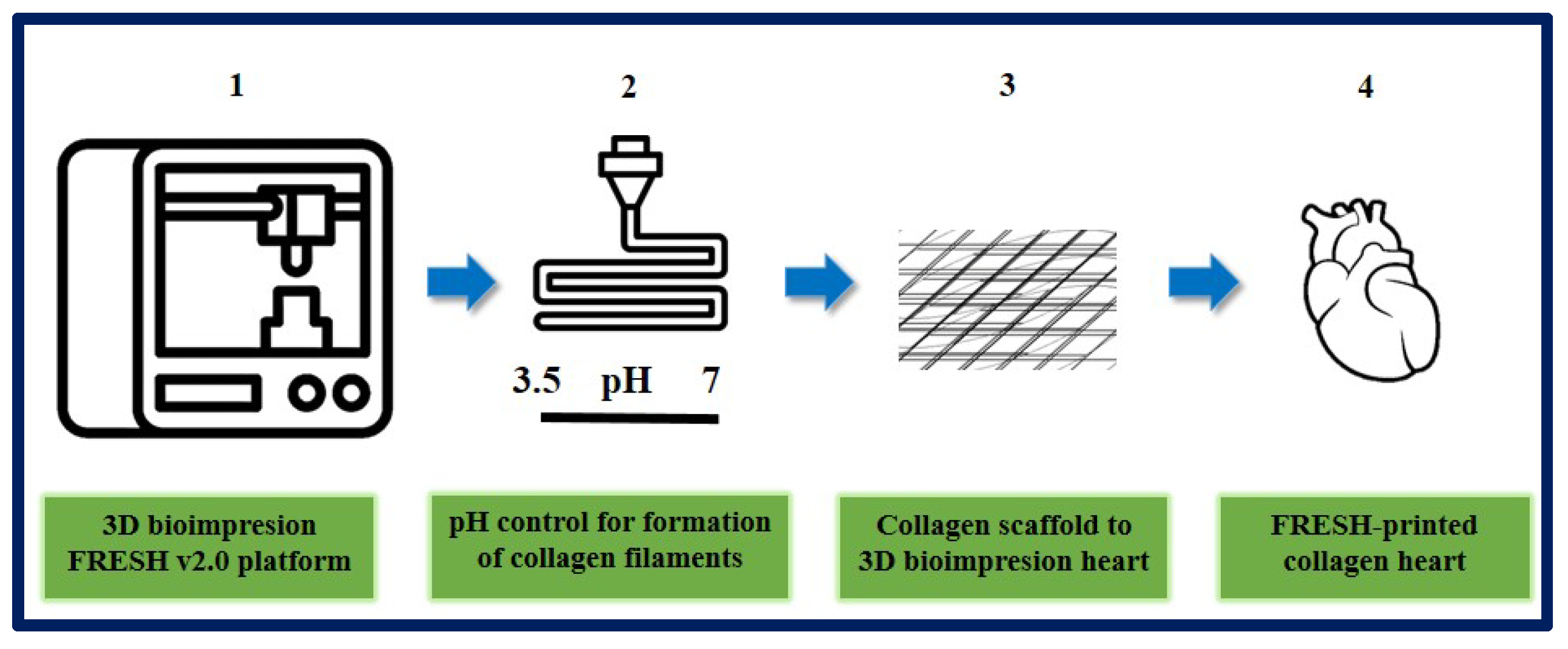
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Zaszczynska, A.; Sajkiewicz, P.; Gradys, A. Piezoelectric Scaffolds as Smart Materials for Neural Tissue Engineering. Polymers 2020, 12, 161. [Google Scholar] [CrossRef]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Zamanian, A.; Kermanian, F.; Shamoosi, A. A bioinspired 3D shape olibanum-collagen-gelatin scaffolds with tunable porous microstructure for efficient neural tissue regeneration. Biotechnol. Prog. 2019, 36, e2918. [Google Scholar] [CrossRef] [PubMed]
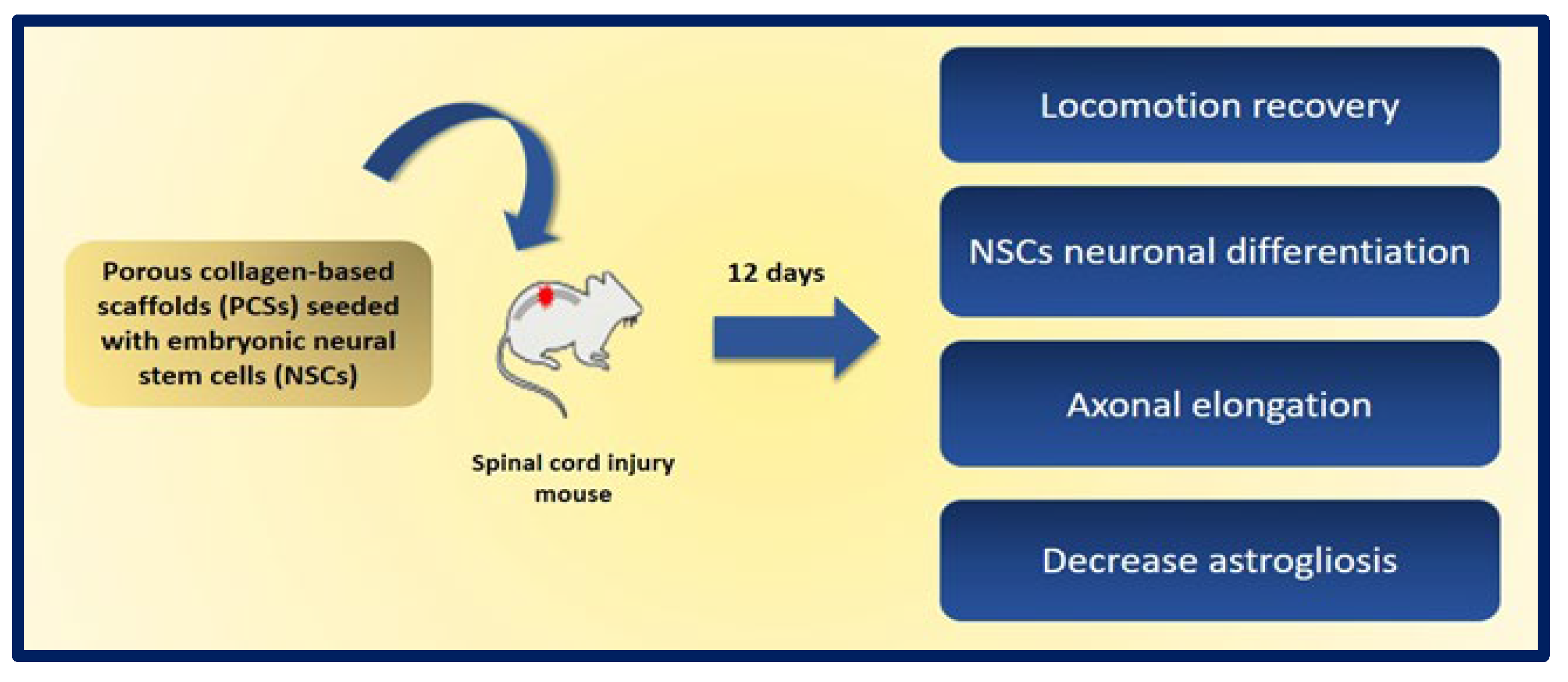
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Louis, F.; Matsusaki, M. Adipose tissue engineering. In Biomaterials for Organ and Tissue Regeneration; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–423. [Google Scholar]
- Nagano, H.; Suematsu, Y.; Takuma, M.; Aoki, S.; Satoh, A.; Takayama, E.; Kinoshita, M.; Morimoto, Y.; Takeoka, S.; Fujie, T.; et al. Enhanced cellular engraftment of adipose-derived mesenchymal stem cell spheroids by using nanosheets as scaffolds. Sci. Rep. 2021, 11, 14500. [Google Scholar] [CrossRef]
- Rodríguez, A.P.; Felice, B.; Sánchez, M.A.; Tsujigiwa, H.; Felice, C.J.; Nagatsuka, H. In Vivo evaluation of adipogenic induction in fibrous and honeycomb-structured atelocollagen scaffolds. Mater. Sci. Eng. C 2016, 63, 125–130. [Google Scholar] [CrossRef]
- Geris, L.; Papantoniou, I. The Third Era of Tissue Engineering: Reversing the Innovation Drivers. Tissue Eng. Part A 2019, 25, 821–826. [Google Scholar] [CrossRef]
- Goh, B.T.; Teh, L.Y.; Tan, D.B.P.; Zhang, Z.; Teoh, S.H. Novel 3D polycaprolactone scaffold for ridge preservation—A pilot randomised controlled clinical trial. Clin. Oral Implant. Res. 2014, 26, 271–277. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, K.S.; Coric, D.; Harrop, J.S.; Theodore, N.; Toselli, R.M. Acute Implantation of a Bioresorbable Polymer Scaffold in Patients with Complete Thoracic Spinal Cord Injury: 24-Month Follow-Up from the INSPIRE Study. Neurosurgery 2022, 90, 668–675. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, K.S.; Coric, D.; Chang, J.J.; Harrop, J.S.; Theodore, N.; Toselli, R.M. A study of probable benefit of a bioresorbable polymer scaffold for safety and neurological recovery in patients with complete thoracic spinal cord injury: 6-month results from the INSPIRE study. J. Neurosurg. Spine 2021, 34, 808–817. [Google Scholar] [CrossRef]
- Suzuki, N.; Kozuma, K.; Nakamura, S.; Aramaki, K.; Saito, S.; Shibata, Y.; Nanasato, M.; Fujii, K.; Kusano, H.; Ediebah, D.; et al. Absorb GT1 Bioresorbable Vascular Scaffold System-1-Year Post-Marketing Surveillance in Japan. Circ. J. 2019, 83, 2460–2465. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kubo, S.; Sasaki, K.; Kawakami, Y.; Oka, S.; Sasaki, H.; Takayama, K.; Tei, K.; Matsushita, T.; Mifune, Y.; et al. Acceleration of Tendon-Bone Healing of Anterior Cruciate Ligament Graft Using Autologous Ruptured Tissue. Am. J. Sports Med. 2012, 40, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- DiDomenico, L.A.; Orgill, D.P.; Galiano, R.D.; Serena, T.E.; Carter, M.J.; Kaufman, J.P.; Young, N.J.; Zelen, C.M. Aseptically processed placental membrane improves healing of diabetic foot ulcerations: Prospective, randomized clinical trial. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1095. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Rinker, B.D.; Weber, R.V.; Chao, J.D.; Ingari, J.V.; Brooks, D.; Buncke, G.M. Functional Outcome Following Nerve Repair in the Upper Extremity Using Processed Nerve Allograft. J. Hand Surg. 2012, 37, 2340–2349. [Google Scholar] [CrossRef]
- El Shazley, N.; Hamdy, A.; El-Eneen, H.A.; El Backly, R.M.; Saad, M.M.; Essam, W.; Moussa, H.; El Tantawi, M.; Jain, H.; Marei, M.K. Bioglass in alveolar bone regeneration in orthodontic patients: Randomized controlled clinical trial. JDR Clin. Trans. Res. 2016, 1, 244–255. [Google Scholar] [CrossRef]
- Ribichini, F.; Pighi, M.; Faggian, G.; Vassanelli, C. Bioresorbable vascular scaffolds in cardiac allograft vasculopathy: A new therapeutic option. Am. J. Med. 2013, 126, e11–e14. [Google Scholar] [CrossRef]
- Pighi, M.; Tomai, F.; Petrolini, A.; De Luca, L.; Tarantini, G.; Barioli, A.; Colombo, P.; Klugmann, S.; Ferlini, M.; Ormezzano, M.F.; et al. Everolimus-Eluting Bioresorbable Vascular Scaffold System in the Treatment of Cardiac Allograft Vasculopathy: The CART (Cardiac Allograft Reparative Therapy) Prospective Multicenter Pilot Study. J. Cardiovasc. Transl. Res. 2015, 9, 40–48. [Google Scholar] [CrossRef]
- Fine, N.A.; Lehfeldt, M.; Gross, J.E.; Downey, S.; Kind, G.M.; Duda, G.; David, K.; Rebecc, H.; Jeff, I.; Mark, J. SERI surgical scaffold, prospective clinical trial of a silk-derived biological scaffold in two-stage breast reconstruction: 1-year data. Plast. Reconstr. Surg. 2015, 135, 339–351. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Gong, J.; Yang, L.; Niu, C.; Ni, X.; Wang, Y.; Peng, S.; Gu, X.; Sun, C.; et al. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials 2017, 134, 64–77. [Google Scholar] [CrossRef]
- Diebold, G.; Lam, P.; Walton, J.; Murrell, G.A.C. Relationship between age and rotator cuff retear: A study of 1,600 consecutive rotator cuff repairs. J. Bone Jt. Surg. Am. 2017, 99, 1198–1205. [Google Scholar] [CrossRef]
- Serruys, P.W.; Chevalier, B.; Sotomi, Y.; Cequier, A.; Carrié, D.; Piek, J.J.; Van Boven, A.J.; Dominici, M.; Dudek, D.; McClean, D.; et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): A 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016, 388, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Ueki, Y.; Souteyrand, G.; Daemen, J.; Wiebe, J.; Nef, H.; Adriaenssens, T.; Loh, J.P.; Lattuca, B.; Wykrzykowska, J.J.; et al. Mechanisms of very late bioresorbable scaffold thrombosis: The INVEST registry. J. Am. Coll. Cardiol. 2017, 70, 2330–2344. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xu, B.; Chen, Y.; Zhou, Y.; Jia, S.; Zhong, Z.; Su, X.; Ma, Y.; Zhang, Q.; Liu, J.; et al. Thinner Strut Sirolimus-Eluting BRS Versus EES in Patients with Coronary Artery Disease: FUTURE-II Trial. JACC Cardiovasc. Interv. 2021, 14, 1450–1462. [Google Scholar] [CrossRef] [PubMed]



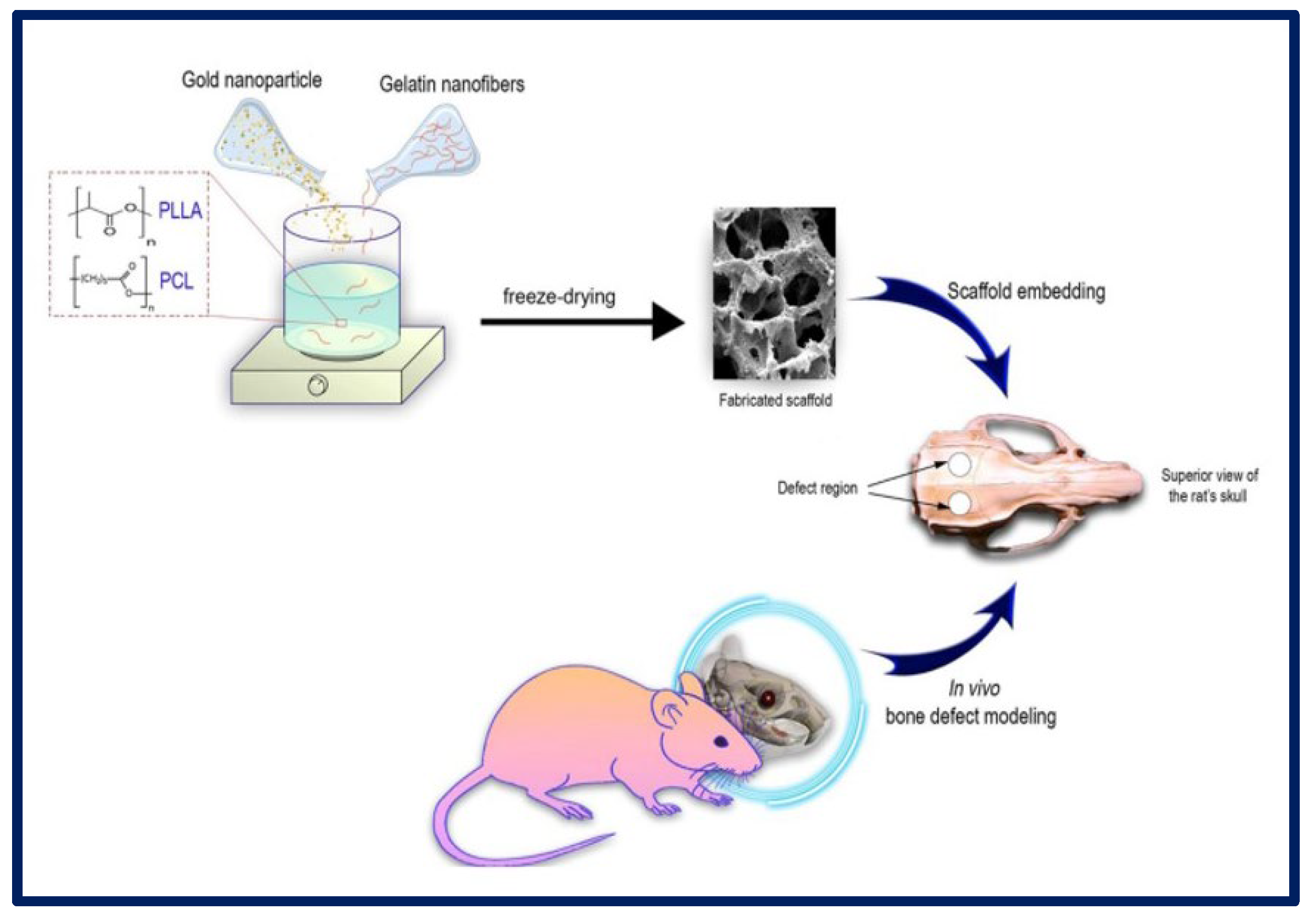
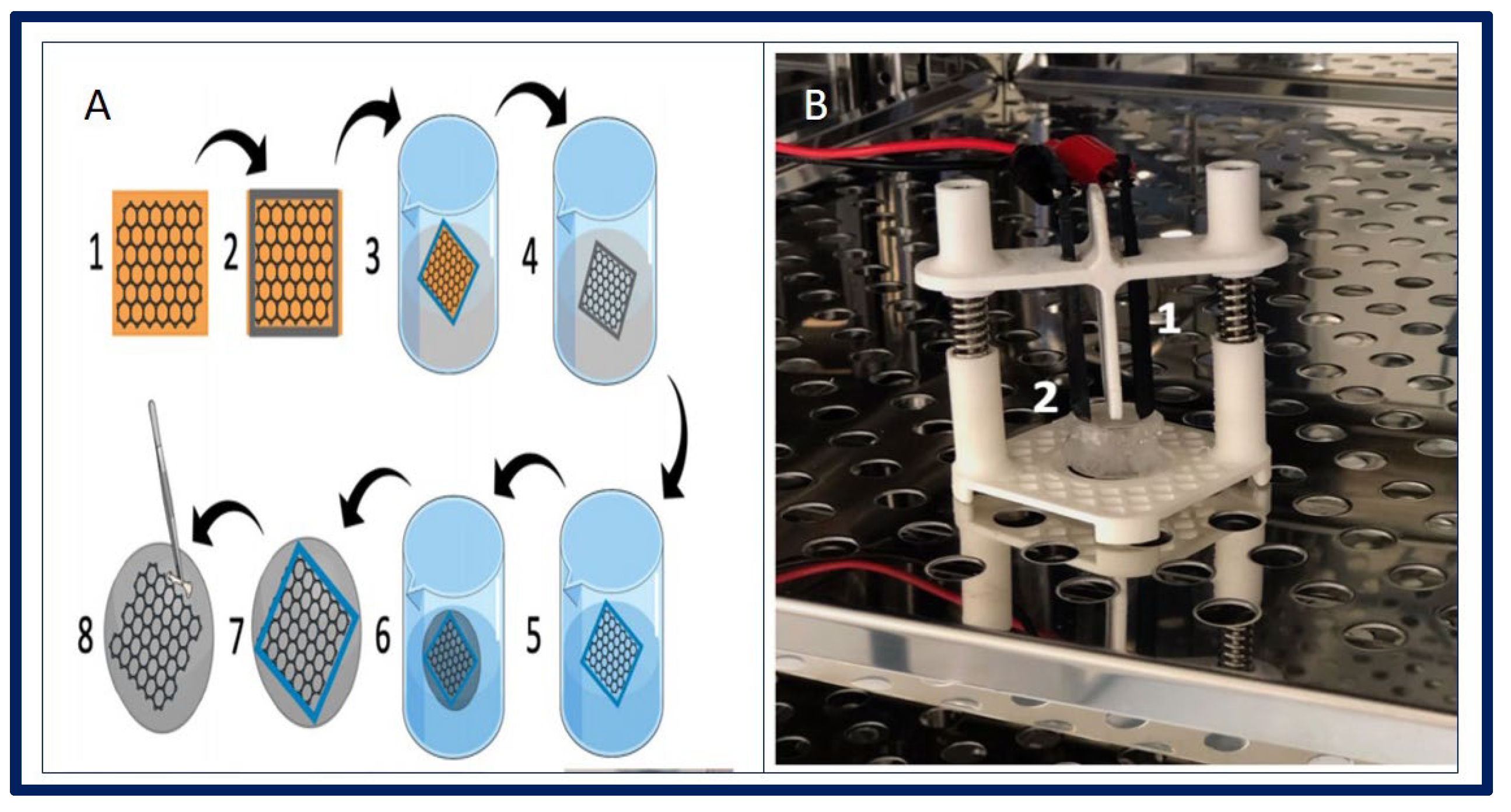
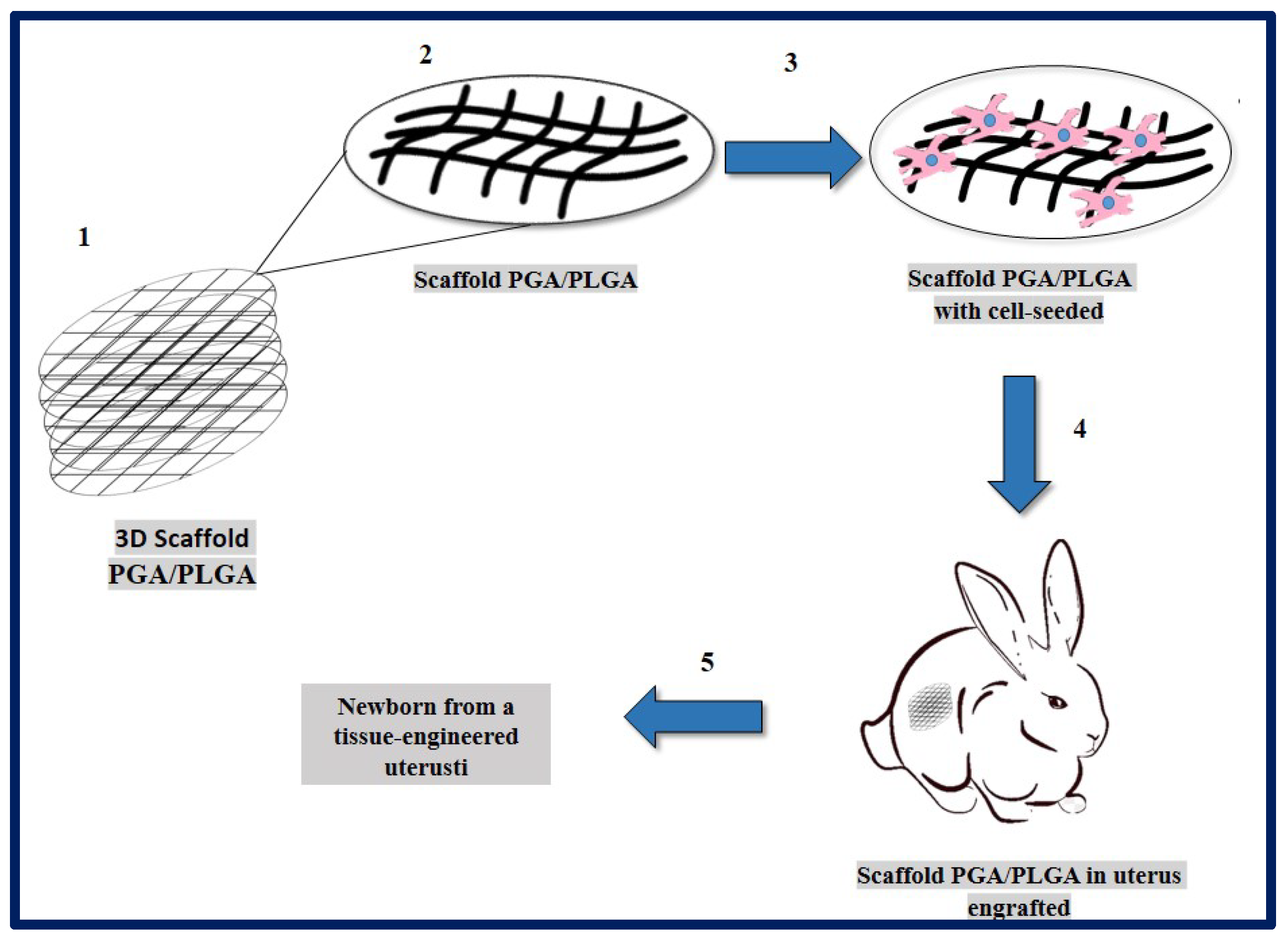
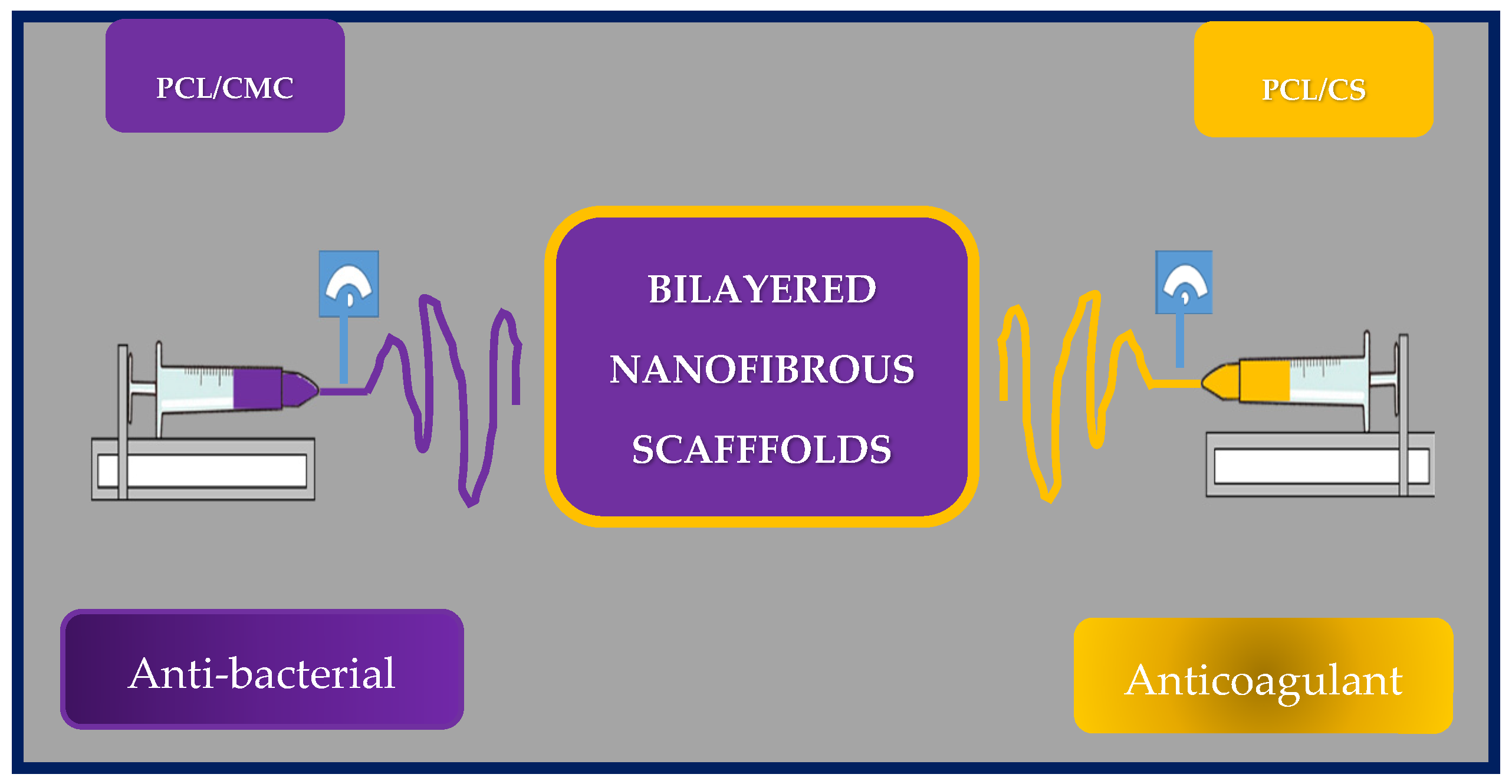

| Polymer | Molecule/Cell | Application | Technique | Ref. |
|---|---|---|---|---|
| PLGA scaffold | CellROX_ Green Reagent, and pHrodoTM Red AM (fluorophores) | Cell behavior study | Encapsulation | [139] |
| Hyaluronic acid-based hydrogel | Anti-Nogo receptor (anti-NgR) RNA aptamer | Spinal cord injury | Encapsulation | [140] |
| Chitosan oligosaccharide/ heparin nanoparticles | Stromal cell-derived factor 1 (SDF-1) and Bone morphogenetic protein 2 (BMP-2) | Bone tissue engineering | Encapsulation | [141] |
| Poly (ε-caprolactone) (PCL)/nano-hydroxyapatite | Mesenchymal stem cell-encapsulated in HPCH (hydroxypropyl chitin hydrogel) | Bone regeneration | Encapsulation | [142] |
| Ethylcellulose and polylactic-co-glycolic acid (PLGA) | Hemoglobin (Hb) and bovine serum albumin (BSA) | Encapsulation efficiency of proteins | Encapsulation | [143] |
| Hyaluronic acid (HA)/poly-L-lysine (PLL) layer-by-layer (LbL) self-assembly coating on β-TCP (β-tricalcium phosphate) | Small extracellular vesicles | Bone regeneration | Surface modification | [144] |
| Chromatin (DNA and histone) supramolecular fibers as scaffold | Murine brain-derived neural stem cells (NSCs) | Neural regenerative medicine | Encapsulation | [145] |
| 3D-printed (3DP) calcium phosphate (CaP) scaffolds | Polydopamine with Cissus Quadrangularis extract | Treatment bone defects | Surface modification | [146] |
| Porous composite scaffold of chitosan, chondroitinsulfate, gelatin | Nano-bioactive glass (nBG) (60% SiO2, 36% CaO, and 4% P2O5) | Bone tissue regeneration | Encapsulation | [147] |
| Polyimide | Aminopropylmethacrylamide, Reactive succinimidyl ester, and Methacrylamide modified gelatin | Ocular diseases: age-related macular degeneration (AMD) | Surface modification | [148] |
| Silk fibroin microparticles | N-(2-aminoethyl)-4-(4-(hydroxymethyl)-2-methoxy-5 nitrosophenoxy) butanamide (NB) | Cartilage regeneration | Surface modification | [149] |
| Methacrylated-Hialuronic Acid | Basic Fibroblastic Growth Factor (bFGF) | Skin wound healing | Surface modification | [150] |
| Poly(glycolic acid)–poly(ethylene glycol)–poly(glycolic acid)-di(but-2-yne-1,4-dithiol) (PdBT) | Hydrophilic bone morphogenetic protein mimetic (BMPm) peptide Hydrophobic N-cadherin (NC) peptide Cartilage-derived glycosaminoglycan macromolecule, chondroitin sulfate (CS) | Mesenchymal stem cell (MSC) encapsulation | Surface modification | [151] |
| Porous 3D PLGA scaffold | Smoothened agonist sterosome | Bone regeneration | Surface modification | [152] |
| Poliuretano | REDV peptide | Improve hemocompatibility by promoting EC attachment, proliferation, and growth | Via an active p -nitrophenyloxycarbonyl group | [153] |
| Potentiality | Cell Type | Source | Features and Mature Cell Lineage |
|---|---|---|---|
| Totipotent stem cells | Embryonic stem cell (ESc) | Zygote [159] | A single cell capable of dividing and forming several differentiated cells. Cells including extraembryonic tissues [159]. |
| Pluripotent stem cells | Embryonic stem cell (ESc) | Isolated from the inner cell mass of the blastocyst [160]. | They give rise to any cell type of the three germ layers. They have the ability to grow indefinitely while maintaining pluripotency [161]. Both types of cells can give rise to teratomas [162]. |
| Induced pluripotent stem cells (iPSc) | Obtained by genetic modification of somatic cells such as fibroblasts, to which specific transcription factors were introduced to induce pluripotency [163]. | ||
| Multipotent stem cells | Adult stem cells | They can be isolated from various tissues, including bone marrow, adipose tissue, umbilical cord, and dental pulp, among others (e.g.,: mesenchymal cells, hematopoietic cells) [164,165]. | They can give rise to a large number of cell lineages [164]. MSCs have immunomodulatory, anti-inflammatory, angiogenic, antiapoptotic, and trophic properties [165]. |
| Oligopotent stem cells | Adult stem cells | They can be isolated from the blood as myeloid and lymphoid cells. | They can give rise to a limited number of cell types. Lymphoid stem cells can only differentiate into basophils, neutrophils, eosinophils, monocytes, and thrombocytes [159]. |
| Unipotent stem cells | Adult stem cells | Epidermal, satellite (SC) | They can give rise to a single cell type. For example, SC are involved in skeletal muscle regeneration and are normally inactive until a stimulus or damage occurs and are activated to trigger the formation of new muscle fibers [166]. |
| Device | Description | Application | Identifier | Ref. |
|---|---|---|---|---|
| 3D-Printed Scaffold | Polycaprolactone Tricalcium Phosphate (PCL-TCP) is a bioactive, biocompatible, and bioabsorbable non-toxic polymer compound. | Ridge preservation after tooth extraction. | NCT03735199 | [198] |
| Neuro-spinal Scaffold | Poly (lactic-co-glycolic acid)-b-poly (L-lysine) scaffold | Thoracic AIS A traumatic spinal cord injury at neurological level of injury of T2-T12. | NCT02138110 | [199,200] |
| Absorb GT1 BVS | Absorb GT1 Bioresorbable Vascular Scaffold (BVS) | Ischemic heart disease, angina pectoris, coronary artery disease, coronary artery occlusion, myocardial ischemia | NCT03409731 | [201] |
| Bio ACL | Collagen based-membrane derived from amniotic tissue | anterior cruciate ligament rupture | NCT03294759 | [202,203] |
| BMAC Nerve Allograft | Decellularized processed peripheral nerve allograft, with autologous bone marrow aspirate concentrate. | Peripheral nerve injury, upper limb | NCT03964129 | [204] |
| Bioactive glass scaffold | Bioactive glass scaffold with multi-scale porosity prepared using the sol-gel technique. | Bone loss, vertical alveolar bone loss, horizontal alveolar bone loss | NCT01878084 | [205] |
| ABSORB scaffold | Everolimus-eluting bioresorbable vascular scaffold | Cardiac allograft vasculopathy | NCT02377648 | [206,207] |
| SERI® Surgical Scaffold | Bioresorbable scaffold derived from silk, developed to provide support and repair of soft tissues | Breast reconstruction | NCT01256502 | [208] |
| Chitosan scaffold | Bilaminar chitosan scaffold | Sellar floor repair in endoscopic endonasal transsphenoidal surgery | NCT03280849 | [209] |
| Nanofiber scaffold | Rotium nanofiber is an FDA approved scaffold. | Rotator cuff tears | NCT04325789 | [210] |
| Bioresorbable vascular scaffold | The bioresorbable vascular scaffold (BVS) has been approved and is used in daily clinical practice. | Coronary thrombosis, tomography, optical coherence drug-eluting stents | NCT03180931 | [211,212] |
| Firesorb | Sirolimus Target Eluting Bioresorbable Vascular Scaffold | Coronary artery disease | NCT02890160 | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socci, M.C.; Rodríguez, G.; Oliva, E.; Fushimi, S.; Takabatake, K.; Nagatsuka, H.; Felice, C.J.; Rodríguez, A.P. Polymeric Materials, Advances and Applications in Tissue Engineering: A Review. Bioengineering 2023, 10, 218. https://doi.org/10.3390/bioengineering10020218
Socci MC, Rodríguez G, Oliva E, Fushimi S, Takabatake K, Nagatsuka H, Felice CJ, Rodríguez AP. Polymeric Materials, Advances and Applications in Tissue Engineering: A Review. Bioengineering. 2023; 10(2):218. https://doi.org/10.3390/bioengineering10020218
Chicago/Turabian StyleSocci, María Cecilia, Gabriela Rodríguez, Emilia Oliva, Shigeko Fushimi, Kiyofumi Takabatake, Hitoshi Nagatsuka, Carmelo José Felice, and Andrea Paola Rodríguez. 2023. "Polymeric Materials, Advances and Applications in Tissue Engineering: A Review" Bioengineering 10, no. 2: 218. https://doi.org/10.3390/bioengineering10020218
APA StyleSocci, M. C., Rodríguez, G., Oliva, E., Fushimi, S., Takabatake, K., Nagatsuka, H., Felice, C. J., & Rodríguez, A. P. (2023). Polymeric Materials, Advances and Applications in Tissue Engineering: A Review. Bioengineering, 10(2), 218. https://doi.org/10.3390/bioengineering10020218