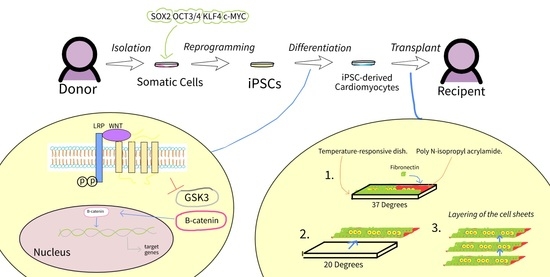
The Exciting Realities and Possibilities of iPS-Derived Cardiomyocytes
Abstract
1. Introduction
2. Differentiation of iPSC-Derived Cardiomyocytes
2.1. iPSC and ESC Differentiation—What Changes?
2.2. Embryoid Body (EB) Differentiation
2.3. Wnt Signaling in Monolayer Differentiation
2.3.1. GSK3 Inhibitors in Wnt Signaling
2.3.2. Only Moderating Regulatory Elements of Wnt Signaling
2.3.3. Other Factors in Wnt Signaling
3. Enhancing iPSC-Derived Cardiomyocytes
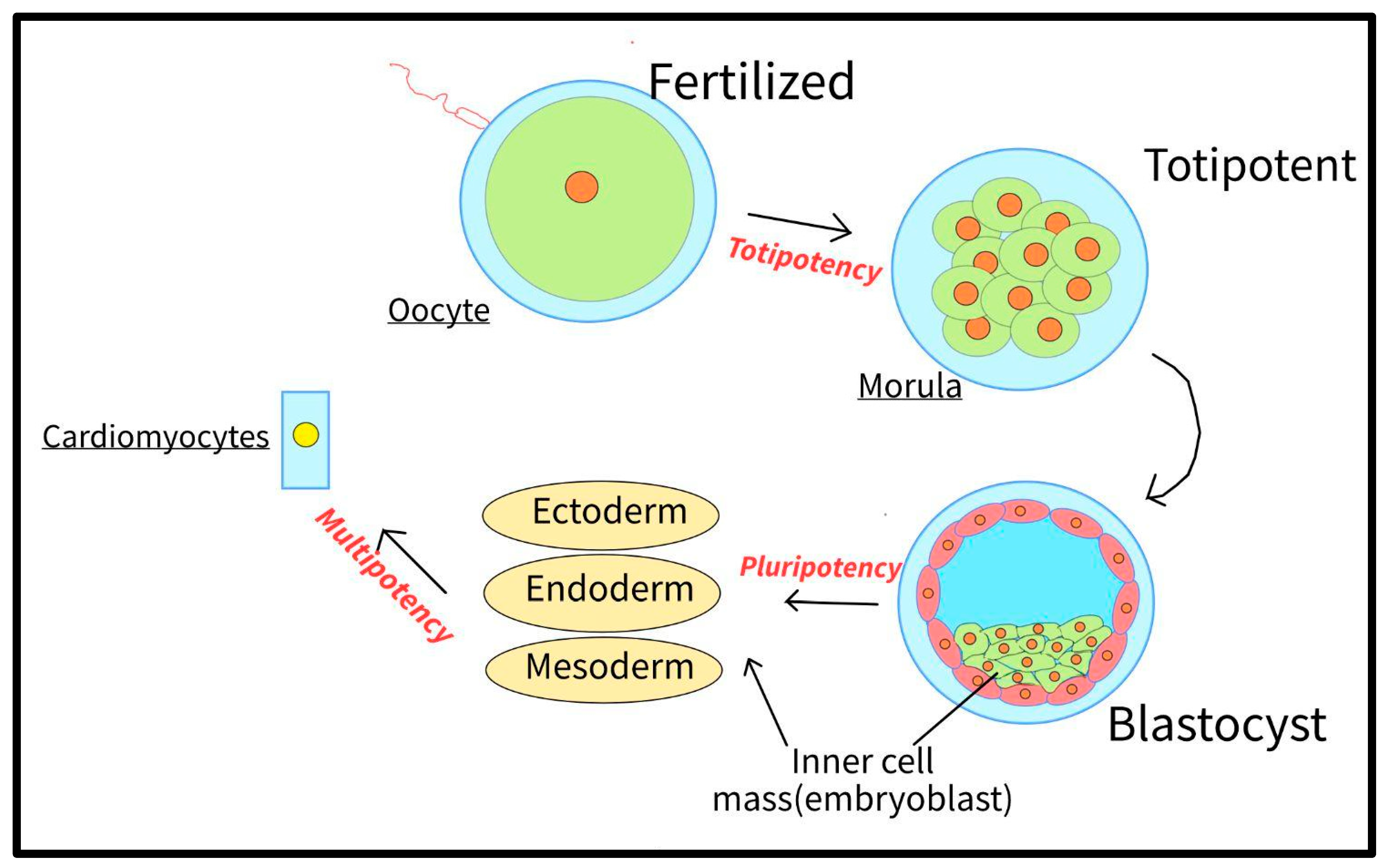
3.1. Pluripotency
3.1.1. Genetic Modifications
3.1.2. Chemical Modifications
3.2. Maturation
3.2.1. Inhibition of MAPK and PI3K/AKT Pathways
3.2.2. Cellular Shape and Environment
3.2.3. Electrical Stimulation
3.2.4. Fatty Acid Metabolism
3.2.5. Epigenetic Priming
4. Applications of iPSC-Derived Cardiomyocytes
4.1. Transplanting the Cardiomyocytes
4.2. Human Cardiac Muscle Patches (hCMP), and iPSC-Derived Cardiomyocytes
4.2.1. iPS-Derived Cardiomyocytes and Cell Sheets
4.2.2. Cell-Free Cardiac Patches
4.2.3. Spheroids and iPSC-Derived Cardiomyocytes
4.2.4. 3D Bioprinting and iPSC-Derived Cardiomyocytes
4.2.5. Transplantation of the hCMP
4.3. Disease Modeling
4.3.1. Disease Modeling for AC Treatments
4.3.2. Disease Modeling for DCM Treatments
4.4. Limitations in iPSCs in Clinical Settings
4.4.1. Transplantation Rejection
4.4.2. Arrhythmias with Cardiomyocyte Cell Sheets and Transplantation
4.4.3. Disease Modeling Challenges
4.4.4. Immaturation
4.4.5. Primed iPSCs Challenges
5. Ethical Considerations and Regulation Challenges of iPSC-Derived Cardiomyocytes
5.1. Human Embryos in hESC Research vs. iPSC Research
5.2. Human iPSC Research Regulation
5.2.1. Regulation in Japan: A Unique Example
5.2.2. Nomenclature Outside of Academia
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Liu, N.; Priori, S.G. Sodium channel mutations and arrhythmias. Nat. Rev. Cardiol. 2009, 6, 337–348. [Google Scholar] [CrossRef]
- Uygur, A.; Lee, R.T. Mechanisms of cardiac regeneration. Dev. Cell 2016, 36, 362–374. [Google Scholar] [CrossRef]
- Plackett, B. Cells or drugs? The race to regenerate the heart. Nature 2021, 594, S16–S17. [Google Scholar] [CrossRef]
- Wang, L.; Serpoohshan, V.; Zhang, J. Engineering human cardiac muscle patch constructs for prevention of post-infarction LV remodeling. Frontiers 2021, 8, 621781. Available online: https://www.frontiersin.org/articles/10.3389/fcvm.2021.621781/full (accessed on 20 December 2022). [CrossRef]
- Awad, M.A.; Shah, A.; Griffith, B.P. Current status and outcomes in heart transplantation: A narrative review. Rev. Cardiovasc. Med. 2022, 23, 11. [Google Scholar] [CrossRef]
- Dumitru, I. Heart Failure Treatment & Management: Approach Considerations, Nonpharmacologic Therapy, Pharmacologic Therapy. eMedicine. July 2022. Available online: https://emedicine.medscape.com/article/163062-treatment#:~:text=Pharmacologic%20therapies%20include%20the%20use (accessed on 20 December 2022).
- Teo, K.K. Effects of Prophylactic Antiarrhythmic Drug Therapy in Acute Myocardial Infarction. JAMA 1993, 270, 1589. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Subačius, H.; Marroquin, O.C.; Beau, S.L.; Simonson, J.; Desai, P.; Betzen, M.; DeLuna, D.; Whitehill, J.; Hatch, J.; et al. One-Year Landmark Analysis of the Effect of Beta-Blocker Dose on Survival After Acute Myocardial Infarction. J. Am. Heart Assoc. 2021, 10, e019017. [Google Scholar] [CrossRef]
- Omole, A.E.; Fakoya, A.O.J. Ten years of progress and promise of induced pluripotent stem cells: Historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ 2018, 6, e4370. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Stocum, D. Somatic Cell Nuclear Transfer|Biology and Technology. 2016. Available online: https://www.britannica.com/science/somatic-cell-nuclear-transfer (accessed on 20 December 2022).
- Gao, L.; Kupfer, M.E.; Jung, J.P.; Yang, L.; Zhang, P.; Da Sie, Y.; Tran, Q.; Ajeti, V.; Freeman, B.T.; Fast, V.G.; et al. Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef]
- Liu, N.; Ye, X.; Yao, B.; Zhao, M.; Wu, P.; Liu, G.; Zhuang, D.; Jiang, H.; Chen, X.; He, Y.; et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2020, 6, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Marote, A.; Salgado, A.; Silva, N. Filling the Gap: NEURAL stem Cells as a Promising Therapy for Spinal Cord Injury. ResearchGate. April 2019. Available online: https://www.researchgate.net/figure/Somatic-cells-reprogramming-using-Takahashi-and-Yamanakas-factors-SOX2-OCT3-4-KLF4_fig1_332735330 (accessed on 20 December 2022).
- Aldrich, S. Solubility Rules|Solubility of Common Ionic Compounds. Sigma Aldrich. 2021. Available online: https://www.sigmaaldrich.com/MX/en/technical-documents/technical-article/genomics/cloning-and-expression/blue-white-screening (accessed on 20 December 2022).
- Leri, A.; Rota, M.; Pasqualini, F.S.; Goichberg, P.; Anversa, P. Origin of cardiomyocytes in the adult heart. Circ. Res. 2015, 116, 150–166. [Google Scholar] [CrossRef]
- Batalov, I.; Feinberg, A.W. Differentiation of cardiomyocytes from human pluripotent stem cells using monolayer culture. Biomark. Insights 2015, 10, BMI-S20050. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Kivelä, R. Cardiomyocyte—Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Front. Cardiovasc. Med. 2018, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Atrial and Ventricular Myocytes. Available online: https://www.ptglab.com/news/blog/atrial-and-ventricular-myocytes/#:~:text=Atrial%20and%20ventricular%20cardiomyocytes%20form (accessed on 20 December 2022).
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzis, K.; Elliott, D.A.; de Sousa Lopes, S.M.C.; Mummery, C.L.; et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef]
- Youssef, A.A.; Ross, E.G.; Bolli, R.; Pepine, C.J.; Leeper, N.J.; Yang, P.C. The promise and challenge of induced pluripotent stem cells for cardiovascular applications. JACC: Basic Transl. Sci. 2016, 1, 510–523. [Google Scholar] [CrossRef]
- Youssef, A. Redirect Notice. 2016. Available online: https://www.google.com/url?q=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4533091/&sa=D&source=docs&ust=1669989389176269&usg=AOvVaw2t_KWAKL7IJr1wqgcyeNdw (accessed on 20 December 2022).
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Zhao, M.-T.; Chen, H.; Liu, Q.; Shao, N.-Y.; Sayed, N.; Wo, H.-T.; Zhang, J.Z.; Ong, S.-G.; Liu, C.; Kim, Y.; et al. Molecular and functional resemblance of differentiated cells derived from isogenic human iPSCs and SCNT-derived ESCs. Proc. Natl. Acad. Sci. USA 2017, 114, E11111–E11120. [Google Scholar] [CrossRef]
- Chin, M.H.; Mason, M.J.; Xie, W.; Volinia, S.; Singer, M.; Peterson, C.; Ambartsumyan, G.; Aimiuwu, O.; Richter, L.; Zhang, J.; et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 2009, 5, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Rungarunlert, S. Embryoid body formation from embryonic and induced pluripotent stem cells: Benefits of bioreactors. World J. Stem Cells 2009, 1, 11. [Google Scholar] [CrossRef]
- Creative Diagnostics. Wnt Signaling Pathway—Creative Diagnostics. Available online: https://www.creative-diagnostics.com/wnt-signaling-pathway.htm (accessed on 20 December 2022).
- Paige, S.L.; Osugi, T.; Afanasiev, O.K.; Pabon, L.; Reinecke, H.; Murry, C.E. Endogenous wnt/β-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE 2010, 5, e11134. [Google Scholar] [CrossRef]
- Schneider, V.A.; Mercola, M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001, 15, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Walthall, J.M.; Hu, J.; Hatzopoulos, A.K. Continuous antagonism by dkk1 counter activates canonical wnt signaling and promotes cardiomyocyte differentiation of embryonic stem cells. Stem Cells Dev. 2012, 21, 54–66. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [PubMed]
- Costello, I.; Pimeisl, I.M.; Dräger, S.; Bikoff, E.K.; Robertson, E.J.; Arnold, S.J. The T-box transcription factor Eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation. Nat. Cell Biol. 2011, 13, 1084–1091. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, Y.; Zhou, Y.; Zhang, J. Deciphering Role of Wnt Signalling in Cardiac Mesoderm and Cardiomyocyte Differentiation from Human iPSCs: Four-dimensional control of Wnt pathway for hiPSC-CMs differentiation. Sci. Rep. 2019, 9, 19389. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Lian, X.; Bao, X.; Zilberter, M.; Westman, M.; Fisahn, A.; Hsiao, C.; Hazeltine, L.B.; Dunn, K.K.; Kamp, T.; Palecek, S.P. Chemically defined, albumin-free human cardiomyocyte generation. Nat. Methods 2015, 12, 595–596. [Google Scholar] [CrossRef]
- Hamad, S.; Derichsweiler, D.; Papadopoulos, S.; Nguemo, F.; Šarić, T.; Sachinidis, A.; Brockmeier, K.; Hescheler, J.; Boukens, B.J.; Pfannkuche, K. Generation of human induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer and scalable 3D suspension bioreactor cultures with reduced batch-to-batch variations. Theranostics 2019, 9, 7222–7238. [Google Scholar] [CrossRef]
- Bruneau, B.G.; Logan, M.; Davis, N.; Levi, T.; Tabin, C.J.; Seidman, J.G.; Seidman, C.E. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev. Biol. 1999, 211, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.T.; Morrisey, E.E.; Anandappa, R.; Sigrist, K.; Lu, M.M.; Parmacek, M.S.; Soudais, C.; Leiden, J.M. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997, 11, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.G.; Lyons, G.E.; Martin, J.F.; Olson, E.N. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development 1994, 120, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Fu, J.-D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef] [PubMed]
- BitSizeBio. Cell Confluency: Why it Matters and 3 Easy Methods. May 2022. Available online: https://bitesizebio.com/63887/cell-confluency/ (accessed on 20 December 2022).
- Balafkan, N.; Mostafavi, S.; Schubert, M.; Siller, R.; Liang, K.X.; Sullivan, G.; Bindoff, L.A. A method for differentiating human induced pluripotent stem cells toward functional cardiomyocytes in 96-well microplates. Sci. Rep. 2020, 10, 18498. [Google Scholar] [CrossRef]
- Siller, R.; Greenhough, S.; Naumovska, E.; Sullivan, G.J. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Rep. 2015, 4, 939–952. [Google Scholar] [CrossRef]
- Siller, R.; Naumovska, E.; Mathapati, S.; Lycke, M.; Greenhough, S.; Sullivan, G.J. Development of a rapid screen for the endodermal differentiation potential of human pluripotent stem cell lines. Sci. Rep. 2016, 6, 37178. [Google Scholar] [CrossRef]
- Mathapati, S.; Siller, R.; Impellizzeri, A.A.; Lycke, M.; Vegheim, K.; Almaas, R.; Sullivan, G.J. Small-Molecule-Directed Hepatocyte-Like cell differentiation of human pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 2016, 38, 1G-6. [Google Scholar] [CrossRef]
- Segers, V.F.M.; Lee, R.T. Stem-cell therapy for cardiac disease. Nature 2008, 451, 937–942. [Google Scholar] [CrossRef]
- Deinsberger, J.; Reisinger, D.; Weber, B. Global trends in clinical trials involving pluripotent stem cells: A systematic multi-database analysis. NPJ Regen. Med. 2020, 5, 15. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in pluripotent stem cells: History, mechanisms, technologies, and applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Pollard, T.D.; Earnshaw, W.C.; Lippincott-Schwartz, J.; Johnson, G. Cell Biology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016; Available online: https://books.google.com/books?hl=en&lr=&id=Th1uDQAAQBAJ&oi=fnd&pg=PP1&ots=5SlhklIjSA&sig=F-u_e7ABICLaFHAdYDFfwTl2INk#v=onepage&q&f=false (accessed on 20 December 2022).
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef]
- Dakhore, S.; Nayer, B.; Hasegawa, K. Human pluripotent stem cell culture: Current status, challenges, and advancement. Stem Cells Int. 2018, 2018, 7396905. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Diomede, F.; Merciaro, I.; Caputi, S.; Tartaro, A.; Guarnieri, S.; Trubiani, O. Endothelial committed oral stem cells as modelling in the relationship between periodontal and cardiovascular disease. J. Cell. Physiol. 2018, 233, 6734–6747. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Mohammadi, M.H.; Rafatian, N.; Toroghi, M.K.; Wang, E.Y.; Zhao, Y.; Korolj, A.; Ahadian, S.; Radisic, M. Cardiovascular disease models: A game changing paradigm in drug discovery and screening. Biomaterials 2019, 198, 3–26. [Google Scholar] [CrossRef]
- Horiguchi, I.; Kino-oka, M. Current developments in the stable production of human induced pluripotent stem cells. Engineering 2021, 7, 144–152. [Google Scholar] [CrossRef]
- MacDonald, A. Cell Potency: Totipotent vs Pluripotent vs Multipotent STEM cells. Cell Science from Technology Networks. May 2018. Available online: https://www.technologynetworks.com/cell-science/articles/cell-potency-totipotent-vs-pluripotent-vs-multipotent-stem-cells-303218 (accessed on 20 December 2022).
- Sommer, C.A.; Mostoslavsky, G. The evolving field of induced pluripotency: Recent progress and future challenges. J. Cell. Physiol. 2012, 228, 267–275. [Google Scholar] [CrossRef]
- Nishi, M.; Akutsu, H.; Masui, S.; Kondo, A.; Nagashima, Y.; Kimura, H.; Perrem, K.; Shigeri, Y.; Toyoda, M.; Okayama, A.; et al. A distinct role for pin1 in the induction and maintenance of pluripotency*. J. Biol. Chem. 2011, 286, 11593–11603. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, H.; Chen, L. A dual role of Erk signaling in embryonic stem cells. Exp. Hematol. 2016, 44, 151–156. [Google Scholar] [CrossRef]
- Kunath, T.; Saba-El-Leil, M.K.; Almousailleakh, M.; Wray, J.; Meloche, S.; Smith, A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 2007, 134, 2895–2902. [Google Scholar] [CrossRef]
- Stadtfeld, M.; Maherali, N.; Breault, D.T.; Hochedlinger, K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2008, 2, 230–240. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, T.; Guan, J.; Zhang, X.; Fu, Y.; Ye, J.; Zhu, J.; Meng, G.; Ge, J.; Yang, S.; et al. A XEN-like state bridges somatic cells to pluripotency during chemical reprogramming. Cell 2015, 163, 1678–1691. [Google Scholar] [CrossRef]
- Garay, B.I.; Givens, S.; Abreu, P.; Liu, M.; Yücel, D.; Baik, J.; Stanis, N.; Rothermel, T.M.; Magli, A.; Abrahante, J.E.; et al. Dual inhibition of MAPK and PI3K/AKT pathways enhances maturation of human iPSC-derived cardiomyocytes. Stem Cell Rep. 2022, 17, 2005–2022. [Google Scholar] [CrossRef]
- Sonnenblick, E.H.; Ross, J.; Covell, J.W.; Spotnitz, H.M.; Spiro, D. The ultrastructure of the heart in systole and diastole. Circ. Res. 1967, 21, 423–431. [Google Scholar] [CrossRef]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef]
- Jimenez-Vazquez, E.N.; Jain, A.; Jones, D.K. Enhancing iPSC-CM maturation using a Matrigel-Coated micropatterned PDMS substrate. Curr. Protoc. 2022, 2, e601. [Google Scholar] [CrossRef]
- Stoppel, W.L.; Kaplan, D.L.; Black, L.D. Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv. Drug Deliv. Rev. 2016, 96, 135–155. [Google Scholar] [CrossRef]
- Xia, Y.; McMillin, J.B.; Lewis, A.; Moore, M.; Zhu, W.G.; Williams, R.; Kellems, R.E. Electrical stimulation of neonatal cardiac myocytes activates the NFAT3 and GATA4 pathways 634 and up-regulates the adenylosuccinate synthetase 1 gene*. J. Biol. Chem. 2000, 275, 1855–1863. [Google Scholar] [CrossRef]
- Guo, Y.; Pu, W.T. Cardiomyocyte Maturation. Circ. Res. 2020, 126, 1086–1106. [Google Scholar] [CrossRef]
- Yang, X.; Rodriguez, M.L.; Leonard, A.; Sun, L.; Fischer, K.A.; Wang, Y.; Ritterhoff, J.; Zhao, L.; Kolwicz, S.C., Jr.; Pabon, L.; et al. Fatty Acids Enhance the Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2019, 13, 657–668. [Google Scholar] [CrossRef]
- Biermann, M.; Cai, W.; Lang, D.; Hermsen, J.; Profio, L.; Zhou, Y.; Czirok, A.; Isai, D.G.; Napiwocki, B.N.; Rodriguez, A.M.; et al. Epigenetic Priming of Human Pluripotent Stem Cell-Derived Cardiac Progenitor Cells Accelerates Cardiomyocyte Maturation. Stem Cells 2019, 37, 910–923. [Google Scholar] [CrossRef]
- Tabei, R.; Kawaguchi, S.; Kanazawa, H.; Tohyama, S.; Hirano, A.; Handa, N.; Hishikawa, S.; Teratani, T.; Kunita, S.; Fukuda, J.; et al. Development of a transplant injection device for optimal distribution and retention of human induced pluripotent stem cell–derived cardiomyocytes. J. Heart Lung Transplant. 2019, 38, 203–214. [Google Scholar] [CrossRef]
- Fujita, J. Development of cardiac regenerative medicine using human iPS cell-derived cardiomyocytes. Keio J. Med. 2020, 70, 53–59. [Google Scholar] [CrossRef]
- Kitsuka, T.; Takahashi, F.; Reinhardt, J.; Watanabe, T.; Ulziibayar, A.; Yimit, A.; Kelly, J.; Shinoka, T. Advances in cardiac tissue engineering. Bioengineering 2022, 9, 696. [Google Scholar] [CrossRef]
- Azevedo-Gaiolla, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.R.; Zornoff, L.A.M. Cardiac remodeling: Concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq. Bras. Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell–Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Wendel, J.S.; Ye, L.; Tao, R.; Zhang, J.; Zhang, J.; Kamp, T.J.; Tranquillo, R.T. Functional effects of a tissue-engineered cardiac patch from human induced pluripotent stem cell-derived cardiomyocytes in a rat infarct model. STEM CELLS Transl. Med. 2015, 4, 1324–1332. [Google Scholar] [CrossRef]
- Riegler, J.; Tiburcy, M.; Ebert, A.D.; Tzatzalos, E.; Raaz, U.; Abilez, O.J.; Shen, Q.; Kooreman, N.G.; Neofytou, E.; Chen, V.C.; et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 2015, 117, 720–730. [Google Scholar] [CrossRef]
- Shadrin, I.Y.; Allen, B.W.; Qian, Y.; Jackman, C.P.; Carlson, A.L.; Juhas, M.E.; Bursac, N. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 2017, 8, 1825. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Electrical coupling of cardiomyocyte sheets occurs rapidly via functional gap junction formation. Biomaterials 2006, 27, 4765–4774. [Google Scholar] [CrossRef]
- Kawamura, M. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012, 126, S29–S37. [Google Scholar] [CrossRef]
- Domae, K.; Miyagawa, S.; Yoshikawa, Y.; Fukushima, S.; Hata, H.; Saito, S.; Kainuma, S.; Kashiyama, N.; Iseoka, H.; Ito, E.; et al. Clinical Outcomes of Autologous Stem Cell–Patch Implantation for Patients With Heart Failure With Nonischemic Dilated Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e008649. [Google Scholar] [CrossRef]
- Merino-González, C.; Zuñiga, F.A.; Escudero, C.; Ormazabal, V.; Reyes, C.; Nova-Lamperti, E.; Salomon, C.; Tapia, C.A. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front. Physiol. 2016, 7, 24. [Google Scholar] [CrossRef]
- Liu, B.; Lee, B.W.; Nakanishi, K.; Villasante, A.; Williamson, R.; Metz, J.; Kim, J.; Kanai, M.; Bi, L.; Brown, K.; et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2018, 2, 293–303. [Google Scholar] [CrossRef]
- Spheroids. Molecular Devices. Available online: https://www.moleculardevices.com/applications/3d-cell-models/spheroids#gref (accessed on 20 December 2022).
- Zhang, J.; Zhu, W.; Radisic, M.; Vunjak-Novakovic, G. Can We Engineer a Human Cardiac Patch for Therapy? Circ. Res. 2018, 123, 244–265. [Google Scholar] [CrossRef]
- Pomeroy, J.E.; Helfer, A.; Bursac, N. Biomaterializing the Promise of Cardiac Tissue Engineering. Biotechnol. Adv. 2020, 42, 107353. [Google Scholar] [CrossRef]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell , P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar]
- Kupfer, M.E.; Lin, W.-H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.B.; Lenz, M.; Ai, J.; Mahutga, R.R.; et al. In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ. Res. 2020, 127, 207–224. [Google Scholar] [CrossRef]
- Anker, S.D.; Coats, A.J.S.; Cristian, G.; Dragomir, D.; Pusineri, E.; Piredda, M.; Bettari, L.; Dowling, R.; Volterrani, M.; Kirwan, B.-A.; et al. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur. Heart J. 2015, 36, 2297–2309. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, T.; Li, Z.; Jia, Z.; Clapcote, S.J.; Liu, C.; Li, S.; Asrar, S.; Pao, A.; Chen, R.; Fan, N.; et al. A mouse model of Down syndrome trisomic for all human chromosome 21 syntenic regions. Hum. Mol. Genet. 2010, 19, 2780–2791. [Google Scholar] [CrossRef]
- Stillitano, F.; Hansen, J.; Kong, C.-W.; Karakikes, I.; Funck-Brentano, C.; Geng, L.; Scott, S.; Reynier, S.; Wu, M.; Valogne, Y.; et al. Modeling susceptibility to drug-induced long QT with a panel of subject-specific induced pluripotent stem cells. eLife 2017, 6, e19406. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Micheu, M.M.; Rosca, A.M. Patient-specific induced pluripotent stem cells as “disease-in-a-dish” models for inherited cardiomyopathies and channelopathies–15 years of research. World J. Stem Cells. 2021, 13, 281–303. [Google Scholar] [CrossRef]
- Park, I.-H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-Specific Induced Pluripotent Stem Cells. Cell 2008, 134, 877–886. [Google Scholar] [CrossRef]
- Funakoshi, S.; Yoshida, Y. Recent progress of iPSC technology in cardiac diseases. Arch. Toxicol. 2021, 95, 3633–3650. [Google Scholar] [CrossRef]
- Veres, A.; Gosis, B.S.; Ding, Q.; Collins, R.; Ragavendran, A.; Brand, H.; Erdin, S.; Cowan, C.A.; Talkowski, M.E.; Musunuru, K. Low Incidence of Off-Target Mutations in Individual CRISPR-Cas9 and TALEN Targeted Human Stem Cell Clones Detected by Whole-Genome Sequencing. Cell Stem Cell 2014, 15, 27–30. [Google Scholar] [CrossRef]
- Musunuru, K. Genome editing of human pluripotent stem cells to generate human cellular disease models. Dis. Model. Mech. 2013, 6, 896–904. [Google Scholar] [CrossRef]
- Smith, C.; Gore, A.; Yan, W.; Abalde-Atristain, L.; Li, Z.; He, C.; Wang, Y.; Brodsky, R.A.; Zhang, K.; Cheng, L.; et al. Whole-Genome Sequencing Analysis Reveals High Specificity of CRISPR/Cas9 and TALEN-Based Genome Editing in Human iPSCs. Cell Stem Cell 2014, 15, 12–13. [Google Scholar] [CrossRef]
- Smith, C.; Gore, A.; Yan, W.; Abalde-Atristain, L.; Li, Z.; He, C.; Wang, Y.; Brodsky, R.A.; Zhang, K.; Cheng, L.; et al. Heart-on-a-chip using human iPSC-derived cardiomyocytes with an integrated vascular endothelial layer based on a culture patch as a potential platform for drug evaluation. Biofabrication 2022, 15, 015010. [Google Scholar] [CrossRef]
- Veldhuizen, J.; Cutts, J.; Brafman, D.A.; Migrino, R.Q.; Nikkhah, M. Engineering anisotropic human stem cell-derived three-dimensional cardiac tissue on-a-chip. Biomaterials 2020, 256, 120195. [Google Scholar] [CrossRef]
- Maoz, B.M.; Herland, A.; Henry, O.Y.F.; Leineweber, W.D.; Yadid, M.; Doyle, J.; Mannix, R.; Kujala, V.J.; FitzGerald, E.A.; Parker, K.K.; et al. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab A Chip 2017, 17, 2294–2302. [Google Scholar] [CrossRef]
- Bernardin, A.A.; Colombani, S.; Rousselot, A.; Andry, V.; Goumon, Y.; Delanoë-Ayari, H.; Pasqualin, C.; Brugg, B.; Jacotot, E.D.; Pasquié, J.-L.; et al. Impact of Neurons on Patient-Derived Cardiomyocytes Using Organ-On-A-Chip and iPSC Biotechnologies. Cells 2022, 11, 3764. [Google Scholar] [CrossRef]
- Sharma, A.; Sances, S.; Workman, M.J.; Svendsen, C.N. Multi-lineage Human iPSC-Derived Platforms for Disease Modeling and Drug Discovery. Cell Stem Cell 2020, 26, 309–329. [Google Scholar] [CrossRef]
- Arrhythmogenic Cardiomyopathy|Boston Children’s Hospital. Available online: https://www.childrenshospital.org/conditions/arrhythmogenic-cardiomyopathy#:~:text=What%20is%20arrhythmogenic%20cardiomyopathy%3F (accessed on 20 December 2022).
- Dilated Cardiomyopathy—Symptoms and Causes. Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/dilated-cardiomyopathy/symptoms-causes/syc-20353149#:~:text=Dilated%20cardiomyopathy%20is%20a%20type (accessed on 20 December 2022).
- Kumar, S.; Curran, J.; Kumar, K.; DeLeon, E.; Leandro, A.; Peralta, J.; Williams-Blangero, S.; Blangero, J. Disease Modeling and Disease Gene Discovery in Cardiomyopathies: A Molecular Study of Induced Pluripotent Stem Cell Generated Cardiomyocytes. Int. J. Mol. Sci. 2021, 22, 3311. [Google Scholar] [CrossRef]
- Austin, K.M.; Trembley, M.A.; Chandler, S.F.; Sanders, S.P.; Saffitz, J.E.; Abrams, D.J.; Pu, W.T. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat. Rev. Cardiol. 2019, 16, 519–537. [Google Scholar] [CrossRef]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.; Ackerman, M.J.; Calkins, H.; Darrieux, F.; Daubert, J.P.; De Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019, 16, e301–e372. [Google Scholar] [CrossRef]
- Corrado, D.; Cipriani, A.; De Lazzari, M.; Perazzolo Marra, M. Right ventricular dilatation in arrhythmogenic right ventricular cardiomyopathy: Need for a revision of the 2010 International Task Force criteria. Eur. Heart J. 2020, 41, 1452–1453. [Google Scholar] [CrossRef]
- Thiene, G. The research venture in arrhythmogenic right ventricular cardiomyopathy: A paradigm of translational medicine. Eur. Heart J. 2015, 36, 837–846. [Google Scholar] [CrossRef]
- Inoue, H.; Nakamura, S.; Higo, S.; Shiba, M.; Kohama, Y.; Kondo, T.; Kameda, S.; Tabata, T.; Okuno, S.; Ikeda, Y.; et al. Modeling reduced contractility and impaired desmosome assembly due to plakophilin-2 deficiency using isogenic iPS cell-derived cardiomyocytes. Stem Cell Rep. 2022, 17, 337–351. [Google Scholar] [CrossRef]
- Kim, C.; Wong, J.; Wen, J.; Wang, S.; Wang, C.; Spiering, S.; Kan, N.G.; Forcales, S.; Puri, P.L.; Leone, T.C.; et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature 2013, 494, 105–110. [Google Scholar] [CrossRef]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Hedges, D.J.; Morales, A. Dilated cardiomyopathy: The complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 2013, 10, 531–547. [Google Scholar] [CrossRef]
- Reichart, D.; Magnussen, C.; Zeller, T.; Blankenberg, S. Dilated cardiomyopathy: From epidemiologic to genetic phenotypes. J. Intern. Med. 2019, 286, 362–372. [Google Scholar] [CrossRef]
- Dai, Y.; Amenov, A.; Ignatyeva, N.; Koschinski, A.; Xu, H.; Soong, P.L.; Tiburcy, M.; Linke, W.A.; Zaccolo, M.; Hasenfuss, G.; et al. Troponin destabilization impairs sarcomere-cytoskeleton interactions in iPSC-derived cardiomyocytes from dilated cardiomyopathy patients. Sci. Rep. 2020, 10, 209. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Schmidt-Hieber, M.; Bertz, H.; Heinz, W.J.; Kiehl, M.; Krüger, W.; Mousset, S.; Neuburger, S.; Neumann, S.; Penack, O.; et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: Prevention and prophylaxis strategy guidelines 2016. Ann. Hematol. 2016, 95, 1435–1455. [Google Scholar] [CrossRef]
- Murata, K.; Ikegawa, M.; Minatoya, K.; Masumoto, H. Strategies for immune regulation in iPS cell-based cardiac regenerative medicine. Inflamm. Regen. 2020, 40, 36. [Google Scholar] [CrossRef]
- Yoshida, S.; Miyagawa, S.; Toyofuku, T.; Fukushima, S.; Kawamura, T.; Kawamura, A.; Kashiyama, N.; Nakamura, Y.; Toda, K.; Sawa, Y. Syngeneic Mesenchymal Stem Cells Reduce Immune Rejection After Induced Pluripotent Stem Cell-Derived Allogeneic Cardiomyocyte Transplantation. Sci. Rep. 2020, 10, 4593. [Google Scholar] [CrossRef]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Tandon, N.; Godier, A.; Maidhof, R.; Marsano, A.; Martens, T.P.; Radisic, M. Challenges in Cardiac Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 169–187. [Google Scholar] [CrossRef]
- Romagnuolo, R.; Masoudpour, H.; Porta-Sánchez, A.; Qiang, B.; Barry, J.; Laskary, A.; Qi, X.; Masse, S.; Magtibay, K.; Kawajiri, H.; et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Rep. 2019, 12, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, H.; Kadota, S.; Kashihara, T.; Yamada, M.; Ito, K.; Kobayashi, H.; Tanaka, Y.; Shiba, N.; Chuma, S.; Tohyama, S.; et al. Increased predominance of the matured ventricular subtype in embryonic stem cell-derived cardiomyocytes in vivo. Sci. Rep. 2020, 10, 11883. [Google Scholar] [CrossRef]
- Nakamura, K.; Neidig, L.E.; Yang, X.; Weber, G.J.; El-Nachef, D.; Tsuchida, H.; Dupras, S.; Kalucki, F.A.; Jayabalu, A.; Futakuchi-Tsuchida, A.; et al. Pharmacologic therapy for engraftment arrhythmia induced by transplantation of human cardiomyocytes. Stem Cell Rep. 2021, 16, 2473–2487. [Google Scholar] [CrossRef] [PubMed]
- Dan, G.-A.; Martinez-Rubio, A.; Agewall, S.; Boriani, G.; Borggrefe, M.; Gaita, F.; Van Gelder, I.; Gorenek, B.; Kaski, J.C.; Kjeldsen, K.; et al. Antiarrhythmic drugs–clinical use and clinical decision making: A consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). EP Eur. 2018, 20, 731–732an. [Google Scholar] [CrossRef]
- Dandulakis, M.G.; Meganathan, K.; Kroll, K.L.; Bonni, A.; Constantino, J.N. Complexities of X chromosome inactivation status in female human induced pluripotent stem cells—A brief review and scientific update for autism research. J. Neurodev. Disord. 2016, 8, 22. [Google Scholar] [CrossRef]
- Jiang, Y.; Park, P.; Hong, S.M.; Ban, K. Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells: Current Strategies and Limitations. Mol. Cells 2018, 41, 613–621. [Google Scholar] [CrossRef]
- Davis, R.P.; Casini, S.; Berg, C.W.V.D.; Hoekstra, M.; Remme, C.A.; Dambrot, C.; Salvatori, D.; Oostwaard, D.W.-V.; Wilde, A.A.M.; Bezzina, C.R.; et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 2012, 125, 3079–3091. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Tran, D.D.; George, S.C. Concise Review: Maturation Phases of Human Pluripotent Stem Cell-Derived Cardiomyocytes. STEM CELLS 2013, 31, 829–837. [Google Scholar] [CrossRef]
- Lee, P.; Klos, M.; Bollensdorff, C.; Hou, L.; Ewart, P.; Kamp, T.J.; Zhang, J.; Bizy, A.; Guerrero-Serna, G.; Kohl, P.; et al. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ. Res. 2012, 110, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Veerman, C.C.; Kosmidis, G.; Mummery, C.L.; Casini, S.; Verkerk, A.O.; Bellin, M. Immaturity of Human Stem-Cell-Derived Cardiomyocytes in Culture: Fatal Flaw or Soluble Problem? Stem Cells Dev. 2015, 24, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pabon, L.; Murry, C.E. Engineering Adolescence. Circ. Res. 2014, 114, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Pedron, S.; van Lierop, S.; Horstman, P.; Penterman, R.; Broer, D.J.; Peeters, E. Stimuli Responsive Delivery Vehicles for Cardiac Microtissue Transplantation. Adv. Funct. Mater. 2011, 21, 1624–1630. [Google Scholar] [CrossRef]
- Scuderi, G.J.; Butcher, J. Naturally Engineered Maturation of Cardiomyocytes. Front. Cell Dev. Biol. 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Chattergoon, N.N.; Giraud, G.D.; Louey, S.; Stork, P.; Fowden, A.L.; Thornburg, K.L. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 397–408. [Google Scholar] [CrossRef]
- Krüger, M.; Sachse, C.; Zimmermann, W.H.; Eschenhagen, T.; Klede, S.; Linke, W.A. Thyroid hormone regulates developmental titin isoform transitions via the phosphatidylinositol-3-kinase/ AKT pathway. Circ. Res. 2008, 102, 439–447. [Google Scholar] [CrossRef]
- Takahashi, S.; Kobayashi, S.; Hiratani, I. Epigenetic differences between naïve and primed pluripotent stem cells. Cell. Mol. Life Sci. 2018, 75, 1191–1203. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, P.; Yan, L.; Li, R.; Hu, B.; Lian, Y.; Yan, J.; Ren, X.; Lin, S.; Li, J.; et al. The DNA methylation landscape of human early embryos. Nature 2014, 511, 606–610. [Google Scholar] [CrossRef]
- Vallot, C.; Patrat, C.; Collier, A.J.; Huret, C.; Casanova, M.; Ali, T.M.L.; Tosolini, M.; Frydman, N.; Heard, E.; Rugg-Gunn, P.J.; et al. XACT Noncoding RNA Competes with XIST in the Control of X Chromosome Activity during Human Early Development. Cell Stem Cell 2017, 20, 102–111. [Google Scholar] [CrossRef]
- Garber, K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat. Biotechnol. 2015, 33, 890–892. [Google Scholar] [CrossRef] [PubMed]
- McNeish, J.; Gardner Jason, P.; Wainger Brian, J.; Woolf Clifford, J.; Eggan, K. From Dish to Bedside: Lessons Learned While Translating Findings from a Stem Cell Model of Disease to a Clinical Trial. Cell Stem Cell 2015, 17, 8–10. [Google Scholar] [CrossRef] [PubMed]
- King, N.M.; Perrin, J. Ethical Issues in Stem Cell Research and Therapy. Stem Cell Res. Ther. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Cebo, D. Public Opinion About Stem Cell Research And Human Cloning—The Bioethics Of Stem Cell Research And Therapy. Available online: https://academic.oup.com/poq/article/68/1/131/1855075 (accessed on 2 December 2022).
- Nisbet, M.C. Public Opinion About Stem Cell Research and Human Cloning. Public Opin. Q. 2004, 68, 131–154. [Google Scholar] [CrossRef]
- Hyun, I. The bioethics of stem cell research and therapy. J. Clin. Investig. 2010, 120, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Godfrey, K.J.; Mathew, B.; Bulman, J.C.; Shah, O.; Clement, S.; Gallicano, G.I. Stem cell-based treatments for Type 1 diabetes mellitus: Bone marrow, embryonic, hepatic, pancreatic and induced pluripotent stem cells. Diabet. Med. 2011, 29, 14–23. [Google Scholar] [CrossRef]
- Agrawal, A. Japan’s Stance on Stem Cell Research Policies—JMTSE. Available online: https://you.stonybrook.edu/jmtse/2021/09/04/japans-stance-on-stem-cell-research-policies/ (accessed on 2 December 2022).
- Verginer, L.; Riccaboni, M. Stem cell legislation and its impact on the geographic preferences of stem cell researchers. Eurasian Bus. Rev. 2021, 11, 163–189. [Google Scholar] [CrossRef]
- Nussbaum, J.; Minami, E.; Laflamme, M.A.; Virag, J.A.I.; Ware, C.B.; Masino, A.; Muskheli, V.; Pabon, L.; Reinecke, H.; Murry, C.E. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 1345–1357. [Google Scholar] [CrossRef]
- Kroon, E.; A Martinson, L.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.G.; Cunningham, J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar] [CrossRef]
- Prokhorova, T.A.; Harkness, L.; Frandsen, U.; Ditzel, N.; Schrøder, H.D.; Burns, J.S.; Kassem, M. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2009, 18, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.R. The significance of induced pluripotent stem cells for basic research and clinical therapy. J. Med. Ethics 2008, 34, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, T.; Rachul, C.; Zarzeczny, A. The Evolution of Policy Issues in Stem Cell Research: An International Survey. Stem Cell Rev. Rep. 2012, 8, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Ginty, P.; McMahon, S.; May, M.; Solomon, S.L.; Kurtz, A.; Stacey, G.N.; Griscelli, A.B.; Li, R.A.; Barry, J.; et al. The Global Alliance for iPSC Therapies (GAiT). Stem Cell Res. 2020, 49, 102036. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Bell, J.; George, C.; Harmon, S.; Munsie, M.; Kaye, J. The European General Data Protection Regulation: Challenges and considerations for iPSC researchers and biobanks. Regen. Med. 2017, 12, 693–703. [Google Scholar] [CrossRef]
- Ilic, D. iPSC in the past decade: The Japanese dominance. Regen. Med. 2016, 11, 747–749. [Google Scholar] [CrossRef]
- Azuma, K.; Yamanaka, S. Recent policies that support clinical application of induced pluripotent stem cell-based regenerative therapies. Regen. Ther. 2016, 4, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B. Embryonic Stem Cell Research: Terminological Ambiguity May Lead to Legal Obscurity. Med. Law 2004, 23, 19. Available online: https://heinonline.org/HOL/LandingPage?handle=hein.journals/mlv23&div=7&id=&page= (accessed on 2 December 2022). [PubMed]
- Weissman, I. Stem Cell Therapies Could Change Medicine… If They Get the Chance. Cell Stem Cell 2012, 10, 663–665. [Google Scholar] [CrossRef]
- Sipp, D. Direct-to-Consumer Stem Cell Marketing and Regulatory Responses. STEM CELLS Transl. Med. 2013, 2, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, J. Human Stem Cell Ethics: Beyond the Embryo. Cell Stem Cell 2008, 2, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Daley, G.Q. The Promise and Perils of Stem Cell Therapeutics. Cell Stem Cell 2012, 10, 740–749. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, F.; Patel, P.; Kitsuka, T.; Arai, K. The Exciting Realities and Possibilities of iPS-Derived Cardiomyocytes. Bioengineering 2023, 10, 237. https://doi.org/10.3390/bioengineering10020237
Takahashi F, Patel P, Kitsuka T, Arai K. The Exciting Realities and Possibilities of iPS-Derived Cardiomyocytes. Bioengineering. 2023; 10(2):237. https://doi.org/10.3390/bioengineering10020237
Chicago/Turabian StyleTakahashi, Fuga, Praneel Patel, Takahiro Kitsuka, and Kenichi Arai. 2023. "The Exciting Realities and Possibilities of iPS-Derived Cardiomyocytes" Bioengineering 10, no. 2: 237. https://doi.org/10.3390/bioengineering10020237
APA StyleTakahashi, F., Patel, P., Kitsuka, T., & Arai, K. (2023). The Exciting Realities and Possibilities of iPS-Derived Cardiomyocytes. Bioengineering, 10(2), 237. https://doi.org/10.3390/bioengineering10020237