The Application of Porous Scaffolds for Cardiovascular Tissues
Abstract
1. Introduction
2. Porous Scaffolds
3. Methods to Create Porous Scaffolds
4. Materials for Porous Scaffolds
4.1. Synthetic Polymer
4.2. Natural Polymer
4.3. Silk, Fibroin, Chitosan
4.4. Composite Material
5. Tissue-Engineered Vascular Graft
5.1. Tissue-Engineered Arterial Graft (TEAG)
5.2. Tissue-Engineered Venous Graft (TEVeG)
6. Tissue-Engineered Heart Valve (TEHV)
7. Cardiac Patch
| Material | Animal Species | Number | Surgery or Intervention | Findings | Reference |
|---|---|---|---|---|---|
| PGA/PLCL with hiPS-CMs | Rat | 6 | RVOT reconstruction, with the cardiomyocyte seeded scaffold. | Seeded cells were not present in the patch after 4 weeks. The seeded cell might affect the host cardiac regeneration at 16 weeks. | [119] |
| PGA/PLCL with hiPS-CPCs | Rat | 3 | LV free wall reconstruction with CPC seeded scaffold. | Seeded cells disappeared at an early stage, no contribution to LV function, possibility of affecting angiogenesis at 9 months. | [120] |
| collagen+G-CSF | Rat AMI model | 5 for each group | engrafting the collagen patch onto the injured myocardium | Effectively grafted, further increase in neovascularization with G-CSF | [122] |
| collagen with BMC+VEGF | Rat | 3-4/group | RV free wall reconstruction with collagen patch. | Promoted cell proliferation within the graft, increased blood vessel density and reduced construct thinning. | [123] |
| Chitosan-hyaluronan/slik fibroin | Rat AMI model | 11 | epicardial placement on the injured area | Improved LV function, reduced LV dilation, also improved angiogenesis. | [124] |
8. Future Prospective
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Truby, L.K.; Rogers, J.G. Advanced heart failure: Epidemiology, diagnosis, and therapeutic approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef]
- Spears, J.; Al-Saiegh, Y.; Goldberg, D.; Manthey, S.; Goldberg, S. TAVR: A review of current practices and considerations in low-risk patients. J. Interv. Cardiol. 2020, 2020, 2582938. [Google Scholar] [CrossRef]
- Mack, M.; Carroll, J.D.; Thourani, V.; Vemulapalli, S.; Squiers, J.; Manandhar, P.; Deeb, G.M.; Batchelor, W.; Herrmann, H.C.; Cohen, D.J.; et al. Transcatheter Mitral valve therapy in the United States: A report from the STS-ACC TVT Registry. J. Am. Coll. Cardiol. 2021, 78, 2316–2353. [Google Scholar] [CrossRef]
- Head, S.J.; Milojevic, M.; Daeman, J.; Ahn, J.M.; Boersma, E.; Christiansen, E.H.; Domanski, M.J.; Farkouh, M.E.; Flather, M.; Fuster, V.; et al. Mortality after coronary artery bypass versus percutaneous coronary intervention with stenting for coronary artery disease: A pooled analysis of individual patient data. Lancet 2018, 391, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F. Lower extremity peripheral artery disease: Contemporary epidemiology, management gaps, and future directions: A scientific statement from the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ishida, N.; Takaoka, M.; Tsujimoto, K.; Kondo, K.; Isoda, R.; Yukawa, T.; Tokunaga, N.; Ishida, A.; Fukazawa, T.; et al. Bioelectrical impedance analysis for perioperative water management in adult cardiovascular valve disease surgery. Surg. Today 2021, 51, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, Y.; Watanabe, T.; Ohashi, I.; Katayama, H.; Ishida, N.; Hayashi, M.; Takaoka, M.; Kuinose, M.; Sugimoto, K.; Toda, Y.; et al. Analysis of water balance for perioperative management in coronary artery bypass grafting. Kawasaki Med. J. 2021, 47, 55–62. [Google Scholar]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Alraies, M.C.; Eckman, P. Adult heart transplant: Indications and outcomes. J. Thorac. Dis. 2014, 6, 1120–1128. [Google Scholar]
- Messer, S.; Large, S. Resuscitating heart transplantation: The donation after circulatory determined death donor. Eur. J. Cardiothorac. Surg. 2016, 49, 1–4. [Google Scholar] [CrossRef]
- Inamdar, A.; Inamdar, C. Heart failure: Diagnosis, management, and utilization. J. Clin. Med. 2016, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R. Clinical burden and health service challenges of chronic heart failure. Br. J. Gen. Pract. 2010, 60, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.; Anker, S.D.; Cleland, J.G.F.; Felker, J.G.M.; Filippatos, G.; Jaarsma, T.; Jourdain, P.; Knight, E.; Massie, B.; Ponikowski, P.; et al. Improving care for patients with acute heart failure: Before, during and after hospitalization. ESC Heart Fail. 2014, 1, 110–145. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Sasha, J.M.; Taylor, A.; Krucoff, M.; Reynolds, M.R.; Gibson, M. A reevaluation of the costs of heart failure and its implications for the allocation of health resources in the United States. Clin. Cardiol. 2014, 37, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Khatibzadeh, S.; Farzadfar, F.; Oliver, J.; Ezzati, M.; Moran, A. Worldwide risk factors for heart failure: A systematic review and pooled analysis. Int. J. Cardiol. 2013, 168, 1186–1194. [Google Scholar] [CrossRef]
- Mamode, N.; Scott, R.N. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst. Rev. 2000, 2, CD001487. [Google Scholar]
- Pugsley, M.K.; Tabrizchi, R. The vascular system. An overview of structure and function. J. Pharmacol. Toxicol. Methods 2000, 44, 333–340. [Google Scholar] [CrossRef]
- Diener, H.; Hellwinklel, O.; Carpenter, S.; Avellaneda, A.L.; Debus, E.S. Homografts and extra-anatomical reconstructions for infected vascular grafts. J. Cardiovasc. Surg. 2014, 55, 217–223. [Google Scholar]
- Kurobe, H.; Maxfield, M.W.; Breuer, C.K.; Shinoka, T. Concise review: Tissue-engineered. Vascular grafts for cardiac surgery: Past, present, and future. Stem Cells Transl. Med. 2012, 1, 566–571. [Google Scholar] [CrossRef]
- O’Donnell, A.; Yutzey, K.E. Mechanisms of heart valve development and diseases. Development 2020, 147, dev183020. [Google Scholar] [CrossRef]
- Salaun, E.; Clavel, M.A.; Rodes-Cabau, J.; Pibarot, P. Bioprosthetic aortic valve durability in the era of transcatheter aortic valve implantation. Heart 2018, 104, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Basu, A.; Di Scenza, G.; Bartlett, J.; Fan, K.S.; Oo, S.; Harky, A. Understanding aortic valve repair through Ozaki procedure: A review of literature evidence. J. Card. Surg. 2022, 37, 5202–5206. [Google Scholar] [CrossRef]
- Westaby, S. Coronary revascularization in ischemic cardiomyopathy. Surg. Clin. N. Am. 2004, 84, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Sayers, J.R.; Riley, P.R. Heart regeneration: Beyond new muscle and vessels. Cardiovasc. Res. 2021, 117, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Lemcke, H.; David, R. Stem cell therapy in heart diseases—Cell types, mechanisms, and improvement strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Atala, A. Tissue Engineering: Toward a New Era of Medicine. Annu. Rev. Med. 2017, 68, 29–40. [Google Scholar] [CrossRef]
- Fukuno, Y.; Usui, M.; Underwood, R.A.; Isenhath, S.; Marshall, A.J.; Hauch, K.D.; Ratner, B.D.; Olerud, J.E.; Fleckman, P. Epidermal and dermal integration into sphere-templated porous poly(2-hydroxyethyl methacrylate) implants in mice. J. Biomed. Mater. Res. A 2010, 94, 1172–1186. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, C.; Khademhosseini, A. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Sharkaway, A.A.; Klitzmann, B.; Truskey, G.A.; Reichert, W.M. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J. Biomed. Mater. Res. 1997, 37, 401–412. [Google Scholar] [CrossRef]
- Galperin, A.; Long, T.J.; Ratner, B.D. Degradable, thermo-sensitive poly(N-isopropyl acrylamide)-based scaffolds with controlled porosity for tissue engineering applications. Biomacromolecules 2010, 11, 2583–2592. [Google Scholar] [CrossRef]
- Sharkawy, A.A.; Klitzman, B.; Truskey, G.A.; Reichart, W.M. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma-tissue exchange properties. J. Biomed. Mater. Res. 1998, 40, 586–597. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Karp, R.D.; Johnson, K.H.; Buoen, L.C.; Ghobrial, H.K.; Brand, I.; Brand, K.G. Tumorigenesis by Millipore filters in mice: Histology and ultrastructure of tissue reactions as related to pore size. Natl. Cancer Inst. 1973, 51, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Brauker, J.H.; CarroBrendel, V.E.; Martinson, L.A.; Crudele, J.; Johnston, W.D.; Johnson, R.C. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 1995, 29, 1517–1524. [Google Scholar] [CrossRef]
- Sayed, E.; Haj-Ahmad, R.; Ruparelia, K.; Arshad, M.S.; Chang, M.; Ahmad, Z. Porous inorganic drug delivery systems—A review. AAPS PharmSciTech 2017, 18, 1507–1525. [Google Scholar] [CrossRef]
- He, F.; Li, D.; He, J.; Liu, Y.; Ahmad, F.; Liu, Y.; Deng, X.; Ye, Y.; Yin, D. A novel layer-structures scaffold with large pore sizes suitable for 3D cell culture prepared by near field electrospinning. Mater. Sci. Eng. C 2018, 86, 18–27. [Google Scholar] [CrossRef]
- Tylek, T.; Blum, C.; Hrynevich, A.; Schlegelmilch, K.; Schilling, T.; Dalton, P.D.; Groll, J. Precisely defined fiber scaffolds with 40um porosity induce elongation driven M2-like polarization of human macrophages. Biofabrication 2020, 12, 025007. [Google Scholar] [CrossRef]
- Li, S.; Deng, B.; Grinthal, A.; Schneider-Yamamura, A.; Kang, J.; Martens, R.S.; Zhang, C.T.; Li, J.; Yu, S.; Bertoldi, K.; et al. Liquid-induced topological transformations of cellular microstructures. Nature 2021, 592, 386–391. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Iwaki, R.; Reinhardt, J.W.; Chang, J.C.; Miyamoto, S.; Kelly, J.; Zbinden, J.; Blum, K.; Mirhaidari, G.; Ulziibayer, A.; et al. The effect of pore diameter on neo-tissue formation in electrospun biodegradable tissue-engineered arterial grafts in a large animal model. Acta Biomater. 2020, 115, 176–184. [Google Scholar] [CrossRef]
- Garg, T.; Goyal, A.K. Biomaterial-based scaffolds—Current status and future directions. Expert Opin. Drug Deliv. 2014, 11, 767–789. [Google Scholar] [CrossRef]
- Braunecker, J.; Baba, M.; Milroy, G.E.; Cameron, R.E. The effects of molecular weight and porosity on the degradation and drug release from polyglycolide. Int. J. Pharm. 2004, 282, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Recum, A.F.; Shannon, C.E.; Cannon, C.E.; Long, K.J.; Kooten, T.G.; Meyle, J. Surface roughness, porosity, and texture as modifiers of cellular adhesion. Tissue Eng. 1996, 2, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Ozpinar, E.W.; Frey, A.L.; Cruse, G.; Freytes, D.O. Mast cell-biomaterial interactions and tissue repair. Tissue Eng. Part B Rev. 2021, 27, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Doloff, J.C.; Veiseh, O.; Mezerville, R.; Sforza, M.; Perry, T.A.; Haupt, J.; Jamiel, M.; Chambers, C.; Nash, A.; Fotovat, A.S.; et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits, and humans. Nat. Biomed. Eng. 2021, 5, 1115–1130. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of critical challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Woodrow, K.A. Medical applications of porous biomaterials: Features of porosity and tissue-specific implications for biocompatibility. Adv. Health Mater. 2022, 11, e2102087. [Google Scholar] [CrossRef]
- Koch, L.; Drenckchan, W.; Stubenrauch, C. Porous polymers via emulsion templating: Pore deformation during solidification cannot be explained by an osmotic transport! Colloid Polym. Sci. 2021, 299, 233–242. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Li, Y.; Wang, X.; Chen, C.; Liu, G. Microfluidic technology for the production of well-ordered porous polymer scaffolds. Polymers 2020, 12, 1863. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications, and challenges. Compos. Part B 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Chou, S.F.; Cardon, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef]
- Stock, U.A.; Mayer, J.E., Jr. Tissue engineering of cardiac valves on the basis of PGA/PLA Copolymers. J. Long Term Eff. Med. Implants. 2001, 11, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Marwani, H.M.; Khan, S.B.; Webster, T.J. Greater cardiomyocyte density on aligned compared with random carbon nanofibers in polymer composites. Int. J. Nanomed. 2014, 9, 5533–5539. [Google Scholar]
- Hibino, N.; Imai, Y.; Shin-Oka, T.; Aoki, M.; Watanabe, M.; Kosaka, Y.; Matsumura, G.; Konuma, T.; Toyama, S.; Murata, A.; et al. First successful clinical application of tissue engineered blood vessel. Kyobu Geka 2002, 55, 368–373. [Google Scholar] [PubMed]
- Naito, Y.; Shinoka, T.; Duncan, D.; Hibino, N.; Solomon, D.; Cleary, M.; Rathore, A.; Fein, C.; Church, S.; Breuer, C. Vascular Tissue Engineering: Towards the next Generation Vascular Grafts. Adv. Drug Deliv. Rev. 2011, 63, 312–323. [Google Scholar] [CrossRef]
- Leal, B.B.J.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts. Front. Cardiovasc. Med. 2021, 7, 592361. [Google Scholar] [CrossRef]
- Ong, C.S.; Fukunishi, T.; Liu, R.H.; Nelson, K.; Zhang, H.; Wieczorek, E.; Palmieri, M.; Ueyama, Y.; Ferris, E.; Geist, G.E.; et al. Bilateral Arteriovenous Shunts as a Method for Evaluating Tissue-Engineered Vascular Grafts in Large Animal Models. Tissue Eng. Part C Methods 2017, 23, 728–735. [Google Scholar] [CrossRef]
- Hu, Y.T.; Pan, X.D.; Zheng, J.; Ma, W.G.; Sun, L.Z. In Vitro and in Vivo Evaluation of a Small-Caliber Coaxial Electrospun Vascular Graft Loaded with Heparin and VEGF. Int. J. Surg. 2017, 44, 244–249. [Google Scholar] [CrossRef]
- Mahara, A.; Somekawa, S.; Kobayashi, N.; Hirano, Y.; Kimura, Y.; Fujisato, T.; Yamaoka, T. Tissue-Engineered Acellular Small Diameter Long-Bypass Grafts With neointima-Inducing Activity. Biomaterials 2015, 58, 54–62. [Google Scholar] [CrossRef]
- Bouchet, M.; Gauthier, M.; Maire, M.; Ajji, A.; Lerouge, S. Towards Compliant Small-Diameter Vascular Grafts: Predictive Analytical Model and Experiments. Mater. Sci. Eng. C 2019, 100, 715–723. [Google Scholar] [CrossRef]
- Fang, Z.; Xiao, Y.; Geng, X.; Jia, L.; Xing, Y.; Ye, L.; Gu, Y.; Zhang, A.-Y.; Feng, Z.-G. Fabrication of Heparinized Small Diameter TPU/PCL Bi-Layered Artificial Blood Vessels and in Vivo Assessment in a Rabbit Carotid Artery Replacement Model. Mater. Sci. Eng. C 2022, 133, 112628. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Jakab, K. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng. Part A 2008, 14, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P.; Lacolley, P. Structural and Genetic Bases of Arterial Stiffness. Hypertension 2005, 45, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Daamen, W.F.; Veerkamp, J.H.; van Hest, J.C.M.; van Kuppevelt, T.H. Elastin as a Biomaterial for Tissue Engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef]
- Chan, A.H.P.; Filipe, E.C.; Tan, R.P.; Santos, M.; Yang, N.; Hung, J.; Feng, J.; Nazir, S.; Benn, A.J.; Ng, M.K.C.; et al. Altered Processing Enhances the Efficacy of Small-Diameter Silk Fibroin Vascular Grafts. Sci. Rep. 2019, 9, 17461. [Google Scholar] [CrossRef] [PubMed]
- Nitta, S.K.; Numata, K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D. L Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Aytemiz, D.; Sakiyama, W.; Suzuki, Y.; Nakaizumi, N.; Tanaka, R.; Ogawa, Y.; Takagi, Y.; Nakazawa, Y.; Asakura, T. Small-Diameter Silk Vascular Grafts (3 Mm Diameter) with a Double-Raschel Knitted Silk Tube Coated with Silk Fibroin Sponge. Adv. Healthc. Mater. 2013, 2, 361–368. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- VandeVord, P.J.; Matthew, H.W.T.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the Biocompatibility of a Chitosan Scaffold in Mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef]
- Zhang, H.; Neau, S.H. In vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Hu, W.; Wang, X.D.; Gu, X.S. The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo. J. Mater. Sci. Mater. Med. 2007, 18, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Fukunishi, T.; Best, C.A.; Sugiura, T.; Shoji, T.; Yi, T.; Udelsman, B.; Ohst, D.; Ong, C.S.; Zhang, H.; Shinoka, T.; et al. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/Chitosan scaffoldsin a sheep model. PLoS ONE 2016, 11, e015855. [Google Scholar]
- Wu, Y.; Qin, Y.; Wang, Z.; Wang, J.; Zhang, C.; Li, C.; Kong, D. The Regeneration of Macro-Porous Electrospun Poly(ε-Caprolactone) Vascular Graft during Long-Term in Situ Implantation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, C.B.; Bell, E. A Blood Vessel Model Constructed from Collagen and Cultured Vascular Cells. Science 1986, 231, 397–400. [Google Scholar] [CrossRef]
- L’Heureux, N.; Pâquet, S.; Labbé, R.; Germain, L.; Auger, F.A. A Completely Biological Tissue-engineered Human Blood Vessel. FASEB J. 1998, 12, 47–56. [Google Scholar]
- Zhang, W.J.; Liu, W.; Cui, L.; Cao, Y. Tissue Engineering of Blood Vessel. J. Cell. Mol. Med. 2007, 11, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Benrashid, E.; Mccoy, C.C.; Youngwirth, L.M.; Kim, J.; Manson, R.J.; Otto, J.C.; Lawson, J.H. Tissue Engineered Vascular Grafts: Origins, Development, and Current Strategies for Clinical Application. Methods 2016, 99, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ben-shaul, S.; Landau, S.; Merdler, U.; Levenberg, S. Mature Vessel Networks in Engineered Tissue Promote Graft–HostAnastomosis and Prevent Graft Thrombosis. Proc. Natl. Acad. Sci. USA 2019, 116, 2955–2960. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Tolba, E.; Dorweiler, B.; Schröder, H.; Link, T.; Diehl-Seifert, B.; Müller, W. Modular Small DiameterVascular Grafts with Bioactive Functionalities. PLoS ONE 2015, 10, e0133632. [Google Scholar]
- DeValence, S.; Tille, J.C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Moller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular graft in a rat aorta replacement model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Shinoka, T.; Imai, Y.; Ikeda, Y. Transplantation of a tissue engineered pulmonary artery. N. Engl. J. Med. 2001, 344, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, D.; Zou, H.; Huang, S.; Yang, Q.; Li, S.; Qing, F.L.; Ye, X.; You, Z.; Zhao, Q. Hybrid electrospun rapamycin-loaded small-diameter decellularized vascular grafts effectively inhibit intimal hyperplasia. Acta Biomater. 2019, 97, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Morton, H.F. Variability of arterial wall shear stress, its dependence on vessel diameter, and implications for Murray’s law. Atherosclerosis 2009, 203, 47–48. [Google Scholar]
- Innocente, F.; Mandracchia, D.; Pektok, E.; Nottelet, B.; Tille, J.C.; de Valence, S.; Faggin, G.; Mazzucco, A.; Kalangos, A.; Gurny, R.; et al. Paclitaxel eluting biodegradable synthetic vascular prostheses: A step to wards reduction of neointima formation? Circulation 2009, 120, S37–S45. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Z.; Song, L.; Zhao, Q.; Zhang, J.; Li, D.; Wang, S.; Han, J.; Zheng, X.L.; Yang, Z.; et al. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials 2012, 33, 2880–2891. [Google Scholar] [CrossRef]
- Khairy, P.; Poirier, N. Is the Extracardiac Conduit the Preferred Fontan Approach for Patients With Univentricular Hearts? Circulation 2012, 126, 2516–2525. [Google Scholar] [CrossRef]
- Bezuska, L.; Lebetkevicius, V.; Sudikiene, R.; Liekiene, D.; Tarutis, V. 30-year experience of Fontan surgery: Single-centre’s data. J. Cardiothorac. Surg. 2017, 12, 67. [Google Scholar] [CrossRef]
- Hibino, N.; McGillicuddy, E.; Matsumura, G.; Ichihara, Y.; Naito, Y.; Breuer, C.; Shinoka, T. Late-term results of tissue-engineeredvascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010, 139, 431–436.e2. [Google Scholar] [CrossRef]
- Sugiura, T.; Matsumura, G.; Miyamoto, S.; Miyachi, H.; Breuer, C.K.; Shinoka, T. Tissue-engineered Vascular Grafts in Children with Congenital Heart Disease: Intermediate Term Follow-up. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 175–179. [Google Scholar] [CrossRef]
- Szafron, J.M.; Khosravi, R.; Reinhardt, J.; Best, C.A.; Bersi, M.R.; Yi, T.; Breuer, C.K.; Humphrey, J.D. Immuno-driven and Mechano-mediated Neotissue Formation in Tissue Engineered Vascular Grafts. Ann. Biomed. Eng. 2018, 46, 1938–1950. [Google Scholar] [CrossRef]
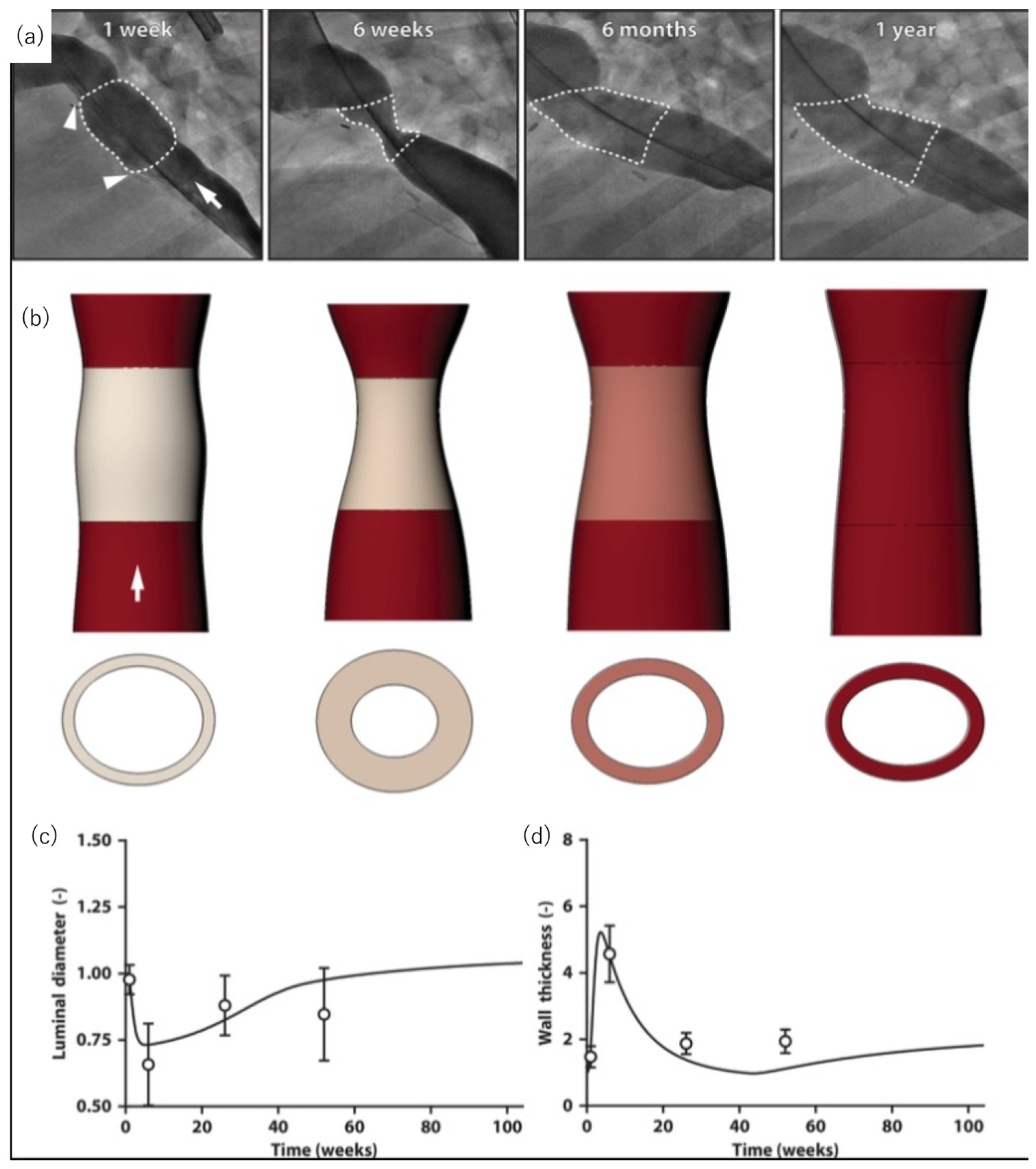
- Drews, J.D.; Pepper, V.K.; Best, C.A.; Szafron, J.M.; Cheatham, J.P.; Yates, A.R.; Hor, K.N.; Zbinden, J.C.; Chang, Y.-C.; Mirhaidari, G.J.M.; et al. Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci. Transl. Med. 2020, 12, eaax6919. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ (accessed on 28 January 2023).
- Dijkman, P.E.; Fioretta, E.S.; Frese, L.; Pasqualini, F.S.; Hoerstrup, S.P. Heart valve replacements with regenerative capacity. Transfus. Med. Hemother. 2016, 43, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Tefft, B.J.; Spoon, D.B.; Simari, R.D. Scaffolds for tissue engineering of cardiac valves. Acta Biomater. 2014, 10, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Kluin, J.; Talacua, H.; Smits, A.; Emmert, M.Y.; Brugmans, M.C.P.; Fioretta, E.S.; Dijkman, P.E.; Sontjens, S.H.M.; Duijvelshoff, R.; Dekker, S.; et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant—From material design to 12 months follow-up in sheep. Biomaterials 2017, 125, 101–117. [Google Scholar] [CrossRef]
- Soliman, O.I.; Miyazaki, Y.; Abdelghani, M.; Brugmans, M.; Witsenburg, M.; Onuma, Y.; Cox, M.; Serruys, P.W. Midterm performance of a novel restorative pulmonary valved conduit: Preclinical results. EuroIntervention 2017, 13, e1418–e1427. [Google Scholar] [CrossRef]
- Capulli, A.K.; Emmert, M.Y.; Pasqualini, F.S.; Kehl, D.; Caliskan, E.; Lind, J.U.; Sheehy, S.P.; Park, S.J.; Ahn, S.; Weber, B.; et al. JetValve: Rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials 2017, 133, 229–241. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Soliman, O.I.I.; Abdelghani, M.; Katsikis, A.; Naz, C.; Lopes, S.; Warnack, B.; Cox, M.; Onuma, Y.; Serruys, P.W. Acute performance of a novel restorative transcatheter aortic valve: Preclinical results. EuroIntervention 2017, 13, e1410–e1417. [Google Scholar] [CrossRef]
- Bennink, G.; Trii, S.; Brugmans, M.; Cox, M.; Svanidze, O.; Ladich, E.; Carrel, T.; Virmani, R. A novel restorative pulmonary valved conduit in a chronic sheep model: Mid-term hemodynamic function and histologic assessment. J. Thorac. Cardiovasc. Surg. 2018, 155, 2591–2601e3. [Google Scholar] [CrossRef]
- Coyan, G.N.; D’Amore, A.; Matsumura, Y.; Pedersen, D.D.; Luketich, S.K.; Shanov, V.; Katz, W.E.; David, T.E.; Wagner, W.R.; Badhwar, V. In vivo functional assessment of a novel degradable metal and elastomeric scaffold-based tissue engineered heart valve. J. Thorac. Cardiovasc. Surg. 2019, 157, 1809–1816. [Google Scholar] [CrossRef]
- Weber, B.; Scherman, J.; Emmert, M.Y.; Gruenenfelder, J.; Verbeek, R.; Bracher, M.; Black, M.; Kortsmit, J.; Franz, T.; Schoenauer, R.; et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: First experiences with a one-step intervention in primates. Eur. Heart J. 2011, 32, 2830–2840. [Google Scholar] [CrossRef] [PubMed]
- Emmert, M.Y.; Weber, B.; Behr, L.; Frauenfelder, T.; Brokopp, C.E.; Grunenfelder, J.; Falk, V.; Hoerstrup, S.P. Transapical aortic implantation of autologous marrow stromal cell-based tissue-engineered heart valves: First experiences in the systemic circulation. JACC Cardiovasc. Interv. 2011, 4, 822–823. [Google Scholar] [CrossRef] [PubMed]
- Emmert, M.Y.; Weber, B.; Wolint, P.; Behr, L.; Sammut, S.; Frauenfelder, T.; Frese, L.; Scherman, J.; Brokopp, C.E.; Templin, C.; et al. Stem cell-based transcatheter aortic valve implantation: First experiences in a preclinical model. JACC Cardiovasc. Interv. 2012, 5, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Emmert, M.Y.; Weber, B.; Behr, L.; Sammut, S.; Frauenfelder, T.; Wolint, P.; Scherman, J.; Bettex, D.; Gruenfelder, J.; Falk, V.; et al. Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: Technical considerations and implications for translational cell-based heart valve concepts. Eur. J. Cardiothorac. Surg. 2014, 45, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fioretta, E.S.; Lintas, V.; Mallone, A.; Motta, S.E.; von Boehmer, L.; Dijkman, P.E.; Cesarovic, N.; Caliskan, E.; Biefer, H.R.C.; Lipiski, M.; et al. Differential leaflet remodeling of bone marrow cell pre-seeded versus nonseeded bioresorbable transcatheter pulmonary valve replacements. JACC Basic Transl. Sci. 2019, 5, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Fioretta, E.; Putti, M.; Fallahi, A.; Mes, T.; Bosman, A.; Caliskan, E.; Cesarovic, N.; Rodriguez, H.; Dnakers, P.; Bouten, C.; et al. A multidisciplinary study to develop a transcatheter aortic valve implantation system for in situ heart valve tissue engineering. Struct. Heart 2020, 4, 57. [Google Scholar] [CrossRef]
- US National Library of Medicine. ClinicalTrials.gov. 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02700100 (accessed on 28 January 2023).
- Morales, D.L.; Herrington, C.; Bacha, E.A.; Morell, V.O.; Prodan, Z.; Mroczek, T.; Sivalingam, S.; Cox, M.; Bennink, G.; Asch, F.M. A novel restorative pulmonary valve conduit: Early outcomes of two clinical trials. Front. Cardiovasc. Med. 2021, 4, 583360. [Google Scholar] [CrossRef]
- US National Library of Medicine. ClinicalTrials.gov. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03022708 (accessed on 23 January 2023).
- Gaetani, R.; Zizzi, E.A.; Deriu, M.A.; Morbiducci, U.; Pesce, M.; Messina, E. When Stiffness Matters: Mechanosensing in Heart Development and Disease. Front. Cell Dev. Biol. 2020, 8, 334. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Darnell, M.; O’Neil, A.; Mao, A.; Gu, L.; Rubin, L.L.; Mooney, D.J. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8368–E8377. [Google Scholar] [CrossRef]
- Seo, B.R.; Chen, X.; Ling, L.; Song, Y.H.; Shimpi, A.A.; Choi, S.; Gonzalez, J.; Sapudom, J.; Wang, K.; Andresen Eguiluz, R.C.; et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 11387–11398. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.; Hnilova, M.; Yang, X.; Nemeth, C.L.; Tsui, J.H.; Smith, A.S.; Jiao, A.; Regnier, M.; Murry, C.E.; Tamerler, C.; et al. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl. Mater. Interfaces 2016, 8, 21923–21932. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Park, Y.; Kim, K. Engineering biomaterials to guide heart cells for matured cardiac tissue. Coatings 2020, 10, 925. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, W.; Radisic, M.; Vunjak-Novakovic, G. Can We Engineer a Human Cardiac Patch for Therapy? Circ. Res. 2018, 123, 244–265. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Sugiura, T.; Hibino, N.; Breuer, C.K.; Shinoka, T. Tissue-engineered cardiac patch seeded with human induced pluripotent stem cell derived cardiomyocytes promoted the regeneration of host cardiomyocytes in a rat model. J. Cardiothorac. Surg. 2016, 11, 163. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Miyamoto, S.; Miyachi, H.; Sugiura, T.; Reinhardt, J.W.; Yu-Chun, C.; Zbinden, J.; Breuer, C.K.; Shinoka, T. The evaluation of a tissue-engineered cardiac patch seeded with hips derived cardiac progenitor cells in a rat left ventricular model. PLoS ONE 2020, 15, e0234087. [Google Scholar] [CrossRef]
- Czosseck, A.; Chen, M.M.; Nguyen, H.; Meeson, A.; Hsu, C.C.; Chen, C.C.; George, T.A.; Ruan, S.C.; Cheng, Y.Y.; Lin, P.J.; et al. Porous scaffold for mesenchymal cell encapsulation and exosome-based therapy of ischemic diseases. J. Control. Release. 2022, 352, 879–892. [Google Scholar] [CrossRef]
- Gaballa, M.A.; Sunkomat, J.N.E.; Thai, H.; Morkin, E.; Ewy, G.; Goldman, S. Grafting an acellular 3-dimensional collagen scaffold onto a non-transmural infarcted myocardium induces neo-angiogenesis and reduces cardiac remodeling. J. Heart Lung Transplant. 2006, 25, 946–954. [Google Scholar] [CrossRef]
- Miyagi, Y.; Chiu, L.L.Y.; Cimini, M.; Weisel, R.D.; Radisic, M.; Li, R. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 2011, 32, 1280–1290. [Google Scholar] [CrossRef]
- Chi, N.; Yang, M.; Chung, T.; Chou, N.; Wang, S. Cardiac repair using chitosan-hyaluronan/silk fibroin patches in a rat heart model with myocardial infarction. Carbohydr. Polym. 2013, 92, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhu, P.; Liu, W.; Gao, Z.; Zhao, L.; Wang, C.; Li, S.; Zhu, M.; Zhang, Q.; Zhang, X.; et al. A conductive bioengineered cardiac patch for myocardial infarction treatment by improving tissue electrical integrity. Adv. Healthc. Mater. 2022, 13, e2201856. [Google Scholar] [CrossRef]
- Engelmayr, G.C., Jr.; Cheng, M.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2018, 4, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y. Rational Design of Smart Hydrogels for Biomedical Applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef] [PubMed]
- Pena, B.; Laughter, M.; Jett, S.; Rowland, T.J.; Taylor, M.R.G.; Mestroni, L.; Park, D. Injectable Hydrogels for Cardiac Tissue Engineering. Macromol. Biosci. 2018, 18, e1800079. [Google Scholar] [CrossRef]
- Smith, R.R.; Marban, E.; Marban, L. Enhancing retention and efficacy of cardiosphere-derived cells administered after myocardial infarction using a hyaluronan-gelatin hydrogel. Biomatter 2013, 3, e24490. [Google Scholar] [CrossRef]
- Wang, L.L.; Liu, Y.; Chung, J.J.; Wang, T.; Gaffey, A.C.; Lu, M.; Cavanaugh, C.A.; Zhou, S.; Kanade, R.; Atluri, P.; et al. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat. Biomed. Eng. 2018, 1, 983–992. [Google Scholar] [CrossRef]
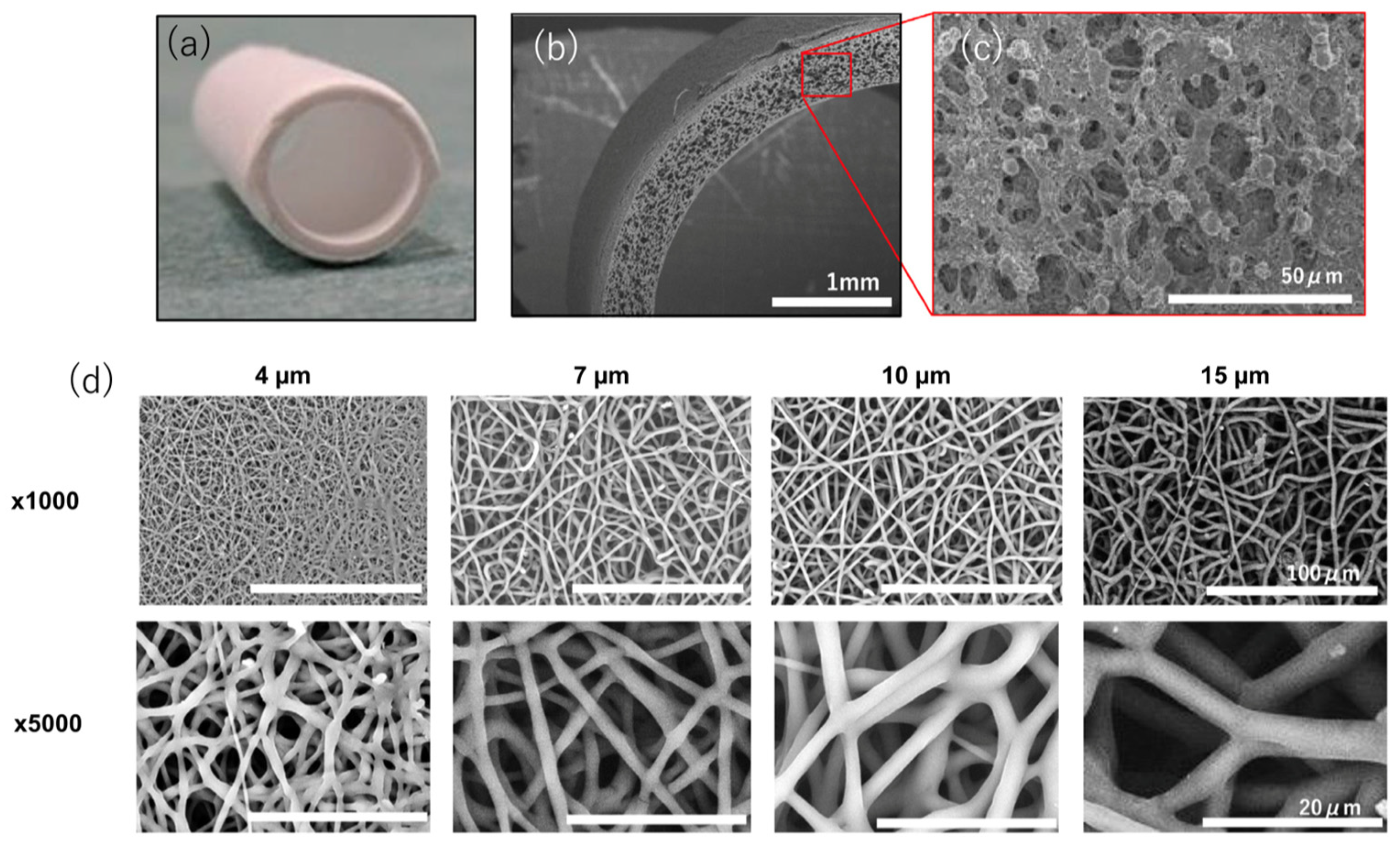
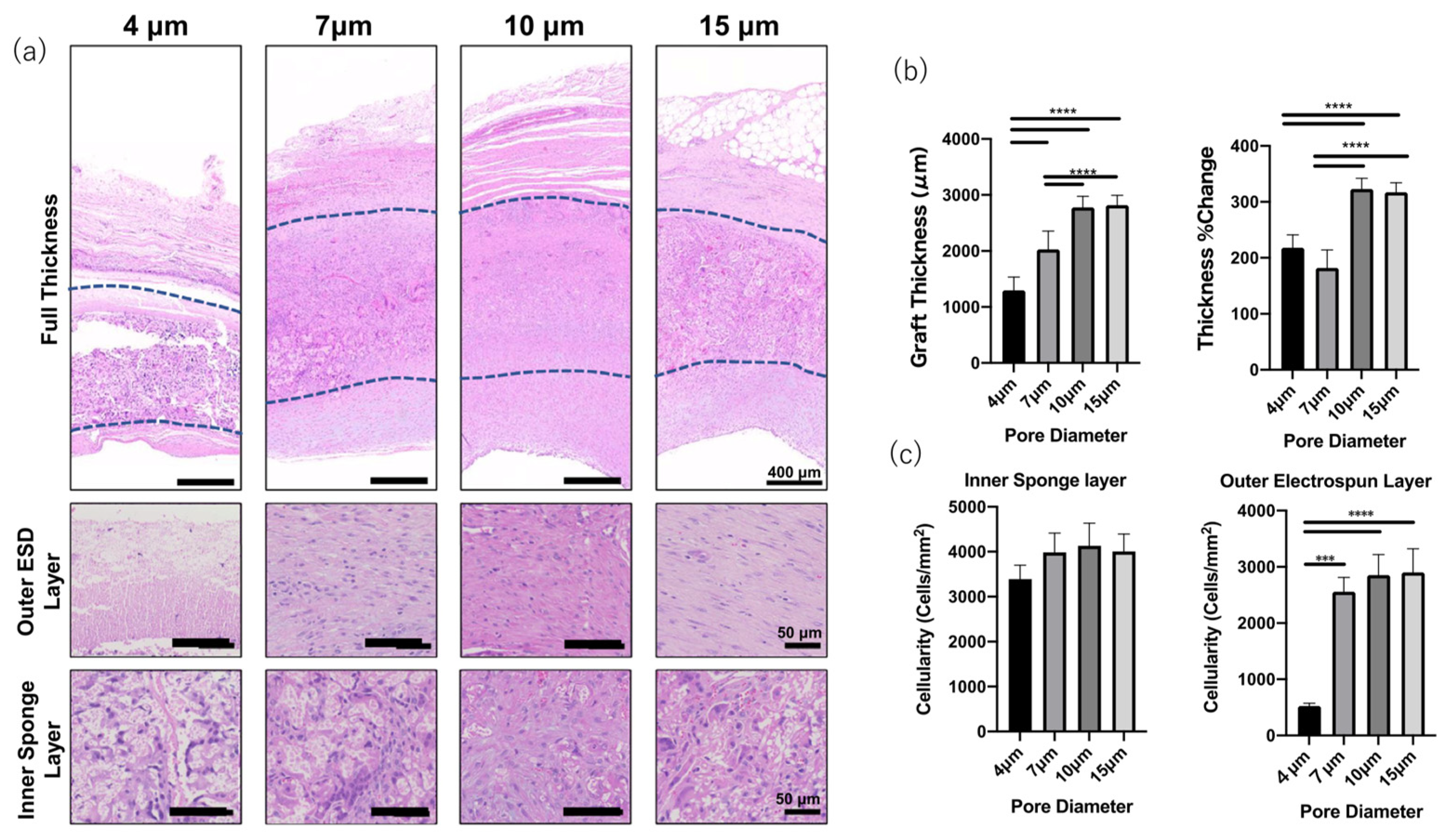
| Response | Ideal Pore Size (um or ↑/↓) | Ideal Porosity (Value or ↑/↓) |
|---|---|---|
| Macrophage polarization | M1: <20 or >60 (at surface), 34 (intrapore) M2: 30–50 (at surface), >360 | M1: ↓ M2: ↑ |
| Angiogenesis | >5, ~40 | ↑ |
| Hemocompatibility | <10 to limit platelet activation | ↓to limit platelet activation; <50 mL H2O min−1 cm2 at 120 mmHg to limit leakage with anti-coagulants |
| Calcification | ↑ | ↑; <5000 mL H2O min−1 cm2 at 120 mmHg to prevent inflammation |
| Reduction of fibrous capsule | 30–40 | ↑ |
| Material | Animal Species | Number | Surgery or Intervention | Findings | Reference |
|---|---|---|---|---|---|
| PCL | rat | 15 | infra-renal abdominal aorta interposition with the graft | Rapid endothelialization, good patency and mechanical properties, insufficient regeneration of the vascular wall on the long term. | [81] |
| PU/PCL | rabbit | 7 | Carotid artery replacement. | Good anti-thrombosis, host cell infiltration, neotissue formation in 5 months. | [60] |
| PLA/PCL and PGA or PLLA | human | 1 | pulmonary artery recontruction | No evidence of graft occlusion or aneurysmal changes in 7 months. | [82] |
| Slik fibroin | dog | 5 | Carotid srtery replacement. | One of the implanted graft showed the pstency more than a year. Development of elastic fiber and reendothelialization. | [68] |
| PCL with decellularized Rat aorta | rat | 6 | infra-renal abdominal aorta interposition with the graft | Reduced neointimal hyperplasia. Progressed reendothelialization at 12 weeks. | [83] |
| Study Phase | Target Disease, or Situations | Scaffold | Original Estimated Enrollment | Outcome Measurement | Follow Up | Status |
|---|---|---|---|---|---|---|
| 1 | Single ventricle cardiac anatomy | synthetic polymer | 4 | Primary: Graft failure requiring intervention Second: Graft growth | 3 years | completed |
| 2 | Vascular conduits for extracardiac total cavopulmonary connections | synthetic polymer | 24 | Primary: Safety and tolerability Secondary: Efficacy of TEVG determined by MRI | 2 years | recruiting |
| 1 | Chronic venous insufficiency | ECM | 15 | Primary: Thrombosis, infection, surgical complications Secondary: symptoms of target disease, QOL, Graft durability, Flow abnormality, wall degeneration | 1 year | recruiting |
| N/A | peripheral arterial disease | Collagen | 20 | Primary: Graft safety and adverse events Secondary: immunoreaction, graft patency, effect to symptoms a d anke-brachial index | 2 years | Active, not recruiting |
| 1 | Hemodialysis access | Collagen | 20 | Primary: graft patency, intervention and adverse evemts Secondary: immunoreaction, patency and interventions | 6 months | completed |
| N/A | Hemodialysis access | Collagen | 40 | Primary: Safety, tolerability and patency rate Secondary: | 57 weeks | Active, not recruiting |
| N/A | Hemodialysis access | synthetic polymer | 110 | Primary: patency rate, freedom from device-related adverse events Secondary: implantation success rate, patency, interventions, infection | 6 months | Recruiting |
| N/A | Hemodialysis access | synthetic polymer | 20 | Primary: device-related adverse events, patency Secondary: patency, adverse events | 5 years | Active, not recruiting |
| N/A | Coronary artery bypass graft | synthetic polymer | 15 | Primary: Procedural success, device-related serious adverse events Secondary: intimal hyperplasia, patency, Major adverse events, mortality | 5 years | Enrolling by invitation |
| Material | Animal Species | Number | Surgery or Intervention | Findings | Reference |
|---|---|---|---|---|---|
| Upy-polyester-urethanes | Sheep | 33 | Transcatheter AVR | Good hemodynamics with acceptable degree of valve regurgitation | [99] |
| Upy-polyester-urethanes | Sheep | 20 | Surgical PVR | Durable hemodynamics, no stenosis or severe regurgitation | [97] |
| Upy-polyester-urethanes | Sheep | 18 | Surgical PVR | Neointima formation was observed, inflamation was peaked at 6 month while degradation peaked at 12 month. | [101] |
| Bisurea polycarbonate | Sheep | 10 | Surgical PVR | Remodeling with collagen and elastin synthesis, incomplete scaffold resorption in 12 months | [96] |
| Polycarbonate urethane urea and AZ31 magnesium alloy stent | Pig | 5 | Surgical PVR | Normal leaflet function in acute phase, no thrombosis or regurgitation | [100] |
| P4HB-gelatin | Sheep | 4 | Transcatheter PVR | Good hemodynamics, competence after implantation | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, T.; Sassi, S.; Ulziibayar, A.; Hama, R.; Kitsuka, T.; Shinoka, T. The Application of Porous Scaffolds for Cardiovascular Tissues. Bioengineering 2023, 10, 236. https://doi.org/10.3390/bioengineering10020236
Watanabe T, Sassi S, Ulziibayar A, Hama R, Kitsuka T, Shinoka T. The Application of Porous Scaffolds for Cardiovascular Tissues. Bioengineering. 2023; 10(2):236. https://doi.org/10.3390/bioengineering10020236
Chicago/Turabian StyleWatanabe, Tatsuya, Salha Sassi, Anudari Ulziibayar, Rikako Hama, Takahiro Kitsuka, and Toshiharu Shinoka. 2023. "The Application of Porous Scaffolds for Cardiovascular Tissues" Bioengineering 10, no. 2: 236. https://doi.org/10.3390/bioengineering10020236
APA StyleWatanabe, T., Sassi, S., Ulziibayar, A., Hama, R., Kitsuka, T., & Shinoka, T. (2023). The Application of Porous Scaffolds for Cardiovascular Tissues. Bioengineering, 10(2), 236. https://doi.org/10.3390/bioengineering10020236






