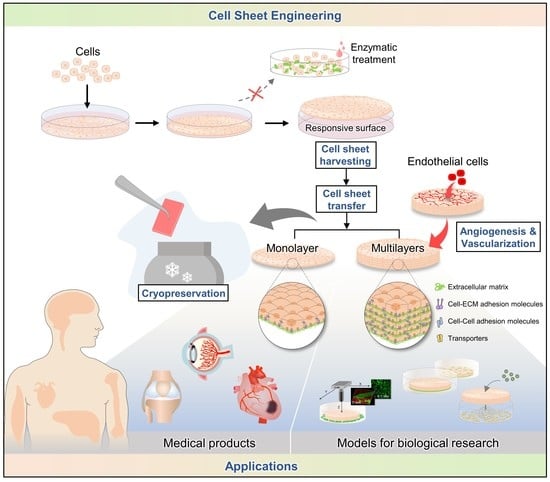
Recent Advances in Cell Sheet Engineering: From Fabrication to Clinical Translation
Abstract
1. Introduction
2. Techniques for Harvesting Cell Sheets
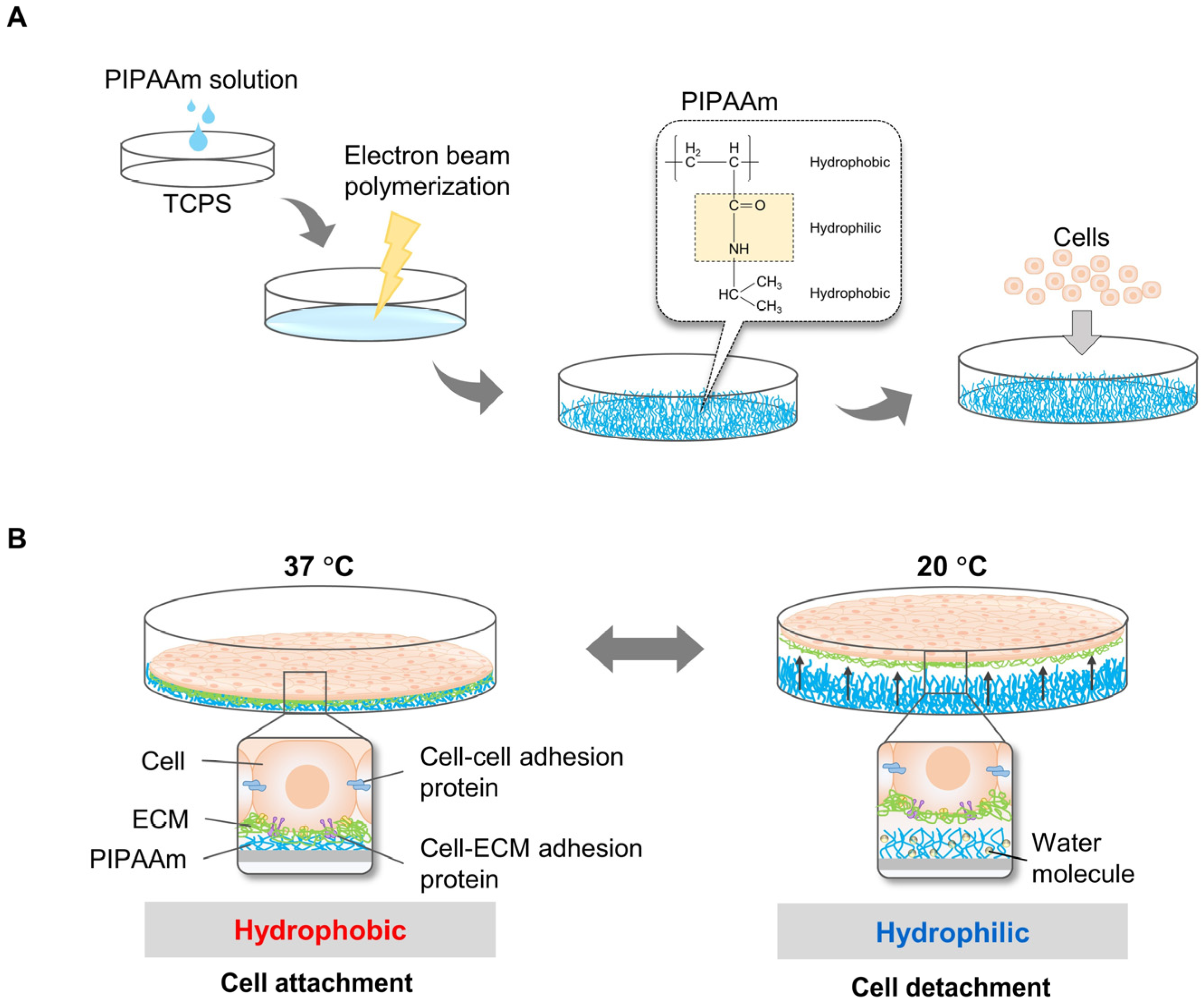
2.1. Temperature-Responsive Systems Using PIPAAm
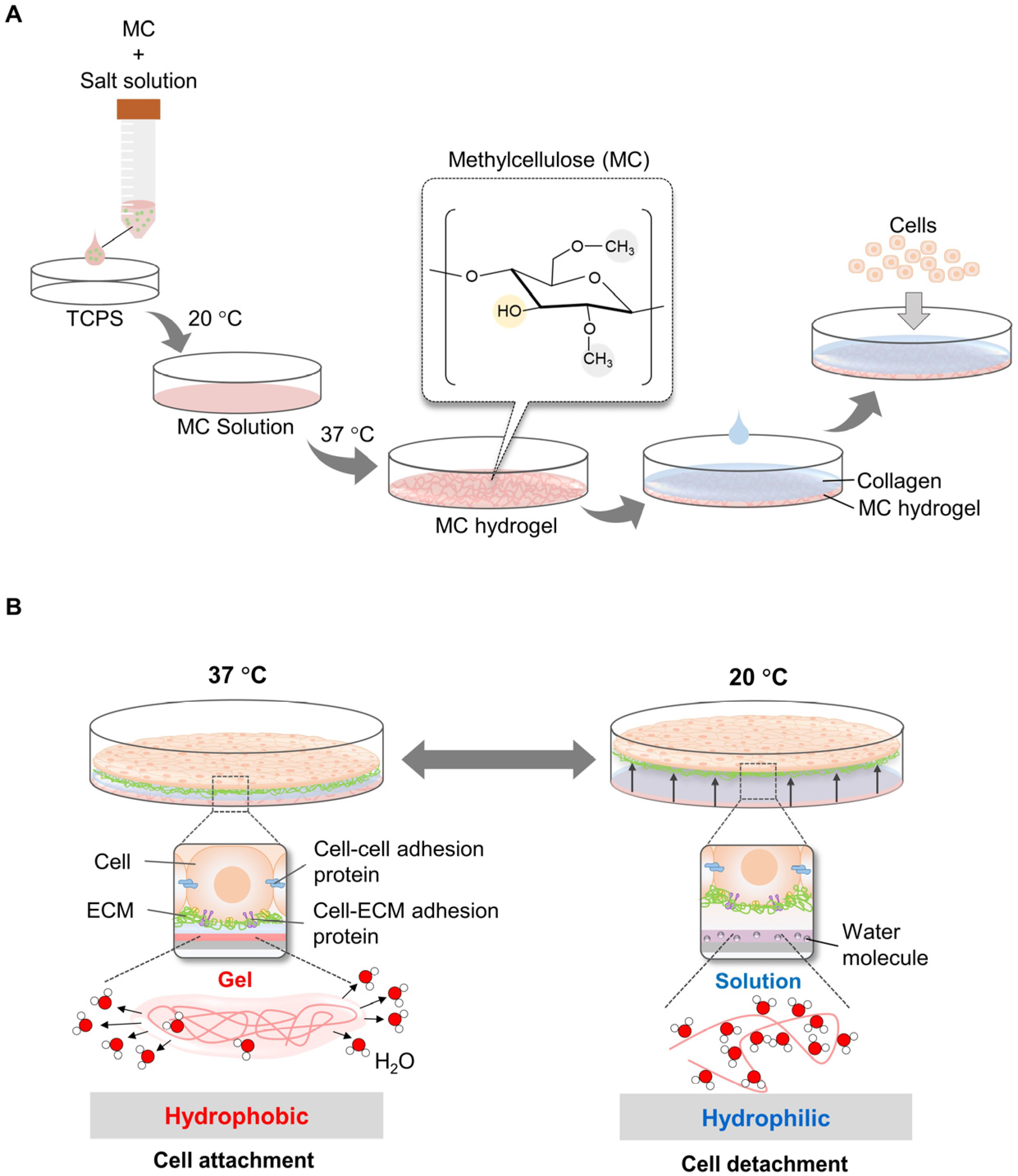
2.2. Temperature-Responsive System Using Methylcellulose (MC)
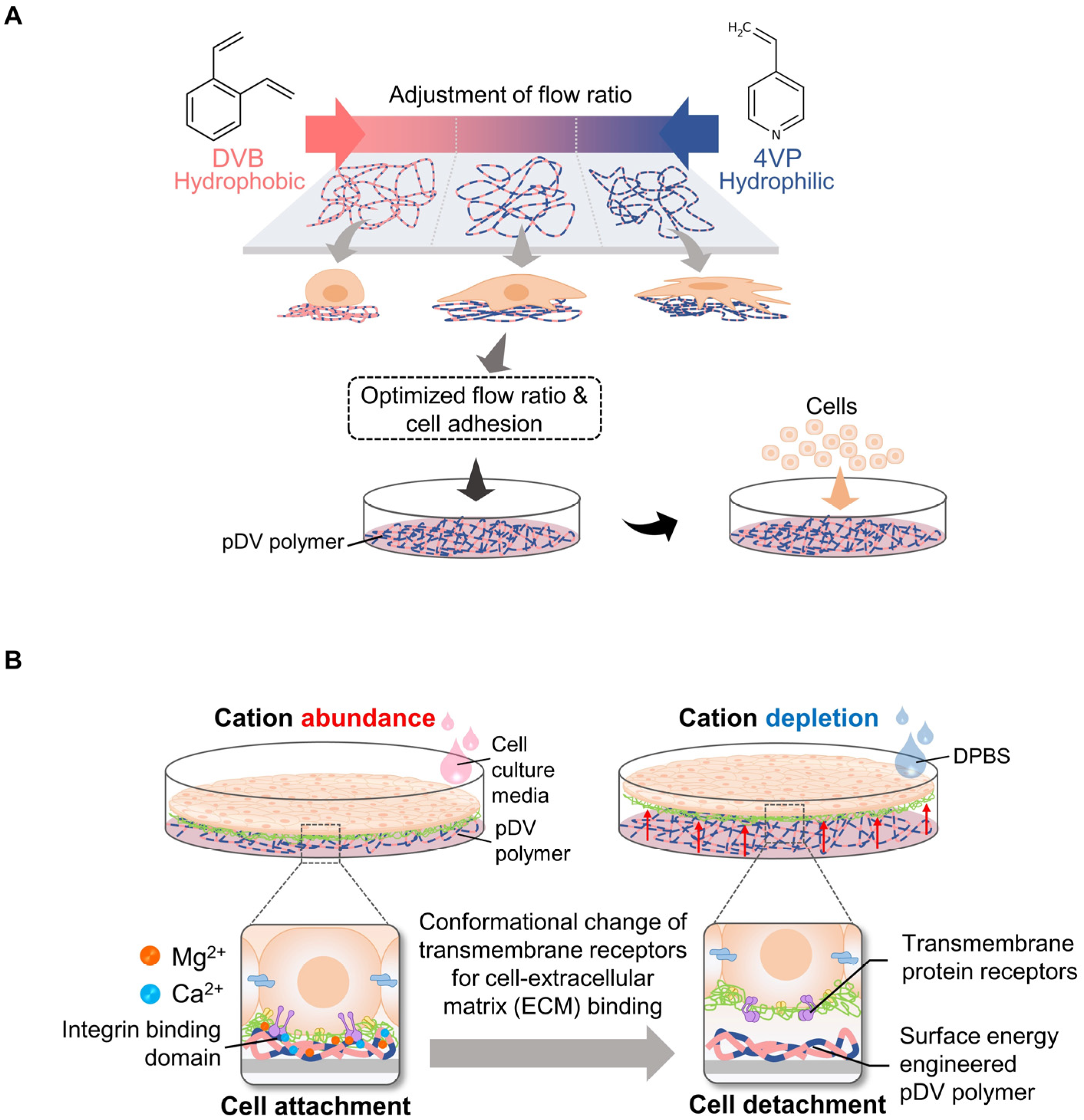
2.3. Non-Temperature Responsive Systems Using Ion-Induced Cell Detachment
2.4. Non-Temperature-Responsive Systems Using Electro-Responsive Surfaces
2.5. Other Systems
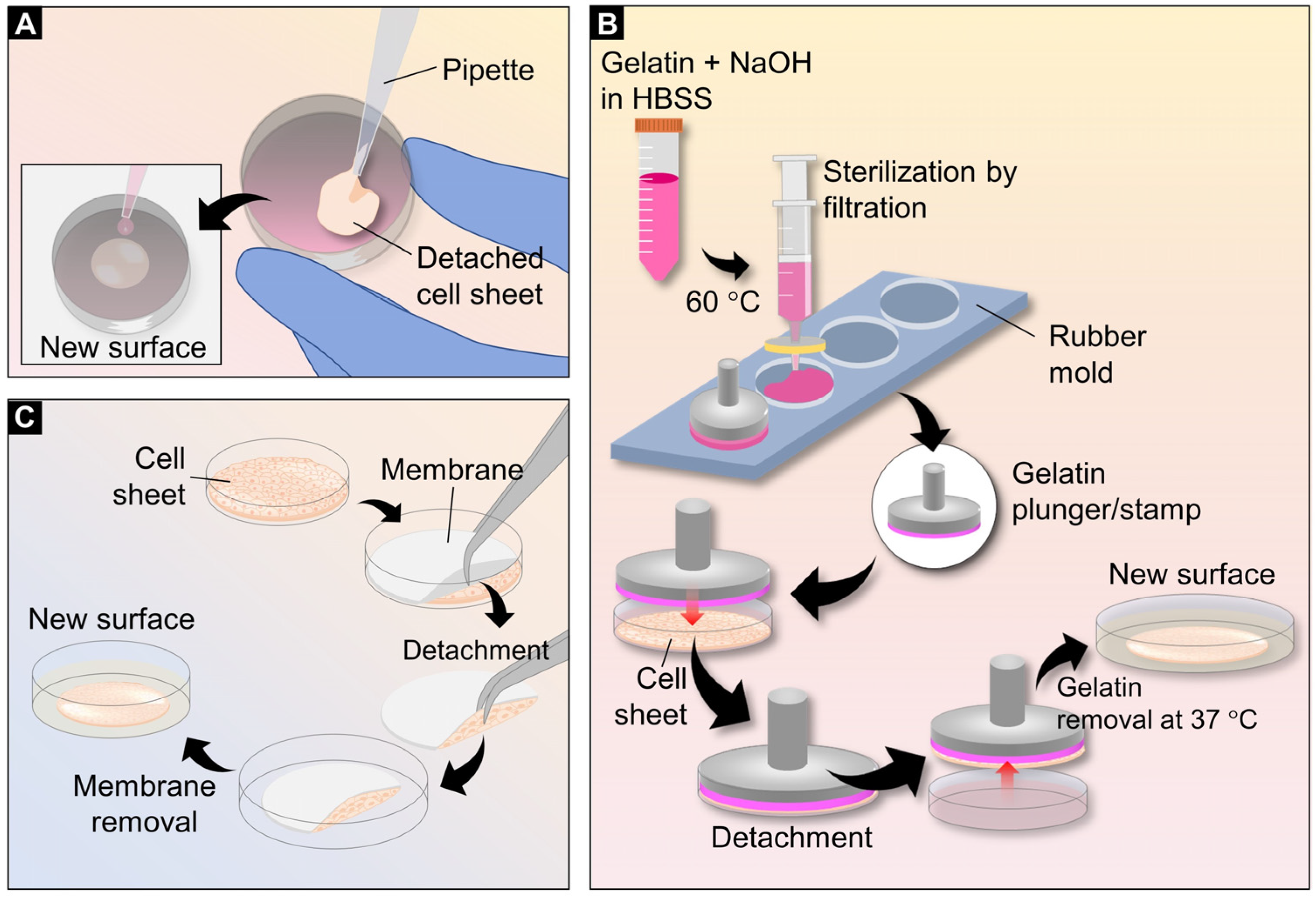
3. Cell Sheet Transferring Techniques
3.1. Simple Pipetting Method
3.2. Gelatin Plungers or Stamps
3.3. Membrane-Assisted Transfer
4. Cryopreservation
| Types of the Cell Sheet | Vitrification Solution Component | Storage Period | Year, Refs |
|---|---|---|---|
| Chondrocyte cell sheet | 20% dimethyl sulfoxide, 20% ethylene glycol (EG), 0.5 M sucrose, and 10% carboxylated poly-L-lysine | 3–6 months | 2013, [84] 2020, [83] |
| Mesenchymal stem-cell sheet | 6.5 M EG, 0.5 M sucrose, and 10% w/w carboxylated poly-l-lysine (COOH-PLL) in PBS | - | 2016, [85] |
| Skeletal-muscle myoblast cell sheet | 6.5 M EG, 0.7 M sucrose, and 10% carboxyl poly-L-lysine | 3 months | 2018, [86] |
| Oral-mucosa epithelial cell sheets | 2.5%, 5%, and 10% of EG | 204 days | 2019, [87] |
| Vitrification solution 1 (1.7% w/v EG, 1.3% w/v formamide, 2.2% w/v DMSO, 0.7% w/v PVP K12, and 0.1% w/v of SuperCool X-1000 and SuperCool Z-1000, Vitrification solution 2 (4.7% w/v EG, 3.6% w/v formamide, 6.2% w/v DMSO, 1.9% w/v PVP K12, and 0.3% w/v of SuperCool X-1000 and SuperCool Z-1000) and Vitrification solution 3 (16.84% w/v EG, 12.86% w/v formamide, 22.3% w/v DMSO, 7% w/v PVP K12, and 1% w/v of SuperCool X-1000 and SuperCool Z-1000) | 204 days | 2019, [87] |
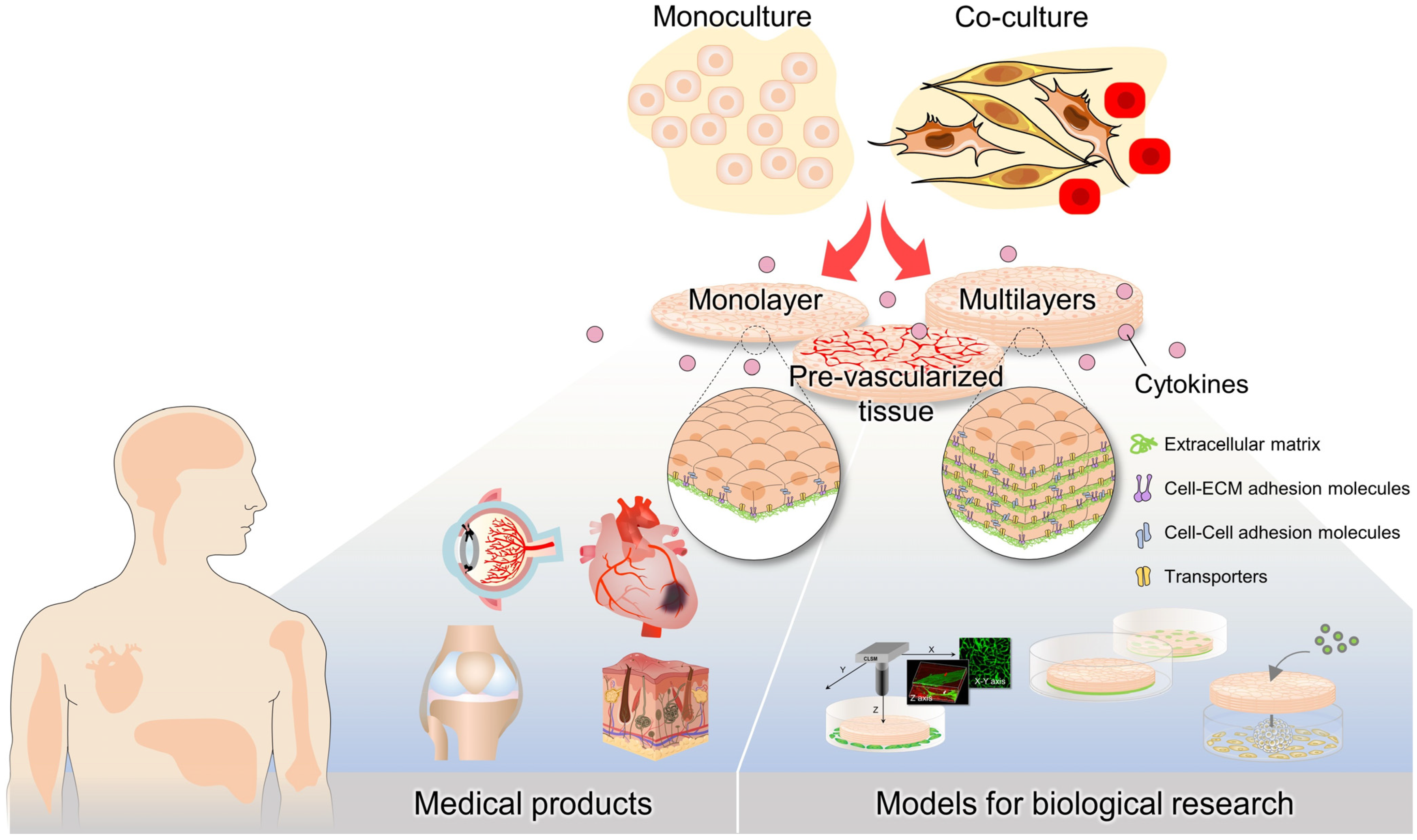
5. Cytokines and Vascularization in Cell Sheets
6. Applications
6.1. Clinical Translation
| Cell Types | Fabrication Methods | Clinical Treatment | Stage of Study | Year, Refs. |
|---|---|---|---|---|
| Skeletal-muscle cells | PIPAAm surface | Ischemic myocardium | Preclinical in vitro | 2006, [104] |
| 2013, [105,106] | ||||
| 2020, [38] | ||||
| Preclinical in vivo | 2005, [107] 2014, [108] | |||
| Clinical study | 2012, [73] | |||
| 2015, [4] | ||||
| 2017, [70] | ||||
| 2021, [109] | ||||
| Myoblasts | PIPAAm surface | Pancreatic fistula | Preclinical in vivo | 2017, [110] |
| Fibroblasts | PIPAAm surface | Ischemic myocardium | Preclinical in vivo | 2008, [111] |
| Wound ulcer | ||||
| Mesenchymal stem cells | PIPAAm surface | Ischemic myocardium | Preclinical in vivo | 2012, [100] |
| 2014, [112] | ||||
| 2016, [113] | ||||
| Ion-induced surface | Ischemic limb | Preclinical in vivo | 2020, [24] | |
| MC surface | Bone regeneration | Preclinical in vitro | 2022, [114] | |
| Thermo-responsive EMDs | Ocular trauma | Preclinical in vitro | 2022, [115] | |
| PIPAAm surface | Diabetic ulcers | Preclinical in vivo | 2015, [116] | |
| Embryonic stem cells | PIPAAm surface | Ischemic myocardium | Preclinical in vivo | 2012, [117] |
| iPS cells | PIPAAm surface | Ischemic myocardium | Preclinical in vitro | 2012, [118] |
| Preclinical in vivo | 2012, [119] | |||
| 2014, [120] | ||||
| 2018, [121] | ||||
| PIPAAm surface | Liver failure | Preclinical in vivo | 2016, [122] | |
| PIPAAm surface | Stroke and brain damage | Preclinical in vivo | 2017, [123] | |
| Osteogenic cells | PIPAAm surface | Bone regeneration | Preclinical in vitro | 2017, [42] |
| MC surface | Bone regeneration | Preclinical in vitro | 2017, [42] | |
| Corneal epithelium | PIPAAm surface | Ocular trauma | Preclinical in vitro | 2004, [124] |
| Oral mucosal epithelial cells | PIPAAm surface | Esophageal ulcer | Preclinical in vitro | 2010, [125] |
| Esophageal ulcer | Preclinical in vivo | 2021, [126] | ||
| Esophageal neoplasm | Clinical study | 2012, [127] | ||
| Ocular trauma | Clinical study | 2004, [6] | ||
| Keratinocytes | PIPAAm surface | Skin defect | Preclinical in vivo | 2017, [128] |
| 2019, [129] | ||||
| Keratinocytes fibroblasts | PIPAAm surface | Burn wound | Preclinical in vitro | 2020, [130] |
| Periodontal-ligament (PDL)-derived cells | PIPAAm surface | Periodontitis | Preclinical in vitro and in vivo Clinical study | 2010, [131] 2018, [132] |
6.2. Models for Biological Research
7. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hofmann, M.; Wollert, K.C.; Meyer, G.P.; Menke, A.; Arseniev, L.; Hertenstein, B.; Ganser, A.; Knapp, W.H.; Drexler, H. Monitoring of Bone Marrow Cell Homing Into the Infarcted Human Myocardium. Circulation 2005, 111, 2198–2202. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Sawa, Y.; Yoshikawa, Y.; Toda, K.; Fukushima, S.; Yamazaki, K.; Ono, M.; Sakata, Y.; Hagiwara, N.; Kinugawa, K.; Miyagawa, S. Safety and Efficacy of Autologous Skeletal Myoblast Sheets (TCD-51073) for the Treatment of Severe Chronic Heart Failure Due to Ischemic Heart Disease. Circ. J. 2015, 79, 991–999. [Google Scholar] [CrossRef]
- Guo, R.; Morimatsu, M.; Feng, T.; Lan, F.; Chang, D.; Wan, F.; Ling, Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res. Ther. 2020, 11, 19. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Sato, M.; Yamato, M.; Hamahashi, K.; Okano, T.; Mochida, J. Articular Cartilage Regeneration Using Cell Sheet Technology. Anat. Rec. 2014, 297, 36–43. [Google Scholar] [CrossRef]
- Takagi, R.; Yamato, M.; Kanai, N.; Murakami, D.; Kondo, M.; Ishii, T.; Ohki, T.; Namiki, H.; Yamamoto, M.; Okano, T. Cell sheet technology for regeneration of esophageal mucosa. World J. Gastroenterol. 2012, 18, 5145–5150. [Google Scholar] [CrossRef]
- Ito, M.; Shichinohe, H.; Houkin, K.; Kuroda, S. Application of cell sheet technology to bone marrow stromal cell transplantation for rat brain infarct. J. Tissue Eng. Regen. Med. 2017, 11, 375–381. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol. Chemie Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999, 45, 355–362. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Yamato, M.; Okano, T. Scaffold-free tissue engineering using cell sheet technology. RSC Adv. 2012, 2, 2184–2190. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M.; et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850–858. [Google Scholar] [CrossRef]
- Sekine, H.; Shimizu, T.; Dobashi, I.; Matsuura, K.; Hagiwara, N.; Takahashi, M.; Kobayashi, E.; Yamato, M.; Okano, T. Cardiac Cell Sheet Transplantation Improves Damaged Heart Function via Superior Cell Survival in Comparison with Dissociated Cell Injection. Tissue Eng. Part A 2011, 17, 2973–2980. [Google Scholar] [CrossRef]
- Kwon, O.H.; Kikuchi, A.; Yamato, M.; Sakurai, Y.; Okano, T. Rapid cell sheet detachment from Poly(N-isopropylacrylamide)-grafted porous cell culture membranes. J. Biomed. Mater. Res. 2000, 50, 82–89. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okuhara, M.; Karikusa, F.; Sakurai, Y.; Okano, T. Two-dimensional manipulation of confluently cultured vascular endothelial cells using temperature-responsive poly(N-isopropylacrylamide)-grafted surfaces. J. Biomater. Sci. Polym. Ed. 1998, 9, 1331–1348. [Google Scholar] [CrossRef]
- Hyeong Kwon, O.; Kikuchi, A.; Yamato, M.; Okano, T. Accelerated cell sheet recovery by co-grafting of PEG with PIPAAm onto porous cell culture membranes. Biomaterials 2003, 24, 1223–1232. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Yamato, M.; Okano, T. Comb-type grafted poly(N-isopropylacrylamide) gel modified surfaces for rapid detachment of cell sheet. Biomaterials 2010, 31, 7435–7443. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, W.I.; Yamato, M.; Okano, T.; Kikuchi, A.; Kwon, O.H. Successive grafting of PHEMA and PIPAAm onto cell culture surface enables rapid cell sheet recovery. Tissue Eng. Regen. Med. 2013, 10, 139–145. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Accelerated cell-sheet recovery from a surface successively grafted with polyacrylamide and poly(N-isopropylacrylamide). Acta Biomater. 2014, 10, 3398–3408. [Google Scholar] [CrossRef]
- Ebara, M.; Yamato, M.; Hirose, M.; Aoyagi, T.; Kikuchi, A.; Sakai, K.; Okano, T. Copolymerization of 2-Carboxyisopropylacrylamide with N-Isopropylacrylamide Accelerates Cell Detachment from Grafted Surfaces by Reducing Temperature. Biomacromolecules 2003, 4, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Tsai, C.-C.; Chen, W.; Mi, F.-L.; Liang, H.-F.; Chen, S.-C.; Sung, H.-W. Novel Living Cell Sheet Harvest System Composed of Thermoreversible Methylcellulose Hydrogels. Biomacromolecules 2006, 7, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Thirumala, S.; Gimble, J.M.; Devireddy, R.V. Methylcellulose Based Thermally Reversible Hydrogel System for Tissue Engineering Applications. Cells 2013, 2, 460–475. [Google Scholar] [CrossRef]
- Baek, J.; Cho, Y.; Park, H.-J.; Choi, G.; Lee, J.S.; Lee, M.; Yu, S.J.; Cho, S.; Lee, E.; Im, S.G. A Surface-Tailoring Method for Rapid Non-Thermosensitive Cell-Sheet Engineering via Functional Polymer Coatings. Adv. Mater. 2020, 32, e1907225. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Cordonier, C.E.J.; Noda, Y.; Nagase, F.; Enomoto, J.; Kageyama, T.; Honma, H.; Maruo, S.; Fukuda, J. Tailored cell sheet engineering using microstereolithography and electrochemical cell transfer. Sci. Rep. 2019, 9, 10415. [Google Scholar] [CrossRef] [PubMed]
- Kenji, K.; Fujishige, S.; Ando, I. Solution properties of poly(N-isopropylacrylamide) in water. Polym. J. 1990, 22, 15–20. [Google Scholar]
- Ono, Y.; Shikata, T. Hydration and Dynamic Behavior of Poly(N-isopropylacrylamide)s in Aqueous Solution: A Sharp Phase Transition at the Lower Critical Solution Temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin Poly(N-isopropylacrylamide) Grafted Layer on Polystyrene Surfaces for Cell Adhesion/Detachment Control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Shimizu, T.; Sekine, H.; Ohashi, K.; Kanzaki, M.; Ohki, T.; Nishida, K.; Okano, T. Reconstruction of functional tissues with cell sheet engineering. Biomaterials 2007, 28, 5033–5043. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Lutz, J.-F. Thermo-Switchable Materials Prepared Using the OEGMA-Platform. Adv. Mater. 2011, 23, 2237–2243. [Google Scholar] [CrossRef]
- Akiyama, Y. Design of Temperature-Responsive Cell Culture Surfaces for Cell Sheet Engineering. Cyborg Bionic Syst. 2021, 2021, 5738457. [Google Scholar] [CrossRef]
- Yamato, M.; Konno, C.; Utsumi, M.; Kikuchi, A.; Okano, T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials 2002, 23, 561–567. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kikuchi, A.; Yamato, M.; Sakurai, Y.; Umezu, M.; Okano, T. Control of cell adhesion and detachment using temperature and thermoresponsive copolymer grafted culture surfaces. J. Biomed. Mater. Res. Part A 2004, 69A, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, T.; Mori, Y.; Yoshizato, K. Cell Culture on a Thermo-Responsive Polymer Surface. Biotechnology 1990, 8, 854–856. [Google Scholar] [CrossRef]
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of (N-isopropyl acrylamide) and Poly(N-isopropyl acrylamide)-coated surfaces. Biointerphases 2013, 8, 19. [Google Scholar] [CrossRef]
- Thummarati, P.; Kino-Oka, M. Effect of Co-culturing Fibroblasts in Human Skeletal Muscle Cell Sheet on Angiogenic Cytokine Balance and Angiogenesis. Front. Bioeng. Biotechnol. 2020, 8, 578140. [Google Scholar] [CrossRef]
- Harimoto, M.; Yamato, M.; Hirose, M.; Takahashi, C.; Isoi, Y.; Kikuchi, A.; Okano, T. Novel approach for achieving double-layered cell sheets co-culture: Overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J. Biomed. Mater. Res. 2002, 62, 464–470. [Google Scholar] [CrossRef]
- Hussain, S.; Keary, C.; Craig, D.Q.M. A thermorheological investigation into the gelation and phase separation of hydroxypropyl methylcellulose aqueous systems. Polymer 2002, 43, 5623–5628. [Google Scholar] [CrossRef]
- Zheng, P.; Li, L.; Hu, X.; Zhao, X. Sol-gel transition of methylcellulose in phosphate buffer saline solutions. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1849–1860. [Google Scholar] [CrossRef]
- Forghani, A.; Kriegh, L.; Hogan, K.; Chen, C.; Brewer, G.; Tighe, T.B.; Devireddy, R.; Hayes, D. Fabrication and characterization of cell sheets using methylcellulose and PNIPAAm thermoresponsive polymers: A comparison Study. J. Biomed. Mater. Res. Part A 2017, 105, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.V.; Wesley, R.A.; Luginbuhl, R.; Denton, D.D.; Ratner, B.D. Plasma Polymerized N-Isopropylacrylamide: Synthesis and Characterization of a Smart Thermally Responsive Coating. Biomacromolecules 2001, 2, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.-S.; Hodneland, C.D.; Mrksich, M. Electroactive Monolayer Substrates that Selectively Release Adherent Cells. Chembiochem 2001, 2, 590–593. [Google Scholar] [CrossRef]
- Hong, Y.; Yu, M.; Weng, W.; Cheng, K.; Wang, H.; Lin, J. Light-induced cell detachment for cell sheet technology. Biomaterials 2013, 34, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Guillaume-Gentil, O.; Semenov, O.V.; Zisch, A.H.; Zimmermann, R.; Voros, J.; Ehrbar, M. pH-controlled recovery of placenta-derived mesenchymal stem cell sheets. Biomaterials 2011, 32, 4376–4384. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chung, Y.-C.; Wang, I.-J.; Young, T.-H. Control of cell attachment on pH-responsive chitosan surface by precise adjustment of medium pH. Biomaterials 2012, 33, 1336–1342. [Google Scholar] [CrossRef]
- Nash, M.E.; Healy, D.; Carroll, W.M.; Elvira, C.; Rochev, Y.A. Cell and cell sheet recovery from pNIPAm coatings; motivation and history to present day approaches. J. Mater. Chem. 2012, 22, 19376–19389. [Google Scholar] [CrossRef]
- Sonna, L.A.; Fujita, J.; Gaffin, S.L.; Lilly, C.M. Invited Review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol. 2002, 92, 1725–1742. [Google Scholar] [CrossRef]
- Lim, J.Y.; Shaughnessy, M.C.; Zhou, Z.; Noh, H.; Vogler, E.A.; Donahue, H.J. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials 2008, 29, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.; Song, W.; Alves, N.M.; Mano, J.F. Chemical modification of bioinspired superhydrophobic polystyrene surfaces to control cell attachment/proliferation. Soft Matter 2011, 7, 8932–8941. [Google Scholar] [CrossRef]
- Decuzzi, P.; Ferrari, M. Modulating cellular adhesion through nanotopography. Biomaterials 2010, 31, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.; Shin, J.; Kim, J.; You, J.B.; Yoo, Y.; Kwak, M.J.; Oh, M.S.; Im, S.G. One-Step Synthesis of Cross-Linked Ionic Polymer Thin Films in Vapor Phase and Its Application to an Oil/Water Separation Membrane. J. Am. Chem. Soc. 2017, 139, 2329–2337. [Google Scholar] [CrossRef]
- Lange, T.S.; Bielinsky, A.K.; Kirchberg, K.; Bank, I.; Herrmann, K.; Krieg, T.; Scharffetter-Kochanek, K. Mg2+ and Ca2+ Differentially Regulate β1 Integrin-Mediated Adhesion of Dermal Fibroblasts and Keratinocytes to Various Extracellular Matrix Proteins. Exp. Cell Res. 1994, 214, 381–388. [Google Scholar] [CrossRef]
- Inaba, R.; Khademhosseini, A.; Suzuki, H.; Fukuda, J. Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials 2009, 30, 3573–3579. [Google Scholar] [CrossRef]
- Enomoto, J.; Mochizuki, N.; Ebisawa, K.; Osaki, T.; Kageyama, T.; Myasnikova, D.; Nittami, T.; Fukuda, J. Engineering thick cell sheets by electrochemical desorption of oligopeptides on membrane substrates. Regen. Ther. 2016, 3, 24–31. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Kagawa, Y.; Hasegawa, A.; Kubo, H.; Shimizu, T. Rapid fabrication of detachable three-dimensional tissues by layering of cell sheets with heating centrifuge. Biotechnol. Prog. 2018, 34, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Shimizu, T.; Sekiya, S.; Haraguchi, Y.; Yamato, M.; Sawa, Y.; Okano, T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 2010, 31, 1646–1654. [Google Scholar] [CrossRef]
- Kino-Oka, M.; Ngo, T.X.; Nagamori, E.; Takezawa, Y.; Miyake, Y.; Sawa, Y.; Saito, A.; Shimizu, T.; Okano, T.; Taya, M. Evaluation of vertical cell fluidity in a multilayered sheet of skeletal myoblasts. J. Biosci. Bioeng. 2012, 113, 128–131. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, H.-B.; Shim, I.K.; Kanai, N.; Okano, T.; Kwon, S.K. Treatment of chemically induced oral ulcer using adipose-derived mesenchymal stem cell sheet. J. Oral Pathol. Med. 2017, 46, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Zurina, I.M.; Presniakova, V.S.; Butnaru, D.V.; Timashev, P.S.; Rochev, Y.A.; Liang, X.-J. Towards clinical translation of the cell sheet engineering: Technological aspects. Smart Mater. Med. 2023, 4, 146–159. [Google Scholar] [CrossRef]
- Venugopal, B.; Shenoy, S.J.; Mohan, S.; Kumar, P.R.A.; Kumary, T.V. Bioengineered corneal epithelial cell sheet from mesenchymal stem cells—A functional alternative to limbal stem cells for ocular surface reconstruction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, Y.; Murakami, D.; Yamato, M.; Hama, T.; Yamamoto, K.; Kojima, H.; Moriyama, H.; Okano, T. Middle ear mucosal regeneration with three-dimensionally tissue-engineered autologous middle ear cell sheets in rabbit model. J. Tissue Eng. Regen. Med. 2016, 10, E188–E194. [Google Scholar] [CrossRef]
- Na Lee, Y.; Yi, H.-J.; Kim, Y.H.; Lee, S.; Oh, J.; Okano, T.; Shim, I.K.; Kim, S.C. Evaluation of Multi-Layered Pancreatic Islets and Adipose-Derived Stem Cell Sheets Transplanted on Various Sites for Diabetes Treatment. Cells 2020, 9, 1999. [Google Scholar] [CrossRef]
- Imamura, T.; Ogawa, T.; Minagawa, T.; Yokoyama, H.; Nakazawa, M.; Nishizawa, O.; Ishizuka, O. Engineered Bone Marrow-Derived Cell Sheets Restore Structure and Function of Radiation-Injured Rat Urinary Bladders. Tissue Eng. Part A 2015, 21, 1600–1610. [Google Scholar] [CrossRef]
- Baimakhanov, Z.; Yamanouchi, K.; Sakai, Y.; Koike, M.; Soyama, A.; Hidaka, M.; Takatsuki, M.; Fujita, F.; Kanetaka, K.; Kuroki, T.; et al. Efficacy of Multilayered Hepatocyte Sheet Transplantation for Radiation-Induced Liver Damage and Partial Hepatectomy in a Rat Model. Cell Transplant. 2016, 25, 549–558. [Google Scholar] [CrossRef]
- Kawecki, M.; Kraut, M.; Klama-Baryła, A.; Łabuś, W.; Kitala, D.; Nowak, M.; Glik, J.; Sieroń, A.L.; Utrata-Wesołek, A.; Trzebicka, B.; et al. Transfer of fibroblast sheets cultured on thermoresponsive dishes with membranes. J. Mater. Sci. Mater. Med. 2016, 27, 111. [Google Scholar] [CrossRef]
- Pouzet, B.; Hagege, A.; Vilquin, J.-T.; Desnos, M.; Duboc, D.; Marolleau, J.P.; Menashé, P. Transplantation of autologous skeletal myoblasts in ischemic cardiac insufficiency. J. Soc. Biol. 2001, 195, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Domae, K.; Yoshikawa, Y.; Fukushima, S.; Nakamura, T.; Saito, A.; Sakata, Y.; Hamada, S.; Toda, K.; Pak, K.; et al. Phase I Clinical Trial of Autologous Stem Cell–Sheet Transplantation Therapy for Treating Cardiomyopathy. J. Am. Heart Assoc. 2017, 6, e003918. [Google Scholar] [CrossRef]
- Menasché, P.; Hagège, A.A.; Vilquin, J.-T.; Desnos, M.; Abergel, E.; Pouzet, B.; Bel, A.; Sarateanu, S.; Scorsin, M.; Schwartz, K.; et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J. Am. Coll. Cardiol. 2003, 41, 1078–1083. [Google Scholar] [CrossRef]
- Dib, N.; Michler, R.E.; Pagani, F.D.; Wright, S.; Kereiakes, D.J.; Lengerich, R.; Binkley, P.; Buchele, D.; Anand, I.; Swingen, C.; et al. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: Four-year follow-up. Circulation 2005, 112, 1748–1755. [Google Scholar] [CrossRef]
- Sawa, Y.; Miyagawa, S.; Sakaguchi, T.; Fujita, T.; Matsuyama, A.; Saito, A.; Shimizu, T.; Okano, T. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: Report of a case. Surg. Today 2012, 42, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Murata, D.; Takao, S.; Verissiomo, A.R.; Nakayama, K. Correction: Cryopreservation method for spheroids and fabrication of scaffold-free tubular constructs. PLoS ONE 2020, 15, e0243249. [Google Scholar] [CrossRef]
- Dong, H.; Li, X.; Chen, K.; Li, N.; Kagami, H. Cryopreserved Spontaneous Spheroids from Compact Bone-Derived Mesenchymal Stromal Cells for Bone Tissue Engineering. Tissue Eng. Part C Methods 2021, 27, 253–263. [Google Scholar] [CrossRef]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplant. 2021, 30. [Google Scholar] [CrossRef]
- Udoh, Y.; Yanaga, H.; Tai, Y.; Kiyokawa, K.; Inoue, Y. Long-term viability of cryopreserved cultured epithelial grafts. Burns 2000, 26, 535–542. [Google Scholar] [CrossRef]
- Hayashi, Y.; Horiguchi, I.; Kino-Oka, M.; Sugiyama, H. Model-based assessment of temperature profiles in slow freezing for human induced pluripotent stem cells. Comput. Chem. Eng. 2020, 144, 107150. [Google Scholar] [CrossRef]
- Yoon, Y.; Jung, T.; Shahid, M.A.; Khan, I.U.; Kim, W.H.; Kweon, O.-K. Frozen-thawed gelatin-induced osteogenic cell sheets of canine adipose-derived mesenchymal stromal cells improved fracture healing in canine model. J. Vet. Sci. 2019, 20, e63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shao, J.; Zhuang, J.; Zhou, S.; Yin, S.; Wu, F.; Hou, J.; Wang, X. Biobanked human foreskin epithelial cell sheets reduce inflammation and promote wound healing in a nude mouse model. BMC Biotechnol. 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hou, J.; Gu, Y.; Shao, J.; Zhou, S.; Zhuang, J.; Song, L.; Wang, X. Cryopreserved skin epithelial cell sheet combined with acellular amniotic membrane as an off-the-shelf scaffold for urethral regeneration. Mater. Sci. Eng. C 2021, 122, 111926. [Google Scholar] [CrossRef] [PubMed]
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature 1985, 313, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Maehara, M.; Uchikura, A.; Matsunari, H.; Matsumura, K.; Hyon, S.-H.; Sato, M.; Nagashima, H. Development of an efficient vitrification method for chondrocyte sheets for clinical application. Regen. Ther. 2020, 14, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Maehara, M.; Sato, M.; Watanabe, M.; Matsunari, H.; Kokubo, M.; Kanai, T.; Sato, M.; Matsumura, K.; Hyon, S.-H.; Yokoyama, M.; et al. Development of a novel vitrification method for chondrocyte sheets. BMC Biotechnol. 2013, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Kawamoto, K.; Takeuchi, M.; Yoshimura, S.; Tanaka, D.; Hyon, S.-H. Cryopreservation of a Two-Dimensional Monolayer Using a Slow Vitrification Method with Polyampholyte to Inhibit Ice Crystal Formation. ACS Biomater. Sci. Eng. 2016, 2, 1023–1029. [Google Scholar] [CrossRef]
- Ohkawara, H.; Miyagawa, S.; Fukushima, S.; Yajima, S.; Saito, A.; Nagashima, H.; Sawa, Y. Development of a vitrification method for preserving human myoblast cell sheets for myocardial regeneration therapy. BMC Biotechnol. 2018, 18, 56. [Google Scholar] [CrossRef]
- Oliva, J.; Florentino, A.; Bardag-Gorce, F.; Niihara, Y. Vitrification and storage of oral mucosa epithelial cell sheets. J. Tissue Eng. Regen. Med. 2019, 13, 1153–1163. [Google Scholar] [CrossRef]
- Vailhé, B.; Vittet, D.; Feige, J.-J. In Vitro Models of Vasculogenesis and Angiogenesis. Lab. Investig. 2001, 81, 439–452. [Google Scholar] [CrossRef]
- Felmeden, D.; Blann, A.; Lip, G. Angiogenesis: Basic pathophysiology and implications for disease. Eur. Heart J. 2003, 24, 586–603. [Google Scholar] [CrossRef]
- Papetti, M.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Physiol. 2002, 282, C947–C970. [Google Scholar] [CrossRef] [PubMed]
- Iruela-Arispe, M.L.; Dvorak, H.F. Angiogenesis: A dynamic balance of stimulators and inhibitors. Thromb. Haemost. 1997, 78, 672–677. [Google Scholar] [CrossRef]
- Bouïs, D.; Kusumanto, Y.; Meijer, C.; Mulder, N.H.; Hospers, G. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol. Res. 2006, 53, 89–103. [Google Scholar] [CrossRef]
- Al Kawas, H.; Saaid, I.; Jank, P.; Westhoff, C.C.; Denkert, C.; Pross, T.; Weiler, K.B.S.; Karsten, M.M. How VEGF-A and its splice variants affect breast cancer development-clinical implications. Cell. Oncol. 2022, 45, 227–239. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef]
- Santos, M.I.; Reis, R.L. Vascularization in Bone Tissue Engineering: Physiology, Current Strategies, Major Hurdles and Future Challenges. Macromol. Biosci. 2010, 10, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, S.; Rouwkema, J.; Macdonald, M.; Garfein, E.S.; Kohane, D.S.; Darland, D.C.; Marini, R.; van Blitterswijk, C.A.; Mulligan, R.C.; D’Amore, P.A.; et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005, 23, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Rücker, M.; Laschke, M.W.; Junker, D.; Carvalho, C.; Schramm, A.; Mülhaupt, R.; Gellrich, N.-C.; Menger, M.D. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials 2006, 27, 5027–5038. [Google Scholar] [CrossRef]
- Anderson, C.R.; Ponce, A.M.; Price, R.J. Immunohistochemical Identification of an Extracellular Matrix Scaffold that Microguides Capillary Sprouting In Vivo. J. Histochem. Cytochem. 2004, 52, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Mahar, M.A.A.; Lakkisto, P.; Tikkanen, I.; Vento, A.; Pätilä, T.; Harjula, A. VEGF overexpression improves mesenchymal stem cell sheet transplantation therapy for acute myocardial infarction. J. Tissue Eng. Regen. Med. 2013, 7, 742–750. [Google Scholar] [CrossRef]
- Lee, J.; Jun, I.; Park, H.-J.; Kang, T.J.; Shin, H.; Cho, S.-W. Genetically Engineered Myoblast Sheet for Therapeutic Angiogenesis. Biomacromolecules 2014, 15, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, S.; Shariatzadeh, S.; Zafari, A.; Majd, A.; Niknejad, H. Recent Advances on Cell-Based Co-Culture Strategies for Prevascularization in Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 745314. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; Alkatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Hata, H.; Matsumiya, G.; Miyagawa, S.; Kondoh, H.; Kawaguchi, N.; Matsuura, N.; Shimizu, T.; Okano, T.; Matsuda, H.; Sawa, Y. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J. Thorac. Cardiovasc. Surg. 2006, 132, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Nagamori, E.; Ngo, T.X.; Takezawa, Y.; Saito, A.; Sawa, Y.; Shimizu, T.; Okano, T.; Taya, M.; Kino-Oka, M. Network formation through active migration of human vascular endothelial cells in a multilayered skeletal myoblast sheet. Biomaterials 2013, 34, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.X.; Nagamori, E.; Kikuchi, T.; Shimizu, T.; Okano, T.; Taya, M.; Kino-Oka, M. Endothelial cell behavior inside myoblast sheets with different thickness. Biotechnol. Lett. 2013, 35, 1001–1008. [Google Scholar] [CrossRef]
- Memon, I.A.; Sawa, Y.; Fukushima, N.; Matsumiya, G.; Miyagawa, S.; Taketani, S.; Sakakida, S.K.; Kondoh, H.; Aleshin, A.; Shimizu, T.; et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J. Thorac. Cardiovasc. Surg. 2005, 130, 1333–1341. [Google Scholar] [CrossRef]
- Terajima, Y.; Shimizu, T.; Tsuruyama, S.; Sekine, H.; Ishii, H.; Yamazaki, K.; Hagiwara, N.; Okano, T. Autologous Skeletal Myoblast Sheet Therapy for Porcine Myocardial Infarction without Increasing Risk of Arrhythmia. Cell Med. 2014, 6, 99–109. [Google Scholar] [CrossRef]
- Araki, K.; Taira, M.; Miyagawa, S.; Kanaya, T.; Okuda, N.; Toda, K.; Kuratani, T.; Ueno, T.; Sawa, Y. Autologous skeletal myoblast sheet implantation for pediatric dilated cardiomyopathy: A case report. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 859–861. [Google Scholar] [CrossRef]
- Tanaka, S.; Kanetaka, K.; Fujii, M.; Ito, S.; Sakai, Y.; Kobayashi, S.; Yamanouchi, K.; Fujita, F.; Kuroki, T.; Eguchi, S. Cell sheet technology for the regeneration of gastrointestinal tissue using a novel gastric perforation rat model. Surg. Today 2017, 47, 114–121. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shimizu, T.; Yamato, M.; Tono, K.; Masuda, H.; Asahara, T.; Kasanuki, H.; Okano, T. Fibroblast sheets co-cultured with endothelial progenitor cells improve cardiac function of infarcted hearts. J. Artif. Organs 2008, 11, 141–147. [Google Scholar] [CrossRef]
- Tano, N.; Narita, T.; Kaneko, M.; Ikebe, C.; Coppen, S.R.; Campbell, N.; Shiraishi, M.; Shintani, Y.; Suzuki, K. Epicardial Placement of Mesenchymal Stromal Cell-sheets for the Treatment of Ischemic Cardiomyopathy; In Vivo Proof-of-concept Study. Mol. Ther. 2014, 22, 1864–1871. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shirasawa, B.; Takeuchi, Y.; Kawamura, D.; Nakamura, T.; Samura, M.; Nishimoto, A.; Ueno, K.; Morikage, N.; Hosoyama, T.; et al. Autologous preconditioned mesenchymal stem cell sheets improve left ventricular function in a rabbit old myocardial infarction model. Am. J. Transl. Res. 2016, 8, 2222–2233. [Google Scholar]
- Berntsen, L.; Forghani, A.; Hayes, D.J. Mesenchymal Stem Cell Sheets for Engineering of the Tendon–Bone Interface. Tissue Eng. Part A 2021, 28, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Zarebkohan, A.; Dianat-Moghadam, H.; Panahi, M.; Andre, H.; Alizadeh, E. Corneal endothelial cell sheet bioengineering from neural crest cell-derived adipose stem cells on novel thermo-responsive elastin-mimetic dendrimers decorated with RGD. Chem. Eng. J. 2022, 429, 132523. [Google Scholar] [CrossRef]
- Kato, Y.; Iwata, T.; Morikawa, S.; Yamato, M.; Okano, T.; Uchigata, Y. Allogeneic Transplantation of an Adipose-Derived Stem Cell Sheet Combined With Artificial Skin Accelerates Wound Healing in a Rat Wound Model of Type 2 Diabetes and Obesity. Diabetes 2015, 64, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Bel, A.; Planat-Bernard, V.; Saito, A.; Bonnevie, L.; Bellamy, V.; Sabbah, L.; Bellabas, L.; Brinon, B.; Vanneaux, V.; Pradeau, P.; et al. Composite Cell Sheets: A further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation 2010, 122 (Suppl. S1), S118–S123. [Google Scholar] [CrossRef]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, Safety, and Therapeutic Efficacy of Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Sheets in a Porcine Ischemic Cardiomyopathy Model. Circulation 2012, 126 (Suppl. S1), S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Uenaka, H.; Saito, A.; Miyagawa, S.; Sakaguchi, T.; Higuchi, T.; Shimizu, T.; Okano, T.; Yamanaka, S.; Sawa, Y. Bioengineered Myocardium Derived from Induced Pluripotent Stem Cells Improves Cardiac Function and Attenuates Cardiac Remodeling Following Chronic Myocardial Infarction in Rats. Stem Cells Transl. Med. 2012, 1, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Ikuno, T.; Takeda, M.; Fukushima, H.; Marui, A.; Katayama, S.; Shimizu, T.; Ikeda, T.; Okano, T.; Sakata, R.; et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci. Rep. 2014, 4, 6716. [Google Scholar] [CrossRef]
- Ishigami, M.; Masumoto, H.; Ikuno, T.; Aoki, T.; Kawatou, M.; Minakata, K.; Ikeda, T.; Sakata, R.; Yamashita, J.K.; Minatoya, K. Human iPS cell-derived cardiac tissue sheets for functional restoration of infarcted porcine hearts. PLoS ONE 2018, 13, e0201650. [Google Scholar] [CrossRef] [PubMed]
- Nagamoto, Y.; Takayama, K.; Ohashi, K.; Okamoto, R.; Sakurai, F.; Tachibana, M.; Kawabata, K.; Mizuguchi, H. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J. Hepatol. 2016, 64, 1068–1075. [Google Scholar] [CrossRef]
- Suzuki, N.; Arimitsu, N.; Shimizu, J.; Takai, K.; Hirotsu, C.; Ueda, Y.; Wakisaka, S.; Fujiwara, N.; Suzuki, T. Neuronal Cell Sheets of Cortical Motor Neuron Phenotype Derived from Human iPSCs. Cell Transplant. 2017, 26, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Maeda, N.; Watanabe, H.; Yamamoto, K.; Nagai, S.; Kikuchi, A.; Tano, Y.; et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 2004, 77, 379–385. [Google Scholar] [CrossRef]
- Takagi, R.; Murakami, D.; Kondo, M.; Ohki, T.; Sasaki, R.; Mizutani, M.; Yamato, M.; Nishida, K.; Namiki, H.; Yamamoto, M.; et al. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest. Endosc. 2010, 72, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Na, H.K.; Lee, J.H.; Shim, I.K.; Jung, H.-Y.; Kim, D.H.; Kim, C.J. Allogeneic epithelial cell sheet transplantation for preventing esophageal stricture after circumferential ESD in a porcine model: Preliminary results. Scand. J. Gastroenterol. 2021, 56, 598–603. [Google Scholar] [CrossRef]
- Ohki, T.; Yamato, M.; Ota, M.; Takagi, R.; Murakami, D.; Kondo, M.; Sasaki, R.; Namiki, H.; Okano, T.; Yamamoto, M. Prevention of Esophageal Stricture After Endoscopic Submucosal Dissection Using Tissue-Engineered Cell Sheets. Gastroenterology 2012, 143, 582–588.e2. [Google Scholar] [CrossRef]
- Osada, A.; Sekine, H.; Soejima, K.; Sakurai, H.; Shimizu, T. Harvesting epithelial keratinocyte sheets from temperature-responsive dishes preserves basement membrane proteins and improves cell survival in a skin defect model. J. Tissue Eng. Regen. Med. 2017, 11, 2516–2524. [Google Scholar] [CrossRef]
- Matsumine, H.; Giatsidis, G.; Osada, A.; Kamei, W.; Fujimaki, H.; Tsukamoto, Y.; Hashimoto, K.; Fujii, K.; Sakurai, H. Keratinocyte sheets prepared with temperature-responsive dishes show enhanced survival after in vivo grafting on acellular dermal matrices in a rat model of staged bi-layered skin reconstruction. Regen. Ther. 2019, 11, 167–175. [Google Scholar] [CrossRef]
- Benchaprathanphorn, K.; Sakulaue, P.; Siriwatwechakul, W.; Muangman, P.; Chinaroonchai, K.; Viravaidya-Pasuwat, K. Preparation and characterization of human keratinocyte–fibroblast cell sheets constructed using PNIAM-co-AM grafted surfaces for burn wound healing. J. Mater. Sci. Mater. Med. 2020, 31, 126. [Google Scholar] [CrossRef] [PubMed]
- Washio, K.; Iwata, T.; Mizutani, M.; Ando, T.; Yamato, M.; Okano, T.; Ishikawa, I. Assessment of cell sheets derived from human periodontal ligament cells: A pre-clinical study. Cell Tissue Res. 2010, 341, 397–404. [Google Scholar] [CrossRef]
- Iwata, T.; Yamato, M.; Washio, K.; Yoshida, T.; Tsumanuma, Y.; Yamada, A.; Onizuka, S.; Izumi, Y.; Ando, T.; Okano, T.; et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets—A safety and efficacy study in ten patients. Regen. Ther. 2018, 9, 38–44. [Google Scholar] [CrossRef]
- Durrani, S.; Konoplyannikov, M.; Ashraf, M.; Haider, K.H. Skeletal myoblasts for cardiac repair. Regen. Med. 2010, 5, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Du, G.Q.; Du, W.J.; Liu, J.J.; Wang, Y.S.; Nie, H.G.; Zhang, M.M.; Yu, B. Wnt1-overexpressing skeletal myoblasts as an improved cell therapy for cardiac repair following myocardial infarction. Panminerva Med. 2015, 57, 153–166. [Google Scholar]
- Dib, N.; McCarthy, P.; Campbell, A.; Yeager, M.; Pagani, F.; Wright, S.; MacLellan, W.R.; Fonarow, G.; Eisen, H.J.; Michler, R.E.; et al. Feasibility and Safety of Autologous Myoblast Transplantation in Patients with Ischemic Cardiomyopathy. Cell Transplant. 2005, 14, 11–19. [Google Scholar] [CrossRef]
- Dib, N.; Campbell, A.; Jacoby, D.B.; Zawadzka, A.; Ratliff, J.; Miedzybrocki, B.M.; Gahremanpour, A.; Diethrich, E.B.; Opie, S.R. Safety and feasibility of percutaneous autologous skeletal myoblast transplantation in the coil-infarcted swine myocardium. J. Pharmacol. Toxicol. Methods 2006, 54, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Fan, T.; Gao, S.; Jin, Y.; Zhang, M.; Ono, M. Application of mesenchymal stem cell sheet to treatment of ischemic heart disease. Stem Cell Res. Ther. 2021, 12, 384. [Google Scholar] [CrossRef]
- Kim, J.-H.; Joo, H.J.; Kim, M.; Choi, S.-C.; Lee, J.I.; Hong, S.J.; Lim, D.-S. Transplantation of Adipose-Derived Stem Cell Sheet Attenuates Adverse Cardiac Remodeling in Acute Myocardial Infarction. Tissue Eng. Part A 2017, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Shibata, R.; Shimizu, Y.; Yamamoto, T.; Kondo, K.; Inoue, Y.; Ouchi, N.; Tanigawa, T.; Kanemura, N.; Ito, A.; et al. Multilayered adipose-derived regenerative cell sheets created by a novel magnetite tissue engineering method for myocardial infarction. Int. J. Cardiol. 2014, 175, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Sekine, K.; Kawaguchi, A.T.; Miyazawa, M.; Hanawa, H.; Matsuda, S.; Tamaki, T.; Asahara, T.; Masuda, H. Transplantation of Fibroblast Sheets with Blood Mononuclear Cell Culture Exerts Cardioprotective Effects by Enhancing Anti-Inflammation and Vasculogenic Potential in Rat Experimental Autoimmune Myocarditis Model. Biology 2022, 11, 106. [Google Scholar] [CrossRef]
- Stöbener, D.D.; Hoppensack, A.; Scholz, J.; Weinhart, M. Endothelial, smooth muscle and fibroblast cell sheet fabrication from self-assembled thermoresponsive poly(glycidyl ether) brushes. Soft Matter 2018, 14, 8333–8343. [Google Scholar] [CrossRef]
- Masumoto, H.; Yamashita, J.K. Human iPS Cell-Derived Cardiac Tissue Sheets: A Platform for Cardiac Regeneration. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Sawa, Y.; Miyagawa, S. Present and Future Perspectives on Cell Sheet-Based Myocardial Regeneration Therapy. BioMed Res. Int. 2013, 2013, 583912. [Google Scholar] [CrossRef] [PubMed]
- Hewett, P.W.; Fujisawa, T.; Sissaoui, S.; Cai, M.; Gueron, G.; Al-Ani, B.; Cudmore, M.; Ahmed, F.S.; Wong, M.K.K.; Wegiel, B.; et al. Carbon monoxide inhibits sprouting angiogenesis and vascular endothelial growth factor receptor-2 phosphorylation. Thromb. Haemost. 2015, 113, 329–337. [Google Scholar] [CrossRef]
- Sekiya, N.; Matsumiya, G.; Miyagawa, S.; Saito, A.; Shimizu, T.; Okano, T.; Kawaguchi, N.; Matsuura, N.; Sawa, Y. Layered implantation of myoblast sheets attenuates adverse cardiac remodeling of the infarcted heart. J. Thorac. Cardiovasc. Surg. 2009, 138, 985–993. [Google Scholar] [CrossRef]
- Campagnoli, C.; Roberts, I.A.G.; Kumar, S.; Bennett, P.R.; Bellantuono, I.; Fisk, N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001, 98, 2396–2402. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Bray, F.; Møller, B. Predicting the future burden of cancer. Nat. Rev. Cancer 2006, 6, 63–74. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Corrigendum: Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, J.; Miller, P. Trial watch: Phase II and phase III attrition rates 2011–2012. Nat. Rev. Drug Discov. 2013, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 2016, 15, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Part 2: Potential Alternatives to the Use of Animals in Preclinical Trials. JACC Basic Transl. Sci. 2020, 5, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Aruga, A.; Kobayashi, H.; Yamato, M.; Yamamoto, M. Development of a Novel In Vivo Cancer Model Using Cell Sheet Engineering. Anticancer. Res. 2014, 34, 4747. [Google Scholar] [PubMed]
- Alshareeda, A.T.; Sakaguchi, K.; Abumaree, M.; Zin, N.K.M.; Shimizu, T. The potential of cell sheet technique on the development of hepatocellular carcinoma in rat models. PLoS ONE 2017, 12, e0184004. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, S.; Ji, Y.; Zhao, L.; Kong, Q.; Zhang, Q. Cell sheet-based multilayered liver tumor models for anti-cancer drug screening. Biotechnol. Lett. 2018, 40, 427–435. [Google Scholar] [CrossRef]
- Lee, J.; Shin, D.; Roh, J.-L. Development of an in vitro cell-sheet cancer model for chemotherapeutic screening. Theranostics 2018, 8, 3964–3973. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Li, M.; Kino-Oka, M. Degradation of endothelial network in disordered tumor-containing cell sheet. J. Biosci. Bioeng. 2017, 123, 748–753. [Google Scholar] [CrossRef]
- Tanaka, R.-I.; Sakaguchi, K.; Yoshida, A.; Takahashi, H.; Haraguchi, Y.; Shimizu, T. Production of scaffold-free cell-based meat using cell sheet technology. npj Sci. Food 2022, 6, 41. [Google Scholar] [CrossRef]
| Cell Types | Responsive System on TCPS | Detachment Temperature/Time | Refs. |
|---|---|---|---|
| BAECs | PIPAAm on TCPS | 20 °C/~75 min | [15,16] |
| PIPAAm/microporous membrane | 20 °C/~30 min | [15] | |
| PIPAAm/PEG/microporous membrane | 20 °C/~19 min | [17] | |
| A comb-type grafted PIPAAm | 20 °C/~25 min | [18] | |
| PIPAAm/PHEMA | 20 °C/~30 min | [19] | |
| PIPAAm/PAAm Poly(IAAm-co-CIPAAm) | 20 °C/~30 min 20 °C/~35 min | [20] [21] | |
| Dermal fibroblast | MC/PBS/Col (8% MC, PBS MW = 77,000–94,000, 10 g/L PBS) | 20 °C/~10–20 min | [22] |
| Human-adipose-tissue-derived stem cells | MC/PBS/Col (12% to 16% MC, MW = 15,000, 1.5 M PBS) | RT (~30 °C)/~2–3 min | [23] |
| Dermal fibroblast, MSC, myoblasts, endothelial cells | DVB/4VP/Ion-induction | 37 °C is possible/~100 s | [24] |
| Dermal fibroblasts | Electrical responsive system | 37 °C is possible/~5 min | [25] |
| Responsive Systems | Advantages | Disadvantages | Refs |
|---|---|---|---|
| PIPAAm-grafted surface |
|
| [10,28,36,38,39,42,43,44] |
| MC-coating surface |
|
| [22,42] |
| Ion-induced cell detachment |
|
| [24] |
| Electro-responsive surface |
|
| [25,45] |
| Photo-responsive surface |
|
| [46] |
| pH-responsive system |
|
| [47,48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thummarati, P.; Laiwattanapaisal, W.; Nitta, R.; Fukuda, M.; Hassametto, A.; Kino-oka, M. Recent Advances in Cell Sheet Engineering: From Fabrication to Clinical Translation. Bioengineering 2023, 10, 211. https://doi.org/10.3390/bioengineering10020211
Thummarati P, Laiwattanapaisal W, Nitta R, Fukuda M, Hassametto A, Kino-oka M. Recent Advances in Cell Sheet Engineering: From Fabrication to Clinical Translation. Bioengineering. 2023; 10(2):211. https://doi.org/10.3390/bioengineering10020211
Chicago/Turabian StyleThummarati, Parichut, Wanida Laiwattanapaisal, Rikiya Nitta, Megumi Fukuda, Artchaya Hassametto, and Masahiro Kino-oka. 2023. "Recent Advances in Cell Sheet Engineering: From Fabrication to Clinical Translation" Bioengineering 10, no. 2: 211. https://doi.org/10.3390/bioengineering10020211
APA StyleThummarati, P., Laiwattanapaisal, W., Nitta, R., Fukuda, M., Hassametto, A., & Kino-oka, M. (2023). Recent Advances in Cell Sheet Engineering: From Fabrication to Clinical Translation. Bioengineering, 10(2), 211. https://doi.org/10.3390/bioengineering10020211