In-Silico and In-Vitro Analysis of the Novel Hybrid Comprehensive Stage II Operation for Single Ventricle Circulation
Abstract
1. Introduction
2. Materials and Methods
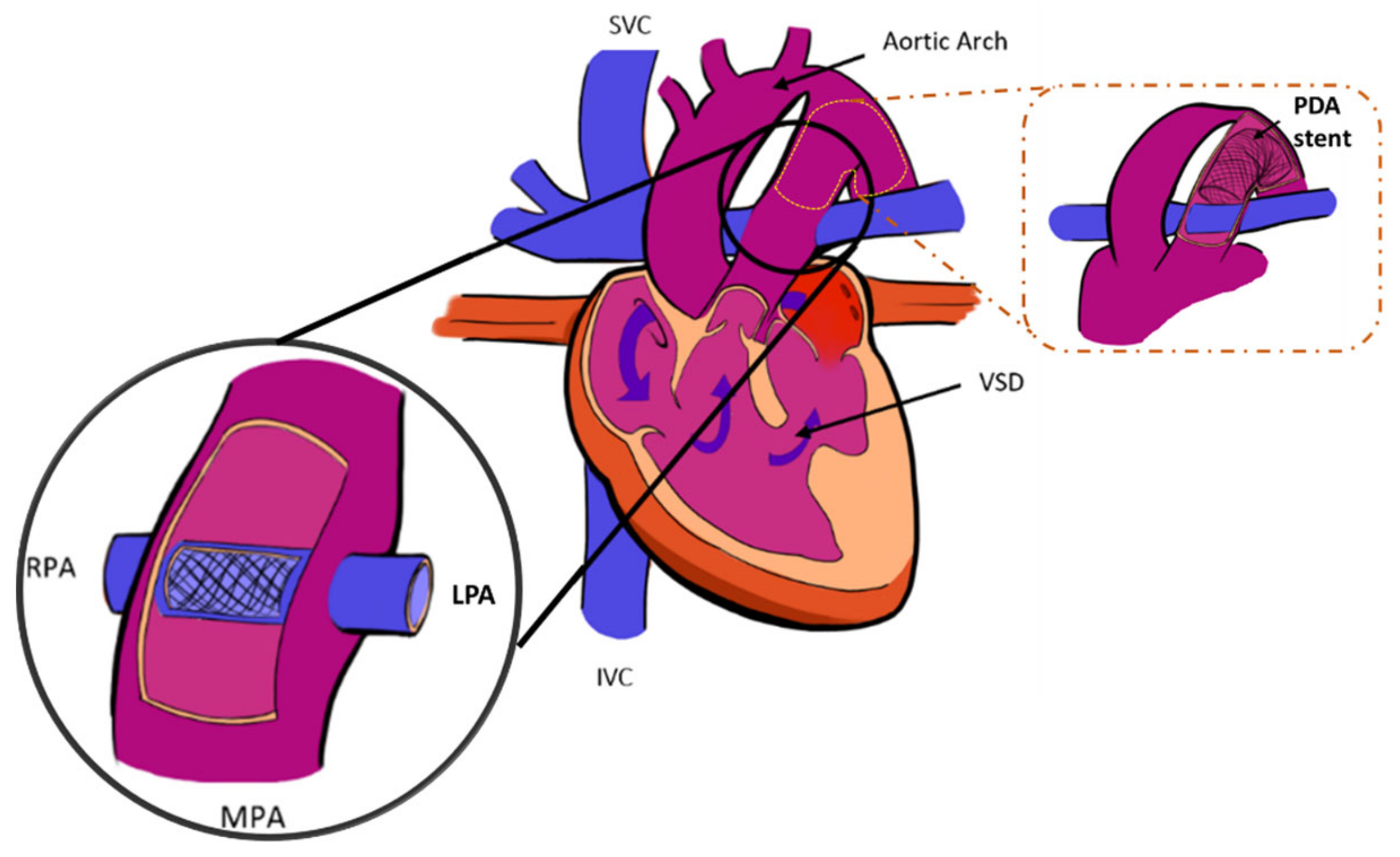
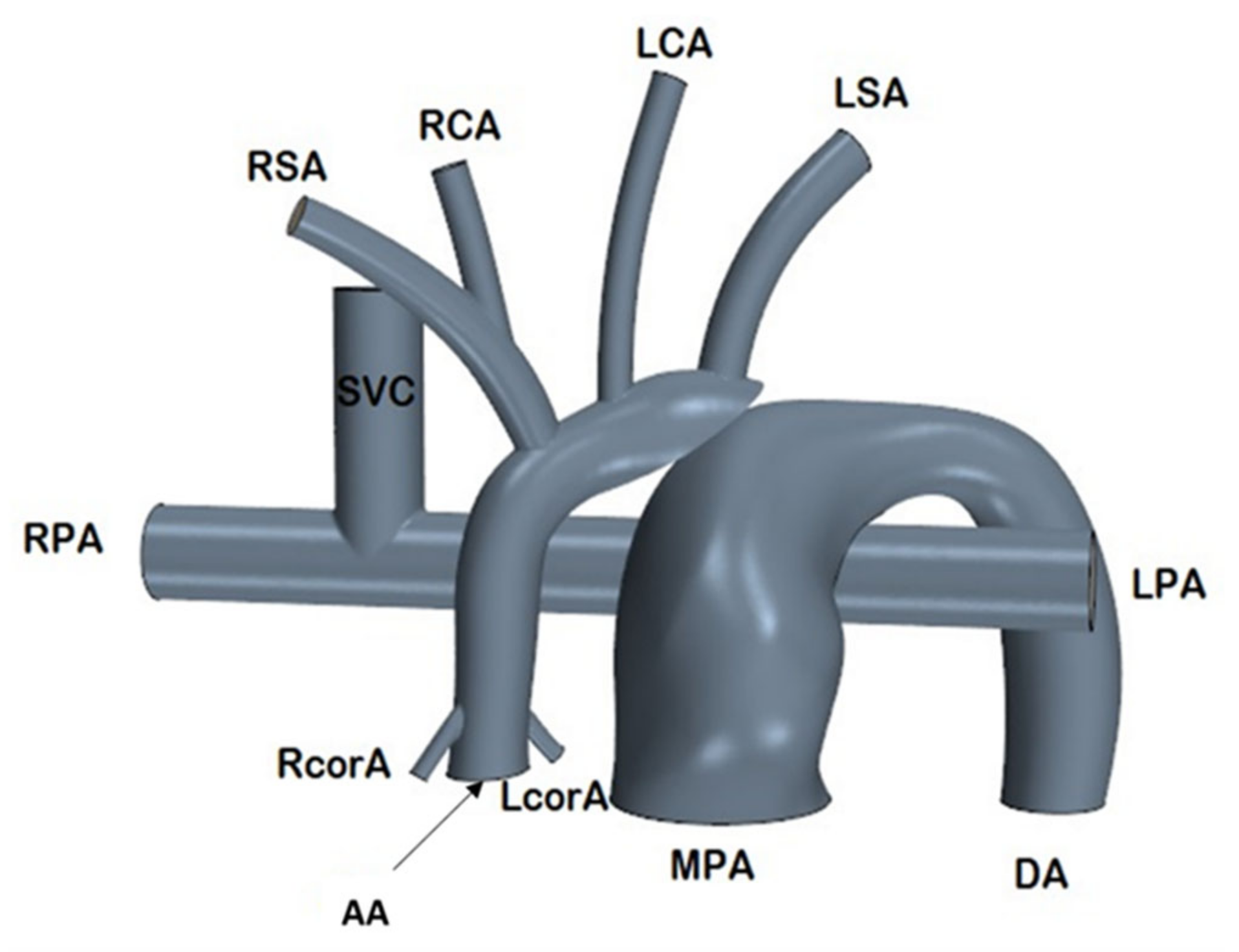
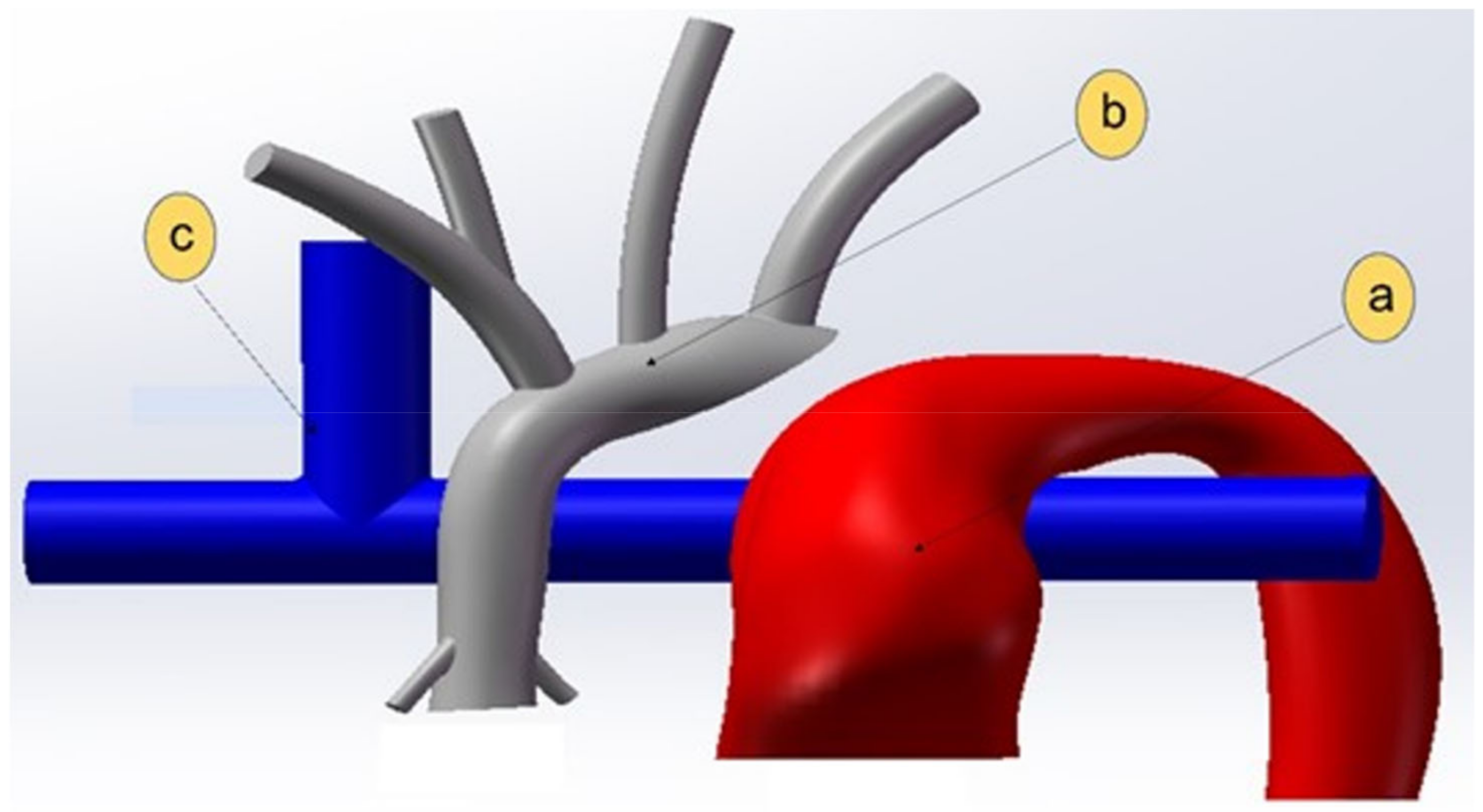
2.1. Anatomical Model
2.1.1. In-Silico Model
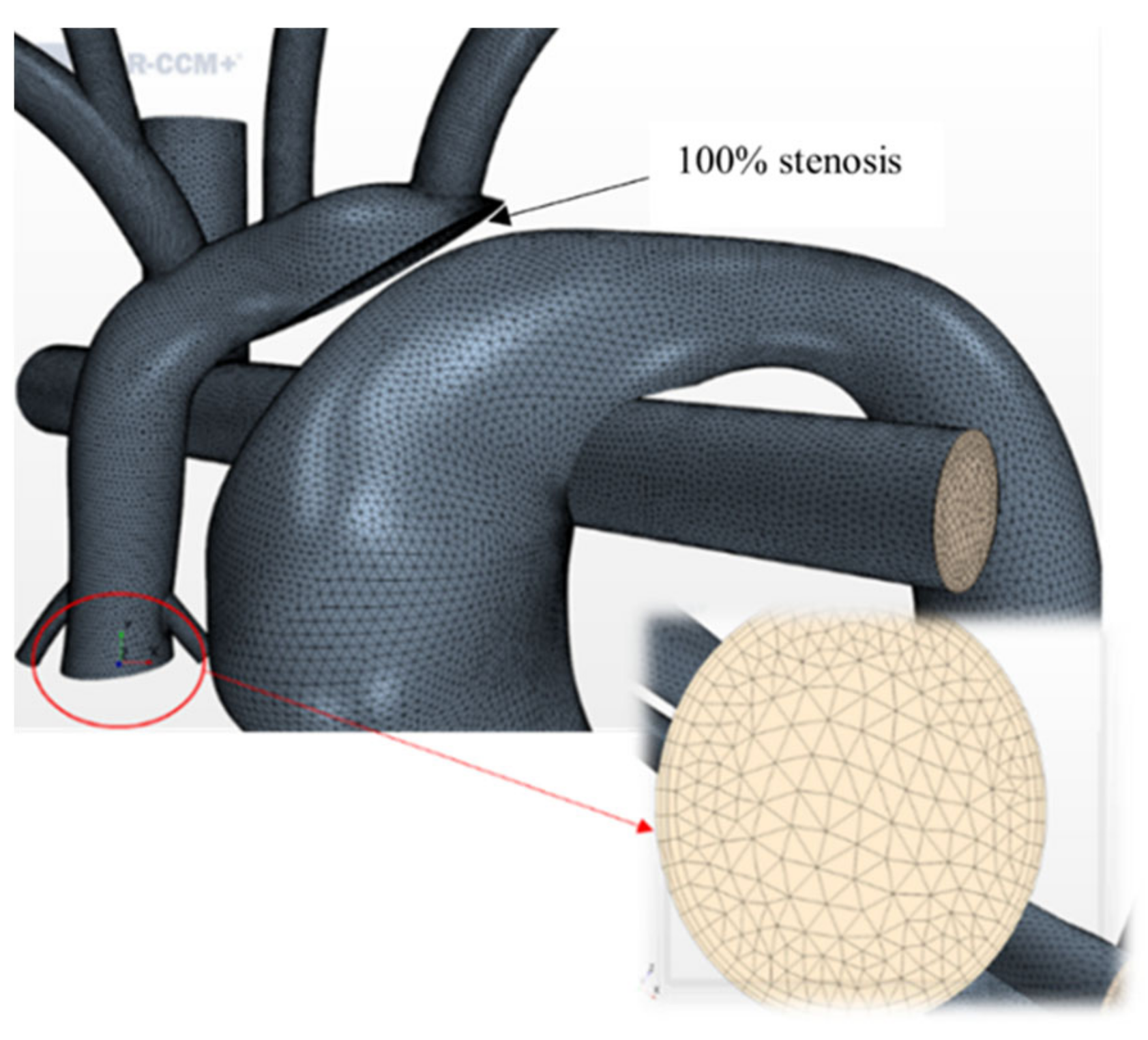
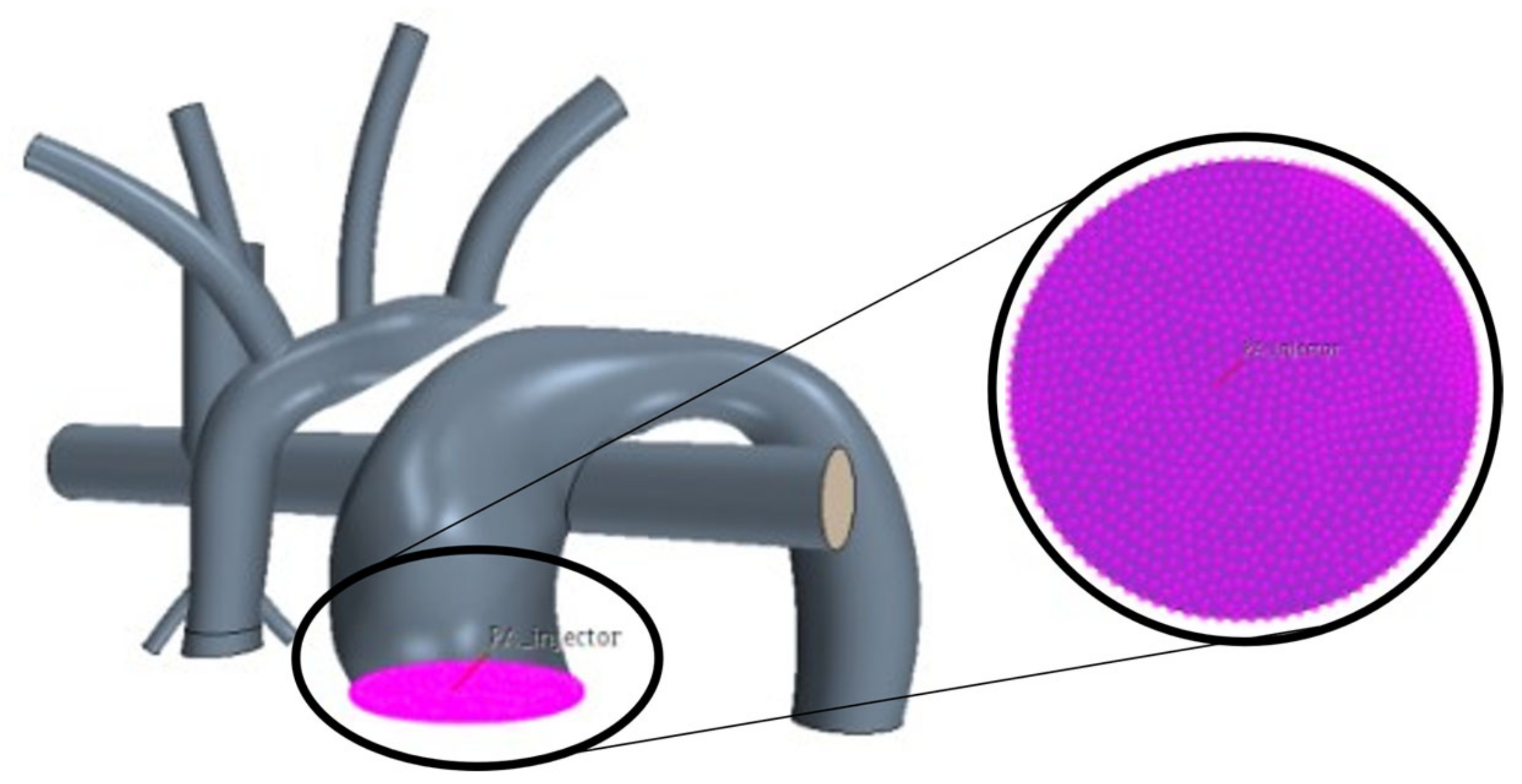
Mesh
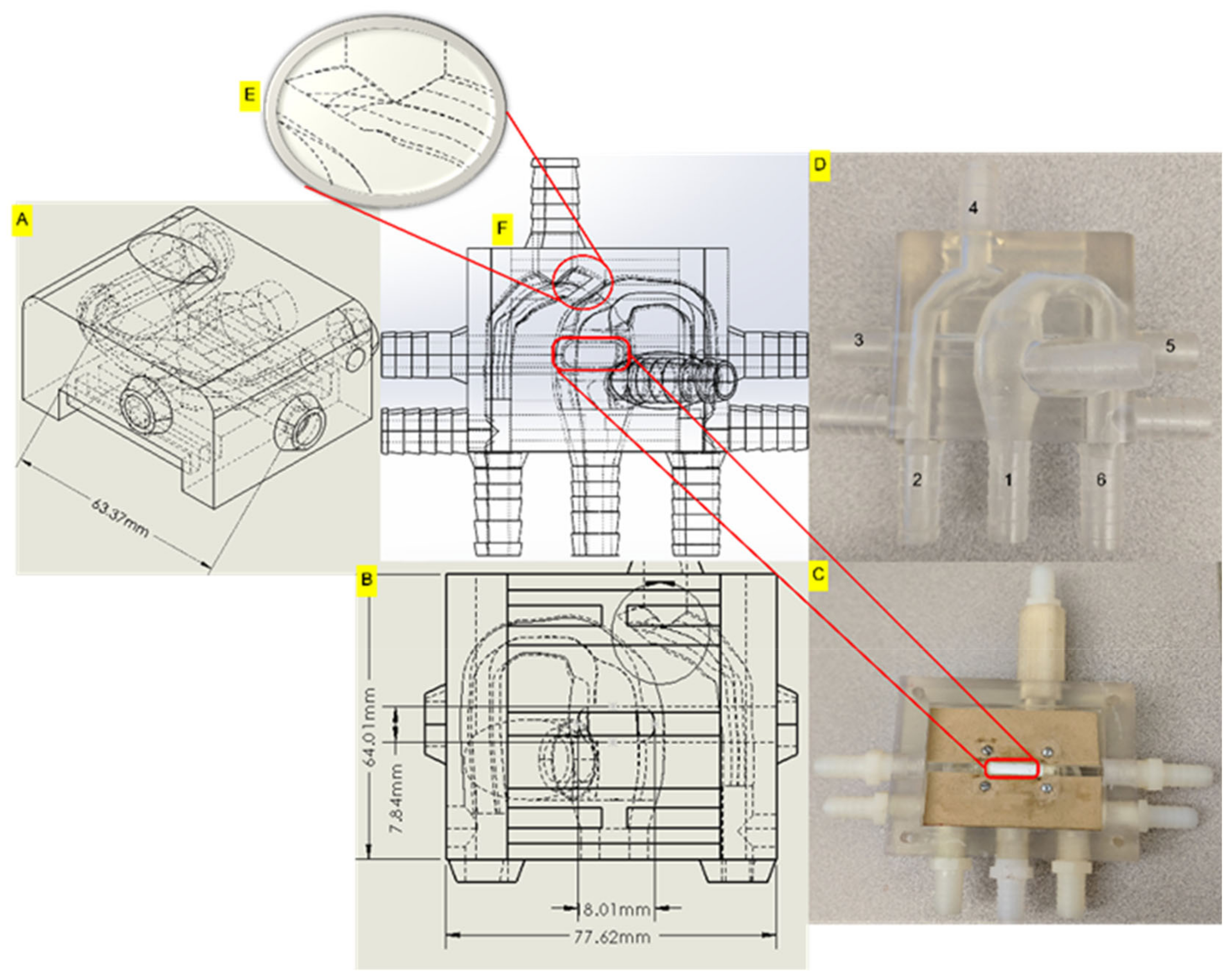
2.1.2. In-Vitro Phantom
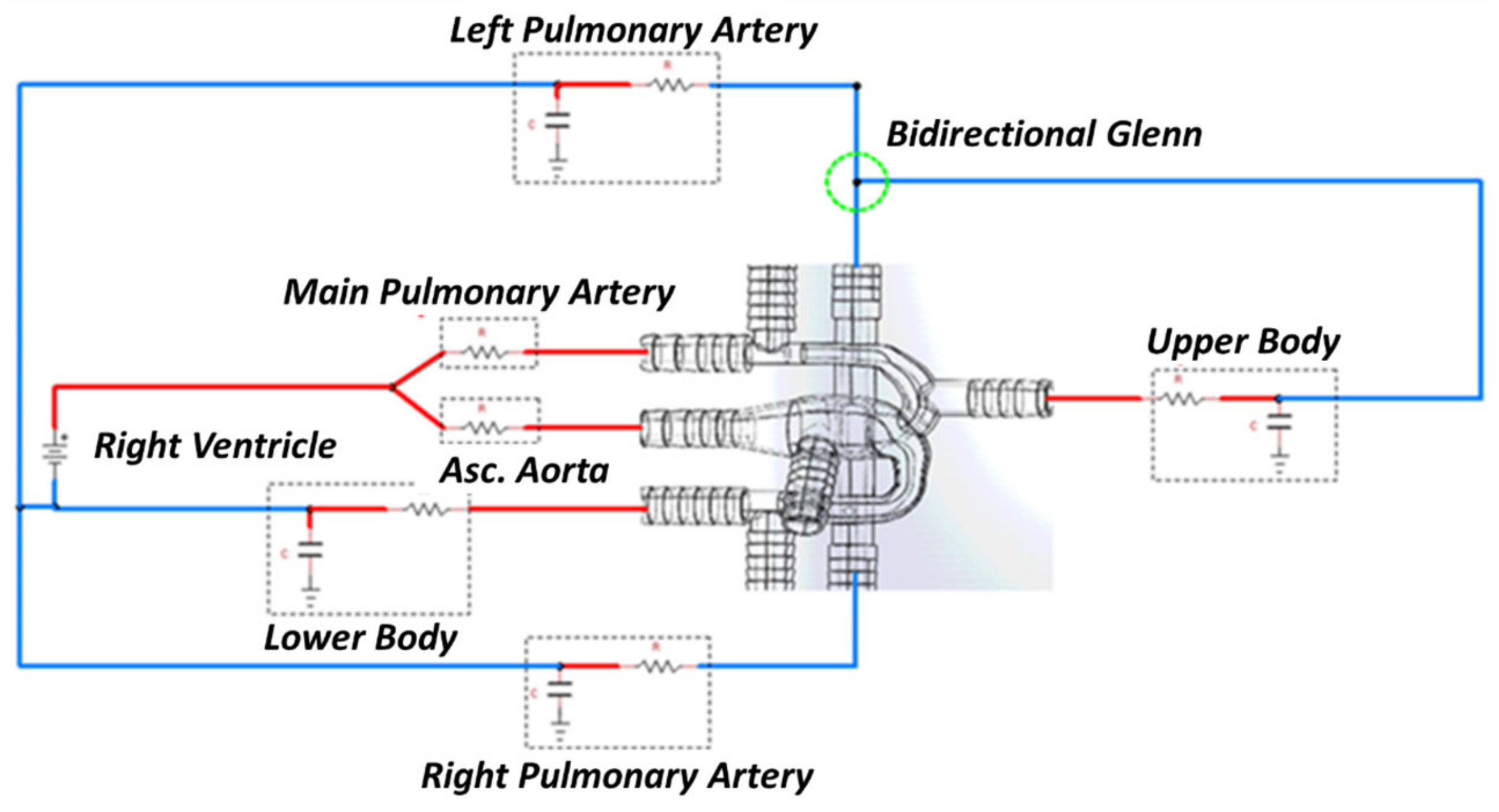
2.2. Lumped Parameter Model
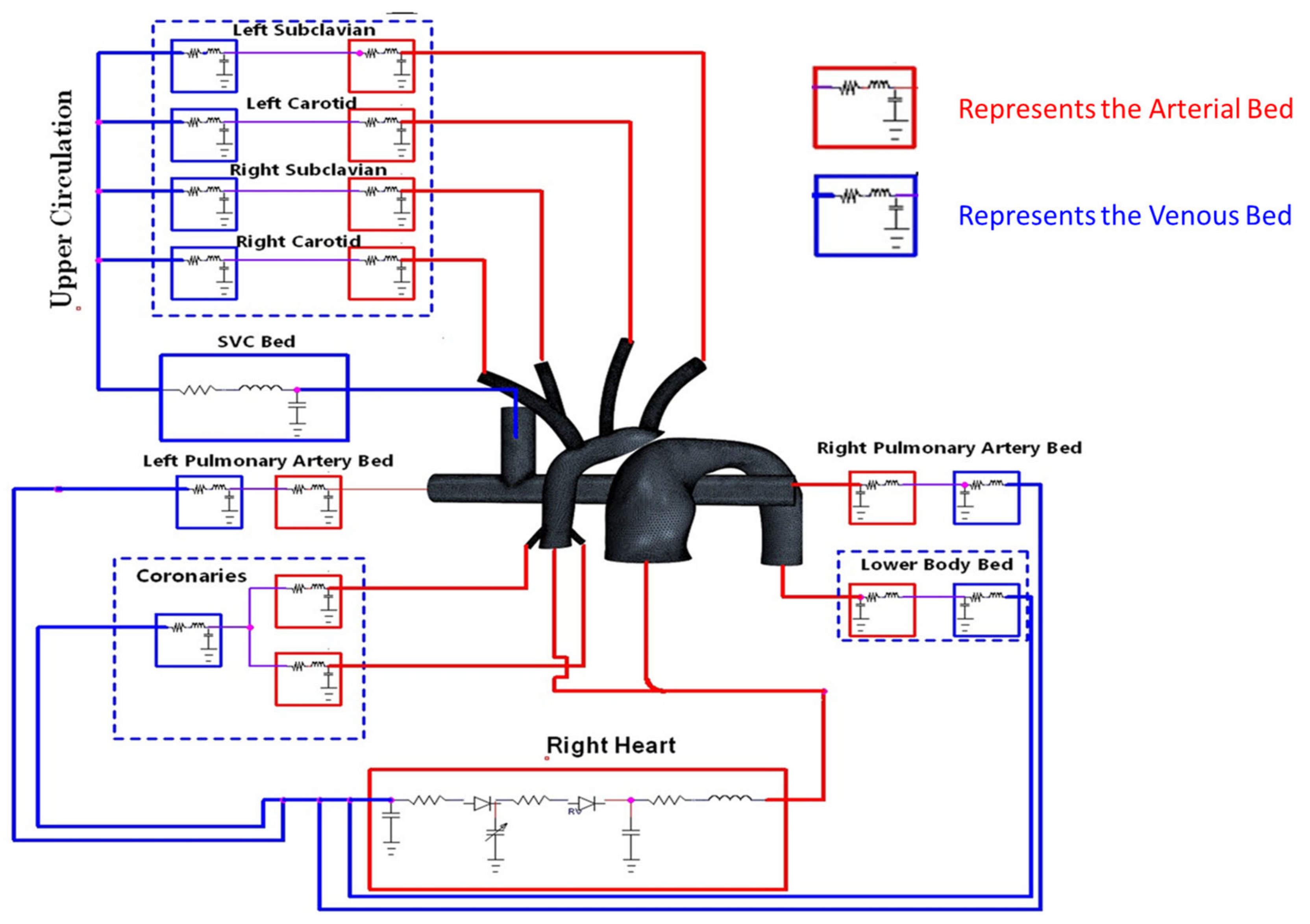
2.2.1. LPM Setup for In-Silico Model
2.2.2. LPM Setup for In-Vitro Model
2.3. CFD-LPM Coupling
2.4. Fluid Domain Solver
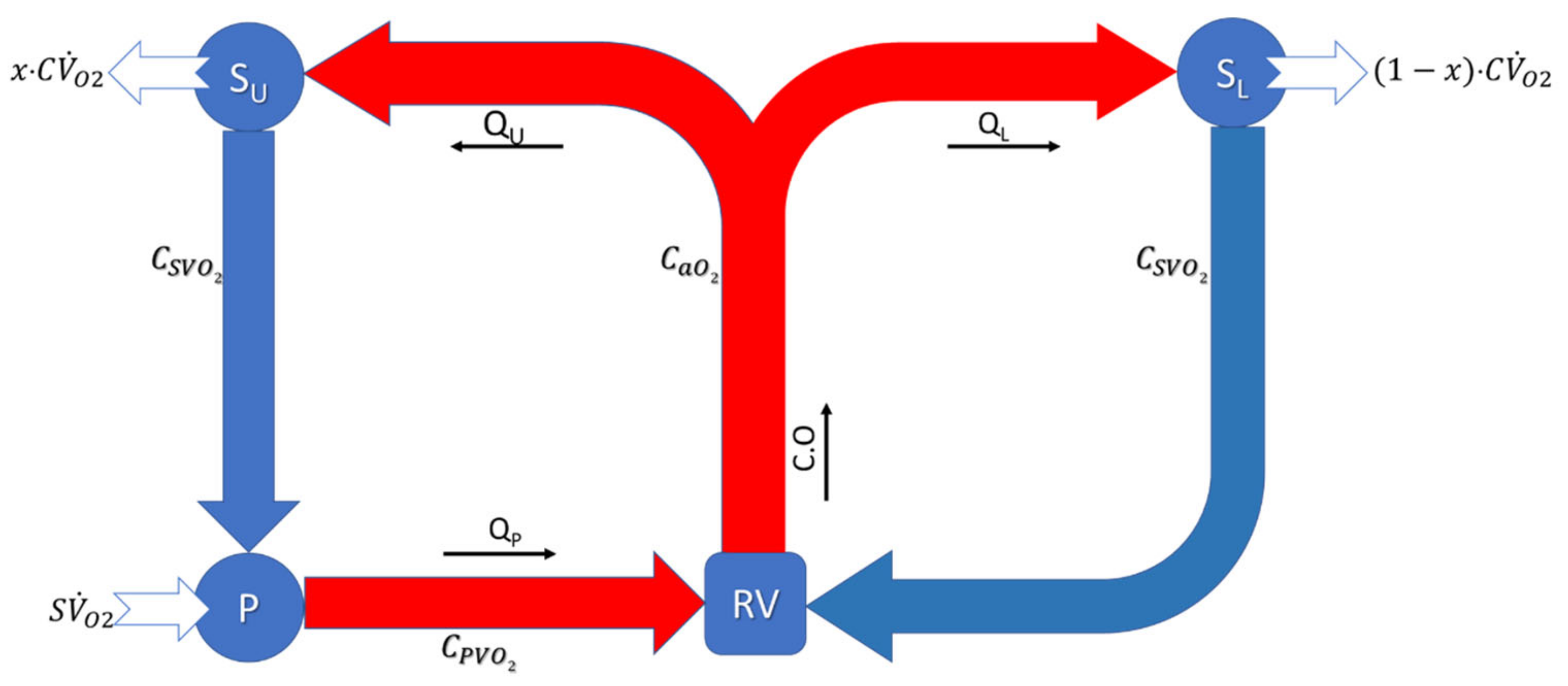
2.5. Oxygen Transport Model
2.6. PowerLoss
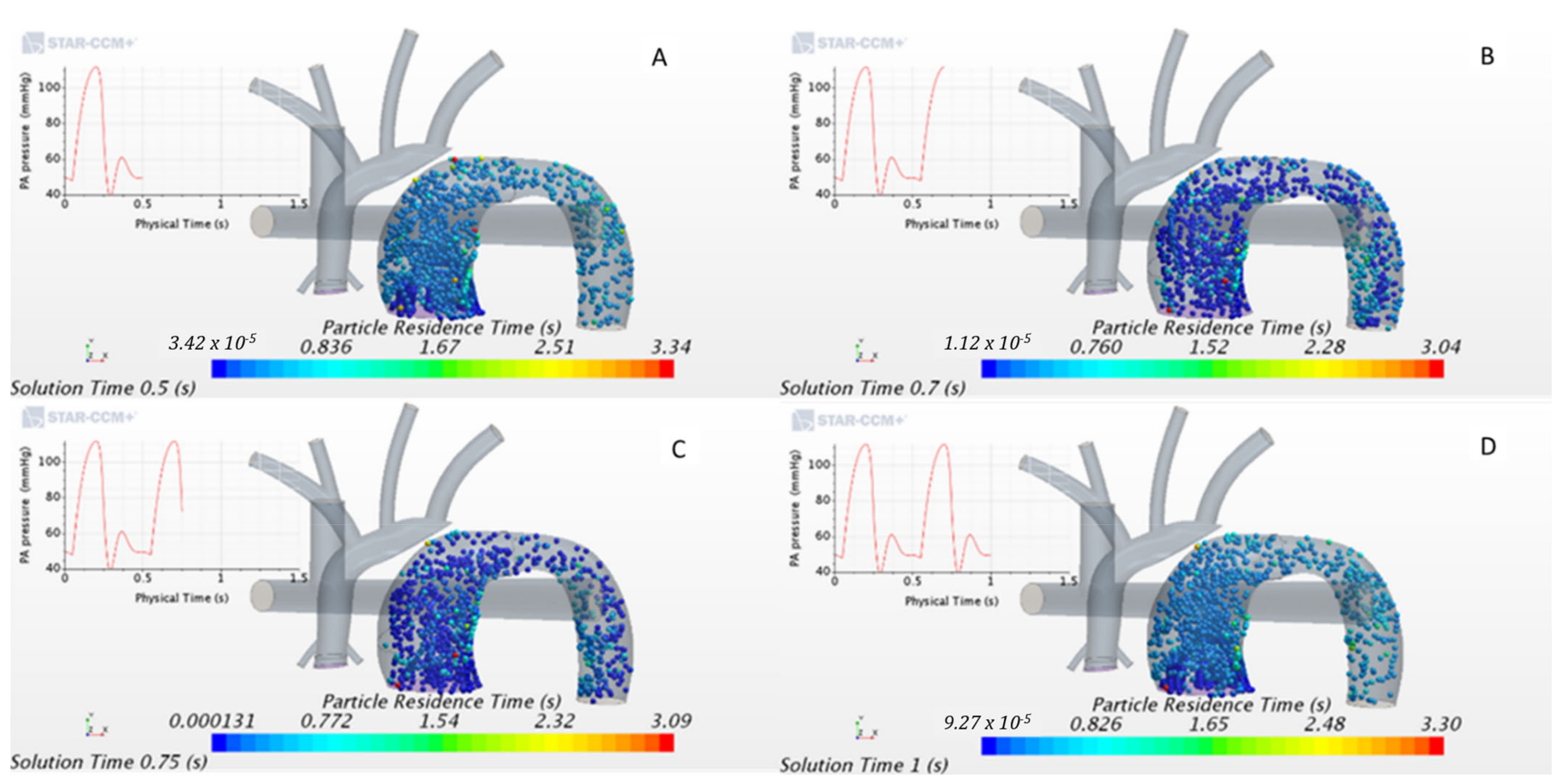
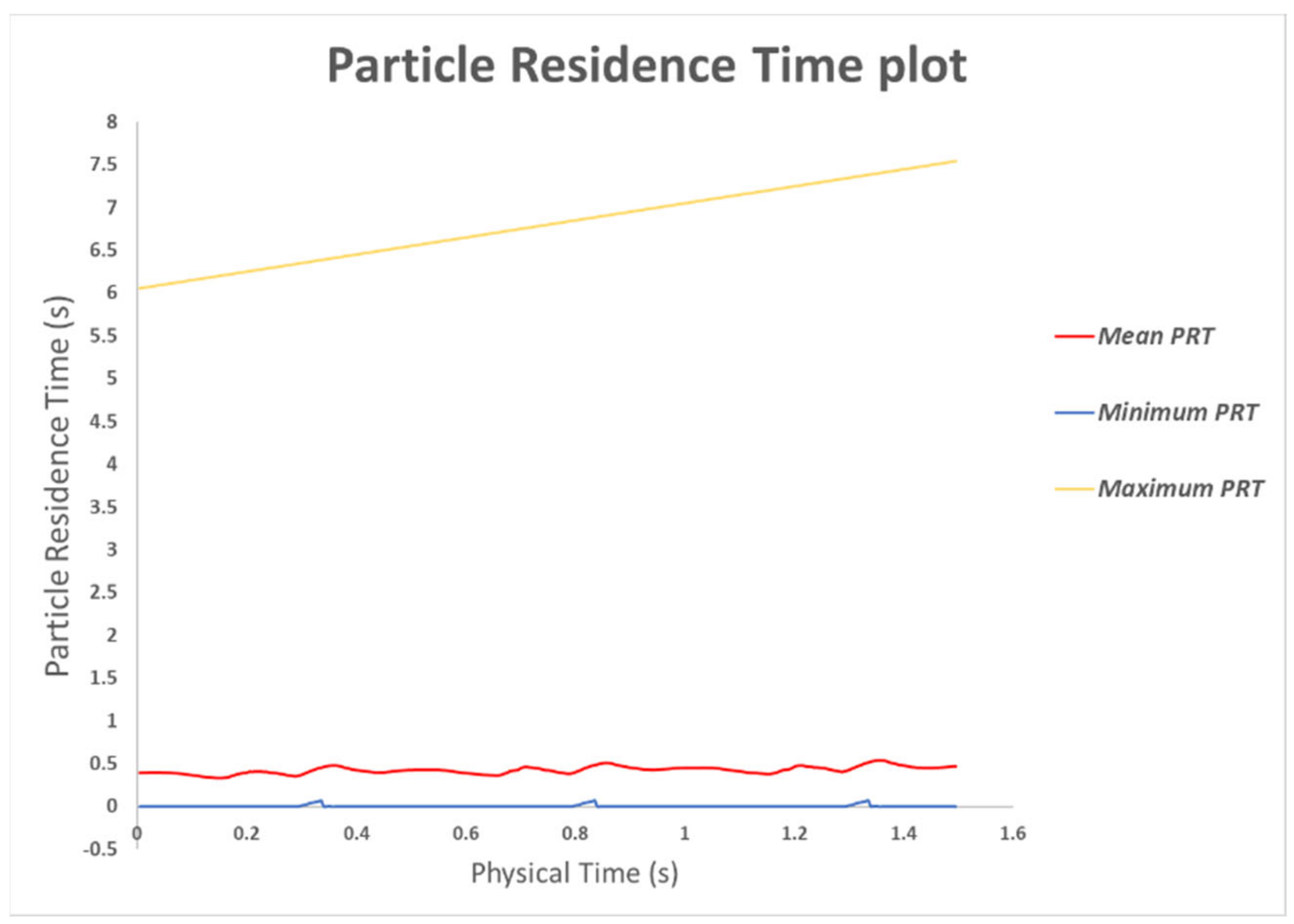
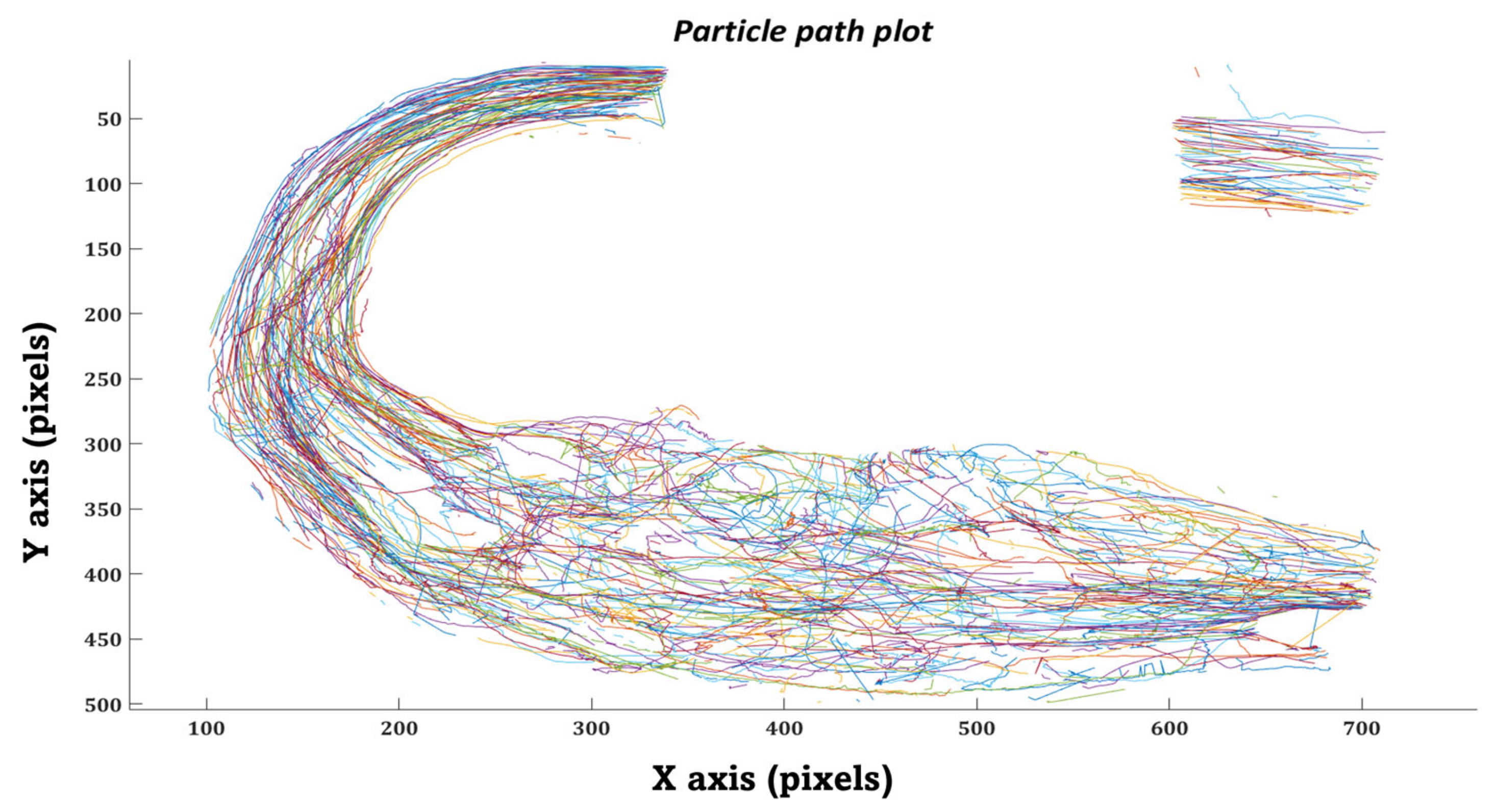
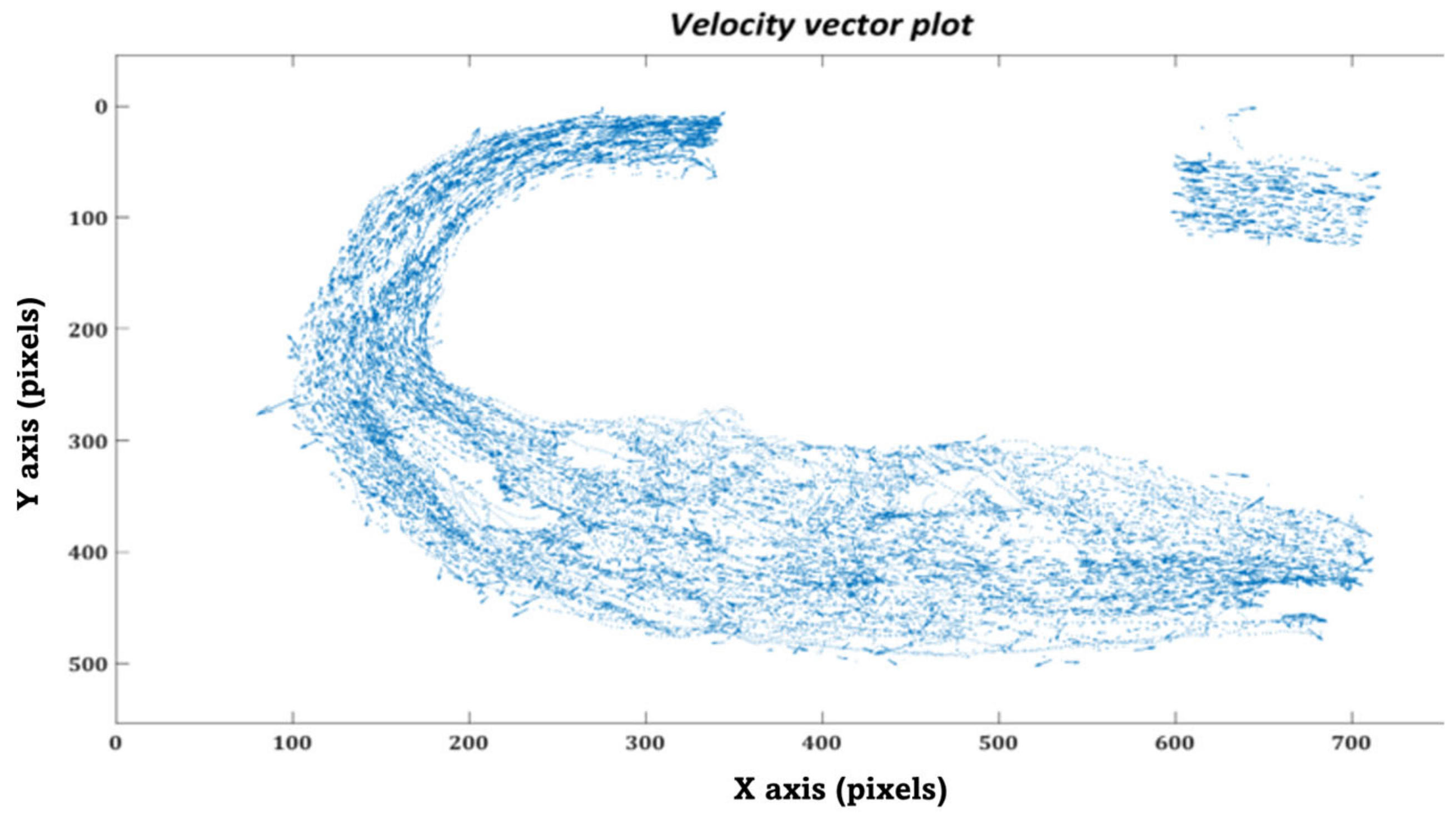
2.7. In-Silico Particle Residence Time
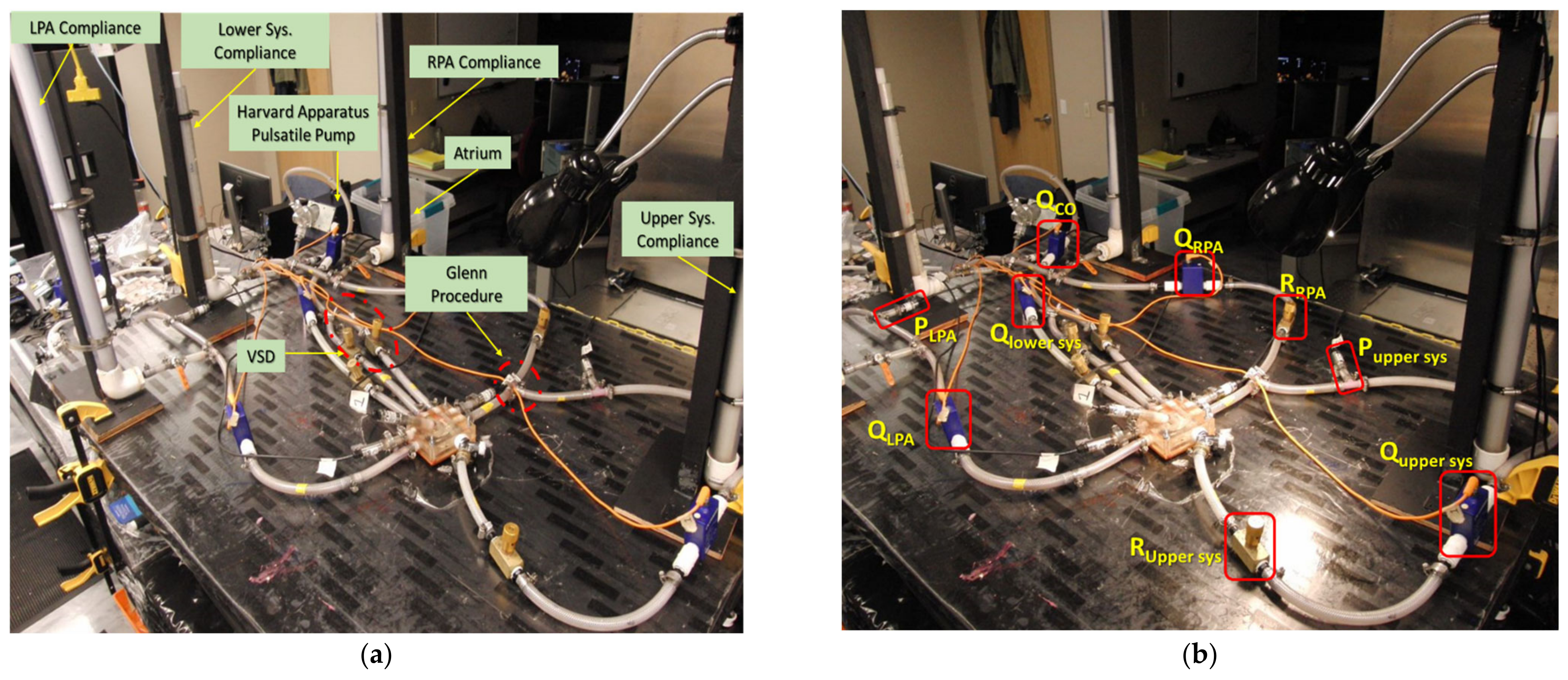
2.8. Experimentation
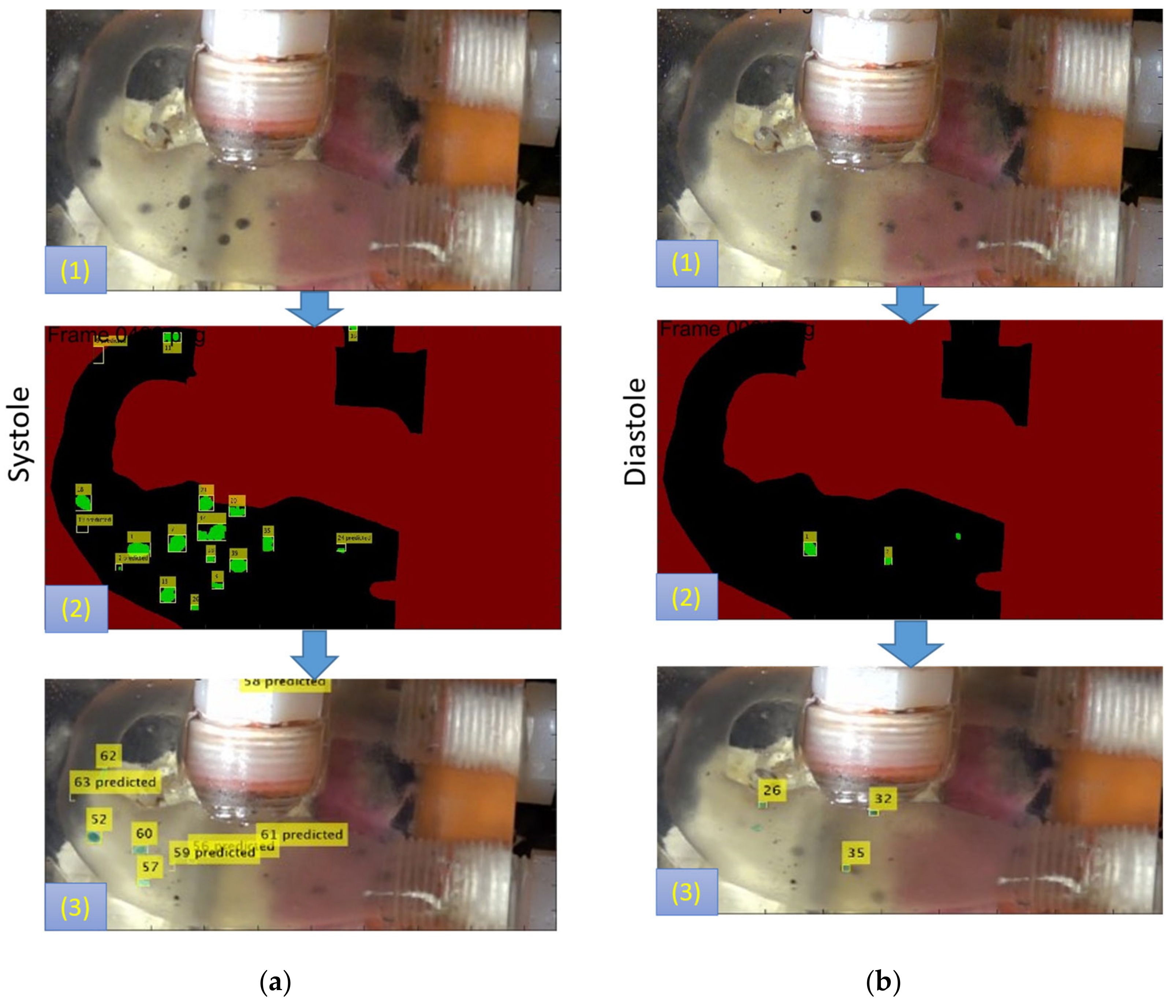
2.8.1. In-Vitro Particle Residence Time
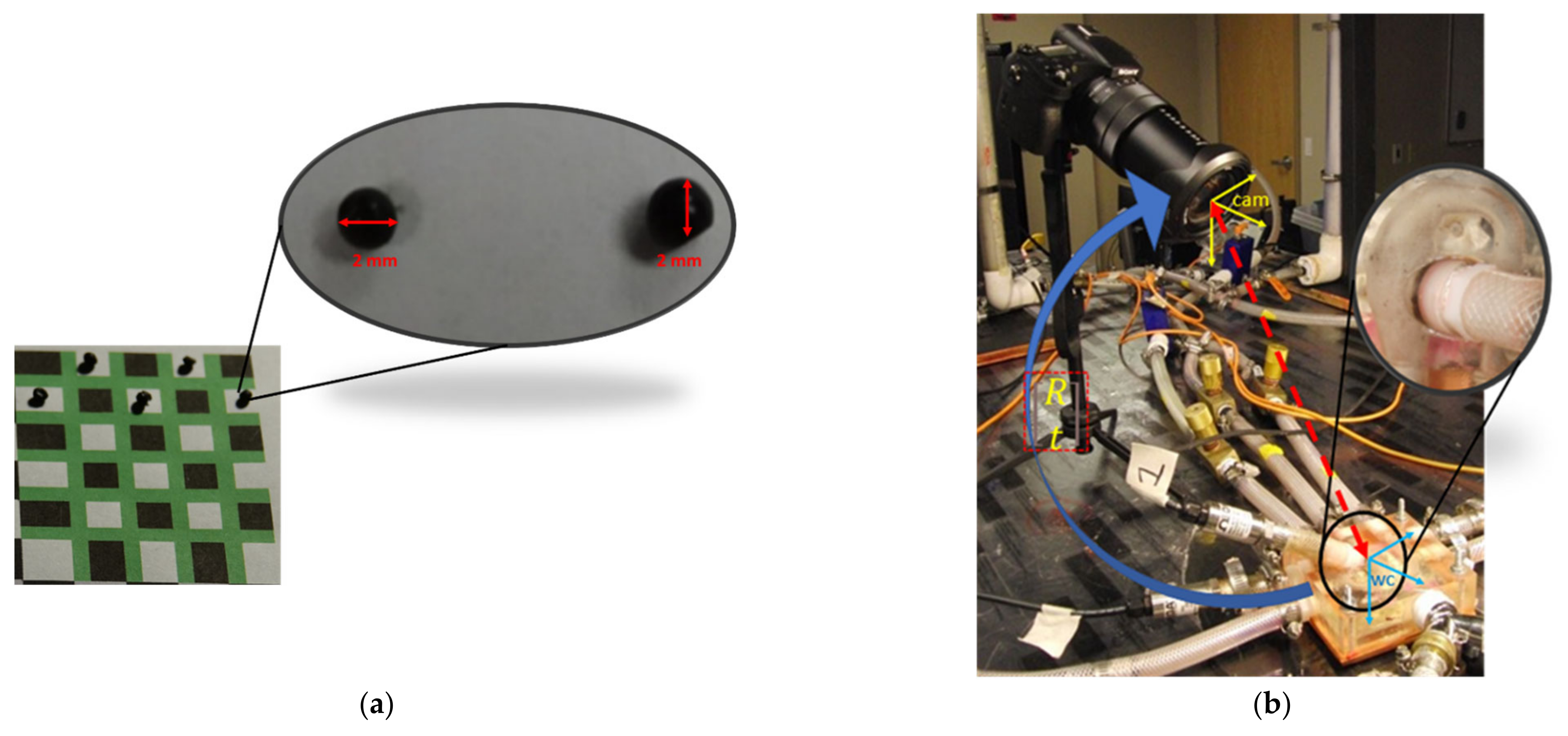
2.8.2. Camera Calibration
2.9. Statistical Analysis
- Paired T-test
- 2.
- One-way ANOVA
3. Results and Discussion
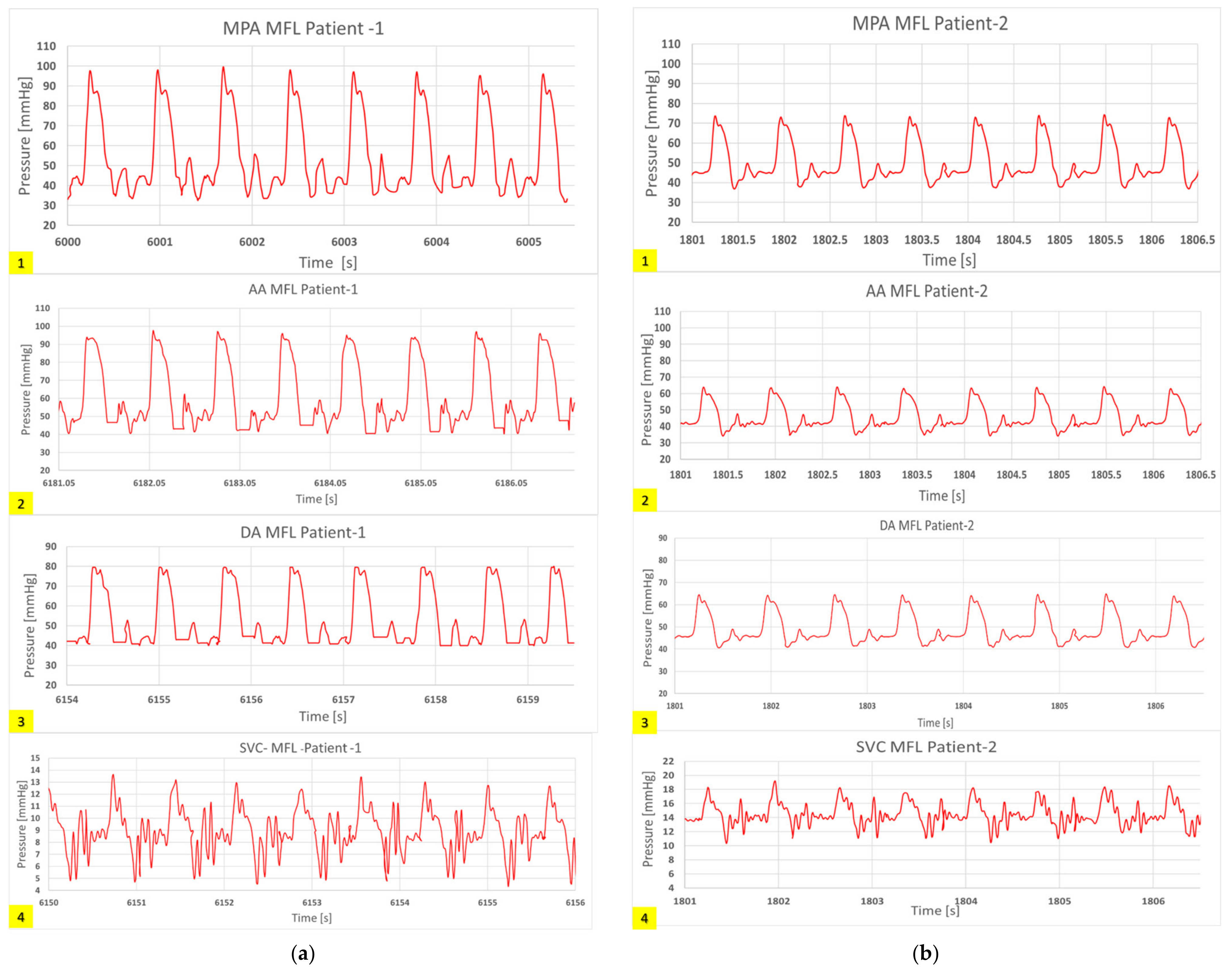
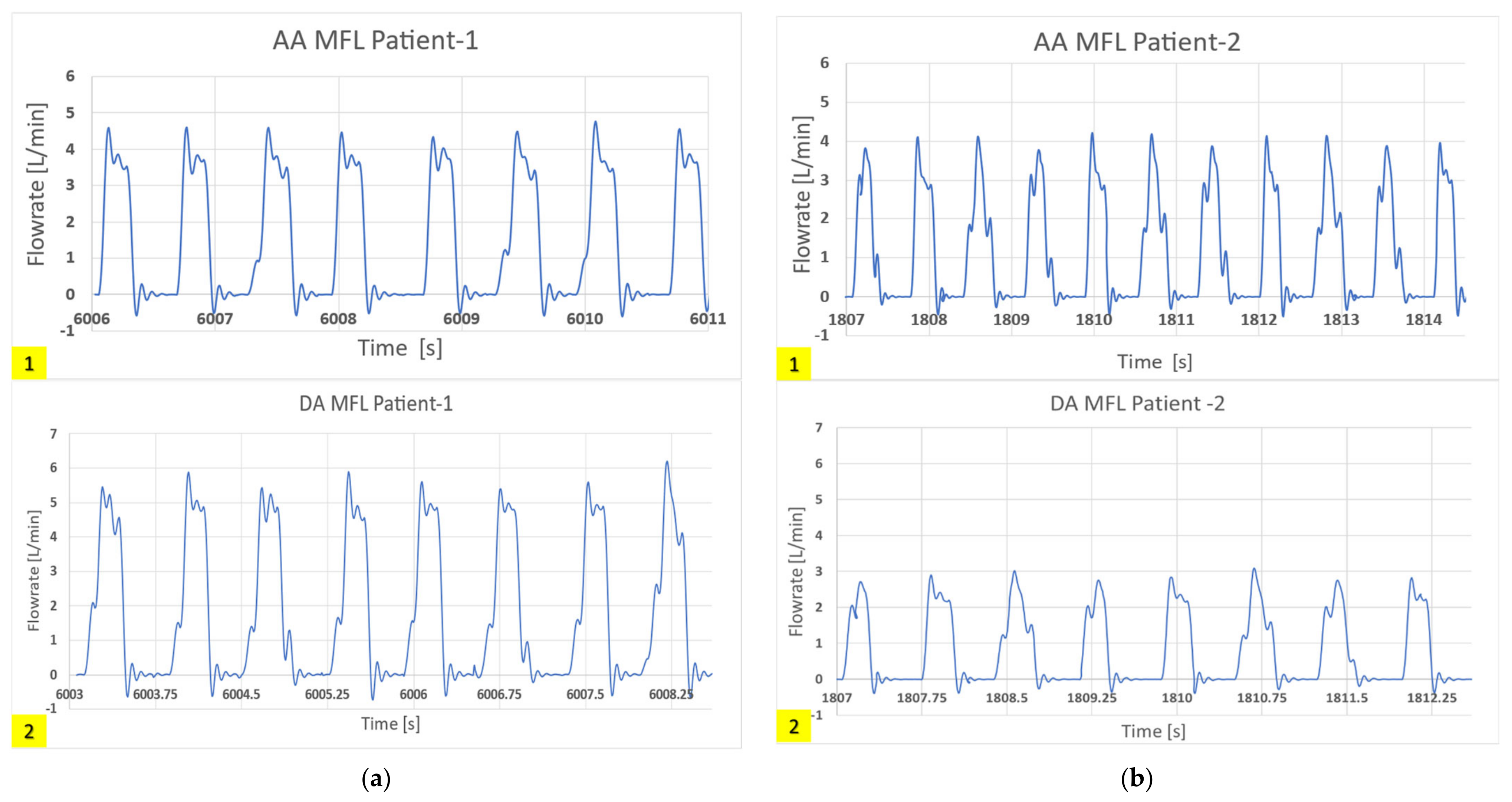
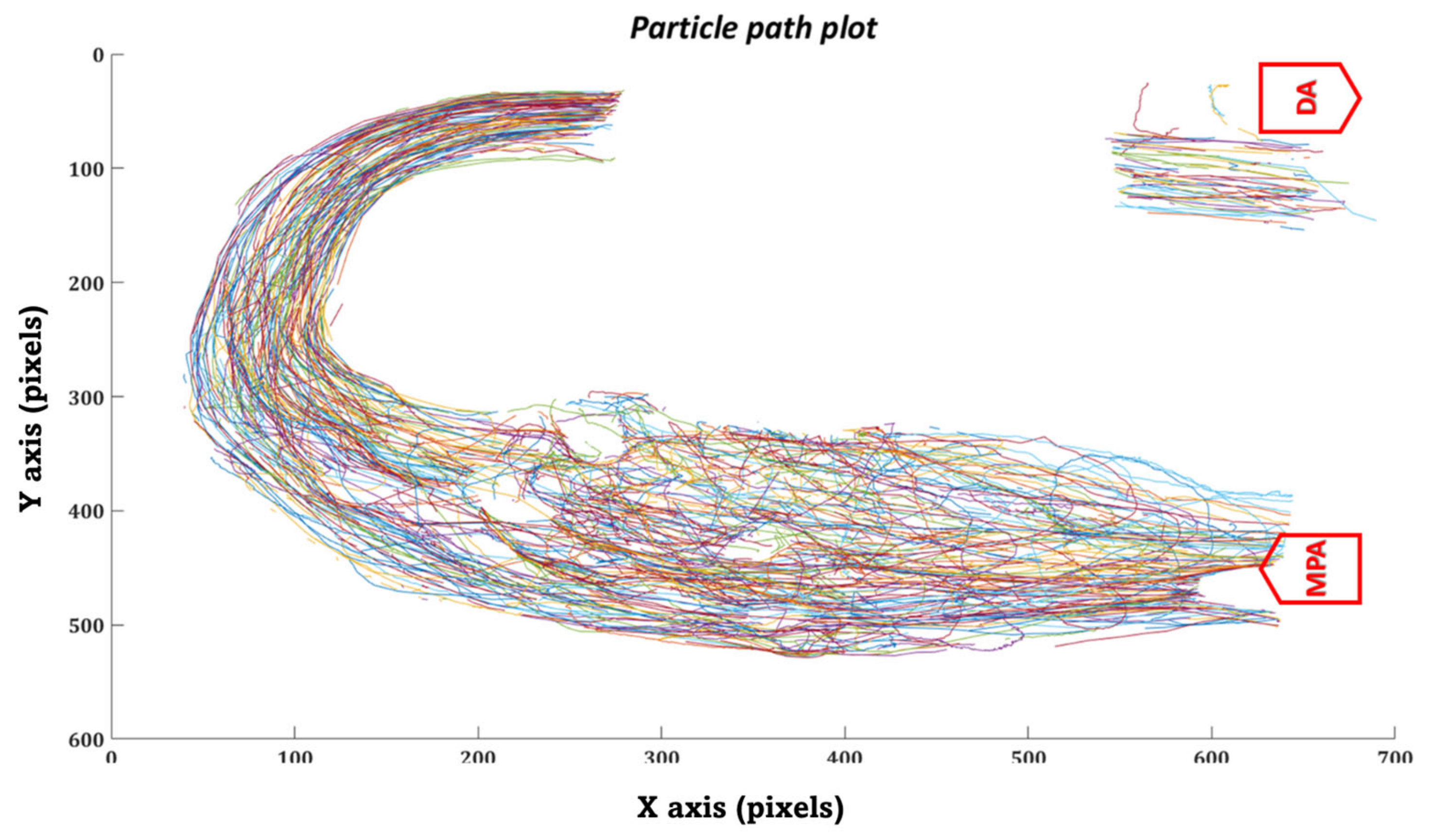
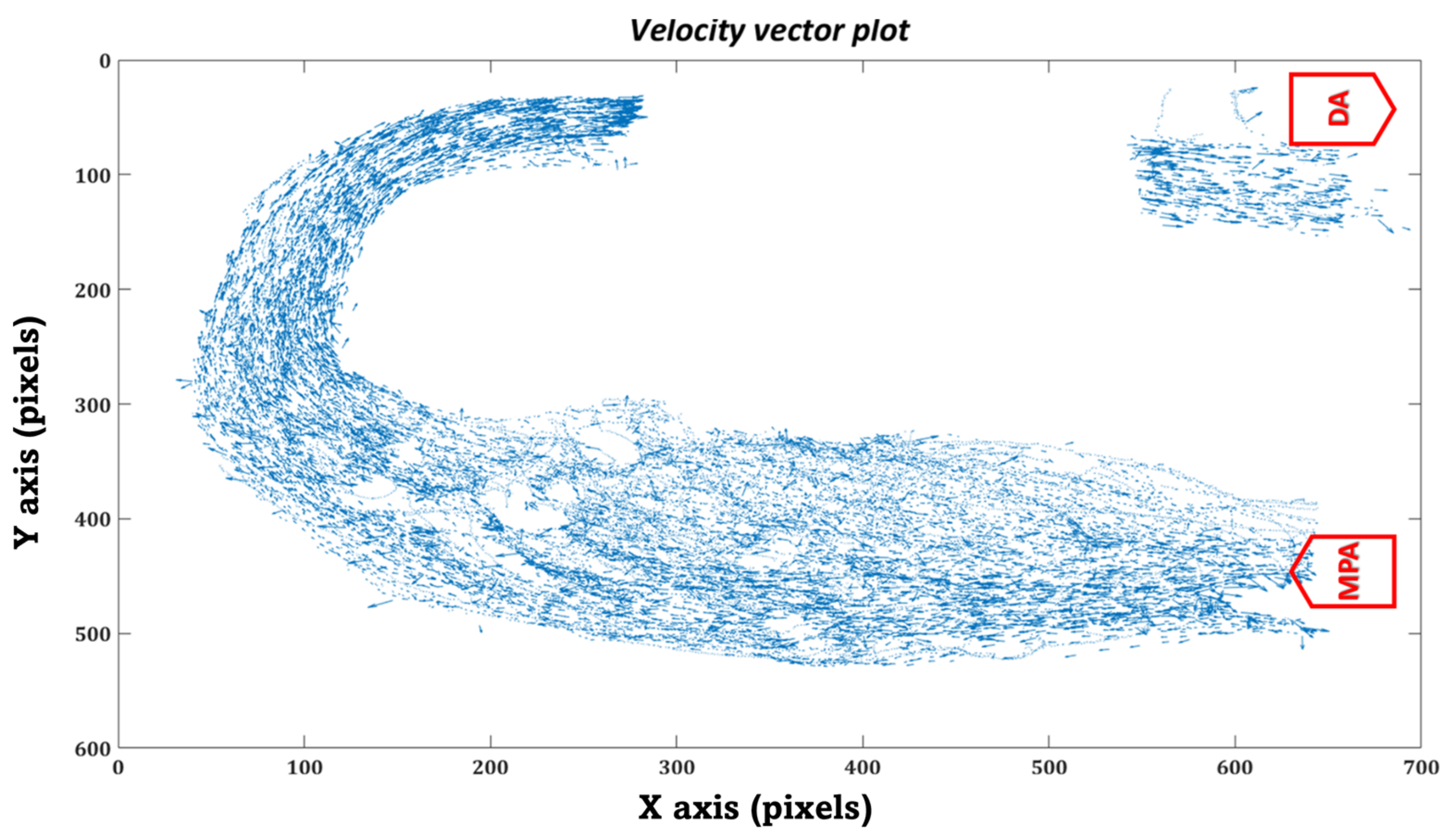
3.1. HCSII Hemodynamics
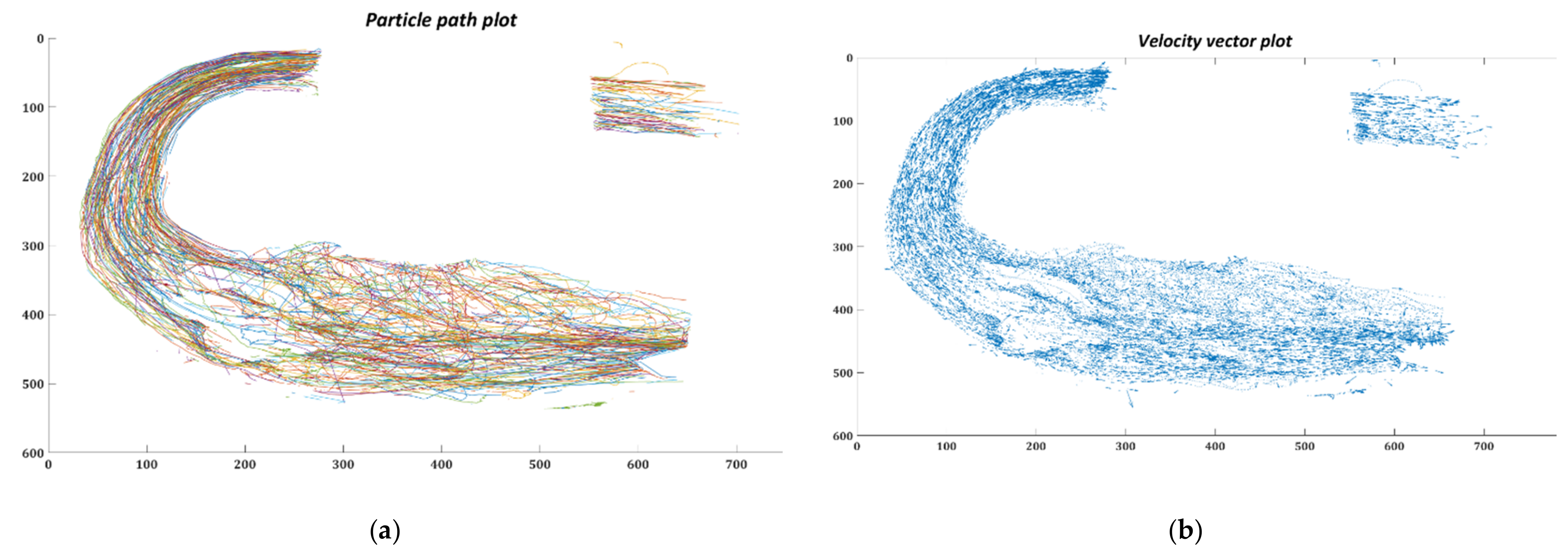
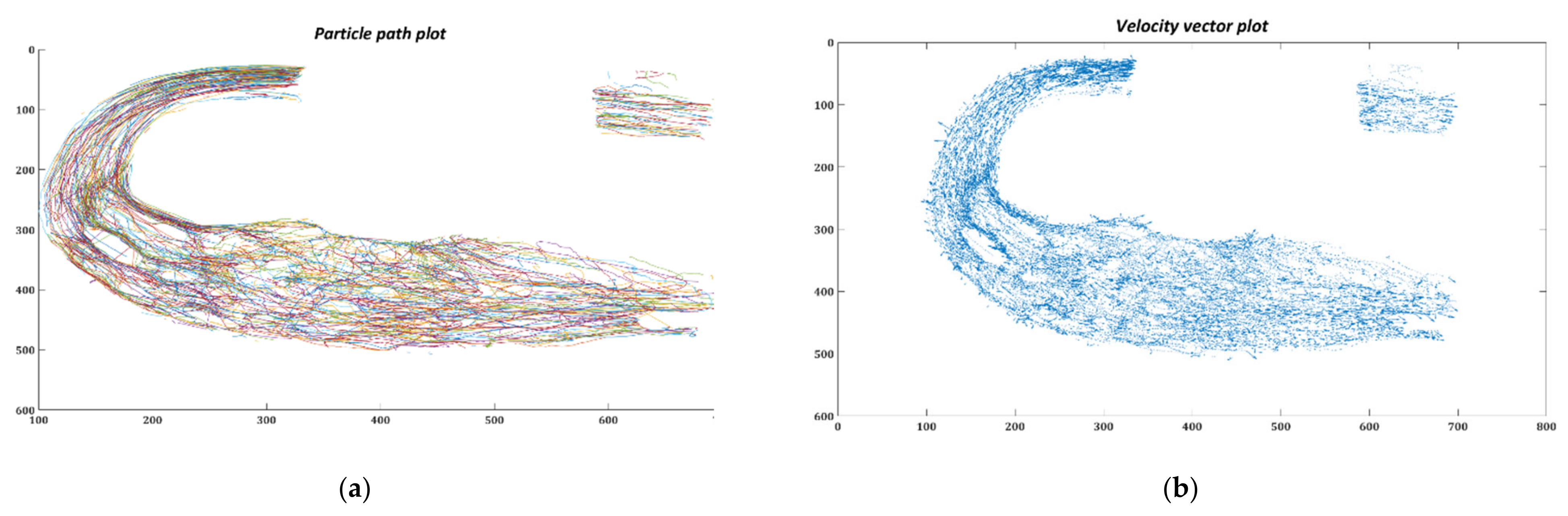
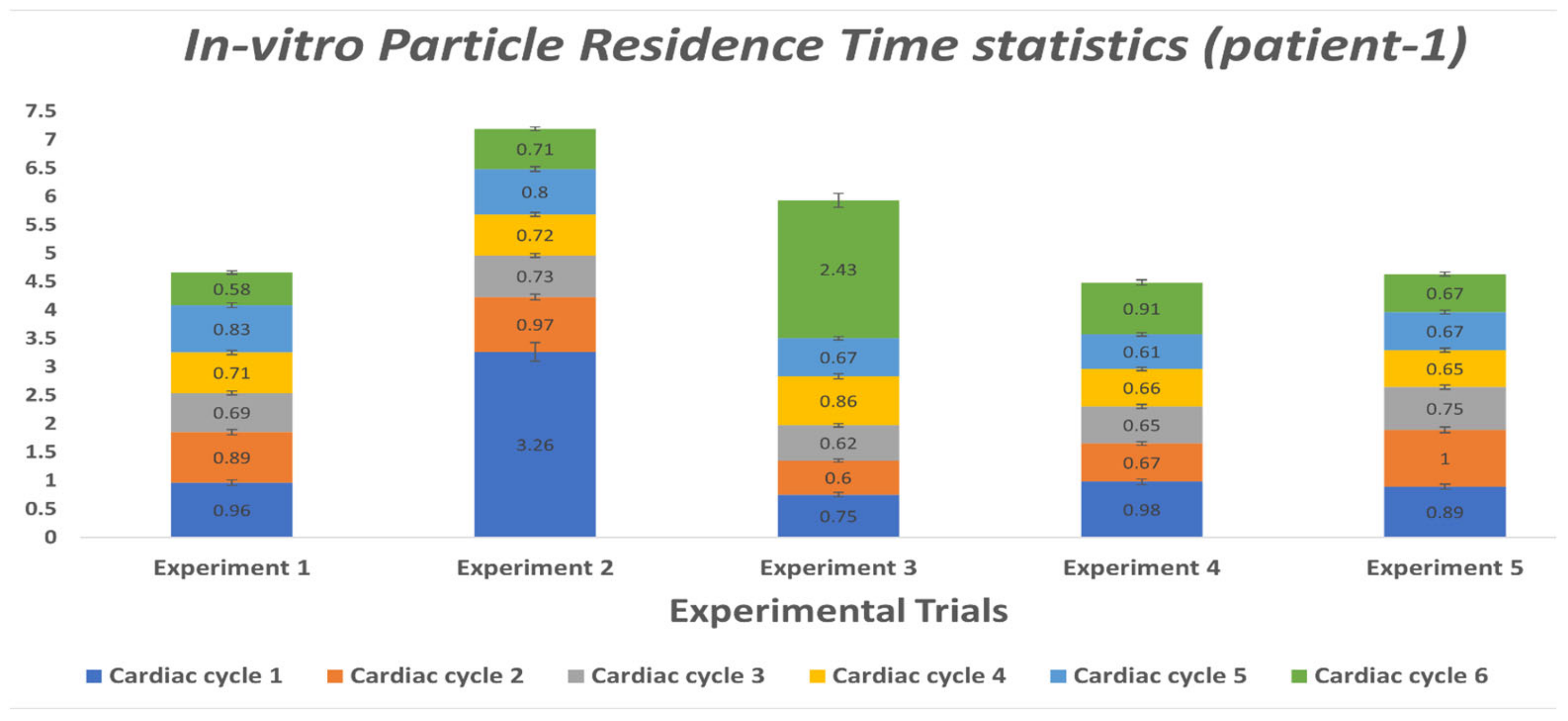
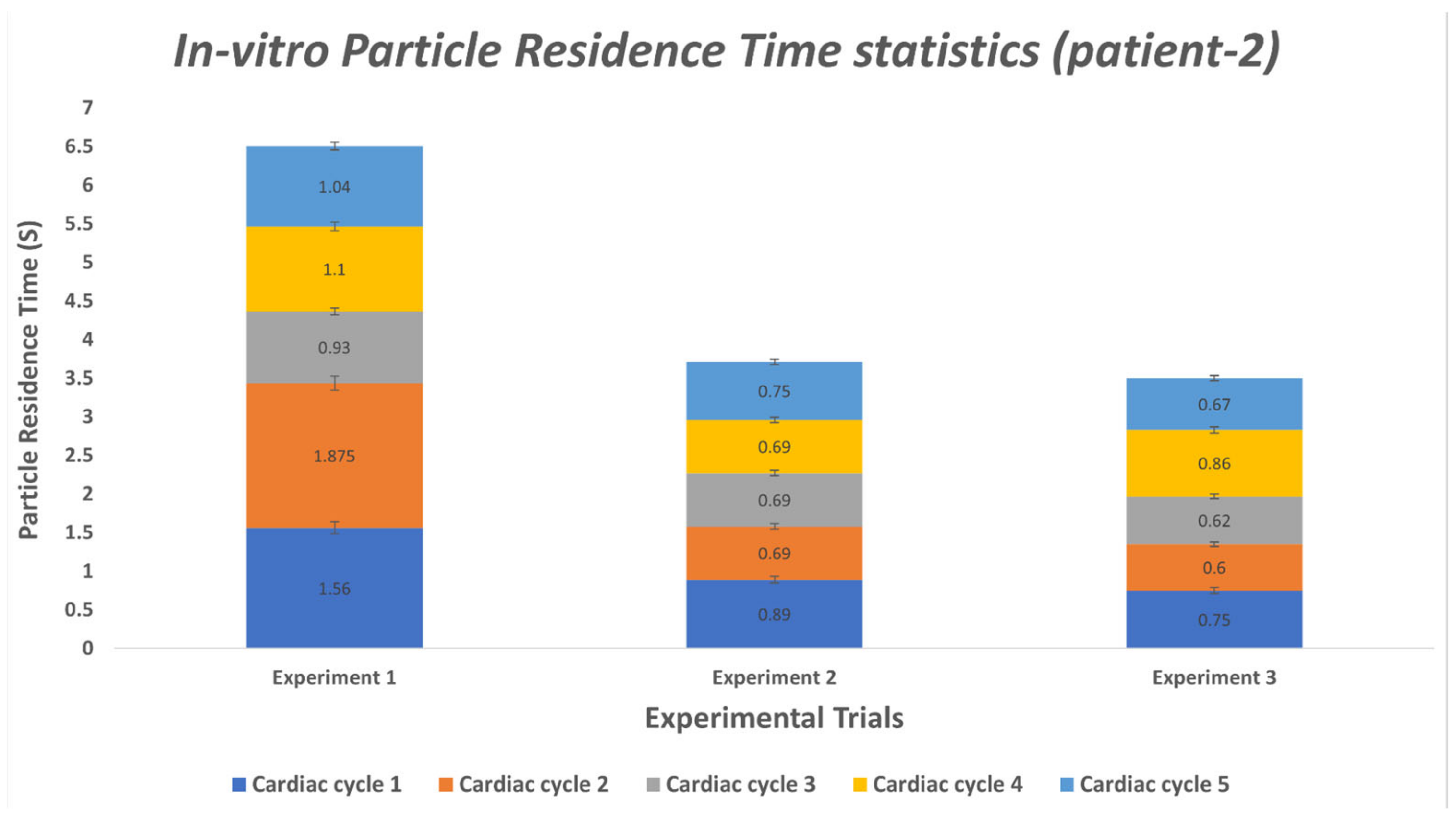
3.2. Particle Residence Time (PRT)
3.2.1. In-silico Study
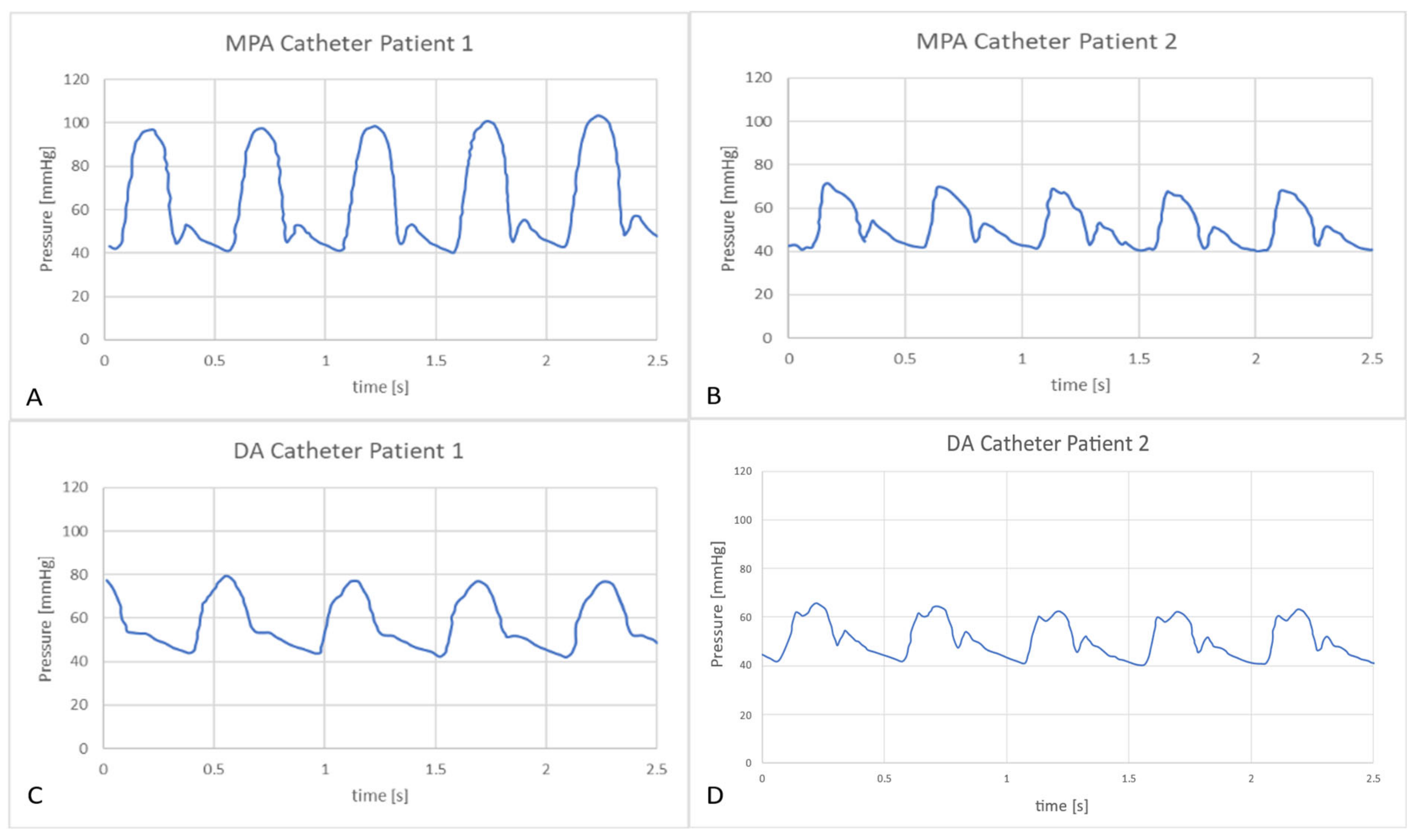
3.2.2. In-vitro Study
- MPA to proximal of the baffle
- Posterior of the baffle.
3.3. Power Loss
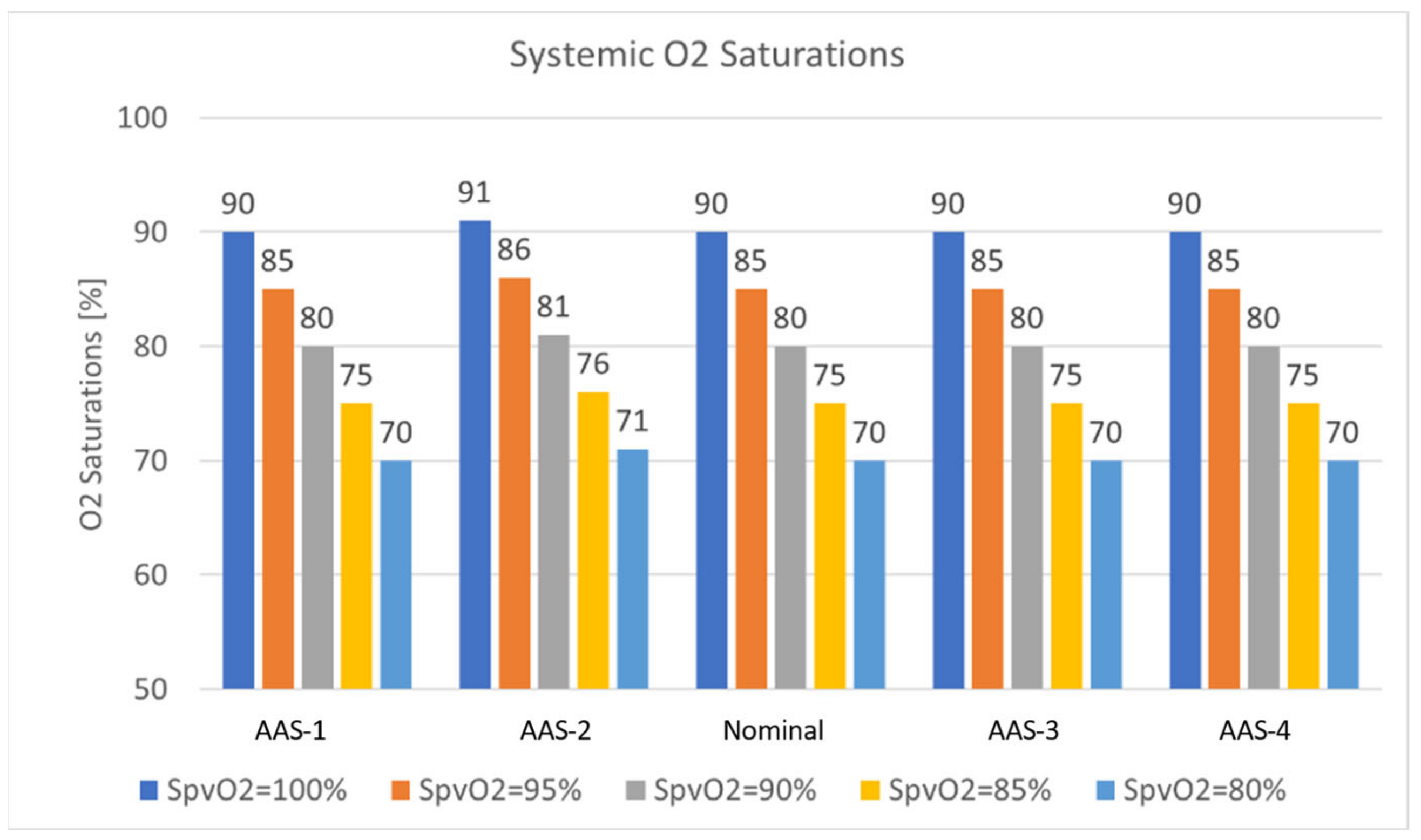
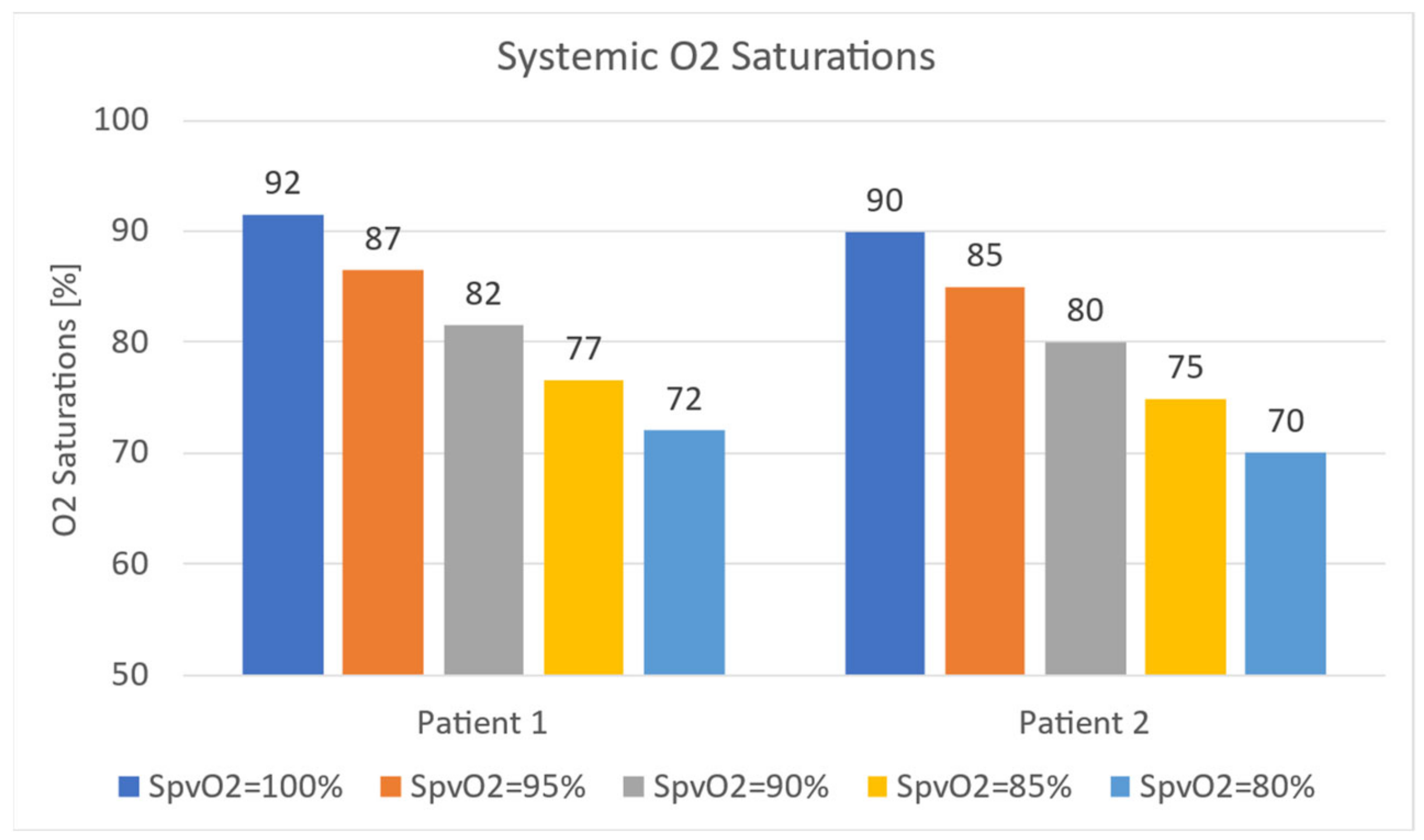
3.4. Oxygen Transport
3.4.1. Effect of Ascending Aorta
3.4.2. Effect of MPA
- The oxygen transport is a function of systemic flow distribution ratio and changes significantly as the systemic flow distribution ratio changes.
- The MPA narrowing, as previously reported, does not cause large pressure gradients, significantly altering flow distribution.
3.4.3. In-Vitro Study
4. Conclusions
- Seeming disturbed flow (MPA to proximal of the baffle)
- Organized flow (Posterior of the baffle to DA)
5. Future Works
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Single ventricle | SV |
| Hybrid comprehensive stage II | HCSII |
| Computational Fluid Dynamics | CFD |
| Mock flow loop | MFL |
| Main pulmonary artery | MPA |
| Congenital Heart Defect | CHD |
| Blalock Taussig Thomas | BTT |
| Bidirectional Glenn | BDG |
| Superior vena cava | SVC |
| Right Atrium | RA |
| Inferior vena cava | IVC |
| Right Ventricle | RV |
| Cardiopulmonary bypass | CPB |
| Left pulmonary artery | LPA |
| Right pulmonary artery | RPA |
| Atrial septal defect | ASD |
| Damus-Kaye-Stansel | DKS |
| Ventricular septal defect | VSD |
| Lumped parameter model | LPM |
| Palmaz Genesis | PG |
| Ascending aorta | AA |
| Descending aorta | DA |
| Main pulmonary artery | MPA |
| Right subclavian arteries | RSA |
| Left subclavian arteries | LSA |
| Right carotid arteries | RCA |
| Left carotid arteries | LCA |
| Right coronary arteries | RcorA |
| Left coronary arteries | LcorA |
| Arnold Palmer Hospital | APH |
| MPA narrowing | H |
| Ascending aorta diameter | DAA |
| Body surface area | BSA |
| Pulmonary Artery | PA |
| Expanded polytetrafluoroethylene | ePTFE |
| Resistors | R |
| Capacitors | C |
| Inductors | L |
| Boundary condition | BC |
| Elastance function | Entn |
| Cardiac Output | CO |
| Courant number | Co |
| Upper Systemic | SL |
| Lower Systemic | SU |
| Power | P |
| Flow rate | Q |
| Power Loss | PL |
| Particle residence time | PRT |
| Beats per minute | BPM |
| World Coordinate | WC |
| Camera coordinate | Cam |
| Rotation vectors | R |
| Translation vectors | t |
| High-speed | HS |
| MFL Setup | Resistor | Compliance | Flow Sensor | Pressure Sensor |
|---|---|---|---|---|
| 3D phantom of HCSII anatomy | RMPA | - | C.O. | PMPA |
| RASC-AORTA | - | - | PASC-AORTA | |
| - | - | - | PDESC-AORTA | |
| Upper Systemic LPM compartment | RUPPER SYS | CUPPER SYS | QUPPER SYS | PUPPER SYS |
| Lower Systemic LPM compartment | RLOWER SYS | CLOWER SYS | QLOWER SYS | PLOWER SYS |
| Left Pulmonary LPM compartment | RLPA | CLPA | QLPA | PLPA |
| Right Pulmonary LPM compartment | RRPA | CRPA | QRPA | PRPA |
Appendix B
References
- Fixler, D.E.; Nembhard, W.N.; Salemi, J.L.; Ethen, M.K.; Canfield, M.A. Mortality in First 5 Years in Infants With Functional Single Ventricle Born in Texas, 1996 to 2003. Circulation 2010, 121, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, J.A.; Benson, D.W.; Dubin, A.M.; Cohen, M.S.; Maxey, D.M.; Mahle, W.T.; Pahl, E.; Villafañe, J.; Bhatt, A.B.; Peng, L.F.; et al. Hypoplastic Left Heart Syndrome. J. Am. Coll. Cardiol. 2012, 59, S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Pekkan, K.; Dasi, L.P.; de Zélicourt, D.; Sundareswaran, K.S.; Fogel, M.A.; Kanter, K.R.; Yoganathan, A.P. Hemodynamic Performance of Stage-2 Univentricular Reconstruction: Glenn vs. Hemi-Fontan Templates. Ann. Biomed. Eng. 2009, 37, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Malec, E.; Januszewska, K.; Kołcz, J.; Pająk, J. Factors Influencing Early Outcome of Norwood Procedure for Hypoplastic Left Heart Syndrome✩. Eur. J. Cardio-Thoracic Surg. 2000, 18, 202–206. [Google Scholar] [CrossRef]
- Razzouk, A.J.; Chinnock, R.E.; Gundry, S.R.; Bailey, L.L. Cardiac Transplantation for Infants with Hypoplastic Left Heart Syndrome. Prog. Pediatr. Cardiol. 1996, 5, 37–47. [Google Scholar] [CrossRef]
- Schidlow, D.N.; Anderson, J.B.; Klitzner, T.S.; Beekman III, R.H.; Jenkins, K.J.; Kugler, J.D.; Martin, G.R.; Neish, S.R.; Rosenthal, G.L.; Lannon, C. Variation in Interstage Outpatient Care after the Norwood Procedure: A Report from the Joint Council on Congenital Heart Disease National Quality Improvement Collaborative. Congenit. Heart Dis. 2011, 6, 98–107. [Google Scholar] [CrossRef]
- Gaynor, J.W.; Mahle, W.T.; Cohen, M.I.; Ittenbach, R.F.; DeCampli, W.M.; Steven, J.M.; Nicolson, S.C.; Spray, T.L. Risk Factors for Mortality after the Norwood Procedure. Eur. J. Cardio-Thoracic Surg. 2002, 22, 82–89. [Google Scholar] [CrossRef]
- Wilder, T.J.; McCrindle, B.W.; Phillips, A.B.; Blackstone, E.H.; Rajeswaran, J.; Williams, W.G.; DeCampli, W.M.; Jacobs, J.P.; Jacobs, M.L.; Karamlou, T.; et al. Survival and Right Ventricular Performance for Matched Children after Stage-1 Norwood: Modified Blalock-Taussig Shunt versus Right-Ventricle-to-Pulmonary-Artery Conduit. J. Thorac. Cardiovasc. Surg. 2015, 150, 1440–1452. [Google Scholar] [CrossRef]
- Honjo, O.; Caldarone, C.A. Hybrid Palliation for Neonates With Hypoplastic Left Heart Syndrome: Current Strategies and Outcomes. Korean Circ. J. 2010, 40, 103–111. [Google Scholar] [CrossRef]
- Fontan, F.; Baudet, E. Surgical Repair of Tricuspid Atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef]
- De Oliveira, N.C.; Ashburn, D.A.; Khalid, F.; Burkhart, H.M.; Adatia, I.T.; Holtby, H.M.; Williams, W.G.; Van Arsdell, G.S. Prevention of Early Sudden Circulatory Collapse After the Norwood Operation. Circulation 2004, 110, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Stasik, C.N.; Goldberg, C.S.; Bove, E.L.; Devaney, E.J.; Ohye, R.G. Current Outcomes and Risk Factors for the Norwood Procedure. J. Thorac. Cardiovasc. Surg. 2006, 131, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.L.; Wren, C.; Watterson, K.G.; Hunter, S.; Hamilton, J.R.L. Stenting of the Arterial Duct Combined with Banding of the Pulmonary Arteries and Atrial Septectomy or Septostomy: A New Approach to Palliation for the Hypoplastic Left Heart Syndrome. Heart 1993, 69, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Akintuerk, H.; Michel-Behnke, I.; Valeske, K.; Mueller, M.; Thul, J.; Bauer, J.; Hagel, K.-J.; Kreuder, J.; Vogt, P.; Schranz, D. Stenting of the Arterial Duct and Banding of the Pulmonary Arteries. Circulation 2002, 105, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Galantowicz, M.; Cheatham, J.P. Lessons Learned from the Development of a New Hybrid Strategy for the Management of Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2005, 26, 307. [Google Scholar] [CrossRef]
- Kern, J.H.; Hayes, C.J.; Michler, R.E.; Gersony, W.M.; Quaegebeur, J.M. Survival and Risk Factor Analysis for the Norwood Procedure for Hypoplastic Left Heart Syndrome. Am. J. Cardiol. 1997, 80, 170–174. [Google Scholar] [CrossRef]
- Cheatham, S.L.; Deyo, G.M. Understanding the Hybrid Stage I Approach for Hypoplastic Left Heart Syndrome. Crit. Care Nurse 2016, 36, 48–55. [Google Scholar] [CrossRef]
- Galantowicz, M.; Cheatham, J.P.; Phillips, A.; Cua, C.L.; Hoffman, T.M.; Hill, S.L.; Rodeman, R. Hybrid Approach for Hypoplastic Left Heart Syndrome: Intermediate Results After the Learning Curve. Ann. Thorac. Surg. 2008, 85, 2063–2071. [Google Scholar] [CrossRef]
- Bacha, E.A.; Daves, S.; Hardin, J.; Abdulla, R.; Anderson, J.; Kahana, M.; Koenig, P.; Mora, B.N.; Gulecyuz, M.; Starr, J.P.; et al. Single-Ventricle Palliation for High-Risk Neonates: The Emergence of an Alternative Hybrid Stage I Strategy. J. Thorac. Cardiovasc. Surg. 2006, 131, 163–171. [Google Scholar] [CrossRef]
- Gil-Jaurena, J.-M.; Zunzunegui, J.-L.; Pérez-Caballero, R.; Pita, A.; Pardo, C.; Calle, C.; Murgoitio, U.; Ballesteros, F.; Rodríguez, A.; Medrano, C. Hybrid Procedures. Opening Doors for Surgeon and Cardiologist Close Collaboration. Front. Pediatr. 2021, 9, 687909. [Google Scholar] [CrossRef]
- DeCampli, W.M.; Fleishman, C.E.; Nykanen, D.G. Hybrid Approach to the Comprehensive Stage II Operation in a Subset of Single-Ventricle Variants. J. Thorac. Cardiovasc. Surg. 2015, 149, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Farias, M.; Fleishman, C.E.; Nykanen, D.; DeCampli, W.M. Clinical Update on the Hybrid Comprehensive Stage II Operation. JTCVS Open 2021, 7, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Prather, R.; Divo, E.; Kassab, A.; Nykanen, D.; Farias, M.; DeCampli, W.M. Computational Fluid Dynamics Investigation of the Novel Hybrid Comprehensive Stage II Operation. JTCVS Open 2021, 7, 308–323. [Google Scholar] [CrossRef]
- Bove, E.L.; de Leval, M.R.; Migliavacca, F.; Guadagni, G.; Dubini, G. Computational Fluid Dynamics in the Evaluation of Hemodynamic Performance of Cavopulmonary Connections after the Norwood Procedure for Hypoplastic Left Heart Syndrome. J. Thorac. Cardiovasc. Surg. 2003, 126, 1040–1047. [Google Scholar] [CrossRef]
- De Leval, M.R.; Dubini, G.; Jalali, H.; Pietrabissa, R. Use of Computational Fluid Dynamics in the Design of Surgical Procedures: Application to the Study of Competitive Flows in Cavopulmonary Connections. J. Thorac. Cardiovasc. Surg. 1996, 111, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Waters, H.; Prather, R.O.; Divo, E.; Kassab, A.J.; Nunez, A.; DeCampli, W.M. Computational Investigation of Magneto-Hydrodynamic Assist Device for Actively Powered Fontan Circulations. In Proceedings of the 5–6th Thermal and Fluids Engineering Conference (TFEC), Virtual, 26–28 May 2021; Begellhouse: Danbury, CT, USA, 2021; pp. 583–594. [Google Scholar]
- Hsia, T.-Y.; Migliavacca, F.; Pittaccio, S.; Radaelli, A.; Dubini, G.; Pennati, G.; De Leval, M. Computational Fluid Dynamic Study of Flow Optimization in Realistic Models of the Total Cavopulmonary Connections. J. Surg. Res. 2004, 116, 305–313. [Google Scholar] [CrossRef]
- Migliavacca, F.; Pennati, G.; Dubini, G.; Fumero, R.; Pietrabissa, R.; Urcelay, G.; Bove, E.L.; Hsia, T.Y.; de Leval, M.R. Modeling of the Norwood Circulation: Effects of Shunt Size, Vascular Resistances, and Heart Rate. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2076–H2086. [Google Scholar] [CrossRef]
- Hameed, M. Computational Fluid Dynamics Investigation of a Novel Hybrid Comprehensive Stage II Operation for Single Ventricle Palliation. Ph.D. Thesis, University of Central Florida, Orlando, FL, USA, 2019. [Google Scholar]
- Prather, R.; Das, A.; Farias, M.; Divo, E.; Kassab, A.; DeCampli, W. Parametric Investigation of an Injection-Jet Self-Powered Fontan Circulation. Sci. Rep. 2022, 12, 2161. [Google Scholar] [CrossRef]
- Das, A.; Prather, R.; Divo, E.; Farias, M.; Kassab, A.; DeCampli, W. In-Vitro Validation of Self-Powered Fontan Circulation for Treatment of Single Ventricle Anomaly. Fluids 2021, 6, 401. [Google Scholar] [CrossRef]
- Das, A. In-Vitro and In-Silico Investigations of Alternative Surgical Techniques for Single Ventricular Disease. Ph.D. Thesis, Embry-Riddle Aeronautical University, Daytona Beach, FL, USA, 2021. [Google Scholar]
- Ni, M.W.; Prather, R.O.; Rodriguez, G.; Quinn, R.; Divo, E.; Fogel, M.; Kassab, A.J.; DeCampli, W.M. Computational Investigation of a Self-Powered Fontan Circulation. Cardiovasc. Eng. Technol. 2018, 9, 202–216. [Google Scholar] [CrossRef]
- Prather, R.O.; Kassab, A.; Ni, M.W.; Divo, E.; Argueta-Morales, R.; DeCampli, W.M. Multi-Scale Pulsatile CFD Modeling of Thrombus Transport in a Patient-Specific LVAD Implantation. Int. J. Numer. Methods Heat Fluid Flow 2017, 27, 1022–1039. [Google Scholar] [CrossRef]
- Wang, C.; Pekkan, K.; de Zélicourt, D.; Horner, M.; Parihar, A.; Kulkarni, A.; Yoganathan, A.P. Progress in the CFD Modeling of Flow Instabilities in Anatomical Total Cavopulmonary Connections. Ann. Biomed. Eng. 2007, 35, 1840–1856. [Google Scholar] [CrossRef] [PubMed]
- Bove, E.L.; Migliavacca, F.; de Leval, M.R.; Balossino, R.; Pennati, G.; Lloyd, T.R.; Khambadkone, S.; Hsia, T.-Y.; Dubini, G. Use of Mathematic Modeling to Compare and Predict Hemodynamic Effects of the Modified Blalock–Taussig and Right Ventricle–Pulmonary Artery Shunts for Hypoplastic Left Heart Syndrome. J. Thorac. Cardiovasc. Surg. 2008, 136, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Hsia, T.-Y.; Cosentino, D.; Corsini, C.; Pennati, G.; Dubini, G.; Migliavacca, F. Use of Mathematical Modeling to Compare and Predict Hemodynamic Effects Between Hybrid and Surgical Norwood Palliations for Hypoplastic Left Heart Syndrome. Circulation 2011, 124, S204–S210. [Google Scholar] [CrossRef] [PubMed]
- Laganà, K.; Balossino, R.; Migliavacca, F.; Pennati, G.; Bove, E.L.; de Leval, M.R.; Dubini, G. Multiscale Modeling of the Cardiovascular System: Application to the Study of Pulmonary and Coronary Perfusions in the Univentricular Circulation. J. Biomech. 2005, 38, 1129–1141. [Google Scholar] [CrossRef]
- Migliavacca, F.; Laganà, K.; Pennati, G.; de Leval, M.R.; Bove, E.L.; Dubini, G. Global mathematical modelling of the norwood circulation: A multiscale approach for the study of the pulmonary and coronary arterial perfusions. Cardiol. Young 2004, 14, 71–76. [Google Scholar] [CrossRef]
- Itatani, K.; Miyaji, K.; Tomoyasu, T.; Nakahata, Y.; Ohara, K.; Takamoto, S.; Ishii, M. Optimal Conduit Size of the Extracardiac Fontan Operation Based on Energy Loss and Flow Stagnation. Ann. Thorac. Surg. 2009, 88, 565–573. [Google Scholar] [CrossRef]
- Stergiopulos, N.; Meister, J.J.; Westerhof, N. Determinants of Stroke Volume and Systolic and Diastolic Aortic Pressure. Am. J. Physiol. Circ. Physiol. 1996, 270, H2050–H2059. [Google Scholar] [CrossRef]
- Ferreira, A.; Chen, S.; Simaan, M.A.; Boston, J.R.; Antaki, J.F. A Nonlinear State-Space Model of a Combined Cardiovascular System and a Rotary Pump. In Proceedings of the 44th IEEE Conference on Decision and Control, Seville, Spain, 15 December 2005; IEEE: Piscataway, NJ, USA, 2005; Volume 2005, pp. 897–902. [Google Scholar]
- Esmaily-Moghadam, M.; Hsia, T.-Y.; Marsden, A.L. The Assisted Bidirectional Glenn: A Novel Surgical Approach for First-Stage Single-Ventricle Heart Palliation. J. Thorac. Cardiovasc. Surg. 2015, 149, 699–705. [Google Scholar] [CrossRef]
- Schiavazzi, D.E.; Kung, E.O.; Marsden, A.L.; Baker, C.; Pennati, G.; Hsia, T.-Y.; Hlavacek, A.; Dorfman, A.L. Hemodynamic Effects of Left Pulmonary Artery Stenosis after Superior Cavopulmonary Connection: A Patient-Specific Multiscale Modeling Study. J. Thorac. Cardiovasc. Surg. 2015, 149, 689–696. [Google Scholar] [CrossRef]
- Sankaran, S.; Esmaily Moghadam, M.; Kahn, A.M.; Tseng, E.E.; Guccione, J.M.; Marsden, A.L. Patient-Specific Multiscale Modeling of Blood Flow for Coronary Artery Bypass Graft Surgery. Ann. Biomed. Eng. 2012, 40, 2228–2242. [Google Scholar] [CrossRef]
- Esmaily Moghadam, M.; Vignon-Clementel, I.E.; Figliola, R.; Marsden, A.L. A Modular Numerical Method for Implicit 0D/3D Coupling in Cardiovascular Finite Element Simulations. J. Comput. Phys. 2013, 244, 63–79. [Google Scholar] [CrossRef]
- Ceballos, A.; Argueta-Morales, I.R.; Divo, E.; Osorio, R.; Caldarone, C.A.; Kassab, A.J.; DeCampli, W.M. Computational Analysis of Hybrid Norwood Circulation with Distal Aortic Arch Obstruction and Reverse Blalock-Taussig Shunt. Ann. Thorac. Surg. 2012, 94, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, A.; Prather, R.; Divo, E.; Kassab, A.; DeCampli, W. Patient-Specific Multi-Scale Model Analysis of Hemodynamics Following the Hybrid Norwood Procedure for Hypoplastic Left Heart Syndrome: Effects of Reverse Blalock–Taussig Shunt Diameter. Cardiovasc. Eng. Technol. 2019, 10, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Good, B.C.; Deutsch, S.; Manning, K.B. Hemodynamics in a Pediatric Ascending Aorta Using a Viscoelastic Pediatric Blood Model. Ann. Biomed. Eng. 2016, 44, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Santamore, W.P.; Barnea, O.; Riordan, C.J.; Ross, M.P.; Austin, E.H. Theoretical Optimization of Pulmonary-to-Systemic Flow Ratio after a Bidirectional Cavopulmonary Anastomosis. Am. J. Physiol. 1998, 274, H694–H700. [Google Scholar] [CrossRef] [PubMed]
- Pekkan, K.; de Zélicourt, D.; Ge, L.; Sotiropoulos, F.; Frakes, D.; Fogel, M.A.; Yoganathan, A.P. Physics-Driven CFD Modeling of Complex Anatomical Cardiovascular Flows-a TCPC Case Study. Ann. Biomed. Eng. 2005, 33, 284–300. [Google Scholar] [CrossRef]
- DeGroff, C.; Birnbaum, B.; Shandas, R.; Orlando, W.; Hertzberg, J. Computational Simulations of the Total Cavo-Pulmonary Connection: Insights in Optimizing Numerical Solutions. Med. Eng. Phys. 2005, 27, 135–146. [Google Scholar] [CrossRef]
- Reininger, A.J.; Reininger, C.B.; Heinzmann, U.; Wurzinger, L.J. Residence Time in Niches of Stagnant Flow Determines Fibrin Clot Formation in an Arterial Branching Model—Detailed Flow Analysis and Experimental Results. Thromb. Haemost. 1995, 74, 916–922. [Google Scholar] [CrossRef]
- Bluestein, D.; Niu, L.; Schoephoerster, R.T.; Dewanjee, M.K. Fluid Mechanics of Arterial Stenosis: Relationship to the Development of Mural Thrombus. Ann. Biomed. Eng. 1997, 25, 344–356. [Google Scholar] [CrossRef]
- Kunov, M.J.; Steinman, D.A.; Ethier, C.R. Particle Volumetric Residence Time Calculations in Arterial Geometries. J. Biomech. Eng. 1996, 118, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Kahn, A.M.; Burns, J.C.; Sankaran, S.; Shadden, S.C.; Marsden, A.L. Image-Based Modeling of Hemodynamics in Coronary Artery Aneurysms Caused by Kawasaki Disease. Biomech. Model. Mechanobiol. 2012, 11, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.-Y.; Les, A.S.; Tenforde, A.S.; Shadden, S.C.; Spilker, R.L.; Yeung, J.J.; Cheng, C.P.; Herfkens, R.J.; Dalman, R.L.; Taylor, C.A. Quantification of Particle Residence Time in Abdominal Aortic Aneurysms Using Magnetic Resonance Imaging and Computational Fluid Dynamics. Ann. Biomed. Eng. 2011, 39, 864–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. A Flexible New Technique for Camera Calibration. IEEE Trans. Pattern Anal. Mach. Intell. 2000, 22, 1330–1334. [Google Scholar] [CrossRef]
- Heikkila, J.; Silven, O. A Four-Step Camera Calibration Procedure with Implicit Image Correction. In Proceedings of the 1997 IEEE Computer Society Conference on Computer Vision and Pattern Recognition, San Juan, PR, USA, 17–19 June 1997; pp. 1106–1112. [Google Scholar]
- Shih, S.-W.; Hung, Y.-P.; Lin, W.-S. Accurate Linear Technique for Camera Calibration Considering Lens Distortion by Solving an Eigenvalue Problem. Opt. Eng. 1993, 32, 138–149. [Google Scholar] [CrossRef]
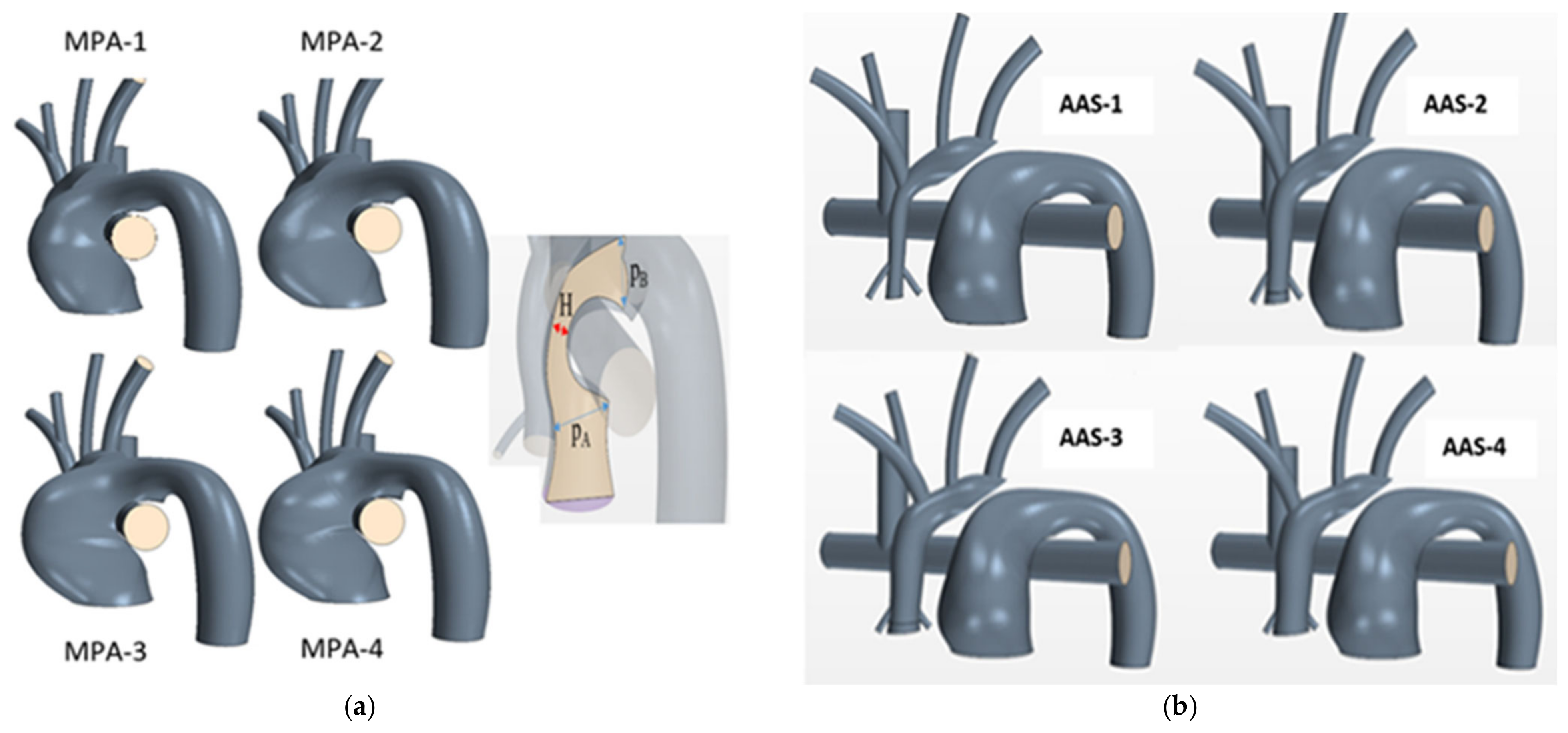







| Case | H [mm] | Case | |
|---|---|---|---|
| MPA-1 | 7.23 | AAS-1 | 5.00 |
| MPA-2 | 8.19 | AAS-2 | 6.00 |
| Nominal | 8.66 | Nominal | 7.00 |
| MPA-3 | 9.09 | AAS-3 | 7.50 |
| MPA-4 | 9.52 | AAS-4 | 8.50 |
| CFD Region | Boundary Condition |
|---|---|
| MPA | Stagnation Pressure Inlet |
| AA | Stagnation Pressure Inlet |
| DA | Mass flow outlet |
| SVC | Mass flow outlet |
| LPA | Mass flow outlet |
| RPA | Mass flow outlet |
| LcorA | Mass flow outlet |
| RcorA | Mass flow outlet |
| LSA | Mass flow outlet |
| RSA | Mass flow outlet |
| LCA | Mass flow outlet |
| RCA | Mass flow outlet |
| Compliance Sections in MFL | Compliance Value (ml/mmHg) |
|---|---|
| C_upper | 0.603 |
| C_lower | 0.735 |
| C_lpa | 0.640 |
| C_rpa | 0.640 |
| Hematocrit [%] | |||
|---|---|---|---|
| 40 | 4.38989 | 8.4248 | 0.3103 |
| Calibration Set | No. of Cells | Cell Dimension (in World Units) | Pattern Type | Mean Projection Error (in Pixel) |
|---|---|---|---|---|
| Calibration Set-1 | 5 × 6 | 7 mm × 7 mm | Binary | 2.40 |
| Calibration Set-2 | 5 × 6 | 7 mm × 7 mm | Color | 2.26 |
| Calibration Set-3 | 8 × 8 | 17 mm × 17 mm | Binary | 10.46 |
| MFL Compartment | Catheter | In-vitro | In-silico |
|---|---|---|---|
| CO [L/min] | 2.99 | 3.15 | 2.99 |
| [L/min] | 1.25 | 1.29 | 1.19 |
| [L/min] | 1.74 | 1.86 | 1.80 |
| [L/min] | 0.63 | 0.64 | 0.60 |
| [L/min] | 0.63 | 0.64 | 0.60 |
| /CO [L/min] | 0.42 | 0.40 | 0.40 |
| MFL Compartment | Catheter | In-vitro | In-silico |
|---|---|---|---|
| MPA [mmHg] | 65 | 62 | 66 |
| AA [mmHg] | 65 | 65 | 65 |
| DA [mmHg] | 58 | 59 | 54 |
| SVC [mmHg] | 9 | 9 | 9 |
| RPA [mmHg] | 9 | 9 | 9 |
| LPA [mmHg] | 8 | 8 | 8 |
| MFL Compartment | Catheter | In-vitro |
|---|---|---|
| CO [L/min] | 1.76 | 1.81 |
| [L/min] | 1.00 | 1.07 |
| [L/min] | 0.76 | 0.74 |
| [L/min] | 0.50 | 0.52 |
| [L/min] | 0.50 | 0.52 |
| /CO [L/min] | 0.57 | 0.59 |
| MFL Compartment | Catheter | In-vitro |
|---|---|---|
| MPA [mmHg] | 52 | 50 |
| AA [mmHg] | 48 | 49 |
| DA [mmHg] | 50 | 49 |
| SVC [mmHg] | 15 | 14 |
| RPA [mmHg] | 9 | 9 |
| LPA [mmHg] | 8 | 9 |
| Hemodynamic Variables | Statistical Analysis | Patient 1 | Patient 2 |
|---|---|---|---|
| Flow rate [L/min] | Pearson Correlation | 0.976 | 0.916 |
| P(T < t) Two tail | 0.854 | 0.170 | |
| Pressure [mmHg] | Pearson Correlation | 0.998 | 0.998 |
| P(T < t) Two tail | 0.675 | 0.713 |
| Hemodynamic Variables | Statistical Analysis | Patient 1 |
|---|---|---|
| Flow rate [L/min] | Pearson Correlation | 0.999 |
| P(T < t) Two tail | 0.657 | |
| Pressure [mmHg] | Pearson Correlation | 0.995 |
| P(T < t) Two tail | 0.872 |
| Cardiac Cycle | Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | Experiment 5 |
|---|---|---|---|---|---|
| 1 | 0.96 | 3.26 | 0.75 | 0.98 | 0.89 |
| 2 | 0.89 | 0.97 | 0.60 | 0.67 | 1 |
| 3 | 0.69 | 0.73 | 0.62 | 0.65 | 0.75 |
| 4 | 0.71 | 0.72 | 0.86 | 0.66 | 0.65 |
| 5 | 0.83 | 0.80 | 0.67 | 0.61 | 0.67 |
| 6 | 0.58 | 0.71 | 2.43 | 0.91 | 0.67 |
| Cardiac Cycle | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|
| 1 | 1.56 | 0.89 | 0.75 |
| 2 | 1.875 | 0.69 | 0.60 |
| 3 | 0.93 | 0.69 | 0.62 |
| 4 | 1.10 | 0.69 | 0.86 |
| 5 | 1.04 | 0.75 | 0.67 |
| Split Ratio | MPA Power [mW] | DA Power [mW] | PL [mW] |
|---|---|---|---|
| 50/50 | 0.338 | 0.307 | 10.33 |
| 60/40 | 0.414 | 0.376 | 12.66 |
| Case | H [mm] | PA [mW] | DA [mW] | PL [mW] | Ƞ |
|---|---|---|---|---|---|
| No baffle | N/A | 0.416 | 0.38 | 10.33 | 0.97 |
| MPA-1 | 7.23 | 0.418 | 0.381 | 12.66 | 0.94 |
| MPA-2 | 8.19 | 0.417 | 0.379 | 12.66 | 0.94 |
| Nominal | 8.66 | 0.413 | 0.375 | 12.33 | 0.97 |
| MPA-3 | 9.09 | 0.411 | 0.374 | 12.33 | 0.97 |
| MPA-4 | 9.52 | 0.407 | 0.37 | 12.33 | 0.97 |
| Case | CO [L/min] | DA [L/min] | LCA [L/min] | LSA [L/min] | LcorA [L/min] | RCA [L/min] | RSA [L/min] | RcorA [L/min] |
|---|---|---|---|---|---|---|---|---|
| AAS-1 | 2.94 | 1.854 | 0.221 | 0.329 | 0.048 | 0.255 | 0.152 | 0.077 |
| AAS-2 | 2.97 | 1.826 | 0.131 | 0.253 | 0.049 | 0.383 | 0.251 | 0.073 |
| Nominal | 2.93 | 1.835 | 0.204 | 0.301 | 0.048 | 0.278 | 0.187 | 0.075 |
| AAS-3 | 2.92 | 1.840 | 0.220 | 0.323 | 0.048 | 0.249 | 0.157 | 0.078 |
| AAS-4 | 2.93 | 1.839 | 0.213 | 0.331 | 0.048 | 0.267 | 0.152 | 0.077 |
| Case | CO [L/min] | DA [L/min] | LCA [L/min] | LSA [L/min] | LcorA [L/min] | RCA [L/min] | RSA [L/min] | RcorA [L/min] |
|---|---|---|---|---|---|---|---|---|
| MPA-1 | 2.94 | 1.854 | 0.220 | 0.329 | 0.048 | 0.255 | 0.152 | 0.077 |
| MPA-2 | 2.92 | 1.868 | 0.214 | 0.326 | 0.048 | 0.244 | 0.138 | 0.079 |
| Nominal | 2.93 | 1.839 | 0.213 | 0.331 | 0.048 | 0.267 | 0.152 | 0.077 |
| MPA-3 | 2.91 | 1.824 | 0.218 | 0.328 | 0.048 | 0.256 | 0.155 | 0.078 |
| MPA-4 | 2.89 | 1.812 | 0.219 | 0.323 | 0.049 | 0.256 | 0.149 | 0.0809 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.; Hameed, M.; Prather, R.; Farias, M.; Divo, E.; Kassab, A.; Nykanen, D.; DeCampli, W. In-Silico and In-Vitro Analysis of the Novel Hybrid Comprehensive Stage II Operation for Single Ventricle Circulation. Bioengineering 2023, 10, 135. https://doi.org/10.3390/bioengineering10020135
Das A, Hameed M, Prather R, Farias M, Divo E, Kassab A, Nykanen D, DeCampli W. In-Silico and In-Vitro Analysis of the Novel Hybrid Comprehensive Stage II Operation for Single Ventricle Circulation. Bioengineering. 2023; 10(2):135. https://doi.org/10.3390/bioengineering10020135
Chicago/Turabian StyleDas, Arka, Marwan Hameed, Ray Prather, Michael Farias, Eduardo Divo, Alain Kassab, David Nykanen, and William DeCampli. 2023. "In-Silico and In-Vitro Analysis of the Novel Hybrid Comprehensive Stage II Operation for Single Ventricle Circulation" Bioengineering 10, no. 2: 135. https://doi.org/10.3390/bioengineering10020135
APA StyleDas, A., Hameed, M., Prather, R., Farias, M., Divo, E., Kassab, A., Nykanen, D., & DeCampli, W. (2023). In-Silico and In-Vitro Analysis of the Novel Hybrid Comprehensive Stage II Operation for Single Ventricle Circulation. Bioengineering, 10(2), 135. https://doi.org/10.3390/bioengineering10020135







