Abstract
Extracellular vesicles (EVs) are small membrane-bound vesicles secreted into the extracellular space by all cell types. EVs transfer their cargo which includes nucleic acids, proteins, and lipids to facilitate cell-to-cell communication. As EVs are released and move from parent to recipient cell, EVs interact with the extracellular matrix (ECM) which acts as a physical scaffold for the organization and function of cells. Recent work has shown that EVs can modulate and act as regulators of the ECM. This review will first discuss EV biogenesis and the mechanism by which EVs are transported through the ECM. Additionally, we discuss how EVs contribute as structural components of the matrix and as components that aid in the degradation of the ECM. Lastly, the role of EVs in influencing recipient cells to remodel the ECM in both pathological and therapeutic contexts is examined.
1. Introduction
Extracellular vesicles (EVs) are released into the extracellular environment by all cell types and function as mediators of cell-to-cell communication [1]. EVs contain cargo including lipids, nucleic acids, and proteins that are reflective of their parent cell phenotype [2]. As such, there has been significant interest in utilizing EVs derived from healthy cell sources as endogenous nanotherapeutics [3,4] and applying EVs to enhance angiogenesis, inhibit fibrosis and apoptosis, regulate the immune microenvironment, and modulate the extracellular matrix (ECM) in the context of promoting tissue regeneration [5,6,7,8,9].
When EVs are released from parent cells into the extracellular space, they may first interact with the surrounding ECM. The ECM is the physical scaffolding for cellular components of tissues and actively participates in cell growth, movement, and differentiation. The major components of the ECM include collagens, laminins, fibronectin, and proteoglycans that are organized into a physically crosslinked network via non-covalent interactions. The ECM interacts with cells through cell adhesion molecules (e.g., integrins, cadherins, and other transmembrane proteoglycans) which enable cells to migrate across the matrix. Importantly, cells can modify the matrix by depositing ECM components or degrading the ECM by secreting matrix-degrading enzymes such as matrix metalloproteases (MMPs). The mechanical and physical properties of the ECM have been demonstrated to be integral to the differentiation, migration, and maintenance of cells within the ECM. These proprieties include (1) mesh size, which is the distance between two crosslinks within a matrix, (2) stiffness, which is the extent to which the matrix resists deformation when stress is applied, and (3) the viscoelastic behavior, or the ability of the matrix to exhibit both viscous and elastic characteristics upon deformation [10]. Together, the components of the ECM and the mechanical characteristics of the matrix are integral to the health and function of the resident cells [11,12,13,14]. As such, EVs released by both healthy and pathological cells can act as mediators of the ECM either through direct EV–ECM interactions or by influencing cell–ECM interactions.
For the receiving cell, EVs also transport through the ECM of the target tissue to reach the recipient cells and deliver their cargo to impart a cellular response. Transport of EVs through the ECM can be a passive, diffusive process dependent on the stiffness and viscoelastic characteristics of the ECM and the deformability of the EV itself [15,16]. EVs can become structural components of the matrix, either as initiators of calcification or as bioactive signaling agents anchored to the matrix, or can modulate the ECM indirectly by inducing recipient cells to promote synthesis or degradation of the ECM [17,18,19,20,21,22].
Given its unstudied but significant role, this review will discuss both the direct and indirect influence of EVs on the ECM. First, the biogenesis and characteristics of EVs and their transport through the ECM will be delineated. Additionally, the role of EVs in modulating the ECM in cancer progression, as structural components during bone/endochondral and vascular calcification, and as ECM-bound bioactive signaling agents will be addressed. Furthermore, how EVs also indirectly modulate the ECM will be explored in both pathological and therapeutic contexts.
2. EV Biology
2.1. EV Biogenesis
EV biogenesis includes vesicle formation, cargo loading, and secretion. Exosome (50–200 nm) biogenesis begins with an early sorting endosome which then matures into a late sorting endosome and finally into a multivesicular body (MVB, Figure 1A). MVB formation is marked by the invagination of the late endosomal membrane forming intraluminal vesicles (ILVs) within the MVB. ILVs are released into the extracellular environment when the MVB merges with the cell membrane and once excreted, they are referred to as exosomes [23]. Exosomes are commonly identified by expression of the membrane-bound tetraspanin marker CD63 and syntenin-1 [24]. Exosome cargo loading is regulated by the endosomal sorting complex required for the transport (ESCRT) pathway, which is comprised of four protein complexes denoted ESCRT-0 through ESCRT-III. ESCRT-0 recruits ubiquitinated protein to the endosomal membrane after which ESCRT-I clusters the cargo and complexes with ESCRT-II [25]. The protein cargo is then sequestered into the endosome and ESCRT-II initiates ILV invagination. Lastly, ESCRT-III de-ubiquinates the protein cargo and finalizes vesicle budding to form ILVs. Additionally, RAB GTPases are associated with exosome formation and support exosome secretion by directing MVBs to the plasma membrane for fusion [26].
Beyond exosomes, microvesicles range in size from 50 to 1000 nm and are released by blebbing of the plasma membrane. Thus, they express markers of the originating cell plasma membrane as well as tetraspanins CD9 and CD81 [27,28]. In addition to surface markers, exosome and microvesicle membranes are comprised of cholesterols, sphingomyelins, and phospholipids [29]. Additionally, apoptotic bodies are large EVs (>1000 nm) released when cells undergo programmed cell death [30]. While apoptotic bodies participate in key processes in cell death, such as the removal of cell contents and delivering information from dying cells to phagocytic cells, the majority of EV biology research has been directed towards small EVs including exosomes and microvesicles which range from 50 to 200 nm in size.
2.2. EV Uptake
Since exosomes and microvesicles participate in cell-to-cell communication via the transfer of encapsulated messenger RNA (mRNA), microRNAs, small nuclear RNA, long non-coding RNAs, and cytosolic and membrane-associated proteins, they are often uptaken by cells to deliver their cargo [31]. Once released into the extracellular environment, multiple energy-dependent pathways have been proposed by which EVs are internalized by the target cell including clatharin-mediated endocytosis (CME), caveolin-dependent endocytosis (CDE), phagocytosis, and membrane fusion (Figure 1B) [28]. Accordingly, previous studies show that inhibition of the CME pathway by the drug chlorpromazine results in a significant decrease in EV uptake [32]. Similarly, suppressing the cavelin-1 protein, which is necessary for the CDE pathway, resulted in decreased EV endocytosis [31]. Special cases for EV uptake include phagocytic cells and within the tumor microenvironment in which EVs are uptaken primarily by phagocytosis [33]. Additionally, EV-cell membrane fusion as an entry mechanism has also been observed in acidic tumor microenvironments [34].
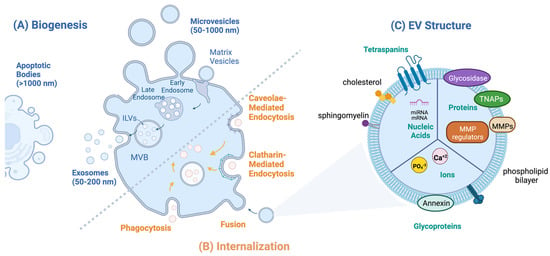
Figure 1.
EV biology: (A) Routes of EV biogenesis: exosomes are formed from multivesicular bodies (MVB); apoptotic bodies are formed from cells undergoing programmed cell death; microvesicles bud off the donor cell plasma membrane and include matrix vesicles. (B) Routes of EV internalization include caveolae-mediated endocytosis, clathrin-mediated endocytosis, micropinocytosis, and phagocytosis, which are internalized via endosomes, and EV fusion with the plasma membrane. (C) EV structure: internal cargo includes nucleic acids, proteins, and ions; surface molecules include glycoproteins, tetraspanins, matrix metalloproteases (MMPs), and TNAPs.
3. EV Transport through the ECM
In order to participate in cell-to-cell communications, EVs must traverse the ECM from the parent cell to the recipient cell. Recent studies regarding stress relaxation of the ECM by Lenzini et al. [15,16] showed that the mechanical properties of the ECM and EVs allow EV diffusion through the extracellular space, despite the larger exosome and microvesicle diameter (50–200 nm) compared to the mesh size of the ECM (~50 nm) [15]. The authors demonstrated approximately 50% of the EVs loaded within decellularized ECM of lung tissue were released after 24 h. By using crosslinked alginate hydrogels with tunable viscoelastic properties, EVs in stiff physically crosslinked alginate hydrogels were found to have significantly higher diffusion coefficients compared to both soft viscoelastic and elastic hydrogels, indicating that the ECM undergoing stress relaxation leads to increased EV diffusion through the matrix. This was true across EVs from a variety of cell sources, implying that the mechanical properties of the matrix play a role in EV transport. In addition, depleting the membrane water channel protein aquaporin-1 on the EV surface increased the stiffness of the EV and reduced the diffusion coefficient by approximately threefold. This indicates that water permeation enables EVs to become more deformable and thus, more easily able to diffuse through the matrix [35].
4. Direct Influence of EVs on the ECM
EVs have been reported to directly interact with the ECM and can contribute as both a physical and bioactive structural component of the matrix. In addition, EVs can directly associate with the ECM and actively degrade the matrix with their surface-associated enzymes [5,16,36,37,38,39]. In each case, EVs play an integral role in the evolution of the ECM in both physiological and pathological states (Table 1).
4.1. EV-Mediated Calcification
4.1.1. Bone Formation and Endochondral Calcification
Mineralization of the ECM is a physiological process during bone formation and endochondral calcification while it is pathological during vascular calcification [40]. The ECM architecture during calcification undergoes a dramatic evolution from a non-crystalline network comprised mainly of type 1 collagen fibrils to a crystalline matrix that is able to support high load and stress [41]. Matrix vesicles (MVs, 100–300 nm) are a subtype of microvesicle that are essential to the mineralization of the ECM. MVs released into the ECM from osteoblast and chondrocytes initiate the formation of hydroxyapatite (Ca10(PO4)6(OH)2) crystals and the transformation of the ECM from an entirely organic architecture to a combination of both an organic and inorganic (i.e., mineralized) structure. During the first phase of mineralization, MVs sequester Ca2+ ions through various calcium-binding molecules concentrated on the MV surface which include the calcium binding proteins annexins II, V, and IV and the membrane phospholipid phosphatidylserine (PS) (Figure 1C and Figure 2A) [42,43,44]. Simultaneously, intra- and extravesicular concentrations of PO43− ions are increased via the following: (1) MV membrane-bound tissue non-specific alkaline phosphate (TNAP), (2) the conversion of ATP to ADP via ATPases, (3) and Pit-1, a sodium-dependent phosphate transporter [44,45,46]. The resultant increased intravesicular Ca2+ and PO43− concentrations leads to precipitation of hydroxyapatite crystals within the MV.
During the second phase of mineralization, hydroxyapatite crystals become large enough to break through the MV into the ECM and continue mineralization of the ECM along the length of type 1 collagen fibrils (Figure 2A). Of note, MV-mediated calcification occurs not only during endochondral calcification during development but also in the constant cycle of bone resorption and formation during adulthood. Thus, MVs are integral in the evolution of the ECM from a viscoelastic network to a fully crystalline structure in physiological calcification processes.
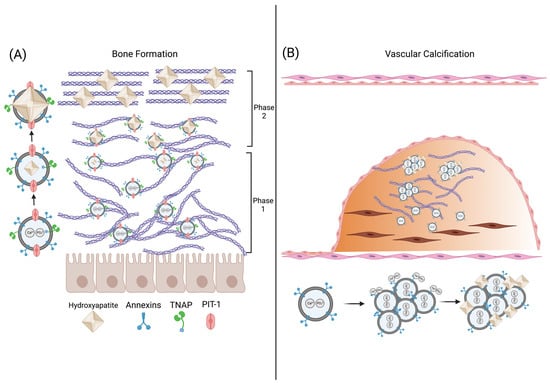
Figure 2.
EV-mediated calcification of bone and the vasculature. (A) During phase 1 of mineralization during physiological bone formation, osteoblasts release matrix vesicles (MVs) which transport calcium and phosphate ions into the vesicle forming hydroxyapatite (HA) crystals. During phase 2 of mineralization, the HA crystals penetrate the MV and orient themselves with the ECM matrix, furthering crystal growth. (B) During vascular calcification, osteogenic-VSMC release EVs that aggregate and anchor to collagen within the ECM. Hydroxyapatite nucleation occurs on the surface of the EV aggregates.
4.1.2. Vascular Calcification
Vascular calcification is the mineralization of ECM in blood vessels and is present in diseases such as atherosclerosis, diabetes mellitus, and chronic kidney disease [47]. Vascular calcification is initiated with the transdifferentiation of VSMCs into an osteoblast-like phenotype. Under physiological conditions, VSMCs release EVs containing calcification inhibitors such as Matrix Gla protein (MGP) and fetuin-A to maintain tissue homeostasis. However, under pathogenic conditions caused by chronic inflammation or aberrant mineral metabolism, osteoblast-like VSMCs release EVs resembling MVs during bone formation [48]. These EVs can destabilize the ECM of the blood vessel by calcifying distinct regions of the vessel wall, which can lead to thrombosis or rupture of the vessel [49].
The mechanism of EV-mediated vascular calcification differs from that of MV-mediated bone or cartilage mineralization. In calcifying conditions, VSMC-derived EVs are enriched in phosphatidylserine and contain annexins I, II, V, and VI similar to MVs. However, unlike MVs, increased extracellular Ca2+ concentration does not lead to an increase in TNAP activity in VSMC-EVs, indicating calcification nucleation is independent of TNAP [50]. Rogers et al. showed that annexin I is enriched in VSMC-derived EVs released during ectopic vascular calcification and facilitates the formation of EV aggregates within collagen fibrils of the ECM. These EV aggregates form mineralization nucleation sites on the surface of the EV which then grow in the presence of increased extracellular Ca2+ and PO43− (Figure 2B). Importantly, the knockdown of annexin-I in osteoblast-like VSMCs inhibited the formation of calcification indicating that annexin-I-mediated EV aggregation is necessary for vascular calcification [51]. Thus, unlike bone or cartilage in which mineralization occurs within the MV, vascular calcification is mediated by the surface phospholipids and annexins of EV aggregates localized in the ECM.
4.2. Matrix-Bound Vesicles Are Integral to the Bioactive Properties of the ECM
In addition to acting as structural components during calcification, EVs embedded within the matrix are an integral part of the bioactive properties of the ECM. Recently discovered is a novel subtype of EVs anchored within the ECM called matrix-bound vesicles (MBV) which act as key biological signaling agents within the ECM [52]. Interestingly, MBVs do not contain any of the common exosome or microvesicle markers CD63, CD9, CD81, or HSP70 suggesting a different biogenesis pathway. Additionally, MBVs have tissue-specific characteristics with distinct lipid, RNA, and protein profiles indicating MBVs are an integral part of the bioactive signaling composition of the ECM and have a function in regulating tissue homeostasis [53,54].
Merwe et al. demonstrated that MBV derived from the ECM of the urinary bladder of healthy rats protected against ischemia-induced injury of retinal ganglion cells (RGC) [55]. As decellularized urinary bladder ECM (UB-ECM) had previously been shown to promote ganglion cell axon growth in coculture with microglia and astrocytes, the authors hypothesized that MBVs were a key biological agent anchored within the UB-ECM that were responsible for the increased cell growth [56]. In vitro treatment of a coculture of microglia, astrocytes, and RGC with UB-ECM-derived EVs suppressed the pro-inflammatory signaling of activated microglia and astrocytes and promoted RGC axon growth. Furthermore, intraocular administration of UB-ECM-derived EVs in an optical ischemia murine model ameliorated hypoxia-induced RGC axon degeneration by approximately 80% compared to the uninjured control. Thus, MBVs are key players responsible for the biological effects, namely promoting cell growth and regulating the immune microenvironment, exerted by the ECM.
Additionally, MBVs have been shown to be integral to ECM-dependent regulation of the immune microenvironment by modulation of immune cells within the matrix. With regards to rheumatoid arthritis, which is an autoimmune disease characterized by chronic inflammation of synovial joints, UB-ECM-derived EVs were shown to prevent acute and chronic arthritis at an efficacy comparable to the clinical standard [57]. Specifically, UB-ECM-derived EVs administered intravenously in an arthritic rat model promoted the transition of pro-inflammatory M1 macrophages to an anti-inflammatory M2 phenotype thereby decreasing inflammation in synovial joints and limiting adverse joint remodeling. Taken together, these studies underscore the indispensable function MBVs have as the bioactive structural component of the ECM.
4.3. EV-Mediated Modulation of the ECM
In contrast to calcification in which EVs aid in the structural development of the ECM, EVs released from cancer cells have been shown to actively degrade the ECM via enzymes such as MMPs and glycosidases present on the EV surface [5,16,36,37,38] (Figure 1C and Figure 3). EVs derived from human G361 melanoma cells and HT-1080 human fibrocarcinoma cells display matrix metalloprotease MT1-MMP (MMP-14), a transmembrane protein that promotes cell migration by degrading ECM components such as collagen, fibronectin, and laminins [58,59]. Similarly, EVs derived from nasopharyngeal cancer (NPC) cells were shown to contain matrix metalloprotease 13 (MMP-13) on the EV surface. Autologous incubation of the MMP-13-expressing EVs with NPC cells in Matrigel led to increased degradation of the matrix compared to both EVs with decreased MMP-13 levels and the untreated control, resulting in increased cell invasion within the matrix [60]. In addition to EVs derived from cancer cells, human vascular endothelial cells (HVECs) also secrete EVs that present MMP-2, MMP-9, and MMP-14 on the membrane surface. Incubating Matrigel with HVEC-EVs resulted in the degradation of the matrix and a threefold increase in HVEC cell migration compared to conditions without EV treatment [61]. As evidenced by these studies, EVs have direct contact and actively degrade the ECM by their surface-bound MMPs, resulting in enhanced cell migration through the matrix.
In addition to MMPs, EVs degrade the ECM by utilizing surface-bound heparinase, a glycosidase that degrades heparan sulfate proteoglycans (HSPC) [36,62,63,64]. HSPC is a is a proteoglycan, which is comprised of a core glycoprotein with multiple heparan sulfate groups that can sequester and bind signaling molecules including growth factors, chemokines, and cytokines to the matrix [65]. EVs derived from myeloma cells have heparinase bound to HSPC on the EV surface [62,63]. When these myeloma-derived EVs were incubated with ECM deposited by endothelial cells, free heparan sulfate chains were detected in the culture media [63]. Since HSPCs sequester bioactive signaling molecules and anchor them to ECM, EV-mediated cleavage of HSPC can modulate the signaling properties of the ECM.
EVs have also been shown to contain the surface-bound ECM-modulating enzymes lysyl oxidase like-2 (LOXL2), elastase, and collagenase [66,67]. Specifically, Jong et al. showed that EVs from human endothelial cells grown in hypoxic conditions present LOXL2, which is responsible for collagen crosslinking, on the EV surface [66]. Incubation of LOXL2-EVs within a collagen gel increased crosslinking leading to approximately twofold higher stiffness. Thus, hypoxic endothelial cells produce EVs which directly promote collagen crosslinking in the ECM. Conversely, Genshmer et al. found that EVs from activated neutrophils contained surface-bound elastase and collagenase [67]. Incubation of these activated neutrophil-derived EVs with both elastin and collagen fibrils led to a 2.5-fold increase in the degradation of elastin and collagen compared to the inactivated neutrophil EVs. As such, EV-surface-associated enzymes can directly influence the ECM by modulating the matrix through multiple ECM targets including collagen, fibronectin, elastin, and heparan sulfate proteoglycans.
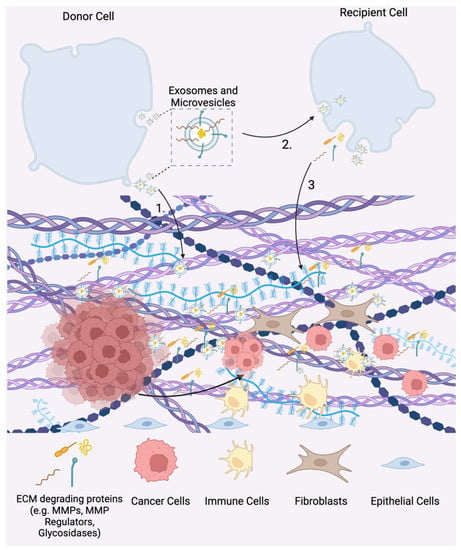
Figure 3.
EV-mediated matrix remodeling in tumor progression. 1. Matrix metalloproteases (MMPs) and glycosidases displayed on EV surfaces promote ECM degradation. 2. EVs released from tumor cells are uptaken by recipient cells in both the tumor microenvironment and the pre-metastatic niche. Tumor-derived EVs promote the release of ECM-degrading proteins (e.g., MMPs and MMP regulators) and EVs in recipient cells. 3. Free and EV-associated ECM degrading proteins promote tumor cell invasion, metastasis, and the development of the pre-metastatic niche.

Table 1.
Direct EV-ECM effects.
Table 1.
Direct EV-ECM effects.
| EV Source | EV Effects on the ECM | Active EV Components | Refs. | |
|---|---|---|---|---|
| EV-mediated calcification | ||||
| Osteoblasts | Promotes calcification of the ECM during bone and endochondral calcification | Annexins II, IV, V, Pit-1, Phosphatidylserine, TNAP | [42,43,44] | |
| Osteochondrogenic vascular smooth muscle cells | Promotes calcification of the vasculature during vascular calcification | Annexins I, II, IV, V, VI | [50,51] | |
| Bioactive signaling Agents | ||||
| Matrix-bound vesicles | Promotes anti-inflammatory signaling and cell growth in retinol ganglion cells and macrophages | N/A | [52,53,54,55,56,57] | |
| Direct modulation of the ECM | ||||
| G361 melanoma cells | Degrades collagen, fibronectin, and laminin; promotes cell migration | MMP-14 | [58] | |
| HT-1080 fibro carcinoma cells | Degrades collagen, fibronectin, and laminin; promotes cell migration | MMP-14 | [59] | |
| Nasal pharyngeal carcinoma cells | Degrades collagen; promotes cell migration | MMP-13 | [60] | |
| Vascular endothelial cells | Degrades collagen, fibronectin, and laminin; promotes cell migration and angiogenesis | MMP-2,9,13 | [61] | |
| Myeloma cells | Degrades heparin sulfate proteoglycans | Heparinase | [62,63,64] | |
| Neutrophils | Degrades collagen and elastase | Collagenase and elastase | [66] | |
| Endothelial cells | Promotes collagen crosslinking | Lysyl oxidase like-2 | [67] |
5. Indirect Influence of EVs on the ECM
While EVs can directly modulate the structural components of the matrix as discussed above, EVs can also indirectly alter the ECM by influencing recipient cells to remodel the ECM. Specifically, EVs released from tumor cells have been shown to direct recipient cells to modulate the matrix to create a favorable tumor niche [36]. In addition, EVs released from mesenchymal stem cells (MSCs) have been found to have therapeutic capability by improving ECM remodeling by fibroblasts and chondrocytes during tissue regeneration which will be further detailed below [52,54,55,57,68] (Table 2).
5.1. Tumor-Derived EVs Mediate Cell–ECM Interactions during Tumor Progression
5.1.1. Tumor Cell-Derived EVs Promote ECM Degradation
In Section 4.2, it was discussed that EVs derived from cancer cells have matrix-degrading enzymes present on their surface which promotes cancer cell migration and growth. However, in addition to directly interacting with the ECM, EVs secreted into the tumor microenvironment also contain cargo such as MMPs and MMP regulators that is transferred to recipient cells inducing cell-mediated remodeling of the ECM (Figure 1C). For example, EVs derived from hypoxic nasopharyngeal carcinoma (NPC) cells contain MMP-13 as cargo, and treatment of normoxic NPC with hypoxic NPC-derived EVs resulted in a 2.5-fold increase in cellular expression of MMP-13. Additionally, hypoxic NPC-derived EV treatment of normoxic NPC cells increased cell migration and invasion by approximately 300% and 200%, respectively, compared to the non-treated control. Furthermore, EV treatment of normoxic NPC cells decreased levels of the tumor suppressor protein E-cadherin by approximately 80% compared to the non-treated control, thereby promoting tumor growth [69]. Similarly, EVs derived from aggressive myeloma patient cells, which bound to both RPMI-8226 plasmacytoma lymphocytes and human endothelial cells via surface-associated fibronectin, increased the expression of MMP-9 in the RPMI-8226 cells and promoted endothelial cell invasion. Specifically, treatment with myeloma-derived EVs promoted increased cell spreading and migration of endothelial cells through a Matrigel basement membrane by 1.5-fold, indicating that the EVs promote modulation of the matrix via MMP-9 and reprogram the healthy cells towards a more cancerous phenotype [64].
Tumor-derived EVs also contain MMP regulators such as the glycoprotein extracellular matrix metalloprotease inducer (EMMPRIN) which is a transmembrane protein that stimulates the expression of MMPs by tumor-associated fibroblasts. EVs derived from epithelioid sarcoma cells contain EMMPRIN and treatment of human fibroblasts with sarcoma-derived EVs resulted in an increased expression of MMP-2 by approximately twofold compared to both the non-treated control and EVs without EMMPRIN [70]. Another MMP regulator that has been associated with poor prognosis in multiple cancer types is TIMP-1 [71,72]. Overexpression of TIMP-1 in human lung adenocarcinoma cells increased the expression and loading of the pro-angiogenic and pro-tumorigenic microRNA-210 into EVs. These EVs induced angiogenic tube formation in human epithelial cells and promoted lung adenocarcinoma cell migration in vitro, both processes in which ECM remodeling occurs [73,74]. Overall, these studies indicate that tumor-cell-derived EVs promote recipient cells toward a cancerous phenotype in which ECM degradation is involved.
5.1.2. Tumor-Derived EVs Facilitate Pre-Metastatic Niche Formation
The pre-metastatic niche is a potential location of metastasis that has been primed for invasion by circulating tumor cells. According to the “seed and soil” model, metastasis requires that the “soil” (receiving microenvironment) be ideal for the “seed” (circulating tumor cells) to populate [75]. Primary tumors release EVs which actively inform distant recipient cells to modify the ECM and prime the pre-metastatic niche for tumor cell invasion and establishment of a metastatic site. The work of Lyden and colleagues unraveled the role of EVs in establishing an organotropic pre-metastatic niche [17]. They first described that melanoma-derived EVs in circulation educate bone marrow progenitor cells towards a phenotype that supports tumor growth and metastasis [17,21]. In addition, they demonstrated that integrins on the surface of tumor-derived EVs are responsible for organotropic accumulation of EVs [76]. Once localized to a specific tissue, resident cells uptake these tumor-derived EVs and are induced to remodel the ECM toward the development of a pre-metastatic niche.
The role of MMPs and other matrix-modulating proteins in the development of the pre-metastatic niche is well established, and the function of EVs in regulating the expression of these matrix-modulating proteins is now being explored [77]. Redzic et al. showed that EVs released from breast cancer, leukemia, and pancreatic cancer cell lines contain EMMPRIN [78]. When these cell lines were treated with their respective EVs, MMP-9 and IL-6, which are downstream targets of EMMPRIN, were upregulated across all cell lines. Additionally, the cells expressed a higher level of EMMPRIN and increased their secretion of EMMPRIN-containing EVs.
Furthermore, melanoma-derived EVs have also been shown to facilitate ECM deposition and promote angiogenesis to prime sentinel lymph nodes for tumor metastasis. Specifically, intravenous injection of melanoma-derived EVs into mice led to an increase in mitogen-activated protein kinase 14, laminin, collagen, and urokinase plasminogen activator, or ECM-modulating factors involved in stroma remodeling that enable invasion by circulating tumor cells. Establishing a pre-metastatic niche in sentinel lymph nodes by intravenous administration of melanoma EVs led to increased selective colocalization of circulating melanoma cells with melanoma EVs in the sentinel lymph nodes [79]. As such, tumor-derived EVs can create an organotropic pre-metastatic niche through influencing recipient cells to modulate the ECM.
5.2. Therapeutic Potential of EVs in Tissue Regeneration
While EVs released from pathogenic cells are involved in disease progression, EVs from healthy cells have become of interest as next-generation therapeutics. Their low immunogenicity, high biocompatibility, endogenous therapeutic cargo, and ability to efficiently transfer bioactive agents to target cells have made them an attractive therapeutic platform in recent years [80]. While there are no current EV therapeutics in clinical trials specifically related to the ECM, multiple studies have reported the therapeutic ability of EVs to promote cell-dependent modulation of the matrix during tissue regeneration.

Table 2.
Indirect effect of EVs on the ECM.
Table 2.
Indirect effect of EVs on the ECM.
| Function | EV Source | EV Target | Cell–ECM Interactions | Active EV Components | Refs. |
|---|---|---|---|---|---|
| Tumor EVs promote ECM degradation | |||||
| Hypoxic nasal pharyngeal carcinoma cells | Normoxic nasal pharyngeal carcinoma cells | Increases cell expression of MMP-13, decreases expression of E-cadherin, promotes cell migration and invasion | MMP-13 | [69] | |
| Human myeloma cells | RPMI-8226 plasmacytoma lymphocytes and human endothelial cells | Increases MMP-9 expression, promotes cell invasion, and migration | MMP-9, fibronectin | [64] | |
| Epithelioid sarcoma cells | Fibroblasts | Increases expression of MMP-2 | EMMPRIN | [70] | |
| Human lung adenocarcinoma cells | Human epithelial cells and lung adenocarcinoma cells | Induces angiogenic tube formation and promotes cell migration | TIMP and miR-210 | [73,74] | |
| Tumor EVs create a pre-metastatic niche | |||||
| Human melanoma cells | Bone marrow progenitor cells | Promotes tumor growth and metastasis | N/A | [17] | |
| Breast cancer MCF-7 cells | Human fibroblasts | Increases expression of MMP-9 and IL-6 | EMMPRIN | [78] | |
| Monocytic leukemia U937 cells | Human fibroblasts | Increases expression of MMP-9 and IL-6 | EMMPRIN | [78] | |
| Pancreatic cancer L3.6pL cells | Human fibroblasts | Increases expression of MMP-9 and IL-6 | EMMPRIN | [78] | |
| Melanoma cells | Sentinel lymph nodes | Increases in mitogen-activated protein kinase 14, laminin, collagen, and urokinase plasminogen activator | N/A | [79] | |
| Mesenchymal stem cell EV-mediated ECM repair | |||||
| Adipose tissue MSCs | Dermal fibroblasts | Increases the ratio of type 3 collagen to type 1 collagen and the ratio of MMP-3 to TIMP-1 | N/A | [81,82] | |
| Adipose tissue MSCs | Chondrocytes | Promotes type 2 collagen synthesis and inhibits the expression of MMP-1, MMP-3, MMP-13, and ADAMTS-5 | N/A | [83] | |
| Bone marrow MSCs | Bone marrow MSCs | Promotes cell adhesion and motility | Hyaluronan | [84] |
Mesenchymal Stem Cell EV-Mediated ECM Repair
EVs derived from mesenchymal stem cells (MSC) have been shown to participate in restoring ECM during tissue regeneration. For example, EVs derived from adipose tissue MSC (AT-MSC) decreased scar formation and aided in wound healing in an in vivo model. Specifically, AT-MSC EVs were administered intravenously to a murine model with a dorsal wound. AT-MSC-EV treatment increased the ratio of type 3 collagen to type 1 collagen and MMP-3 to TIMP-1 in dermal fibroblasts, thereby aiding in matrix remodeling and limiting scar formation [81]. Interestingly, fibroblasts isolated from women with stress urinary incontinence that had significantly decreased expression of type 1 collagen, treated with AT-MSC EVs had increased expression of type 1 collagen and TIMP-1/3, and downregulation of MMP-1/2 [82]. Increased deposition of collagen along with inhibition and decreased expression of MMPs led to the restoration of ECM stiffness that is lost during stress urinary incontinence. Woo et al. demonstrated that AT-MSC EVs could also attenuate the progression of osteoarthritis, which is a chronic degenerative disease of articular cartilage. In vitro, AT-MSC EV treatment promoted chondrocyte migration and proliferation while simultaneously promoting type 2 collagen synthesis and inhibiting the expression of MMP-1, MMP-3, MMP-13, and ADAMTS-5. Additionally, intra-articular injections of AT-MSC EVs inhibited osteoarthritis progression in a murine model by protecting the cartilage matrix both by increased collagen deposition and inhibition of ECM-degrading proteases [83]. In addition to MMPs, human bone marrow (BM) MSC secretes hyaluronan-coated EVs. Hyaluronan (HA) is a glycosaminoglycan that acts as a critical bioactive factor within the ECM that plays roles in cell adhesion, motility, proliferation, and differentiation. HA-coated EVs were shown to be secreted from BM-MSC and anchored onto the ECM providing bioactive signaling cues within the matrix [84]. Taken together, these studies suggest that stem-cell-derived EVs have promise as therapeutics for tissue regeneration via modulation of the ECM.
6. Conclusions
During pathological processes such as tumor progression, EVs function via both the direct (i.e., EV-surface-associated enzymes) and indirect mechanisms (i.e., influencing recipient cells) to aid in ECM degradation, tumor progression, and the development of the pre-metastatic niche. Similarly, in physiological processes such as bone formation and tissue regeneration, EVs from osteoblasts and MSCs can directly evolve the structure of the ECM through calcification or by directing recipient cells to remodel the ECM during tissue repair, respectively. More knowledge regarding EV–ECM interactions during pathological processes such as cancer will allow for new therapeutic and diagnostic approaches targeting EV-mediated tumor progression and metastases. Similarly, characterizing the effect of EVs on the ECM during physiological processes such as tissue regeneration presents the opportunity to create novel or augment current therapeutic approaches while overcoming translational limitations in tissue repair.
Author Contributions
Conceptualization, N.J.P. and E.J.C.; writing-original draft preparation, N.J.P., A.A. and E.J.C.; writing-review and editing, N.J.P., A.A. and E.J.C.; funding acquisition, E.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the financial support from the National Science Foundation Graduate Student Fellowship awarded to N.J.P., and USC Women in Science and Engineering (WiSE), Ming Hsieh Institute for Research on Engineering-Medicine for Cancer, and NSF EAGER (DMR 2132744) awarded to E.J.C.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created or analyzed. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no competing interests.
References
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef]
- Kalfon, T.; Loewenstein, S.; Gerstenhaber, F.; Leibou, S.; Geller, H.; Sher, O.; Nizri, E.; Lahat, G. Gastric Cancer-Derived Extracellular Vesicles (EVs) Promote Angiogenesis via Angiopoietin-2. Cancers 2022, 14, 2953. [Google Scholar] [CrossRef] [PubMed]
- Brigstock, D.R. Extracellular Vesicles in Organ Fibrosis: Mechanisms, Therapies, and Diagnostics. Cells 2021, 10, 1596. [Google Scholar] [CrossRef]
- Xie, F.; Zhou, X.; Fang, M.; Li, H.; Su, P.; Tu, Y.; Zhang, L.; Zhou, F. Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Adv. Sci. 2019, 6, 1901779. [Google Scholar] [CrossRef] [PubMed]
- Sanwlani, R.; Gangoda, L. Role of Extracellular Vesicles in Cell Death and Inflammation. Cells 2021, 10, 2663. [Google Scholar] [CrossRef]
- Padhi, A.; Nain, A.S. ECM in Differentiation: A Review of Matrix Structure, Composition and Mechanical Properties. Ann. Biomed. Eng. 2020, 48, 1071–1089. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Lenzini, S.; Bargi, R.; Chung, G.; Shin, J.-W. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 2020, 15, 217–223. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Mooney, D.J. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015, 6, 6365. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.; Li, S.; Chin, A.R.; et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef]
- Nogués, L.; Benito-Martin, A.; Hergueta-Redondo, M.; Peinado, H. The influence of tumour-derived extracellular vesicles on local and distal metastatic dissemination. Mol. Asp. Med. 2018, 60, 15–26. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Raza, A.; Ahmed, E.L.; Khan, A.Q.; Prabhu, K.S.; Kuttikrishnan, S.; Mateo, J.M.; Zayed, H.; Rasul, K.; Azizi, F.; et al. The role of extracellular vesicles as modulators of the tumor microenviornment, metastasis and drug resistance in colorectal cancer. Cancers 2019, 11, 746. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef]
- Henne, W.M.; Stenmark, H.; Emr, S.D. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb. Perspect. Biol. 2013, 5, a016766. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.; Vidal, M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 2018, 9, 95–106. [Google Scholar] [CrossRef]
- Ståhl, A.L.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2019, 34, 11–30. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef]
- Dellar, E.R.; Hill, C.; Melling, G.E.; Carter, D.R.F.; Baena-Lopez, L.A. Unpacking extracellular vesicles: RNA cargo loading and function. J. Extracell. Biol. 2022, 1, e40. [Google Scholar] [CrossRef]
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Clarke-Bland, C.E.; Bill, R.M.; Devitt, A. Emerging roles for AQP in mammalian extracellular vesicles. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2022, 1864, 183826. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, R.D.; Bandari, S.K.; Vlodavsky, I. Proteases and glycosidases on the surface of exosomes: Newly discovered mechanisms for extracellular remodeling. Matrix Biol. 2019, 75–76, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.T.; Tan, S.; Agreiter, C.; Auer, K.; Bachmayr-Heyda, A.; Aust, S.; Pecha, N.; Mandorfer, M.; Pils, D.; Brisson, A.R.; et al. EV-Associated MMP9 in High-Grade Serous Ovarian Cancer Is Preferentially Localized to Annexin V-Binding EVs. Dis. Mrk. 2017, 2017, 9653194. [Google Scholar] [CrossRef]
- Dolo, V.; D’Ascenzo, S.; Violini, S.; Pompucci, L.; Festuccia, C.; Ginestra, A.; Vittorelli, M.L.; Canevari, S.; Pavan, A. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin. Exp. Metastasis 1999, 17, 131–140. [Google Scholar] [CrossRef]
- Sung, B.H.; Ketova, T.; Hoshino, D.; Zijlstra, A.; Weaver, A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015, 6, 7164. [Google Scholar] [CrossRef]
- Murshed, M.; Schinke, T.; McKee, M.D.; Karsenty, G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J. Cell Biol. 2004, 165, 625–630. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharm. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Veschi, E.A.; Bolean, M.; Strzelecka-Kiliszek, A. Bandorowicz-Pikula, J.; Pikula, S.; Granjon, T.; Mebarek, S.; Magne, D.; Ramos, A.P.; Rosato, N.; et al. Localization of Annexin A6 in Matrix Vesicles During Physiological Mineralization. Int. J. Mol. Sci. 2020, 21, 1367. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.; Arispe, N.; Haigler, H.T.; Burns, A.L.; Pollard, H.B. Identification of annexins as calcium channels in biological membranes. Bone Miner. 1992, 17, 214–218. [Google Scholar] [CrossRef]
- Golub, E.E. Role of matrix vesicles in biomineralization. Biochim. Biophys. Acta 2009, 1790, 1592–1598. [Google Scholar] [CrossRef]
- Suzuki, A.; Ghayor, C.; Guicheux, J.; Magne, D.; Quillard, S.; Kakita, A.; Ono, Y.; Miura, Y.; Oiso, Y.; Itoh, M.; et al. Enhanced Expression of the Inorganic Phosphate Transporter Pit-1 Is Involved in BMP-2–Induced Matrix Mineralization in Osteoblast-Like Cells. J. Bone Miner. Res. 2006, 21, 674–683. [Google Scholar] [CrossRef]
- Anderson, H.C. Molecular biology of matrix vesicles. Clin. Orthop. Relat. Res. 1995, 314, 266–280. [Google Scholar] [CrossRef]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular calcification: An update on mechanisms and challenges in treatment. Calcif Tissue Int 2013, 93, 365–373. [Google Scholar] [CrossRef]
- Krohn, J.B.; Hutcheson, J.D.; Martínez-Martínez, E.; Aikawa, E. Extracellular vesicles in cardiovascular calcification: Expanding current paradigms. J. Physiol. 2016, 594, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.L.; Weinbaum, S.; Aikawa, E.; Hutcheson, J.D. Zooming in on the genesis of atherosclerotic plaque microcalcifications. J. Physiol. 2016, 594, 2915–2927. [Google Scholar] [CrossRef] [PubMed]
- Kapustin, A.N.; Davies, J.D.; Reynolds, J.L.; McNair, R.; Jones, G.T.; Sidibe, A.; Schurgers, L.J.; Skepper, J.N.; Proudfoot, D.; Mayr, M.; et al. Calcium Regulates Key Components of Vascular Smooth Muscle Cell–Derived Matrix Vesicles to Enhance Mineralization. Circ. Res. 2011, 109, e1–e12. [Google Scholar] [CrossRef]
- Rogers, M.A.; Buffolo, F.; Schlotter, F.; Atkins, S.K.; Lee, L.H.; Halu, A.; Blaser, M.C.; Tsolaki, E.; Higashi, H.; Luther, K.; et al. Annexin A1-dependent tethering promotes extracellular vesicle aggregation revealed with single-extracellular vesicle analysis. Sci. Adv. 2020, 6, eabb1244. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Quijano, L.M.; Hussey, G.S.; Jiang, P.; Badylak, S.F. Matrix Bound Nanovesicles Have Tissue-Specific Characteristics That Suggest a Regulatory Role. Tissue Eng. Part A 2022, 28, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Pineda Molina, C.; Cramer, M.C.; Tyurina, Y.Y.; Tyurin, V.A.; Lee, Y.C.; El-Mossier, S.O.; Murdock, M.H.; Timashev, P.S.; Kagan, V.E.; et al. Lipidomics and RNA sequencing reveal a novel subpopulation of nanovesicle within extracellular matrix biomaterials. Sci. Adv. 2020, 6, eaay4361. [Google Scholar] [CrossRef]
- van der Merwe, Y.; Faust, A.E.; Sakalli, E.T.; Westrick, C.C.; Hussey, G.; Chan, K.C.; Conner, I.P.; Fu, V.L.N.; Badylak, S.F.; Steketee, M.B. Matrix-bound nanovesicles prevent ischemia-induced retinal ganglion cell axon degeneration and death and preserve visual function. Sci. Rep. 2019, 9, 3482. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Weng, Z.; Brown, B.N.; Yan, H.; Ma, X.M.; Vosler, P.S.; Badylak, S.F.; Dixon, C.E.; Cui, X.T.; et al. Effect of an inductive hydrogel composed of urinary bladder matrix upon functional recovery following traumatic brain injury. Tissue Eng. Part A 2013, 19, 1909–1918. [Google Scholar] [CrossRef]
- Crum, R.J.; Hall, K.; Molina, C.P.; Hussey, G.S.; Graham, E.; Li, H.; Badylak, S.F. Immunomodulatory matrix-bound nanovesicles mitigate acute and chronic pristane-induced rheumatoid arthritis. Npj Regen. Med. 2022, 7, 13. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Hakulinen, J.; Sankkila, L.; Sugiyama, N.; Lehti, K.; Keski-Oja, J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J. Cell. Biochem. 2008, 105, 1211–1218. [Google Scholar] [CrossRef]
- You, Y.; Shan, Y.; Chen, J.; Yue, H.; You, B.; Shi, S.; Li, X.; Cao, X. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015, 106, 1669–1677. [Google Scholar] [CrossRef]
- Taraboletti, G.; D’Ascenzo, S.; Borsotti, P.; Giavazzi, R.; Pavan, A.; Dolo, V. Shedding of the Matrix Metalloproteinases MMP-2, MMP-9, and MT1-MMP as Membrane Vesicle-Associated Components by Endothelial Cells. Am. J. Pathol. 2002, 160, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.A.; Purushothaman, A.; Ramani, V.C.; Vlodavsky, I.; Sanderson, R.D. Heparanase Regulates Secretion, Composition, and Function of Tumor Cell-derived Exosomes. J. Biol. Chem. 2013, 288, 10093–10099. [Google Scholar] [CrossRef] [PubMed]
- Bandari, S.K.; Purushothaman, A.; Ramani, V.C.; Brinkley, G.J.; Chandrashekar, D.S.; Varambally, S.; Mobley, J.A.; Zhang, Y.; Brown, E.E.; Vlodavsky, I.; et al. Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biol. 2018, 65, 104–118. [Google Scholar] [CrossRef]
- Purushothaman, A.; Bandari, S.K.; Liu, J.; Mobley, J.A.; Brown, E.E.; Sanderson, R.D. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. J. Biol. Chem. 2016, 291, 1652–1663. [Google Scholar] [CrossRef]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef]
- de Jong, O.G.; van Balkom, B.W.; Gremmels, H.; Verhaar, M.C. Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. J. Cell Mol. Med. 2016, 20, 342–350. [Google Scholar] [CrossRef]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Abdul Roda, M.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e115. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Lee, Y.C.; Bartolacci, J.G.; Behun, M.; Turnquist, H.R.; Badylak, S.F. Matrix bound nanovesicle-associated IL-33 activates a pro-remodeling macrophage phenotype via a non-canonical, ST2-independent pathway. J. Immunol Regen. Med. 2019, 3, 26–35. [Google Scholar] [CrossRef]
- Shan, Y.; You, B.; Shi, S.; Shi, W.; Zhang, Z.; Zhang, Q.; Gu, M.; Chen, J.; Bao, L.; Liu, D.; et al. Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes from Nasopharyngeal Carcinoma Enhances Metastases. Cell Death Dis. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Koga, K.; Hamasaki, M.; Egawa, N.; Nabeshima, K. Emmprin, released as a microvesicle in epithelioid sarcoma, interacts with fibroblasts. Int. J. Oncol. 2017, 50, 2229–2235. [Google Scholar] [CrossRef]
- Song, G.; Xu, S.; Zhang, H.; Wang, Y.; Xiao, C.; Jiang, T.; Wu, L.; Zhang, T.; Sun, X.; Zhong, L.; et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J. Exp. Clin. Cancer Res. 2016, 35, 148. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Scott, E.; Lu, R.; Xu, Y.; Oh, W.K.; Yu, Q. TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS ONE 2013, 8, e77366. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Myers, K.A. The role of hypoxia-induced miR-210 in cancer progression. Int. J. Mol. Sci. 2015, 16, 6353–6372. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Seubert, B.; Stahl, E.; Dietz, H.; Reuning, U.; Moreno-Leon, L.; Ilie, M.; Hofman, P.; Nagase, H.; Mari, B.; et al. Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene 2015, 34, 3640–3650. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Redzic, J.S.; Kendrick, A.A.; Bahmed, K.; Dahl, K.D.; Pearson, C.G.; Robinson, W.A.; Robinson, S.E.; Graner, M.W.; Eisenmesser, E.Z. Extracellular Vesicles Secreted from Cancer Cell Lines Stimulate Secretion of MMP-9, IL-6, TGF-β1 and EMMPRIN. PLoS ONE 2013, 8, e71225. [Google Scholar] [CrossRef]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes Released by Melanoma Cells Prepare Sentinel Lymph Nodes for Tumor Metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef]
- Klyachko, N.L.; Arzt, C.J.; Li, S.M.; Gololobova, O.A.; Batrakova, E.V. Extracellular Vesicle-Based Therapeutics: Preclinical and Clinical Investigations. Pharmaceutics 2020, 12, 1171. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Wu, S.; Hao, Q.; Li, Y.; Guo, Z.; Wang, W. Exosomes secreted by adipose-derived mesenchymal stem cells regulate type I collagen metabolism in fibroblasts from women with stress urinary incontinence. Stem. Cell Res. Ther. 2018, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.H.; Kim, H.K.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; Han, J.; Lee, J.; Kim, W.S.; Choi, J.S.; et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 2020, 9, 1735249. [Google Scholar] [CrossRef] [PubMed]
- Arasu, U.T.; Kärnä, R.; Härkönen, K.; Oikari, S.; Koistinen, A.; Kröger, H.; Qu, C.; Lammi, M.J.; Rilla, K. Human mesenchymal stem cells secrete hyaluronan-coated extracellular vesicles. Matrix Biol. 2017, 64, 54–68. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).