Towards Optimizing Sub-Normothermic Machine Perfusion in Fasciocutaneous Flaps: A Large Animal Study
Abstract
:1. Introduction
2. Materials and Methods
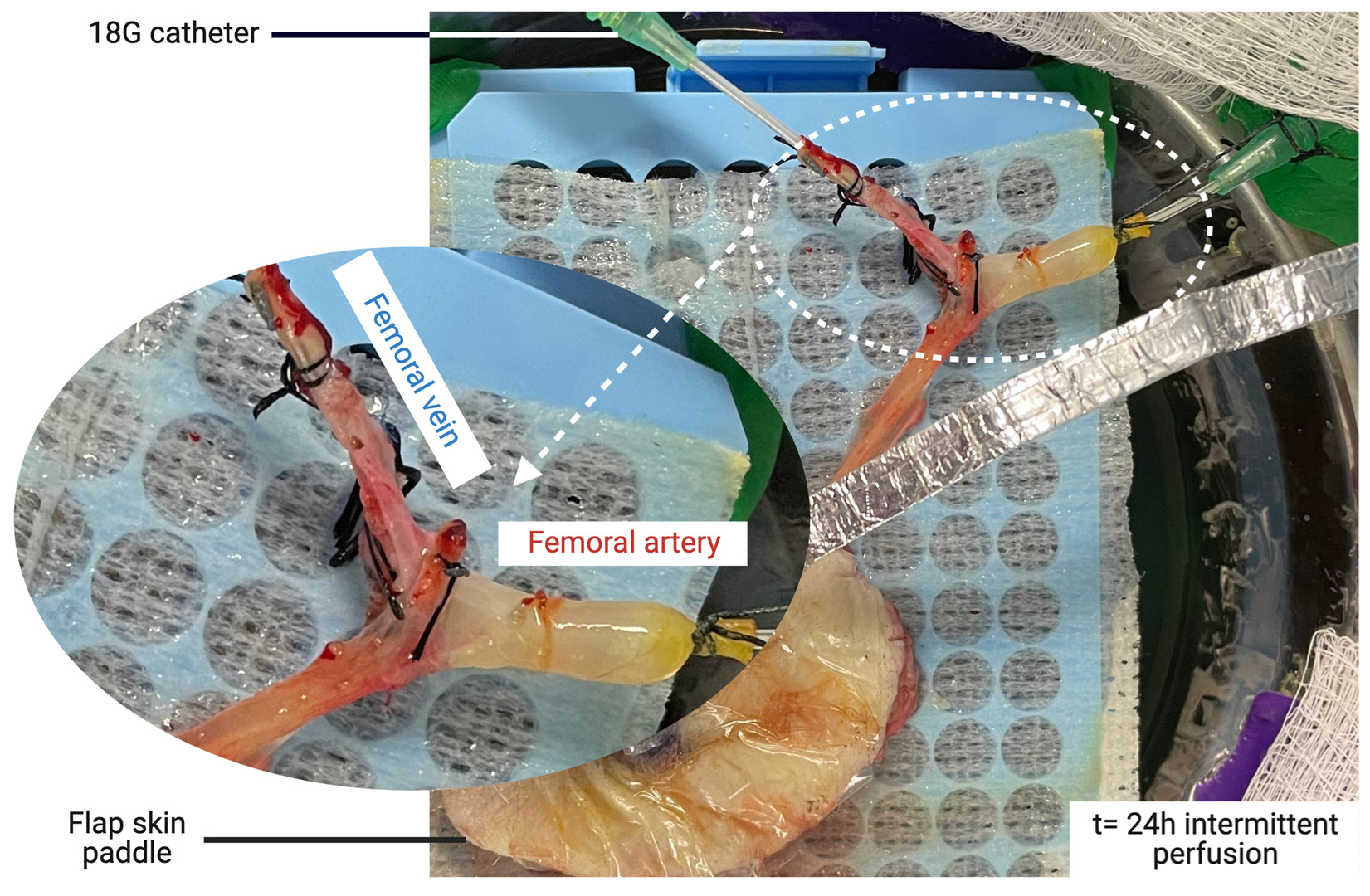
2.1. Flap Procurement Procedure
2.2. Machine Perfusion System
2.3. Perfusate Solution
2.4. Perfusion Monitoring
- –
- Weight gain of the flap every 6 h;
- –
- Perfusion parameters, including flow (mL/min) and measured and corrected pressures (mmHg);
- –
- Resistances were calculated according to the formula R = P/Q (R: resistance (mmHg.min/mL), P: corrected pressure (mmHg), and Q: flow rate (mL/min));
- –
- Biochemical parameters were repeatedly measured using a handheld analyzer (iStat 1, Abbott, Chicago, IL, USA). Inflow and outflow samples were collected for each time point and assessed the following measurements: pH, pO2 (mmHg), pCO2 (mmHg), lactate (mmol/L), [K+] (mmol/L), [Na+] (mmol/L), [HCO3−] (mmol/L), base excess (mmol/L), and glucose (mmol/L). Oxygen consumption was measured based on the difference in partial pressure between the inflow and outflow, the flow rate, and the initial weight using a modified Fick equation [35]. Similarly, glucose consumption was estimated as the inflow–outflow difference.
2.5. Determination of Flow Rates for Experiments
2.6. Continuous Versus Intermittent Perfusion Protocols
2.7. Statistical Analysis
3. Results
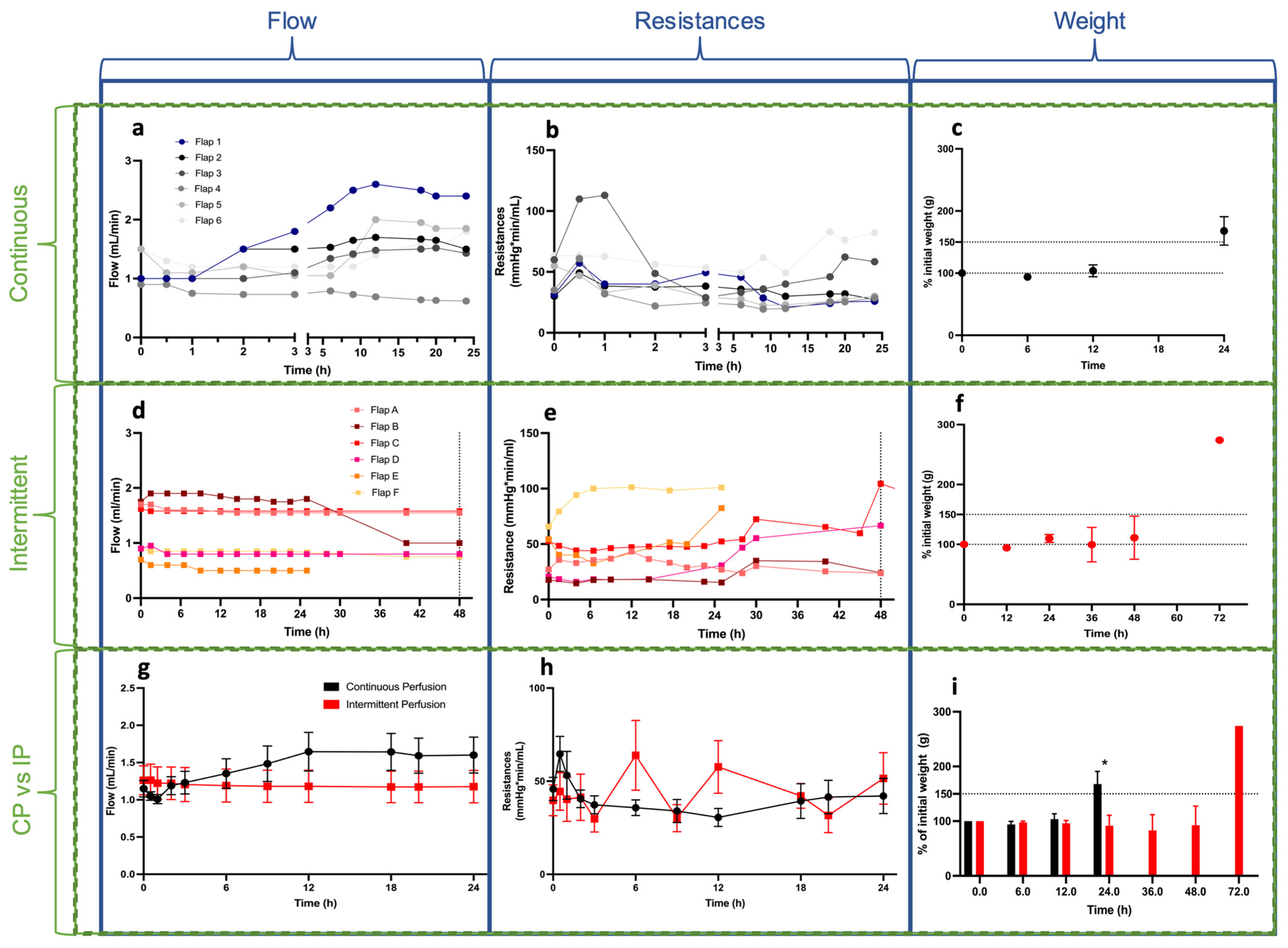
3.1. Perfusion Parameters
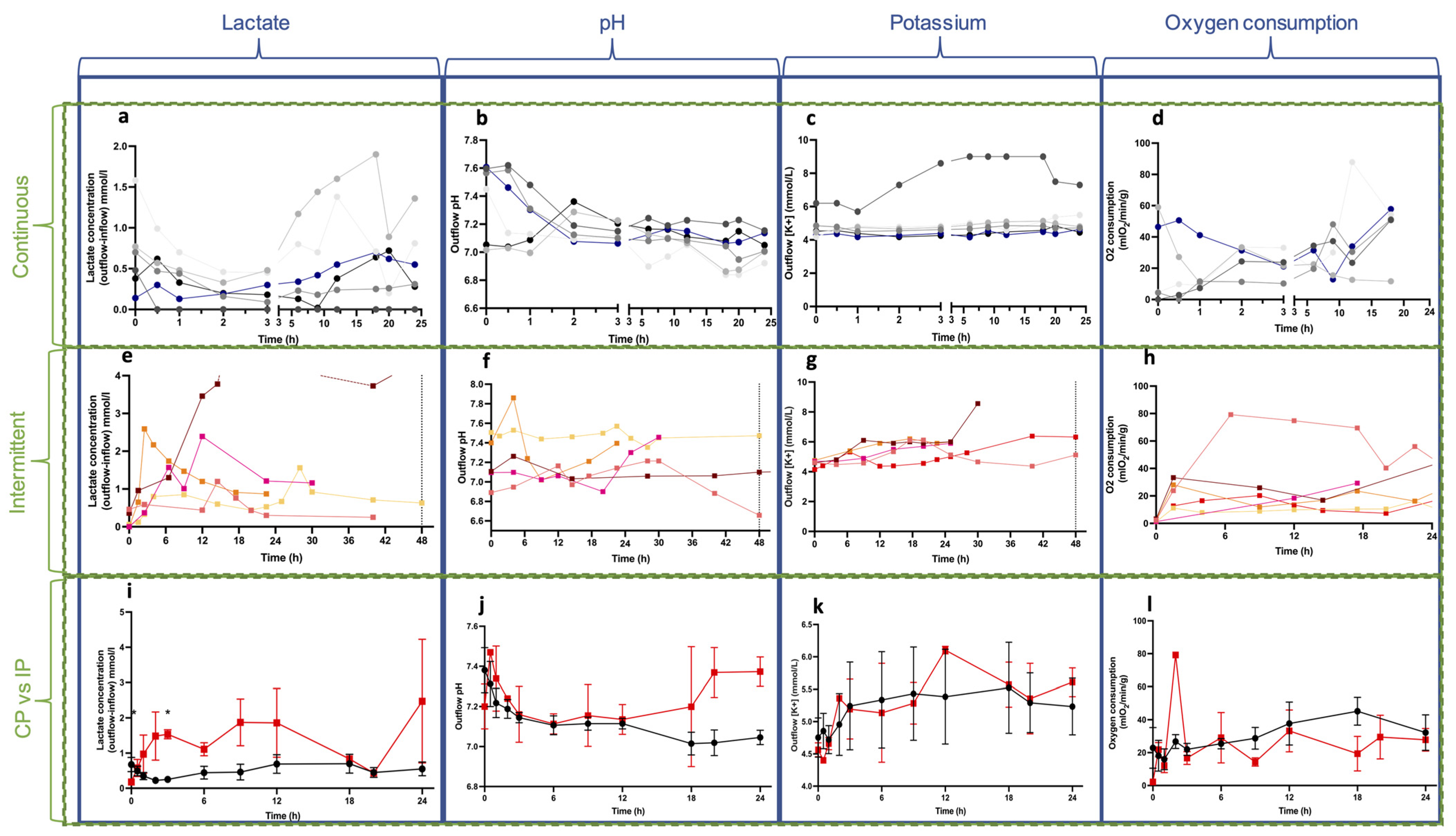
3.2. Biochemical Parameters
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- (1)
- Perfusion quality assessment through vascular angiography.
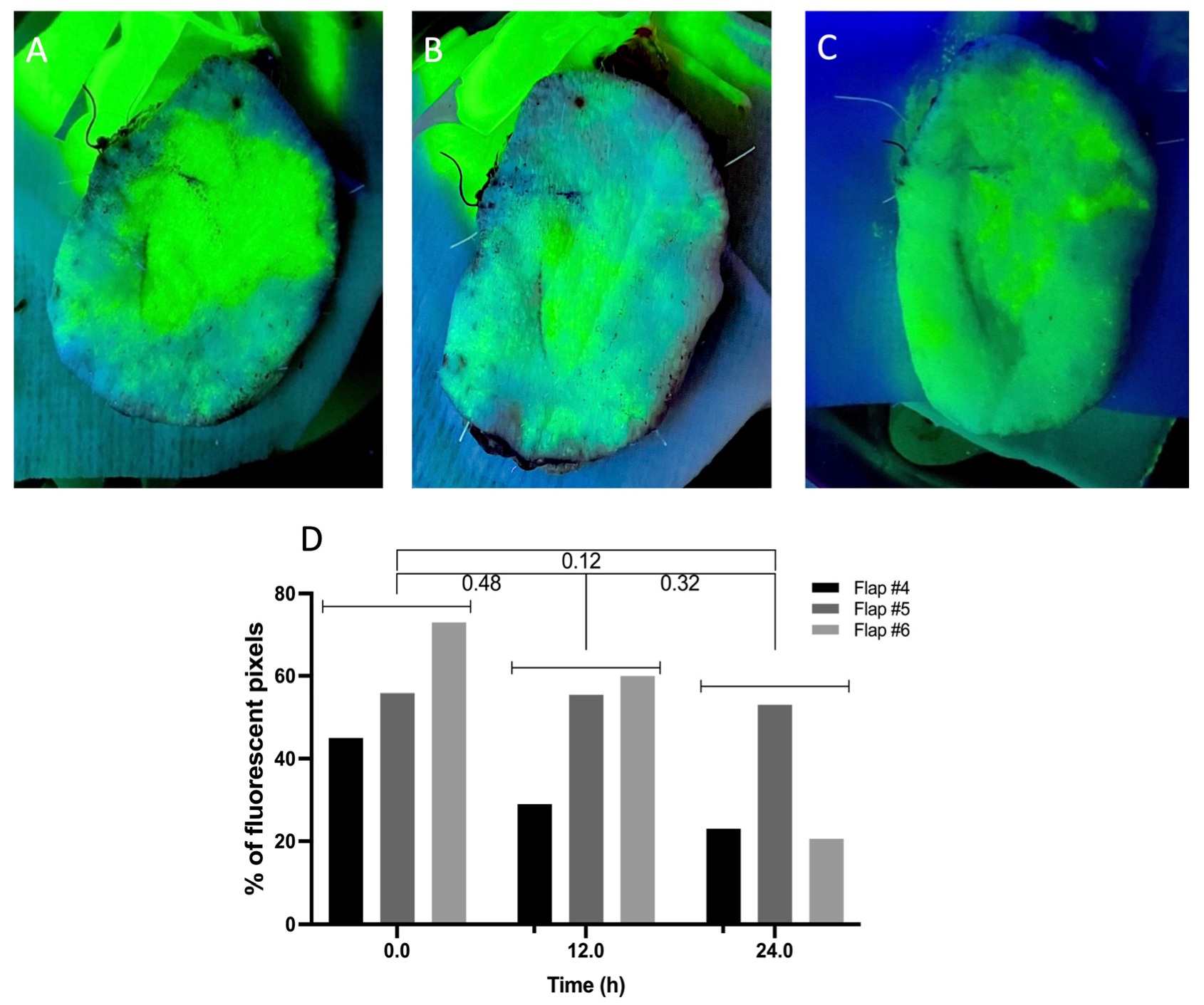
- (2)
- Tissue samples and Histology:
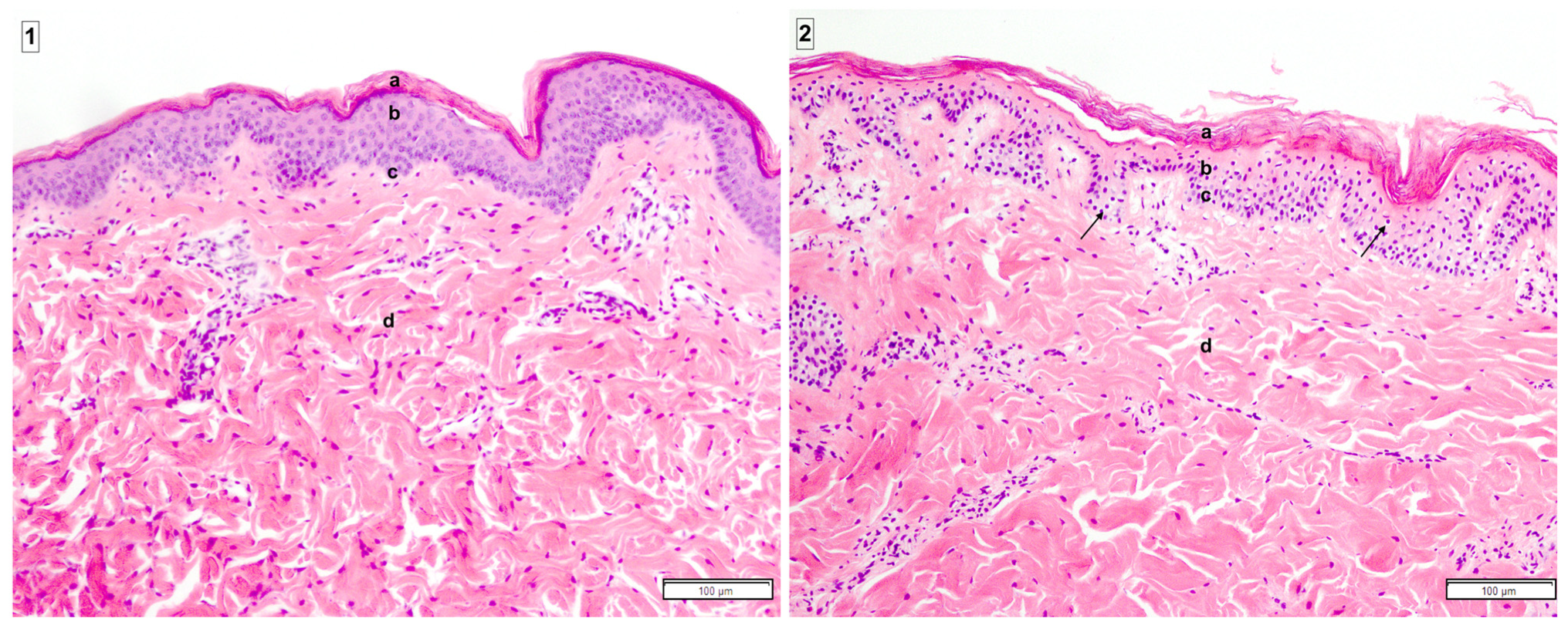
References
- Chan, J.K.-K.M.; Harry, L.M.; Williams, G.; Nanchahal, J.P. Soft-tissue reconstruction of open fractures of the lower limb: Muscle versus fasciocutaneous flaps. Plast. Reconstr. Surg. 2012, 130, 284e–295e. [Google Scholar] [CrossRef]
- Fox, C.M.; Beem, H.M.; Wiper, J.; Wagels, M.; Leong, J.C.; Rozen, W.M. Muscle versus fasciocutaneous free flaps in heel reconstruction: Systematic review and meta-analysis. J. Reconstr. Microsurg. 2015, 31, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Koshima, I.; Soeda, S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br. J. Plast. Surg. 1989, 42, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Chrelias, T.; Berkane, Y.; Rousson, E.; Uygun, K.; Meunier, B.; Kartheuser, A.; Watier, E.; Duisit, J.; Bertheuil, N. Gluteal Propeller Perforator Flaps: A Paradigm Shift in Abdominoperineal Amputation Reconstruction. J. Clin. Med. 2023, 12, 4014. [Google Scholar] [CrossRef] [PubMed]
- Alabdulkareem, M.; Berkane, Y.M.; Le Bras, E.; Rousson, E.; Chrelias, T.; Beaufils, T.; Leclere, F.-M.; Watier, E.; Bertheuil, N. Axillary Hidradenitis Suppurativa: A Comparison between Two Perforator Flap Reconstructive Approaches after Radical Surgical Management. Plast. Reconstr. Surg.-Glob. Open 2023, 11, e5301. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, C.; Berkane, Y.; Lupon, E.; Atlan, M.; Rousseau, P.; Lellouch, A.G.; Duisit, J.; Bertheuil, N. Outcomes and Reliability of Perforator Flaps in the Reconstruction of Hidradenitis Suppurativa Defects: A Systemic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5813. [Google Scholar] [CrossRef] [PubMed]
- Momoh, A.O.; Colakoglu, S.; Westvik, T.S.; Curtis, M.S.; Yueh, J.H.; de Blacam, C.; Tobias, A.M.; Lee, B.T. Analysis of complications and patient satisfaction in pedicled transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flap breast reconstruction. Ann. Plast. Surg. 2012, 69, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.S.; Lee, H.S.; Hong, J.P. Diabetic foot reconstruction using free flaps increases 5-year-survival rate. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 243–250. [Google Scholar] [CrossRef]
- Copelli, C.; Tewfik, K.; Cassano, L.; Pederneschi, N.; Catanzaro, S.; Manfuso, A.; Cocchi, R. Management of free flap failure in head and neck surgery. Acta Otorhinolaryngol. Ital. 2017, 37, 387–392. [Google Scholar] [CrossRef]
- Lese, I.; Biedermann, R.; Constantinescu, M.; Grobbelaar, A.O.; Olariu, R. Predicting risk factors that lead to free flap failure and vascular compromise: A single unit experience with 565 free tissue transfers. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 512–522. [Google Scholar] [CrossRef]
- Wang, W.; Ong, A.; Vincent, A.G.; Shokri, T.; Scott, B.; Ducic, Y. Flap Failure and Salvage in Head and Neck Reconstruction. Semin. Plast. Surg. 2020, 34, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, C.L.M.M.; Drolet, B.C.; Kassis, S.H.; Thayer, W.P.; Higdon, K.K.; Perdikis, G. Breast Reconstruction Free Flap Failure: National Outcomes based on Preoperative Comorbidities. Plast. Reconstr. Surg.-Glob. Open 2022, 10, 5. [Google Scholar] [CrossRef]
- Crawley, M.B.; Sweeny, L.; Ravipati, P.; Heffelfinger, R.; Krein, H.; Luginbuhl, A.; Goldman, R.; Curry, J. Factors Associated with Free Flap Failures in Head and Neck Reconstruction. Otolaryngol. Neck Surg. 2019, 161, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.K.; Nguyen, T.J.; Peric, M.; Shahabi, A.; Vidar, E.N.; Hwang, B.H.; Leilabadi, S.N.; Chan, L.S.; Urata, M.M. Analysis of risk factors associated with microvascular free flap failure using a multi-institutional database. Microsurgery 2015, 35, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, S.; Schoedel, S.; Spanier, G.; Mueller, K.; Meier, J.K.; Reichert, T.E.; Ettl, T. A decade of reconstructive surgery: Outcome and perspectives of free tissue transfer in the head and neck. Experience of a single center institution. Oral Maxillofac. Surg. 2020, 24, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.-D. New aspects in free flap surgery: Mini-perforator flaps and extracorporeal flap perfusion. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 238–241. [Google Scholar] [CrossRef]
- Brouwers, K.; Kruit, A.S.; van Midden, D.; Rijpma, S.R.; Schuijt, T.J.P.; Koers, E.J.; Zegers, H.J.H.; Hummelink, S.; Ulrich, D.J.O.M. Ex Vivo Machine Thrombolysis Reduces Rethrombosis Rates in Salvaged Thrombosed Myocutaneous Flaps in Swine. Plast. Reconstr. Surg. 2022, 150, 81–90. [Google Scholar] [CrossRef]
- Brouwers, K.; Thijssen, M.F.; Kruit, A.S.; van Midden, D.; Koers, E.J.B.; Zegers, H.J.I.; Hummelink, S.; Ulrich, D.J. 24-h Perfusion of Porcine Myocutaneous Flaps Mitigates Reperfusion Injury: A 7-day Follow-up Study. Plast. Reconstr. Surg.-Glob. Open 2022, 10, e4123. [Google Scholar] [CrossRef] [PubMed]
- Küntscher, M.V.M.; Erdmann, D.M.; Homann, H.-H.M.; Steinau, H.-U.M.; Levin, S.L.M.; Germann, G.M. The concept of fillet flaps: Classification, indications, and analysis of their clinical value. Plast. Reconstr. Surg. 2001, 108, 885–896. [Google Scholar] [CrossRef]
- Lauritzen, E.; Ibrahim, R.M.; Jensen, L.T.; Gámiz, R.C. Reconstruction by means of fillet flaps. Ugeskr. Laeger 2019, 181, V09180648. [Google Scholar]
- Halen, J.P.M.V.; Yu, P.M.; Skoracki, R.J.M.; Chang, D.W.M. Reconstruction of massive oncologic defects using free fillet flaps. Plast. Reconstr. Surg. 2010, 125, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.S.; Eloy, J.A.; Park, E.; Roman, B.; Genden, E.M. Vessel-depleted neck: Techniques for achieving microvascular reconstruction. Head Neck 2008, 30, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Manrique, O.J.; Bishop, S.N.; Ciudad, P.; Adabi, K.; Martinez-Jorge, J.; Moran, S.L.; Huang, T.; Vijayasekaran, A.; Chen, S.-H.; Chen, H.-C. Lower Extremity Limb Salvage with Cross Leg Pedicle Flap, Cross Leg Free Flap, and Cross Leg Vascular Cable Bridge Flap. J. Reconstr. Microsurg. 2018, 34, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Sagar, A.; Friend, P.; Friend, P. Multi-day perfusion of transplant organs: The how and the why. Med 2022, 3, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.-D.; Ritschl, L.M.; von Bomhard, A.; Braun, C.; Wolff, C.; Fichter, A.M. In vivo perfusion of free skin flaps using extracorporeal membrane oxygenation. J. Cranio-Maxillofac. Surg. 2020, 48, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.-D.; Mücke, T.; von Bomhard, A.; Ritschl, L.M.; Schneider, J.; Humbs, M.; Fichter, A.M. Free flap transplantation using an extracorporeal perfusion device: First three cases. J. Cranio-Maxillofac. Surg. 2016, 44, 148–154. [Google Scholar] [CrossRef]
- Brouwers, K.; Kruit, A.S.; Koers, E.J.; Zegers, H.J.H.; Hummelink, S.; Ulrich, D.J.O. Ex Vivo Thrombolysis to Salvage Free Flaps Using Machine Perfusion: A Pilot Study in a Porcine Model. J. Reconstr. Microsurg. 2022, 38, 757–765. [Google Scholar] [CrossRef]
- Kruit, A.S.; Schreinemachers, M.-C.J.; Koers, E.J.; Zegers, H.J.; Hummelink, S.; Ulrich, D.J. Successful Long-term Extracorporeal Perfusion of Free Musculocutaneous Flaps in a Porcine Model. J. Surg. Res. 2019, 235, 113–123. [Google Scholar] [CrossRef]
- Lellouch, A.G.; Karimian, N.; Ng, Z.Y.; Mert, S.; Geerts, S.; Uygun, K.; Cetrulo, C.L. 2517: Ex-vivo subnormothermic oxygenated machine perfusion of swine forelimbs enables prolonged graft preservation prior to transplantation. Vasc. Compos. Allotransplant. 2016, 3, 38. [Google Scholar] [CrossRef]
- Berkane, Y.; Goutard, M.; Tawa, P.; von Reiterdank, I.F.; Lancia, H.; Andrews, A. Abstract #436—24h Acellular Subnormothermic Machine Perfusion for VCA Preservation. Am. J. Transplant. 2023, 23, 614–1200. [Google Scholar]
- Burlage, L.C.; Lellouch, A.G.; Taveau, C.B.; Tratnig-Frankl, P.; Pendexter, C.A.; Randolph, M.A.; Porte, R.J.; Lantieri, L.A.; Tessier, S.N.; Cetrulo, C.L.; et al. Optimization of Ex Vivo Machine Perfusion and Transplantation of Vascularized Composite Allografts. J. Surg. Res. 2022, 270, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Goutard, M.; de Vries, R.J.; Tawa, P.; Pendexter, C.A.; Rosales, I.A.; Tessier, S.N.; Burlage, L.C.; Lantieri, L.; Randolph, M.A.; Lellouch, A.G.; et al. Exceeding the Limits of Static Cold Storage in Limb Transplantation Using Subnormothermic Machine Perfusion. J. Reconstr. Microsurg. 2022, 39, 350–360. [Google Scholar] [CrossRef]
- Pozzo, V.; Romano, G.; Goutard, M.; Lupon, E.; Tawa, P.; Acun, A.; Andrews, A.R.; Taveau, C.B.; Uygun, B.E.; Randolph, M.A.; et al. A Reliable Porcine Fascio-Cutaneous Flap Model for Vascularized Composite Allografts Bioengineering Studies. J. Vis. Exp. 2022, 181, e63557. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- de Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Supercooling extends preservation time of human livers. Nat. Biotechnol. 2019, 37, 1131–1136. [Google Scholar] [CrossRef]
- Fichter, A.M.; Ritschl, L.M.; Rau, A.; Schwarzer, C.; von Bomhard, A.; Wagenpfeil, S.; Wolff, K.-D.; Mücke, T. Free flap rescue using an extracorporeal perfusion device. J. Cranio-Maxillofac. Surg. 2016, 44, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Kruit, A.S.; Winters, H.; van Luijk, J.; Schreinemachers, M.-C.J.; Ulrich, D.J. Current insights into extracorporeal perfusion of free tissue flaps and extremities: A systematic review and data synthesis. J. Surg. Res. 2018, 227, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.; Wong, C.; Schaverien, M.; Mojallal, A.; Rohrich, R.J. The perforasome theory: Vascular anatomy and clinical implications. Plast. Reconstr. Surg. 2009, 124, 1529–1544. [Google Scholar] [CrossRef]
- Veraza, R.; Merlo, J.; Bunegin, L. 24-h Rat Hind Limb Preservation Using a 3D-Printed Subnormothermic Portable Machine Perfusion Device. J. Transplant. Technol. Res. 2021, 11, 2021. [Google Scholar]
- Beckler, A.D.; Ezzat, W.H.; Seth, R.; Nabili, V.; Blackwell, K.E. Assessment of Fibula Flap Skin Perfusion in Patients Undergoing Oromandibular Reconstruction: Comparison of Clinical Findings, Fluorescein, and Indocyanine Green Angiography. JAMA Facial Plast. Surg. 2015, 17, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Goudot, G.; Berkane, Y.; de Clermont-Tonnerre, E.; Guinier, C.; von Reiterdank, I.F.; van Kampen, A.; Uygun, K.; Cetrulo, C.L.; Uygun, B.E.; Dua, A.; et al. Microvascular assessment of fascio-cutaneous flaps by ultrasound: A large animal study. Front. Physiol. 2022, 13, 1063240. [Google Scholar] [CrossRef]
- Lodhi, S.; Stone, J.P.; Entwistle, T.R.; Fildes, J.E. The Use of Hemoglobin-Based Oxygen Carriers in Ex Vivo Machine Perfusion of Donor Organs for Transplantation. ASAIO J. 2022, 68, 461–470. [Google Scholar] [CrossRef]
- Pendexter, C.A.; Haque, O.; Mojoudi, M.; Maggipinto, S.; Goutard, M.; Baicu, S.; Lellouch, A.G.; Markmann, J.F.; Brandacher, G.; Yeh, H.; et al. Development of a rat forelimb vascularized composite allograft (VCA) perfusion protocol. PLoS ONE 2023, 18, e0266207. [Google Scholar] [CrossRef] [PubMed]
- Burlage, L.C.; Hessels, L.; van Rijn, R.; Matton, A.P.; Fujiyoshi, M.; Berg, A.P.v.D.; Reyntjens, K.M.; Meyer, P.; de Boer, M.T.; de Kleine, R.H.; et al. Opposite acute potassium and sodium shifts during transplantation of hypothermic machine perfused donor livers. Am. J. Transplant. 2019, 19, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Ishikawa, J.; Yoshimoto, S.; Hakamata, Y.; Kobayashi, E. A rat model of dual-flow liver machine perfusion system. Acta Cirúrgica Bras. 2023, 38, e387723. [Google Scholar] [CrossRef] [PubMed]
- Agius, T.; Songeon, J.; Klauser, A.; Allagnat, F.; Longchamp, G.; Ruttimann, R.B.; Lyon, A.; Ivaniesevic, J.; Meier, R.; Déglise, S.; et al. Subnormothermic Ex Vivo Porcine Kidney Perfusion Improves Energy Metabolism: Analysis Using 31P Magnetic Resonance Spectroscopic Imaging. Transplant. Direct 2022, 8, e1354. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.; Pandey, S.; Kopparthy, V.; Sadeghi, P.; Clark, R.C.; Figueroa, B.; Dasarathy, S.; Brunengraber, H.; Papay, F.; Rampazzo, A.; et al. Weight gain is an early indicator of injury in ex vivo normothermic limb perfusion (EVNLP). Artif. Organs 2023, 47, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Haug, V.; Kollar, B.; Endo, Y.; Kadakia, N.; Veeramani, A.; Kauke, M.; Tchiloemba, B.; Klasek, R.; Pomahac, B. Comparison of Acellular Solutions for Ex-situ Perfusion of Amputated Limbs. Mil. Med. 2020, 185, e2004–e2012. [Google Scholar] [CrossRef]
- Lee, Z.-H.; Abdou, S.A.; Daar, D.A.; Anzai, L.; Stranix, J.T.; Thanik, V.; Levine, J.P.; Saadeh, P.B. Comparing Outcomes for Fasciocutaneous versus Muscle Flaps in Foot and Ankle Free Flap Reconstruction. J. Reconstr. Microsurg. 2019, 35, 646–651. [Google Scholar] [CrossRef]
- Kruit, A.S.; Smits, L.; Pouwels, A.; Schreinemachers, M.-C.J.; Hummelink, S.L.; Ulrich, D.J. Ex-vivo perfusion as a successful strategy for reduction of ischemia-reperfusion injury in prolonged muscle flap preservation—A gene expression study. Gene 2019, 701, 89–97. [Google Scholar] [CrossRef]
- Hölzle, F.; Rau, A.; Loeffelbein, D.; Mücke, T.; Kesting, M.; Wolff, K.-D. Results of monitoring fasciocutaneous, myocutaneous, osteocutaneous and perforator flaps: 4-year experience with 166 cases. Int. J. Oral Maxillofac. Surg. 2010, 39, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kovar, A.; Colakoglu, S.; Iorio, M.L. A Systematic Review of Muscle and Fasciocutaneous Flaps in the Treatment of Extremity Osteomyelitis: Evidence for Fasciocutaneous Flap Use. Plast. Reconstr. Surg.-Glob. Open 2019, 7, 1–2. [Google Scholar] [CrossRef]
- Ozturk, M.B.; Aksan, T.; Ozcelik, I.B.; Ertekin, C.; Akcakoyunlu, B.; Ozkanli, S.S.; Tezcan, M. Extracorporeal Free Flap Perfusion Using Extracorporeal Membrane Oxygenation Device: An Experimental Model. Ann. Plast. Surg. 2019, 83, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Wu, G.B.; Chu, C.; Li, X.; Zou, Q.; Cao, Y.B.; Zhu, L.B. Standardized Skin Flap Warming Effectively Improves Flap Survival without Obstructing Temperature Monitoring after DIEP. Plast. Reconstr. Surg.-Glob. Open 2022, 10, e4153. [Google Scholar] [CrossRef]
- Spetzler, V.N.; Goldaracena, N.; Echiverri, J.; Kaths, J.M.; Louis, K.S.; Adeyi, O.A.; Yip, P.M.; Grant, D.R.; Selzner, N.; Selzner, M. Subnormothermic ex vivo liver perfusion is a safe alternative to cold static storage for preserving standard criteria grafts. Liver Transplant. 2016, 22, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Leber, B.; Schlechter, S.; Weber, J.; Rohrhofer, L.; Niedrist, T.; Aigelsreiter, A.; Stiegler, P.; Schemmer, P. Experimental long-term sub-normothermic machine perfusion for non-allocable human liver grafts: First data towards feasibility. Eur. Surg. 2022, 54, 150–155. [Google Scholar] [CrossRef]
- De Vries, Y.; Brüggenwirth, I.M.A.B.; Karangwa, S.A.; von Meijenfeldt, F.A.B.; van Leeuwen, O.B.; Burlage, L.C.; de Jong, I.E.M.; Gouw, A.S.H.; de Meijer, V.E.; Lisman, T.; et al. Dual Versus Single Oxygenated Hypothermic Machine Perfusion of Porcine Livers: Impact on Hepatobiliary and Endothelial Cell Injury. Transplant. Direct 2021, 7, e741. [Google Scholar] [CrossRef]
- Michel, S.G.; LaMuraglia, G.M.; Madariaga, M.L.L.; Titus, J.S.; Selig, M.K.; Farkash, E.A.; Allan, J.S.; Anderson, L.M.; Madsen, J.C. Twelve-Hour Hypothermic Machine Perfusion for Donor Heart Preservation Leads to Improved Ultrastructural Characteristics Compared to Conventional Cold Storage. J. Heart Lung Transplant. 2015, 20, 461–468. [Google Scholar] [CrossRef]
- Akcal, A.; Sirvan, S.S.; Karsidag, S.; Görgülü, T.; Akcal, M.A.; Ozagari, A.; Tatlidede, S. Combination of ischemic preconditioning and postconditioning can minimise skin flap loss: Experimental study. J. Plast. Surg. Hand Surg. 2016, 50, 233–238. [Google Scholar] [CrossRef]
- Berkane, Y.; Shamlou, A.A.; Reyes, J.; Lancia, H.H.; von Reiterdank, I.F.; Bertheuil, N.; Uygun, B.E.; Uygun, K.; Austen, W.G., Jr.; Cetrulo, C.L., Jr.; et al. The Superficial Inferior Epigastric Artery Axial Flap to Study Ischemic Preconditioning Effects in a Rat Model. J. Vis. Exp. 2023, 191, e64980. [Google Scholar] [CrossRef]
- Küntscher, M.V.; Schirmbeck, E.U.; Menke, H.; Klar, E.; Gebhard, M.M.; Germann, G. Ischemic preconditioning by brief extremity ischemia before flap ischemia in a rat model. Plast. Reconstr. Surg. 2002, 109, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Rosales, I.A.; Foreman, R.K.; DeFazio, M.; Sachs, D.H.; Cetrulo, C.L., Jr.; Leonard, D.A.; Colvin, R.B. Systematic pathological component scores for skin-containing vascularized composite allografts. Vasc. Compos. Allotransplant. 2016, 3, 62–74. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berkane, Y.; Lellouch, A.G.; Goudot, G.; Shamlou, A.; Filz von Reiterdank, I.; Goutard, M.; Tawa, P.; Girard, P.; Bertheuil, N.; Uygun, B.E.; et al. Towards Optimizing Sub-Normothermic Machine Perfusion in Fasciocutaneous Flaps: A Large Animal Study. Bioengineering 2023, 10, 1415. https://doi.org/10.3390/bioengineering10121415
Berkane Y, Lellouch AG, Goudot G, Shamlou A, Filz von Reiterdank I, Goutard M, Tawa P, Girard P, Bertheuil N, Uygun BE, et al. Towards Optimizing Sub-Normothermic Machine Perfusion in Fasciocutaneous Flaps: A Large Animal Study. Bioengineering. 2023; 10(12):1415. https://doi.org/10.3390/bioengineering10121415
Chicago/Turabian StyleBerkane, Yanis, Alexandre G. Lellouch, Guillaume Goudot, Austin Shamlou, Irina Filz von Reiterdank, Marion Goutard, Pierre Tawa, Paul Girard, Nicolas Bertheuil, Basak E. Uygun, and et al. 2023. "Towards Optimizing Sub-Normothermic Machine Perfusion in Fasciocutaneous Flaps: A Large Animal Study" Bioengineering 10, no. 12: 1415. https://doi.org/10.3390/bioengineering10121415
APA StyleBerkane, Y., Lellouch, A. G., Goudot, G., Shamlou, A., Filz von Reiterdank, I., Goutard, M., Tawa, P., Girard, P., Bertheuil, N., Uygun, B. E., Randolph, M. A., Duisit, J., Cetrulo, C. L., Jr., & Uygun, K. (2023). Towards Optimizing Sub-Normothermic Machine Perfusion in Fasciocutaneous Flaps: A Large Animal Study. Bioengineering, 10(12), 1415. https://doi.org/10.3390/bioengineering10121415









