State of the Art in Immersive Interactive Technologies for Surgery Simulation: A Review and Prospective
Abstract
:1. Introduction
2. Haptic Rendering
3. Tracking
3.1. Vision-Based Tracking
3.2. Non-Vision-Based Tracking
4. Minimally Invasive Surgery Simulation
5. Open Surgery Simulation
| Author and Year | Surgical Procedure | Immersive Interaction Type | Description | Device and/or Method |
|---|---|---|---|---|
| Lin et al., 2015 [68] | Dental implant | AR | Development of an augmented reality-based dental implant simulation system | Sony® HMZ-T1 personal 3D viewer, CCD and marker tracking |
| Watanabe et al., 2016 [69] | Tumor resection surgery | AR | An augmented reality-based navigation system was developed to realize the full tracking function by overlaying MRI and CT images | Tablet and VICON® tracking system with 6 cameras |
| Yoon et al., 2017 [75] | Parieto-occipital ventriculoperitoneal shunt placement (Ventricular Catheter Placement) | AR | Practice of surgical assistance via a wearable flat screen monitor mounted on a magnifying glass | Google® glasses |
| Weidert et al., 2019 [71] | Distal Interlocking | AR | Evaluating the Feasibility of a Video-Augmented Fluoroscopy (VAF) Technique for Distal Interlocking of Intramedullary Nails Using a Camera-Enhanced Mobile C-Arm (CamC) | Marker-based tracking and video-augmented instrument tracking |
| Coelho et al., 2020 [72] | Metopic craniosynostosis | AR | Development of a preoperative planning method combining hybrid modeling and augmented reality (AR) for correction of deviated cephalic deformity | Cell phones with AR applications and vision tracking method |
| Golse et al., 2021 [73] | Liver section surgery | AR | Practice of hepatic resection by markerless visual tracking technique | Markerless tracking and 3D-CT scanning |
| Fushima and Kobayashi 2016 [74] | Orthognathic | MR | Presentation and evaluation of a mandibular motion tracking system | Three-dimensional computed tomography and device tracking technology |
| Ameri et al., 2017 [17] | Internal jugular vein cannulation | MR | Development of a mixed reality ultrasound guidance system tailored to central line insertions | Ultrasound-assisted visual tracking |
| McJunkin et al., 2018 [70] | Lateral skull base anatomy | MR | An MR device-based system was developed for three-dimensional (3D) visualization of interactive holograms fixed at specific points in physical space for lateral skull base dissection | Microsoft® HoloLens and marker-based vision tracking method |
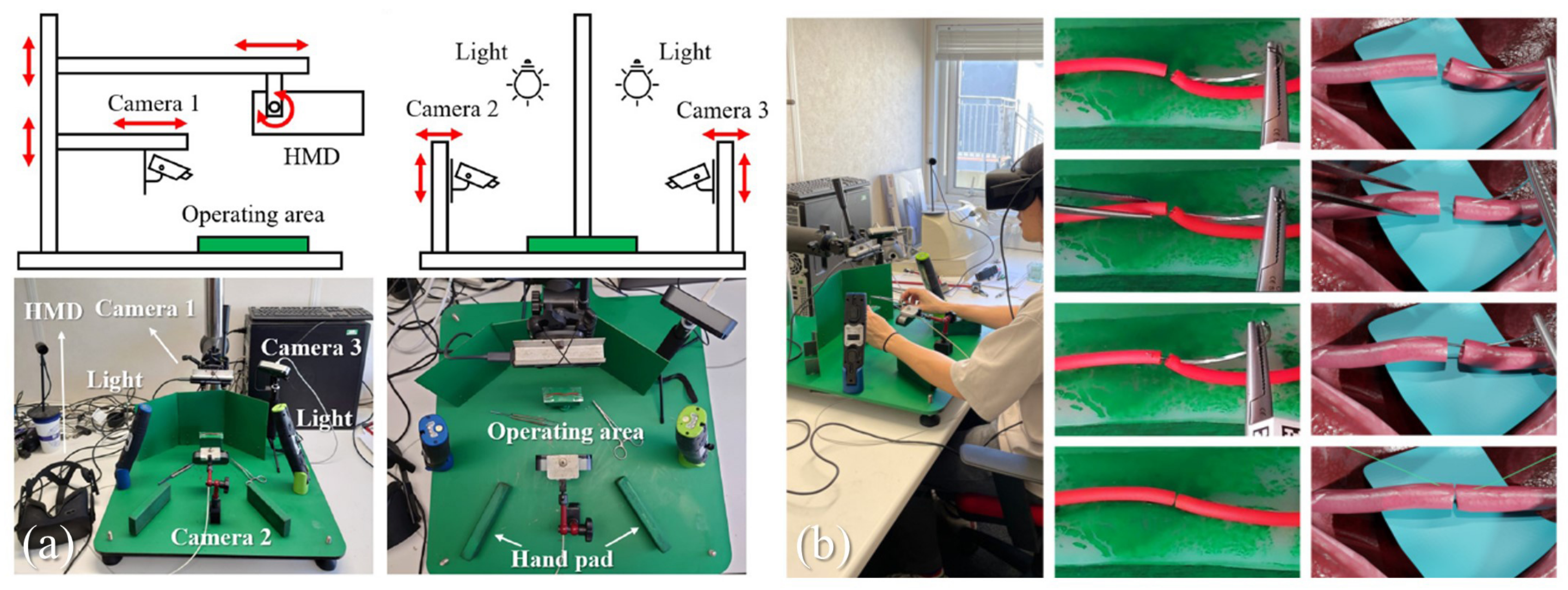
| Xiang et al., 2023 [11] | Microvascular anastomosis | MR | A vision-based tracking system is proposed to simultaneously track surgical instruments and artificial blood vessels | TsFPS [79] based high accuracy surgical instrument tracking |
| Alaraj et al., 2015 [76] | Aneurysm clipping surgery | VR | Development of a real-time sensory haptic feedback virtual reality aneurysm clipping simulator | Immersive Touch® platform with haptic feedback technique |
| Azarnoush et al., 2015 [77] | Tumor section surgery | VR | Evaluating the Effectiveness of Metrics Extracted from the NeuroTouch Platform for Brain Tumor Surgery | NeuroTouch Platform with haptic feedback |
| Pulijala et al., 2018 [78] | Orthognathic surgery | VR | Development and validation of a novel immersive virtual reality (iVR)-based Le Fort I osteotomy training tool based on Oculus® Rift and Leap® Motion devices | Oculus® Rift and Leap® Motion VR platform |
6. Discussion
6.1. Challenges
6.2. Future Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, B.; Dasgupta, P. 3D printing technology and its role in urological training. World J. Urol. 2020, 38, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Satava, R.M. Surgical education and surgical simulation. World J. Surg. 2001, 25, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Ramesh, K.; Okamura, A.M. Modeling of tool-tissue interactions for computer-based surgical simulation: A literature review. Presence 2008, 17, 463–491. [Google Scholar] [CrossRef] [PubMed]
- Lungu, A.J.; Swinkels, W.; Claesen, L.; Tu, P.; Egger, J.; Chen, X. A review on the applications of virtual reality, augmented reality and mixed reality in surgical simulation: An extension to different kinds of surgery. Expert Rev. Med. Devices 2021, 18, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Krummel, T.M. Surgical Simulation and Virtual Reality: The Coming Revolution. Ann. Surg. 1998, 228, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Robison, R.A.; Liu, C.Y.; Apuzzo, M.L. Man, Mind, and Machine: The Past and Future of Virtual Reality Simulation in Neurologic Surgery. World Neurosurg. 2011, 76, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, H.; Haavisto, E.; Havola, S.; Koivisto, M. Graduating nursing students’ user experiences of the immersive virtual reality simulation in learning—A qualitative descriptive study. Nurs Open. 2023, 10, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Y.; Lu, L.; Chen, Y.; Long, H.; Wang, J. Virtual simulation in undergraduate medical education: A scoping review of recent practice. Front. Med. 2022, 9, 855403. [Google Scholar] [CrossRef]
- Chan, W.Y.; Matteucci, P.; Southern, S.J. Validation of microsurgical models in microsurgery training and competence: A review. Microsurg. Off. J. Int. Microsurg. Soc. Eur. Fed. Soc. Microsurg. 2007, 27, 494–499. [Google Scholar] [CrossRef]
- Javid, P.; Aydın, A.; Mohanna, P.N.; Dasgupta, P.; Ahmed, K. Current status of simulation and training models in microsurgery: A systematic review. Microsurgery 2019, 39, 655–668. [Google Scholar] [CrossRef]
- Xiang, N.; Liang, H.N.; Yu, L.; Yang, X.; Zhang, J.J. A mixed reality framework for microsurgery simulation with visual-tactile perception. Vis. Comput. 2023, 39, 3661–3673. [Google Scholar] [CrossRef]
- Zackoff, M.; Real, F.; Sahay, R.; Fei, L.; Guiot, A.; Lehmann, C.; Tegtmeyer, K.; Klein, M. Impact of an Immersive Virtual Reality Curriculum on Medical Students’ Clinical Assessment of Infants With Respiratory Distress. Pediatr. Crit. Care Med. 2020, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Hernon, O.; McSharry, E.; MacLaren, I.; Carr, P.J. The use of educational technology in teaching and assessing clinical psychomotor skills in nursing and midwifery education: A state-of-the-art literature review. J. Prof. Nurs. 2023, 45, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S. Metaverse and Immersive Interaction Technology. In Metaverse: Concept, Content and Context; Springer: Cham, Switzerland, 2023; pp. 47–81. [Google Scholar]
- Abu-Salih, B. MetaOntology: Toward developing an ontology for the metaverse. Front. Big Data 2022, 5, 998648. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Kim, Y.O. Virtual reality and augmented reality in plastic surgery: A review. Arch. Plast. Surg. 2017, 44, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ameri, G.; Baxter, J.S.; Bainbridge, D.; Peters, T.M.; Chen, E.C. Mixed reality ultrasound guidance system: A case study in system development and a cautionary tale. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Overtoom, E.M.; Horeman, T.; Jansen, F.W.; Dankelman, J.; Schreuder, H.W. Haptic feedback, force feedback, and force-sensing in simulation training for laparoscopy: A systematic overview. J. Surg. Educ. 2019, 76, 242–261. [Google Scholar] [CrossRef]
- Yang, H.; Shao, L.; Zheng, F.; Wang, L.; Song, Z. Recent advances and trends in visual tracking: A review. Neurocomputing 2011, 74, 3823–3831. [Google Scholar] [CrossRef]
- Medicine, S. What Are the Different Methods of Surgery? Available online: https://stanfordhealthcare.org/medical-treatments/g/general-surgery/types.html (accessed on 6 October 2023).
- Gunalan, A.; Mattos, L.S. Towards OCT-Guided Endoscopic Laser Surgery & mdash; A Review. Diagnostics 2023, 13, 677. [Google Scholar] [CrossRef]
- Babalola, A.; Manu, P.; Cheung, C.; Yunusa-Kaltungo, A.; Bartolo, P. A systematic review of the application of immersive technologies for safety and health management in the construction sector. J. Saf. Res. 2023, 85, 66–85. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, Y.; Men, J.; Ma, Z.R.; Jiang, H.Z.; Liu, C.Y.; Feng, W. Application of Virtual and Augmented Reality Technology in Hip Surgery: Systematic Review. J. Med. Internet Res. 2023, 25, e37599. [Google Scholar] [CrossRef] [PubMed]
- Găină, M.A.; Szalontay, A.S.; Ștefănescu, G.; Bălan, G.G.; Ghiciuc, C.M.; Boloș, A.; Găină, A.M.; Ștefănescu, C. State-of-the-art review on immersive virtual reality interventions for colonoscopy-induced anxiety and pain. J. Clin. Med. 2022, 11, 1670. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.; Ramirez, O.A.D.; Vega, V.P.; Oliver, J.P.O. Phantom omni haptic device: Kinematic and manipulability. In Proceedings of the 2009 Electronics, Robotics and Automotive Mechanics Conference (CERMA), Cuernavaca, Mexico, 22–25 September 2009; pp. 193–198. [Google Scholar]
- Haptic Devices | 3D Systems. Available online: https://www.3dsystems.com/scanners-haptics. (accessed on 15 October 2023).
- Lin, M.C.; Otaduy, M. Haptic Rendering: Foundations, Algorithms, and Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- McNeely, W.A.; Puterbaugh, K.D.; Troy, J.J. Six degree-of-freedom haptic rendering using voxel sampling. In Proceedings of the ACM SIGGRAPH 2005 Courses, Los Angeles, CA, USA, 31 July–4 August 2005. [Google Scholar]
- Pacchierotti, C.; Prattichizzo, D.; Kuchenbecker, K.J. Cutaneous Feedback of Fingertip Deformation and Vibration for Palpation in Robotic Surgery. IEEE Trans. Biomed. Eng. 2016, 63, 278–287. [Google Scholar] [CrossRef]
- Sariturk, B.; Seker, D.Z. A Residual-Inception U-Net (RIU-Net) Approach and Comparisons with U-Shaped CNN and Transformer Models for Building Segmentation from High-Resolution Satellite Images. Sensors 2022, 22, 7624. [Google Scholar] [CrossRef] [PubMed]
- Xia, P. New advances for haptic rendering: State of the art. Vis. Comput. 2018, 34, 271–287. [Google Scholar] [CrossRef]
- Cirio, G.; Marchal, M.; Hillaire, S.; Lecuyer, A. Six Degrees-of-Freedom Haptic Interaction with Fluids. IEEE Trans. Vis. Comput. Graph. 2011, 17, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Ho Ba Tho, M.C.; Dao, T.T. A Systematic Review of Real-Time Medical Simulations with Soft-Tissue Deformation: Computational Approaches, Interaction Devices, System Architectures, and Clinical Validations. Appl. Bionics Biomech. 2020, 2020, 5039329. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, S.; Deng, Y.; Zhang, Y.; Yin, L.; Zheng, W. Construction of force haptic reappearance system based on Geomagic Touch haptic device. Comput. Methods Programs Biomed. 2020, 190, 105344. [Google Scholar] [CrossRef]
- Booth, S.; Angelis, F.; Schmidt-Tjarksen, T. The influence of changing haptic refresh-rate on subjective user experiences-lessons for effective touch-based applications. In Proceedings of the Eurohaptics, Dublin, Ireland, 6–9 July 2003; pp. 374–383. [Google Scholar]
- Laycock, S.; Day, A. A Survey of Haptic Rendering Techniques. Comput. Graph. Forum 2007, 26, 50–65. [Google Scholar] [CrossRef]
- Viglialoro, R.M.; Esposito, N.; Condino, S.; Cutolo, F.; Guadagni, S.; Gesi, M.; Ferrari, M.; Ferrari, V. Augmented reality to improve surgical simulation: Lessons learned towards the design of a hybrid laparoscopic simulator for cholecystectomy. IEEE Trans. Biomed. Eng. 2018, 66, 2091–2104. [Google Scholar] [CrossRef]
- Gadwe, A.; Ren, H. Real-time 6dof pose estimation of endoscopic instruments using printable markers. IEEE Sens. J. 2018, 19, 2338–2346. [Google Scholar] [CrossRef]
- Liu, X.; Plishker, W.; Shekhar, R. Hybrid electromagnetic-ArUco tracking of laparoscopic ultrasound transducer in laparoscopic video. J. Med. Imaging 2021, 8, 015001. [Google Scholar] [CrossRef] [PubMed]
- Shono, N.; Kin, T.; Nomura, S.; Miyawaki, S.; Saito, T.; Imai, H.; Nakatomi, H.; Oyama, H.; Saito, N. Microsurgery simulator of cerebral aneurysm clipping with interactive cerebral deformation featuring a virtual arachnoid. Oper. Neurosurg. 2018, 14, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Ding, H.; Wang, J.; Liu, P.; Ling, Q.; Chen, J.; Xu, J.; Zhang, S.; Xu, R. Designing a wearable navigation system for image-guided cancer resection surgery. Ann. Biomed. Eng. 2014, 42, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, H.; Song, G.; Zhao, Y.; Han, J. Augmented reality system training for minimally invasive spine surgery. In Proceedings of the 2017 IEEE International Conference on Robotics and Biomimetics (ROBIO), Macau, Macao, 5–8 December 2017; pp. 1200–1205. [Google Scholar]
- Wang, J.; Suenaga, H.; Hoshi, K.; Yang, L.; Kobayashi, E.; Sakuma, I.; Liao, H. Augmented reality navigation with automatic marker-free image registration using 3-D image overlay for dental surgery. IEEE Trans. Biomed. Eng. 2014, 61, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Suenaga, H.; Yang, L.; Kobayashi, E.; Sakuma, I. Video see-through augmented reality for oral and maxillofacial surgery. Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13, e1754. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. An Overview and Application of Deep Convolutional Neural Networks for Medical Image Segmentation. In Proceedings of the 2023 Third International Conference on Artificial Intelligence and Smart Energy (ICAIS), Coimbatore, India, 2–4 February 2023; pp. 722–728. [Google Scholar]
- Li, X.; Dick, A.; Wang, H.; Shen, C.; van den Hengel, A. Graph mode-based contextual kernels for robust SVM tracking. In Proceedings of the 2011 International Conference on Computer Vision, Barcelona, Spain, 6–13 November 2011; pp. 1156–1163. [Google Scholar]
- He, K.; Gkioxari, G.; Dollár, P.; Girshick, R. Mask r-cnn. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 2961–2969. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015: 18th International Conference, Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar]
- Ahmad, N.; Ghazilla, R.A.R.; Khairi, N.M.; Kasi, V. Reviews on various inertial measurement unit (IMU) sensor applications. Int. J. Signal Process. Syst. 2013, 1, 256–262. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, N.M.; Kumar, A. Hybrid method for 3D instrument reconstruction and tracking in laparoscopy surgery. In Proceedings of the 2013 International Conference on Control, Automation and Information Sciences (ICCAIS), Nha Trang, Vietnam, 31 May 2013; pp. 36–41. [Google Scholar] [CrossRef]
- Nakamoto, M.; Sato, Y.; Miyamoto, M.; Nakamjima, Y.; Konishi, K.; Shimada, M.; Hashizume, M.; Tamura, S. 3D Ultrasound System Using a Magneto-optic Hybrid Tracker for Augmented Reality Visualization in Laparoscopic Liver Surgery. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI, Tokyo, Japan, 25–28 September 2002; pp. 148–155. [Google Scholar]
- Basdogan, C.; Sedef, M.; Harders, M.; Wesarg, S. VR-based simulators for training in minimally invasive surgery. IEEE Comput. Graph. Appl. 2007, 27, 54–66. [Google Scholar] [CrossRef]
- Medicine, Y. Minimally Invasive Surgery. Available online: https://www.yalemedicine.org/conditions/minimally-invasive-surgery. (accessed on 12 October 2023).
- Pan, J.; Zhang, L.; Yu, P.; Shen, Y.; Wang, H.; Hao, H.; Qin, H. Real-time VR simulation of laparoscopic cholecystectomy based on parallel position-based dynamics in GPU. In Proceedings of the 2020 IEEE Conference on Virtual Reality and 3D User Interfaces (VR), Atlanta, GA, USA, 22–26 March 2020; pp. 548–556. [Google Scholar]
- Qian, K.; Bai, J.; Yang, X.; Pan, J.; Zhang, J. Virtual reality based laparoscopic surgery simulation. In Proceedings of the 21st ACM Symposium on Virtual Reality Software and Technology, Beijing, China, 13–15 November 2015; pp. 69–78. [Google Scholar]
- Botden, S.M.; de Hingh, I.H.; Jakimowicz, J.J. Meaningful assessment method for laparoscopic suturing training in augmented reality. Surg. Endosc. 2009, 23, 2221–2228. [Google Scholar] [CrossRef]
- Zátonyi, J.; Paget, R.; Székely, G.; Grassi, M.; Bajka, M. Real-time synthesis of bleeding for virtual hysteroscopy. Med. Image Anal. 2005, 9, 255–266. [Google Scholar] [CrossRef]
- von Websky, M.W.; Vitz, M.; Raptis, D.A.; Rosenthal, R.; Clavien, P.; Hahnloser, D. Basic laparoscopic training using the Simbionix LAP Mentor: Setting the standards in the novice group. J. Surg. Educ. 2012, 69, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.R.; Lohani, S.; Manjila, S.; Natsupakpong, S.; Brown, N.; Cavusoglu, M.C. Virtual reality simulation: Basic concepts and use in endoscopic neurosurgery training. Child’s Nerv. Syst. 2013, 29, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Breimer, G.E.; Haji, F.A.; Bodani, V.; Cunningham, M.S.; Lopez-Rios, A.L.; Okrainec, A.; Drake, J.M. Simulation-based education for endoscopic third ventriculostomy: A comparison between virtual and physical training models. Oper. Neurosurg. 2017, 13, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Bai, J.; Yang, X.; Pan, J.; Zhang, J. Essential techniques for laparoscopic surgery simulation. Comput. Animat. Virtual Worlds 2017, 28, e1724. [Google Scholar] [CrossRef]
- Matsuo, A.; Hamada, H.; Oba, H.; Shibata, K. Virtual reality head-mounted display for endoscopically-assisted implant surgery. Br. J. Oral Maxillofac. Surg. 2018, 56, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, J.G.; Sørensen, S.M.D.; Konge, L.; Svendsen, M.B.S.; Nobel-Jørgensen, M.; Bjerrum, F.; Andersen, S.A.W. Cognitive load and performance in immersive virtual reality versus conventional virtual reality simulation training of laparoscopic surgery: A randomized trial. Surg. Endosc. 2020, 34, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Koizumi, T.; Mansour, D.A.; Fujimori, A.; Kusano, T.; Matsuda, K.; Nogaki, K.; Tashiro, Y.; Hakozaki, T.; Wada, Y.; et al. Virtual reality with three-dimensional image guidance of individual patients’ vessel anatomy in laparoscopic distal pancreatectomy. Langenbeck’s Arch. Surg. 2020, 405, 381–389. [Google Scholar] [CrossRef]
- Tai, Y.; Shi, J.; Pan, J.; Hao, A.; Chang, V. Augmented reality-based visual-haptic modeling for thoracoscopic surgery training systems. Virtual Real. Intell. Hardw. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Lohre, R.; Wang, J.C.; Lewandrowski, K.U.; Goel, D.P. Virtual reality in spinal endoscopy: A paradigm shift in education to support spine surgeons. J. Spine Surg. 2020, 6, S208. [Google Scholar] [CrossRef]
- Long, A.S.; Almeida, M.N.; Chong, L.; Prsic, A. Live Virtual Surgery and Virtual Reality in Surgery: Potential Applications in Hand Surgery Education. J. Hand Surg. 2023, 48, 499–505. [Google Scholar] [CrossRef]
- Lin, Y.K.; Yau, H.T.; Wang, I.C.; Zheng, C.; Chung, K.H. A novel dental implant guided surgery based on integration of surgical template and augmented reality. Clin. Implant. Dent. Relat. Res. 2015, 17, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Satoh, M.; Konno, T.; Hirai, M.; Yamaguchi, T. The trans-visible navigator: A see-through neuronavigation system using augmented reality. World Neurosurg. 2016, 87, 399–405. [Google Scholar] [CrossRef] [PubMed]
- McJunkin, J.L.; Jiramongkolchai, P.; Chung, W.; Southworth, M.; Durakovic, N.; Buchman, C.A.; Silva, J.R. Development of a mixed reality platform for lateral skull base anatomy. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2018, 39, e1137. [Google Scholar] [CrossRef] [PubMed]
- Weidert, S.; Wang, L.; Landes, J.; Sandner, P.; Suero, E.M.; Navab, N.; Kammerlander, C.; Euler, E.; von Der Heide, A. Video-augmented fluoroscopy for distal interlocking of intramedullary nails decreased radiation exposure and surgical time in a bovine cadaveric setting. Int. J. Med. Robot. Comput. Assist. Surg. 2019, 15, e1995. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.; Rabelo, N.N.; Vieira, E.; Mendes, K.; Zagatto, G.; de Oliveira, R.S.; Raposo-Amaral, C.E.; Yoshida, M.; de Souza, M.R.; Fagundes, C.F.; et al. Augmented reality and physical hybrid model simulation for preoperative planning of metopic craniosynostosis surgery. Neurosurg. Focus 2020, 48, E19. [Google Scholar] [CrossRef] [PubMed]
- Golse, N.; Petit, A.; Lewin, M.; Vibert, E.; Cotin, S. Augmented reality during open liver surgery using a markerless non-rigid registration system. J. Gastrointest. Surg. 2021, 25, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Fushima, K.; Kobayashi, M. Mixed-reality simulation for orthognathic surgery. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 1–12. [Google Scholar] [CrossRef]
- Yoon, J.W.; Chen, R.E.; ReFaey, K.; Diaz, R.J.; Reimer, R.; Komotar, R.J.; Quinones-Hinojosa, A.; Brown, B.L.; Wharen, R.E. Technical feasibility and safety of image-guided parieto-occipital ventricular catheter placement with the assistance of a wearable head-up display. Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13, e1836. [Google Scholar] [CrossRef]
- Alaraj, A.; Luciano, C.J.; Bailey, D.P.; Elsenousi, A.; Roitberg, B.Z.; Bernardo, A.; Banerjee, P.P.; Charbel, F.T. Virtual reality cerebral aneurysm clipping simulation with real-time haptic feedback. Neurosurgery 2015, 11, 52. [Google Scholar] [CrossRef]
- Azarnoush, H.; Alzhrani, G.; Winkler-Schwartz, A.; Alotaibi, F.; Gelinas-Phaneuf, N.; Pazos, V.; Choudhury, N.; Fares, J.; DiRaddo, R.; Del Maestro, R.F. Neurosurgical virtual reality simulation metrics to assess psychomotor skills during brain tumor resection. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 603–618. [Google Scholar] [CrossRef]
- Pulijala, Y.; Ma, M.; Pears, M.; Peebles, D.; Ayoub, A. An innovative virtual reality training tool for orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2018, 47, 1199–1205. [Google Scholar] [CrossRef]
- Xiang, N.; Yang, X.; Zhang, J.J. Tsfps: An accurate and flexible 6dof tracking system with fiducial platonic solids. In Proceedings of the 29th ACM International Conference on Multimedia, Virtual, 20–24 October 2021; pp. 4454–4462. [Google Scholar]
- Hazarika, A.; Rahmati, M. Towards an Evolved Immersive Experience: Exploring 5G- and Beyond-Enabled Ultra-Low-Latency Communications for Augmented and Virtual Reality. Sensors 2023, 23, 3682. [Google Scholar] [CrossRef]


| Author and Year | Surgical Procedure | Immersive Interaction Type | Description | Device and/or Method |
|---|---|---|---|---|
| Botden et al., 2009 [56] | Laparoscopic surgery | AR | Validation of newly developed laparoscopic suturing on the ProMIS augmented reality simulator | ProMIS v2.0 augmented reality (AR) simulator with visual tracking solution |
| Viglialoro et al., 2019 [37] | Laparoscopic cholecystectomy | AR | Reported results of the long-term development phase of a hybrid simulator for laparoscopic cholecystectomy | Electromagnetic emitter/visual tracking-based AR device |
| Zátonyi et al., 2005 [57] | Hysteroscopic surgery | VR | A state-of-the-art graphical blood flow simulation designed to meet the specific requirements of virtual hysteroscopic surgical bleeding simulation | 3D Fluid Simulation |
| von WebSky et al., 2012 [58] | Laparoscopic surgery | VR | Compiled a set of criteria to demonstrate the performance and feasibility of the Simbionix LAP Mentor for basic laparoscopic training for novice surgeons | Simbionix® LAP Mentor with haptic feedback device |
| Cohen et al., 2013 [59] | Endoscopic neurosurgery | VR | A review of basic concepts and applications in endoscopic neurosurgery training | Unspecified haptic feedback device |
| Breimer et al., 2017 [60] | Endoscopic neurosurgery | VR | Evaluation of the Educational Benefits of Virtual Reality (VR) and Physical Simulation Models for Endoscopic Third Ventriculostomy (ETV) | PHANTOM® Omni (haptic feedback device) and NeuroTouch |
| Qian et al., 2017 [61] | Laparoscopic surgery | VR | Proposed a set of tailored key technologies for laparoscopic surgery simulation | Unspecified haptic feedback device |
| Matsuo et al., 2018 [62] | Endoscopically assisted implant surgery | VR | Established a system of endoscopically assisted VR for implant surgery using a head-mounted display | Sony® HMS-3000MT and Olympus 4mm nasoscope |
| Frederiksen et al., 2019 [63] | Laparoscopic surgery | VR | Cognitive load of immersive VR laparoscopic simulation of surgery was assessed | Simball 4D joysticks (haptic feedback device) and Oculus Rift |
| Aoki et al., 2020 [64] | Laparoscopic distal pancreatectomy | VR | Evaluate the impact of 3DVE guidance in laparoscopic distal pancreatectomy (LDP) | SYNAPSE VINCENT Volume Analyzer |
| Pan et al., 2020 [54] | Laparoscopic cholecystectomy surgery | VR | A VR simulation framework based on PBD for cholecystectomy that has been applied to laparoscopic cholecystectomy training in several hospitals | Unspecified haptic feedback device |
| Tai et al., 2021 [65] | Thoracoscopic surgery | VR, AR | Presented the AR visual rendering and haptic modeling to study the potential benefits of thoracoscope surgical skills | Unspecified haptic feedback device |
| Lohre et al., 2020 [66] | Spinal endoscopic surgery | VR, AR, MR | Reviewed work on endoscopic spinal surgery | Multiple works on different devices are reviewed, such as H3D and Volume Haptics Toolkit, PHANTOM® haptic device graphical user interface and various unspecified haptic devices |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Xiang, N.; Pan, J. State of the Art in Immersive Interactive Technologies for Surgery Simulation: A Review and Prospective. Bioengineering 2023, 10, 1346. https://doi.org/10.3390/bioengineering10121346
Deng Z, Xiang N, Pan J. State of the Art in Immersive Interactive Technologies for Surgery Simulation: A Review and Prospective. Bioengineering. 2023; 10(12):1346. https://doi.org/10.3390/bioengineering10121346
Chicago/Turabian StyleDeng, Zihan, Nan Xiang, and Junjun Pan. 2023. "State of the Art in Immersive Interactive Technologies for Surgery Simulation: A Review and Prospective" Bioengineering 10, no. 12: 1346. https://doi.org/10.3390/bioengineering10121346
APA StyleDeng, Z., Xiang, N., & Pan, J. (2023). State of the Art in Immersive Interactive Technologies for Surgery Simulation: A Review and Prospective. Bioengineering, 10(12), 1346. https://doi.org/10.3390/bioengineering10121346








