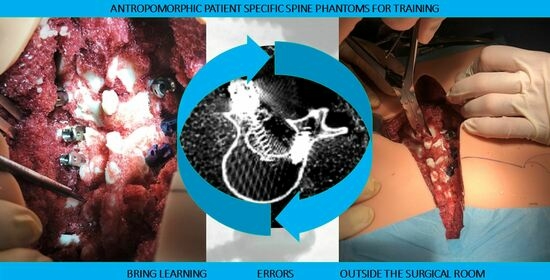
Design, Fabrication, and Preliminary Validation of Patient-Specific Spine Section Phantoms for Use in Training Spine Surgeons Outside the Operating Room/Theatre
Abstract
:1. Introduction
2. Materials and Methods
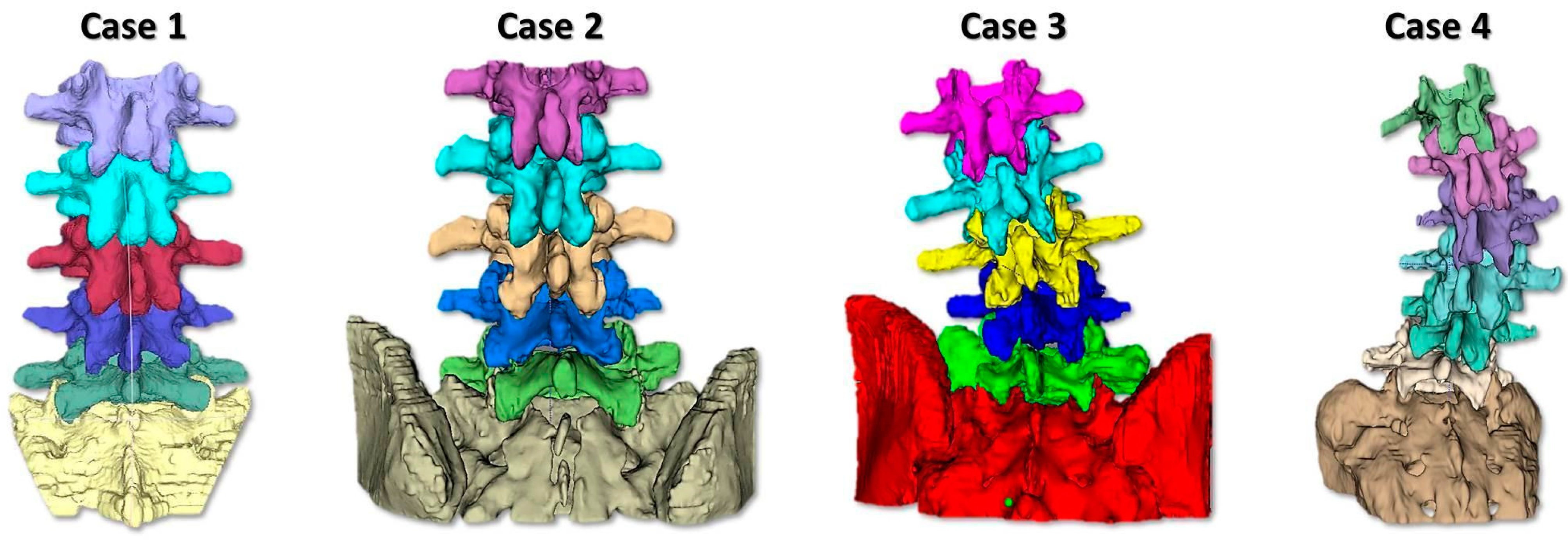
2.1. Phantom Design and Realization
- -
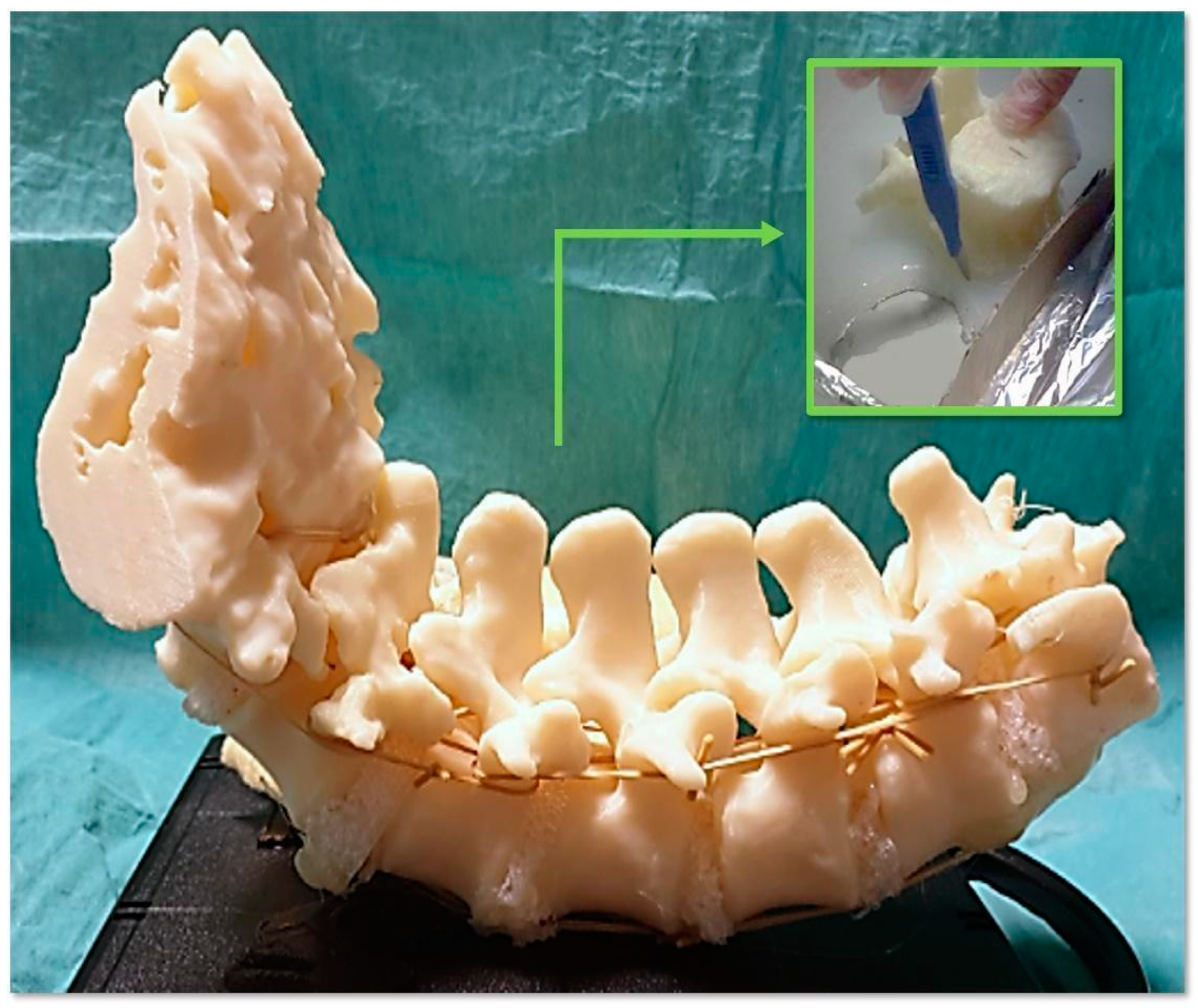
- To simulate the spine instrumentation and the challenges related to this action, our phantoms included patient-specific bone replicas with a correct replication of the cortico–cancellous interface (thoracic and lumbar vertebrae of actual pathological patients); flexible intervertebral discs to mimic intervertebral natural movements; and a flexible anterior longitudinal ligament to hold the vertebrae together, stabilize the spine, and allow physiological motion. An additional feature of our phantoms was the replication of realistic radiodensities for bony structures.
- -
- Patient-specific deepness of the operating room/theater. No efforts were made for the accurate replication of muscle structures, as surgical access challenges were not the intended use of the phantoms. In any case, to provide trainees with the opportunity to experience the same challenges as are faced in the confined space of the operating room/theatre, the replica of the spine was sunk in a soft material that replicated the colors and bulkiness of muscles, providing the trainee with a realistic operating field. Moreover, a skin-like covering allowed an accurate simulation of palpation and surgical incisions.
2.2. Testing Setup and Structure
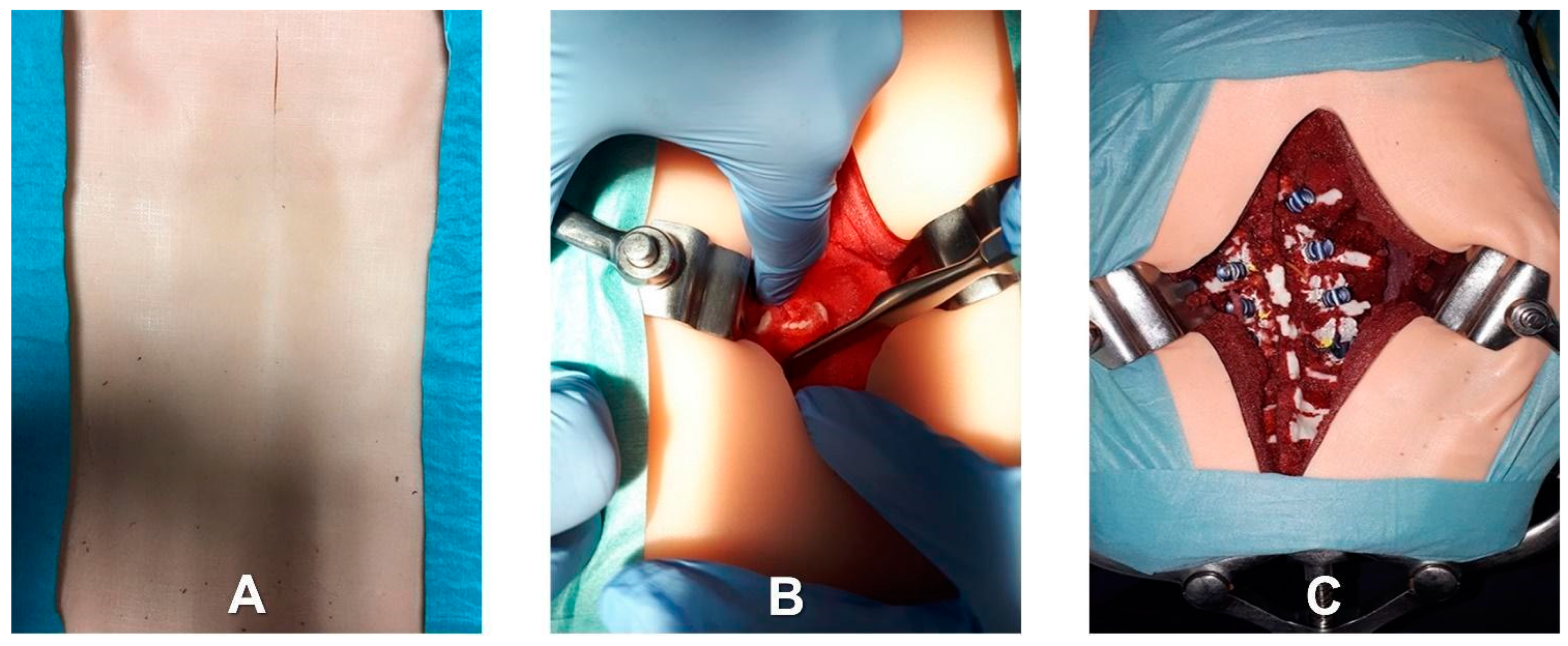
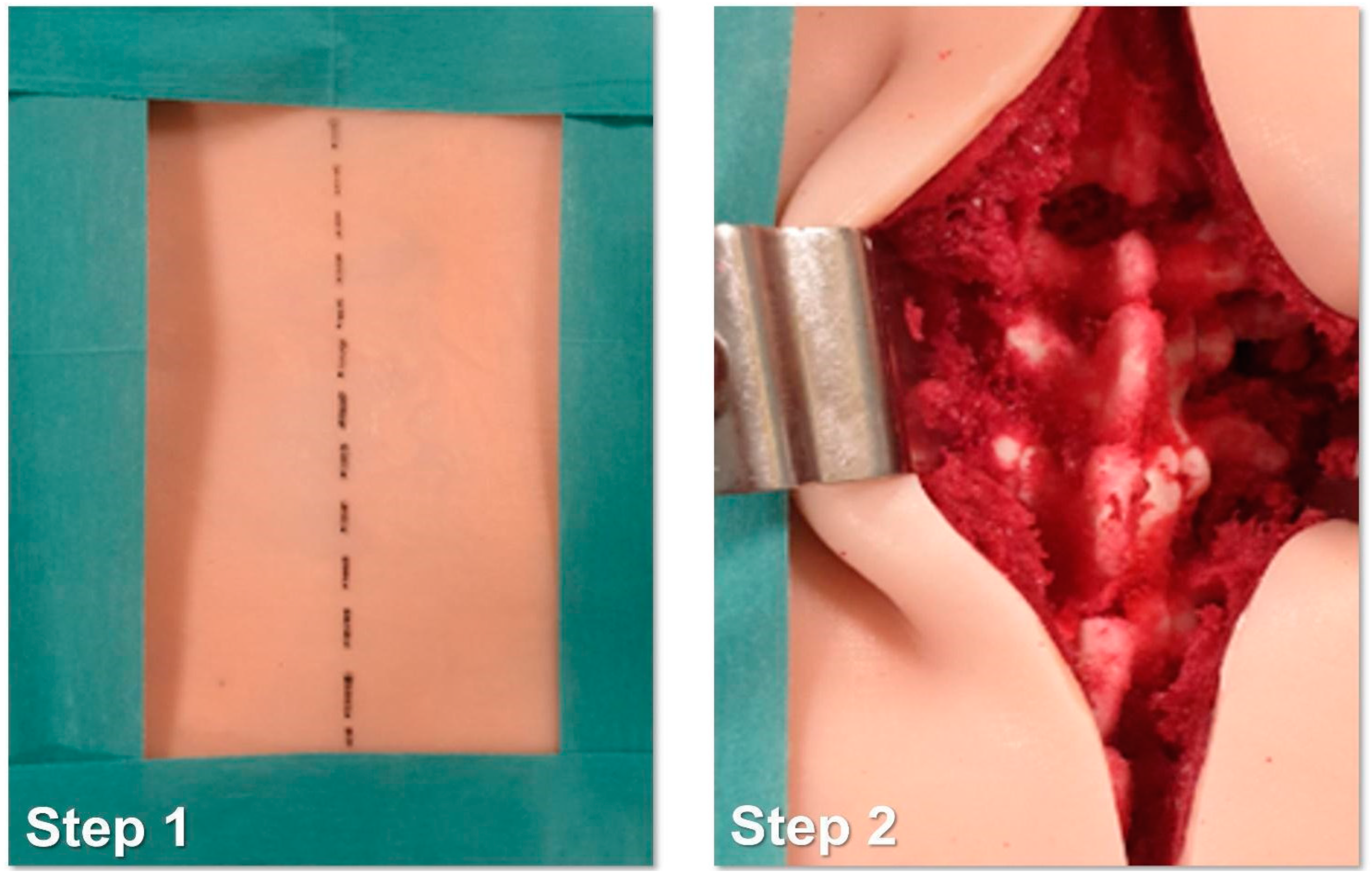
- Identification of the longitudinal surgical access point, determined after locating the spinous processes through deep palpation (Figure 4).
- Exposure of anatomical landmarks such as facet joints, transverse processes, and the lateral portion of the pars interarticularis following incision, divarication, and the removal of soft tissue from the exposed surface (Figure 4).
- Creation of the cortical breach and placement of the bone probe after the identification of the entry point. The latter corresponded with the point where the major axis of the transverse process met the line passing through the lateral margin of the superior facial joint (Figure 5).
- Navigation of the bone probe into the pedicle along the ideal trajectory. This was achieved by aiming for the contralateral transverse process, thus aligning the screw with the superior endplate. To assess the integrity of the hole walls, a probe was inserted (Figure 5).
- Insertion of the selected screw (ensuring it passed through the canal in the pedicle and affected 2/3 of the vertebral body’s depth (Figure 5).
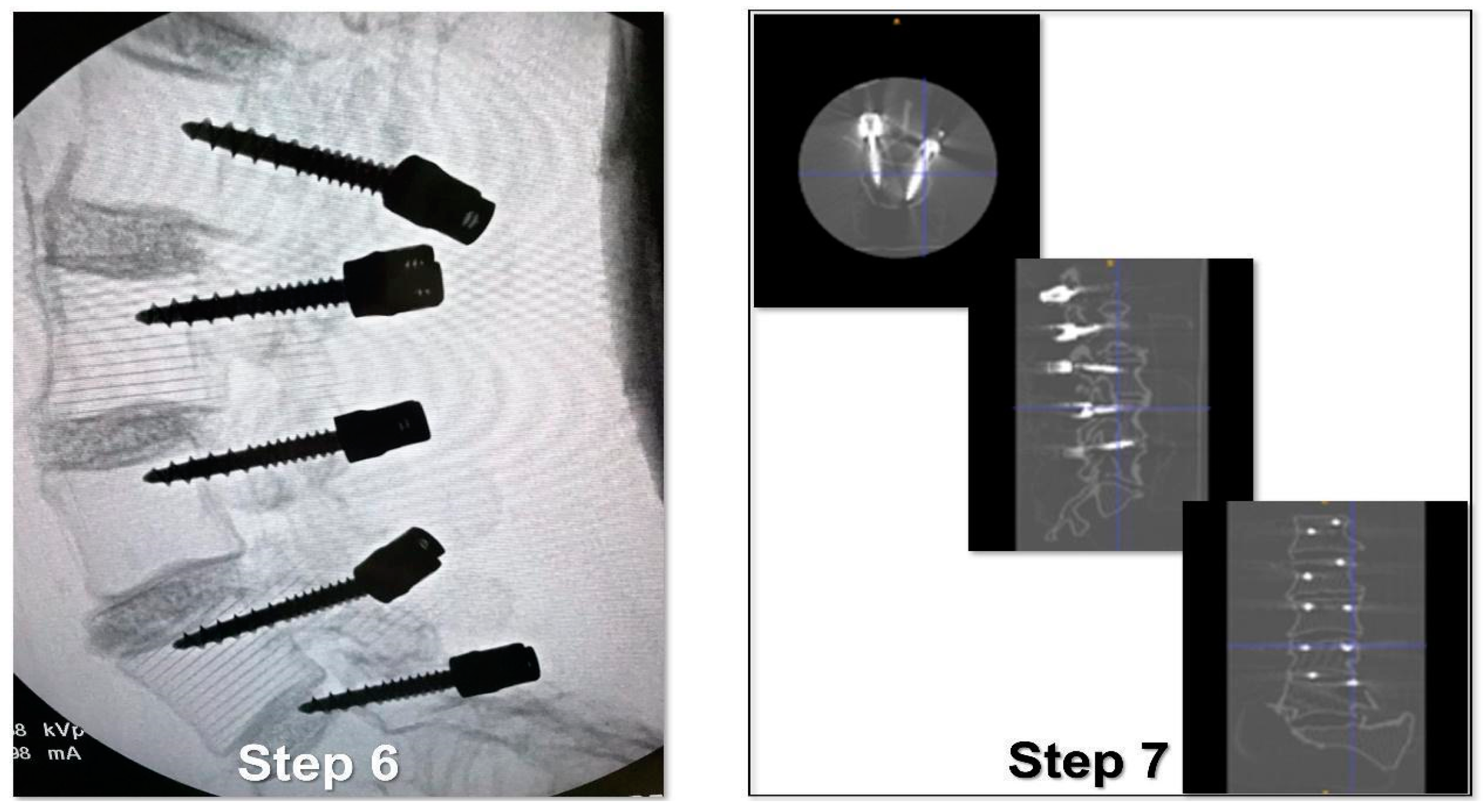
- Verification of the PS placement under RX control (Figure 6).
- Classification of the PS placement, according to the degree of possible pedicle wall violation under CT control (Figure 6).
- -
- In the first session, R0 worked on Phantoms 1 and 2 (simulating low-complexity level cases) but under active supervision (the expert surgeon tutored him/her for each screw insertion, guiding R0 and putting hands on the phantom).
- -
- In the second session, R0 repeated the work on Phantoms 1 and 2 (low-complexity level) but under passive supervision (the expert surgeon guided R0 by talking to him/her and eventually advising and/or correcting R0’s gestures, but without putting hands on the phantom).
- -
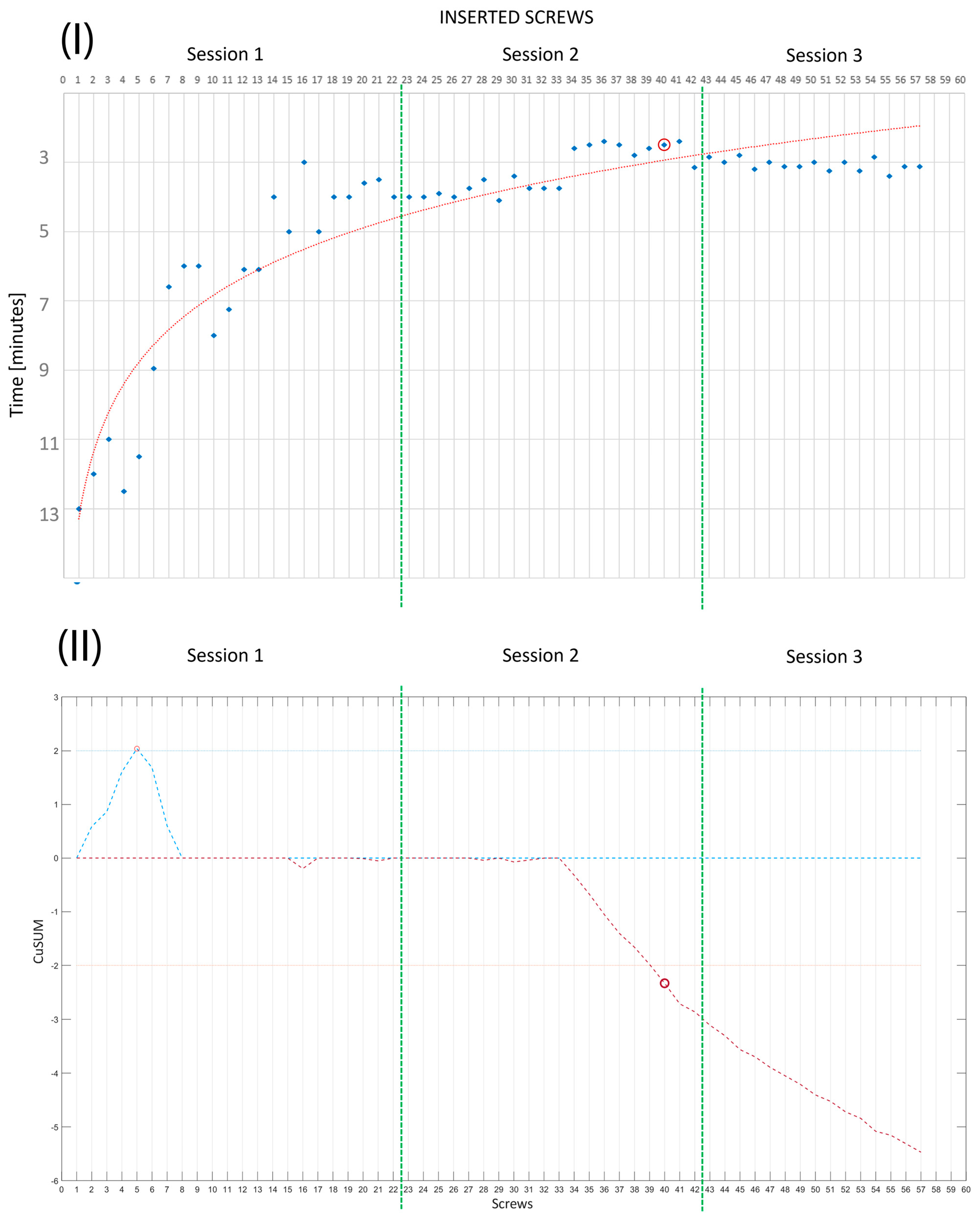
- In the third session, R0 worked on Phantoms 3 and 4 (simulating medium-complexity cases) without any supervision; that is, the expert surgeon was present but did not intervene during the session. Two performance measurements were conducted. These were PS placement accuracy, evaluated by the degree of pedicle wall violation on CT images according to the classification of Gertzbein and Robbins [39], and the time taken to place each implanted screw.
- The anthropomorphic phantom accurately replicated the surgical field and necessary anatomy for the simulation of posterior pedicle screw insertions.
- The anthropomorphic phantom provided a surgical field closely resembling an actual one in terms of confined space, anatomical structure footprint, and visibility.
- The feedback on hands and surgical instruments during the surgical tasks was realistic.
- The anthropomorphic phantom, when arranged in a simulation course with increasing complexity, was a valuable platform for the teaching of how to perform posterior pedicle screw fixation.
3. Results
3.1. Quantitative Evaluation
3.2. Qualitative Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crawford, N.; Johnson, N.; Theodore, N. Ensuring navigation integrity using robotics in spine surgery. J. Robot. Surg. 2020, 14, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Burström, G.; Persson, O.; Edström, E.; Elmi-Terander, A. Augmented reality navigation in spine surgery: A systematic review. Acta Neurochir. 2021, 163, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.H.; McDonald, D.; Araghi, K.; Araghi, T.; Chutkan, N.; Araghi, A. The Clinical Impact of Image Guidance and Robotics in Spinal Surgery: A Review of Safety, Accuracy, Efficiency, and Complication Reduction. Int. J. Spine Surg. 2021, 15, S10–S20. [Google Scholar] [CrossRef]
- Perfetti, D.C.; Kisinde, S.; Rogers-LaVanne, M.P.; Satin, A.M.; Lieberman, I.H. Robotic Spine Surgery: Past, Present, and Future. Spine 2022, 47, 909–921. [Google Scholar] [CrossRef]
- Shafi, K.A.; Pompeu, Y.A.; Vaishnav, A.S.; Mai, E.; Sivaganesan, A.; Shahi, P.; Qureshi, S.A. Does robot-assisted navigation influence pedicle screw selection and accuracy in minimally invasive spine surgery? Neurosurg. Focus 2022, 52, E4. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Wang, M.Y. Minimally Invasive Spinal Deformity Surgery: Current State and Future Direction. Neurosurgery 2016, 63 (Suppl. S1), 43–51. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Hussain, I.; Sommer, F.; Hartl, R.; Elowitz, E. The Future of Minimally Invasive Spinal Surgery. World Neurosurg. 2022, 163, 233–240. [Google Scholar] [CrossRef]
- Jain, A.; Dai, T.B.K.; Myers, C.G. COVID-19 created an elective surgery backlog: How can hospitals get back on track. Harv. Bus. Rev. 2020, 10. [Google Scholar]
- Losco, M.; Familiari, F.; Giron, F.; Papalia, R. Use and Effectiveness of the Cadaver-Lab in Orthopaedic and Traumatology Education: An Italian Survey. Joints 2017, 5, 197–201. [Google Scholar] [CrossRef]
- Daniels, A.H.; Ames, C.P.; Garfin, S.R.; Shaffrey, C.I.; Riew, K.D.; Smith, J.S.; Anderson, P.A.; Hart, R.A. Spine surgery training: Is it time to consider categorical spine surgery residency? Spine J. 2015, 15, 1513–1518. [Google Scholar] [CrossRef]
- Herkowitz, H.N.; Connolly, P.J.; Gundry, C.R.; Varlotta, G.P.; Zdeblick, T.A.; Truumees, E. Resident and fellowship guidelines: Educational guidelines for resident training in spinal surgery. Spine 2000, 25, 2703–2707. [Google Scholar] [CrossRef]
- Yamagata, T.; Chataigner, H.; Longis, P.-M.; Takami, T.; Delecrin, J. Posterior instrumented fusion surgery for adult spinal deformity: Correction rate and total balance. J. Craniovertebral Junction Spine 2019, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Castro, W.H.; Halm, H.; Jerosch, J.; Malms, J.; Steinbeck, J.; Blasius, S. Accuracy of pedicle screw placement in lumbar vertebrae. Spine 1996, 21, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Maruo, K.; Arizumi, F.; Kishima, K.; Yoshie, N.; Kusukawa, T.; Tachibana, T. Patient-specific guide systems decrease the major perforation rate of pedicle screw placement in comparison to the freehand technique for adolescent idiopathic scoliosis. Eur. Spine J. 2023, 32, 3105–3112. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Bhushan, M.; Aiyer, S.; Kanna, R.; Shetty, A.P. Accuracy of pedicle screw insertion by AIRO(A (R)) intraoperative CT in complex spinal deformity assessed by a new classification based on technical complexity of screw insertion. Eur. Spine J. 2018, 27, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, V.; Parchi, P.; Condino, S.; Carbone, M.; Baluganti, A.; Ferrari, M.; Mosca, F.; Lisanti, M. An optimal design for patient-specific templates for pedicle spine screws placement. Int. J. Med. Robot. Comput. Assist. Surg. 2013, 9, 298–304. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Song, X.; Liu, S.; Zhang, Y.; Jiang, S.; Ji, X.; Zhang, T.; Xu, F.; Hu, L.; et al. Accuracy Evaluation of a Novel Spinal Robotic System for Autonomous Laminectomy in Thoracic and Lumbar Vertebrae: A Cadaveric Study. J. Bone Jt. Surg. Am. 2023, 105, 943–950. [Google Scholar] [CrossRef]
- Gonzalvo, A.; Fitt, G.; Liew, S.; de la Harpe, D.; Turner, P.; Ton, L.; Rogers, M.A.; Wilde, P.H. The Learning Curve of Pedicle Screw Placement: How Many Screws Are Enough? Spine 2009, 34, E761–E765. [Google Scholar] [CrossRef]
- Condino, S.; Turini, G.; Parchi, P.D.; Viglialoro, R.M.; Piolanti, N.; Gesi, M.; Ferrari, M.; Ferrari, V. How to Build a Patient-Specific Hybrid Simulator for Orthopaedic Open Surgery: Benefits and Limits of Mixed-Reality Using the Microsoft HoloLens. J. Healthc. Eng. 2018, 2018, 5435097. [Google Scholar] [CrossRef]
- Clifton, W.; Damon, A.; Valero-Moreno, F.; Nottmeier, E.; Pichelmann, M. The SpineBox: A Freely Available, Open-access, 3D-printed Simulator Design for Lumbar Pedicle Screw Placement. Cureus 2020, 12, e7738. [Google Scholar] [CrossRef]
- Yang, P.; Ju, Y.; Hu, Y.; Xie, X.; Fang, B.; Lei, L. Emerging 3D bioprinting applications in plastic surgery. Biomater. Res. 2023, 27, 1. [Google Scholar] [CrossRef]
- Parchi, P.; Condino, S.; Carbone, M.; Gesi, M.; Ferrari, V.; Ferrari, M.; Lisanti, M. Total hip replacement simulators with virtual planning and physical replica for surgical training and reharsal. In Proceedings of the 12th IASTED International Conference on Biomedical Engineering, BioMed 2016, Innsbruck, Austria, 15–17 February 2016; pp. 97–101. [Google Scholar]
- Condino, S.; Carbone, M.; Barderi, S.; Piazza, R.; Ferrari, V.; Parchi, P. Simulation in spinal surgery: State of the art and future perspectives of simulation systems for surgical training. Minerva Orthop. 2021, 72, 365–375. [Google Scholar] [CrossRef]
- Agyeman, K.D.; Summers, S.H.; Massel, D.H.; Mouhanna, J.; Aiyer, A.; Dodds, S.D. Innovation in orthopaedic surgery education: Novel tools for modern times. J. Am. Acad. Orthop. Surg. 2020, 28, e782–e792. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, G.; Rodrigo, A.; Besa, P.; Irarrázaval, S. Orthopaedic residents’ transfer of knee arthroscopic abilities from the simulator to the operating room. J. Am. Acad. Orthop. Surg. 2020, 28, 194–199. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, Z.; Bao, J.; Wen, L.; Zhang, L. C2 pedicle screw placement on 3D-printed models for the performance assessment of CTA-based screw preclusion. J. Orthop. Surg. Res. 2023, 18, 7. [Google Scholar] [CrossRef]
- Hong, J.-k.; Bae, I.-S.; Kang, H.I.; Kim, J.H.; Jwa, C. Development of a Pedicle Screw Fixation Simulation Model for Surgical Training Using a 3-Dimensional Printer. World Neurosurg. 2023, 171, e554–e559. [Google Scholar] [CrossRef]
- Clifton, W.; Pichelmann, M.; Vlasak, A.; Damon, A.; ReFaey, K.; Nottmeier, E. Investigation and Feasibility of Combined 3D Printed Thermoplastic Filament and Polymeric Foam to Simulate the Cortiocancellous Interface of Human Vertebrae. Sci. Rep. 2020, 10, 2912. [Google Scholar] [CrossRef] [PubMed]
- Munshi, F.; Lababidi, H.; Alyousef, S. Low-versus high-fidelity simulations in teaching and assessing clinical skills. J. Taibah Univ. Med. Sci. 2015, 10, 12–15. [Google Scholar] [CrossRef]
- Stirling, E.R.; Lewis, T.L.; Ferran, N.A. Surgical skills simulation in trauma and orthopaedic training. J. Orthop. Surg. Res. 2014, 9, 126. [Google Scholar] [CrossRef]
- Schoenherr, J.R.; Hamstra, S.J. Beyond Fidelity: Deconstructing the Seductive Simplicity of Fidelity in Simulator-Based Education in the Health Care Professions. Simul. Healthc. 2017, 12, 117–123. [Google Scholar] [CrossRef]
- Ferrari, V.; Carbone, M.; Cappelli, C.; Boni, L.; Melfi, F.; Ferrari, M.; Mosca, F.; Pietrabissa, A. Value of multidetector computed tomography image segmentation for preoperative planning in general surgery. Surg. Endosc. 2012, 26, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Penumakala, P.K.; Santo, J.; Thomas, A. A critical review on the fused deposition modeling of thermoplastic polymer composites. Compos. Part B Eng. 2020, 201, 108336. [Google Scholar] [CrossRef]
- Turek, P.; Budzik, G.; Przeszłowski, Ł. Assessing the Radiological Density and Accuracy of Mandible Polymer Anatomical Structures Manufactured Using 3D Printing Technologies. Polymers 2020, 12, 2444. [Google Scholar] [CrossRef] [PubMed]
- So-Yeon, P.; Noorie, C.; Byeong Geol, C.; Dong Myung, L.; Na Young, J. Radiological Characteristics of Materials Used in 3-Dimensional Printing with Various Infill Densities. Prog. Med. Phys. 2019, 30, 155–159. [Google Scholar] [CrossRef]
- Tino, R.; Leary, M.; Yeo, A.; Brandt, M.; Kron, T. Gyroid structures for 3D-printed heterogeneous radiotherapy phantoms. Phys Med. Biol. 2019, 64, 21NT05. [Google Scholar] [CrossRef]
- Condino, S.; Carbone, M.; Ferrari, V.; Faggioni, L.; Peri, A.; Ferrari, M.; Mosca, F. How to build patient-specific synthetic abdominal anatomies. An innovative approach from physical toward hybrid surgical simulators. Int. J. Med. Robot. 2011, 7, 202–213. [Google Scholar] [CrossRef]
- Gertzbein, S.D.; Robbins, S.E. Accuracy of pedicular screw placement in vivo. Spine 1990, 15, 11–14. [Google Scholar] [CrossRef]
- McDougall, E.M. Validation of surgical simulators. J. Endourol. 2007, 21, 244–247. [Google Scholar] [CrossRef]
- Samia, H.; Khan, S.; Lawrence, J.; Delaney, C.P. Simulation and Its Role in Training. Clin. Colon Rectal Surg. 2013, 26, 47–55. [Google Scholar] [CrossRef]
- Schout, B.M.; Hendrikx, A.J.; Scheele, F.; Bemelmans, B.L.; Scherpbier, A.J. Validation and implementation of surgical simulators: A critical review of present, past, and future. Surg. Endosc. 2010, 24, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Zheng, F.; Shin, M.; Liu, X.; Oh, D.; D’Attilio, D. CUSUM learning curves: What they can and can’t tell us. Surg. Endosc. 2023, 37, 7991–7999. [Google Scholar] [CrossRef] [PubMed]
- Bohl, M.A.; McBryan, S.; Pais, D.; Chang, S.W.; Turner, J.D.; Nakaji, P.; Kakarla, U.K. The Living Spine Model: A Biomimetic Surgical Training and Education Tool. Oper. Neurosurg. 2020, 19, 98–106. [Google Scholar] [CrossRef]
- Li, H.-M.; Zhang, R.-J.; Shen, C.-L. Accuracy of pedicle screw placement and clinical outcomes of robot-assisted technique versus conventional freehand technique in spine surgery from nine randomized controlled trials: A meta-analysis. Spine 2020, 45, E111–E119. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Ammanuel, S.; Gillan, D.; Shah, V. Efficacy of using a 3D printed lumbosacral spine phantom in improving trainee proficiency and confidence in CT-guided spine procedures. 3D Print. Med. 2018, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Wang, C.; Choi, K.H.; Kim, H.N. Use of a life-size three-dimensional-printed spine model for pedicle screw instrumentation training. J. Orthop. Surg. Res. 2018, 13, 86. [Google Scholar] [CrossRef]

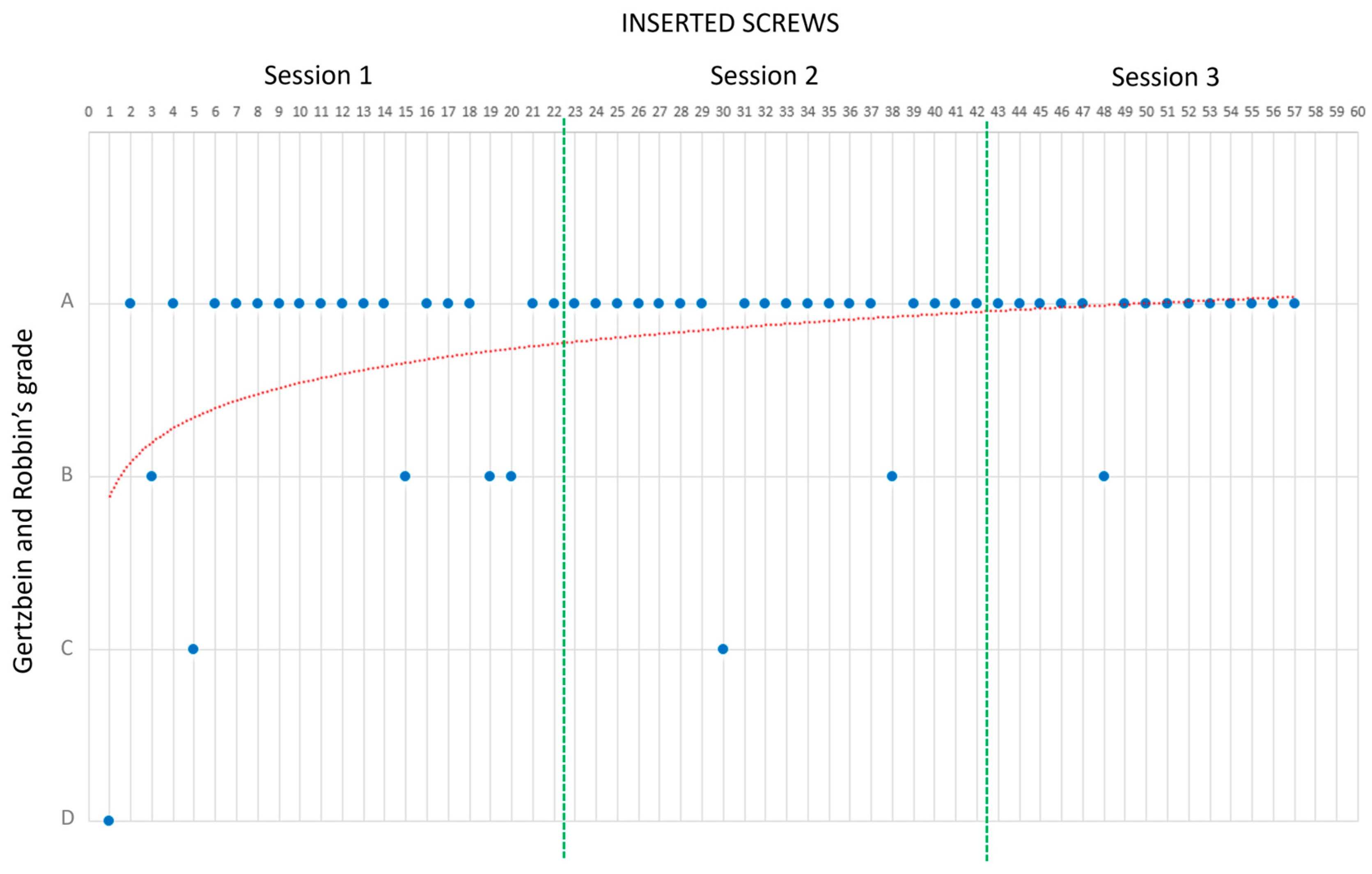
| Percentage of Screws per Grade | ||||
|---|---|---|---|---|
| Session | Grade A | Grade B | Grade C | Grade D |
| 1 | 73% | 18% | 5% | 5% |
| 2 | 90% | 5% | 5% | |
| 3 | 93% | 5% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, M.; Viglialoro, R.M.; Stagnari, S.; Condino, S.; Gesi, M.; Scaglione, M.; Parchi, P.D. Design, Fabrication, and Preliminary Validation of Patient-Specific Spine Section Phantoms for Use in Training Spine Surgeons Outside the Operating Room/Theatre. Bioengineering 2023, 10, 1345. https://doi.org/10.3390/bioengineering10121345
Carbone M, Viglialoro RM, Stagnari S, Condino S, Gesi M, Scaglione M, Parchi PD. Design, Fabrication, and Preliminary Validation of Patient-Specific Spine Section Phantoms for Use in Training Spine Surgeons Outside the Operating Room/Theatre. Bioengineering. 2023; 10(12):1345. https://doi.org/10.3390/bioengineering10121345
Chicago/Turabian StyleCarbone, Marina, Rosanna Maria Viglialoro, Sara Stagnari, Sara Condino, Marco Gesi, Michelangelo Scaglione, and Paolo Domenico Parchi. 2023. "Design, Fabrication, and Preliminary Validation of Patient-Specific Spine Section Phantoms for Use in Training Spine Surgeons Outside the Operating Room/Theatre" Bioengineering 10, no. 12: 1345. https://doi.org/10.3390/bioengineering10121345
APA StyleCarbone, M., Viglialoro, R. M., Stagnari, S., Condino, S., Gesi, M., Scaglione, M., & Parchi, P. D. (2023). Design, Fabrication, and Preliminary Validation of Patient-Specific Spine Section Phantoms for Use in Training Spine Surgeons Outside the Operating Room/Theatre. Bioengineering, 10(12), 1345. https://doi.org/10.3390/bioengineering10121345