Evaluation of Cytocompatibility of PEEK-Based Composites as a Function of Manufacturing Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Fillers
2.2. Composite Materials
2.3. Processing Technologies
2.3.1. Extrusion Compounding
2.3.2. Injection of Specimens for Mechanical Characterization
2.3.3. Filament Preparation
2.3.4. Filament Fused Fabrication
2.4. Surface Morphology
2.5. Mechanical Properties
2.6. Cytocompatibility
2.7. Composite Materials for Bioactivity Studies
2.8. Cell Type
2.9. Assays for Changes in Osteoblast Viability
2.10. Assays of Cell Morphology and Organization of the Actin Cytoskeleton
2.11. Statistical Analysis
3. Results
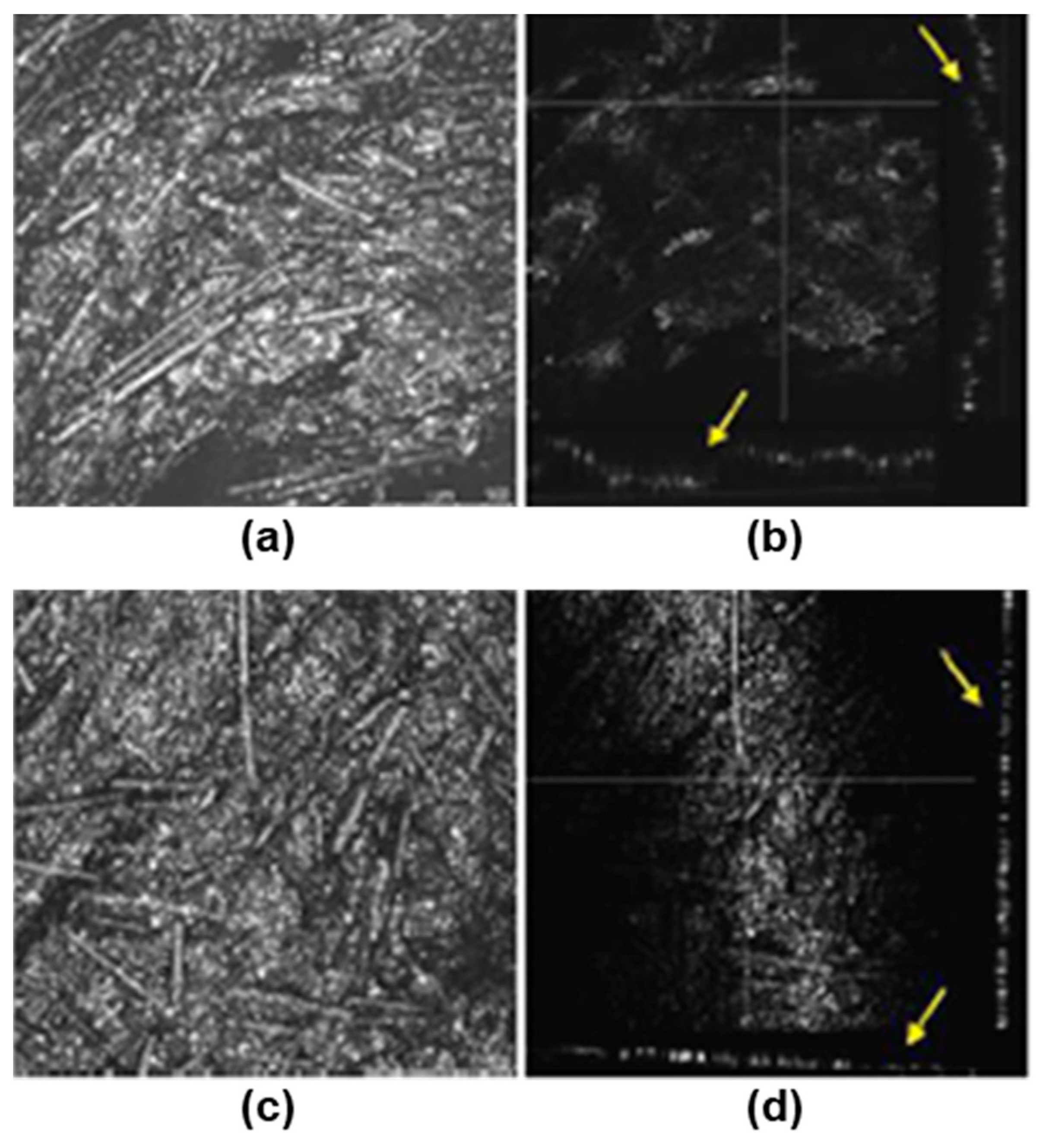
3.1. Surface Characterization
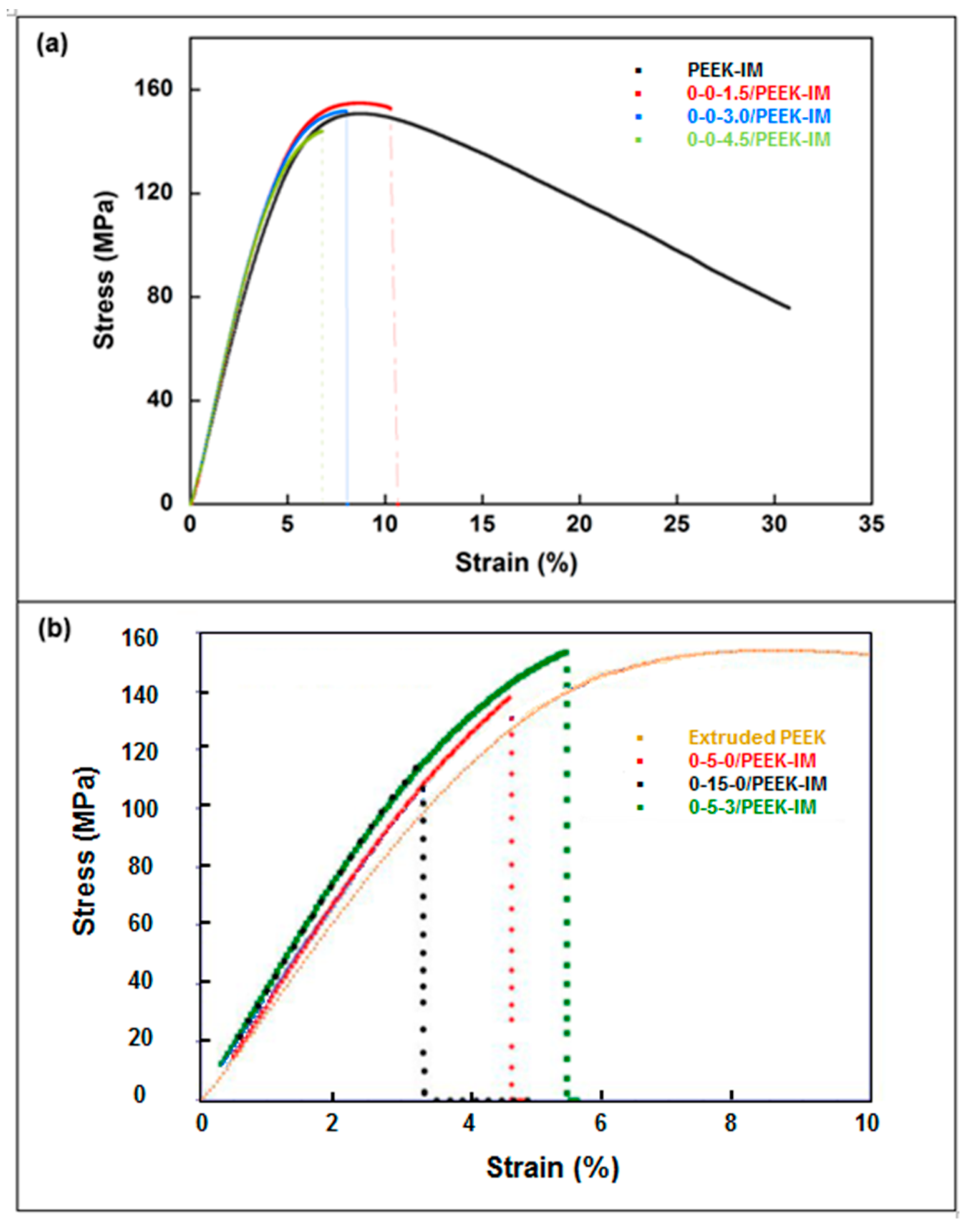
3.2. Mechanical Properties
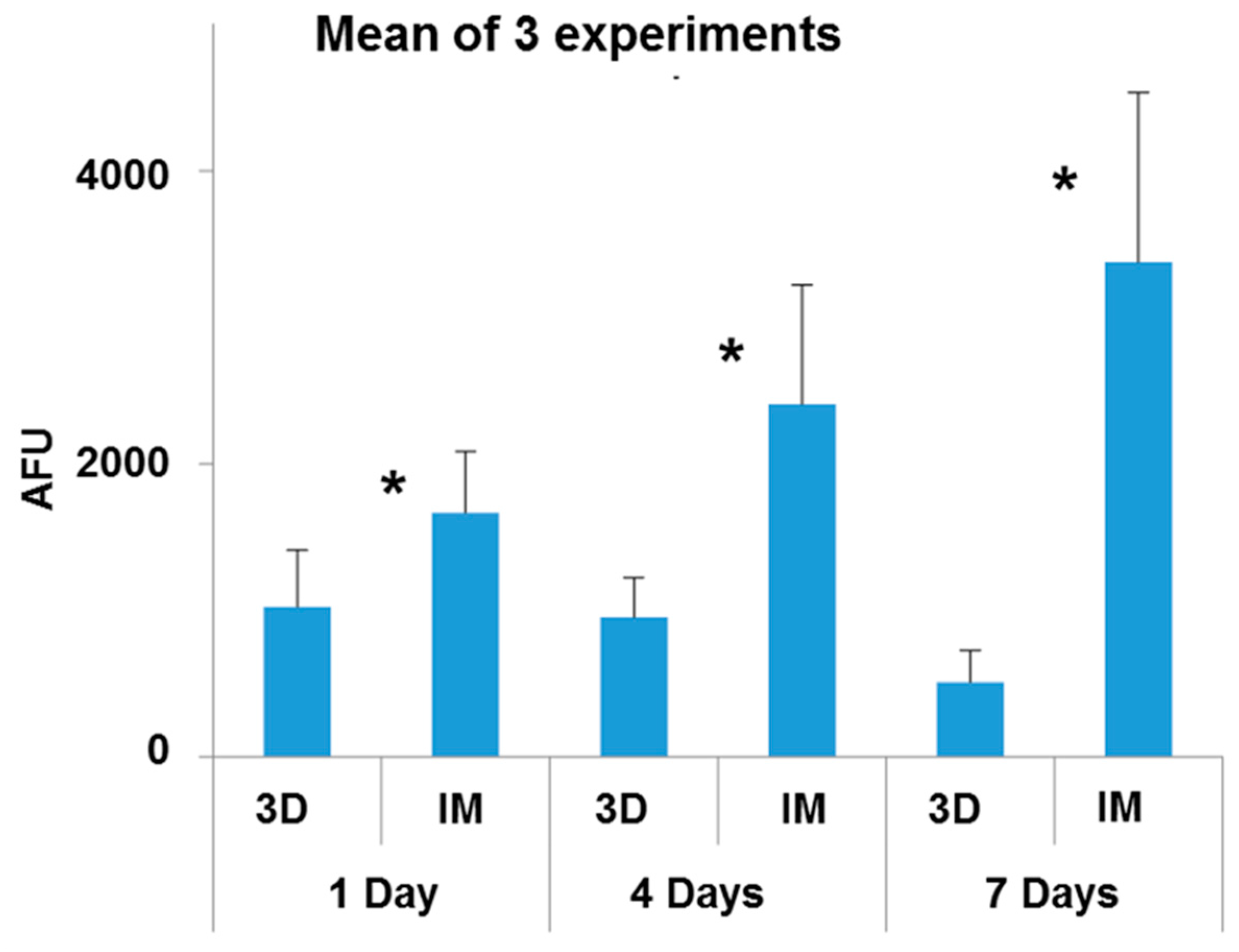
3.3. Osteoblast Viability Assays
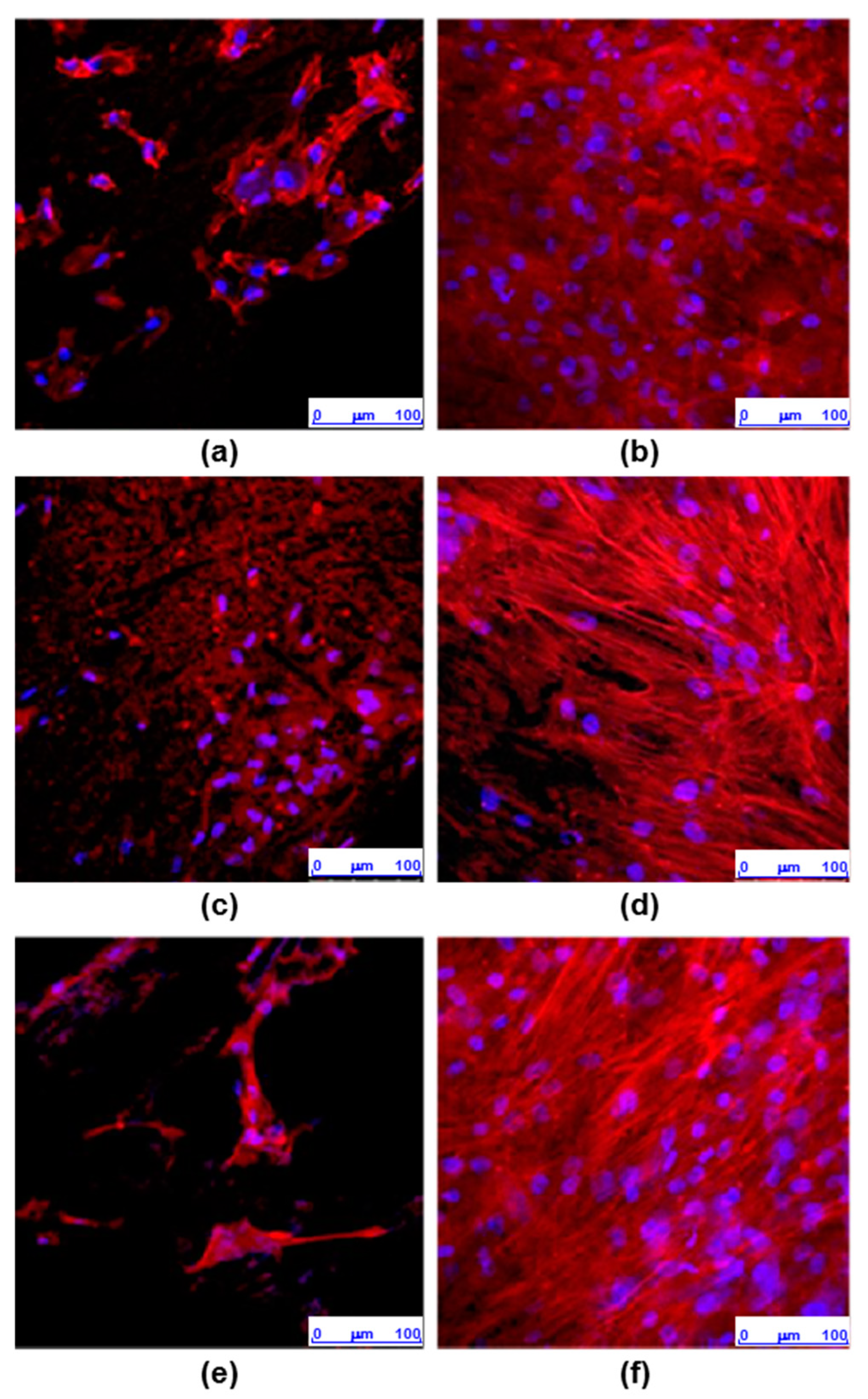
3.4. Cell Morphology and Actin Cytoskeleton Assays
4. Discussion
5. Conclusions
- -
- Two different PEEK composites were prepared using IM and a 3D printing technique based on the FFF process.
- -
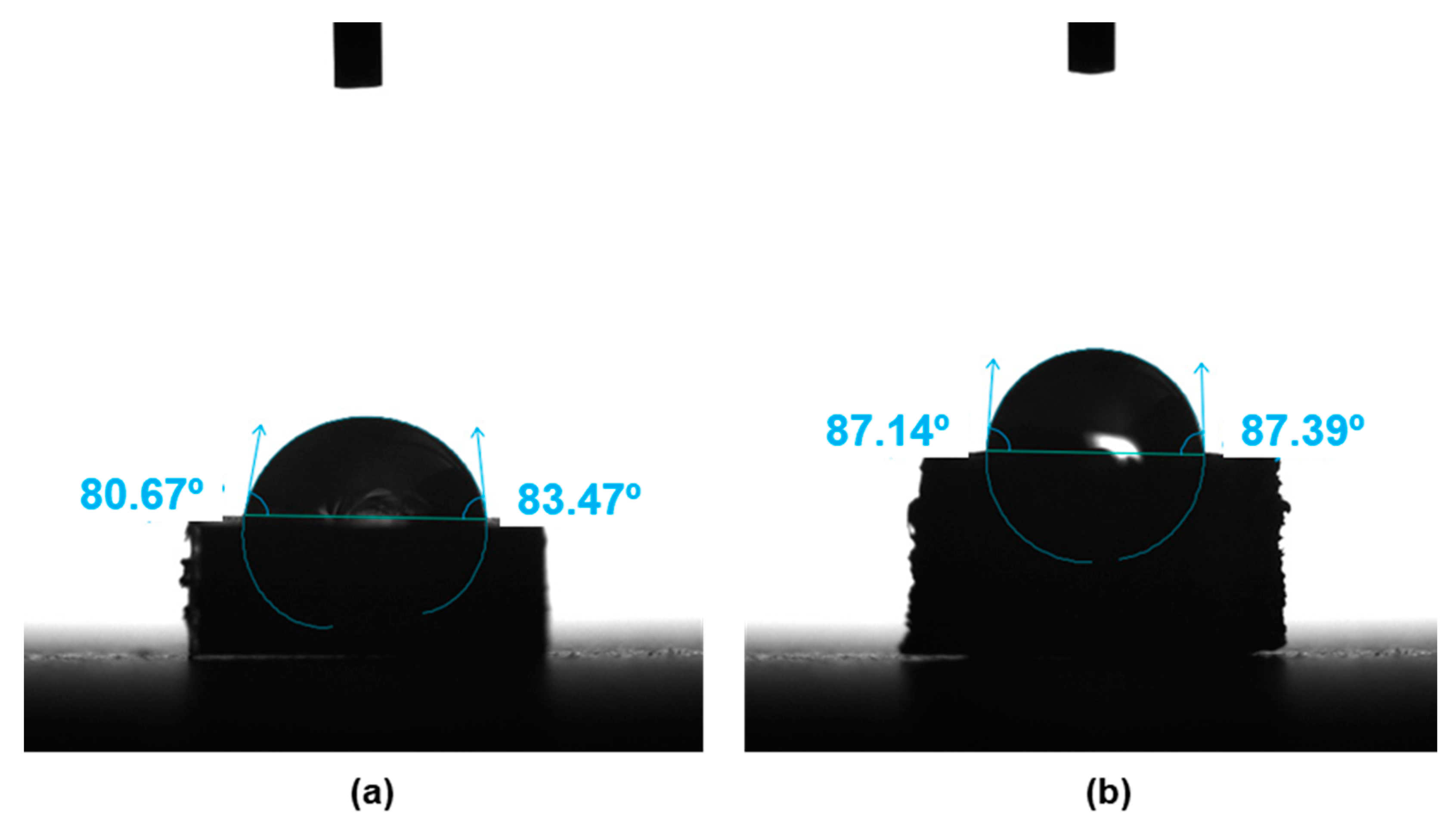
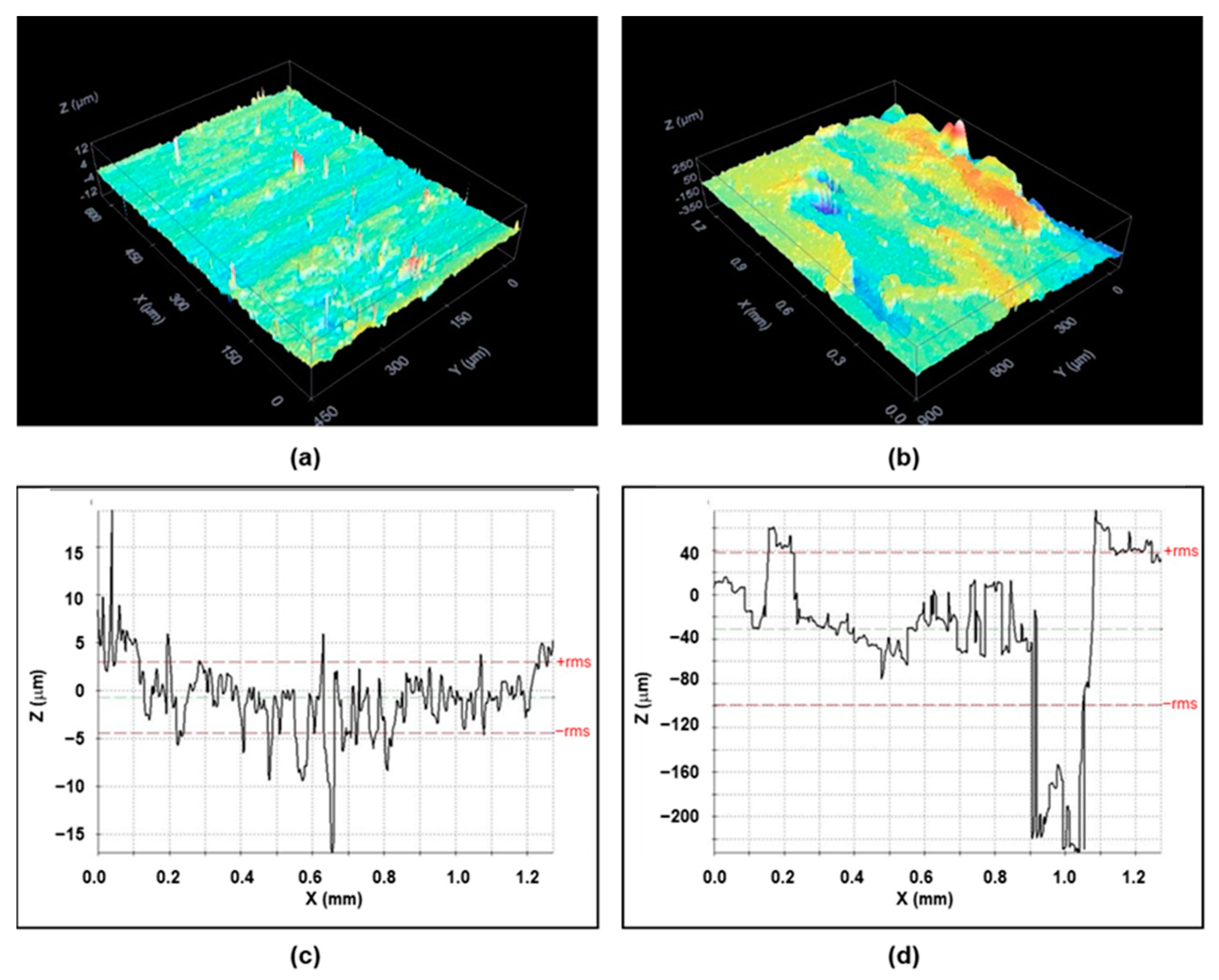
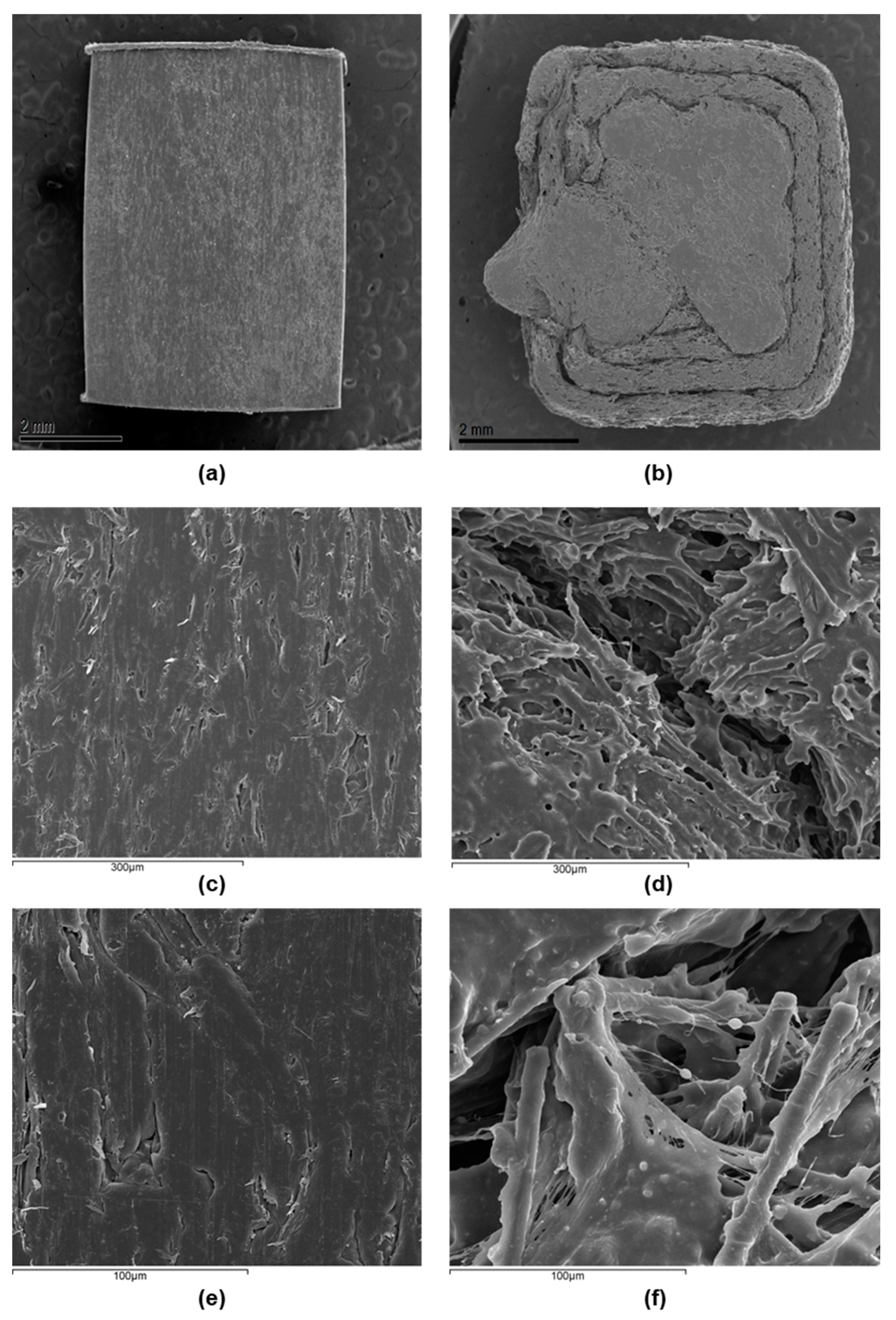
- The contact angle data indicated no significant differences in the surface wettability between the two types of surface, while the average roughness of the 3D printing sample was an order of magnitude higher than that of the sample obtained by IM. Confocal microscopy and SEM images showed the presence of small holes in both composites, with the appearance of strong cracks in the samples generated by FFF, which mostly affect the toughness behavior of the composite.
- -
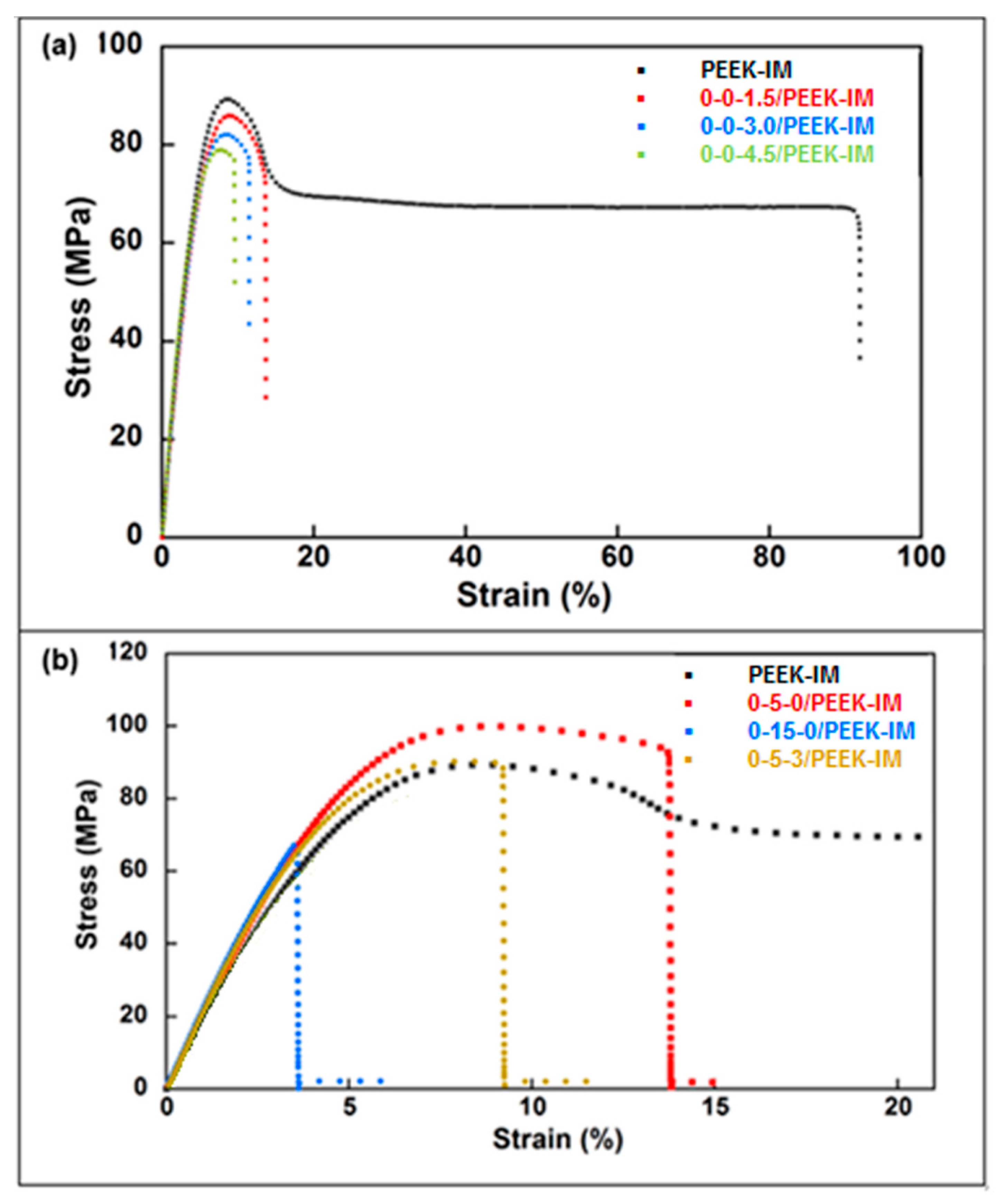
- Mechanical properties are strongly affected by the presence of any type of filler in the PEEK matrix. Ultimate strain and toughness undergo a sharp downturn, leading to brittle fracture behavior.
- -
- The adhesion capacity of osteoblasts is higher on PEEK-HA surfaces obtained by IM than on those produced by 3D printing.
- -
- The viability of osteoblasts grown on PEEK-HA surfaces obtained by IM improves by increasing the culture time up to 7 days, while it decreases on surfaces obtained by 3D printing.
- -
- Osteoblasts cultured for 7 days on surfaces obtained by IM form a confluent monolayer, with an elongated morphology with a well-organized actin cytoskeleton, while on surfaces obtained by 3D printing the cells are arranged in isolated clusters showing less definite cytoskeleton.
- -
- In summary, we can conclude that the CF-HA-GNP/PEEK surfaces obtained by IM allow the adhesion of human osteoblasts and their proliferation over time. In contrast, the surfaces generated by 3D printing, despite allowing the adhesion of osteoblasts, interfere with their growth, which could be explained by their topographic characteristics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godara, A.; Raabe, D.; Green, S. The influence of sterilization processes on the micromechanical properties of carbon fiber-reinforced PEEK composites for bone implant applications. Acta Biomater. 2007, 3, 209–220. [Google Scholar] [CrossRef]
- Kurtz, S.M. (Ed.) PEEK Biomaterials Handbook, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Abdullah, M.R.; Goharian, A.; Kadir, M.R.A.; Wahit, M.U. Biomechanical and bioactivity concepts of polyetheretherketone composites for use in orthopedic implants—A review. J. Biomed. Mater. Res. Part A 2015, 103, 3689–3702. [Google Scholar] [CrossRef] [PubMed]
- Stepashkin, A.A.; Chukov, D.I.; Senatov, F.S.; Salimon, A.I.; Korsunsky, A.M.; Kaloshkin, S.D. 3D-printed PEEK-carbon fiber (CF) composites: Structure and thermal properties. Compos. Sci. Technol. 2018, 164, 319–326. [Google Scholar] [CrossRef]
- Han, X.; Sharma, N.; Xu, Z.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F.; Thieringer, F.M.; Spintzyk, S. An In Vitro Study of Osteoblast Response on Fused-Filament Fabrication 3D Printed PEEK for Dental and Cranio-Maxillofacial Implants. J. Clin. Med. 2019, 8, 771. [Google Scholar] [CrossRef]
- Basgul, C.; Yu, T.; MacDonald, D.W.; Siskey, R.; Marcolongo, M.; Kurtz, S.M. Does annealing improve the interlayer adhesion and structural integrity of FFF 3D printed PEEK lumbar spinal cages? In Proceedings of the 4th International PEEK Meeting, Washington, DC, USA, 25–26 April 2019; p. 58. [Google Scholar]
- Spece, H.; Yu, T.; Law, A.W.; Marcolongo, M.; Kurtz, S.M. Invitro response to FFF printed porous PEEK surfaces. In Proceedings of the 4th International PEEK Meeting, Washington, DC, USA, 25–26 April 2019; pp. 40–41. [Google Scholar]
- Elhattab, K.; Sikder, P.; Walker, J.M.; Bottino, M.; Bhaduri, S. Fabrication and evaluation of 3-D printed PEEK scaffolds containing Macropores by design. Mater. Lett. 2020, 263, 127227. [Google Scholar] [CrossRef]
- Shuai, C.; Peng, S.; Wu, P.; Gao, C.; Huang, W.; Deng, Y.; Xiao, T.; Feng, P. A nano-sandwich construct built with graphene nanosheets and carbon nanotubes enhances mechanical properties of hydroxyapatite–polyetheretherketone scaffolds. Int. J. Nanomed. 2016, 11, 3487–3500. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Fonseca, J.; Peixinho, N.; Alves, N.; Gasik, M.; Silva, F.; Miranda, G. Predicting the output dimensions, porosity and elastic modulus of additive manufactured biomaterial structures targeting orthopedic implants. J. Mech. Behav. Biomed. Mater. 2019, 99, 104–117. [Google Scholar] [CrossRef]
- Hollinger, J.; Battistone, G.C. Biodegradable bone repair materials. Synthetic polymers and ceramics. Clin Orthop. Relat. Res. 1986, 207, 290–305. [Google Scholar] [CrossRef]
- Walsh, W.R.; Pelletier, M.H.; Bertollo, N.; Christou, C.; Tan, C. Does PEEK/HA enhance bone formation compared with PEEK in a sheep cervical fusion model? Clin. Orthop. Relat. Res. 2016, 474, 2364–2372. [Google Scholar] [CrossRef]
- Ma, R.; Tang, S.; Tan, H.; Lin, W.; Wang, Y.; Wei, J.; Zhao, L.; Tang, T. Preparation, characterization, and in vitro osteoblast functions of a nano-hydroxyapatite/polyetheretherketone biocomposite as orthopedic implant material. Int. J. Nanomed. 2014, 9, 3949. [Google Scholar] [CrossRef]
- Ma, R.; Guo, D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019, 14, 32. [Google Scholar] [CrossRef]
- Prabhu, B.; Wood, A.; Bandi, S.; Knebel, M.; Ross, K.; Patel, H.; Bodhak, S.; Dadsetan, M. Next generation VESTAKEEP PEEK. In Proceedings of the 4th International PEEK Meeting, Washington, DC, USA, 25–26 April 2019; p. 17. [Google Scholar]
- Li, E.; Guo, W.; Wang, H.; Xu, B.; Liu, X. Research on Tribological Behavior of PEEK and Glass Fiber Reinforced PEEK Composite. In Proceedings of the International Federation for Heat Treatment and Surface Engineering (20th Congress), Chennai, India, 16–18 May 2013; Volume 50, pp. 453–460. [Google Scholar]
- Wang, A.; Lin, R.; Polineni, V.; Essner, A.; Stark, C.; Dumbleton, J. Carbon fiber reinforced polyether ether ketone composite as a bearing surface for total hip replacement. Tribol. Int. 1998, 31, 661–667. [Google Scholar] [CrossRef]
- Zheng, J.; Kang, J.; Sun, C.; Yang, C.; Wang, L.; Li, D. Effects of printing path and material components on mechanical properties of 3D-printed polyether-ether-ketone/hydroxyapatite composites. J. Mech. Behav. Biomed. Mater. 2021, 118, 104475. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Liao, C.Z.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Preparation of polyetheretherketone composites with nanohydroxyapatite rods and carbon nanofibers having high strength, good biocompatibility and excellent thermal stability. RSC Adv. 2016, 6, 19417–19429. [Google Scholar] [CrossRef]
- Yaragalla, S.; Zahid, M.; Panda, J.K.; Tsagarakis, N.; Cingolani, R.; Athanassiou, A. Comprehensive Enhancement in Thermomechanical Performance of Melt-Extruded PEEK Filaments by Graphene Incorporation. Polymers 2021, 13, 1425. [Google Scholar] [CrossRef]
- Jeon, I.S.; Lee, M.H.; Choi, H.-H.; Lee, S.; Chon, J.W.; Chung, D.J.; Park, J.H.; Jho, J.Y. Mechanical Properties and Bioactivity of Polyetheretherketone/Hydroxyapatite/Carbon Fiber Composite Prepared by the Mechanofusion Process. Polymers 2021, 13, 1978. [Google Scholar] [CrossRef]
- Puértolas, J.; Castro, M.; Morris, J.; Ríos, R.; Ansón-Casaos, A. Tribological and mechanical properties of graphene nanoplatelet/PEEK composites. Carbon 2019, 141, 107–122. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Omigbodun, F.T. 3D printing of PEEK and its composite to increase biointerfaces as a biomedical material- A review. Colloids Surf. B Biointerfaces 2021, 203, 111726. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A. Improving bioactivity and strength of PEEK composite polymer for bone application. Mater. Chem. Phys. 2021, 266, 124485. [Google Scholar] [CrossRef]
- Verma, S.; Sharma, N.; Kango, S.; Sharma, S. Developments of PEEK (Polyetheretherketone) as a biomedical material: A focused review. Eur. Polym. J. 2021, 147, 110295. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, P.; Liu, X.; Wang, L.; Xiong, X.; Tang, Z.; Wei, J.; Wei, S. Preparation, characterization, cellular response and in vivo osseointegration of polyetherether-ketone/nano-hydroxyapatite/carbon fiber ternary biocomposite. Colloids Surf. B Biointerfaces 2015, 136, 64–73. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Wen, J.; Zhang, X.; Cao, L.; Zeng, D.; Liu, X.; Jiang, X. Novel vascular strategies on polyetheretherketone modification in promoting osseointegration in ovariectomized rats. Mater. Des. 2021, 202, 109526. [Google Scholar] [CrossRef]
- He, Y.; Wang, W.; Lin, S.; Yang, Y.; Song, L.; Jing, Y.; Chen, L.; He, Z.; Li, W.; Xiong, A.; et al. Fabrication of a bio-instructive scaffold conferred with a favorable microenvironment allowing for superior implant osseointegration and accelerated in situ vascularized bone regeneration via type H vessel formation. Bioact. Mater. 2021, 9, 491–507. [Google Scholar] [CrossRef]
- UNE-EN ISO 572-2; ASTM D638 M Standard. Asociación Española de Normalización y Certificación: Madrid, Spain, 2012.
- UNE-EN ISO 178:2011/A1:2013; BS EN ISO 178:2010+A1:2013 Standard. Asociación Española de Normalización y Certificación: Madrid, Spain, 2020.
- Saldaña, L.; Bensiamar, F.; Boré, A.; Vilaboa, N. In search of representative models of human bone-forming cells for cytocompatibility studies. Acta Biomater. 2011, 7, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-H.; Wang, C.-H.; Li, K.-W.; Zhang, Q.; Yang, M.; Di-Wu, W.-L.; Yan, M.; Song, Y.; Ba, J.-J.; Bi, L.; et al. Enhancement of surface bioactivity on carbon fiber-reinforced polyether ether ketone via graphene modification. Int. J. Nanomed. 2018, 13, 3425–3440. [Google Scholar] [CrossRef] [PubMed]
- Cicala, G.; Latteri, A.; Del Curto, B.; Russo, A.L.; Recca, G.; Farè, S. Engineering Thermoplastics for Additive Manufacturing: A Critical Perspective with Experimental Evidence to Support Functional Applications. J. Appl. Biomater. Funct. Mater. 2017, 15, 10–18. [Google Scholar] [CrossRef]

| (a) | ||||||
| Composite Material Nomenclature | ||||||
| Component | 0-0-1.5/PEEK-IM | 0-0-3/PEEK-IM | 0-0-4.5/PEEK-IM | 0-5-0/PEEK-IM | 0-15-0/PEEK-IM | 0-5-3/PEEK-IM |
| CF | 0 | 0 | 0 | 0 | 0 | 0 |
| HA | 0 | 0 | 0 | 5 | 15 | 5 |
| GNP | 1.5 | 3 | 4.5 | 0 | 0 | 3 |
| (b) | ||||||
| Composite Material Nomenclature | ||||||
| Component | 30-0-0/PEEK-IM | 30-8-2/PEEK-IM | 30-8-2/PEEK-3D | |||
| CF | 30 | 30 | 30 | |||
| HA | 0 | 8 | 8 | |||
| GNP | 0 | 2 | 2 | |||
| Sample | Incubation Period |
|---|---|
| 3D samples + osteoblasts | 1 day |
| IM samples + osteoblast | 1 day |
| 3D samples + osteoblasts | 4 days |
| IM samples + osteoblasts | 4 days |
| 3D samples + osteoblasts | 7 days |
| IM samples + osteoblasts | 7 days |
| Sample | Incubation Period |
|---|---|
| 3D samples + osteoblasts | 7 days |
| IM samples + osteoblasts | 7 days |
| Material | E (GPa) | σts (MPa) | ε* (%) | W (MJ/m3) |
|---|---|---|---|---|
| Virgin PEEK | 3.9 | 68.8 | 41.9 | 41.9 |
| Extruded PEEK | 3.6 ± 0.2 | 89.5 ± 0.1 | 94.7 ± 4.3 | 65.0 ± 3.6 |
| 0-0-1.5/PEEK-IM | 3.6 ± 0.2 | 85.8 ± 0.3 | 13.9 ± 0.9 | 9.15 ± 0.5 |
| 0-0-3.0/PEEK-IM | 3.5 ± 0.1 | 82.5 ± 0.4 | 11.6 ± 0.4 | 7.33 ± 0.3 |
| 0-0-4.5/PEEK-IM | 3.9 ± 0.3 | 79.6 ± 0.7 | 9.7 ± 0.2 | 5.76 ± 0.2 |
| 0-5-0/PEEK-IM | 4.0 ± 0.1 | 99.1 ± 1.1 | 13.2 ± 1.9 | 10.2 ± 1.8 |
| 0-15-0/PEEK-IM | 4.4 ± 0.5 | 65.6 ± 3.4 | 3.6 ± 0.2 | 1.3 ± 0.2 |
| 0-5-3/PEEK-IM | 4.2 ± 0.2 | 90.8 ± 0.4 | 9.1 ± 0.1 | 6.0 ± 0.1 |
| Material | Eb (GPa) | σb (MPa) | ε* (%) |
|---|---|---|---|
| Virgin PEEK | 3.1 ± 0.1 | 153 ± 1 | 31.5 ± 1.9 |
| Extruded PEEK | 3.1 ± 0.1 | 152 ± 2 | 30.3 ± 0.3 |
| 0-0-1.5/PEEK-IM | 3.4 ± 0.1 | 155 ± 1 | 9.8 ± 0.8 |
| 0-0-3.0/PEEK-IM | 3.4 ± 0.1 | 152 ± 1 | 7.9 ± 0.1 |
| 0-0-4.5/PEEK-IM | 3.5 ± 0.1 | 144 ± 1 | 6.6 ± 0.2 |
| 0-5-0/PEEK-IM | 3.5 ± 0.1 | 159 ± 10 | 7.0 ± 2.0 |
| 0-15-0/PEEK-IM | 3.8 ± 0.1 | 104 ± 10 | 2.9 ± 0.3 |
| 0-5-3/PEEK-IM | 3.8 ± 0.1 | 156 ± 2 | 5.8 ± 0.4 |
| Material | Eb (GPa) | σb (MPa) | ε* (%) |
|---|---|---|---|
| Extruded PEEK | 3.1 ± 0.1 | 152 ± 2.0 | 30.3 ± 0.3 |
| 30-0-0/PEEK-IM | 16.2 ± 0.3 | 319 ± 9.0 | 2.2 ± 0.1 |
| 30-8-2/PEEK-IM | 16.1 ± 0.1 | 295 ± 8.0 | 2.3 ± 0.1 |
| 30-8-2/PEEK-3D | 4.2 | 57.3 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Albarova, J.; Martínez-Morlanes, M.J.; Fernández, J.M.; Castell, P.; Gracia, L.; Puértolas, J.A. Evaluation of Cytocompatibility of PEEK-Based Composites as a Function of Manufacturing Processes. Bioengineering 2023, 10, 1327. https://doi.org/10.3390/bioengineering10111327
Gil-Albarova J, Martínez-Morlanes MJ, Fernández JM, Castell P, Gracia L, Puértolas JA. Evaluation of Cytocompatibility of PEEK-Based Composites as a Function of Manufacturing Processes. Bioengineering. 2023; 10(11):1327. https://doi.org/10.3390/bioengineering10111327
Chicago/Turabian StyleGil-Albarova, Jorge, María José Martínez-Morlanes, José Miguel Fernández, Pere Castell, Luis Gracia, and José Antonio Puértolas. 2023. "Evaluation of Cytocompatibility of PEEK-Based Composites as a Function of Manufacturing Processes" Bioengineering 10, no. 11: 1327. https://doi.org/10.3390/bioengineering10111327
APA StyleGil-Albarova, J., Martínez-Morlanes, M. J., Fernández, J. M., Castell, P., Gracia, L., & Puértolas, J. A. (2023). Evaluation of Cytocompatibility of PEEK-Based Composites as a Function of Manufacturing Processes. Bioengineering, 10(11), 1327. https://doi.org/10.3390/bioengineering10111327








