Preoperative Grading of Rectal Cancer with Multiple DWI Models, DWI-Derived Biological Markers, and Machine Learning Classifiers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Examinations
2.3. Image Analysis
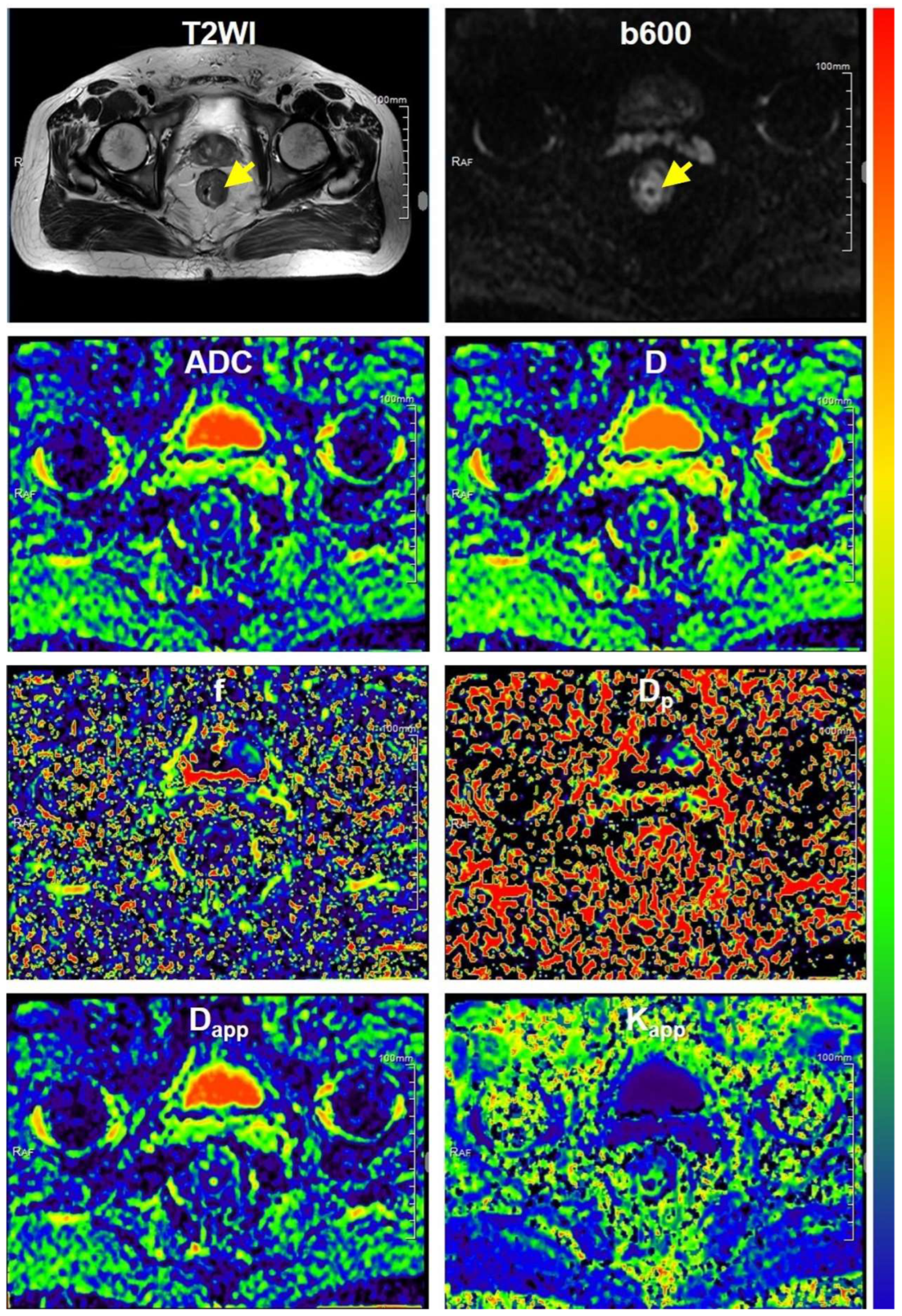
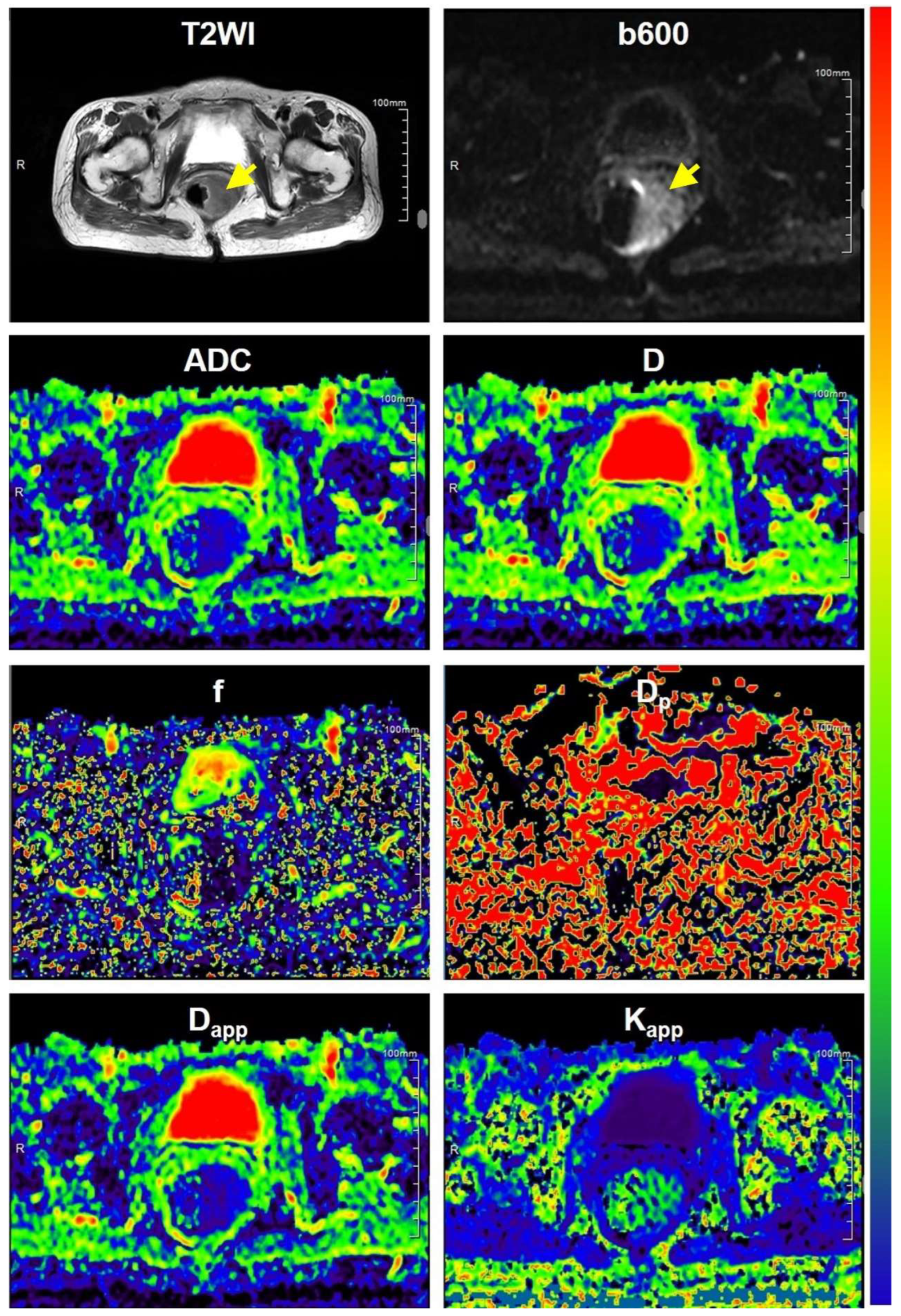
2.3.1. DWI Parametric Maps
2.3.2. Definition of Volume of Interests (VOIs)
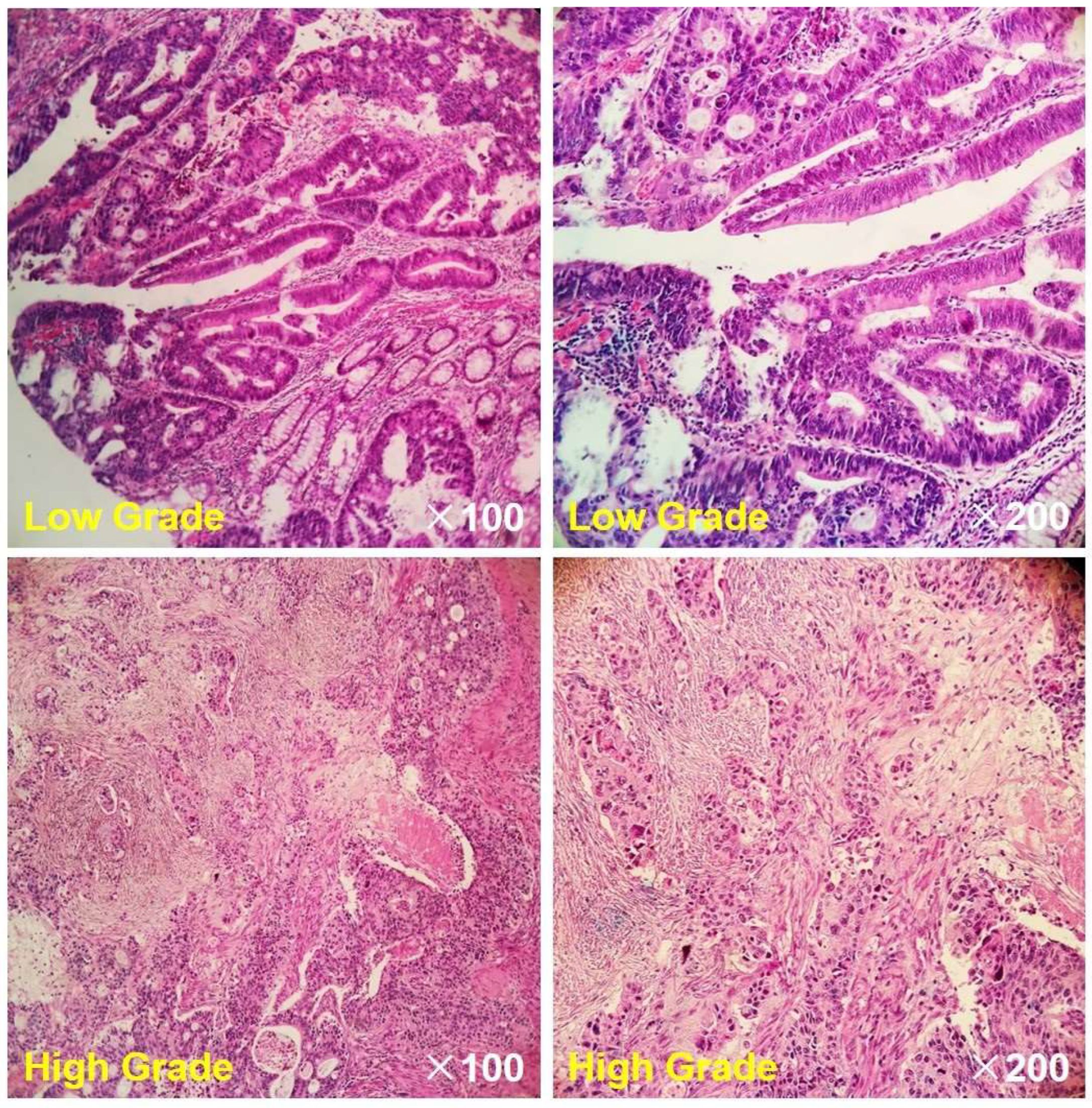
2.4. Histopathological Evaluation
2.5. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics and MR Images
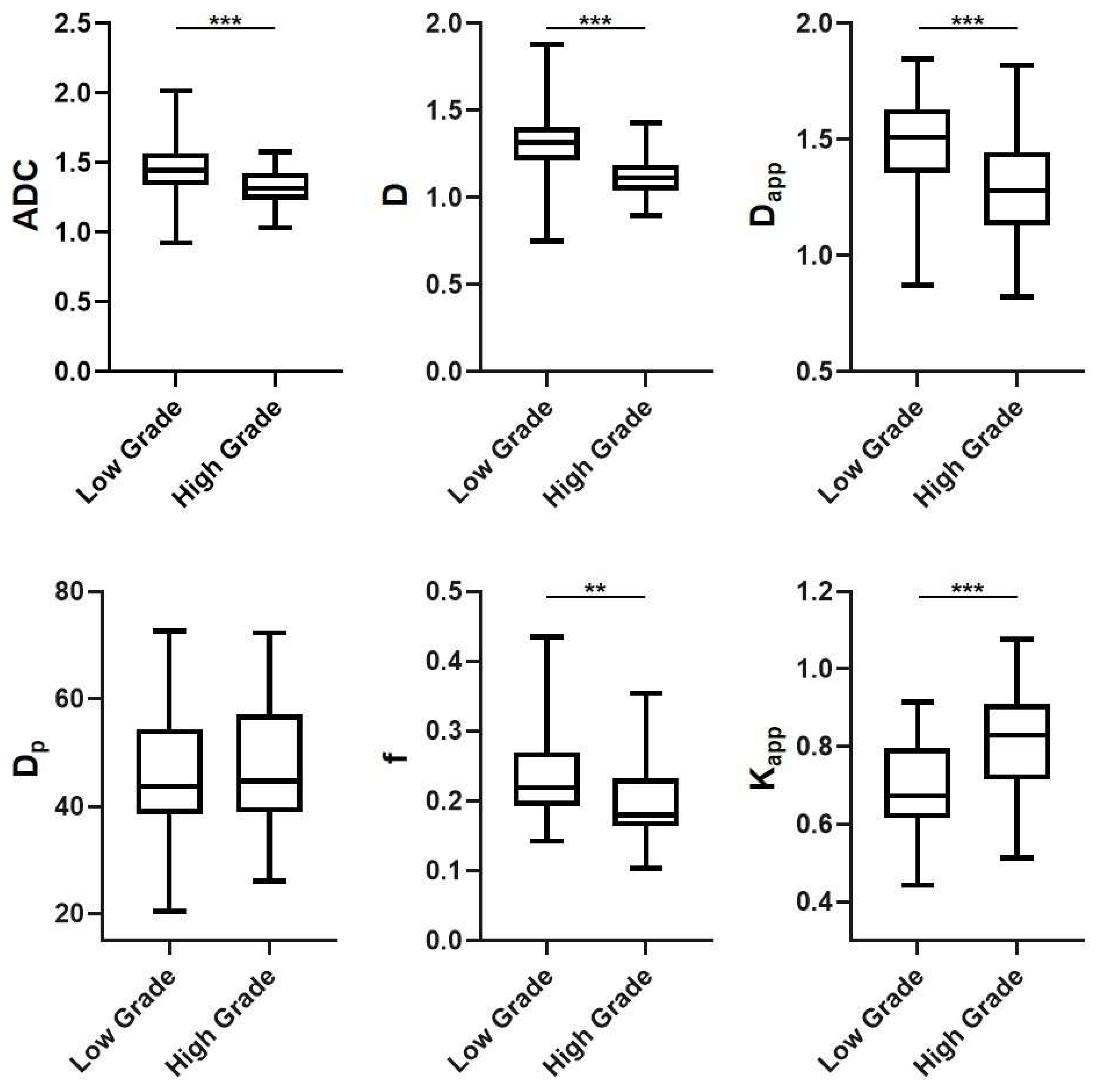
3.2. DWI-Derived Parameters in Different Subgroups
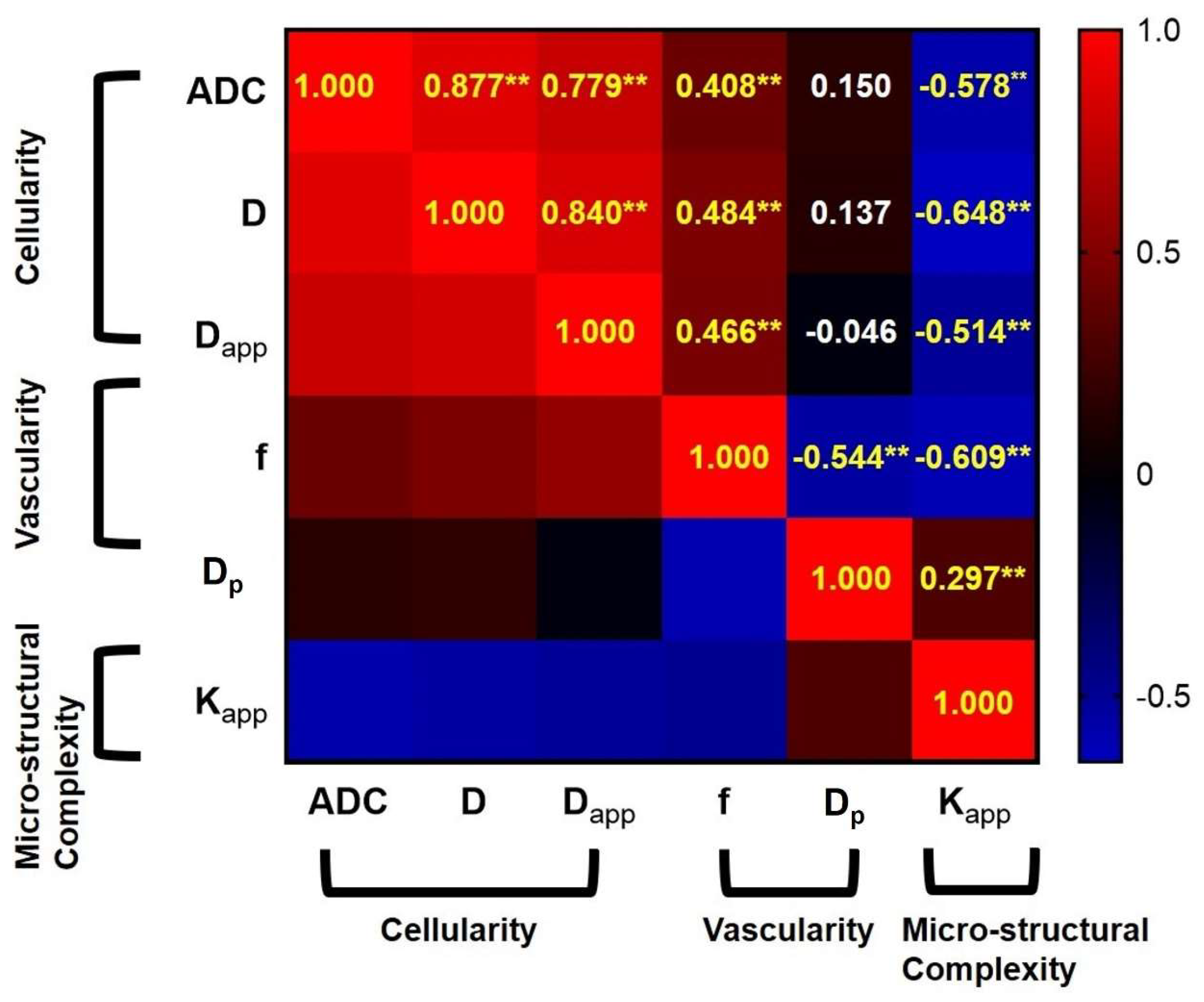
3.3. The Correlations among the Different DWI-Derived Biological Markers
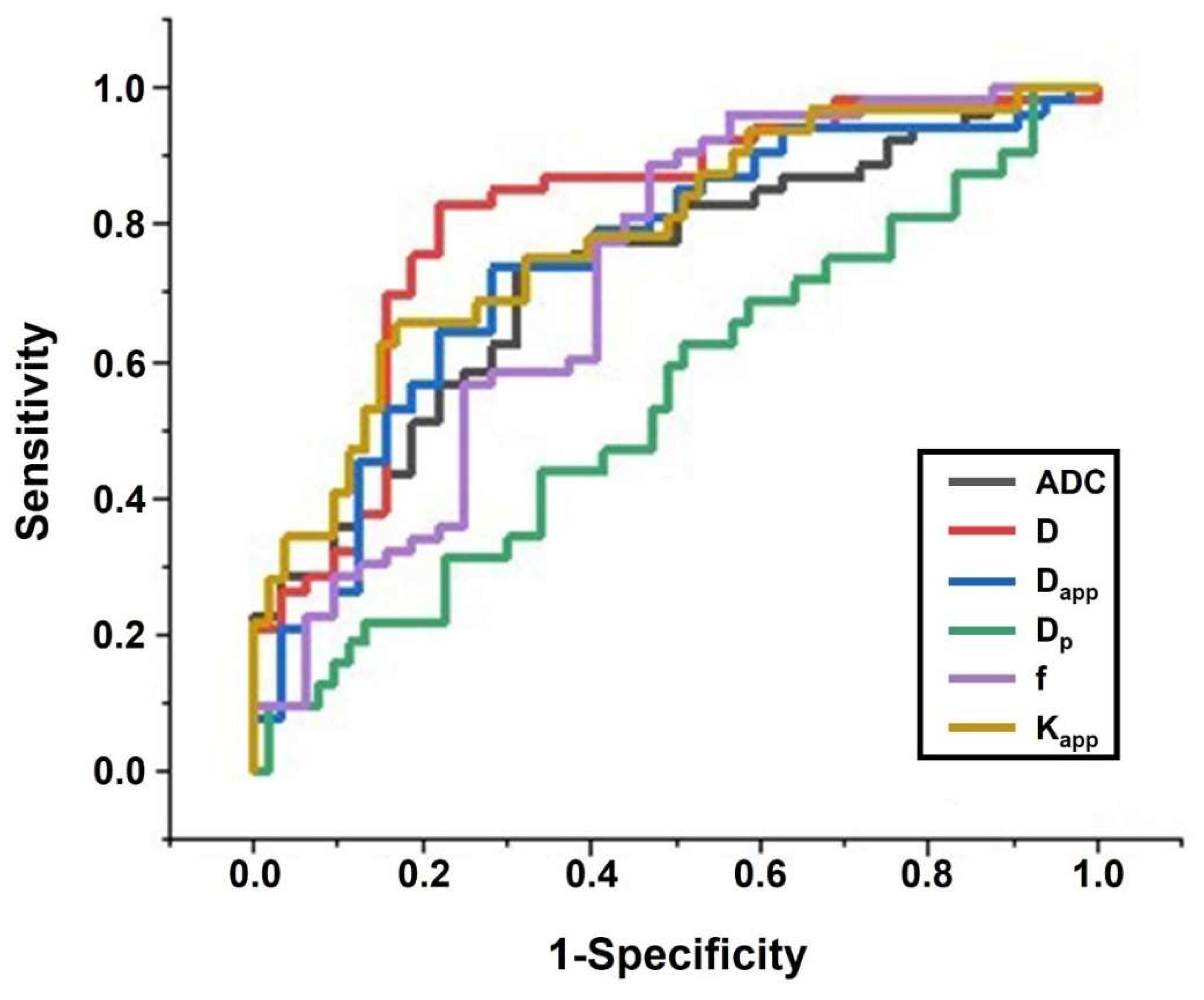
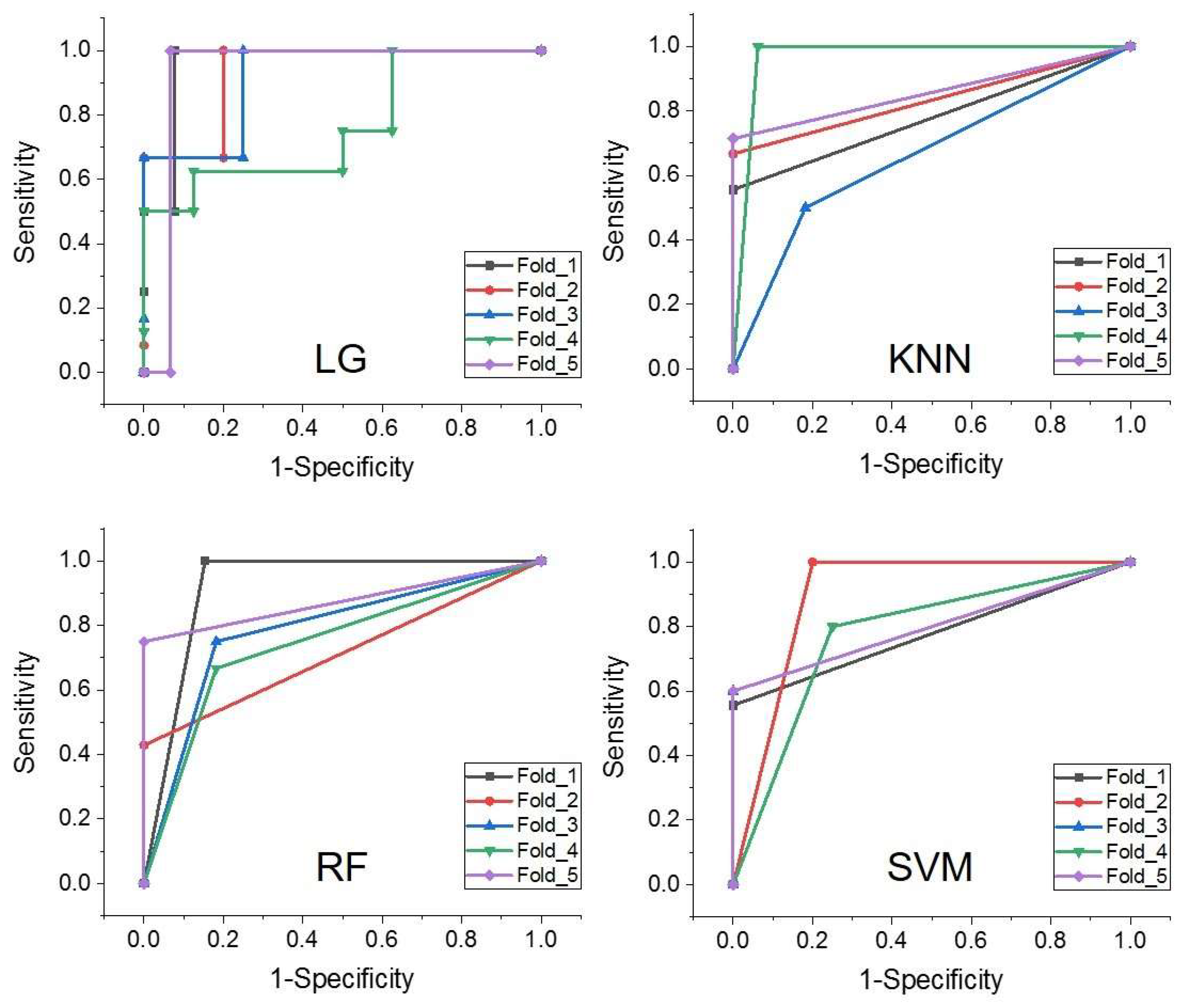
3.4. Diagnostic Performance Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boras, Z.; Kondza, G.; Sisljagic, V.; Busic, Z.; Gmajnic, R.; Istvanic, T. Prognostic factors of local recurrence and survival after curative rectal cancer surgery: A single institution experience. Coll. Antropol. 2012, 36, 1355–1361. [Google Scholar]
- Freedman, L.S.; Macaskill, P.; Smith, A.N. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet 1984, 2, 733–736. [Google Scholar] [CrossRef]
- Bown, E.J.; Lloyd, G.M.; Boyle, K.M.; Miller, A.S. Rectal cancer: Prognostic indicators of long-term outcome in patients considered for surgery. Int. J. Color. Dis. 2014, 29, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wibe, A.; Law, W.; Fazio, V.; Delaney, C.J.C.D. Tailored rectal cancer treatment—A time for implementing contemporary prognostic factors? Color. Dis. 2013, 15, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Compton, C.C.; Shellito, P.C.; Wood, W.C.; Landry, J.; Machuta, S.R.; Kaufman, D.; Ancukiewicz, M.; Willett, C.G. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann. Surg. 1999, 230, 49. [Google Scholar] [CrossRef]
- Feng, Z.; Shi, X.; Zhang, Q.; Zhang, X.; Li, X.; Chen, Z.; Liu, D.; Sun, B.; Zuo, Y.; Ren, S. Analysis of clinicopathological features and prognosis of 1315 cases in colorectal cancer located at different anatomical subsites. Pathol. Res. Pract. 2019, 215, 152560. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Pan, Z.; Ma, Q.; Yang, W.; Shi, H.; Fu, C.; Yan, X.; Du, L.; Yan, F.; Zhang, H. Diffusion Kurtosis Imaging Study of Rectal Adenocarcinoma Associated with Histopathologic Prognostic Factors: Preliminary Findings. Radiology 2017, 284, 66–76. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, X.; Du, X.; Zhuo, Z.; Xin, L.; Cheng, X. Whole-tumour diffusion kurtosis MR imaging histogram analysis of rectal adenocarcinoma: Correlation with clinical pathologic prognostic factors. Eur. Radiol. 2018, 28, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Hohn, A.K.; Behrmann, C.; Wienke, A.; Spielmann, R.P.; Garnov, N. Correlations between intravoxel incoherent motion (IVIM) parameters and histological findings in rectal cancer: Preliminary results. Oncotarget 2017, 8, 21974–21983. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.Y.; Kim, C.K.; Kim, J.H.; Im, G.H.; Park, B.K.; Lee, J.H. Assessment of early therapeutic response to sorafenib in renal cell carcinoma xenografts by dynamic contrast-enhanced and diffusion-weighted MR imaging. Br. J. Radiol. 2015, 88, 20150163. [Google Scholar] [CrossRef]
- Xing, Z.; Yang, X.; She, D.; Lin, Y.; Zhang, Y.; Cao, D. Noninvasive Assessment of IDH Mutational Status in World Health Organization Grade II and III Astrocytomas Using DWI and DSC-PWI Combined with Conventional MR Imaging. AJNR Am. J. Neuroradiol. 2017, 38, 1138–1144. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, X.J. Diffusion MRI of cancer: From low to high b-values. J. Magn. Reson. Imaging 2019, 49, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Y.; Guo, Y.; Jiang, M.; Zhang, Y.; Dai, Y.; Yao, X. Preoperative assessment of microvascular invasion of hepatocellular carcinoma using non-Gaussian diffusion-weighted imaging with a fractional order calculus model: A pilot study. Magn. Reson. Imaging 2023, 95, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, D.; Guo, Y.; Zhang, Y.; Guo, Y.; Jiang, M.; Dai, Y.; Yao, X. Preoperative identification of cytokeratin 19 status of hepatocellular carcinoma based on diffusion kurtosis imaging. Abdom. Radiol. 2023, 48, 579–589. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, Y.; Yin, S.; Lian, S.; He, H.; Li, H.; Xie, C.; Dai, Y. Preoperatively Grading Rectal Cancer with the Combination of Intravoxel Incoherent Motions Imaging and Diffusion Kurtosis Imaging. Contrast Media Mol. Imaging 2020, 2020, 2164509. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, J.; Zhang, Y.; Guo, Y.; Jiang, M.; Dai, Y.; Yao, X. Differentiating Cytokeratin 19 expression of hepatocellular carcinoma by using multi-b-value diffusion-weighted MR imaging with mono-exponential, stretched exponential, intravoxel incoherent motion, diffusion kurtosis imaging and fractional order calculus models. Eur. J. Radiol. 2022, 150, 110237. [Google Scholar] [CrossRef]
- Qin, Y.; Tang, C.; Hu, Q.; Zhang, Y.; Yi, J.; Dai, Y.; Ai, T. Quantitative Assessment of Restriction Spectrum MR Imaging for the Diagnosis of Breast Cancer and Association With Prognostic Factors. J. Magn. Reson. Imaging 2023, 57, 1832–1841. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Zhang, Y.; Dai, Y.; Yang, D.; Wang, C.; Li, J. Differentiation of benign and malignant breast lesions using diffusion-weighted imaging with a fractional-order calculus model. Eur. J. Radiol. 2023, 159, 110646. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Geng, Z.; Lian, S.; Yin, S.; Xu, G.; Zhang, Y.; Dai, Y.; Zhao, J.; Ma, L.; Liu, X.; et al. Discriminating rectal cancer grades using restriction spectrum imaging. Abdom. Radiol. 2022, 47, 2014–2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Liang, L.; Shi, Z.; Zhu, S.; Chen, C.; Dai, Y.; Zeng, M. Preliminary Experience of 5.0 T Higher Field Abdominal Diffusion-Weighted MRI: Agreement of Apparent Diffusion Coefficient With 3.0 T Imaging. J. Magn. Reson. Imaging 2022, 56, 1009–1017. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Sheng, R.; Dai, Y.; Zeng, M. Preoperatively Identify the Microvascular Invasion of Hepatocellular Carcinoma with the Restricted Spectrum Imaging. Acad Radiol 2023, 30 (Suppl. S1), S30–S39. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Y.; Song, A.; Shi, K.; Wang, W. Intravoxel incoherent motion MRI of rectal cancer: Correlation of diffusion and perfusion characteristics with prognostic tumor markers. Am. J. Roentgenol. 2018, 210, W139–W147. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tang, H.; Meng, X.; Shen, Y.; Hu, D.; Kamel, I.; Li, Z. Histological grades of rectal cancer: Whole-volume histogram analysis of apparent diffusion coefficient based on reduced field-of-view diffusion-weighted imaging. Quant. Imaging Med. Surg. 2020, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef]
- Lu, H.; Jensen, J.H.; Ramani, A.; Helpern, J.A. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006, 19, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Woo, S.; Lee, J.M.; Yoon, J.H.; Joo, I.; Han, J.K.; Choi, B.I. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: Correlation with enhancement degree and histologic grade. Radiology 2013, 270, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lin, Y.; Tian, J.; Shi, D.; Cheng, J.; Haacke, E.M.; Hong, X.; Ma, B.; Zhou, J.; Wang, M. Grading of Gliomas by Using Monoexponential, Biexponential, and Stretched Exponential Diffusion-weighted MR Imaging and Diffusion Kurtosis MR Imaging. Radiology 2016, 278, 496–504. [Google Scholar] [CrossRef]
- Sui, Y.; Wang, H.; Liu, G.; Damen, F.W.; Wanamaker, C.; Li, Y.; Zhou, X.J. Differentiation of low-and high-grade pediatric brain tumors with high b-value diffusion-weighted MR imaging and a fractional order calculus model. Radiology 2015, 277, 489–496. [Google Scholar] [CrossRef]
- Karaman, M.M.; Sui, Y.; Wang, H.; Magin, R.L.; Li, Y.; Zhou, X.J. Differentiating low-and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values. Magn. Reson. Med. 2016, 76, 1149–1157. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lee, S.S.; Sung, Y.S.; Cheong, H.; Lee, H.; Byun, J.H.; Kim, S.Y.; Lee, S.J.; Shin, Y.M.; Lee, M.G. Intravoxel incoherent motion diffusion-weighted imaging for characterizing focal hepatic lesions: Correlation with lesion enhancement. J. Magn. Reson. Imaging 2017, 45, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, F.; Wang, M.; Huang, Z.; Tang, H.; Li, J.; Wang, Y.; Zhang, T.; Wei, X.; Zheng, D.; et al. Intravoxel incoherent motion diffusion-weighted imaging for assessment of histologic grade of hepatocellular carcinoma: Comparison of three methods for positioning region of interest. Eur. Radiol. 2019, 29, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, C.-J.; Bao, M.-L.; Zhang, J.; Wang, X.-N.; Zhang, Y.-D. Machine learning-based analysis of MR radiomics can help to improve the diagnostic performance of PI-RADS v2 in clinically relevant prostate cancer. Eur. Radiol. 2017, 27, 4082–4090. [Google Scholar] [CrossRef]
- Vidic, I.; Egnell, L.; Jerome, N.P.; Teruel, J.R.; Sjobakk, T.E.; Ostlie, A.; Fjosne, H.E.; Bathen, T.F.; Goa, P.E. Support vector machine for breast cancer classification using diffusion-weighted MRI histogram features: Preliminary study. J. Magn. Reson. Imaging 2018, 47, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, L.; Luan, S.; Xiong, J.; Geng, D.; Yin, B. The diagnostic value of texture analysis in predicting WHO grades of meningiomas based on ADC maps: An attempt using decision tree and decision forest. Eur. Radiol. 2019, 29, 1318–1328. [Google Scholar] [CrossRef]

| Characteristics | Low Grade (n = 53) | High Grade (n = 32) | p Value |
|---|---|---|---|
| Age: mean ± SD (years) | 59.13 ± 10.75 | 61.27 ± 11.54 | 0.612 |
| Gender | 0.703 | ||
| Men | 28 | 17 | |
| Women | 25 | 15 | |
| Size of tumor (mm) | 24.1 ± 8.79 | 38.3 ± 9.25 | 0.085 |
| pT stage | 0.047 | ||
| T1 | 3 | 2 | |
| T2 | 28 | 7 | |
| T3 | 15 | 13 | |
| T4 | 7 | 10 | |
| pN stage | 0.452 | ||
| N0 | 20 | 15 | |
| N1 | 17 | 10 | |
| N2 | 16 | 7 | |
| CA199 | 0.251 | ||
| ≤20 U/mL | 36 | 13 | |
| >20 U/mL | 17 | 19 | |
| CEA | 0.632 | ||
| ≤5 ng/mL | 30 | 10 | |
| >5 ng/mL | 23 | 22 |
| ICC | Lower Bound of 95% CI | Upper Bound of 95% CI | |
|---|---|---|---|
| ADC | 0.862 | 0.821 | 0.883 |
| D | 0.827 | 0.787 | 0.840 |
| Dp | 0.751 | 0.734 | 0.792 |
| f | 0.802 | 0.761 | 0.829 |
| Dapp | 0.836 | 0.801 | 0.855 |
| Kapp | 0.843 | 0.817 | 0.862 |
| Sensitivity | Specificity | AUC | 95% CI of AUC | Youden Index | |
|---|---|---|---|---|---|
| KNN | 0.687 | 0.951 | 0.819 | 0.590–0.964 | 0.638 |
| LG | 0.925 | 0.856 | 0.902 | 0.754–1.000 | 0.781 |
| RF | 0.719 | 0.897 | 0.808 | 0.628–0.975 | 0.616 |
| SVM | 0.711 | 0.910 | 0.811 | 0.628–0.982 | 0.621 |
| ADC | 0.686 | 0.736 | 0.729 | 0.620–0.838 | 0.423 |
| D | 0.781 | 0.830 | 0.811 | 0.711–0.911 | 0.611 |
| Kapp | 0.656 | 0.830 | 0.782 | 0.681–0.884 | 0.486 |
| Dapp | 0.719 | 0.736 | 0.746 | 0.635–0.856 | 0.455 |
| f | 0.531 | 0.887 | 0.718 | 0.598–0.838 | 0.418 |
| Dp | 0.625 | 0.490 | 0.543 | 0.415–0.671 | 0.116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Wang, Q.; Feng, H.; Wang, L.; Zhang, Y.; Liu, H. Preoperative Grading of Rectal Cancer with Multiple DWI Models, DWI-Derived Biological Markers, and Machine Learning Classifiers. Bioengineering 2023, 10, 1298. https://doi.org/10.3390/bioengineering10111298
Song M, Wang Q, Feng H, Wang L, Zhang Y, Liu H. Preoperative Grading of Rectal Cancer with Multiple DWI Models, DWI-Derived Biological Markers, and Machine Learning Classifiers. Bioengineering. 2023; 10(11):1298. https://doi.org/10.3390/bioengineering10111298
Chicago/Turabian StyleSong, Mengyu, Qi Wang, Hui Feng, Lijia Wang, Yunfei Zhang, and Hui Liu. 2023. "Preoperative Grading of Rectal Cancer with Multiple DWI Models, DWI-Derived Biological Markers, and Machine Learning Classifiers" Bioengineering 10, no. 11: 1298. https://doi.org/10.3390/bioengineering10111298
APA StyleSong, M., Wang, Q., Feng, H., Wang, L., Zhang, Y., & Liu, H. (2023). Preoperative Grading of Rectal Cancer with Multiple DWI Models, DWI-Derived Biological Markers, and Machine Learning Classifiers. Bioengineering, 10(11), 1298. https://doi.org/10.3390/bioengineering10111298







