Evaluation of Augmented Reality Surgical Navigation in Percutaneous Endoscopic Lumbar Discectomy: Clinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. System Components and Functions
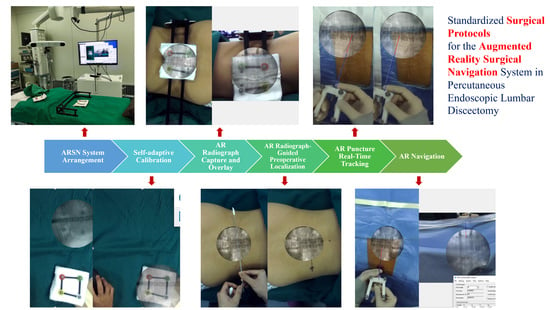
2.3. Standardized Surgical Protocols for the ARSN System in PELD
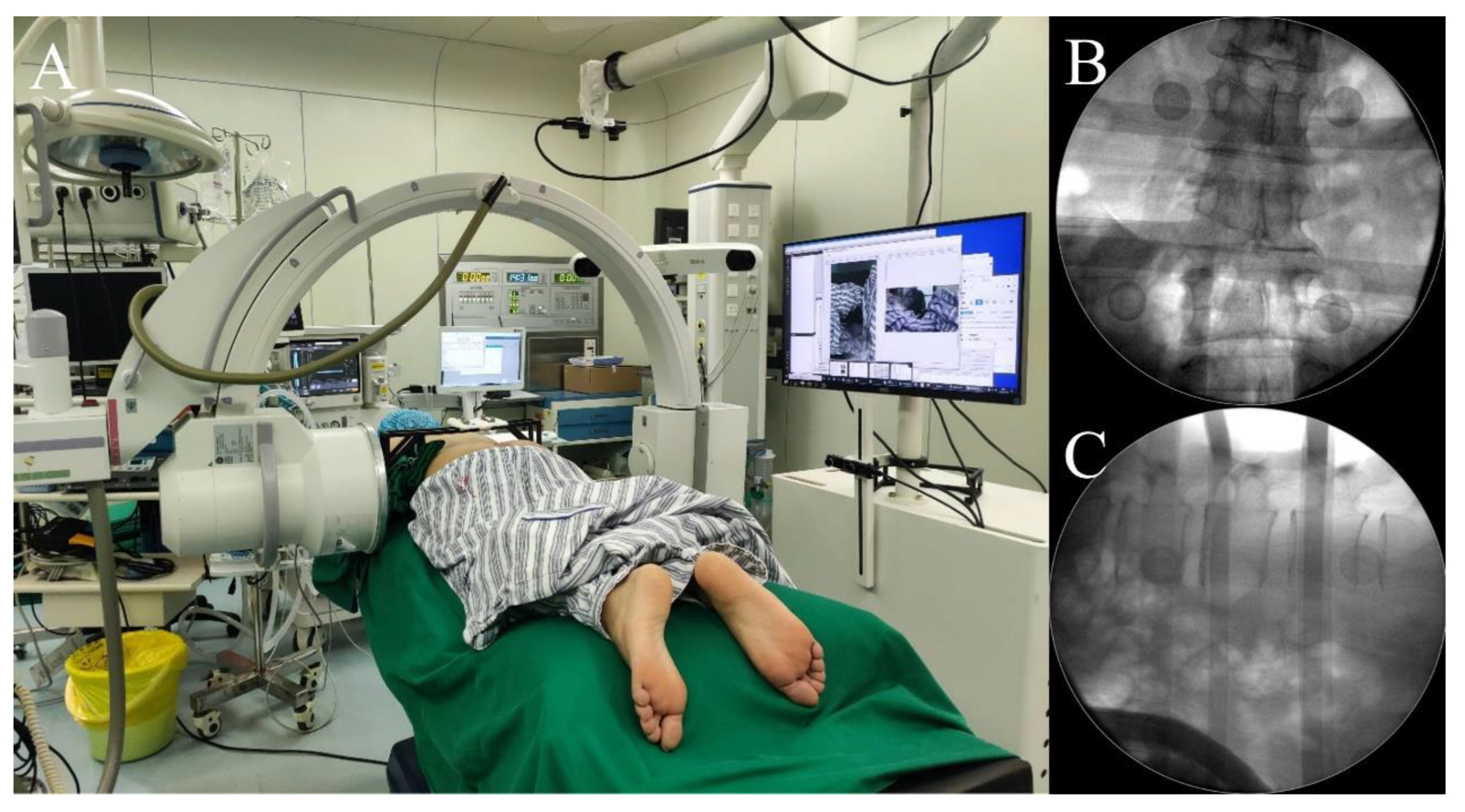
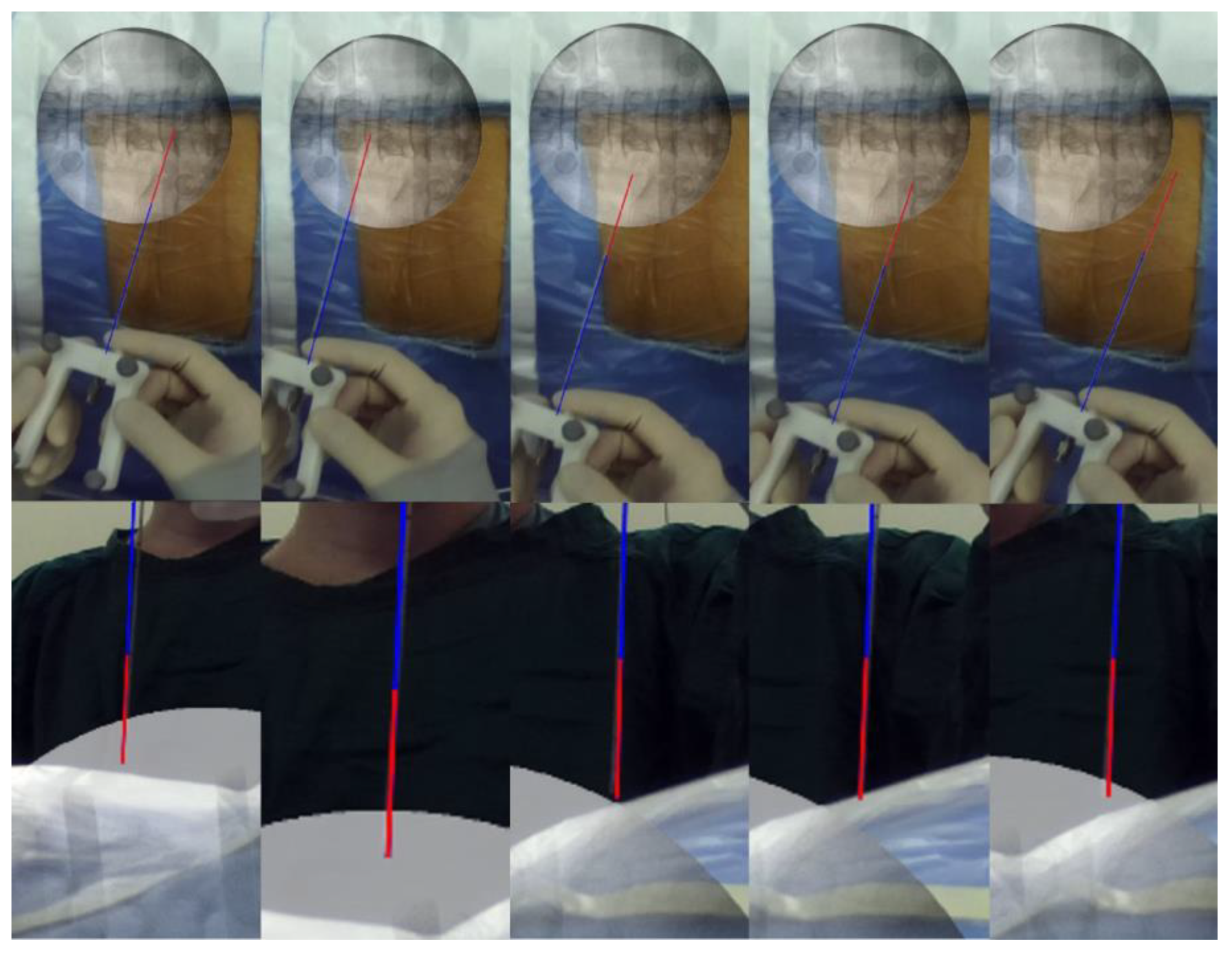
2.3.1. ARSN System Arrangement in the Operating Room
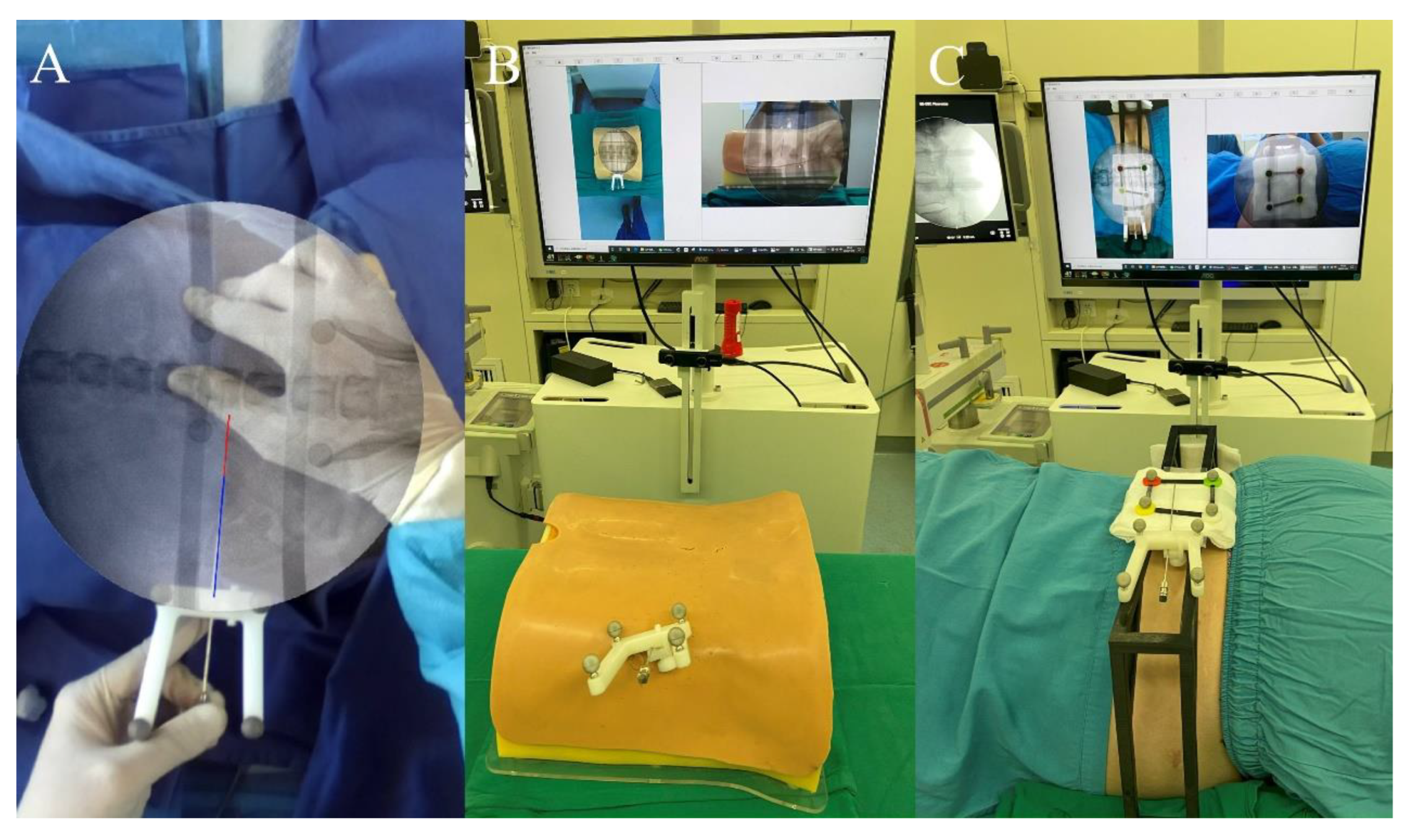
2.3.2. Self-Adaptive Calibration
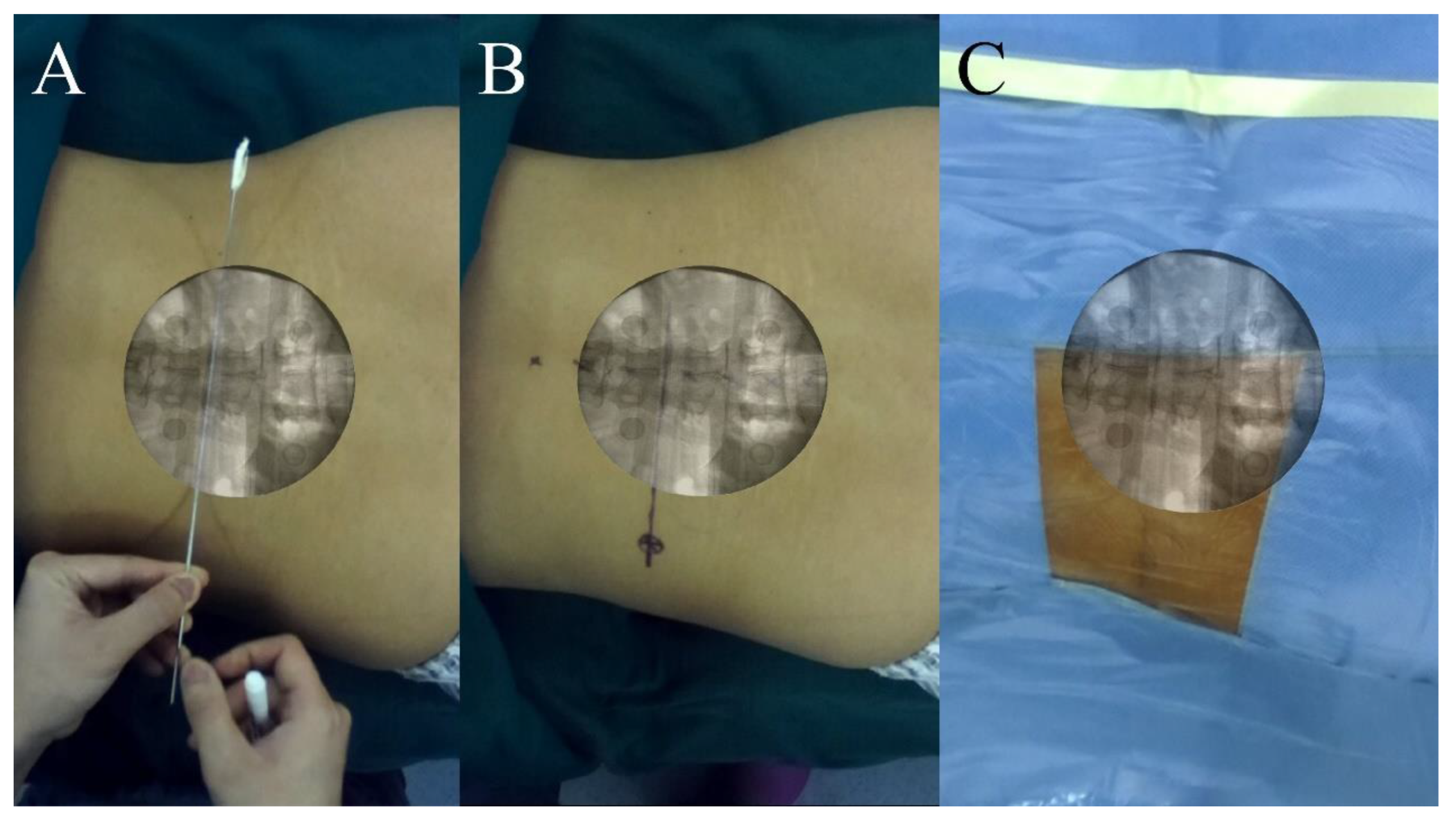
2.3.3. AR Radiograph Capture
2.3.4. AR Radiograph Overlay
2.3.5. AR Radiograph-Guided Preoperative Localization
2.3.6. AR Puncture Real-Time Tracking: External Test before Puncture
2.3.7. AR-Navigated Puncture
2.4. Control Group
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| PELD | Percutaneous Endoscopic Lumbar Discectomy |
| AR | Augmented Reality |
| ARSN | Augmented Reality Surgical Navigation |
| AP | Anteroposterior |
| SVD | Singular Value Decomposition |
References
- Chen, F.; Yang, G.; Wang, J.; Ge, Z.; Wang, H.; Guo, Y.; Yang, H.; Jing, X.; Liu, X.; Cui, X. Clinical Characteristics of Minimal Lumbar Disc Herniation and Efficacy of Percutaneous Endoscopic Lumbar Discectomy via Transforaminal Approach: A Retrospective Study. J. Pers. Med. 2023, 13, 552. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Li, Q.; Li, S.; Mao, H.; Meng, B.; Zhou, F.; Yang, H. Percutaneous endoscopic lumbar discectomy: Indications and complications. Pain Physician 2020, 23, 49–56. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, S.; Son, S.; Kim, H.; Kim, J.E. Learning Curve for Transforaminal Percutaneous Endoscopic Lumbar Discectomy: A Systematic Review. World Neurosurg. 2020, 143, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Chang, S.J.; Yang, S.S.; Chai, C.L. Learning curve of full-endoscopic lumbar discectomy. Eur. Spine J. 2013, 22, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.T.; Tsou, P.M. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine 2002, 27, 722–731. [Google Scholar] [CrossRef]
- Morgenstern, R.; Morgenstern, C.; Yeung, A.T. The learning curve in foraminal endoscopic discectomy: Experience needed to achieve a 90% success rate. SAS J. 2007, 1, 100–107. [Google Scholar] [CrossRef]
- Iprenburg, M.; Wagner, R.; Godschalx, A.; Telfeian, A.E. Patient radiation exposure during transforaminal lumbar endoscopic spine surgery: A prospective study. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef]
- Yu, E.; Khan, S.N. Does less invasive spine surgery result in increased radiation exposure? A systematic review. Clin. Orthop. Relat. Res. 2014, 472, 1738–1748. [Google Scholar] [CrossRef]
- Tezuka, F.; Sakai, T.; Abe, M.; Yamashita, K.; Takata, Y.; Higashino, K.; Chikawa, T.; Nagamachi, A.; Sairyo, K. Anatomical considerations of the iliac crest on percutaneous endoscopic discectomy using a transforaminal approach. Spine J. 2017, 17, 1875–1880. [Google Scholar] [CrossRef]
- Choi, I.; Ahn, J.O.; So, W.S.; Lee, S.J.; Choi, I.J.; Kim, H. Exiting root injury in transforaminal endoscopic discectomy: Preoperative image considerations for safety. Eur. Spine J. 2013, 22, 2481–2487. [Google Scholar] [CrossRef]
- Kim, H.S.; Ju, C.I.; Kim, S.W.; Kim, J.G. Huge Psoas Muscle Hematoma due to Lumbar Segmental Vessel Injury Following Percutaneous Endoscopic Lumbar Discectomy. J. Korean Neurosurg. Soc. 2009, 45, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y. Transforaminal percutaneous endoscopic lumbar discectomy: Technical tips to prevent complications. Expert Rev. Med. Devices 2012, 9, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Gubian, A.; Kausch, L.; Neumann, J.O.; Kiening, K.; Ishak, B.; Maier-Hein, K.; Unterberg, A.; Scherer, M. CT-Navigated Spinal Instrumentations–Three-Dimensional Evaluation of Screw Placement Accuracy in Relation to a Screw Trajectory Plan. Medicina 2022, 58, 1200. [Google Scholar] [CrossRef] [PubMed]
- Móga, K.; Ferencz, A.; Haidegger, T. What Is Next in Computer-Assisted Spine Surgery? Advances in Image-Guided Robotics and Extended Reality. Robotics 2023, 12, 1. [Google Scholar] [CrossRef]
- Wu, B.; Wei, T.; Yao, Z.; Yang, S.; Yao, Y.; Fu, C.; Xu, F.; Xiong, C. A real-time 3D electromagnetic navigation system for percutaneous transforaminal endoscopic discectomy in patients with lumbar disc herniation: A retrospective study. BMC Musculoskelet. Disord. 2022, 23, 57. [Google Scholar] [CrossRef]
- Otomo, N.; Funao, H.; Yamanouchi, K.; Isogai, N.; Ishii, K. Computed Tomography-Based Navigation System in Current Spine Surgery: A Narrative Review. Medicina 2022, 58, 241. [Google Scholar] [CrossRef]
- Härtl, R.; Lam, K.S.; Wang, J.; Korge, A.; Kandziora, F.; Audigé, L. Worldwide survey on the use of navigation in spine surgery. World Neurosurg. 2013, 79, 162–172. [Google Scholar] [CrossRef]
- Makhataeva, Z.; Varol, H.A. Augmented reality for robotics: A review. Robotics 2020, 9, 21. [Google Scholar] [CrossRef]
- Burström, G.; Persson, O.; Edström, E.; Elmi-Terander, A. Augmented reality navigation in spine surgery: A systematic review. Acta Neurochir. 2021, 163, 843–852. [Google Scholar] [CrossRef]
- Ghaednia, H.; Fourman, M.S.; Lans, A.; Detels, K.; Dijkstra, H.; Lloyd, S.; Sweeney, A.; Oosterhoff, J.H.F.; Schwab, J.H. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021, 21, 1617–1625. [Google Scholar] [CrossRef]
- Molina, C.A.; Phillips, F.M.; Colman, M.W.; Ray, W.Z.; Khan, M.; Orru’, E.; Poelstra, K.; Khoo, L. A cadaveric precision and accuracy analysis of augmented reality–mediated percutaneous pedicle implant insertion. J. Neurosurg. Spine 2020, 34, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, X.; Zhu, B.; Hou, X.; Hai, B.; Yu, D.; Zheng, W.; Li, R.; Pan, J.; Yao, Y.; et al. Augmented Reality Surgical Navigation in Minimally Invasive Spine Surgery: A Preclinical Study. Bioengineering 2023, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Sato, S.; Kato, K.; Hyakumachi, T.; Yanagibashi, Y.; Ito, M.; Abumi, K. A novel 3D guidance system using augmented reality for percutaneous vertebroplasty. J. Neurosurg. Spine 2013, 19, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Elmi-Terander, A.; Nachabe, R.; Skulason, H.; Pedersen, K.; Söderman, M.; Racadio, J.; Babic, D.; Gerdhem, P.; Edström, E. Feasibility and accuracy of thoracolumbar minimally invasive pedicle screw placement with augmented reality navigation technology. Spine 2018, 43, 1018–1023. [Google Scholar] [CrossRef]
- Kosterhon, M.; Gutenberg, A.; Kantelhardt, S.R.; Archavlis, E.; Giese, A. Navigation and image injection for control of bone removal and osteotomy planes in spine surgery. Oper. Neurosurg. 2017, 13, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Carl, B.; Bopp, M.; Saß, B.; Pojskic, M.; Voellger, B.; Nimsky, C. Spine Surgery Supported by Augmented Reality. Glob. Spine J. 2020, 10 (Suppl. S2), 41S–55S. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, V.; Sheikh, S.; Schmidt, E.; Steinmetz, M. Imaging Technologies in Spine Surgery. Neurosurg. Clin. N. Am. 2020, 31, 93–101. [Google Scholar] [CrossRef]




| Characteristics | ARSN Group | Control Group | p-Values |
|---|---|---|---|
| Age (years) | 46.9 ± 3.7 | 43.5 ± 4.7 | 0.57 |
| Gender | 0.5 | ||
| Male | 5 | 6 | 1 |
| Female | 5 | 4 | |
| BMI (kg/m2) | 24.3 ± 0.5 | 25.6 ± 1.0 | 0.26 |
| Operative level | 1 | ||
| L2/3 | 0 | 1 | |
| L3/4 | 1 | 1 | |
| L4/5 | 4 | 3 | |
| L5/S1 | 5 | 5 |
| Characteristics | ARSN Group | Control Group | p-Values |
|---|---|---|---|
| Number of Puncture Attempts | 2.0 ± 0.4 | 6.9 ± 0.5 | 0.000 |
| Overall Number of Fluoroscopies | 10.6 ± 0.9 | 18.5 ± 1.6 | 0.000 |
| 5.2 ± 0.6 | 9.1 ± 0.8 | 0.001 |
| 5.4 ± 0.6 | 9.4 ± 0.8 | 0.001 |
| Number of Localization Fluoroscopies | 6.6 ± 0.6 | 4.8 ± 0.9 | 0.095 |
| 3.2 ± 0.5 | 2.2 ± 0.4 | 0.117 |
| 3.4 ± 0.3 | 2.6 ± 0.5 | 0.202 |
| Number of Puncture Fluoroscopies | 4.0 ± 0.8 | 13.7 ± 1.0 | 0.000 |
| 2.0 ± 0.4 | 6.9 ± 0.5 | 0.000 |
| 2.0 ± 0.4 | 6.8 ± 0.5 | 0.000 |
| Operation Time (min) | 75.8 ± 11.2 | 74.3 ± 7.2 | 0.911 |
| Characteristics/Metrics | ARSN Group | Control Group | p-Values |
|---|---|---|---|
| Preoperative Metrics: | |||
| VAS | 7.3 ± 0.3 | 7.8 ± 0.2 | 0.247 |
| ODI | 31.0 ± 1.8 | 29.5 ± 2.8 | 0.661 |
| Post-Surgery Metrics (1 Week): | |||
| VAS | 1.3 ± 0.4 | 1.4 ± 0.5 | 0.866 |
| ODI | 8.2 ± 1.9 | 9.3 ± 2.4 | 0.724 |
| Post-Surgery Metrics (1 Month): | |||
| VAS | 0.8 ± 0.3 | 1.1 ± 0.3 | 0.517 |
| ODI | 6.6 ± 1.4 | 7.9 ± 1.5 | 0.532 |
| Complication Rate | 0 | 0 | 1 |
| Modified Macnab Scores | 1 | ||
| Excellent | 5 | 4 | |
| Good | 4 | 5 | |
| Fair | 1 | 1 | |
| Poor | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Liu, X.; Zhu, B.; Hou, X.; Hai, B.; Li, S.; Yu, D.; Zheng, W.; Li, R.; Pan, J.; et al. Evaluation of Augmented Reality Surgical Navigation in Percutaneous Endoscopic Lumbar Discectomy: Clinical Study. Bioengineering 2023, 10, 1297. https://doi.org/10.3390/bioengineering10111297
Huang X, Liu X, Zhu B, Hou X, Hai B, Li S, Yu D, Zheng W, Li R, Pan J, et al. Evaluation of Augmented Reality Surgical Navigation in Percutaneous Endoscopic Lumbar Discectomy: Clinical Study. Bioengineering. 2023; 10(11):1297. https://doi.org/10.3390/bioengineering10111297
Chicago/Turabian StyleHuang, Xin, Xiaoguang Liu, Bin Zhu, Xiangyu Hou, Bao Hai, Shuiqing Li, Dongfang Yu, Wenhao Zheng, Ranyang Li, Junjun Pan, and et al. 2023. "Evaluation of Augmented Reality Surgical Navigation in Percutaneous Endoscopic Lumbar Discectomy: Clinical Study" Bioengineering 10, no. 11: 1297. https://doi.org/10.3390/bioengineering10111297
APA StyleHuang, X., Liu, X., Zhu, B., Hou, X., Hai, B., Li, S., Yu, D., Zheng, W., Li, R., Pan, J., Yao, Y., Dai, Z., & Zeng, H. (2023). Evaluation of Augmented Reality Surgical Navigation in Percutaneous Endoscopic Lumbar Discectomy: Clinical Study. Bioengineering, 10(11), 1297. https://doi.org/10.3390/bioengineering10111297