Accelerating High b-Value Diffusion-Weighted MRI Using a Convolutional Recurrent Neural Network (CRNN-DWI)
Abstract
1. Introduction
2. Materials and Methods
2.1. CRNN-DWI
2.1.1. CRNN-i Layer
2.1.2. CRNN-b-i Layer
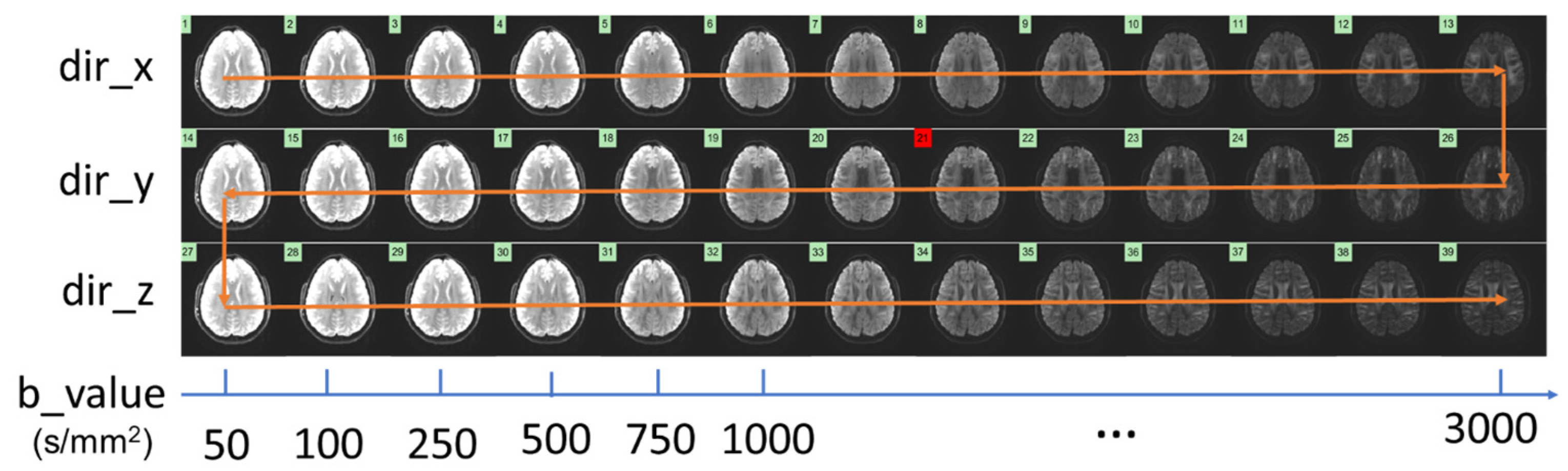
2.2. Data Acquisition and Image Reconstruction
2.3. CTRW Model Fitting
2.4. Image and Statistical Analysis
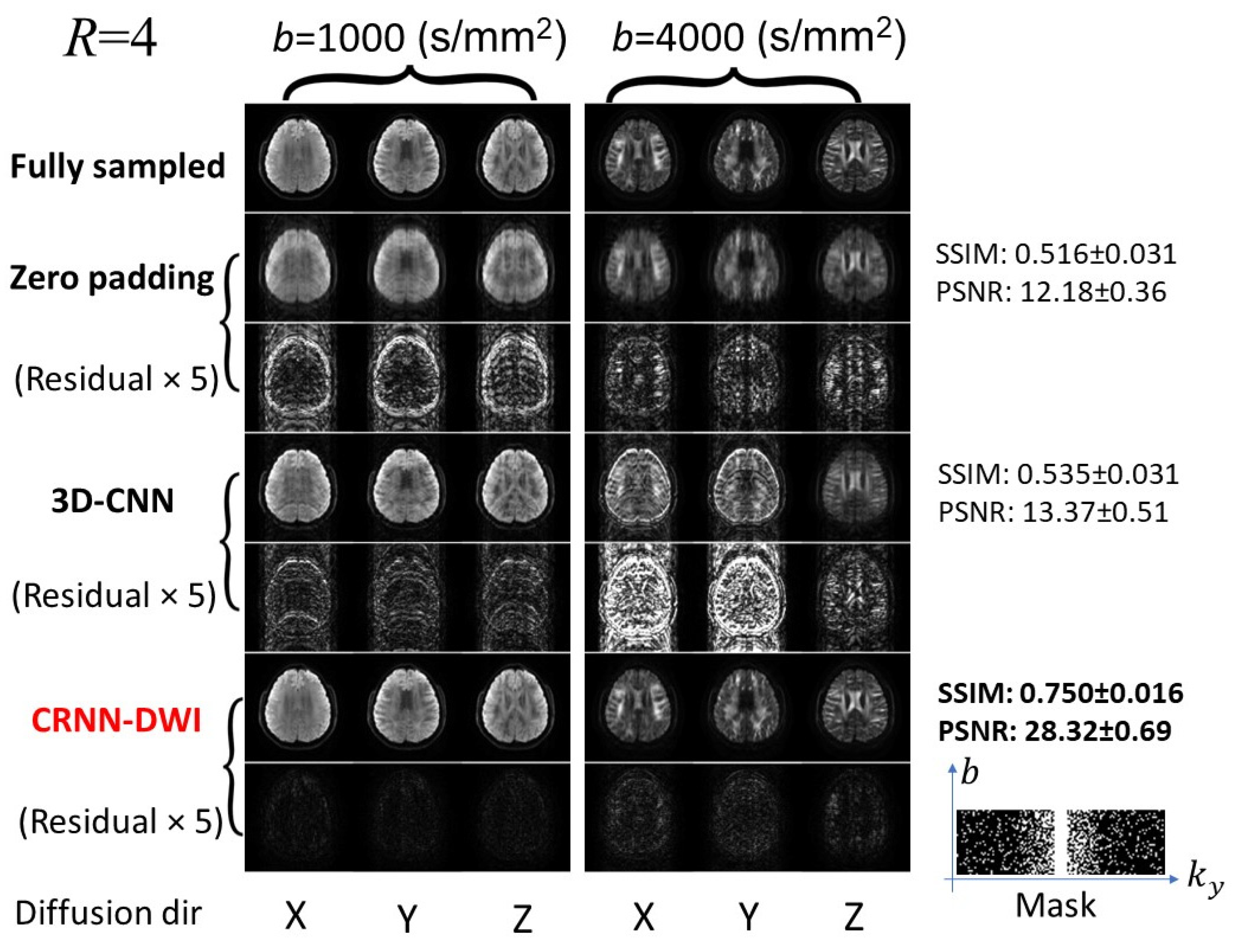
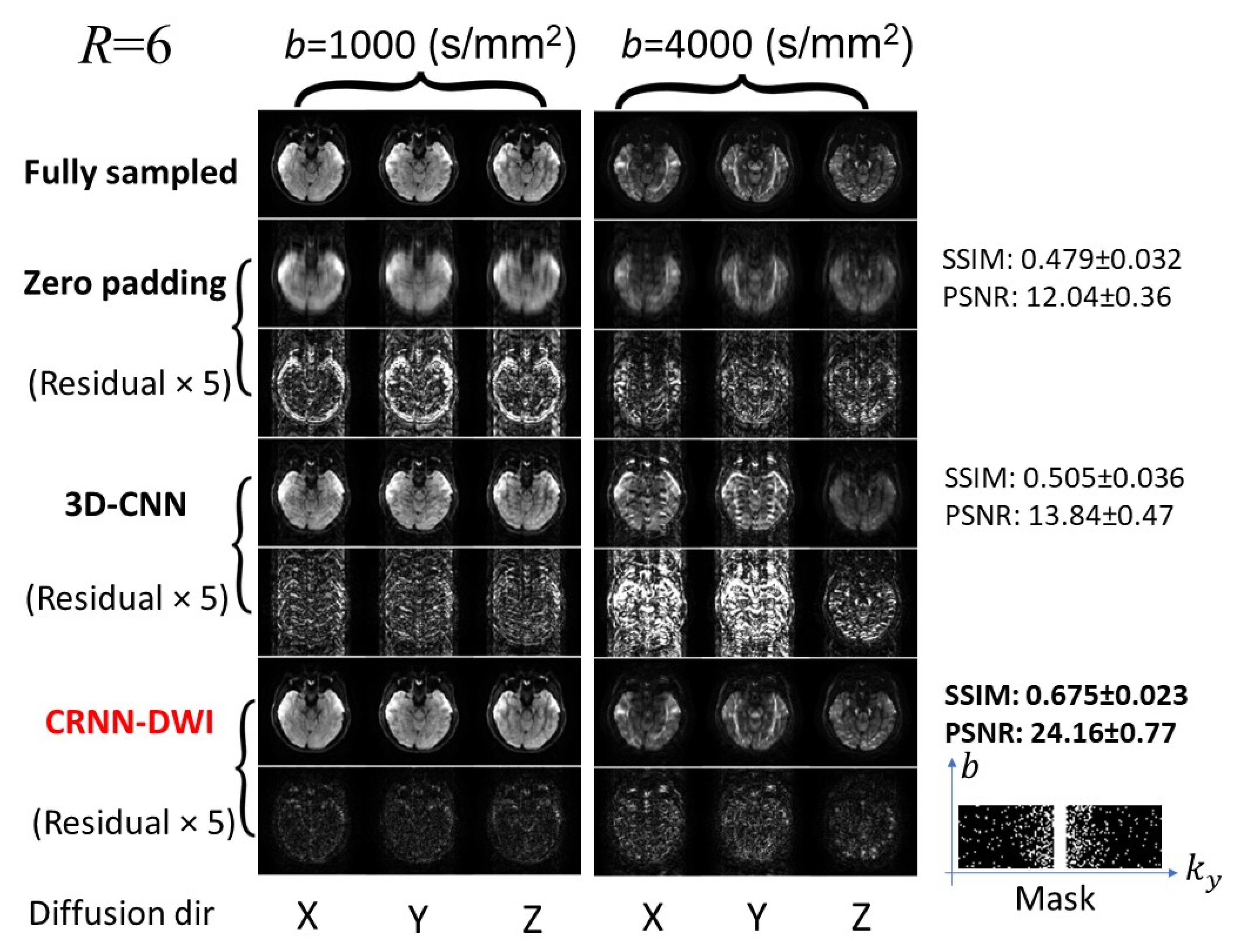
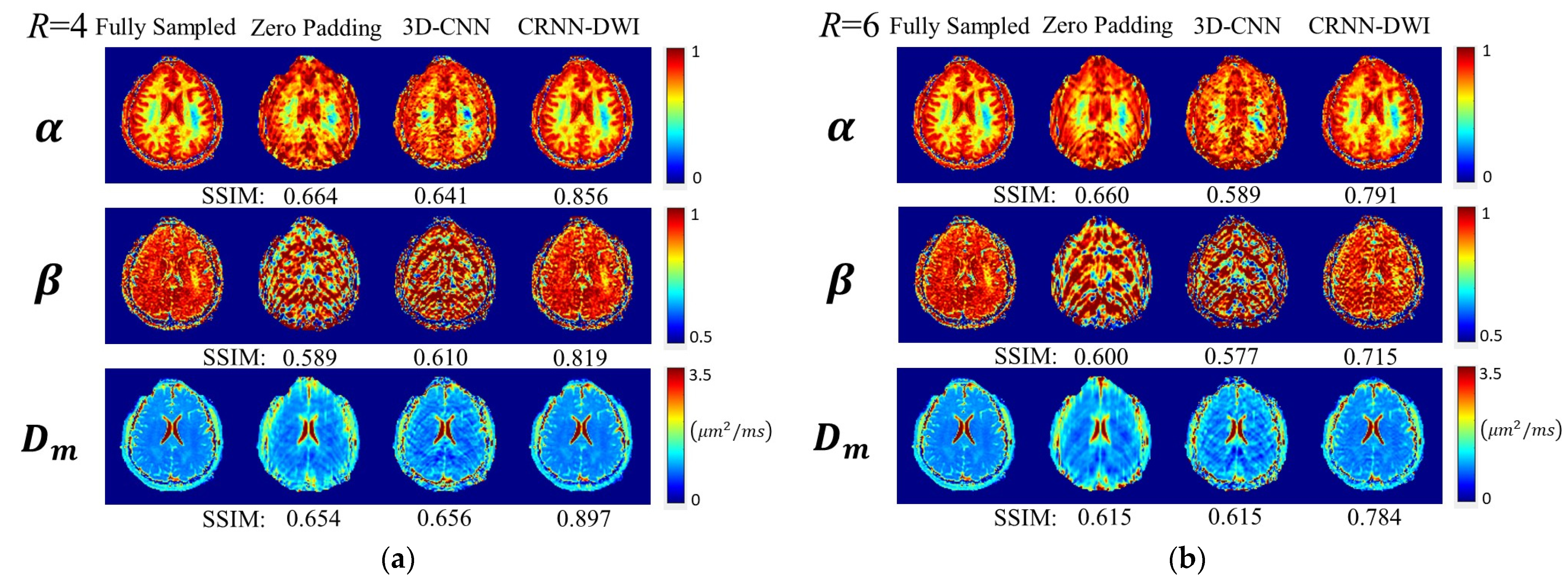
3. Results
3.1. Reconstructed Images
3.2. Diffusion Parameter Maps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorensen, A.G.; Buonanno, F.S.; Gonzalez, R.G.; Schwamm, L.H.; Lev, M.H.; Huang-Hellinger, F.R.; Reese, T.G.; Weisskoff, R.M.; Davis, T.L.; Suwanwela, N.; et al. Hyperacute stroke: Evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996, 199, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wang, H.; Liu, G.; Damen, F.W.; Wanamaker, C.; Li, Y.; Zhou, X.J. Differentiation of Low- and High-Grade Pediatric Brain Tumors with High b-Value Diffusion-weighted MR Imaging and a Fractional Order Calculus Model. Radiology 2015, 277, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Seo, N.; Chung, Y.E.; Park, M.-S.; Choi, J.-Y.; Kim, M.-J. Characterization of focal liver lesions using the stretched exponential model: Comparison with monoexponential and biexponential diffusion-weighted magnetic resonance imaging. Eur. Radiol. 2019, 29, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, P.; Mann, R.M.; Iima, M.; Sigmund, E.E.; Clauser, P.; Gilbert, F.J.; Martincich, L.; Partridge, S.C.; Patterson, A.; Pinker, K.; et al. Diffusion-weighted imaging of the breast—A consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur. Radiol. 2020, 30, 1436–1450. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Spraker, M.B.; Prodoehl, J.; Abraham, I.; Corcos, D.M.; Zhou, X.J.; Comella, C.L.; Little, D.M. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 2009, 72, 1378–1384. [Google Scholar] [CrossRef]
- Niendorf, T.; Dijkhuizen, R.M.; Norris, D.G.; van Lookeren Campagne, M.; Nicolay, K. Biexponential diffusion attenuation in various states of brain tissue: Implications for diffusion-weighted imaging. Magn. Reson. Med. 1996, 36, 847–857. [Google Scholar] [CrossRef]
- Bennett, K.M.; Schmainda, K.M.; Tong, R.B.; Rowe, D.B.; Lu, H.; Hyde, J.S. Characterization of continuously distributed cortical water diffusion rates with a stretched-exponential model. Magn. Reson. Med. 2003, 50, 727–734. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, X.J. Diffusion MRI of cancer: From low to high b-values: High b-Value Diffusion MRI of Cancer. J. Magn. Reson. Imaging 2019, 49, 23–40. [Google Scholar] [CrossRef]
- Zhou, X.J.; Gao, Q.; Abdullah, O.; Magin, R.L. Studies of anomalous diffusion in the human brain using fractional order calculus. Magn. Reson. Med. 2010, 63, 562–569. [Google Scholar] [CrossRef]
- Zhong, Z.; Merkitch, D.; Karaman, M.M.; Zhang, J.; Sui, Y.; Goldman, J.G.; Zhou, X.J. High-Spatial-Resolution Diffusion MRI in Parkinson Disease: Lateral Asymmetry of the Substantia Nigra. Radiology 2019, 291, 149–157. [Google Scholar] [CrossRef]
- Karaman, M.M.; Sui, Y.; Wang, H.; Magin, R.L.; Li, Y.; Zhou, X.J. Differentiating low- and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values. Magn. Reson. Med. 2016, 76, 1149–1157. [Google Scholar] [CrossRef]
- Karaman, M.M.; Zhou, C.Y.; Zhang, J.; Zhong, Z.; Wang, K.; Zhu, W. Percentile-Based Analysis of Non-Gaussian Diffusion Parameters for Improved Glioma Grading. Investig. Magn. Reson. Imaging 2022, 26, 104. [Google Scholar] [CrossRef]
- Karaman, M.M.; Zhang, J.; Xie, K.L.; Zhu, W.; Zhou, X.J. Quartile histogram assessment of glioma malignancy using high b-value diffusion MRI with a continuous-time random-walk model. NMR Biomed. 2021, 34, e4485. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.A.; King, K.F.; Zhou, X.J. Handbook of MRI Pulse Sequences; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Tamada, T.; Kido, A.; Ueda, Y.; Takeuchi, M.; Kanki, A.; Neelavalli, J.; Yamamoto, A. Comparison of single-shot EPI and multi-shot EPI in prostate DWI at 3.0 T. Sci. Rep. 2022, 12, 16070. [Google Scholar] [CrossRef]
- Banerjee, S.; Nishimura, D.G.; Shankaranarayanan, A.; Saritas, E.U. Reduced field-of-view DWI with robust fat suppression and unrestricted slice coverage using tilted 2DRF excitation: rFOV DWI with Tilted 2D RF Excitation. Magn. Reson. Med. 2016, 76, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Finsterbusch, J. Improving the performance of diffusion-weighted inner field-of-view echo-planar imaging based on 2D-Selective radiofrequency excitations by tilting the excitation plane. J. Magn. Reson. Imaging 2012, 35, 984–992. [Google Scholar] [CrossRef]
- Sun, K.; Zhong, Z.; Xu, Z.; Dan, G.; Karaman, M.M.; Zhou, X.J. In-plane simultaneous multisegment imaging using a 2D RF pulse. Magn. Reson. Med. 2022, 87, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Pipe, J.G. Non-EPI Pulse Sequences for Diffusion MRI. In Diffusion MRI; Jones, D.K., Ed.; Oxford University Press: Singapore, 2010; pp. 203–217. [Google Scholar] [CrossRef]
- Lee, D.; Yoo, J.; Tak, S.; Ye, J.C. Deep Residual Learning for Accelerated MRI using Magnitude and Phase Networks. arXiv 2018, arXiv:1804.00432. [Google Scholar] [CrossRef] [PubMed]
- Eo, T.; Jun, Y.; Kim, T.; Jang, J.; Lee, H.; Hwang, D. KIKI-net: Cross-domain convolutional neural networks for reconstructing undersampled magnetic resonance images. Magn. Reson. Med. 2018, 80, 2188–2201. [Google Scholar] [CrossRef]
- Qin, C.; Schlemper, J.; Caballero, J.; Price, A.N.; Hajnal, J.V.; Rueckert, D. Convolutional Recurrent Neural Networks for Dynamic MR Image Reconstruction. IEEE Trans. Med. Imaging 2019, 38, 280–290. [Google Scholar] [CrossRef]
- Schlemper, J.; Caballero, J.; Hajnal, J.V.; Price, A.N.; Rueckert, D. A Deep Cascade of Convolutional Neural Networks for Dynamic MR Image Reconstruction. IEEE Trans. Med. Imaging 2018, 37, 491–503. [Google Scholar] [CrossRef]
- Guo, P.; Valanarasu, J.M.J.; Wang, P.; Zhou, J.; Jiang, S.; Patel, V.M. Over-and-Under Complete Convolutional RNN for MRI Reconstruction. In Medical Image Computing and Computer Assisted Intervention—MICCAI 2021; De Bruijne, M., Cattin, P.C., Cotin, S., Padoy, N., Speidel, S., Zheng, Y., Essert, C., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 12906, pp. 13–23. [Google Scholar] [CrossRef]
- Zhang, Y.; She, H.; Du, Y.P. Dynamic MRI of the abdomen using parallel non-Cartesian convolutional recurrent neural networks. Magn. Reson. Med. 2021, 86, 964–973. [Google Scholar] [CrossRef]
- Pipe, J.G.; Farthing, V.G.; Forbes, K.P. Multishot diffusion-weighted FSE using PROPELLER MRI. Magn. Reson. Med. 2002, 47, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Xiong, Y.; Jiang, J.; Karaman, M.M.; Xie, K.L.; Zhu, W.; Zhou, X.J. Differentiation of Low- and High-Grade Gliomas Using High b-Value Diffusion Imaging with a Non-Gaussian Diffusion Model. Am. J. Neuroradiol. 2016, 37, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Sui, Y.; Zhong, Z.; Damen, F.C.; Li, J.; Shen, L.; Sun, Y.; Zhou, X.J. Non-Gaussian diffusion imaging with a fractional order calculus model to predict response of gastrointestinal stromal tumor to second-line sunitinib therapy: A FROC Diffusion Model to Predict GIST Response. Magn. Reson. Med. 2018, 79, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.M.; Tang, L.; Li, Z.; Sun, Y.; Li, J.-Z.; Zhou, X.J. In vivo assessment of Lauren classification for gastric adenocarcinoma using diffusion MRI with a fractional order calculus model. Eur. Radiol. 2021, 31, 5659–5668. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Y.; Dan, G.; Zhong, Z.; Karaman, M.M.; Li, Z.; Hu, D.; Zhou, X.J. Evaluation of a fractional-order calculus diffusion model and bi-parametric VI-RADS for staging and grading bladder urothelial carcinoma. Eur. Radiol. 2022, 32, 890–900. [Google Scholar] [CrossRef]
- Li, Z.; Dan, G.; Tammana, V.; Johnson, S.; Zhong, Z.; Rabiee, B.; Zhou, X.J.; Xie, K.L. Predicting the aggressiveness of peripheral zone prostate cancer using a fractional order calculus diffusion model. Eur. J. Radiol. 2021, 143, 109913. [Google Scholar] [CrossRef]
- Borkowski, K.; Krzyżak, A.T. Analysis and correction of errors in DTI-based tractography due to diffusion gradient inhomogeneity. J. Magn. Reson. 2018, 296, 5–11. [Google Scholar] [CrossRef]
- Krzyzak, A.; Borkowski, K. Theoretical analysis of phantom rotations in BSD-DTI. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 410–413. [Google Scholar] [CrossRef]
- Sandino, C.M.; Ong, F.; Vasanawala, S.S. Deep Subspace Learning: Enhancing Speed and Scalability of Deep Learning-Based Reconstruction of Dynamic Imaging Data; International Society for Magnetic Resonance in Medicine: Concord, CA, USA, 2020; Volume 0599. [Google Scholar]

| CRNN-DWI | 3D-CNN | Zero-Filling | |||||||
|---|---|---|---|---|---|---|---|---|---|
| α | β | Dm | α | β | Dm | α | β | Dm | |
| R = 4 | 0.84 ± 0.04 | 0.82 ± 0.04 | 0.9 ± 0.03 | 0.62 ± 0.06 | 0.62 ± 0.05 | 0.64 ± 0.06 | 0.66 ± 0.06 | 0.62 ± 0.05 | 0.65 ± 0.06 |
| R = 6 | 0.75 ± 0.06 | 0.71 ± 0.05 | 0.77 ± 0.05 | 0.6 ± 0.06 | 0.6 ± 0.05 | 0.61 ± 0.06 | 0.64 ± 0.06 | 0.61 ± 0.05 | 0.62 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Z.; Ryu, K.; Mao, J.; Sun, K.; Dan, G.; Vasanawala, S.S.; Zhou, X.J. Accelerating High b-Value Diffusion-Weighted MRI Using a Convolutional Recurrent Neural Network (CRNN-DWI). Bioengineering 2023, 10, 864. https://doi.org/10.3390/bioengineering10070864
Zhong Z, Ryu K, Mao J, Sun K, Dan G, Vasanawala SS, Zhou XJ. Accelerating High b-Value Diffusion-Weighted MRI Using a Convolutional Recurrent Neural Network (CRNN-DWI). Bioengineering. 2023; 10(7):864. https://doi.org/10.3390/bioengineering10070864
Chicago/Turabian StyleZhong, Zheng, Kanghyun Ryu, Jonathan Mao, Kaibao Sun, Guangyu Dan, Shreyas S. Vasanawala, and Xiaohong Joe Zhou. 2023. "Accelerating High b-Value Diffusion-Weighted MRI Using a Convolutional Recurrent Neural Network (CRNN-DWI)" Bioengineering 10, no. 7: 864. https://doi.org/10.3390/bioengineering10070864
APA StyleZhong, Z., Ryu, K., Mao, J., Sun, K., Dan, G., Vasanawala, S. S., & Zhou, X. J. (2023). Accelerating High b-Value Diffusion-Weighted MRI Using a Convolutional Recurrent Neural Network (CRNN-DWI). Bioengineering, 10(7), 864. https://doi.org/10.3390/bioengineering10070864









