Cost-Effective Full-Color 3D Dental Imaging Based on Close-Range Photogrammetry
Abstract
:1. Introduction
2. Materials and Methods
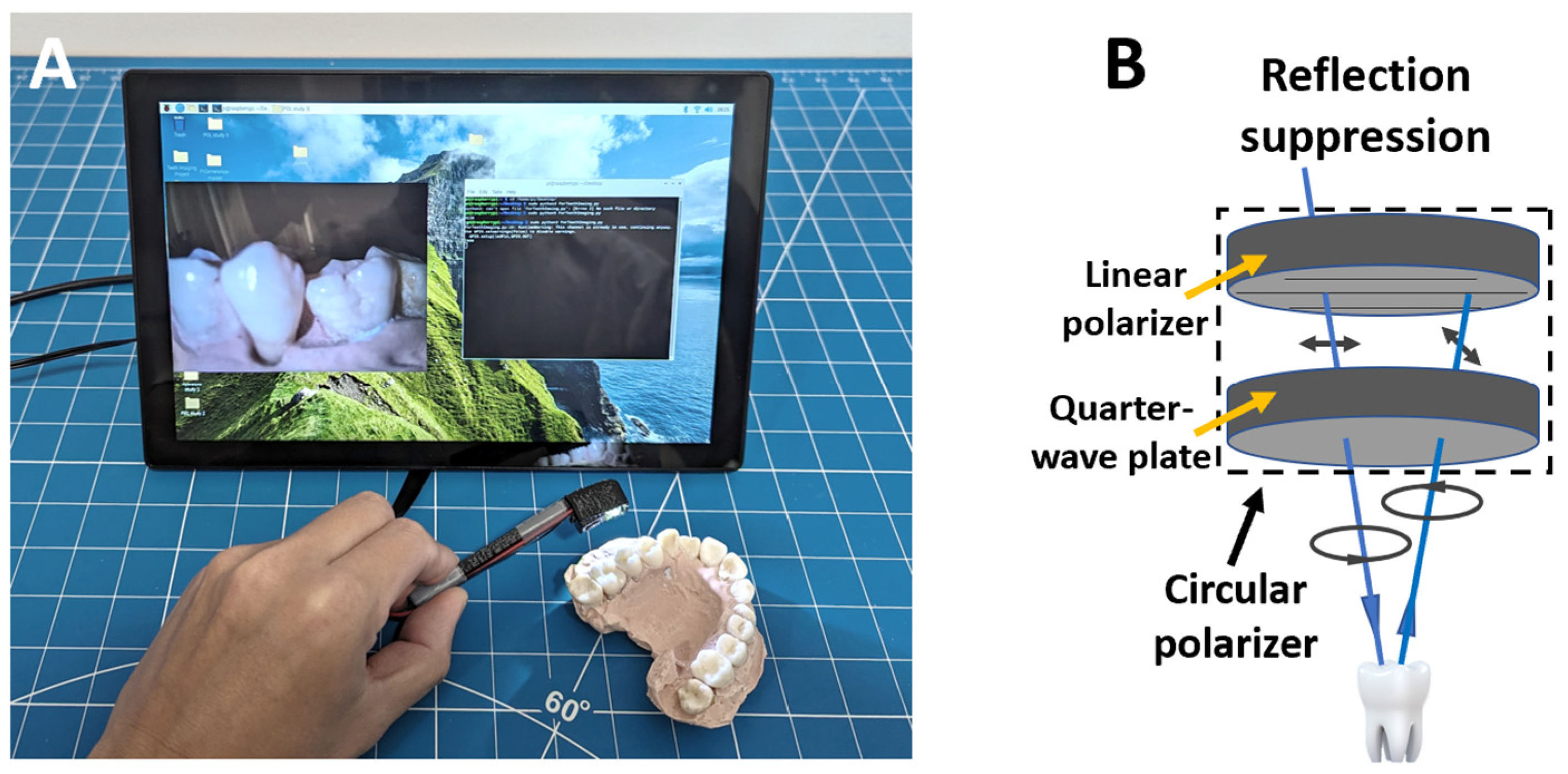
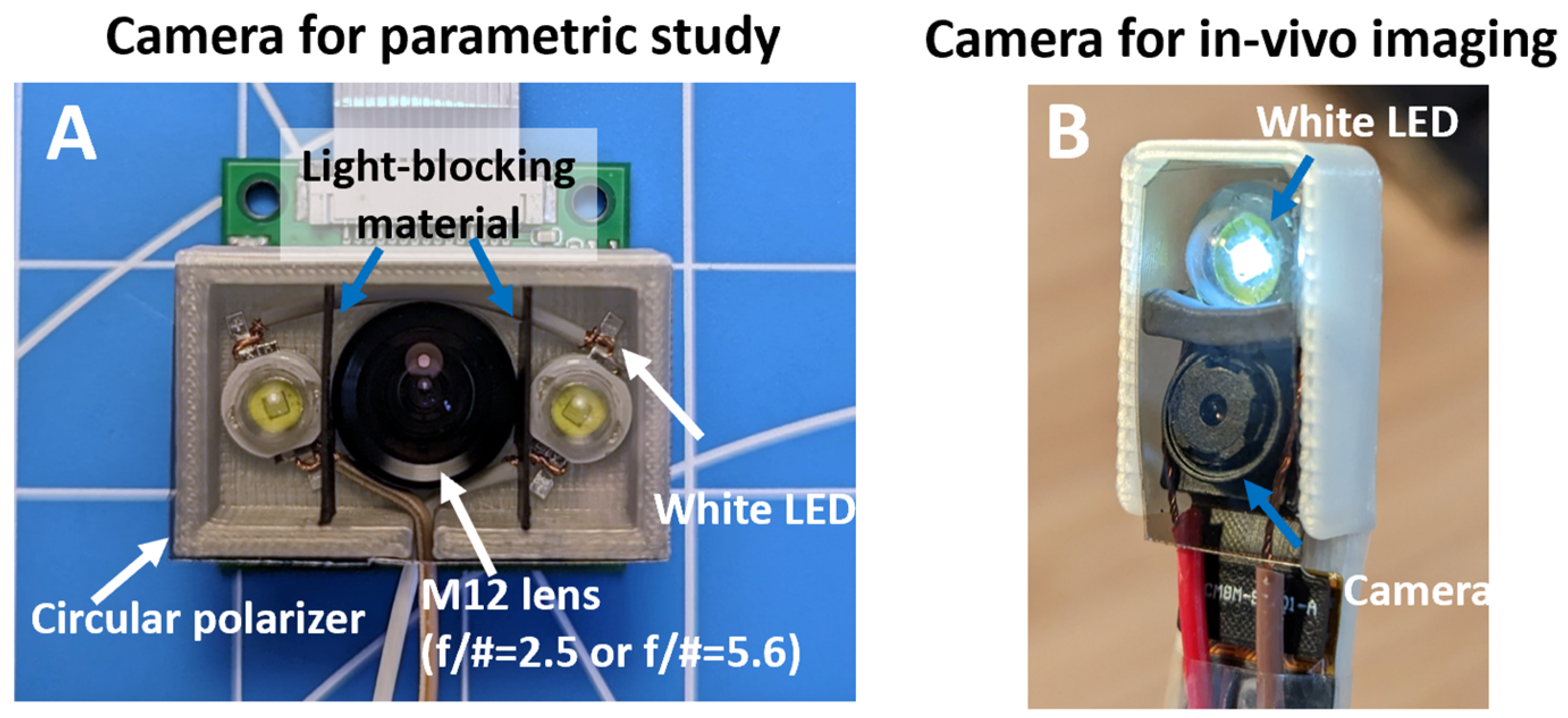
2.1. Imaging Device Development
2.2. Parametric Studies of the Effects of Depth-of-Field and Surface Reflection on Reconstruction Quality
2.3. In Vivo Teeth Imaging
2.4. Photogrammetry-Based 3D Reconstruction
2.5. Reconstruction and Trueness Analysis
3. Results
3.1. Imaging Device Development
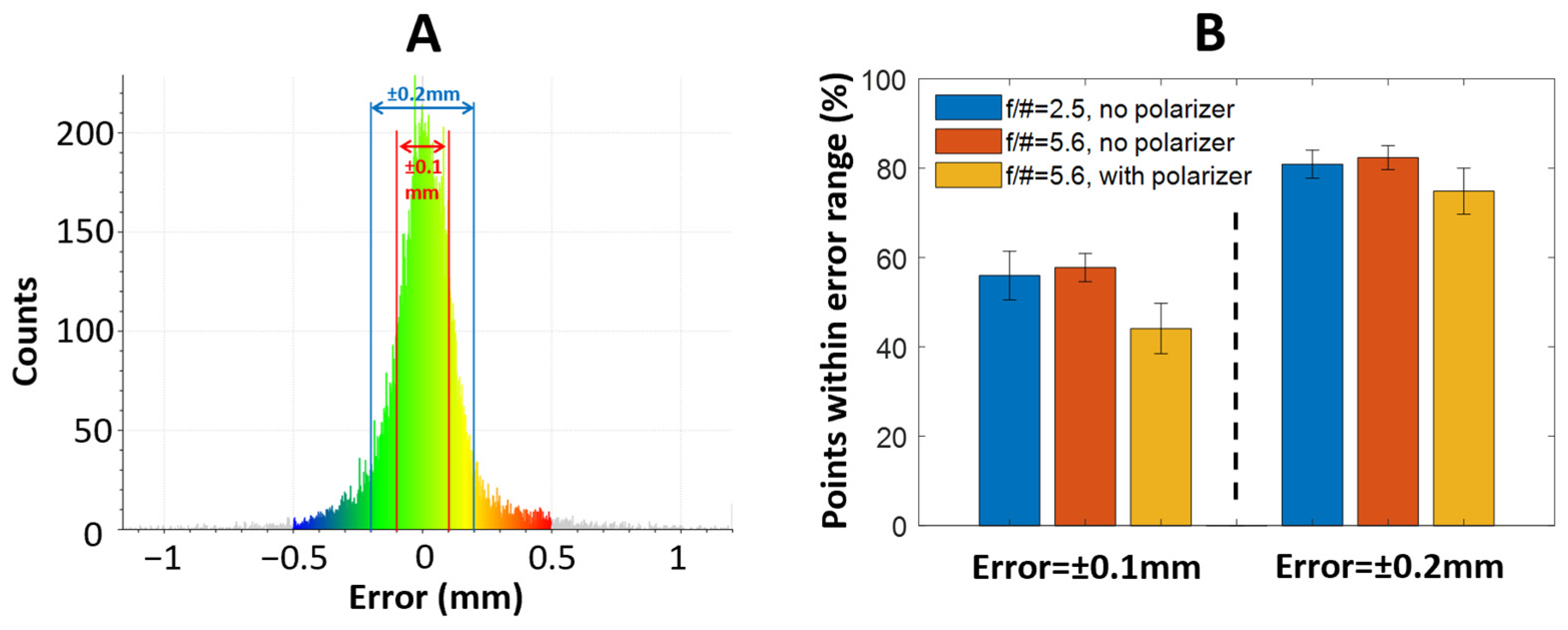
3.2. Parametric Studies of the Effects of Depth-of-Field and Surface Reflection on Reconstruction Quality
3.3. In Vivo Teeth Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, I. Digital dental photography. Part 1: An overview. Br. Dent. J. 2009, 206, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I. Digital dental photography. Part 2: Purposes and uses. Br. Dent. J. 2009, 206, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Casaglia, A.; De Dominicis, P.; Arcuri, L.; Gargari, M.; Ottria, L. Dental photography today. Part 1: Basic concepts. Oral Implantol. 2015, 8, 122. [Google Scholar]
- Mladenović, D.; Mladenović, L.; Mladenović, S. Importance of digital dental photography in the practice of dentistry. Sci. J. Fac. Med. Niš 2010, 27, 75–79. [Google Scholar]
- Birnbaum, N.S.; Aaronson, H.B.; Stevens, C.; Cohen, B. 3D digital scanners: A high-tech approach to more accurate dental impressions. Inside Dent. 2009, 5, 70–74. [Google Scholar]
- Logozzo, S.; Zanetti, E.M.; Franceschini, G.; Kilpelä, A.; Mäkynen, A. Recent advances in dental optics–Part I: 3D intraoral scanners for restorative dentistry. Opt. Lasers Eng. 2014, 54, 203–221. [Google Scholar]
- Jedliński, M.; Mazur, M.; Grocholewicz, K.; Janiszewska-Olszowska, J. 3D scanners in orthodontics—Current knowledge and future perspectives—A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 1121. [Google Scholar] [CrossRef]
- Taneva, E.; Kusnoto, B.; Evans, C.A. 3D scanning, imaging, and printing in orthodontics. Issues Contemp. Orthod. 2015, 148, 862–867. [Google Scholar]
- Dreiseidler, T.; Neugebauer, J.; Ritter, L.; Lingohr, T.; Rothamel, D.; Mischkowski, R.A.; Zöller, J.E. Accuracy of a newly developed integrated system for dental implant planning. Clin. Oral Implant. Res. 2009, 20, 1191–1199. [Google Scholar] [CrossRef]
- Flügge, T.V.; Nelson, K.; Schmelzeisen, R.; Metzger, M.C. Three-dimensional plotting and printing of an implant drilling guide: Simplifying guided implant surgery. J. Oral Maxillofac. Surg. 2013, 71, 1340–1346. [Google Scholar]
- Sarment, D.P.; Sukovic, P.; Clinthorne, N. Accuracy of implant placement with a stereolithographic surgical guide. Int. J. Oral Maxillofac. Implant. 2003, 18, 571–577. [Google Scholar]
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral scanners in dentistry: A review of the current literature. BMC Oral Health 2017, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Park, A.; Kim, J.W.; Lee, B.H.; Eom, J.B. Development of three-dimensional dental scanning apparatus using structured illumination. Sensors 2017, 17, 1634. [Google Scholar] [CrossRef] [PubMed]
- Geng, J. Structured-light 3D surface imaging: A tutorial. Adv. Opt. Photonics 2011, 3, 128–160. [Google Scholar] [CrossRef]
- Wang, J.; Suenaga, H.; Hoshi, K.; Yang, L.; Kobayashi, E.; Sakuma, I.; Liao, H. Augmented reality navigation with automatic marker-free image registration using 3-D image overlay for dental surgery. IEEE Trans. Biomed. Eng. 2014, 61, 1295–1304. [Google Scholar] [CrossRef]
- Orban, G.A.; Janssen, P.; Vogels, R. Extracting 3D structure from disparity. Trends Neurosci. 2006, 29, 466–473. [Google Scholar] [CrossRef]
- Aguilar, J.-J.; Torres, F.; Lope, M. Stereo vision for 3D measurement: Accuracy analysis, calibration and industrial applications. Measurement 1996, 18, 193–200. [Google Scholar] [CrossRef]
- Jiang, R.; Jáuregui, D.V.; White, K.R. Close-range photogrammetry applications in bridge measurement: Literature review. Measurement 2008, 41, 823–834. [Google Scholar] [CrossRef]
- Luhmann, T. Close range photogrammetry for industrial applications. ISPRS J. Photogramm. Remote Sens. 2010, 65, 558–569. [Google Scholar] [CrossRef]
- Luhmann, T.; Robson, S.; Kyle, S.; Boehm, J. Close-Range Photogrammetry and 3D Imaging; Walter de Gruyter: Berlin, Germany, 2013. [Google Scholar]
- Hartley, R.I.; Sturm, P. Triangulation. Comput. Vis. Image Underst. 1997, 68, 146–157. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Qian, S.-J.; Lai, H.-C.; Shi, J.-Y. Accuracy of photogrammetric imaging versus conventional impressions for complete-arch implant-supported fixed dental prostheses: A comparative clinical study. J. Prosthet. Dent. 2021, 130, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Peng, C.; Li, Z.; Liu, S.; Tan, M.; Song, J. The application of multi-baseline digital close-range photogrammetry in three-dimensional imaging and measurement of dental casts. PLoS ONE 2017, 12, e0178858. [Google Scholar] [CrossRef]
- Zotti, F.; Rosolin, L.; Bersani, M.; Poscolere, A.; Pappalardo, D.; Zerman, N. Digital dental models: Is photogrammetry an alternative to dental extraoral and intraoral scanners? Dent. J. 2022, 10, 24. [Google Scholar] [CrossRef]
- Morgan, S.P.; Stockford, I.M. Surface-reflection elimination in polarization imaging of superficial tissue. Opt. Lett. 2003, 28, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Collett, E. Field Guide to Polarization; SPIE: Bellingham, WA, USA, 2005. [Google Scholar]
- Colomina, I.; Molina, P. Unmanned aerial systems for photogrammetry and remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2014, 92, 79–97. [Google Scholar] [CrossRef]
- Konecny, G. Geoinformation: Remote Sensing, Photogrammetry and Geographic Information Systems; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Honkavaara, E.; Arbiol, R.; Markelin, L.; Martinez, L.; Cramer, M.; Bovet, S.; Chandelier, L.; Ilves, R.; Klonus, S.; Marshal, P. Digital airborne photogrammetry—A new tool for quantitative remote sensing?—A state-of-the-art review on radiometric aspects of digital photogrammetric images. Remote Sens. 2009, 1, 577–605. [Google Scholar] [CrossRef]
- Remondino, F. Heritage recording and 3D modeling with photogrammetry and 3D scanning. Remote Sens. 2011, 3, 1104–1138. [Google Scholar] [CrossRef]
- Portalés, C.; Lerma, J.L.; Navarro, S. Augmented reality and photogrammetry: A synergy to visualize physical and virtual city environments. ISPRS J. Photogramm. Remote Sens. 2010, 65, 134–142. [Google Scholar] [CrossRef]
- Lindeberg, T. Scale invariant feature transform. Digit. Vetensk. Ark. 2012, 7, 10491. [Google Scholar] [CrossRef]
- Tomita, Y.; Uechi, J.; Konno, M.; Sasamoto, S.; Iijima, M.; Mizoguchi, I. Accuracy of digital models generated by conventional impression/plaster-model methods and intraoral scanning. Dent. Mater. J. 2018, 37, 628–633. [Google Scholar] [CrossRef]
- Mathys, A.; Semal, P.; Brecko, J.; Van den Spiegel, D. Improving 3D photogrammetry models through spectral imaging: Tooth enamel as a case study. PLoS ONE 2019, 14, e0220949. [Google Scholar] [CrossRef] [PubMed]
- Zotti, F.; Rosolin, L.; Simoncelli, F.; Pappalardo, D.; Cominziolli, A.; Zerman, N. Telediagnosis of dental caries: Possible or impossible? A pilot cross-sectional study. Clin. Exp. Dent. Res. 2022, 8, 1614–1622. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Schinke, J.; Rastegar, A.; Tanyeri, M.; Viator, J.A. Cost-Effective Full-Color 3D Dental Imaging Based on Close-Range Photogrammetry. Bioengineering 2023, 10, 1268. https://doi.org/10.3390/bioengineering10111268
Yang B, Schinke J, Rastegar A, Tanyeri M, Viator JA. Cost-Effective Full-Color 3D Dental Imaging Based on Close-Range Photogrammetry. Bioengineering. 2023; 10(11):1268. https://doi.org/10.3390/bioengineering10111268
Chicago/Turabian StyleYang, Bin, Jennifer Schinke, Amir Rastegar, Melikhan Tanyeri, and John A. Viator. 2023. "Cost-Effective Full-Color 3D Dental Imaging Based on Close-Range Photogrammetry" Bioengineering 10, no. 11: 1268. https://doi.org/10.3390/bioengineering10111268
APA StyleYang, B., Schinke, J., Rastegar, A., Tanyeri, M., & Viator, J. A. (2023). Cost-Effective Full-Color 3D Dental Imaging Based on Close-Range Photogrammetry. Bioengineering, 10(11), 1268. https://doi.org/10.3390/bioengineering10111268







