Fabrication of a Reflective Optical Imaging Device for Early Detection of Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Methods
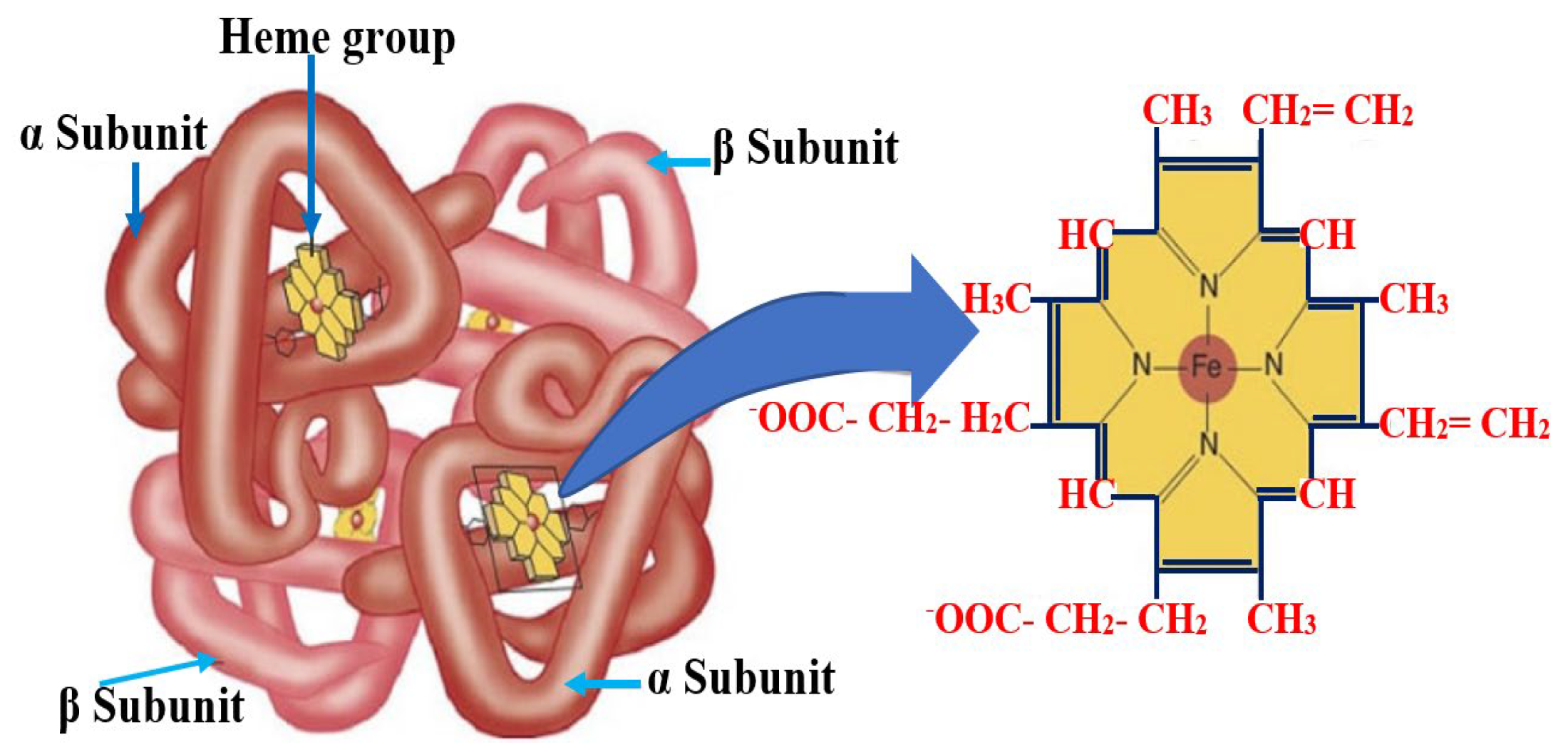
2.2. Physical Basis and Operating Principle of BKA-06
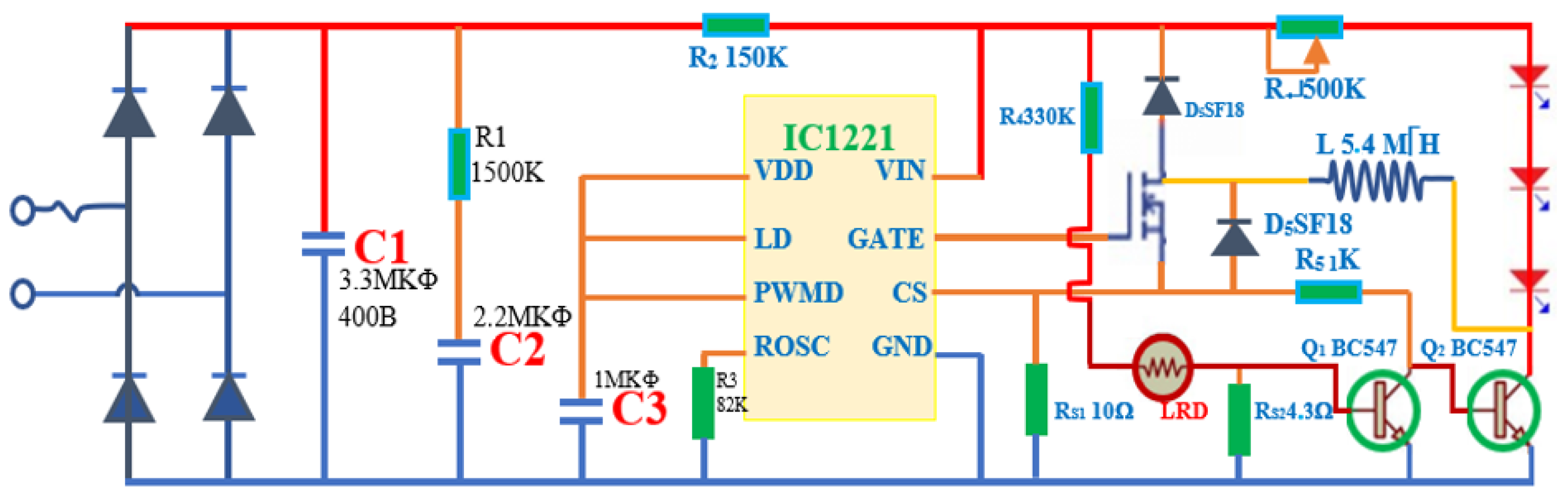
2.3. Techniques
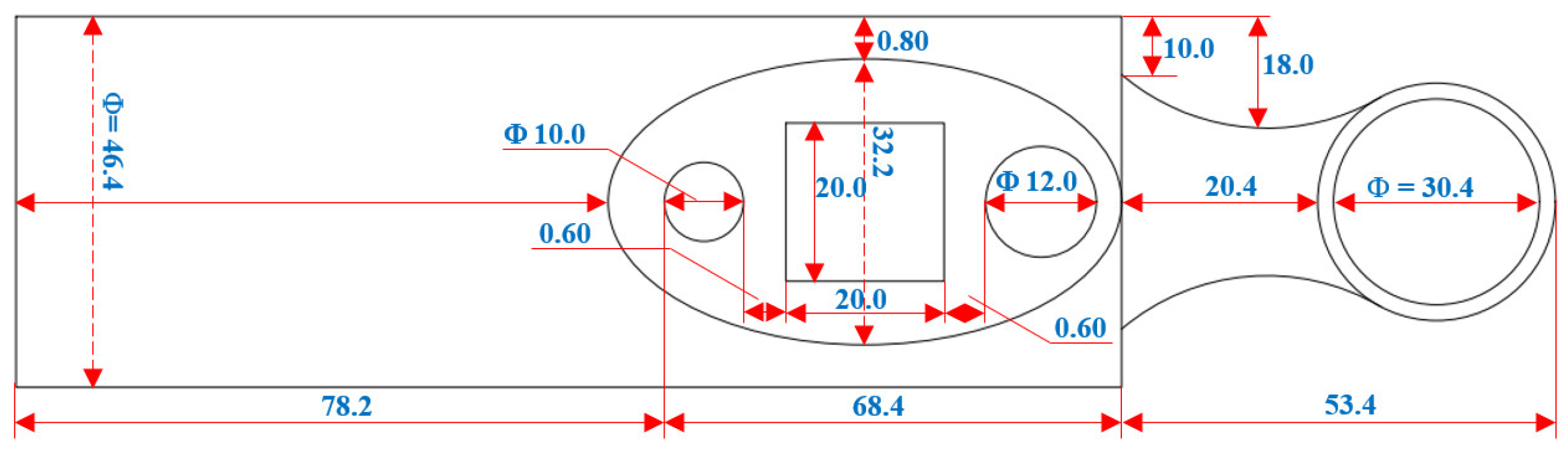
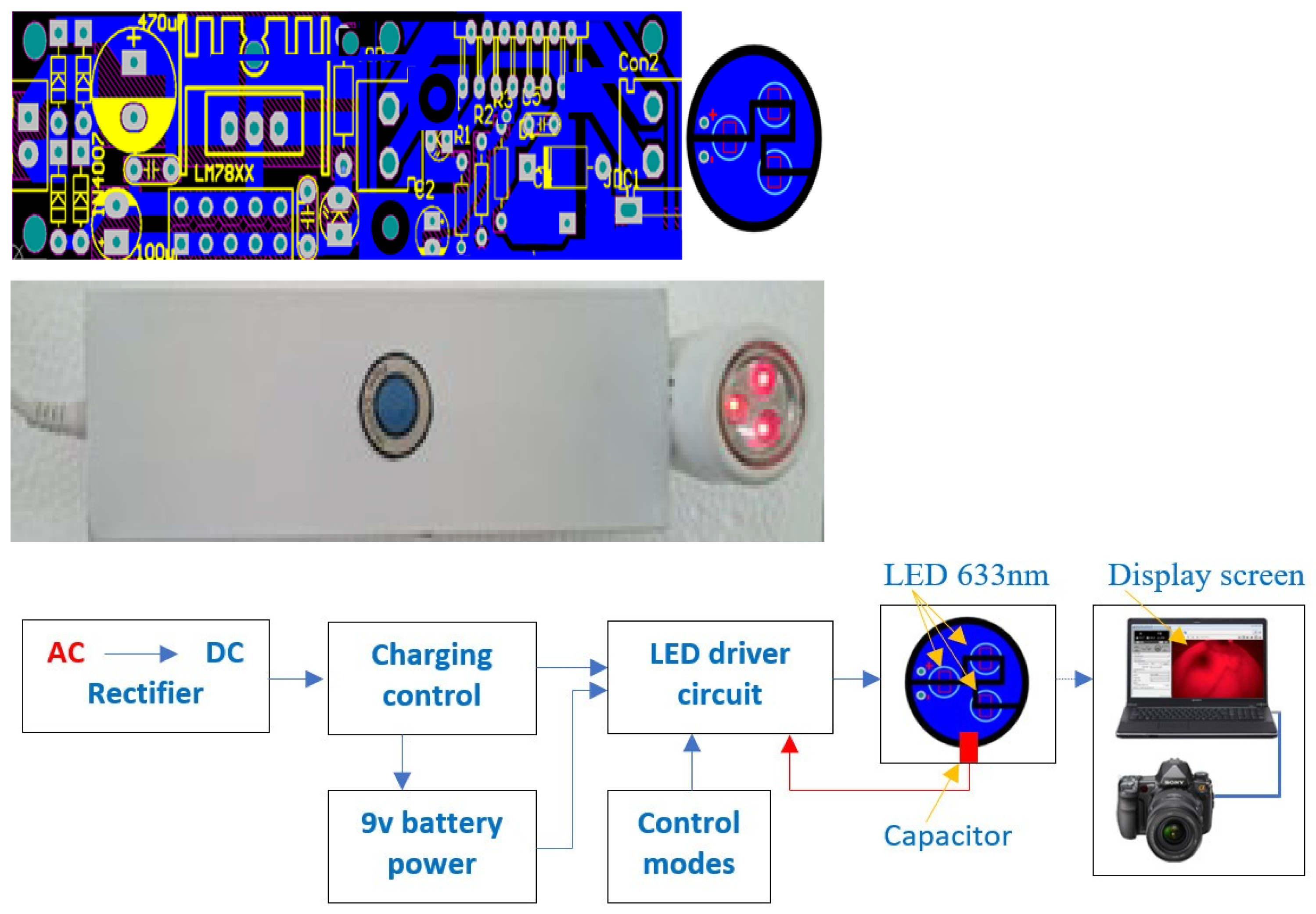
2.4. Fabrication Design
2.5. Measurement Process
3. Results
3.1. Studies of Basic Parameters of BKA-06
3.1.1. Device Specifications
3.1.2. Measure Brightness
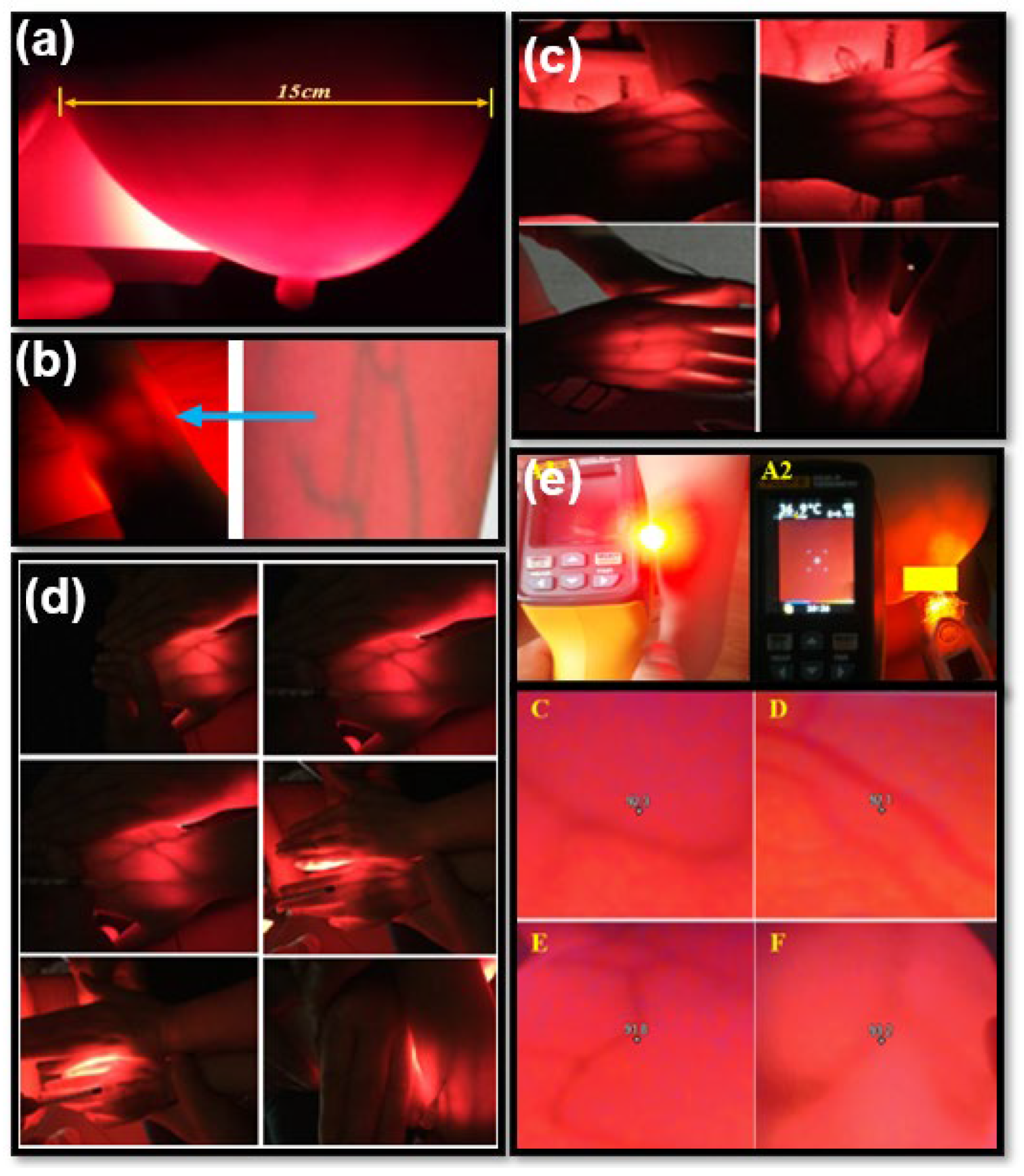
3.2. Vascular Results in Adults and Children
3.3. Breast Test Results on Volunteers and Patients
3.3.1. Volunteers
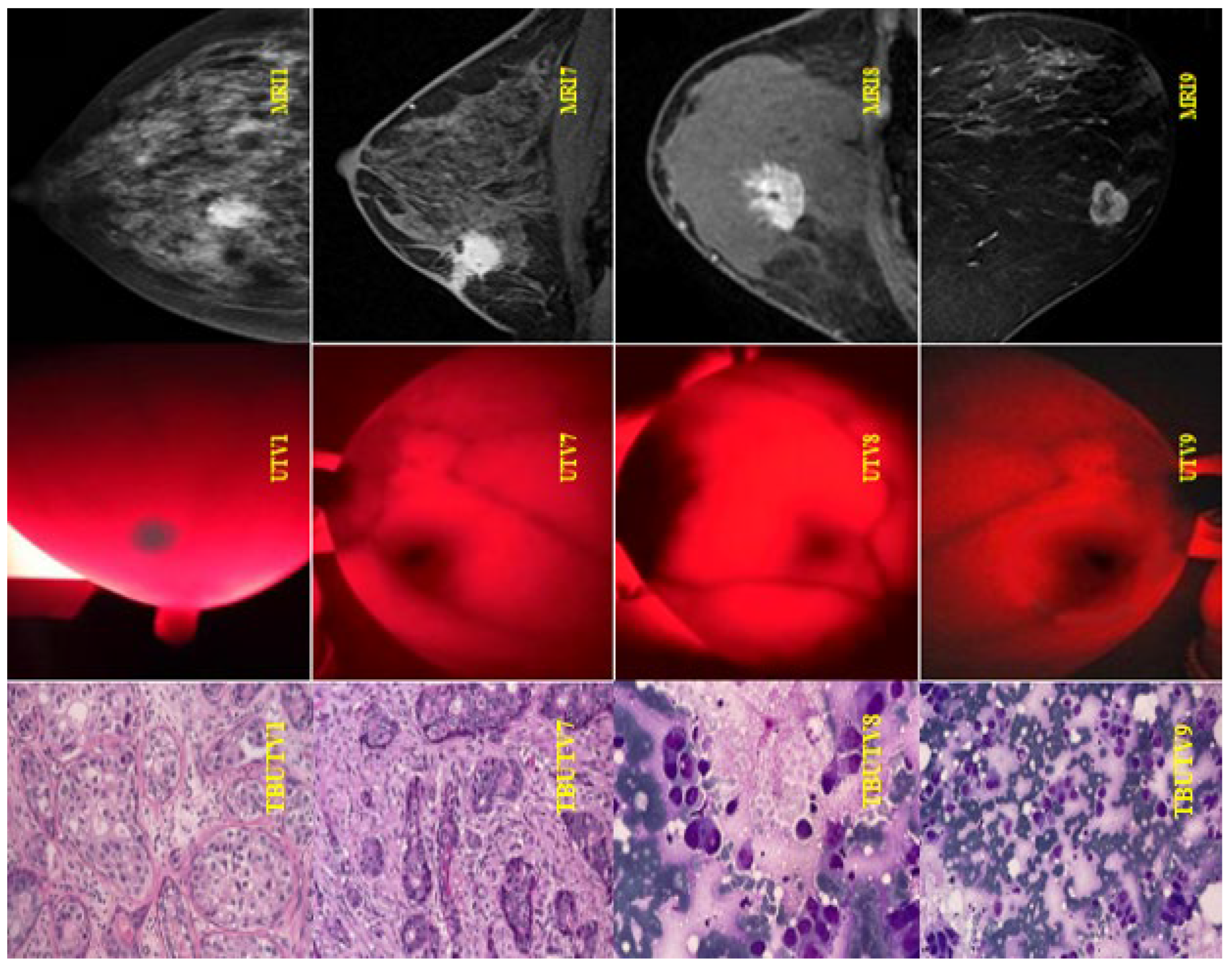
3.3.2. Patient Participation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duong Thi Tuan, A. Assessment of the Actual Situation of Performing Intravenous Injection Procedures by Nurses in Construction Hospitals; KHKT Publisher: Hanoi, Vietnam, 2011; pp. 6–11,13–20. [Google Scholar]
- Dang Thi Thanh, T. Knowledge, Skills to Practice Safe Injection and Some Related Factors of Students at Kon Tum Medical Secondary School in 2016; KHKT Publisher: Hanoi, Vietnam, 2016; pp. 12–14. [Google Scholar]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.O.; Ilbawi, A.M.; Fidarova, E.; Weiderpass, E.; Stevens, L.; Abdel-Wahab, M.; Mikkelsen, B. The Global Breast Cancer Initiative: A strategic collaboration to strengthen health care for non-communicable diseases. Lancet. Oncol. 2021, 22, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Migowski, A. Early detection of breast cancer and the interpretation of results of survival studies. Cienc. Saude Coletiva 2015, 20, 1309. [Google Scholar] [CrossRef]
- Breast Cancer. Early Detection and Prompt Treatment are Critical, Mayo Clinic Health Letter, English ed.; Mayo Clinic: Rochester, MN, USA, 2013; pp. 1–8. [Google Scholar]
- Onega, T.; Goldman, L.E.; Walker, R.L.; Miglioretti, D.L.; Buist, D.S.; Taplin, S.; Geller, B.M.; Hill, D.A.; Smith-Bindman, R. Facility Mammography Volume in Relation to Breast Cancer Screening Outcomes. J. Med. Screen. 2016, 23, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.C.; Pizzitola, V.J.; Giurescu, M.E.; Eversman, W.G.; Lorans, R.; Robinson, K.A.; Patel, B.K. Contrast-enhanced Digital Mammography: A Single-Institution Experience of the First 208 Cases. Breast J. 2017, 23, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; El-Shenawee, M. Review of electromagnetic techniques for breast cancer detection. IEEE Rev. Biomed. Eng. 2011, 4, 103–118. [Google Scholar] [CrossRef]
- Xu, P.; Peng, Y.; Sun, M.; Yang, X. SU-E-I-81: Targeting of HER2-Expressing Tumors with Dual PET-MR Imaging Probes. Med. Phys. 2015, 42, 3260. [Google Scholar] [CrossRef]
- Poneros, J.M.; Brand, S.; Bouma, B.E.; Tearney, G.J.; Compton, C.C.; Nishioka, N.S. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology 2001, 120, 7–12. [Google Scholar] [CrossRef]
- Arridge, S.R. Philosophical Transactions of the Royal Society A: Mathematical. Phys. Eng. Sci. 2011, 369, 4558–4576. [Google Scholar]
- Ren, K.; Bal, G.; Hielscher, A.H. Diffuse optical tomography using the one-way radiative transfer equation. J. Sci. Comput. 2006, 28, 1463–1489. [Google Scholar]
- Cerussi, A.E.; Berger, A.J.; Bevilacqua, F.; Shah, N.; Jakubowski, D.; Butler, J.; Holcombe, R.F.; Tromberg, B.J. Sources of absorption and scattering contrast for near-infrared optical mammography. Acad. Radiol. 2001, 8, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, A.; Swartling, J.; Chikoidze, E.; Torricelli, A.; Taroni, P.; Bassi, A.; Andersson-Engels, S.; Cubeddu, R. Spectroscopic time-resolved diffuse reflectance and transmittance measurements of the female breast at different interfiber distances. J. Biomed. Opt. 2004, 9, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Grosenick, D.; Rinneberg, H.; Cubeddu, R.; Taroni, P. Review of optical breast imaging and spectroscopy. J. Biomed. Opt. 2016, 21, 091311. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, A.; Contini, D.; Mora, A.D.; Farina, A.; Spinelli, L.; Torricelli, A. Palatino Linotype. New frontier in time-domain diffuse optics, a review. J. Biomed. Opt. 2016, 21, 091310. [Google Scholar] [CrossRef]
- Pogue, B.W.; Testorf, M.; McBride, T.; Osterberg, U.; Paulsen, K. Instrumentation and design of a frequency-domain diffuse optical tomography imager for breast cancer detection. Opt. Express 1997, 1, 391–403. [Google Scholar] [CrossRef]
- Siegel, A.; Marota, J.; Boas, D.A. Design and development of a continuous-wave diffuse optical tomography system. Opt. Express 1999, 4, 287–298. [Google Scholar] [CrossRef]
- Flexman, M.L.; Kim, H.K.; Gunther, J.E.; Lim, E.A.; Alvarez, M.C.; Desperito, E.; Kalinsky, K.; Hershman, D.L.; Hielscher, A.H. Optical biomarkers for breast cancer derived from dynamic diffuse optical tomography. J. Biomed. Opt. 2013, 18, 096012. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H.; Anderson, B.; Jiang, H. Multispectral breast imaging using a ten-wavelength, source/detector channels silicon photodiode-based diffuse optical tomography system. Med. Phys. 2006, 33, 627–636. [Google Scholar] [CrossRef]
- Schmitz, C.H.; Klemer, D.P.; Hardin, R.; Katz, M.S.; Pei, Y.; Graber, H.L.; Levin, M.B.; Levina, R.D.; Franco, N.A.; Solomon, W.B. Design and implementation of dynamic near-infrared optical tomographic imaging instrumentation for simultaneous dual-breast measurements. Appl. Opt. 2005, 41, 2140–2153. [Google Scholar] [CrossRef]
- El-Ghussein, F.; Mastanduno, M.A.; Jiang, S.; Pogue, B.W.; Paulsen, K.D. Medical hyperspectral imaging. J. Biomed. Opt. 2014, 19, 10901. [Google Scholar]
- Higgins, C. Hemoglobin and its measurement. Hemoglobin 2005. Available online: https://acutecaretesting.org/en/articles/hemoglobin-and-its-measurement (accessed on 13 August 2023).
- Chandra, F.; Wahyudianto, A.; Yasin, M. Design of vein finder with multi tuning wavelength using RGB LED. J. Phys. Conf. Ser. 2017, 853, 012019. [Google Scholar] [CrossRef]
- Delvo, E.D.; Guin, P. Implementa, on of Near—Infrared Technology (AccuVein AV—400®) To Facilitate Successful PIV Cannula; UFHealth: Leesburg, FL, USA, 2012; Available online: https://www.accuvein.com/articles/implementation-of-near-infrared-technology-accuvein-av-400-to-facilitate-successful-piv-cannulation/ (accessed on 13 August 2023).
- Wang, Y.; Bower, B.A.; Izatt, J.A.; Tan, O.; Huang, D. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J. Biomed. Opt. 2008, 13, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Chiao, F.B.; Resta-Flarer, F.; Lesser, J.; Ng, J.; Ganz, A.; Pino-Luey, D.; Bennett, H.; Perkins, C., Jr.; Witek, B. Vein visualization: Patient characteristic factors and efficacy of a new infrared vein finder technology. Br. J. Anaesth. 2013, 110, 966–971. [Google Scholar] [CrossRef] [PubMed]
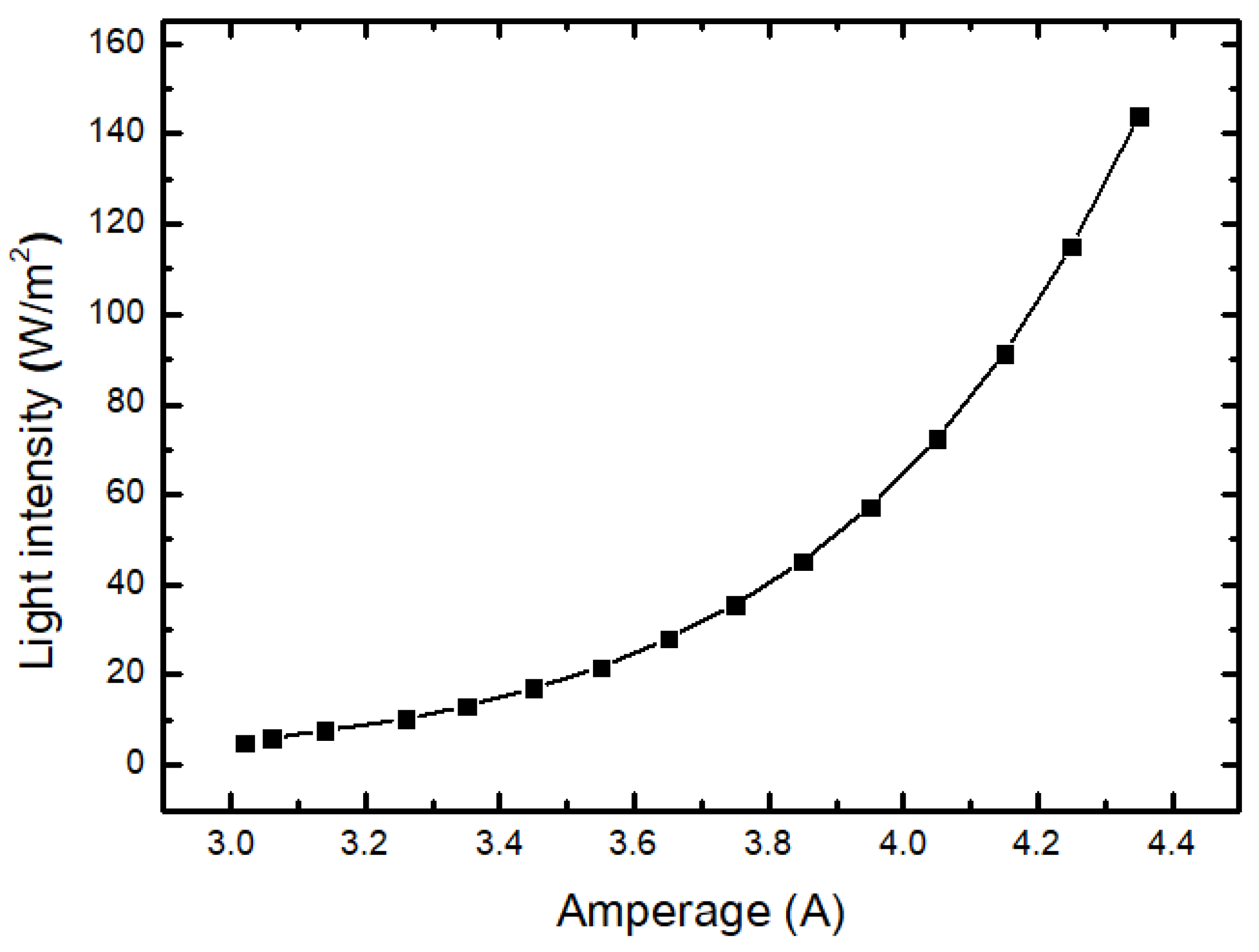





| Amperage I (A) | Voltage U (V) | Power P (W) | Light Intensity (W/m2) |
|---|---|---|---|
| 3.02 | 0.75 | 2.27 | 5.03 |
| 3.06 | 0.82 | 2.50 | 6.07 |
| 3.14 | 0.90 | 2.82 | 7.70 |
| 3.26 | 1.00 | 3.25 | 10.24 |
| 3.35 | 1.10 | 3.68 | 13.16 |
| 3.45 | 1.21 | 4.17 | 17.07 |
| 3.55 | 1.33 | 4.73 | 21.73 |
| 3.65 | 1.46 | 5.35 | 28.08 |
| 3.75 | 1.61 | 6.04 | 35.59 |
| 3.85 | 1.77 | 6.81 | 45.22 |
| 3.95 | 1.94 | 7.67 | 57.33 |
| 4.05 | 2.13 | 8.63 | 72.49 |
| 4.15 | 2.33 | 9.69 | 91.31 |
| 4.25 | 2.56 | 10.87 | 114.99 |
| 4.35 | 2.80 | 12.19 | 143.93 |
| No. | Full Name | Age | Code |
|---|---|---|---|
| 1 | Volunteer 1 | 43 | CV1 |
| 2 | Volunteer 2 | 31 | CV2 |
| 3 | Volunteer 3 | 28 | CV3 |
| 4 | Volunteer 4 | 41 | CV4 |
| 5 | Volunteer 5 | 55 | CV5 |
| 6 | Volunteer 6 | 30 | CV6 |
| 7 | Volunteer 7 | 60 | CV7 |
| 8 | Volunteer 8 | 40 | CV8 |
| 9 | Volunteer 9 | 65 | CV9 |
| 10 | Volunteer 10 | 40 | CV10 |
| 11 | Volunteer 11 | 18 | CV11 |
| 12 | Volunteer 12 | 42 | CV12 |
| 13 | Volunteer 13 | 36 | CV13 |
| 14 | Volunteer 14 | 25 | CV14 |
| 15 | Volunteer 15 | 58 | CV15 |
| Number | Full Name | Age | Encrypted Image |
|---|---|---|---|
| 1 | Patient 1 | 25 | UHV1–MRI1 |
| 2 | Patient 2 | 67 | UHV1–MRI2 |
| 3 | Patient 3 | 45 | UHV3–MRI3 |
| 4 | Patient 4 | 65 | UHV4–MRI4 |
| 5 | Patient 5 | 45 | UHV5–MRI5 |
| 6 | Patient 6 | 58 | UHV6–MRI6 |
| 7 | Patient 7 | 64 | UHV7–MRI7 |
| 8 | Patient 8 | 52 | UHV8–MRI8 |
| 9 | Patient 9 | 46 | UHV9–MRI9 |
| 10 | Patient 10 | 43 | UHV10–MRI10 |
| 11 | Patient 11 | 59 | UHV11–MRI11 |
| 12 | Patient 12 | 58 | UHV12–MRI12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, H.T.; Ngo, D.Q.; Nguyen, H.P.T.; La, D.D. Fabrication of a Reflective Optical Imaging Device for Early Detection of Breast Cancer. Bioengineering 2023, 10, 1272. https://doi.org/10.3390/bioengineering10111272
Mai HT, Ngo DQ, Nguyen HPT, La DD. Fabrication of a Reflective Optical Imaging Device for Early Detection of Breast Cancer. Bioengineering. 2023; 10(11):1272. https://doi.org/10.3390/bioengineering10111272
Chicago/Turabian StyleMai, Huu Thuan, Duc Quan Ngo, Hong Phuong Thi Nguyen, and Duong Duc La. 2023. "Fabrication of a Reflective Optical Imaging Device for Early Detection of Breast Cancer" Bioengineering 10, no. 11: 1272. https://doi.org/10.3390/bioengineering10111272
APA StyleMai, H. T., Ngo, D. Q., Nguyen, H. P. T., & La, D. D. (2023). Fabrication of a Reflective Optical Imaging Device for Early Detection of Breast Cancer. Bioengineering, 10(11), 1272. https://doi.org/10.3390/bioengineering10111272








