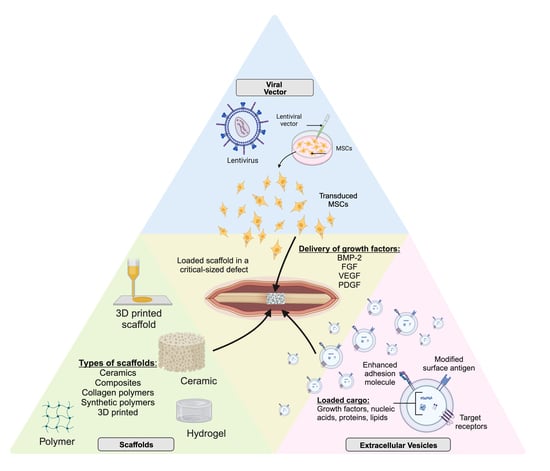
Delivery of Growth Factors to Enhance Bone Repair
Abstract
1. Introduction
1.1. Bone Loss Challenge
1.2. Growth Factors for Treatment
1.3. FDA-Approved Growth Factors for Bone Repair
2. Carriers of Bone Morphogenetic Protein and Other Growth Factors
2.1. Collagen Polymers
2.2. Ceramics, Composites, and Applications of 3D Printing
2.3. Synthetic Polymers
3. Extracellular Vesicles
3.1. Role of Extracellular Vesicles in Growth Factor Delivery and Bone Repair
In Vivo Extracellular Vesicle Mediated Delivery of Growth Factors
3.2. Engineering Extracellular Vesicles to Optimize Growth Factor Delivery
3.2.1. Endogenous Engineering of Parent Cells
3.2.2. Exogenous Engineering of Extracellular Vesicles
3.2.3. Optimizing Extracellular Vesicles for Growth Factor Delivery
4. Role of Regional Gene Therapy in Growth Factor Delivery
4.1. In Vivo Versus Ex Vivo Regional Gene Therapy
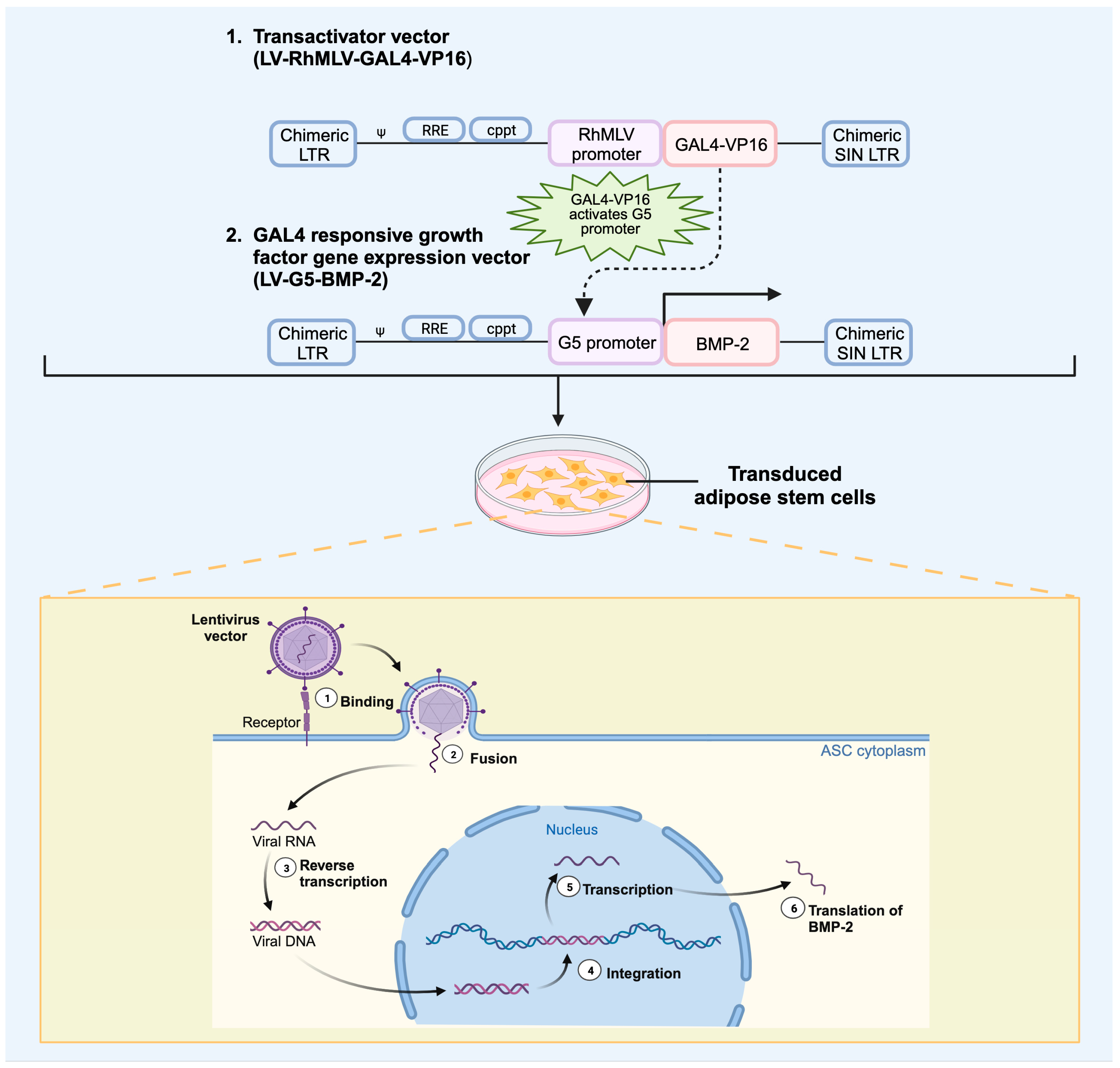
4.2. Viral Vectors
Viral Vector Delivery of Growth Factor in Bone Repair
4.3. Nonviral Vector Delivery of Growth Factor in Bone Repair
4.4. Role of Scaffolds in Gene Therapy
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinker, M.R.; O’Connor, D.P. The biological basis for nonunions. JBJS Rev. 2016, 4, e3. [Google Scholar] [CrossRef] [PubMed]
- Carofino, B.C.; Lieberman, J.R. Gene Therapy Applications for Fracture-Healing. JBJS 2008, 90, 99. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; Lee, M.; Gardner, M.J.; Brinker, M.R.; Warner, S.J.; Tornetta, P.; Leucht, P. Principles of Nonunion Management: State of the Art. J. Orthop. Trauma 2018, 32, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Garrison, K.; Shemilt, I.; Donell, S.; Ryder, J.; Mugford, M.; Harvey, I.; Song, F. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst. Rev. 2008, 2010, CD006950. [Google Scholar] [CrossRef]
- Gulabi, D.; Erdem, M.; Cecen, G.S.; Avci, C.C.; Saglam, N.; Saglam, F. Ilizarov Fixator Combined With an Intramedullary Nail for Tibial Nonunions With Bone Loss: Is It Effective? Clin. Orthop. Relat. Res. 2014, 472, 3892–3901. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Faour, O.; Goff, T.; Kanakaris, N.; Dimitriou, R. Masquelet technique for the treatment of bone defects: Tips-tricks and future directions. Injury 2011, 42, 591–598. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, M.P.G.; Seigerman, D.A. The Clinical Use of Allografts, Demineralized Bone Matrices, Synthetic Bone Graft Substitutes and Osteoinductive Growth Factors: A Survey Study. HSS J. 2005, 1, 9–18. [Google Scholar] [CrossRef]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 340516. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Mao, J. Bone tissue engineering and regeneration: From discovery to the clinic—An overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef]
- Watson, T.J. Distraction Osteogenesis. JAAOS J. Am. Acad. Orthop. Surg. 2006, 14, S168–S174. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Strates, B.S. Bone Morphogenetic Protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H.; Anderson, W.A. Collagenous bone matrix-induced endochondral ossification hemopoiesis. J. Cell Biol. 1976, 69, 557–572. [Google Scholar] [CrossRef]
- Muthukumaran, N.; Ma, S.; Reddi, A.H. Dose-dependence of and threshold for optimal bone induction by collagenous bone matrix and osteogenin-enriched fraction. Coll. Relat. Res. 1988, 8, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Daluiski, A.; Stevenson, S.; Jolla, L.; Wu, L.; McAllister, P.; Lee, Y.P.; Kabo, J.M.; Finerman, G.A.; Berk, A.J.; et al. The Effect of Regional Gene Therapy with Bone Morphogenetic Protein-2-Producing Bone-Marrow Cells on the Repair of Segmental Femoral Defects in Rats. JBJS 1999, 81, 905–917. [Google Scholar] [CrossRef]
- ElHawary, H.; Baradaran, A.; Abi-Rafeh, J.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Bone Healing and Inflammation: Principles of Fracture and Repair. Semin. Plast. Surg. 2021, 35, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef]
- FDA. Recombinant Human Bone Morphogenetic Protein; FDA: Silver Spring, MD, USA, 2023. [Google Scholar]
- DiGiovanni, C.W.; Petricek, J.M. The evolution of rhPDGF-BB in musculoskeletal repair and its role in foot and ankle fusion surgery. Foot Ankle Clin. 2010, 15, 621–640. [Google Scholar] [CrossRef]
- Daniels, T.R.; Younger, A.S.E.; Penner, M.J.; Wing, K.J.; Le, I.L.D.; Russell, I.S.; Lalonde, K.-A.; Evangelista, P.T.; Quiton, J.D.; Glazebrook, M.; et al. Prospective Randomized Controlled Trial of Hindfoot and Ankle Fusions Treated With rhPDGF-BB in Combination With a β-TCP-Collagen Matrix. Foot Ankle Int. 2015, 36, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Anderson, J.; Swords, M.P.; Maislin, G.; Donahue, R.; Pinsker, E.; Quiton, J.D. Recombinant Human Platelet-Derived Growth Factor BB in Combination With a Beta-Tricalcium Phosphate (rhPDGF-BB/β-TCP)-Collagen Matrix as an Alternative to Autograft. Foot Ankle Int. 2019, 40, 1068–1078. [Google Scholar] [CrossRef]
- Digiovanni, C.W.; Baumhauer, J.; Lin, S.S.; Berberian, W.S.; Flemister, A.S.; Enna, M.J.; Evangelista, P.; Newman, J. Prospective, randomized, multi-center feasibility trial of rhPDGF-BB versus autologous bone graft in a foot and ankle fusion model. Foot Ankle Int. 2011, 32, 344–354. [Google Scholar] [CrossRef]
- Street, J.; Bao, M.; DeGuzman, L.; Bunting, S.; Peale, F.V.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef]
- Keramaris, N.C.; Calori, G.M.; Nikolaou, V.S.; Schemitsch, E.H.; Giannoudis, P.V. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury 2008, 39, S45–S57. [Google Scholar] [CrossRef]
- Eckardt, H.; Ding, M.; Lind, M.; Hansen, E.S.; Christensen, K.S.; Hvid, I. Recombinant human vascular endothelial growth factor enhaces bone healing in an experimental nonunion model. J. Bone Jt. Surg. Ser. B 2005, 87, 1434–1438. [Google Scholar] [CrossRef]
- Kawai, M.; Rosen, C.J. The Insulin-Like Growth Factor System in Bone: Basic and Clinical Implications. Endocrinol. Metab. Clin. N. Am. 2012, 41, 323–333. [Google Scholar] [CrossRef]
- Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells In Vitro. Int. J. Mol. Sci. 2018, 19, 1674. [Google Scholar] [CrossRef]
- Fei, Q.; Li, J.; Lin, J.; Li, D.; Wang, B.; Meng, H.; Wang, Q.; Su, N.; Yang, Y. Risk Factors for Surgical Site Infection After Spinal Surgery: A Meta-Analysis. World Neurosurg. 2016, 95, 507–515. [Google Scholar] [CrossRef]
- Radomsky, M.L.; Aufdemorte, T.B.; Swain, L.D.; Fox, W.C.; Spiro, R.C.; Poser, J.W. Novel formulation of fibroblast growth factor-2 in a hyaluronan gel accelerates fracture healing in nonhuman primates. J. Orthop. Res. 1999, 17, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Charoenlarp, P.; Rajendran, A.K.; Iseki, S. Role of fibroblast growth factors in bone regeneration. Inflamm. Regen. 2017, 37, 10. [Google Scholar] [CrossRef] [PubMed]
- Rosier, R.N.; O’Keefe, R.J.; Hicks, D.G. The Potential Role of Transforming Growth Factor Beta in Fracture Healing. Clin. Orthop. Relat. Res. (1976–2007) 1998, 355, S294–S300. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; Ristevski, B.; Li, R.; Schemitsch, E.H. Growth factors and bone regeneration: How much bone can we expect? Injury 2011, 42, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, D. The BMP signaling and in vivo bone formation. Gene 2005, 357, 1–8. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Wang, A.; Zhu, Z.; Li, Y.; Zhu, C.; Che, Z.; Liu, T.; Liu, H.; Huang, L. Application of BMP in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 810880. [Google Scholar] [CrossRef]
- Gipson, G.R.; Goebel, E.J.; Hart, K.N.; Kappes, E.C.; Kattamuri, C.; McCoy, J.C.; Thompson, T.B. Structural perspective of BMP ligands and signaling. Bone 2020, 140, 115549. [Google Scholar] [CrossRef]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef]
- Sampath, T.K.; Reddi, A.H. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc. Natl. Acad. Sci. USA 1981, 78, 7599–7603. [Google Scholar] [CrossRef] [PubMed]
- Cahill, K.S.; Chi, J.H.; Day, A.; Claus, E.B. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 2009, 302, 58–66. [Google Scholar] [CrossRef]
- Kelly, M.P.; Savage, J.W.; Bentzen, S.M.; Hsu, W.K.; Ellison, S.A.; Anderson, P.A. Cancer risk from bone morphogenetic protein exposure in spinal arthrodesis. J. Bone Jt. Surg. Am. 2014, 96, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.S.; Kou, T.D. Risk of Cancer Following Lumbar Fusion Surgery With Recombinant Human Bone Morphogenic Protein-2 (rhBMP-2): An Analysis Using a Commercially Insured Patient Population. Int. J. Spine Surg. 2018, 12, 260–268. [Google Scholar] [CrossRef]
- Chrastil, J.; Low, J.B.; Whang, P.G.; Patel, A.A. Complications associated with the use of the recombinant human bone morphogenetic proteins for posterior interbody fusions of the lumbar spine. Spine 2013, 38, E1020–E1027. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, A.; Buchowski, J.M.; Zebala, L.P.; Bakhsh, W.R.; Aronson, A.B.; Fogelson, J.L.; Hershman, S.; Kim, H.J.; Ahmad, A.; Bridwell, K.H. High-dose rhBMP-2 for adults: Major and minor complications: A study of 502 spine cases. J. Bone Jt. Surg. Am. 2013, 95, 1546–1553. [Google Scholar] [CrossRef]
- Smucker, J.D.; Rhee, J.M.; Singh, K.; Yoon, S.T.; Heller, J.G. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine 2006, 31, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-D.; Jiang, W.-M.; Yang, H.-L.; Shi, J.-H. Exploratory meta-analysis on dose-related efficacy and complications of rhBMP-2 in anterior cervical discectomy and fusion: 1,539,021 cases from 2003 to 2017 studies. J. Orthop. Transl. 2020, 24, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Woo, B.H.; Fink, B.F.; Page, R.; Schrier, J.A.; Jo, Y.W.; Jiang, G.; DeLuca, M.; Vasconez, H.C.; DeLuca, P.P. Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm. Res. 2001, 18, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef]
- Visser, R.; Arrabal, P.M.; Becerra, J.; Rinas, U.; Cifuentes, M. The effect of an rhBMP-2 absorbable collagen sponge-targeted system on bone formation in vivo. Biomaterials 2009, 30, 2032–2037. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Arias-Betancur, A.; Badilla-Wenzel, N.; Astete-Sanhueza, Á.; Farfán-Beltrán, N.; Dias, F.J. Carrier systems for bone morphogenetic proteins: An overview of biomaterials used for dentoalveolar and maxillofacial bone regeneration. Jpn. Dent. Sci. Rev. 2022, 58, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A. Recombinant Human Bone Morphogenetic Protein-2 for Treatment of Open Tibial Fractures. J. Bone Jt. Surg.-Am. Vol. 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. Rev. J. 2021, 146, 100641. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Gornet, M.F.; Dickman, C.A.; Zdeblick, T.A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J. Spinal Disord. Tech. 2002, 15, 337–349. [Google Scholar] [CrossRef]
- Burkus, J.K.; Haid, R.W.; Traynelis, V.C.; Mummaneni, P.V. Long-term clinical and radiographic outcomes of cervical disc replacement with the Prestige disc: Results from a prospective randomized controlled clinical trial. J. Neurosurg. Spine 2010, 13, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kigami, R.; Sato, S.; Tsuchiya, N.; Yoshimakai, T.; Arai, Y.; Ito, K. FGF-2 angiogenesis in bone regeneration within critical-sized bone defects in rat calvaria. Implant Dent. 2013, 22, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Hong, L.; Yamamoto, M.; Shigeno, K.; Inoue, M.; Toba, T.; Yoshitani, M.; Nakamura, T.; Tabata, Y.; Shimizu, Y. Use of collagen sponge incorporating transforming growth factor-beta1 to promote bone repair in skull defects in rabbits. Biomaterials 2002, 23, 1003–1010. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, S.-K.; Kim, B.-S.; Im, G.-I.; Cho, K.-S.; Kim, C.-S. Controlled release of BMP-2 using a heparin-conjugated carrier system reduces in vivo adipose tissue formation. J. Biomed. Mater. Res. A 2015, 103, 545–554. [Google Scholar] [CrossRef]
- García-García, P.; Reyes, R.; Pérez-Herrero, E.; Arnau, M.R.; Évora, C.; Delgado, A. Alginate-hydrogel versus alginate-solid system. Efficacy in bone regeneration in osteoporosis. Mater. Sci. Eng. C 2020, 115, 111009. [Google Scholar] [CrossRef]
- Itoh, T.; Mochizuki, M.; Nishimura, R.; Matsunaga, S.; Kadosawa, T.; Kokubo, S.; Yokota, S.; Sasaki, N. Repair of Ulnar Segmental Defect by Recombinant Human Bone Morphogenetic Protein-2 in Dogs. J. Vet. Med. Sci. 1998, 60, 451–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Itoh, S.; Kikuchi, M.; Takakuda, K.; Nagaoka, K.; Koyama, Y.; Tanaka, J.; Shinomiya, K. Implantation study of a novel hydroxyapatite/collagen (HAp/col) composite into weight-bearing sites of dogs. J. Biomed. Mater. Res. 2002, 63, 507–515. [Google Scholar] [CrossRef]
- Lee, S.S.; Huang, B.J.; Kaltz, S.R.; Sur, S.; Newcomb, C.J.; Stock, S.R.; Shah, R.N.; Stupp, S.I. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials 2013, 34, 452–459. [Google Scholar] [CrossRef]
- Docherty-Skogh, A.-C.; Bergman, K.; Waern, M.J.; Ekman, S.; Hultenby, K.; Ossipov, D.; Hilborn, J.; Bowden, T.; Engstrand, T. Bone Morphogenetic Protein-2 Delivered by Hyaluronan-Based Hydrogel Induces Massive Bone Formation and Healing of Cranial Defects in Minipigs. Plast. Reconstr. Surg. 2010, 125, 1383–1392. [Google Scholar] [CrossRef]
- Mabilleau, G.; Aguado, E.; Stancu, I.C.; Cincu, C.; Baslé, M.F.; Chappard, D. Effects of FGF-2 release from a hydrogel polymer on bone mass and microarchitecture. Biomaterials 2008, 29, 1593–1600. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef] [PubMed]
- den Boer, F.C.; Wippermann, B.W.; Blokhuis, T.J.; Patka, P.; Bakker, F.C.; Haarman, H.J.T.M. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-I or autologous bone marrow. J. Orthop. Res. 2003, 21, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Seeherman, H.J.; Bouxsein, M.; Kim, H.; Li, R.; Li, X.J.; Aiolova, M.; Wozney, J.M. Recombinant human bone morphogenetic protein-2 delivered in an injectable calcium phosphate paste accelerates osteotomy-site healing in a nonhuman primate model. J. Bone Jt. Surg. Am. 2004, 86, 1961–1972. [Google Scholar] [CrossRef]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Mohd Roslan, M.R.; Mohd Kamal, N.L.; Abdul Khalid, M.F.; Mohd Nasir, N.F.; Cheng, E.M.; Beh, C.Y.; Tan, J.S.; Mohamed, M.S. The State of Starch/Hydroxyapatite Composite Scaffold in Bone Tissue Engineering with Consideration for Dielectric Measurement as an Alternative Characterization Technique. Materials 2021, 14, 1960. [Google Scholar] [CrossRef]
- Weber, F.E.; Eyrich, G.; Grätz, K.W.; Maly, F.E.; Sailer, H.F. Slow and continuous application of human recombinant bone morphogenetic protein via biodegradable poly(lactide-co-glycolide) foamspheres. Int. J. Oral Maxillofac. Surg. 2002, 31, 60–65. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. Evaluation of BMP-2 and VEGF loaded 3D printed hydroxyapatite composite scaffolds with enhanced osteogenic capacity in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110893. [Google Scholar] [CrossRef]
- Tsurushima, H.; Marushima, A.; Suzuki, K.; Oyane, A.; Sogo, Y.; Nakamura, K.; Matsumura, A.; Ito, A. Enhanced bone formation using hydroxyapatite ceramic coated with fibroblast growth factor-2. Acta Biomater. 2010, 6, 2751–2759. [Google Scholar] [CrossRef]
- Komaki, H.; Tanaka, T.; Chazono, M.; Kikuchi, T. Repair of segmental bone defects in rabbit tibiae using a complex of beta-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials 2006, 27, 5118–5126. [Google Scholar] [CrossRef]
- Mayfield, C.K.; Ayad, M.; Lechtholz-Zey, E.; Chen, Y.; Lieberman, J.R. 3D-Printing for Critical Sized Bone Defects: Current Concepts and Future Directions. Bioengineering 2022, 9, 680. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Huang, Y.-W.; Semon, J.A.; Leu, M.C. 3D-printed Biomimetic Bioactive Glass Scaffolds for Bone Regeneration in Rat Calvarial Defects. Int. J. Bioprint. 2020, 6, 274. [Google Scholar] [CrossRef]
- Teotia, A.K.; Dienel, K.; Qayoom, I.; van Bochove, B.; Gupta, S.; Partanen, J.; Seppälä, J.; Kumar, A. Improved Bone Regeneration in Rabbit Bone Defects Using 3D Printed Composite Scaffolds Functionalized with Osteoinductive Factors. ACS Appl. Mater. Interfaces 2020, 12, 48340–48356. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Weber, F.E.; Schmoekel, H.G.; Schense, J.C.; Kohler, T.; Müller, R.; Hubbell, J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 2003, 21, 513–518. [Google Scholar] [CrossRef]
- Rizzi, S.C.; Ehrbar, M.; Halstenberg, S.; Raeber, G.P.; Schmoekel, H.G.; Hagenmüller, H.; Müller, R.; Weber, F.E.; Hubbell, J.A. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part II: Biofunctional characteristics. Biomacromolecules 2006, 7, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Pratt, A.B.; Weber, F.E.; Schmoekel, H.G.; Müller, R.; Hubbell, J.A. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol. Bioeng. 2004, 86, 27–36. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Zhang, Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J. Dent. Res. 2018, 97, 859–868. [Google Scholar] [CrossRef]
- Patois, E.; Osorio-da Cruz, S.; Tille, J.-C.; Walpoth, B.; Gurny, R.; Jordan, O. Novel thermosensitive chitosan hydrogels: In vivo evaluation. J. Biomed. Mater. Res. A 2009, 91, 324–330. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Lauer-Fields, J.L.; Schmoekel, H.G.; Metters, A.T.; Weber, F.E.; Fields, G.B.; Hubbell, J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. USA 2003, 100, 5413–5418. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Kim, S.W.; Sun, B.K.; Woo, D.G.; Yang, H.N.; Chung, H.M.; Park, K.H. Osteogenic differentiation of rabbit mesenchymal stem cells in thermo-reversible hydrogel constructs containing hydroxyapatite and bone morphogenic protein-2 (BMP-2). Biomaterials 2007, 28, 2631–2637. [Google Scholar] [CrossRef]
- Garbern, J.C.; Hoffman, A.S.; Stayton, P.S. Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules 2010, 11, 1833–1839. [Google Scholar] [CrossRef]
- Kim, H.K.; Shim, W.S.; Kim, S.E.; Lee, K.-H.; Kang, E.; Kim, J.-H.; Kim, K.; Kwon, I.C.; Lee, D.S. Injectable in situ-forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng. Part A 2009, 15, 923–933. [Google Scholar] [CrossRef]
- Puddu, P.; Puddu, G.M.; Cravero, E.; Muscari, S.; Muscari, A. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can. J. Cardiol. 2010, 26, e140–e145. [Google Scholar] [CrossRef]
- Davies, O.G. Extracellular vesicles: From bone development to regenerative orthopedics. Mol. Ther. J. Am. Soc. Gene Ther. 2023, 31, 1251–1274. [Google Scholar] [CrossRef]
- Ren, J.; He, W.; Zheng, L.; Duan, H. From structures to functions: Insights into exosomes as promising drug delivery vehicles. Biomater. Sci. 2016, 4, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Shahabipour, F.; Barati, N.; Johnston, T.P.; Derosa, G.; Maffioli, P.; Sahebkar, A. Exosomes: Nanoparticulate tools for RNA interference and drug delivery. J. Cell. Physiol. 2017, 232, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Kempen, D.H.R.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J.A. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef]
- Sharmin, F.; Adams, D.; Pensak, M.; Dukas, A.; Lieberman, J.; Khan, Y. Biofunctionalizing devitalized bone allografts through polymer-mediated short and long term growth factor delivery: Biofunctionalizing Devitalized Bone Allografts. J. Biomed. Mater. Res. A 2015, 103, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, F.; O’Sullivan, M.; Malinowski, S.; Lieberman, J.R.; Khan, Y. Large scale segmental bone defect healing through the combined delivery of VEGF and BMP-2 from biofunctionalized cortical allografts. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.; Pérez-Campo, F. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J. Stem Cells 2020, 12, 1529–1552. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.-F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, K.; Hazrati, A.; Zahar, M.; Markov, A.; Zekiy, A.O.; Navashenaq, J.G.; Roshangar, L.; Ahmadi, M. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes for Orthopedic Diseases Treatment. Stem Cell Rev. Rep. 2022, 18, 933–951. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, R.; Wu, C.; Wang, L.; Zhang, C. Exosome: A Novel Approach to Stimulate Bone Regeneration through Regulation of Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2016, 17, 712. [Google Scholar] [CrossRef] [PubMed]
- van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2022, 11, 2100639. [Google Scholar] [CrossRef] [PubMed]
- Niedermair, T.; Lukas, C.; Li, S.; Stöckl, S.; Craiovan, B.; Brochhausen, C.; Federlin, M.; Herrmann, M.; Grässel, S. Influence of Extracellular Vesicles Isolated From Osteoblasts of Patients with Cox-Arthrosis and/or Osteoporosis on Metabolism and Osteogenic Differentiation of BMSCs. Front. Bioeng. Biotechnol. 2020, 8, 615520. [Google Scholar] [CrossRef]
- Ren, J.; Yu, R.; Xue, J.; Tang, Y.; Su, S.; Liao, C.; Guo, Q.; Guo, W.; Zheng, J. How Do Extracellular Vesicles Play a Key Role in the Maintenance of Bone Homeostasis and Regeneration? A Comprehensive Review of Literature. Int. J. Nanomed. 2022, 17, 5375–5389. [Google Scholar] [CrossRef]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef]
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e0225472. [Google Scholar] [CrossRef]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl. Mater. Interfaces 2021, 13, 18472–18487. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Thomsen, P. Mesenchymal stem cell–derived small extracellular vesicles and bone regeneration. Basic Clin. Pharmacol. Toxicol. 2021, 128, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xi, J.; Cheng, Y.; Sun, D.; Shu, P.; Chi, S.; Tian, S.; Ye, S. Reprogrammed mesenchymal stem cells derived from iPSCs promote bone repair in steroid-associated osteonecrosis of the femoral head. Stem Cell Res. Ther. 2021, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Treatment of Patients with Bone Tissue Defects Using Mesenchymal Stem Cells Enriched by Extracellular Vesicles. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05520125 (accessed on 10 August 2023).
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Li, P.; Fan, Y.; Zhang, L.; Ma, X.; Sun, R.; Liu, Y.; Li, W. microRNA-935-modified bone marrow mesenchymal stem cells-derived exosomes enhance osteoblast proliferation and differentiation in osteoporotic rats. Life Sci. 2021, 272, 119204. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.; Li, C.; Zhou, S.; Tian, D.; Xiao, D.; Zhang, H.; Gao, F.; Huang, J. Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol. Int. 2017, 41, 1379–1390. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Cui, Y.; Luan, J.; Li, H.; Zhou, X.; Han, J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016, 590, 185–192. [Google Scholar] [CrossRef]
- Uenaka, M.; Yamashita, E.; Kikuta, J.; Morimoto, A.; Ao, T.; Mizuno, H.; Furuya, M.; Hasegawa, T.; Tsukazaki, H.; Sudo, T.; et al. Osteoblast-derived vesicles induce a switch from bone-formation to bone-resorption in vivo. Nat. Commun. 2022, 13, 1066. [Google Scholar] [CrossRef]
- Eichholz, K.F.; Woods, I.; Riffault, M.; Johnson, G.P.; Corrigan, M.; Lowry, M.C.; Shen, N.; Labour, M.-N.; Wynne, K.; O’Driscoll, L.; et al. Human bone marrow stem/stromal cell osteogenesis is regulated via mechanically activated osteocyte-derived extracellular vesicles. Stem Cells Transl. Med. 2020, 9, 1431–1447. [Google Scholar] [CrossRef]
- Lv, P.-Y.; Gao, P.-F.; Tian, G.-J.; Yang, Y.-Y.; Mo, F.-F.; Wang, Z.-H.; Sun, L.; Kuang, M.-J.; Wang, Y.-L. Osteocyte-derived exosomes induced by mechanical strain promote human periodontal ligament stem cell proliferation and osteogenic differentiation via the miR-181b-5p/PTEN/AKT signaling pathway. Stem Cell Res. Ther. 2020, 11, 295. [Google Scholar] [CrossRef]
- Wang, C.-L.; Xiao, F.; Wang, C.-D.; Zhu, J.-F.; Shen, C.; Zuo, B.; Wang, H.; Li, D.; Wang, X.-Y.; Feng, W.-J.; et al. Gremlin2 Suppression Increases the BMP-2-Induced Osteogenesis of Human Bone Marrow-Derived Mesenchymal Stem Cells Via the BMP-2/Smad/Runx2 Signaling Pathway. J. Cell. Biochem. 2017, 118, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Macías, I.; Alcorta-Sevillano, N.; Infante, A.; Rodríguez, C.I. Cutting Edge Endogenous Promoting and Exogenous Driven Strategies for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 7724. [Google Scholar] [CrossRef]
- Gholami, L.; Nooshabadi, V.T.; Shahabi, S.; Jazayeri, M.; Tarzemany, R.; Afsartala, Z.; Khorsandi, K. Extracellular vesicles in bone and periodontal regeneration: Current and potential therapeutic applications. Cell Biosci. 2021, 11, 16. [Google Scholar] [CrossRef]
- Han, Z.; Liu, S.; Pei, Y.; Ding, Z.; Li, Y.; Wang, X.; Zhan, D.; Xia, S.; Driedonks, T.; Witwer, K.W.; et al. Highly efficient magnetic labelling allows MRI tracking of the homing of stem cell-derived extracellular vesicles following systemic delivery. J. Extracell. Vesicles 2021, 10, e12054. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.H.; Tjio, C.K.E.; Wong, J.R.Y.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal Stem Cell Exosomes for Cartilage Regeneration: A Systematic Review of Preclinical In Vivo Studies. Tissue Eng. Part B Rev. 2021, 27, 1–13. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Y.; Li, S.; Li, A.; Li, Y.; Pei, D. Engineering exosomes for bone defect repair. Front. Bioeng. Biotechnol. 2022, 10, 1091360. [Google Scholar] [CrossRef]
- Bei, H.P.; Hung, P.M.; Yeung, H.L.; Wang, S.; Zhao, X. Bone-a-Petite: Engineering Exosomes towards Bone, Osteochondral, and Cartilage Repair. Small 2021, 17, 2101741. [Google Scholar] [CrossRef]
- Xing, Y.; Yerneni, S.S.; Wang, W.; Taylor, R.E.; Campbell, P.G.; Ren, X. Engineering pro-angiogenic biomaterials via chemoselective extracellular vesicle immobilization. Biomaterials 2022, 281, 121357. [Google Scholar] [CrossRef]
- Gupta, D.; Zickler, A.M.; El Andaloussi, S. Dosing extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113961. [Google Scholar] [CrossRef]
- Hertel, F.C.; da Silva, A.S.; de Paula Sabino, A.; Valente, F.L.; Reis, E.C.C. Preconditioning Methods to Improve Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Bone Regeneration—A Systematic Review. Biology 2022, 11, 733. [Google Scholar] [CrossRef]
- Hnatiuk, A.P.; Ong, S.-G.; Olea, F.D.; Locatelli, P.; Riegler, J.; Lee, W.H.; Jen, C.H.; De Lorenzi, A.; Giménez, C.S.; Laguens, R.; et al. Allogeneic Mesenchymal Stromal Cells Overexpressing Mutant Human Hypoxia-Inducible Factor 1-α (HIF1-α) in an Ovine Model of Acute Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003714. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Man, K.; Eisenstein, N.M.; Hoey, D.A.; Cox, S.C. Bioengineering extracellular vesicles: Smart nanomaterials for bone regeneration. J. Nanobiotechnol. 2023, 21, 137. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Hsu, C.-Y.; Gao, Y.; Meyers, C.A.; Chang, L.; Zhang, L.; Broderick, K.; Ding, C.; Peault, B.; et al. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. eLife 2019, 8, e48191. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Jie Zhang, W.; Chen, Z. Extracellular Vesicle-functionalized Decalcified Bone Matrix Scaffolds with Enhanced Pro-angiogenic and Pro-bone Regeneration Activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef]
- Luo, Z.-W.; Li, F.-X.-Z.; Liu, Y.-W.; Rao, S.-S.; Yin, H.; Huang, J.; Chen, C.-Y.; Hu, Y.; Zhang, Y.; Tan, Y.-J.; et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 2019, 11, 20884–20892. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes with Hydroxyapatite-Embedded Hyaluronic Acid-Alginate Hydrogel for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Esposti, M.D.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials 2020, 10, 432. [Google Scholar] [CrossRef]
- Yan, H.-C.; Yu, T.-T.; Li, J.; Qiao, Y.-Q.; Wang, L.-C.; Zhang, T.; Li, Q.; Zhou, Y.-H.; Liu, D.-W. The Delivery of Extracellular Vesicles Loaded in Biomaterial Scaffolds for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 1015. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Lee, C.; Lee, M. Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration. Bioengineering 2021, 8, 137. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing; Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef]
- Collon, K.; Gallo, M.C.; Lieberman, J.R. Musculoskeletal tissue engineering: Regional gene therapy for bone repair. Biomaterials 2021, 275, 120901. [Google Scholar] [CrossRef] [PubMed]
- Bougioukli, S.; Alluri, R.; Pannell, W.; Sugiyama, O.; Vega, A.; Tang, A.; Skorka, T.; Park, S.H.; Oakes, D.; Lieberman, J.R. Ex vivo gene therapy using human bone marrow cells overexpressing BMP-2: “Next-day” gene therapy versus standard “two-step” approach. Bone 2019, 128, 115032. [Google Scholar] [CrossRef] [PubMed]
- Vakhshori, V.; Bougioukli, S.; Sugiyama, O.; Kang, H.P.; Tang, A.H.; Park, S.-H.; Lieberman, J.R. Ex vivo regional gene therapy with human adipose-derived stem cells for bone repair. Bone 2020, 138, 115524. [Google Scholar] [CrossRef]
- Virk, M.S.; Sugiyama, O.; Park, S.H.; Gambhir, S.S.; Adams, D.J.; Drissi, H.; Lieberman, J.R. Same Day Ex-vivo regional gene therapy: A novel strategy to enhance bone repair. Mol. Ther. 2011, 19, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana-Calvo, M.; Payen, E.; Negre, O.; Wang, G.; Hehir, K.; Fusil, F.; Down, J.; Denaro, M.; Brady, T.; Westerman, K.; et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 2010, 467, 318–322. [Google Scholar] [CrossRef]
- Aiuti, A.; Biasco, L.; Scaramuzza, S.; Ferrua, F.; Cicalese, M.P.; Baricordi, C.; Dionisio, F.; Calabria, A.; Giannelli, S.; Castiello, M.C.; et al. Lentiviral hematopoietic stem cell gene therapy in patients with wiskott-aldrich syndrome. Science 2013, 341, 1233151. [Google Scholar] [CrossRef]
- Sessa, M.; Lorioli, L.; Fumagalli, F.; Acquati, S.; Redaelli, D.; Baldoli, C.; Canale, S.; Lopez, I.D.; Morena, F.; Calabria, A.; et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: An ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet 2016, 388, 476–487. [Google Scholar] [CrossRef]
- Keeler, A.M.; Flotte, T.R. Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annu. Rev. Virol. 2019, 6, 601–621. [Google Scholar] [CrossRef]
- Vorburger, S.A.; Hunt, K.K. Adenoviral Gene Therapy. Oncologist 2002, 7, 46–59. [Google Scholar] [CrossRef]
- Mcconnell, M.J.; Imperiale, M.J. Biology of Adenovirus and Its Use as a Vector for Gene Therapy. Hum. Gene Ther. 2004, 15, 1022–1033. [Google Scholar] [CrossRef]
- Turgeman, G.; Pittman, D.D.; Müller, R.; Gowda Kurkalli, B.; Zhou, S.; Pelled, G.; Peyser, A.; Zilberman, Y.; Moutsatsos, I.K.; Gazit, D. Engineered human mesenchymal stem cells: A novel platform for skeletal cell mediated gene therapy. J. Gene Med. 2001, 3, 240–251. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Le, L.Q.; Wu, L.; Finerman, G.A.M.; Berk, A.; Witte, O.N.; Stevenson, S. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J. Orthop. Res. 1998, 16, 330–339. [Google Scholar] [CrossRef]
- Feeley, B.T.; Conduah, A.H.; Sugiyama, O.; Krenek, L.; Chen, I.S.Y.; Lieberman, J.R. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J. Orthop. Res. 2006, 24, 1709–1721. [Google Scholar] [CrossRef]
- Virk, M.S.; Conduah, A.; Park, S.H.; Liu, N.; Sugiyama, O.; Cuomo, A.; Kang, C.; Lieberman, J.R. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone 2008, 42, 921–931. [Google Scholar] [CrossRef]
- Musgrave, D.S.; Bosch, P.; Ghivizzani, S.; Robbins, P.D.; Evans, C.H.; Huard, J. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone 1999, 24, 541–547. [Google Scholar] [CrossRef]
- Kaihara, S.; Bessho, K.; Okubo, Y.; Sonobe, J.; Kawai, M.; Iizuka, T. Simple and effective osteoinductive gene therapy by local injection of a bone morphogenetic protein-2-expressing recombinant adenoviral vector and FK506 mixture in rats. Gene Ther. 2004, 11, 439–447. [Google Scholar] [CrossRef]
- Alden, T.D.; Pittman, D.D.; Hankins, G.R.; Beres, E.J.; Engh, J.A.; Das, S.; Hudson, S.B.; Kerns, K.M.; Kallmes, D.F.; Helm, G.A. In Vivo Endochondral Bone Formation Using a Bone Morphogenetic Protein 2 Adenoviral Vector. Hum. Gene Ther. 2004, 10, 2245–2253. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358. [Google Scholar] [CrossRef]
- Bougioukli, S.; Chateau, M.; Morales, H.; Vakhshori, V.; Sugiyama, O.; Oakes, D.; Longjohn, D.; Cannon, P. Limited potential of AAV-mediated gene therapy in transducing human mesenchymal stem cells for bone repair applications. Gene Ther. 2021, 28, 729–739. [Google Scholar] [CrossRef]
- Buchschacher, G.L.; Wong-Staal, F. Development of lentiviral vectors for gene therapy for human diseases. Blood 2000, 95, 2499–2504. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Rothe, M.; Modlich, U.; Schambach, A. Biosafety challenges for use of lentiviral vectors in gene therapy. Curr. Gene Ther. 2013, 13, 453–468. [Google Scholar] [CrossRef]
- Miyazaki, M.; Sugiyama, O.; Zou, J.; Yoon, S.H.; Wei, F.; Morishita, Y.; Sintuu, C.; Virk, M.S.; Lieberman, J.R.; Wang, J.C. Comparison of lentiviral and adenoviral gene therapy for spinal fusion in rats. Spine 2008, 33, 1410–1417. [Google Scholar] [CrossRef]
- Miyazaki, M.; Sugiyama, O.; Tow, B.; Zou, J.; Morishita, Y.; Wei, F.; Napoli, A.; Sintuu, C.; Lieberman, J.R.; Wang, J.C. The effects of lentiviral gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J. Spinal Disord. Tech. 2008, 21, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Alden, T.D.; Pittman, D.D.; Beres, E.J.; Hankins, G.R.; Kallmes, D.F.; Wisotsky, B.M.; Kerns, K.M.; Helm, G.A. Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J. Neurosurg. Spine 1999, 90, 109–114. [Google Scholar] [CrossRef]
- Hidaka, C.; Goshi, K.; Rawlins, B.; Boachie-Adjei, O.; Crystal, R.G. Enhancement of spine fusion using combined gene therapy and tissue engineering BMP-7-expressing bone marrow cells and allograft bone. Spine 2003, 28, 2049–2057. [Google Scholar] [CrossRef]
- Dumont, R.J.; Dayoub, H.; Li, J.Z.; Dumont, A.S.; Kallmes, D.F.; Hankins, G.R.; Helm, G.A. Ex Vivo Bone Morphogenetic Protein-9 Gene Therapy Using Human Mesenchymal Stem Cells Induces Spinal Fusion in Rodents. Neurosurgery 2002, 51, 1239–1244. [Google Scholar] [CrossRef]
- Laurent, J.J.; Webb, K.M.; Beres, E.J.; McGee, K.; Li, J.; Van Rietbergen, B.; Helm, G.A. The use of bone morphogenetic protein—6 gene therapy for percutaneous spinal fusion in rabbits. J. Neurosurg. Spine 2004, 1, 90–94. [Google Scholar] [CrossRef]
- Helm, G.A.; Alden, T.D.; Beres, E.J.; Hudson, S.B.; Das, S.; Engh, J.A.; Pittman, D.D.; Kerns, K.M.; Kallmes, D.F. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J. Neurosurg. 2000, 92, 191–196. [Google Scholar] [CrossRef]
- Wang, J.C.; Kanim, L.E.; Yoo, S.; Campbell, P.A.; Berk, A.J.; Lieberman, J.R. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. JBJS 2003, 85, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Peng, H.; Usas, A.; Musgrave, D.; Cummins, J.; Pelinkovic, D.; Jankowski, R.; Huard, J.; Lee, J.Y.; Peng, H.; et al. Enhancement of Bone Healing Based on Ex Vivo Gene Therapy Using Human Muscle-Derived Cells Expressing Bone Morphogenetic Protein 2. Hum. Gene Ther. 2004, 13, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Alluri, R.; Jakus, A.; Bougioukli, S.; Pannell, W.; Sugiyama, O.; Tang, A.; Shah, R.; Lieberman, J.R. 3D printed hyperelastic “bone” scaffolds and regional gene therapy: A novel approach to bone healing. J. Biomed. Mater. Res. A 2018, 106, 1104–1110. [Google Scholar] [CrossRef]
- Miyazaki, M.; Zuk, P.A.; Zou, J.; Yoon, S.H.; Wei, F.; Morishita, Y.; Sintuu, C.; Wang, J.C. Comparison of human mesenchymal stem cells derived from adipose tissue and bone marrow for ex vivo gene therapy in rat spinal fusion model. Spine 2008, 33, 863–869. [Google Scholar] [CrossRef]
- Kang, H.P.; Ihn, H.; Robertson, D.M.; Chen, X.; Sugiyama, O.; Tang, A.; Hollis, R.; Skorka, T.; Longjohn, D.; Oakes, D.; et al. Regional gene therapy for bone healing using a 3D printed scaffold in a rat femoral defect model. J. Biomed. Mater. Res. A 2021, 109, 2346–2356. [Google Scholar] [CrossRef]
- Rundle, C.H.; Miyakoshi, N.; Kasukawa, Y.; Chen, S.T.; Sheng, M.H.C.; Wergedal, J.E.; Lau, K.H.W.; Baylink, D.J. In vivo bone formation in fracture repair induced by direct retroviral-based gene therapy with bone morphogenetic protein-4. Bone 2003, 32, 591–601. [Google Scholar] [CrossRef]
- Baltzer, A.W.A.; Lattermann, C.; Whalen, J.D.; Wooley, P.; Weiss, K.; Grimm, M.; Ghivizzani, S.C.; Robbins, P.D.; Evans, C.H. Genetic enhancement of fracture repair: Healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000, 7, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wright, V.; Usas, A.; Gearhart, B.; Shen, H.C.; Cummins, J.; Huard, J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J. Clin. Investig. 2002, 110, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Koefoed, M.; Tiyapatanaputi, P.; Gromov, K.; Goater, J.J.; Carmouche, J.; Zhang, X.; Rubery, P.T.; Rabinowitz, J.; Samulski, R.J.; et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat. Med. 2005, 11, 291–297. [Google Scholar] [CrossRef]
- Chang, P.C.; Seol, Y.J.; Cirelli, J.A.; Pellegrini, G.; Jin, Q.; Franco, L.M.; Goldstein, S.A.; Chandler, L.A.; Sosnowski, B.; Giannobile, W.V. PDGF-B Gene Therapy Accelerates Bone Engineering and Oral Implant Osseointegration. Gene Ther. 2010, 17, 95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Dosari, M.S.; Gao, X. Nonviral Gene Delivery: Principle, Limitations, and Recent Progress. AAPS J. 2009, 11, 671–681. [Google Scholar] [CrossRef]
- Bez, M.; Sheyn, D.; Tawackoli, W.; Avalos, P.; Shapiro, G.; Giaconi, J.C.; Da, X.; David, S.B.; Gavrity, J.; Awad, H.A.; et al. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci. Transl. Med. 2017, 9, eaal3128. [Google Scholar] [CrossRef]
- Bez, M.; Foiret, J.; Shapiro, G.; Pelled, G.; Ferrara, K.W.; Gazit, D. Nonviral ultrasound-mediated gene delivery in small and large animal models. Nat. Protoc. 2019, 14, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Feichtinger, G.A.; Hofmann, A.T.; Slezak, P.; Schuetzenberger, S.; Kaipel, M.; Schwartz, E.; Neef, A.; Nomikou, N.; Nau, T.; Van Griensven, M.; et al. Sonoporation increases therapeutic efficacy of inducible and constitutive BMP2/7 in vivo gene delivery. Hum. Gene Ther. Methods 2014, 25, 57–71. [Google Scholar] [CrossRef]
- Curtin, C.M.; Tierney, E.G.; McSorley, K.; Cryan, S.-A.; Duffy, G.P.; O’Brien, F.J.; Curtin, C.M.; Tierney, E.G.; McSorley, K.; Duffy, G.P. Combinatorial Gene Therapy Accelerates Bone Regeneration: Non-Viral Dual Delivery of VEGF and BMP2 in a Collagen-Nanohydroxyapatite Scaffold. Adv Healthc Mater. 2014, 4, 223–227. [Google Scholar] [CrossRef]
- Alluri, R.; Song, X.; Bougioukli, S.; Pannell, W.; Vakhshori, V.; Sugiyama, O.; Tang, A.; Park, S.H.; Chen, Y.; Lieberman, J.R. Regional gene therapy with 3D printed scaffolds to heal critical sized bone defects in a rat model. J. Biomed. Mater. Res. A 2019, 107, 2174–2182. [Google Scholar] [CrossRef]
- Bozo, I.Y.; Deev, R.V.; Drobyshev, A.Y.; Isaev, A.A.; Eremin, I.I. World’s First Clinical Case of Gene-Activated Bone Substitute Application. Case Rep. Dent. 2016, 2016, 8648949. [Google Scholar] [CrossRef]
- Bozo, I.Y.; Drobyshev, A.Y.; Redko, N.A.; Komlev, V.S.; Isaev, A.A.; Deev, R.V. Bringing a Gene-Activated Bone Substitute Into Clinical Practice: From Bench to Bedside. Front. Bioeng. Biotechnol. 2021, 9, 599300. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Li, C.; Du, Y.; Zhang, T.; Wang, H.; Hou, Z.; Zhang, Y.; Cui, W.; Chen, W. “Genetic scissors” CRISPR/Cas9 genome editing cutting-edge biocarrier technology for bone and cartilage repair. Bioact. Mater. 2023, 22, 254–273. [Google Scholar] [CrossRef]
- Ghadakzadeh, S.; Mekhail, M.; Aoude, A.; Hamdy, R.; Tabrizian, M. Small Players Ruling the Hard Game: siRNA in Bone Regeneration. J. Bone Miner. Res. 2016, 31, 475–487. [Google Scholar] [CrossRef]
- Leng, Q.; Chen, L.; Lv, Y. RNA-based scaffolds for bone regeneration: Application and mechanisms of mRNA, miRNA and siRNA. Theranostics 2020, 10, 3190. [Google Scholar] [CrossRef]
- Rose, L.; Uludaǧ, H. Realizing the potential of gene-based molecular therapies in bone repair. J. Bone Miner. Res. 2013, 28, 2245–2262. [Google Scholar] [CrossRef]
- Liu, F.; Ferreira, E.; Porter, R.M.; Glatt, V.; Schinhan, M.; Shen, Z.; Randolph, M.A.; Kirker-Head, C.A.; Wehling, C.; Vrahas, M.S.; et al. Rapid and reliable healing of critical size bone defects with genetically modified sheep muscle. Eur. Cells Mater. 2015, 30, 118–130; discussion 130–131. [Google Scholar] [CrossRef]
- De La Vega, R.E.; De Padilla, C.L.; Trujillo, M.; Quirk, N.; Porter, R.M.; Evans, C.H.; Ferreira, E. Contribution of Implanted, Genetically Modified Muscle Progenitor Cells Expressing BMP-2 to New Bone Formation in a Rat Osseous Defect. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 208–218. [Google Scholar] [CrossRef] [PubMed]





| Delivery Method | Advantages | Challenges |
|---|---|---|
| Scaffolds |
|
|
| Extracellular Vesicles |
|
|
| Regional Gene Therapy |
|
|
| Systems | Indication | Approval Date |
|---|---|---|
| INFUSE® Bone Graft |
| March 2007 |
| October 2009 | |
| INFUSE™ Bone Graft/LT-CAGE™ Lumbar Tapered Fusion Device |
| July 2002 |
| July 2004 | |
| October 2009 | |
| INFUSE™ Bone Graft/Medtronic Interbody Fusion Device |
| December 2015 |
| Author | Parent Cell | EV Dose | Scaffold Carrier | Cargo; Pathway | Growth Factor, Protein, Gene | Model | Outcome |
|---|---|---|---|---|---|---|---|
| Takeuchi et al. [111] | hBMMSCs | 30 μg | Atelocollagen sponge | miRNA undefined; unspecified | ↑ VEGF, ANG1, ANG2, COLI, OCN, Runx2 | Wistar rat calvarial defect | In vitro: anti-VEGF antibody decreased expression of osteogenic and angiogenic-related genes; EVs promoted hMSC migration In vivo: anti-VEGF antibody impaired bone formation |
| Zhang et al. [117] | Rat BMMSCs | 100 μL (1010 particles) | Local injection | BMP; BMP-2/Smad1/Runx2 and HIF1-1α/VEGF pathways | ↑ OCN, OPN, OGN, BMP2, Smad1, Runx2 | Wistar rat femoral nonunion | In vitro: BMMSC-EVs + promoted proliferation and migration of HUVECs and osteoblast precursors In vivo: BMMSC-EVs + enhanced osteogenesis, angiogenesis, and fracture healing |
| Zhang et al. [118] | hBMMSCs | 25 μg/mL | Local injection | miR-935; inhibition of STAT1 | ↑ Runx2, ATF4 | Sprague–Dawley ovariectomized, osteoporotic rats | In vitro: increased ALP activity, enhanced levels of Runx2 and ATF4, enhanced osteoblast proliferation and differentiation In vivo: increased BMD, BV/TV, TbN, Tb.Th |
| Li et al. [100] | hADSCs | 25 μg/mL | PLGA/pDA | miR-218; unspecified | ↑ Runx2, ALP, COL1a1 | Murine critical-sized calvarial defect | In vitro: enhanced expression of osteoblastogenesis-related genes In vivo: significantly more new bone formation and recruitment of host MSCs |
| Li et al. [119] | hADSCs | 0.8 mg/mL | GNP hydrogel | miR-451a; unspecified | ↑ M2 marker (CD206) ↓ M1 marker (iNOS) | Sprague–Dawley rat calvarial defect | In vitro: miR-451a promotes the polarization of macrophage phenotypes through the inhibition of MIF In vivo: immunoregulated bone microenvironment, promoted osteogenesis |
| Zhang et al. [120] | hUCMSCs | 100 μL/mL | HyStem-HP hydrogel | HIF-1α, VEGF; unspecified | ↑ HIF-1α, VEGF, OCN, COL1a1 | Rat femoral fracture | In vitro: Upregulation of osteogenic- and angiogenic-related gene expression levels In vivo: promoted angiogenesis and fracture healing through the proliferation of HUVECs |
| Qi et al. [121] | hiPSC-MSCs | 100 µg | β-TCP | unspecified | ↑ OPN, COL1, Runx2 | Sprague–Dawley ovariectomized rats with calvarial defect | In vitro: increased ALP activity and expression levels of osteoblast-related genes and increased proliferation of rBMSCs In vivo: enhanced BV/TV and angiogenesis in a dose-dependent manner |
| Cui et al. [122] | MC3T3-E1 | 100 µg | ---- | miR-1192, miR-680, miR-302a; Wnt pathway | ↑ Runx2, ALP, β-catenin ↓ Axin1 | Murine bone marrow-derived stromal cell line (ST2) | In vitro: increased osteoblast differentiation and matrix mineralization |
| Uenaka et al. [123] | MC3T3-E1, Mature osteoblasts | 1–5 × 109 particles | Gelatin-hydrogel sheet | miR-143-3p; targeting of Cbfb | ↑ Rankl ↓ Runx2, Sp7 | Murine critical-sized calvarial defect | In vitro: Inhibition of osteoblast differentiation and promotion of osteoclastogenesis through the suppression of osteoblastic gene expression In vivo: inhibition of bone repair and promotion of bone resorption |
| Eichholz et al. [124] | MLO-Y4 osteocyte-like cells | 1 μg | ---- | Annexin A5, Histone H4; inhibition of RANKL-RANK | ↑ (CM-F): Histone H4, COX2, OCN, OPN, Runx2, OSX, ALP | hMSCs | In vitro: CM-F treatment groups enhanced osteogenesis, osteoblastogenesis |
| Lv et al. [125] | MLO-Y4 osteocyte-like cells | 10 μL | ---- | miR181b-5p; PTEN/AKT pathway | ↑ ALP, BMP2, Runx2 | hPDLSC | In vitro: promoted osteogenic proliferation and differentiation in mechanically strained MLO-Y4 cells |
| Author | Vector | Cell | Carrier | Model | Results |
|---|---|---|---|---|---|
| Lieberman et al. [160] | Ad-BMP2 | W-20 (murine stromal) | DBM | SCID Mouse 8 mm Femoral Defect | Radiographic healing at 8 wks. Histologic demonstration of lamellar bone formation |
| Lieberman et al. [20] | Ad-BMP2 | Rat Bone Marrow Cells | DBM | Lewis Rat 8 mm Femoral Defect | Radiographic healing at 8 wks with course trabecular bone with remodeling. Equivalent mechanics between operated and non-operated femurs that healed |
| Dumont et al. [176] | Ad-BMP9 | Human Mesenchymal Stem Cells | ---- | Athymic Nude Rat Lumbar Fusion | MicroCT evidence of ectopic bone formation with histologic demonstration of fusion with posterior spinal elements |
| Wang et al. [179] | Ad-BMP2 | Rat Bone Marrow Cells | DBM or collagen sponge | Lewis Rat Lumbar Fusion | Radiograph, histologic, and mechanical testing demonstrate spinal fusion in both carriers |
| Hidaka et al. [175] | Ad-BMP7 | Rat Bone Marrow Cells | Allogeneic Allograft | Lewis Rat Lumbar Fusion | Radiograph, histologic, and mechanical testing demonstrate spinal fusion |
| Lee et al. [180] | Ad-BMP2 | Human Myocytes | Collagen Matrix | SCID Mouse 5 mm Calvarial Defect | Bridging bone appears at 2 wks postoperatively with significant healing and periosteum at 4 wks |
| Feeley et al. [161] | LV-BMP2 or Ad-BMP2 | Rat Bone Marrow Cells | Collagen Sponge | SCID Mouse 4 mm Radial Defect | LV-BMP2 continued BMP2 production at 12 wks compared to only 4 wks for Ad-BMP2, with radiographic and histologic healing for both vectors |
| Virk et al. [162] | LV-BMP2 or Ad-BMP2 | Rat Bone Marrow Cells | Calcium Phosphate plus Type I Collagen | Lewis Rat 8 mm Femoral Defect | Higher rates of healing on radiographs and microCT with improved mechanical properties for LV-BMP2 vs. Ad-BMP2 |
| Virk et al. [152] | LV-BMP2 | “Same-Day” Rat Bone Marrow Cells vs. Traditional | Calcium Phosphate plus Type I Collagen | Lewis Rat 8 mm Femoral Defect | “Same-Day” ex vivo technique resulting in faster rates of healing with increased bone formation and improved biomechanics compared to traditional methods |
| Alluri et al. [181] | LV-BMP2 | Rat Bone Marrow Cells | 3D-printed Tricalcium Phosphate Scaffold | Lewis Rat 6 mm Femoral Defect | Radiographic healing with histology demonstrating trabecular bone circumferentially |
| Miyazaki et al. [173] | LV-BMP2 | Rat Bone Marrow Cells | Collagen Carrier | Lewis Rat Lumbar Fusion | Radiographic, microCT, and histologic evidence of healing with resorption of collagen sponge |
| Miyazaki et al. [172] | LV-BMP2 or Ad-BMP2 | Rat Bone Marrow Cell | Collagen Carrier | Lewis Rat Lumbar Fusion | Improved spinal fusion with LV-BMP2 seen on radiographs, microCT, and histology vs. Ad-BMP2 |
| Miyazaki et al. [182] | Ad-BMP2 | Human Bone Marrow or Adipose Stem Cells | Collagen Carrier | Athymic Nude Rat Lumbar Fusion | Equivalent fusion on radiographics, microCT, histology, and mechanical testing between bone marrow and stem cell groups |
| Vakhshori et al. [151] | LV-BMP2 | Human Adipose Stem Cell | Tricalcium phosphate/Hydroxyapatite | Athymic Nude Rat 6 mm Femoral Defect | Equivalent radiograph, microCT, histological, and biomechanical testing compared to rhBMP |
| Kang et al. [183] | LV-BMP2 | Rat Bone Marrow Cells | 3D Printed Hyperelastic bone | Lewis Rat 6 mm Femoral Defect | Radiographic and histologic evidence of healing with bony ingrowth on scaffold |
| Author | Vector | Model | Results |
|---|---|---|---|
| Rundle et al. [184] | MLV-BMP2/BMP4 hybrid | Sprague–Dawley Femur Fracture | Significant callous formation early post-injection; however, mostly found to be extra-periosteal. Histology failed to demonstrate significant differences in remodeling compared to controls. |
| Baltzer et al. [185] | AV-BMP2 | New Zealand White Rabbit Femoral Defect | Defects were 75% restored after 7 wks and healed after 12 wks in the experimental group only. Histology demonstrated a bridging callus present at 8 wks post-injection. |
| Helm et al. [178] | AV-BMP9 | Athymic Nude Rat Lumbar Fusion | 16 wks post-injection microCT demonstrated fusion mass in direct contact with posterior spinal elements without canal compromise. Histological evidence of lamellar bone with marrow cavities developed. |
| Alden et al. [174] | AV-BMP2 | Athymic Nude Rat Lumbar Fusion | 12 wks post-injection microCT demonstrated fusion mass in direct contact with posterior spinal elements. Sharp borders observed on histology but no adverse reaction in surrounding paraspinal musculature. |
| Laurent et al. [177] | AV-BMP6 | New Zealand White Rabbit Lumbar Fusion | 14 wks post-injection microCT demonstrated fusion mass in direct contact with posterior spinal elements. Histological evidence of bony bridging between transverse processes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ball, J.R.; Shelby, T.; Hernandez, F.; Mayfield, C.K.; Lieberman, J.R. Delivery of Growth Factors to Enhance Bone Repair. Bioengineering 2023, 10, 1252. https://doi.org/10.3390/bioengineering10111252
Ball JR, Shelby T, Hernandez F, Mayfield CK, Lieberman JR. Delivery of Growth Factors to Enhance Bone Repair. Bioengineering. 2023; 10(11):1252. https://doi.org/10.3390/bioengineering10111252
Chicago/Turabian StyleBall, Jacob R., Tara Shelby, Fergui Hernandez, Cory K. Mayfield, and Jay R. Lieberman. 2023. "Delivery of Growth Factors to Enhance Bone Repair" Bioengineering 10, no. 11: 1252. https://doi.org/10.3390/bioengineering10111252
APA StyleBall, J. R., Shelby, T., Hernandez, F., Mayfield, C. K., & Lieberman, J. R. (2023). Delivery of Growth Factors to Enhance Bone Repair. Bioengineering, 10(11), 1252. https://doi.org/10.3390/bioengineering10111252