In Vivo Evaluation of Safety and Efficacy of Ethyl Cellulose-Ethanol Tissue Ablation in a Swine Cervix Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort of Swine
2.2. Devices and Consumables
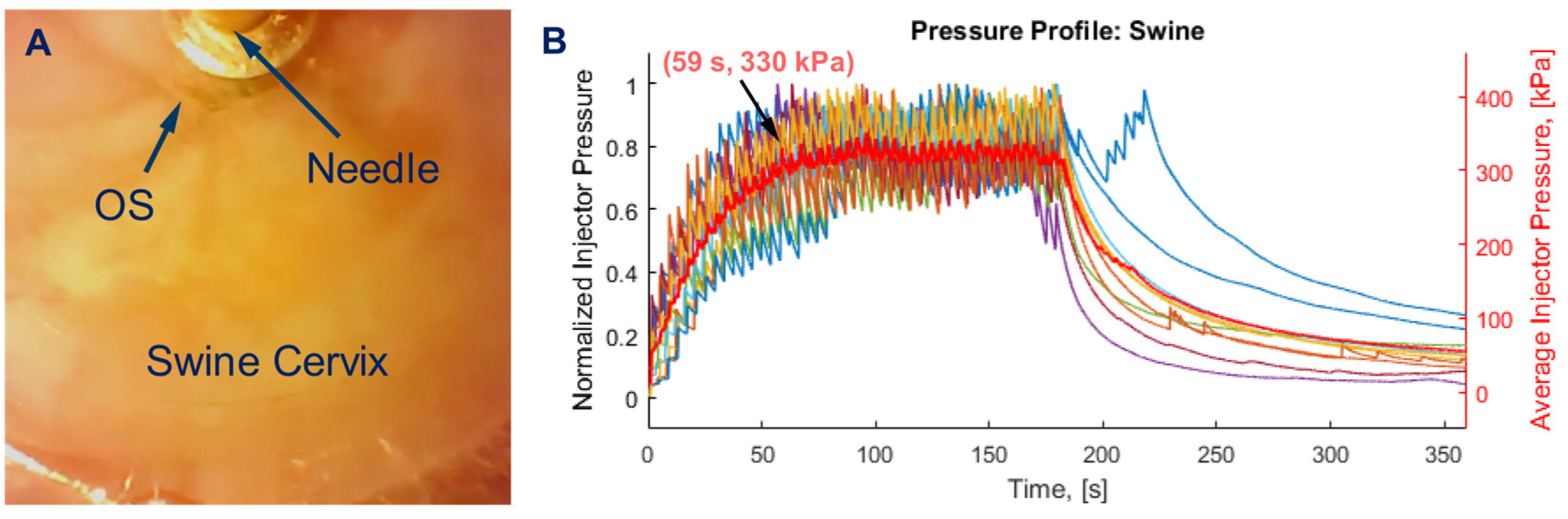
2.3. Protocol for EC-Ethanol Ablation and Thermocoagulation Procedures in Swine Cervix
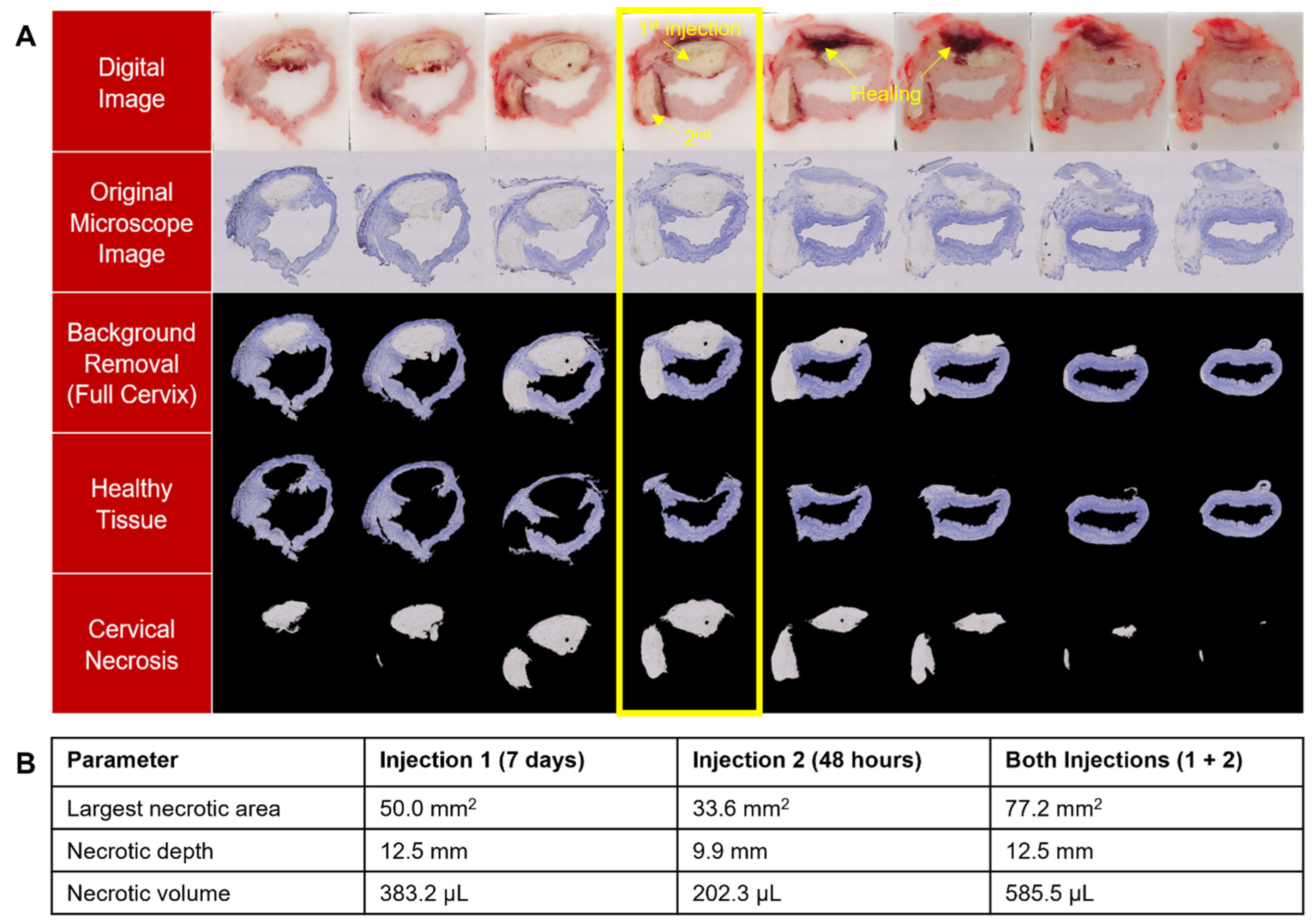
2.4. Processing for Histopathological Assessment
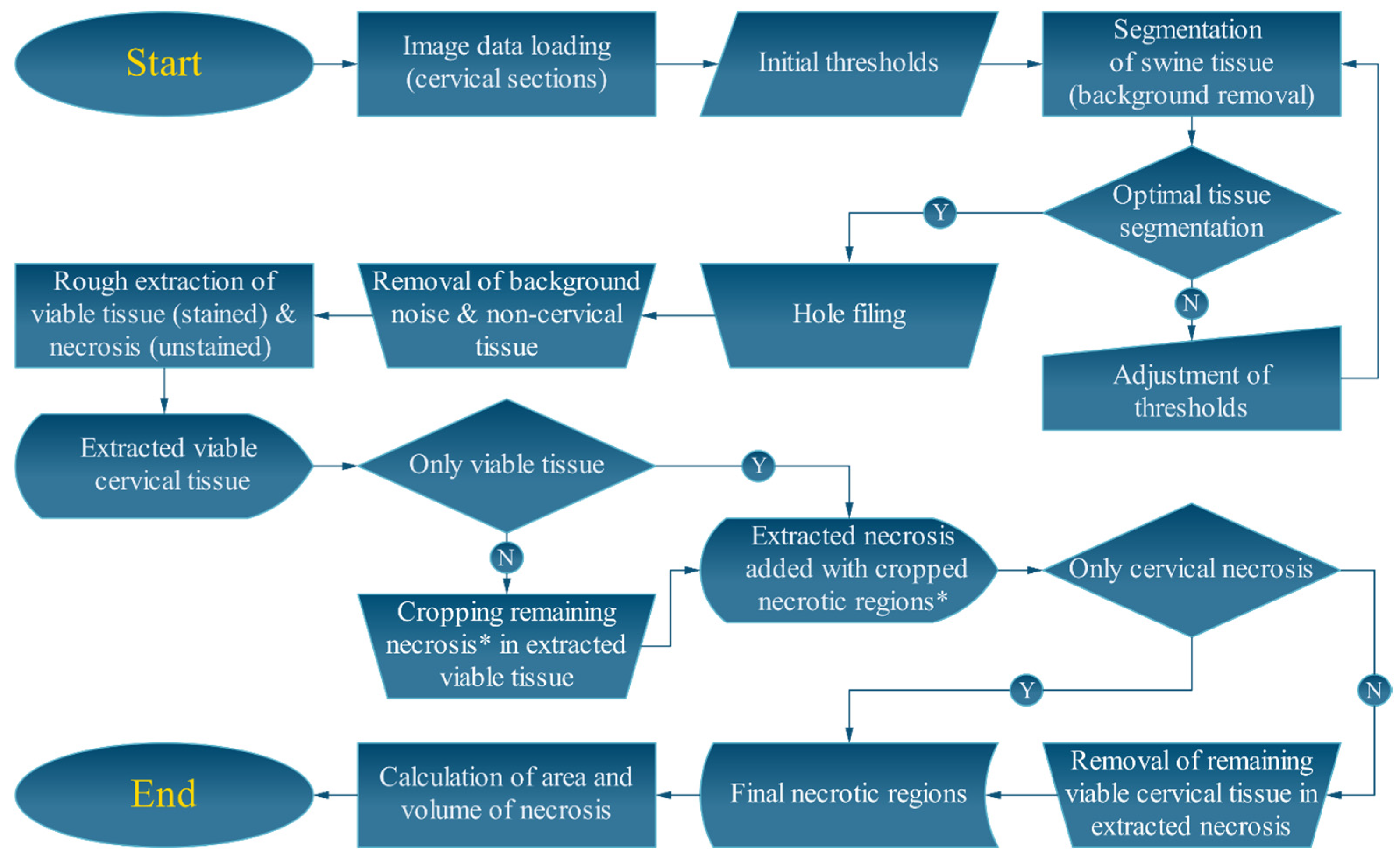
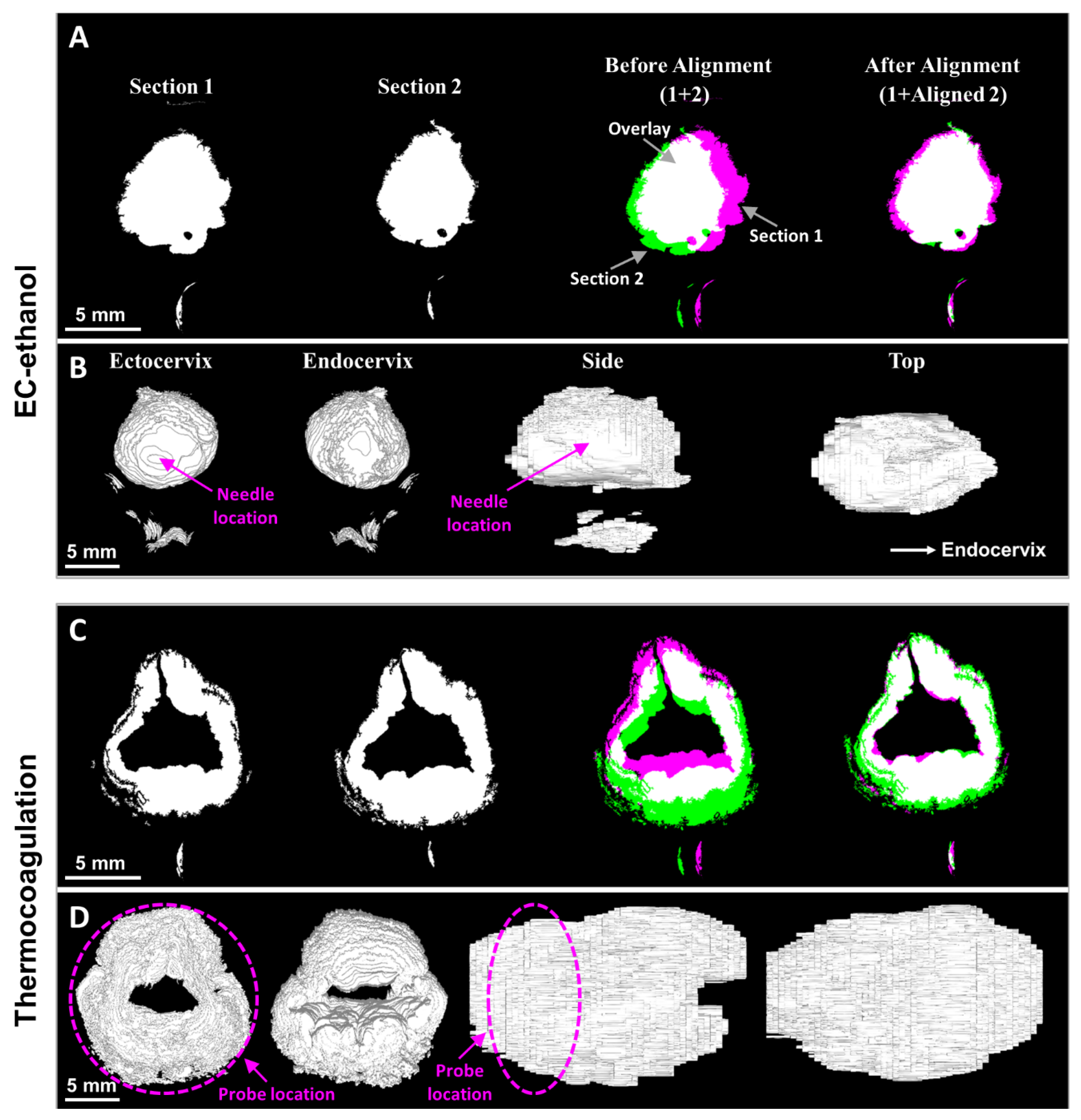
2.5. Semi-Automated Image Processing Algorithms
2.6. Image Alignment and Volume Reconstruction
2.7. Statistical Analysis
2.8. Data Availability
3. Results
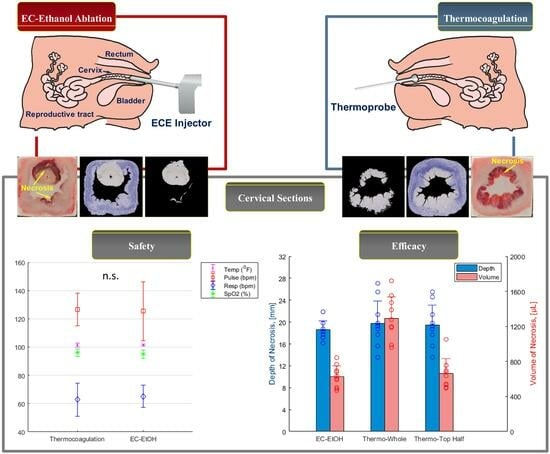
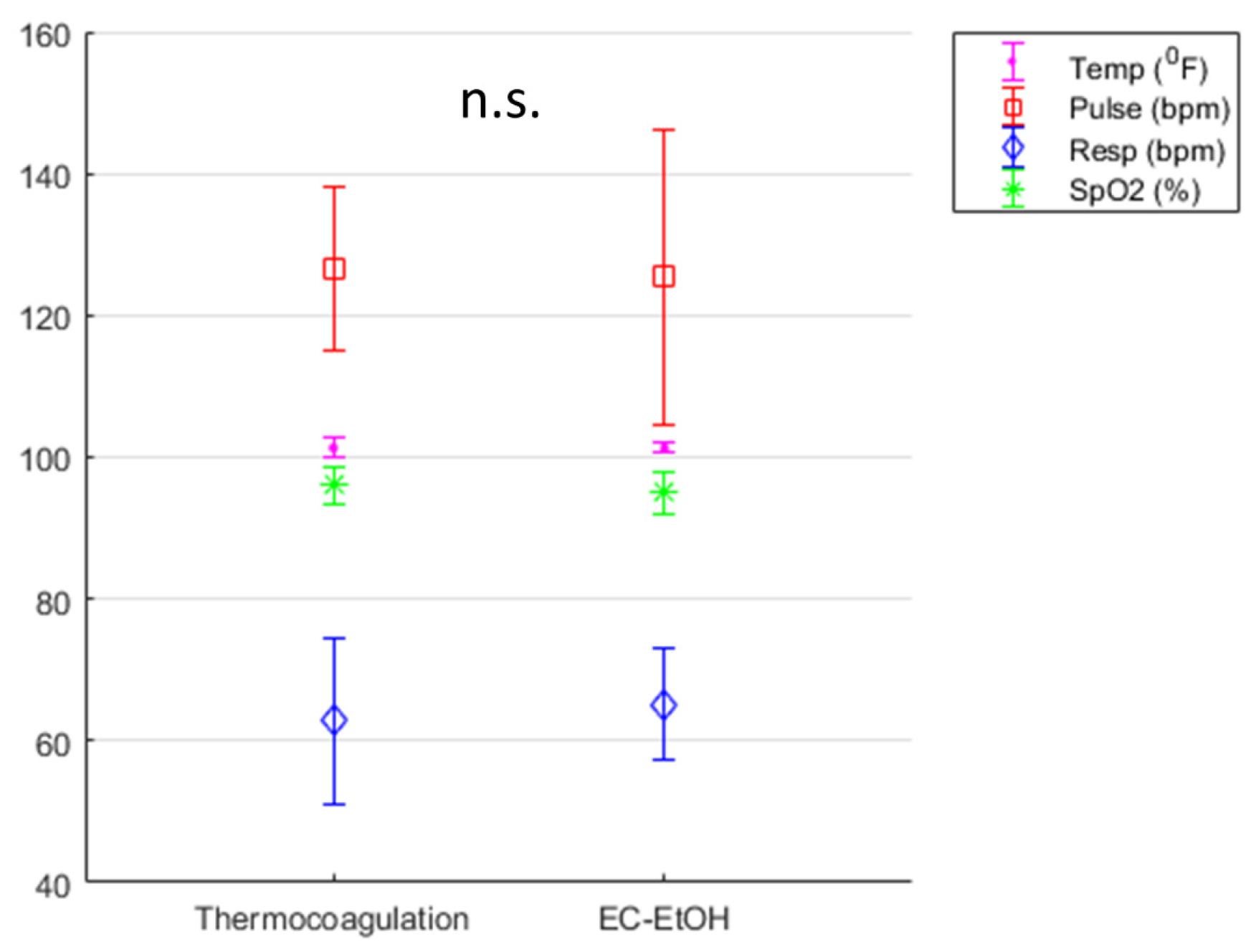
3.1. EC-Ethanol Ablation Achieved Comparable Acute Safety Endpoints to That of Thermocoagulation
3.2. EC-Ethanol Ablation Achieved Comparable Depth and Volume of Necrosis in the Upper Half of the Cervix as Compared to That of Thermocoagulation
3.3. EC Deposits Remain in Cervix for at Least 7 Days and Multiple EC-Ethanol Injections Can Induce Necrosis at Different Positions in the Cervix
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. 2020. Available online: https://gco.iarc.fr/today (accessed on 1 May 2023).
- Lee, F.; Desravines, N.; Recknagel, J.; Singleton, M.; Muñoz, R.; Rahangdale, L. History of Surgical Treatment for Cervical Intraepithelial Neoplasia. J. Gynecol. Surg. 2022, 38, 17–23. [Google Scholar] [CrossRef]
- Smith, J.S.; Sanusi, B.; Swarts, A.; Faesen, M.; Levin, S.; Goeieman, B.; Ramotshela, S.; Rakhombe, N.; Williamson, A.L.; Michelow, P. A randomized clinical trial comparing cervical dysplasia treatment with cryotherapy vs loop electrosurgical excision procedure in HIV-seropositive women from Johannesburg, South Africa. Am. J. Obstet. Gynecol. 2017, 217, 183.e1–183.e11. [Google Scholar] [CrossRef] [PubMed]
- Cubie, H.A.; Campbell, C. Cervical cancer screening—The challenges of complete pathways of care in low-income countries: Focus on Malawi. Women’s Health 2020, 16, 1745506520914804. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for the Use of Thermal Ablation for Cervical Pre-Cancer Lesions; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- de Fouw, M.; Oosting, R.M.; Rutgrink, A.; Dekkers, O.M.; Peters, A.A.W.; Beltman, J.J. A systematic review and meta-analysis of thermal coagulation compared with cryotherapy to treat precancerous cervical lesions in low-and middle-income countries. Int. J. Gynecol. Obstet. 2019, 147, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Chigbu, C.O.; Onwudiwe, E.N.; Onyebuchi, A.K. Thermo-coagulation versus cryotherapy for treatment of cervical precancers: A prospective analytical study in a low-resource African setting. J. Obstet. Gynaecol. Res. 2020, 46, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Pinder, L.F.; Parham, G.P.; Basu, P.; Muwonge, R.; Lucas, E.; Nyambe, N.; Sauvaget, C.; Mwanahamuntu, M.H.; Sankaranarayanan, R.; Prendiville, W. Thermal ablation versus cryotherapy or loop excision to treat women positive for cervical precancer on visual inspection with acetic acid test: Pilot phase of a randomised controlled trial. Lancet Oncol. 2020, 21, 175–184. [Google Scholar] [CrossRef]
- Banerjee, D.; Mandal, R.; Mandal, A.; Ghosh, I.; Mittal, S.; Muwonge, R.; Lucas, E.; Basu, P. A Prospective Randomized Trial to Compare Safety, Acceptability and Efficacy of Thermal Ablation and Cryotherapy in a Screen and Treat Setting. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1391. [Google Scholar] [CrossRef]
- Maza, M.; Schocken, C.M.; Bergman, K.L.; Randall, T.C.; Cremer, M.L. Cervical Precancer Treatment in Low- and Middle-Income Countries: A Technology Overview. J. Glob. Oncol. 2017, 3, 400–408. [Google Scholar] [CrossRef]
- de Fouw, M.; Oosting, R.; Eijkel, B.; van Altena, P.; Peters, A.; Dankelman, J.; Beltman, J. Comparison of the tissue interaction between thermal ablation and cryotherapy as treatment for cervical precancerous lesions in an ex-vivo model. Health Technol. 2020, 10, 1275–1281. [Google Scholar] [CrossRef]
- Sandoval, M.; Slavkovsky, R.; Bansil, P.; Jeronimo, J.; Lim, J.; Figueroa, J.; de Sanjose, S. Acceptability and safety of thermal ablation for the treatment of precancerous cervical lesions in Honduras. Trop. Med. Int. Health 2019, 24, 1391–1399. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.; Bagherpour, A.; Irfan, M. 99—Nonthermal Ablation of Liver Lesions. In Image-Guided Interventions, 3rd ed.; Mauro, M.A., Murphy, K.P.J., Venbrux, A.C., Morgan, R.A., Eds.; Elsevier: Boston, MA, USA, 2020; pp. 802–810.e2. [Google Scholar] [CrossRef]
- Mosquera-Klinger, G.; Gutierrez, J.J.C. Endoscopic ultrasound-guided ethanol ablation for the management of a symptomatic pancreatic insulinoma. Rev. Esp. Enfermadades Dig. (REED) 2021, 113, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ludena, L.; Consiglieri, C.-F.; Gornals, J.-B. EUS-guided ethanol ablation therapy for gastric stromal tumors. Rev. Española Enfermedades Dig. 2018, 110, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.W. Surgery of the Thyroid and Parathyroid Glands E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Cesareo, R.; Tabacco, G.; Naciu, A.M.; Crescenzi, A.; Bernardi, S.; Romanelli, F.; Deandrea, M.; Trimboli, P.; Palermo, A.; Castellana, M. Long-term efficacy and safety of percutaneous ethanol injection (PEI) in cystic thyroid nodules. A systematic review and meta-analysis. Clin. Endocrinol. 2021, 96, 97–106. [Google Scholar] [CrossRef]
- Morhard, R.; Nief, C.; Barrero Castedo, C.; Hu, F.; Madonna, M.; Mueller, J.L.; Dewhirst, M.W.; Katz, D.F.; Ramanujam, N. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Sci. Rep. 2017, 7, 8750. [Google Scholar] [CrossRef]
- Shiina, S.; Tagawa, K.; Niwa, Y.; Unuma, T.; Komatsu, Y.; Yoshiura, K.; Hamada, E.; Takahashi, M.; Shiratori, Y.; Terano, A. Percutaneous ethanol injection therapy for hepatocellular carcinoma: Results in 146 patients. AJR Am. J. Roentgenol. 1993, 160, 1023–1028. [Google Scholar] [CrossRef]
- Nief, C.; Morhard, R.; Chelales, E.; Alvarez, D.; Bourla, I.; Lam, C.; Crouch, B.; Sag, A.; Mueller, J.; Katz, D.; et al. Polymer-assisted intratumoral delivery of ethanol: Preclinical investigation of safety and efficacy in a murine breast cancer model. PLoS ONE, 2021; in press. [Google Scholar] [CrossRef]
- Morhard, R.; Mueller, J.L.; Tang, Q.; Nief, C.; Chelales, E.; Lam, C.T.; Alvarez, D.A.; Rubinstein, M.; Katz, D.F.; Ramanujam, N. Understanding factors governing distribution volume of ethyl cellulose-ethanol to optimize ablative therapy in the liver. IEEE Trans. Biomed. Eng. 2019, 67, 2337–2348. [Google Scholar] [CrossRef]
- Chelales, E.; Morhard, R.; Nief, C.; Crouch, B.; Sag, A.; Ramanujam, N. Radiologic-Pathologic Analysis of Increased Ethanol Localization and Ablative Extent Achieved by Ethyl Cellulose. Sci. Rep. 2021, 11, 20700. [Google Scholar] [CrossRef]
- Lorenzen, E.; Follmann, F.; Jungersen, G.; Agerholm, J.S. A review of the human vs. porcine female genital tract and associated immune system in the perspective of using minipigs as a model of human genital Chlamydia infection. Vet. Res. 2015, 46, 116. [Google Scholar] [CrossRef]
- Mueller, J.L.; Morhard, R.; DeSoto, M.; Chelales, E.; Yang, J.; Nief, C.; Crouch, B.; Everitt, J.; Previs, R.; Katz, D. Optimizing ethyl cellulose-ethanol delivery towards enabling ablation of cervical dysplasia. Sci. Rep. 2021, 11, 16869. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.K.; Baker, M.; Jaffers, O.; Pearle, M.S.; Lindberg, G.L.; Cadeddu, J.A. Time course of nicotinamide adenine dinucleotide diaphorase staining after renal radiofrequency ablation influences viability assessment. J. Endourol. 2007, 21, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Koda, M.; Okamoto, K.; Miyoshi, Y.; Kawasaki, H. Hepatic vascular and bile duct injury after ethanol injection therapy for hepatocellular carcinoma. Gastrointest. Radiol. 1992, 17, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Dompmartin, A.; Blaizot, X.; Théron, J.; Hammer, F.; Chene, Y.; Labbé, D.; Barrellier, M.T.; Gaillard, C.; Leroyer, R.; Chedru, V.; et al. Radio-opaque ethylcellulose-ethanol is a safe and efficient sclerosing agent for venous malformations. Eur. Radiol. 2011, 21, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Cremer, M.L.; Conzuelo-Rodriguez, G.; Cherniak, W.; Randall, T. Ablative therapies for cervical intraepithelial neoplasia in low-resource settings: Findings and key questions. J. Glob. Oncol. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Mueller, J.L.; Asma, E.; Lam, C.T.; Krieger, M.S.; Gallagher, J.E.; Erkanli, A.; Hariprasad, R.; Malliga, J.; Muasher, L.C.; Mchome, B. International image concordance study to compare a point of care tampon colposcope to a standard-of-care colposcope. J. Low. Genit. Tract Dis. 2017, 21, 112. [Google Scholar] [CrossRef]
- Mueller, J.L.; Lam, C.T.; Dahl, D.; Asiedu, M.N.; Krieger, M.S.; Bellido-Fuentes, Y.; Kellish, M.; Peters, J.; Erkanli, A.; Ortiz, E.J. Portable Pocket colposcopy performs comparably to standard-of-care clinical colposcopy using acetic acid and Lugol’s iodine as contrast mediators: An investigational study in Peru. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1321–1329. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quang, T.T.; Yang, J.; Kaluzienski, M.L.; Parrish, A.; Farooqui, A.; Katz, D.; Crouch, B.; Ramanujam, N.; Mueller, J.L. In Vivo Evaluation of Safety and Efficacy of Ethyl Cellulose-Ethanol Tissue Ablation in a Swine Cervix Model. Bioengineering 2023, 10, 1246. https://doi.org/10.3390/bioengineering10111246
Quang TT, Yang J, Kaluzienski ML, Parrish A, Farooqui A, Katz D, Crouch B, Ramanujam N, Mueller JL. In Vivo Evaluation of Safety and Efficacy of Ethyl Cellulose-Ethanol Tissue Ablation in a Swine Cervix Model. Bioengineering. 2023; 10(11):1246. https://doi.org/10.3390/bioengineering10111246
Chicago/Turabian StyleQuang, Tri T., Jeffrey Yang, Michele L. Kaluzienski, Anna Parrish, Asma Farooqui, David Katz, Brian Crouch, Nimmi Ramanujam, and Jenna L. Mueller. 2023. "In Vivo Evaluation of Safety and Efficacy of Ethyl Cellulose-Ethanol Tissue Ablation in a Swine Cervix Model" Bioengineering 10, no. 11: 1246. https://doi.org/10.3390/bioengineering10111246
APA StyleQuang, T. T., Yang, J., Kaluzienski, M. L., Parrish, A., Farooqui, A., Katz, D., Crouch, B., Ramanujam, N., & Mueller, J. L. (2023). In Vivo Evaluation of Safety and Efficacy of Ethyl Cellulose-Ethanol Tissue Ablation in a Swine Cervix Model. Bioengineering, 10(11), 1246. https://doi.org/10.3390/bioengineering10111246