A Glutaraldehyde-Free Crosslinking Method for the Treatment of Collagen-Based Biomaterials for Clinical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Processing of Tissue
2.2. Tissue Treatment
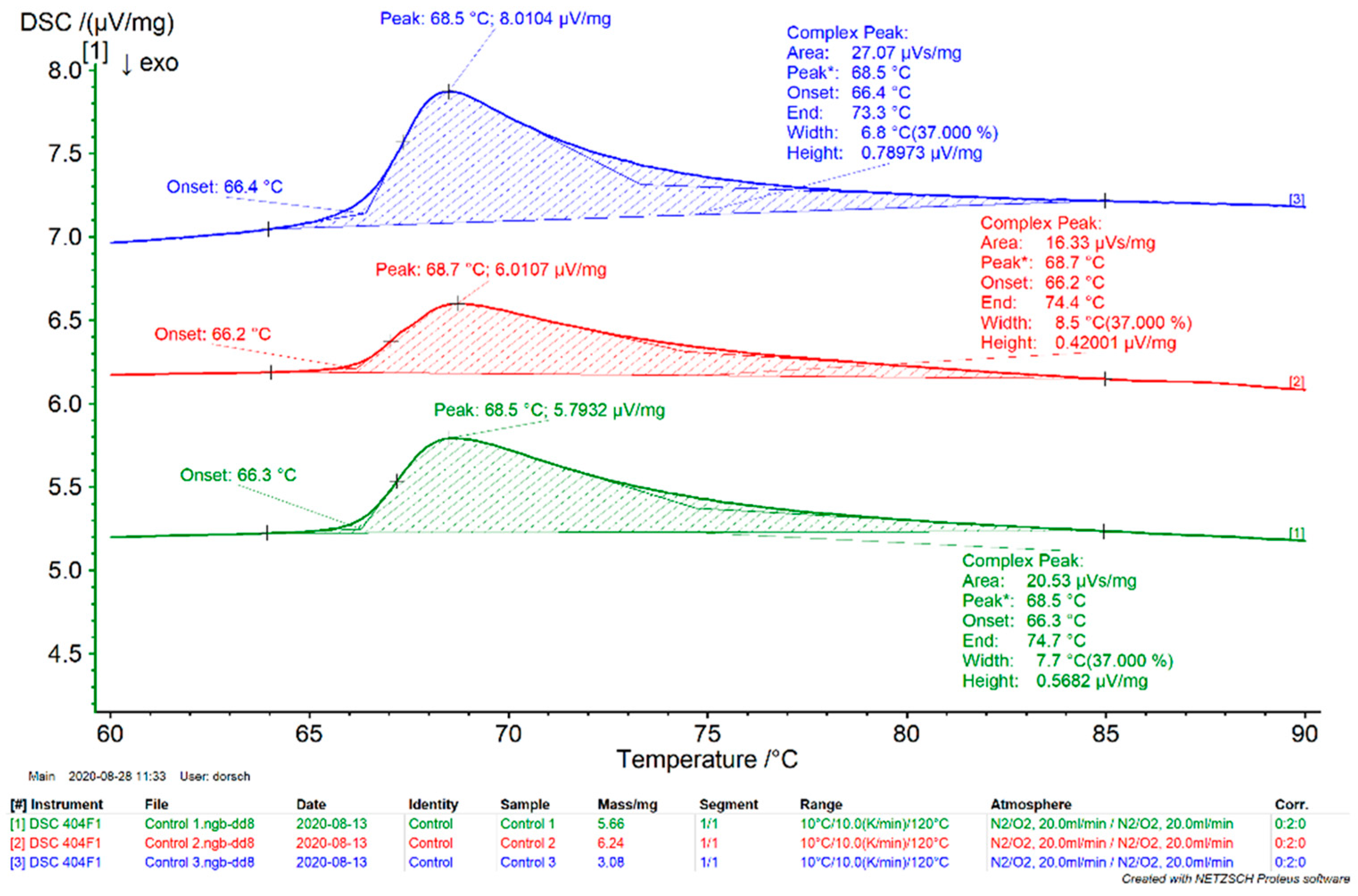
2.3. Thermoanalytic Stability Testing (Differential Scanning Calorimetry)
2.4. Enzymatic Stability Testing (Collagenase Assay)
2.5. Protein Structure (Fourier-Transform Infrared Spectroscopy)
2.6. Two-Photon Microscopy
2.7. Biomechanical Characterization (Low Strain Rate Uniaxial Tensile Testing)
2.8. Statistical Analysis
3. Results
3.1. Denaturation Profile
3.2. Enzymatic Stability Assessment
3.3. Protein Fingerprint Region Analysis
3.4. Multi-Photon Microscopy
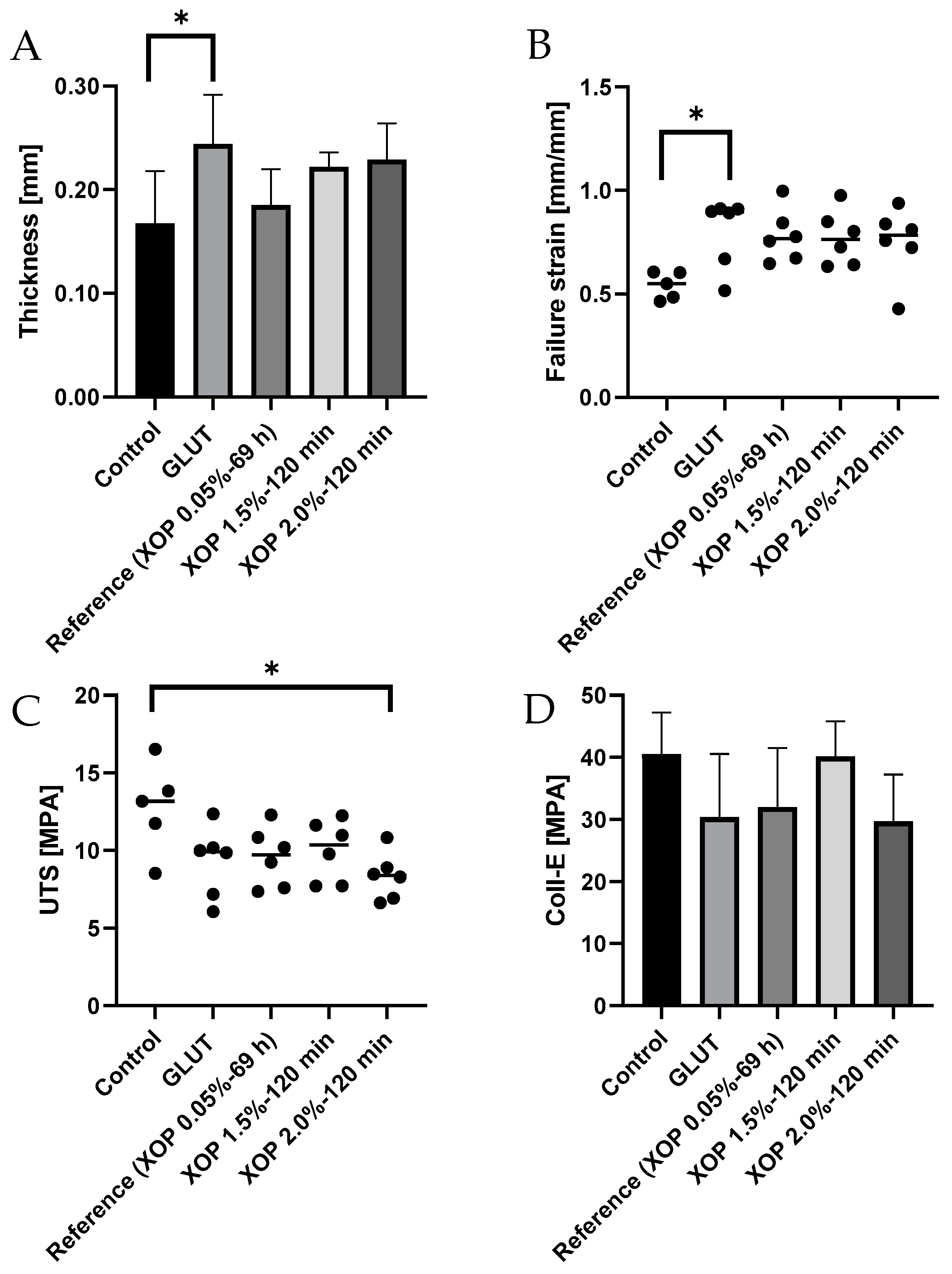
3.5. Biomechanical Characterization of Cross-Linked Tissue
4. Discussion
- (i)
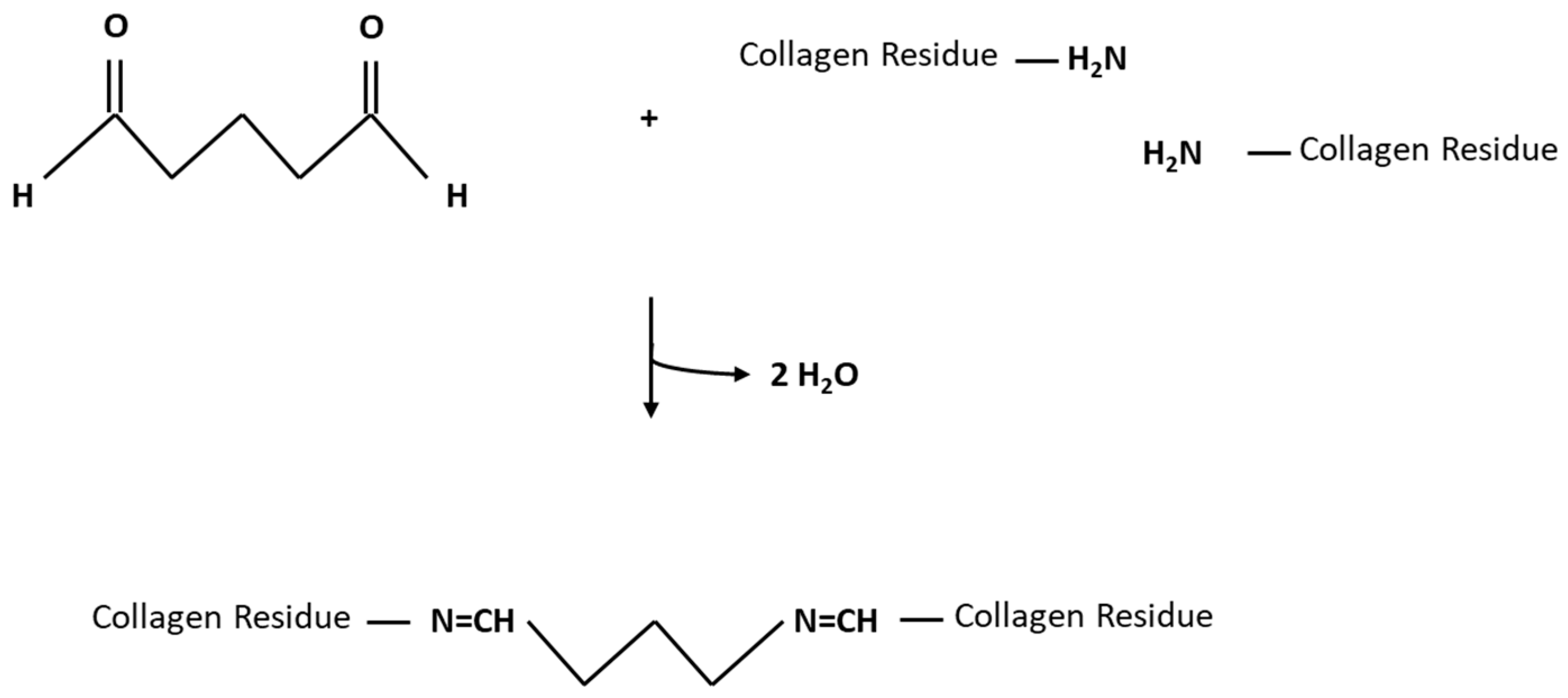
- A higher denaturation or shrinkage temperature of cross-linked tissue is due to the introduction of additional chemical covalent crosslinks, which disrupt at significantly higher temperatures [40] than the fewer existing crosslinks in untreated tissue.
- (ii)
- Increased resistance to collagenase due to the introduction of inter- and intra-molecular crosslinks, leading to a change in the collagen’s protein-structure. Subsequently, enzymatic access to particular cleavage sites is impeded [41].
- (iii)
- The introduction of new crosslinks and the resulting altered fiber alignment also lead to an alteration of the α-helical and β-sheet structures, which was observed in the change of the secondary structure. This also explains an alteration in the fiber alignment observed in the microscopic assessment.
- (iv)
- An increase in compliance and a decrease in ultimate tensile strength are due to the introduction of additional crosslinks, leading to increased rotational stiffness and compression of the fibers and a change in the fiber alignment, resulting in a reduced capacity of the fibers to shear.
5. Conclusions
6. Limitations
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations/Nomenclature
| Abbreviation/Nomenclature | Meaning |
| α-Gal | Galα1,3-Galβ1-4GlcNAc-R |
| Ampho B | Amphotericin B |
| Coll-e | Collagen Phase Modulus |
| DSC | Differential Scanning Calorimetry |
| ECM | Extracellular Matrix |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GLUT | Glutaraldehyde |
| MPa | Megapascal |
| PBS | Phosphate-Buffered Solution |
| Pen-Strep | Penicillin-Streptomycin |
| PICs | Pericardial Interstitial Cells |
| R | Ratio of Absorbance Value |
| RT | Room Temperature |
| SHG | Second Harmonic Generation |
| Td | Denaturation Temperature |
| Tonset | Endothermic Onset Temperature |
| UTS | Ultimate Tensile Strength |
| XOP | Novel phenolic crosslinking agent |
Appendix A
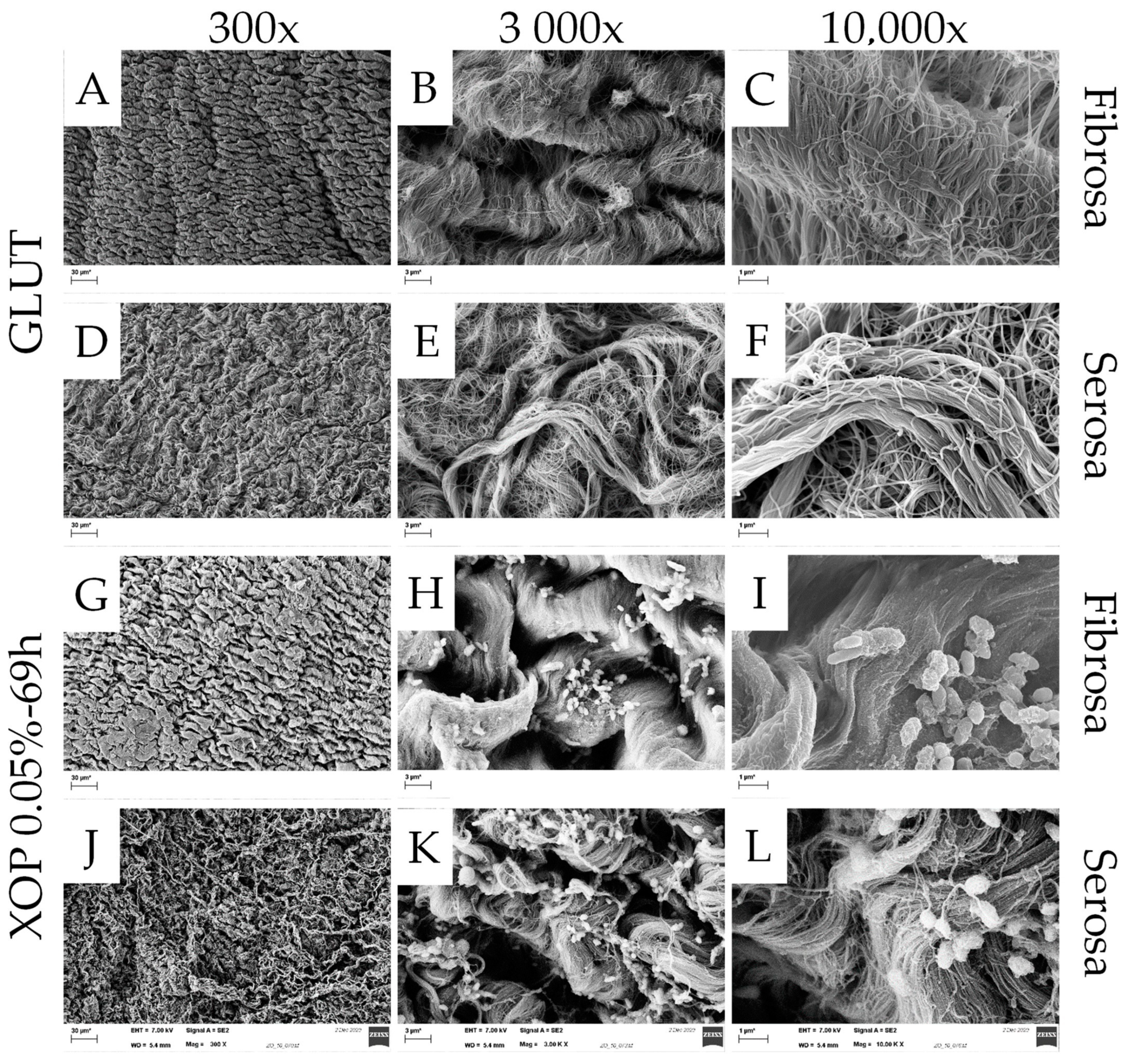
Appendix A.1. Scanning Electron Microscopy
Appendix A.1.1. Materials/Method
Appendix A.1.2. Results

Appendix B

| Materials | Devices | |
|---|---|---|
| Tissue Treatment |
|
|
| Differential scanning calorimetry |
|
|
| Collagenase assay |
|
|
| Fourier-transform-infrared-spectroscopy |
|
|
| Two-Photon microscopy |
| |
| Uniaxial tensile loading to failure testing |
| |
| Scanning electron microscope |
|
|
References
- Rodriguez, E.R.; Tan, C.D. Structure and Anatomy of the Human Pericardium. Prog. Cardiovasc. Dis. 2017, 59, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Straka, F.; Schornik, D.; Masin, J.; Filova, E.; Mirejovsky, T.; Burdikova, Z.; Svindrych, Z.; Chlup, H.; Horny, L.; Daniel, M.; et al. A human pericardium biopolymeric scaffold for autologous heart valve tissue engineering: Cellular and extracellular matrix structure and biomechanical properties in comparison with a normal aortic heart valve. J. Biomater. Sci. Polym. Ed. 2018, 29, 599–634. [Google Scholar] [CrossRef] [PubMed]
- Remi, E.; Khelil, N.; Di, I.; Roques, C.; Ba, M.; Medjahed-Hamidi, F.; Chaubet, F.; Letourneur, D.; Lansac, E.; Meddahi-Pelle, A. Pericardial Processing: Challenges, Outcomes and Future Prospects. In Biomaterials Science and Engineering; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Balguid, A.; Rubbens, M.P.; Mol, A.; Bank, R.A.; Bogers, A.J.; van Kats, J.P.; de Mol, B.A.; Baaijens, F.P.T.; Bouten, C.V.C. The Role of Collagen Cross-Links in Biomechanical Behavior of Human Aortic Heart Valve Leaflets—Relevance for Tissue Engineering. Tissue Eng. 2007, 13, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M.; Allen, S.P.; Yacoub, M.H. Phenotypic and Functional Characterization of Interstitial Cells from Human Heart Valves, Pericardium and Skin. J. Heart Valve Dis. 2000, 9, 150–158. [Google Scholar] [PubMed]
- Sheehy, E.J.; Cunniffe, G.M.; O’Brien, F.J. Collagen-Based Biomaterials for Tissue Regeneration and Repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Woodhead Publishing: Sawston, UK, 2018; pp. 127–150. [Google Scholar] [CrossRef]
- Zeeman, R. Cross-Linking of Collagen-Based Materials. Doctoral Thesis, University of Twente, Enschede, The Netherlands, 1998. [Google Scholar]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of Bioprosthetic Heart Valves: Update 2020. J. Am. Heart Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2020, 18, 92–116. [Google Scholar] [CrossRef]
- Klein, A.A.; Lewis, C.J.; Madsen, J.C. (Eds.) Organ Transplatation—A Clinical Guide; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Badria, A.F.; Koutsoukos, P.G.; Mavrilas, D. Decellularized tissue-engineered heart valves calcification: What do animal and clinical studies tell us? J. Mater. Sci. Mater. Med. 2020, 31, 132. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lim, H.-G.; Kim, Y.J. Calcification of decellularized and alpha-galactosidase-treated bovine pericardial tissue in an alpha-Gal knock-out mouse implantation model: Comparison with primate pericardial tissue. Eur. J. Cardio-Thorac. Surg. 2015, 49, 894–900. [Google Scholar] [CrossRef]
- Galili, U. The α-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol. Cell Biol. 2005, 83, 674–686. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To Cross-Link or Not to Cross-Link? Cross-Linking Associated Foreign Body Response of Collagen-Based Devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Sun, X.; Kiekenap, J.F.S.; Emeis, J.; Steitz, M.; Breitenstein-Attach, A.; Berger, F.; Schmitt, B. Transcatheter Pulmonary Valve Replacement from Autologous Pericardium with a Self-Expandable Nitinol Stent in an Adult Sheep Model. J. Vis. Exp. 2022, 184, e63661. [Google Scholar] [CrossRef]
- Ozaki, S.; Kawase, I.; Yamashita, H.; Uchida, S.; Nozawa, Y.; Takatoh, M.; Hagiwara, S. A total of 404 cases of aortic valve reconstruction with glutaraldehyde-treated autologous pericardium. J. Thorac. Cardiovasc. Surg. 2014, 147, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Ghez, O.; Saeed, I.; Serrato, M.; Quintero, D.B.; Kreitmann, B.; Fraisse, A.; Uemura, H.; Seale, A.; Daubeney, P.; McCarthy, K.; et al. Surgical repair of pulmonary artery branches. Multimed. Man. Cardio-Thorac. Surg. 2013, 2013, mmt014. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Nakaya, M.; Otsu, M.; Sunazawa, T.; Wakabayashi, Y. Autologous Pericardial Patch Repair for Papillary Fibroelastoma on an Aortic Valve Leaflet. Tex. Heart Inst. J. 2017, 44, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Lausberg, H.F.; Aicher, D.; Langer, F.; Schäfers, H.-J. Aortic valve repair with autologous pericardial patch? Eur. J. Cardio-Thorac. Surg. 2006, 30, 244–249. [Google Scholar] [CrossRef]
- Hiester, E.D.; Sacks, M.S. Optimal Bovine Pericardial Tissue Selection Sites. I. Fiber Architecture and Tissue Thickness Measurements. J. Biomed. Mater. Res. 1998, 39, 207–214. [Google Scholar] [CrossRef]
- Bezuidenhout, D.; Oosthuysen, A.; Human, P.; Weissenstein, C.; Zilla, P. The effects of cross-link density and chemistry on the calcification potential of diamine-extended glutaraldehyde-fixed bioprosthetic heart-valve materials. Biotechnol. Appl. Biochem. 2009, 54, 133–140. [Google Scholar] [CrossRef]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef]
- Loke, W.K.; Khor, E. Validation of the shrinkage temperature of animal tissue for bioprosthetic heart valve application by differential scanning calorimetry. Biomaterials 1995, 16, 251–258. [Google Scholar] [CrossRef]
- Lei, Y.; Jin, W.; Luo, R.; Li, G.; Guo, G.; Wang, Y. Bioprosthetic heart valves’ structural integrity improvement through exogenous amino donor treatments. J. Mater. Res. 2018, 33, 2576–2585. [Google Scholar] [CrossRef]
- Liu, J.; Jing, H.; Qin, Y.; Li, B.; Sun, Z.; Kong, D.; Leng, X.; Wang, Z. Nonglutaraldehyde Fixation for off the Shelf Decellularized Bovine Pericardium in Anticalcification Cardiac Valve Applications. ACS Biomater. Sci. Eng. 2019, 5, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Rivera, A.; Oldenhof, H.; Hilfiker, A.; Wolkers, W.F. Spectral fingerprinting of decellularized heart valve scaffolds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Rivera, A.; Oldenhof, H.; Dipresa, D.; Goecke, T.; Kouvaka, A.; Will, F.; Haverich, A.; Korossis, S.; Hilfiker, A.; Wolkers, W.F. Use of sucrose to diminish pore formation in freeze-dried heart valves. Sci. Rep. 2018, 8, 12982. [Google Scholar] [CrossRef] [PubMed]
- Korossis, S. Structure-Function Relationship of Heart Valves in Health and Disease. In Structural Insufficiency Anomalies in Cardiac Valves; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Caliskan, S.; Oldenhof, H.; Brogna, R.; Rashidfarokhi, B.; Sieme, H.; Wolkers, W.F. Spectroscopic assessment of oxidative damage in biomolecules and tissues. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 246, 119003. [Google Scholar] [CrossRef]
- Croce, A.; Bottiroli, G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 2461. [Google Scholar] [CrossRef]
- Sherlock, B.E.; Harvestine, J.N.; Mitra, D.; Haudenschild, A.; Hu, J.; Athanasiou, K.A.; Leach, J.K.; Marcu, L. Nondestructive assessment of collagen hydrogel cross-linking using time-resolved autofluorescence imaging. J. Biomed. Opt. 2018, 23, 036004–036009. [Google Scholar] [CrossRef]
- Lutz, V.; Sattler, M.; Gallinat, S.; Wenck, H.; Poertner, R.; Fischer, F. Impact of collagen crosslinking on the second harmonic generation signal and the fluorescence lifetime of collagen autofluorescence. Ski. Res. Technol. 2011, 18, 168–179. [Google Scholar] [CrossRef]
- Holzapfel, G.A. Biomechanics of Soft Tissue. In Handbook of Material Behavior; Biomech Preprint Series; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Filova, E.; Burdikova, Z.; Stankova, L.; Hadraba, D.; Svindrych, Z.; Schornik, D.; Bacakova, L.; Chlup, H.; Gultova, E.; Vesely, J.; et al. Collagen Structures in Pericardium and Aortic Heart Valves and Their Significance for Tissue Engineering. In Proceedings of the 2013 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2013. [Google Scholar] [CrossRef]
- Duncan, A.C.; Boughner, D. Effect of dynamic glutaraldehyde fixation on the viscoelastic properties of bovine pericardial tissue. Biomaterials 1998, 19, 777–783. [Google Scholar] [CrossRef]
- Freitas, F.S.; Gonçalves, A.S.; De Morais, A.; Benedetti, J.E.; Nogueira, A.F. Graphene-like MoS2 as a Low-Cost Counter Electrode Material for Dye-Sensitized Solar Cells. J. NanoGe J. Energy Sustain. 2012, 1, 11002–11003. [Google Scholar]
- Constable, M.; Burton, H.E.; Lawless, B.M.; Gramigna, V.; Buchan, K.G.; Espino, D.M. Effect of glutaraldehyde based cross-linking on the viscoelasticity of mitral valve basal chordae tendineae. Biomed. Eng. Online 2018, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Spiesz, E.M.; Thorpe, C.T.; Thurner, P.J.; Screen, H.R. Structure and collagen crimp patterns of functionally distinct equine tendons, revealed by quantitative polarised light microscopy (qPLM). Acta Biomater. 2018, 70, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.L.; Paik, D.C.; Holmes, J.W. Structural Mechanism for Alteration of Collagen Gel Mechanics by Glutaraldehyde Crosslinking. Connect. Tissue Res. 2011, 53, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Krasselt, K.; Frommelt, C.; Brunner, R.; Rauscher, F.G.; Francke, M.; Körber, N. Various cross-linking methods inhibit the collagenase I degradation of rabbit scleral tissue. BMC Ophthalmol. 2020, 20, 488. [Google Scholar] [CrossRef]
- Tripi, D.R.; Vyavahare, N.R. Neomycin and pentagalloyl glucose enhanced cross-linking for elastin and glycosaminoglycans preservation in bioprosthetic heart valves. J. Biomater. Appl. 2013, 28, 757–766. [Google Scholar] [CrossRef]
- Tam, H.; Zhang, W.; Feaver, K.R.; Parchment, N.; Sacks, M.S.; Vyavahare, N. A novel crosslinking method for improved tear resistance and biocompatibility of tissue based biomaterials. Biomaterials 2015, 66, 83–91. [Google Scholar] [CrossRef]


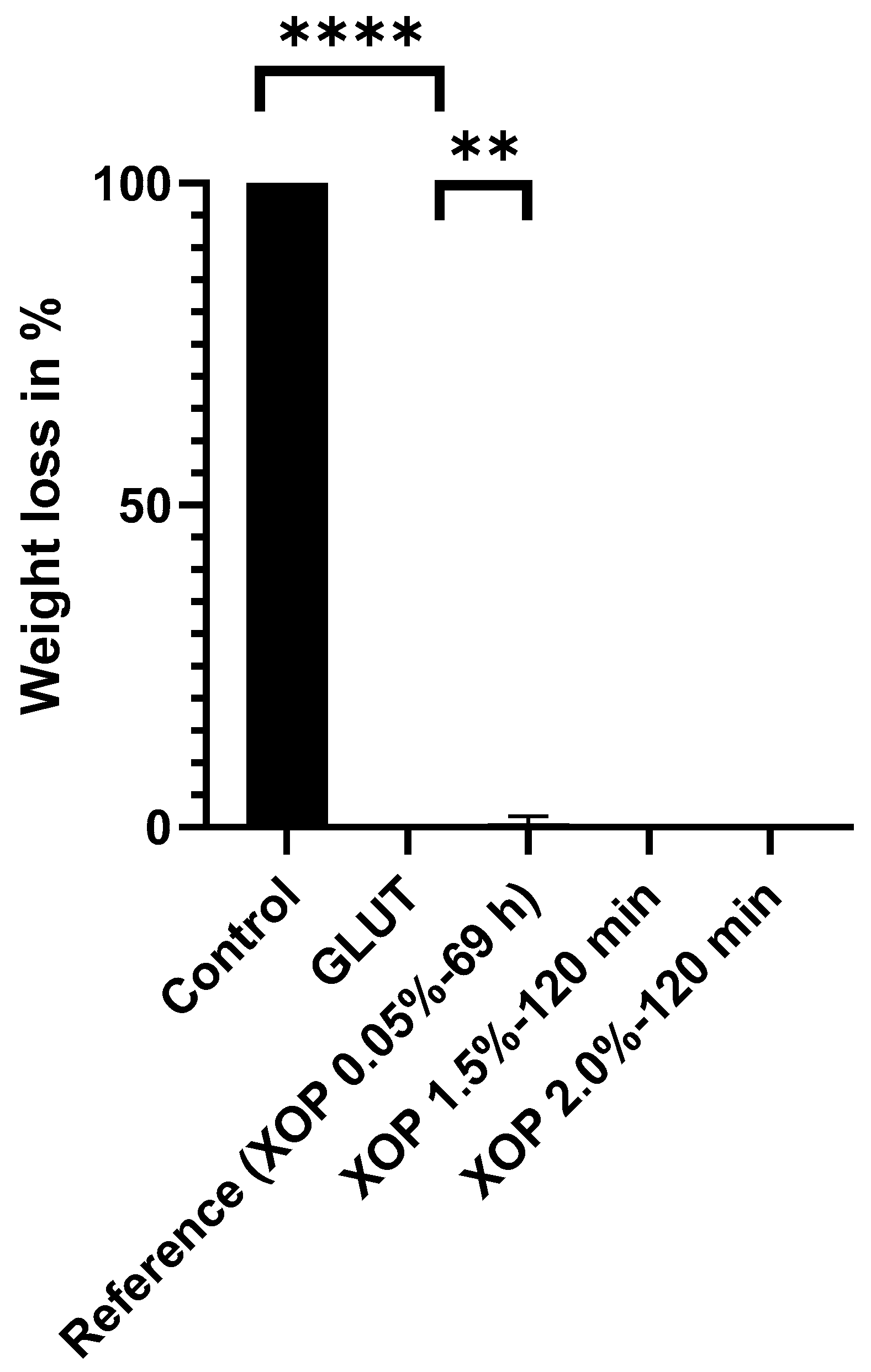


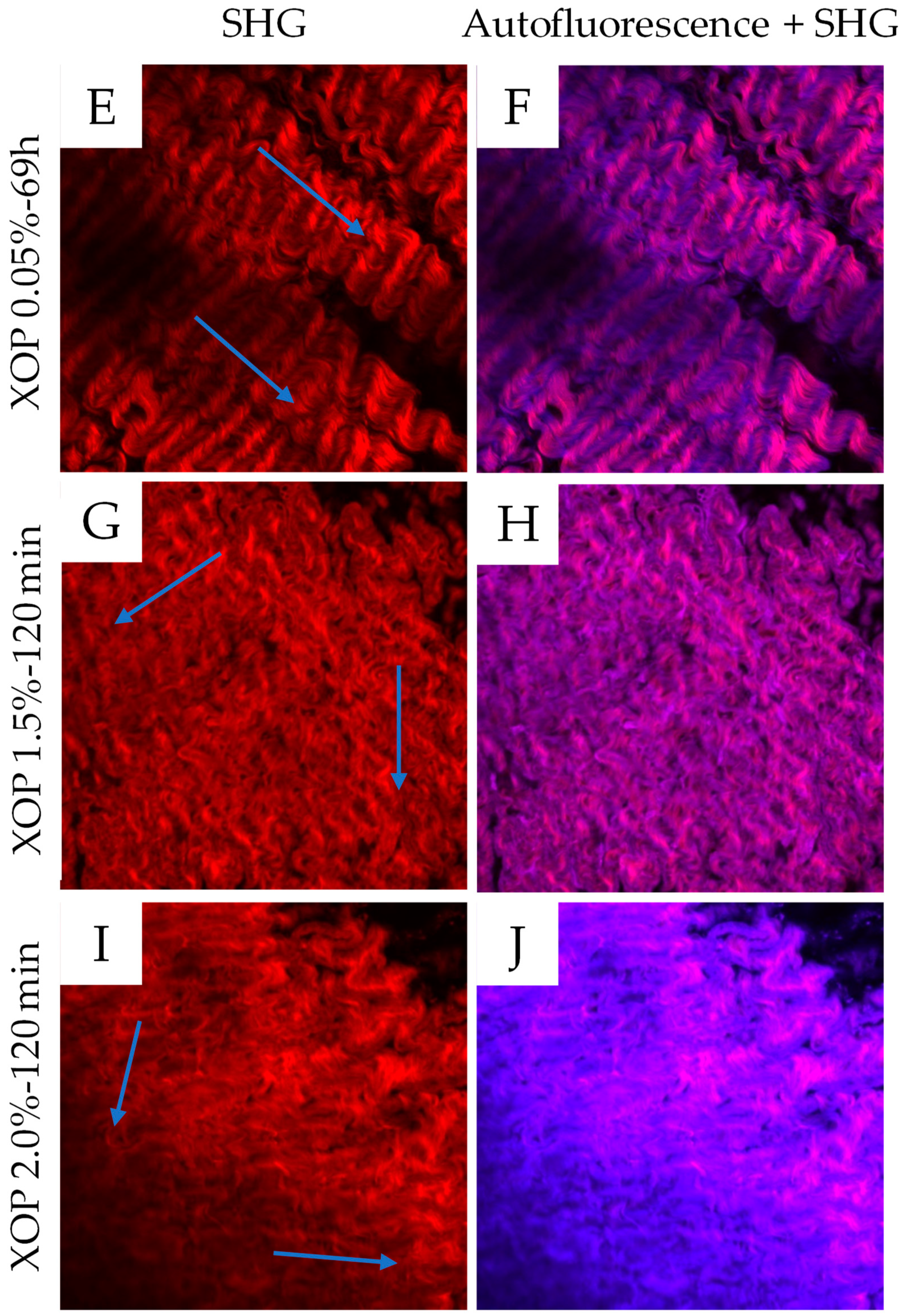
| XOP-Concentration | XOP 0.05% | XOP 0.5% | XOP 1.0% | XOP 1.5% | XOP 2.0% |
|---|---|---|---|---|---|
| Incubation-Time | 69 h | 120 min | 120 min | 120 min | 120 min |
| Biomechanical Properties | Control | GLUT | XOP 0.05%-69h | XOP 1.5%-120 min | XOP 2.0%-120 min |
|---|---|---|---|---|---|
| Thickness in mm | 0.17 ± 0.05 | 0.24 ± 0.05 | 0.19 ± 0.04 | 0.22 ± 0.01 | 0.23 ± 0.04 |
| Failure Strain in mm/mm | 0.54 ± 0.07 | 0.80 ± 0.17 | 0.78 ± 0.13 | 0.77 ± 0.13 | 0.75 ± 0.17 |
| UTS in MPa | 12.76 ± 2.93 | 9.27 ± 2.28 | 9.59 ± 1.91 | 10.00 ± 1.95 | 8.34 ± 1.51 |
| Coll-e in MPa | 40.54 ± 6.62 | 30.36 ± 10.19 | 31.96 ± 9.58 | 40.16 ± 5.61 | 29.67 ± 7.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steitz, M.; Zouhair, S.; Khan, M.B.; Breitenstein-Attach, A.; Fritsch, K.; Tuladhar, S.R.; Wulsten, D.; Wolkers, W.-F.; Sun, X.; Hao, Y.; et al. A Glutaraldehyde-Free Crosslinking Method for the Treatment of Collagen-Based Biomaterials for Clinical Application. Bioengineering 2023, 10, 1247. https://doi.org/10.3390/bioengineering10111247
Steitz M, Zouhair S, Khan MB, Breitenstein-Attach A, Fritsch K, Tuladhar SR, Wulsten D, Wolkers W-F, Sun X, Hao Y, et al. A Glutaraldehyde-Free Crosslinking Method for the Treatment of Collagen-Based Biomaterials for Clinical Application. Bioengineering. 2023; 10(11):1247. https://doi.org/10.3390/bioengineering10111247
Chicago/Turabian StyleSteitz, Marvin, Sabra Zouhair, Mahamuda Badhon Khan, Alexander Breitenstein-Attach, Katharina Fritsch, Sugat Ratna Tuladhar, Dag Wulsten, Willem-Frederik Wolkers, Xiaolin Sun, Yimeng Hao, and et al. 2023. "A Glutaraldehyde-Free Crosslinking Method for the Treatment of Collagen-Based Biomaterials for Clinical Application" Bioengineering 10, no. 11: 1247. https://doi.org/10.3390/bioengineering10111247
APA StyleSteitz, M., Zouhair, S., Khan, M. B., Breitenstein-Attach, A., Fritsch, K., Tuladhar, S. R., Wulsten, D., Wolkers, W.-F., Sun, X., Hao, Y., Emeis, J., Lange, H.-E., Berger, F., & Schmitt, B. (2023). A Glutaraldehyde-Free Crosslinking Method for the Treatment of Collagen-Based Biomaterials for Clinical Application. Bioengineering, 10(11), 1247. https://doi.org/10.3390/bioengineering10111247









