Artificial Vision: The High-Frequency Electrical Stimulation of the Blind Mouse Retina Decay Spike Generation and Electrogenically Clamped Intracellular Ca2+ at Elevated Levels
Abstract
1. Introduction
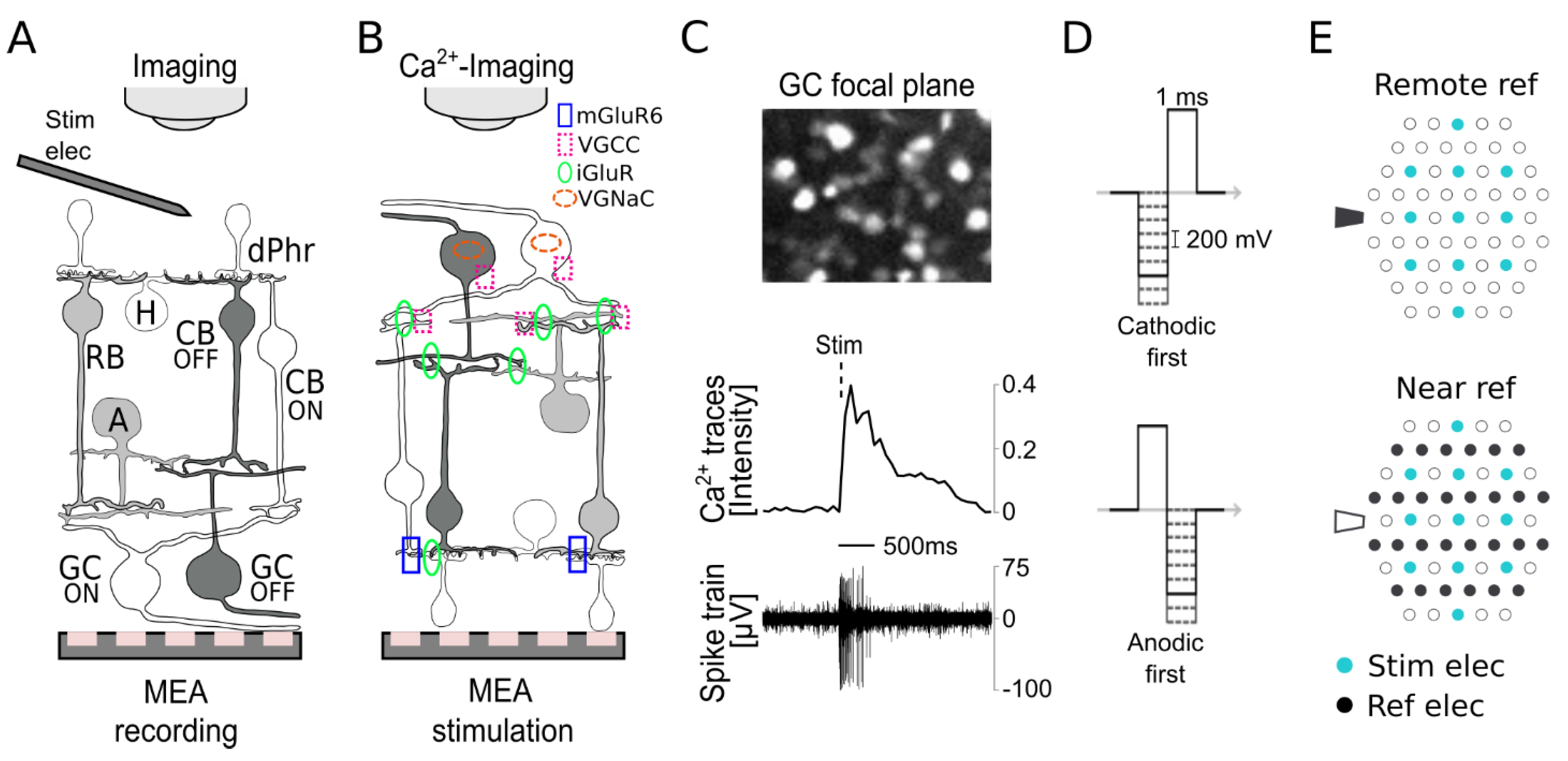
2. Materials and Methods
2.1. Animals
2.2. Tissue Preparation and Loading
2.3. Electrical Stimulation and Recording
2.4. Data Analysis
2.4.1. Ca2+ Imaging
2.4.2. MEA Recordings
2.5. Pharmacology
3. Results
3.1. Subretinal Electrical Stimulation and Ca2+ Imaging of Ganglion Cell Responses
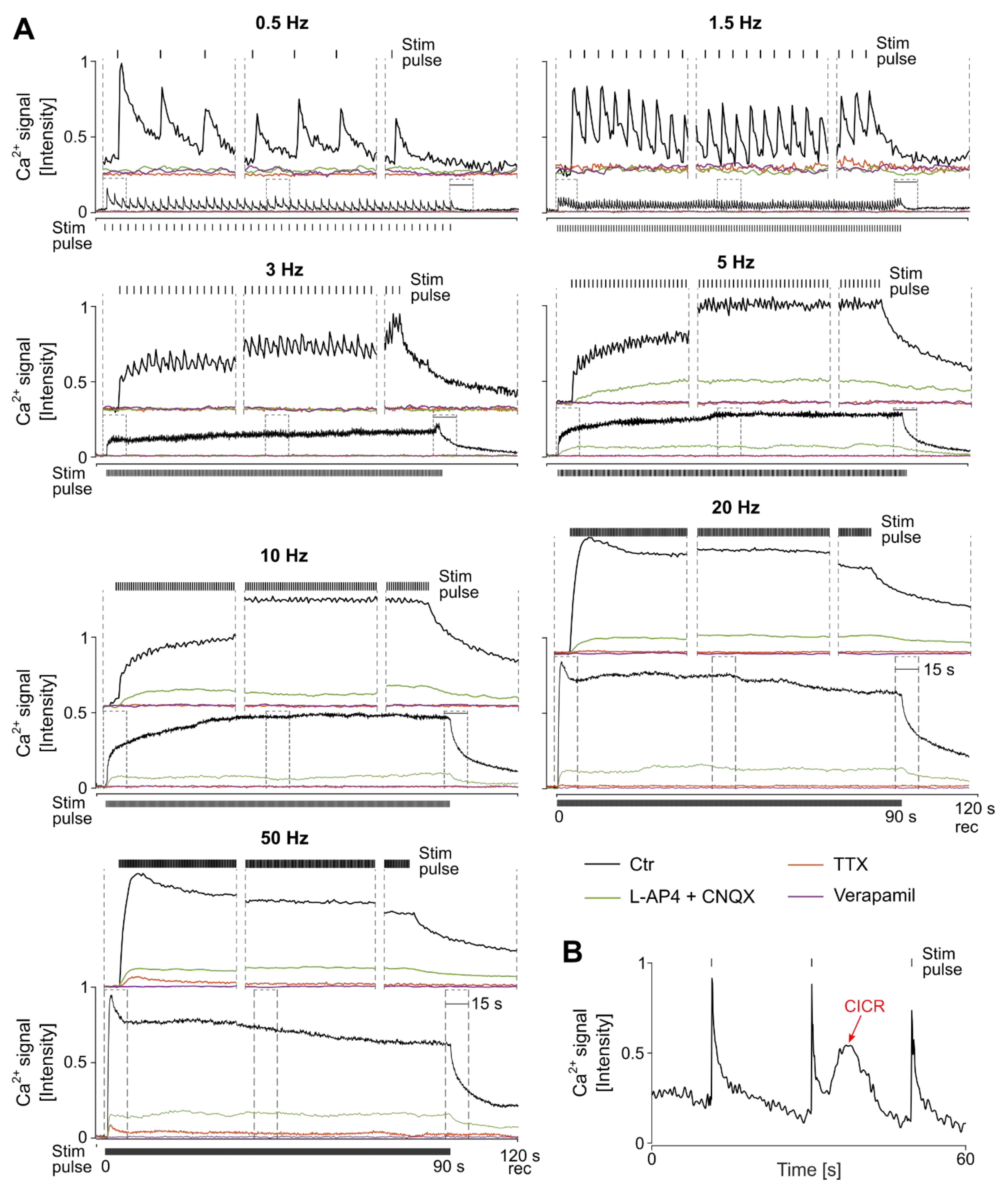
3.2. Variation in the Electrical Stimulation Paradigm Modulates the Strength of the Ganglion Cell Ca2+ Responses
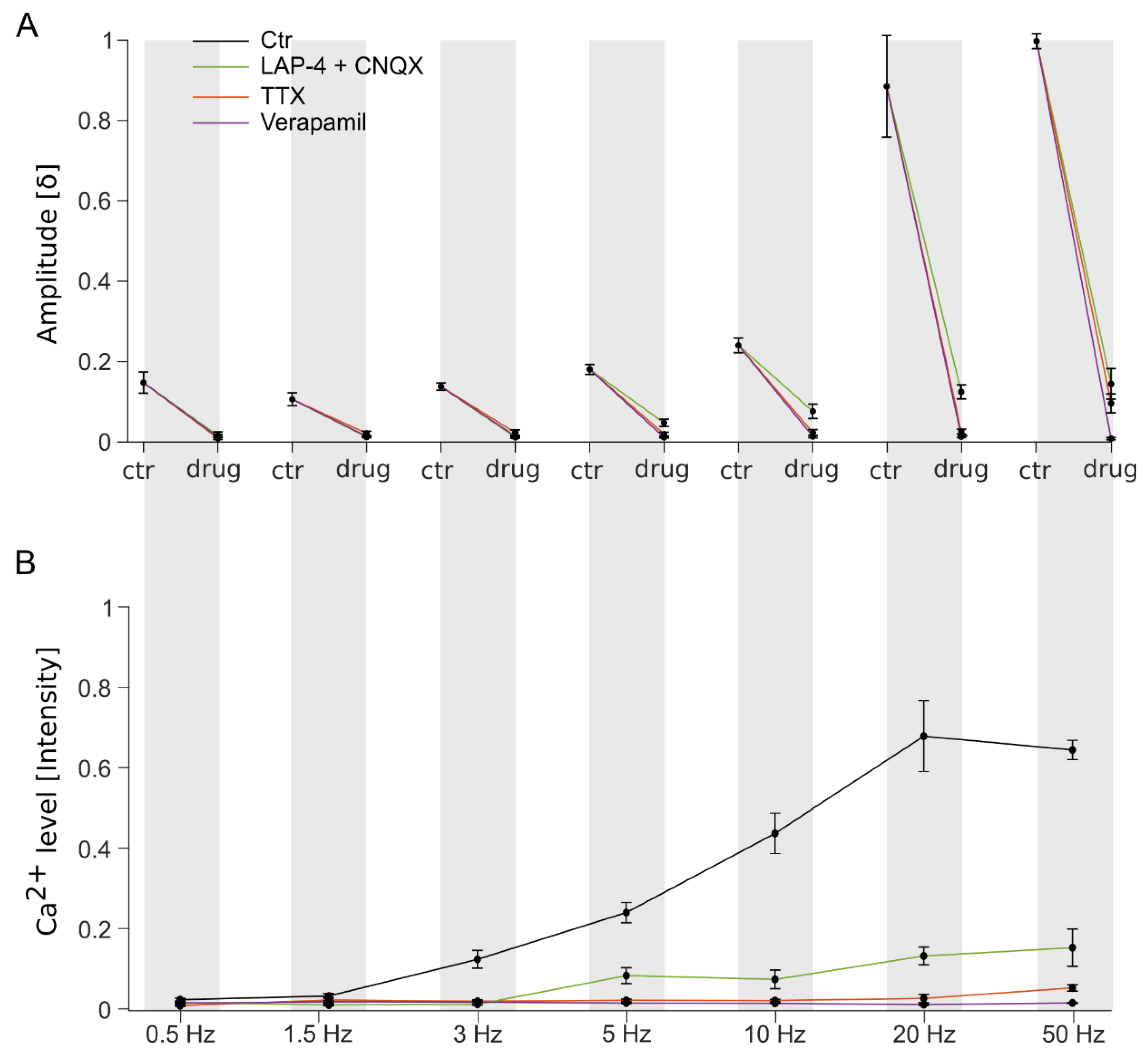
3.3. The Characteristics of Ganglion Cell Ca2+ Responses Are Modulated by Electrical Stimulation Frequencies
3.4. Stimulation Frequency-Dependent Activation of Ganglion Cells: Network-Mediated or Direct Activation
3.5. Continuous High-Frequency Electrical Stimulation Does Not Generate Pulse-Correlated Spikes in Ganglion Cells but Clamps Intracellular Ca2+ at Elevated Levels
4. Discussion
4.1. Switch from Network-Mediated to the Direct Stimulation of Ganglion Cells
4.2. High-Frequency Stimulation Electrogenically Modulates the Desensitization of the Ganglion Cells
4.3. Implications of Electrical-Stimulation-Mediated Modulation of Cellular Ca2+ Dynamics for the Development of Strategies for Electrical Implants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACSF | Artificial cerebrospinal fluid |
| BC | Bipolar cells |
| Ca2+ | Calcium |
| CB OFF | OFF-type cone bipolar cell |
| CB ON | ON-type cone bipolar cell |
| CICR | Calcium-induced calcium release |
| CNQX | 6-Cyano-7-nitroquinoxaline-2,3-dione |
| ctr | Control |
| elec | Electrode |
| ER | Endoplasmic reticulum |
| GC | Ganglion cell |
| Hz | Hertz |
| iGluR | Ionotropic glutamate receptors |
| K+ | Potassium |
| kHz | Kilo hertz |
| L-AP4 | L-2-amino-4-phosphonobutyric acid |
| MEA | Multielectrode array |
| mGluR6 | Metabotropic glutamate receptor 6 |
| mM | Milli molar |
| Na+ | Sodium |
| NCX | Sodium/calcium exchanger |
| OGB-1 | Oregon Green 488 BAPTA-1 |
| PMCA | Plasma membrane calcium ATP-pump |
| rd1 | C3H/rd1 (C3H Pde6brd1/rd1) |
| ref | Reference |
| SEM | Standard error mean |
| stim | Electrical stimulation |
| TTX | Tetrodotoxin |
| V | Volt |
| VGC | Voltage-gated channels |
| VGCC | Voltage-gated calcium channels |
| VGNaC | Voltage-gated sodium channels |
| δ | Delta (difference) |
| τ | Tau |
| µV | Microvolt |
| ∆ | Rate (change over time) |
References
- Tsang, S.H.; Sharma, T. Retinitis Pigmentosa (Non-syndromic). Adv. Exp. Med. Biol. 2018, 1085, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Ragi, S.D.; Tsang, S.H. Therapy in Rhodopsin-Mediated Autosomal Dominant Retinitis Pigmentosa. Mol. Ther. 2020, 28, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, T.B.; Luther, E.E. Retinitis Pigmentosa; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Van Hook, M.J.; Nawy, S.; Thoreson, W.B. Voltage- and calcium-gated ion channels of neurons in the vertebrate retina. Prog. Retin. Eye Res. 2019, 72, 100760. [Google Scholar] [CrossRef] [PubMed]
- Farnum, A.; Pelled, G. New Vision for Visual Prostheses. Front. Neurosci. 2020, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, K.; Loudin, J.; Goetz, G.; Huie, P.; Wang, L.; Kamins, T.I.; Galambos, L.; Smith, R.; Harris, J.S.; Sher, A.; et al. Photovoltaic Retinal Prosthesis with High Pixel Density. Nat. Photonics 2012, 6, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Lorach, H.; Goetz, G.; Smith, R.; Lei, X.; Mandel, Y.; Kamins, T.; Mathieson, K.; Huie, P.; Harris, J.; Sher, A.; et al. Photovoltaic restoration of sight with high visual acuity. Nat. Med. 2015, 21, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Weiland, J.D.; Roska, B.; Humayun, M.S. Retinal stimulation strategies to restore vision: Fundamentals and systems. Prog. Retin. Eye Res. 2016, 53, 21–47. [Google Scholar] [CrossRef]
- Stingl, K.; Schippert, R.; Bartz-Schmidt, K.U.; Besch, D.; Cottriall, C.L.; Edwards, T.L.; Gekeler, F.; Greppmaier, U.; Kiel, K.; Koitschev, A.; et al. Interim Results of a Multicenter Trial with the New Electronic Subretinal Implant Alpha AMS in 15 Patients Blind from Inherited Retinal Degenerations. Front. Neurosci. 2017, 11, 445. [Google Scholar] [CrossRef]
- Humayun, M.S.; Dorn, J.D.; da Cruz, L.; Dagnelie, G.; Sahel, J.A.; Stanga, P.E.; Cideciyan, A.V.; Duncan, J.L.; Eliott, D.; Filley, E.; et al. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology 2012, 119, 779–788. [Google Scholar] [CrossRef]
- da Cruz, L.; Coley, B.F.; Dorn, J.; Merlini, F.; Filley, E.; Christopher, P.; Chen, F.K.; Wuyyuru, V.; Sahel, J.; Stanga, P.; et al. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br. J. Ophthalmol. 2013, 97, 632–636. [Google Scholar] [CrossRef]
- Lowery, A.J.; Rosenfeld, J.V.; Lewis, P.M.; Browne, D.; Mohan, A.; Brunton, E.; Yan, E.; Maller, J.; Mann, C.; Rajan, R.; et al. Restoration of vision using wireless cortical implants: The Monash Vision Group project. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 2015, pp. 1041–1044. [Google Scholar] [CrossRef]
- Pouratian, N.; Yoshor, D.; Niketeghad, S.; Dornm, J.; Greenberg, R. Early Feasibility Study of a Neurostimulator to Create Artificial Vision. Neurosurgery 2019, 66, 310-146. [Google Scholar] [CrossRef]
- Fernandez, E.; Alfaro, A.; Soto-Sanchez, C.; Gonzalez-Lopez, P.; Lozano, A.M.; Pena, S.; Grima, M.D.; Rodil, A.; Gomez, B.; Chen, X.; et al. Visual percepts evoked with an intracortical 96-channel microelectrode array inserted in human occipital cortex. J. Clin. Investig. 2021, 131, e151331. [Google Scholar] [CrossRef] [PubMed]
- Stingl, K.; Bartz-Schmidt, K.U.; Besch, D.; Chee, C.K.; Cottriall, C.L.; Gekeler, F.; Groppe, M.; Jackson, T.L.; MacLaren, R.E.; Koitschev, A.; et al. Subretinal Visual Implant Alpha IMS—Clinical trial interim report. Vis. Res. 2015, 111, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, G.; Christopher, P.; Arditi, A.; da Cruz, L.; Duncan, J.L.; Ho, A.C.; Olmos de Koo, L.C.; Sahel, J.A.; Stanga, P.E.; Thumann, G.; et al. Performance of real-world functional vision tasks by blind subjects improves after implantation with the Argus(R) II retinal prosthesis system. Clin. Exp. Ophthalmol. 2017, 45, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Chaumette, C. New rehabilitation strategies for patients with Argus II retinal implants. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4573. [Google Scholar]
- Farvardin, M.; Afarid, M.; Attarzadeh, A.; Johari, M.K.; Mehryar, M.; Nowroozzadeh, M.H.; Rahat, F.; Peyvandi, H.; Farvardin, R.; Nami, M. The Argus-II Retinal Prosthesis Implantation; From the Global to Local Successful Experience. Front. Neurosci. 2018, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Cehajic Kapetanovic, J.; Troelenberg, N.; Edwards, T.L.; Xue, K.; Ramsden, J.D.; Stett, A.; Zrenner, E.; MacLaren, R.E. Highest reported visual acuity after electronic retinal implantation. Acta Ophthalmol. 2020, 98, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Wilke, R.G.H.; Moghadam, G.K.; Lovell, N.H.; Suaning, G.J.; Dokos, S. Electric crosstalk impairs spatial resolution of multi-electrode arrays in retinal implants. J. Neural Eng. 2011, 8, 046016. [Google Scholar] [CrossRef]
- Fornos, A.P.; Sommerhalder, J.; da Cruz, L.; Sahel, J.A.; Mohand-Said, S.; Hafezi, F.; Pelizzone, M. Temporal Properties of Visual Perception on Electrical Stimulation of the Retina. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2720–2731. [Google Scholar] [CrossRef][Green Version]
- Jensen, R.J.; Rizzo, J.F., III. Responses of ganglion cells to repetitive electrical stimulation of the retina. J. Neural Eng. 2007, 4, S1. [Google Scholar] [CrossRef]
- Freeman, D.K.; Fried, S.I. Multiple Components of Ganglion Cell Desensitization in Response to Prosthetic Stimulation. J. Neural Eng. 2011, 8, 016008. [Google Scholar] [CrossRef]
- Weiland, J.D.; Walston, S.T.; Humayun, M.S. Electrical Stimulation of the Retina to Produce Artificial Vision. Annu. Rev. Vis. Sci. 2016, 2, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Haq, W.; Basavaraju, S.; Speck, A.; Zrenner, E. Nature-inspired saccadic-like electrical stimulation paradigm promotes sustained retinal ganglion cell responses by spatiotemporally alternating activation of contiguous multi-electrode patterns. J. Neural Eng. 2022, 19, 055001. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, N.; Kilgore, K.L. Direct current electrical conduction block of peripheral nerve. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.K.; Jeng, J.S.; Kelly, S.K.; Hartveit, E.; Fried, S.I. Calcium channel dynamics limit synaptic release in response to prosthetic stimulation with sinusoidal waveforms. J. Neural Eng. 2011, 8, 046005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kameneva, T.; Maturana, M.I.; Hadjinicolaou, A.E.; Cloherty, S.L.; Ibbotson, M.R.; Grayden, D.B.; Burkitt, A.N.; Meffin, H. Retinal ganglion cells: Mechanisms underlying depolarization block and differential responses to high frequency electrical stimulation of ON and OFF cells. J. Neural Eng. 2016, 13, 016017. [Google Scholar] [CrossRef] [PubMed]
- Haq, W.; Dietter, J.; Zrenner, E. Electrical activation of degenerated photoreceptors in blind mouse retina elicited network-mediated responses in different types of ganglion cells. Sci. Rep. 2018, 8, 16998. [Google Scholar] [CrossRef] [PubMed]
- Keeler, C.E. The Inheritance of a Retinal Abnormality in White Mice. Proc. Natl. Acad. Sci. USA 1924, 10, 329–333. [Google Scholar] [CrossRef]
- Briggman, K.L.; Euler, T. Bulk electroporation and population calcium imaging in the adult mammalian retina. J. Neurophysiol. 2011, 105, 2601–2609. [Google Scholar] [CrossRef]
- Baden, T.; Berens, P.; Franke, K.; Roman Roson, M.; Bethge, M.; Euler, T. The functional diversity of retinal ganglion cells in the mouse. Nature 2016, 529, 345–350. [Google Scholar] [CrossRef]
- Gleichmann, M.; Mattson, M.P. Neuronal calcium homeostasis and dysregulation. Antioxid. Redox Signal. 2011, 14, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.M.; Miller, M.W.; Brayman, A.A.; Carstensen, E.L. Electric field-induced inhibition of electrogenic pumping in Nitella: Analysis for transmembrane potential hypo- and hyperpolarization effects. Environ. Exp. Bot. 1993, 33, 209–223. [Google Scholar] [CrossRef]
- Beurrier, C.; Bioulac, B.; Audin, J.; Hammond, C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J. Neurophysiol. 2001, 85, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- von Gersdorff, H.; Matthews, G. Calcium-dependent inactivation of calcium current in synaptic terminals of retinal bipolar neurons. J. Neurosci. 1996, 16, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Borde, M.; Bonansco, C.; Fernandez de Sevilla, D.; Le Ray, D.; Buno, W. Voltage-clamp analysis of the potentiation of the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. Hippocampus 2000, 10, 198–206. [Google Scholar] [CrossRef]
- Sah, P.; Davies, P. Calcium-activated potassium currents in mammalian neurons. Clin. Exp. Pharmacol. Physiol. 2000, 27, 657–663. [Google Scholar] [CrossRef]
- Kovacs-Oller, T.; Szarka, G.; Ganczer, A.; Tengolics, A.; Balogh, B.; Volgyi, B. Expression of Ca2+-Binding Buffer Proteins in the Human and Mouse Retinal Neurons. Int. J. Mol. Sci. 2019, 20, 2229. [Google Scholar] [CrossRef]
- Mattson, M.P.; LaFerla, F.M.; Chan, S.L.; Leissring, M.A.; Shepel, P.N.; Geiger, J.D. Calcium signaling in the ER: Its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000, 23, 222–229. [Google Scholar] [CrossRef]
- Hajieva, P.; Baeken, M.W.; Moosmann, B. The role of Plasma Membrane Calcium ATPases (PMCAs) in neurodegenerative disorders. Neurosci. Lett. 2018, 663, 29–38. [Google Scholar] [CrossRef]
- Lee, J.I.; Werginz, P.; Fried, S.I. Variability in Depolarization Sensitivity Underlies Differential Responses to High-frequency Stimulation of ON and OFF RGCs. In Proceedings of the 2023 11th International IEEE/EMBS Conference on Neural Engineering (NER), Baltimore, MD, USA, 24–27 April 2023; pp. 1–4. [Google Scholar]
- Borde, M.; Cazalets, J.R.; Buno, W. Activity-dependent response depression in rat hippocampal CA1 pyramidal neurons in vitro. J. Neurophysiol. 1995, 74, 1714–1729. [Google Scholar] [CrossRef] [PubMed]
- Borde, M.; Bonansco, C.; Buno, W. The activity-dependent potentiation of the slow Ca2+-activated K+ current regulates synaptic efficacy in rat CA1 pyramidal neurons. Pflugers Arch. 1999, 437, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Sekirnjak, C.; Hottowy, P.; Sher, A.; Dabrowski, W.; Litke, A.M.; Chichilnisky, E.J. Electrical stimulation of mammalian retinal ganglion cells with multielectrode arrays. J. Neurophysiol. 2006, 95, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Theis, L.; Berens, P.; Froudarakis, E.; Reimer, J.; Rosón, M.R.; Baden, T.; Euler, T.; Tolias, A.S.; Bethge, M. Supervised learning sets benchmark for robust spike rate inference from calcium imaging signals. bioRxiv 2015, arXiv:1503.00135. [Google Scholar]
- Pachitariu, M.; Stringer, C.; Harris, K.D. Robustness of Spike Deconvolution for Neuronal Calcium Imaging. J. Neurosci. 2018, 38, 7976–7985. [Google Scholar] [CrossRef] [PubMed]
- Fleming, W.; Jewell, S.; Engelhard, B.; Witten, D.M.; Witten, I.B. Inferring spikes from calcium imaging in dopamine neurons. PLoS ONE 2021, 16, e0252345. [Google Scholar] [CrossRef] [PubMed]
- Werginz, P.; Benav, H.; Zrenner, E.; Rattay, F. Modeling the response of ON and OFF retinal bipolar cells during electric stimulation. Vision Res. 2015, 111, 170–181. [Google Scholar] [CrossRef]
- Rattay, F.; Bassereh, H.; Stiennon, I. Compartment models for the electrical stimulation of retinal bipolar cells. PLoS ONE 2018, 13, e0209123. [Google Scholar] [CrossRef]
- Werginz, P.; Rattay, F. The impact of calcium current reversal on neurotransmitter release in the electrically stimulated retina. J. Neural Eng. 2016, 13, 046013. [Google Scholar] [CrossRef]
- Jensen, R.J.; Rizzo, J.F., 3rd. Activation of ganglion cells in wild-type and rd1 mouse retinas with monophasic and biphasic current pulses. J. Neural Eng. 2009, 6, 035004. [Google Scholar] [CrossRef]
- Sekhar, S.; Jalligampala, A.; Zrenner, E.; Rathbun, D.L. Tickling the retina: Integration of subthreshold electrical pulses can activate retinal neurons. J. Neural Eng. 2016, 13, 046004. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Haji Ghaffari, D.; Misra, R.; Weiland, J.D. Retinal ganglion cell desensitization is mitigated by varying parameter constant excitation pulse trains. Front. Cell. Neurosci. 2022, 16, 897146. [Google Scholar] [CrossRef] [PubMed]
- Chenais, N.A.L.; Airaghi Leccardi, M.J.I.; Ghezzi, D. Naturalistic spatiotemporal modulation of epiretinal stimulation increases the response persistence of retinal ganglion cell. J. Neural Eng. 2021, 18, 016016. [Google Scholar] [CrossRef] [PubMed]
- Power, M.; Das, S.; Schutze, K.; Marigo, V.; Ekstrom, P.; Paquet-Durand, F. Cellular mechanisms of hereditary photoreceptor degeneration—Focus on cGMP. Prog. Retin. Eye Res. 2020, 74, 100772. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peiroten, L.; Zrenner, E.; Haq, W. Artificial Vision: The High-Frequency Electrical Stimulation of the Blind Mouse Retina Decay Spike Generation and Electrogenically Clamped Intracellular Ca2+ at Elevated Levels. Bioengineering 2023, 10, 1208. https://doi.org/10.3390/bioengineering10101208
Peiroten L, Zrenner E, Haq W. Artificial Vision: The High-Frequency Electrical Stimulation of the Blind Mouse Retina Decay Spike Generation and Electrogenically Clamped Intracellular Ca2+ at Elevated Levels. Bioengineering. 2023; 10(10):1208. https://doi.org/10.3390/bioengineering10101208
Chicago/Turabian StylePeiroten, Lucia, Eberhart Zrenner, and Wadood Haq. 2023. "Artificial Vision: The High-Frequency Electrical Stimulation of the Blind Mouse Retina Decay Spike Generation and Electrogenically Clamped Intracellular Ca2+ at Elevated Levels" Bioengineering 10, no. 10: 1208. https://doi.org/10.3390/bioengineering10101208
APA StylePeiroten, L., Zrenner, E., & Haq, W. (2023). Artificial Vision: The High-Frequency Electrical Stimulation of the Blind Mouse Retina Decay Spike Generation and Electrogenically Clamped Intracellular Ca2+ at Elevated Levels. Bioengineering, 10(10), 1208. https://doi.org/10.3390/bioengineering10101208





