Potential of Porous Substrate Bioreactors for Removal of Pollutants from Wastewater Using Microalgae
Abstract
:1. Introduction
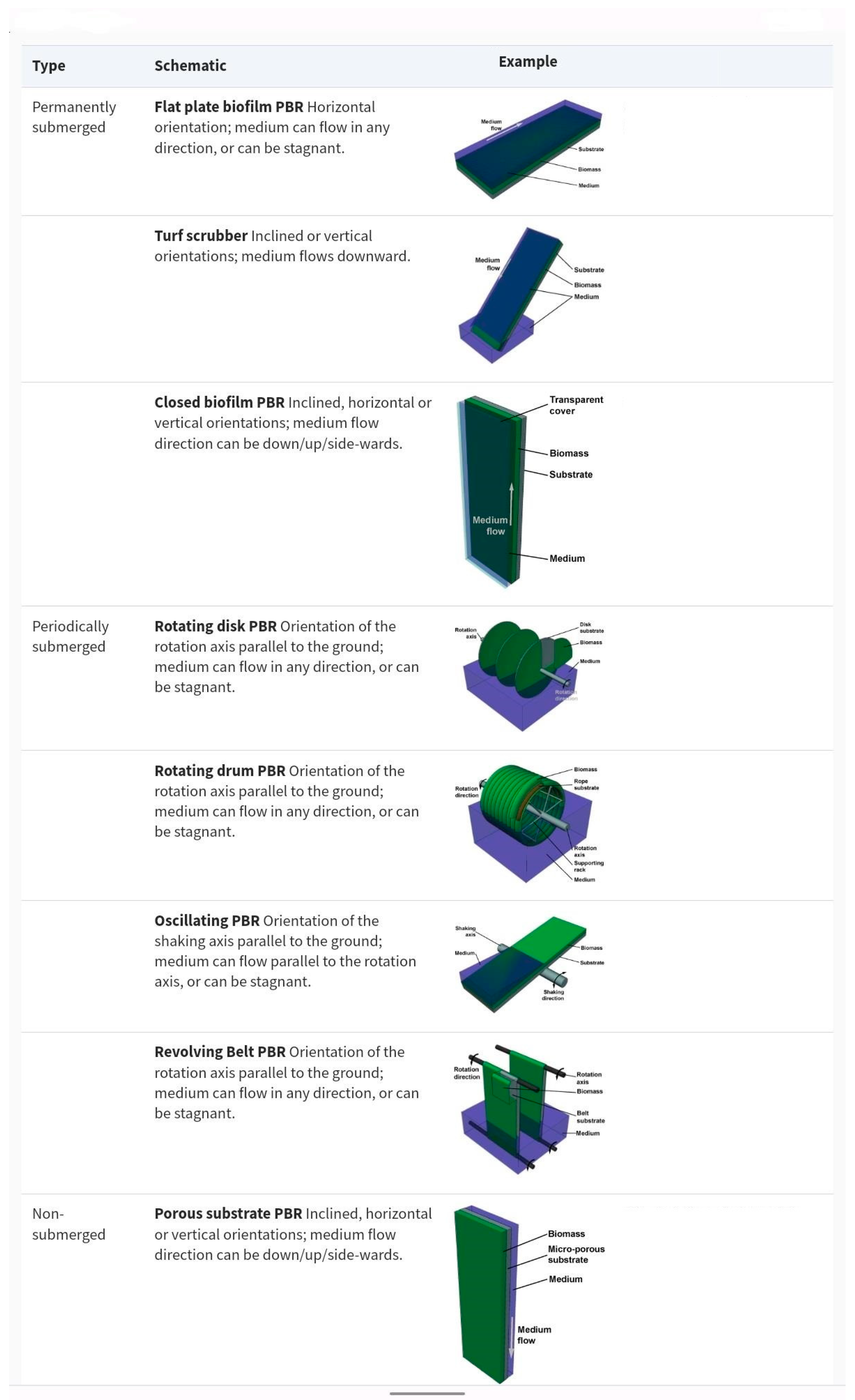
2. General Facts about the Use of PSBRs in the Removal of Pollutants from Wastewater
3. Removal of Pollutants from WW
| Ammonium | Phosphorus | |||||||
|---|---|---|---|---|---|---|---|---|
| Microalgae | Medium/ Substrate | Initial Value mg L−1 | Final Value mg L−1 | Final Removal Efficiency % | Initial Value mg L−1 | Final Value mg L−1 | Final Removal Efficiency % | References |
| Scenedesmus (Halochlorella) rubescens | SWW | 20 | 1.16 | 94 | 3 | 0.2 | 93 | Shi et al. [37] |
| Chlorella vulgaris | SWW | 20 | 0.87 | 96 | 3 | 0.1 | 96 | Shi et al. [37] |
| Desmodesmus abundans | Human urine | 5.76 | 4.8 | 13 | 0.29 | 0.02 | 94 | Piltz and Melkonian [52] |
| Chlorella pyrenoidosa | SWIW | 409 | 98 | 76 | 35 | 10 | 71 | Cheng et al. [43] |
| Scenedesmus sp. | SYSWIW + antibiotics | 50 | 9 | 83 | / | / | / | Cheng et al. [53] |
| Chlorella sp. | ADSW-5D | 134 | 2.67 | 94 | 6.65 | 1.05 | 84.3 | Cheng et al. [54] |
| Scenedesmus sp. | RWW | 47.04 | 12 | 85 | 5.08 | 1.88 | 97 | Saleem et al. [55] |
| Scenedesmus sp. | WTWW | 25 | 0 | 80 | 1.70 | 0.3 | 83 | Saleem et al. [55] |
| Tetradesmus obliquus | PWW | 110 | 0 | 98 | 12.56 | 1 | 27 | Sohail et al. [56] |
| Tetradesmus obliquus | AD | 104 | 0 | 90 | 10.61 | 0.5 | 16 | Sohail et al. [56] |
| Tetradesmus obliquus | SWL | 104 | 0 | 93 | 16.93 | 9 | 2.4 | Sohail et al. [56] |
| Tetradesmus obliquus | MWW | 47 | 0.3 | 97 | 9.07 | 0 | 100 | Sohail et al. [56] |
| P. maculatum | PRNM | 14.02 | 0.29 | 97 | 8.84 | 1.5 | 97 | Meril et al. [57] |
| P. maculatum | PP | 14.02 | 3 | 78 | 8.84 | 2.77 | 69 | Meril et al. [57] |
| P.maculatum | NM | 14.02 | 2 | 85 | 8.84 | 2 | 77 | Meril et al. [57] |
| Nitrate | COD | |||||||
| Scenedesmus (Halochlorella) rubescens | SWW | 3 | 0.2 | 96 | / | / | / | Shi et al. [37] |
| Chlorella vulgaris | SWW | 3 | 0.2 | 96 | / | / | / | Shi et al. [37] |
| Scenedesmus (Halochlorella) rubescens | MWW | 6.2 | 0.11 | 98 | / | / | / | Shi et al. [37] |
| Chlorella vulgaris | MWW | 6.2 | 0.22 | 96 | / | / | / | Shi et al. [37] |
| Chlorella pyrenoidosa | SWIW | / | / | / | 601 | 152 | 74 | Cheng et al. [43] |
| Chlorella sp. | ADSW-5D | 14.3 | 2.29 | 85.5 | 116 | 16.12 | 86.8 | Cheng et al. [54] |
| Scenedesmus sp. | RWW | 0.0287 | 0.0 | 100 | 458 | 350 | 25 | Saleem et al. [55] |
| Scenedesmus sp. | WTWW | 1.73 | 0.7 | 70 | 296 | 180 | 40 | Saleem et al. [55] |
| P. maculatum | PRNM | 6.09 | 0.45 | 92 | / | / | / | Meril et al. [57] |
| P. maculatum | PP | 6.09 | 1.45 | 0.76 | / | / | / | Meril et al. [57] |
| P. maculatum | NM | 6.09 | 0.8 | 86 | / | / | / | Meril et al. [57] |

4. Removal of Heavy Metals
5. Prototype and Pilot Scale PSBRs for WW Treatment
6. Utilization of Microalgal Biomass after WW Treatment
7. Outlook and Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Sustainability: Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef]
- van Vliet, M.T.H.; Jones, E.R.; Flörke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global water scarcity including surface water quality and expansions of clean water technologies. Environ. Res. Lett. 2021, 16, 24020. [Google Scholar] [CrossRef]
- Falkenmark, M.; Rockström, J. The new blue and green water paradigm: Breaking new ground for water resources planning and management. J. Water Res. Plan. Manag. 2006, 132, 129–132. [Google Scholar] [CrossRef]
- Hightower, M.; Pierce, S. The energy challenge. Nature 2008, 452, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. Available online: https://www.science.org/doi/full/10.1126/science.1185383 (accessed on 27 September 2023). [CrossRef] [PubMed]
- Choudhary, M.; Peter, C.N.; Shukla, S.K.; Govender, P.P.; Joshi, G.M.; Wang, R. Environmental issues: A challenge for wastewater treatment. In Green Materials for Wastewater Treatment. Environmental Chemistry for a Sustainable World; Naushad, M., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; Volume 38. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef] [PubMed]
- Jetten, M.S.M.; Horn, S.J.; Van Loosdrecht, M.C.M. Towards a more sustainable municipal wastewater treatment system. Water Sci. Technol. 1997, 35, 171–180. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Liu, J.; Pemberton, B.; Lewis, J.; Scales, P.J.; Martin, G.J.O. Wastewater treatment using filamentous algae—A review. Bioresour. Technol. 2020, 298, 22556. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. Microalgae cultivation in wastewater. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Muñoz, R., Gonzalez-Fernandez, C., Eds.; Woodhead Publishing Elsevier: Duxford, UK, 2017; pp. 67–91. [Google Scholar] [CrossRef]
- Plöhn, M.; Spain, O.; Sirin, S.; Silva, M.; Escudero-Oñate, C.; Ferrando-Climent, L.; Allahverdiyeva, Y.; Funk, C. Wastewater treatment by microalgae. Physiol. Plantarum. 2021, 173, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W.J.; Gotaas, M. Photosynthesis in sewage. Treat. Transact. 1957, 12, 2–73. [Google Scholar] [CrossRef]
- Young, P.; Taylor, M.; Fallowfield, H.J. Mini-review: High-rate algal ponds, flexible systems for sustainable wastewater treatment. World J. Microbiol. Biotechnol. 2017, 33, 117. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Ekelhof, A.; Herrero, N.; Siles, J.Á.; Podola, B.; Chica, A.F.; Ángeles Martín, M.; Melkonian, M.; Izquierdo, C.G.; Gómez, J.M. Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production. Water Sci. Technol. 2021, 82, 1044–1061. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.L.; Li, M.; Hao Ngo, H. Non-suspended microalgae cultivation for wastewater refinery and biomass production. Bioresour. Technol. 2020, 308, 123320. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tan, Y.; Zhu, L.; Zhou, C.; Yan, X.; Xu, Q.; Ruan, R.; Cheng, P. The intrinsic characteristics of microalgae biofilm and their potential applications in pollutants removal—A review. Algal Res. 2022, 68, 102849. [Google Scholar] [CrossRef]
- Saini, S.; Tewari, S.; Dwivedi, J.; Sharma, V. Biofilm-mediated wastewater treatment: A comprehensive review. Mater. Adv. 2023, 4, 1415–1443. Available online: https://pubs.rsc.org/en/content/articlehtml/2023/ma/d2ma00945e. (accessed on 27 September 2023). [CrossRef]
- Naumann, T.; Çebi, Z.; Podola, B.; Melkonian, M. Growing microalgae as aquaculture feeds on twin-layers: A novel solid-state photobioreactor. J. Appl. Phycol. 2013, 25, 1413–1420. [Google Scholar] [CrossRef]
- Podola, B.; Li, T.; Melkonian, M. Porous Substrate Bioreactors: A paradigm shift in microalgal biotechnology? Trends Biotechnol. 2017, 35, 121–132. [Google Scholar] [CrossRef]
- Carbone, D.A.; Olivieri, G.; Pollio, A.; Gabriele; Melkonian, M. Growth and biomass productivity of Scenedesmus vacuolatus on a twin layer system and a comparison with other types of cultivations. Appl. Microbiol. Biotechnol. 2017, 101, 8321–8329. [Google Scholar] [CrossRef]
- Miranda, A.F.; Ramkumar, N.; Andriotis, C.; Höltkemeier, T.; Yasmin, A.; Rochfort, S.; Wlodkowic, D.; Morrison, P.; Roddick, F.; Spangenberg, G.; et al. Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol. Biofuels 2017, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, T.Y.; Dao, G.H.; Xu, X.Q.; Wang, X.X.; Hu, H.Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Mallick, N. Biotechnological potential of immobilized algae for wastewater N, P and metal removal: A review. BioMetals 2002, 15, 377–390. [Google Scholar] [CrossRef]
- Shaker, S.; Nemati, A.; Montazeri-Najafabady, N.; Mobasher, M.A.; Morowvat, M.H.; Ghasemi, Y. Treating urban wastewater: Nutrient removal by using immobilized green algae in batch cultures. Int. J. Phytoremed. 2015, 17, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Güereca, D.A.; Sánchez-Saavedra, M.P. Growth and phosphorus removal by Synechococcus elongatus co-immobilized in alginate beads with Azospirillum brasilense. J. Appl. Phycol. 2016, 28, 1501–1507. [Google Scholar] [CrossRef]
- Emparan, Q.; Jye, Y.S.; Danquah, M.K.; Harun, R. Cultivation of Nannochloropsis sp. microalgae in palm oil mill effluent (POME) media for phycoremediation and biomass production: Effect of microalgae cells with and without beads. J. Water Proc. Eng. 2020, 33, 101043. [Google Scholar] [CrossRef]
- Kesaano, M.; Sims, R.C. Algal biofilm based technology for wastewater treatment. Algal Res. 2014, 5, 231–240. [Google Scholar] [CrossRef]
- Hu, Y.; Xiao, Y.; Liao, K.; Leng, Y.; Lu, Q. Development of microalgal biofilm for wastewater remediation: From mechanism to practical application. J. Chem. Technol. Biotechnol. 2021, 96, 2993–3008. [Google Scholar] [CrossRef]
- Craggs, R.L. Wastewater treatment by algal turf scrubbing. Water Sci. Technol. 2001, 44, 427–433. [Google Scholar] [CrossRef]
- Hassard, F.; Biddle, J.; Cartmell, E.; Stephenson, T. Mesh rotating reactors for biofilm pre-treatment of wastewaters—Influence of media type on microbial activity, viability and performance. Proc. Saf. Environ. Prot. 2016, 103, 69–75. [Google Scholar] [CrossRef]
- Li, T.; Strous, M.; Melkonian, M. Biofilm-based photobioreactors: Their design and improving productivity through efficient supply of dissolved inorganic carbon. FEMS Microbiol. Lett. 2017, 24, fnx218. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.E.; Berberoglu, H. Flux balancing of light and nutrients in a biofilm photobioreactor for maximizing photosynthetic productivity. Biotechnol. Prog. 2014, 30, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Podola, B.; Melkonian, M. A long-term operating algal biosensor for the rapid detection of volatile toxic compounds. J. Appl. Phycol. 2003, 15, 415–424. [Google Scholar] [CrossRef]
- Nowack, E.C.M.; Podola, B.; Melkonian, M. The 96-well twin-layer system: A novel approach in the cultivation of microalgae. Protist 2005, 156, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Podola, B.; Melkonian, M. Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: An experimental study. J. Appl. Phycol. 2007, 19, 417–423. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Hu, Q.; Cheng, P.; Ji, B.; Liu, J.; Chen, Y.; Zhang, W.; Chen, X.; Chen, L.; et al. Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresour. Technol. 2013, 127, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.L.; Yu, D.; Zhang, J.; Liu, F.F.; Wu, Y.H.; Zhang, T.Y.; Dao, G.-H.; Hu, H.Y. The characteristics and influencing factors of the attached microalgae cultivation: A review. Renew. Sustain. Energy Rev. 2018, 94, 1110–1119. [Google Scholar] [CrossRef]
- Gross, M.; Henry, W.; Michael, C.; Wen, Z. Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour. Technol. 2013, 150, 195–201. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Liu, T. Biofilm based attached cultivation technology for microalgal biorefineries—A review. Bioresour. Technol. 2017, 244, 1245–1253. [Google Scholar] [CrossRef]
- Murshid, S.; Antonysamy, A.; Dhakshinamoorthy, G.; Jayaseelan, A.; Arivalagan, P. A review on biofilm-based reactors for wastewater treatment: Recent advancements in biofilm carriers, kinetics, reactors, economics, and future perspectives. Sci. Total Environ. 2023, 892, 164796. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Liu, T.; Liu, D. Biofilm attached cultivation of Chlorella pyrenoidosa is a developed system for swine wastewater treatment and lipid production. Front. Plant Sci. 2017, 8, 1594. [Google Scholar] [CrossRef] [PubMed]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Zhou, W.; Min, M.; Li, Y.; Hu, B.; Ma, X.; Cheng, Y.; Liu, Y.; Chen, P.; Ruan, R. A hetero-photoautotrophic two-stage cultivation process to improve wastewater nutrient removal and enhance algal lipid accumulation. Bioresour. Technol. 2012, 110, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Podola, B.; Melkonian, M. Application of a prototype-scale twin-layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour. Technol. 2014, 154, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Halder, G.; Oinam, G.; Indrama, T.; Tiwari, O.N. Bioremediation of organic and inorganic pollutants using microalgae. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–235. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.M.; Wurtsbaugh, W.A.; Paerl, H.W. Rationale for control of anthropogenic nitrogen and phosphorus to reduce eutrophication of inland waters. Environ. Sci. Technol. 2011, 45, 10300–10305. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, S.; Stephenson, T.; Lester, J.N.; Perry, R. The removal of phosphorus during wastewater treatment: A review. Environ. Pollut. 1988, 49, 183–233. [Google Scholar] [CrossRef]
- Zhou, G.J.; Ying, G.G.; Liu, S.; Zhou, L.J.; Chen, Z.F.; Peng, F.Q. Simultaneous removal of inorganic and organic compounds in wastewater by freshwater green microalgae. Environ. Sci. Process. Impacts 2014, 16, 2018–2027. [Google Scholar] [CrossRef]
- Piltz, B.; Melkonian, M. Immobilized microalgae for nutrient recovery from source-separated human urine. J. Appl. Phycol. 2017, 30, 421–429. [Google Scholar] [CrossRef]
- Cheng, P.; Osei-Wusu, D.; Zhou, C.; Wang, Y.; Xu, Z.; Chang, T.; Huo, S. The effects of refractory pollutants in swine wastewater on the growth of Scenedesmus sp. with biofilm attached culture. Int. J. Phytorem. 2020, 22, 241–250. [Google Scholar] [CrossRef]
- Cheng, P.; Chu, R.; Zhang, X.; Song, L.; Chen, D.; Zhou, C.; Yan, X.; Cheng, J.J.; Ruan, R. Screening of the dominant Chlorella pyrenoidosa for biofilm attached culture and feed production while treating swine wastewater. Bioresour. Technol. 2020, 318, 124054. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Iftikhar, R.; Arshad, M.; Zeeshan, M.; Hassan, M. Operation of microalgal horizontal twin layer system for treatment of real wastewater and production of lipids. J. Water Proc. Eng. 2022, 48, 102932. [Google Scholar] [CrossRef]
- Sohail, N.F.; Iftikhar, R.; Saleem, S. Microalgal treatment of high-nutrient wastewater using twin layer cultivation system. J. Environ. Chem. Eng. 2023, 11, 109248. [Google Scholar] [CrossRef]
- Meril, D.; Piliyan, R.; Perumal, S.; Selvakumaran, J.; Sundarraj, D.K.; Ayyanar, S.D.; Samraj, A. Performance analysis of Picochlorum maculatum reared on a twin-layer recirculation system in nutrient recovery from aquaculture effluents. Biomass Conver. Bioref. 2022. [CrossRef]
- Schwartz, T.; Kohnen, W.; Jansen, B.; Obst, U. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol. Ecol. 2003, 43, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Significance of antibiotics in the environment. J. Antimicrob. Chemother. 2003, 52, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.Q.; Zheng, H.S.; Li, S.; Du, J.S.; Feng, X.C.; Yin, R.L.; Wu, Q.L.; Ren, N.Q.; Chang, J.S. Removal of cephalosporin antibiotics 7-ACA from wastewater during the cultivation of lipid-accumulating microalgae. Bioresour. Technol. 2016, 221, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. Available online: https://academicjournals.org/journal/IJPS/article-abstract/59CA35213127. (accessed on 27 September 2023).
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Eccles, H. Treatment of metal-contaminated wastes: Why select a biological process? Trends Biotechnol. 1999, 17, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef] [PubMed]
- Crist, R.H.; Oberholser, K.; Shank, N.; Nguyen, M. Nature of bonding between metallic ions and algal cell walls. Environ. Sci. Technol. 1981, 15, 1212–1217. [Google Scholar] [CrossRef]
- Kaplan, D. Absorption and adsorption of heavy metals by microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 602–611. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Liu, T. Effect of cobalt enrichment on growth and hydrocarbon accumulation of Botryococcus braunii with immobilized biofilm attached cultivation. Bioresour. Technol. 2015, 177, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lin, G.; Podola, B.; Melkonian, M. Continuous removal of zinc from wastewater and mine dump leachate by a microalgal biofilm PSBR. J. Hazard. Mater. 2015, 297, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.; Kolattukudy, P.E. A cobalt-porphyrin enzyme converts a fatty aldehyde to a hydrocarbon and CO. Proc. Natl. Acad. Sci. USA 1992, 89, 5306–5310. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo-Salces, B.; Riaño, B.; Hernández, D.; Cruz García-González, M. Microalgae and wastewater treatment: Advantages and disadvantages. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 505–533. [Google Scholar] [CrossRef]
- Carbone, D.A.; Olivieri, G.; Pollio, A.; Melkonian, M. Biomass and phycobiliprotein production of Galdieria sulphuraria, immobilized on a twin-layer porous substrate photobioreactor. Appl. Microbiol. Biotechnol. 2020, 104, 3109–3119. [Google Scholar] [CrossRef]
- Carbone, D.A.; Olivieri, G.; Pollio, A.; Melkonian, M. Comparison of Galdieria growth and photosynthetic activity in different culture systems. AMB Express 2020, 10, 170. [Google Scholar] [CrossRef]

| Microalgae | Initial Value (g m−2) | Final Value (g m−2) | Biomass Productivity (g m−2 d−1) | Days | Medium | PSBRs | References |
|---|---|---|---|---|---|---|---|
| Scenedesmus (Halochlorella) rubescens | 1.4 | 7.7 | 0.9 | 9 | SWW | TW-S | Shi et al. [37] |
| Chlorella vulgaris | 1.4 | 11.9 | 1.1 | 9 | SWW | TW-S | Shi et al. [37] |
| Desmodesmus abundans | 2.5 | 75 | 8 | 9 | Human urine | TW-S | Piltz and Melkonian [52] |
| Chlorella pyrenoidosa | 8 | 48 | 3.3 | 8 | SWIW | A-T | Cheng et al. [43] |
| Scenedesmus sp. | 8 | 55 | 5.8 | 8 | SYSWIW | A-T | Cheng et al. [53] |
| Scenedesmus sp. | 8 | 60 | 6.5 | 8 | SYSWIW + Tetracycline | A-T | Cheng et al. [53] |
| Scenedesmus sp. | 8 | 58 | 6.2 | 8 | SYSWIW + Norfloxacin | A-T | Cheng et al. [53] |
| Scenedesmus sp. | 8 | 55 | 5.8 | 8 | SYSWIW + Sulfadimidine | A-T | Cheng et al. [53] |
| Chlorella sp. | 8 | 45 | 4.6 | 8 | ADSW | A-T | Cheng et al. [54] |
| Scenedesmus sp. | 5 | 39 | 5.6 | RWW | TW-S | Saleem et al. [55] | |
| Scenedesmus sp. | 5 | 32 | 4.7 | WTWW | TW-S | Saleem et al. [55] | |
| Tetradesmus obliquus | 3 | 92.83 | 5.13 | 18 | PWW | TW-S | Sohail et al. [56] |
| Tetradesmus obliquus | 3 | 87.7 | 4.87 | 18 | AD | TW-S | Sohail et al. [56] |
| Tetradesmus obliquus | 3 | 84.5 | 4.69 | 18 | SWL | TW-S | Sohail et al. [56] |
| Tetradesmus obliquus | 3 | 67.8 | 3.48 | 18 | MWW | TW-S | Sohail et al. [56] |
| P. maculatum | 3 | 15 | 4.2 | 15 | PRNM | TW-S | Meril et al. [57] |
| P. maculatum | 3 | 8 | 3.7 | 15 | NM | TW-S | Meril et al. [57] |
| P. maculatum | 3 | 5 | 3 | 15 | PP | TW-S | Meril et al. [57] |
| Microalgae | Initial Value (g m−2) | Final Value (g m−2) | Biomass Productivity (g m−2 d−1) | Days | Medium | PSBRs | References |
|---|---|---|---|---|---|---|---|
| Scenedesmus (Halochlorella) rubescens | 1.4 | 7.7 | 0.9 | 9 | SWW | TW-S | Shi et al. [37] |
| Chlorella vulgaris | 1.4 | 11.9 | 1.1 | 9 | SWW | TW-S | Shi et al. [37] |
| Desmodesmus abundans | 2.5 | 75 | 8 | 9 | Human urine | TW-S | Piltz and Melkonian [52] |
| Chlorella pyrenoidosa | 8 | 48 | 3.3 | 8 | SWIW | A-T | Cheng et al. [43] |
| Scenedesmus sp. | 8 | 55 | 5.8 | 8 | SYSWIW | A-T | Cheng et al. [53] |
| Scenedesmus sp. | 8 | 60 | 6.5 | 8 | SYSWIW + Tetracycline | A-T | Cheng et al. [53] |
| Scenedesmus sp. | 8 | 58 | 6.2 | 8 | SYSWIW + Norfloxacin | A-T | Cheng et al. [53] |
| Scenedesmus sp. | 8 | 55 | 5.8 | 8 | SYSWIW + Sulfadimidine | A-T | Cheng et al. [53] |
| Chlorella sp. | 8 | 45 | 4.6 | 8 | ADSW | A-T | Cheng et al. [54] |
| Microalgae | Heavy Metal | Initial Values mg L−1 | Final Values mg L−1 | Removal Efficiency % | QE mg g −1 | PSBRs | References |
|---|---|---|---|---|---|---|---|
| Botryococcus braunii | cobalt | 4.5 | 0.68 | 85 | 84 | A-T | Cheng et al. [69] |
| Stichococcus bacillaris | zinc | 2 | 0.4 | 80 | 126 | TW-S | Li et al. [70] |
| Stichococcus bacillaris | zinc | 3 | 0.6 | 80 | 106 | TW-S | Li et al. [70] |
| Stichococcus bacillaris | zinc | 2 | 0.3 | 85 | 118 | Multi-TW-S | Li et al. [70] |
| Stichococcus bacillaris | zinc | 3 | 0.45 | 85 | 72 | Multi-TW-S | Li et al. [70] |
| Stichococcus bacillaris | zinc | 3.3 | 0.2 | 96 | 155 | TW-S | Li et al. [70] |
| Chlorella pyrenoidosa | zinc | 2.8 | 0.96 | 66 | 38 | A-T | Cheng et al. [43] |
| Chlorella pyrenoidosa | copper | 2 | 1 | 50 | 24 | A-T | Cheng et al. [43] |
| Chlorella pyrenoidosa | iron | 1.8 | 0.75 | 58 | 22 | A-T | Cheng et al. [43] |
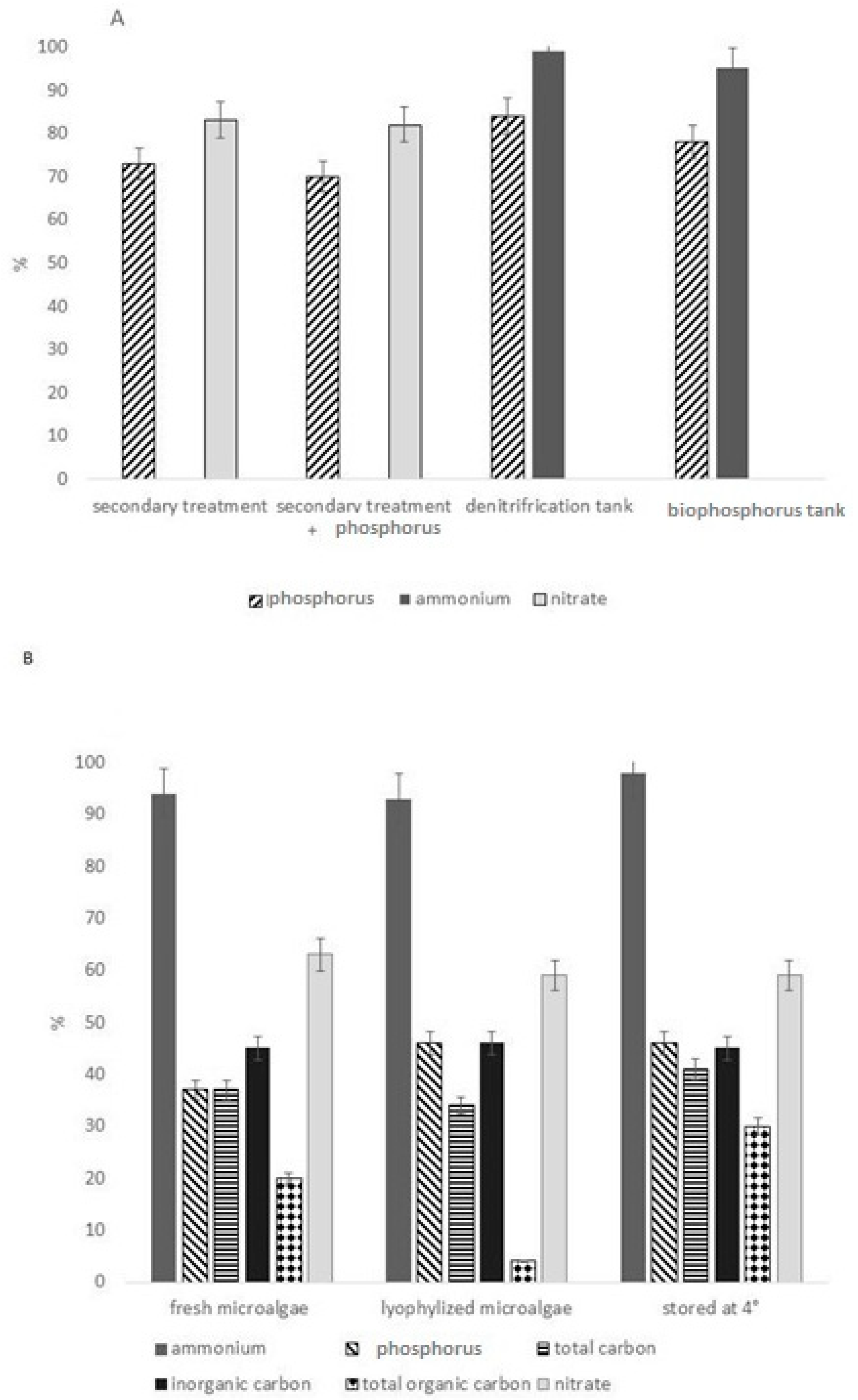
| Ammonium | Phosphorus | Nitrate | ||||||
|---|---|---|---|---|---|---|---|---|
| Microalgae | WW Type | Initial Values mg mL−1 | Final Values mg mL−1 | Initial Values mg mL−1 | Final Values mg mL−1 | Initial Values mg mL−1 | Final Values mg mL−1 | References |
| Scenedesmus (Halochlorella) rubescens | After secondary treatment | 0.10 | 0.6 | 0.61 | 0.2 | 7.51 | 0.6 | Shi et al. [46] |
| Scenedesmus (Halochlorella) rubescens | After secondary treatment and phosphorus addition | n.d | 2 | 0.2 | 5.85 | 0.5 | Shi et al. [46] | |
| Scenedesmus (Halochlorella) rubescens | After secondary treatment and denitrification tank | 1.79 | 0.8 | 1.95 | 0.3 | 0.52 | 0.3 | Shi et al. [46] |
| Scenedesmus (Halochlorella) rubescens | After secondary treatment and bio-phosphorus tank | 11.3 | 0.6 | 3.81 | 0.2 | 0.14 | 0.4 | Shi et al. [46] |
| Scenedesmus sp. Fresh microalgae | After secondary treatment | 24 | 1.4 | 24 | 1.7 | 24 | 1 | González-Camejo et al. [16] |
| Scenedesmus sp. Lyophilized microalgae | After secondary treatment | 5.4 | 3.4 | 5.4 | 2.9 | 5.4 | 2.9 | González-Camejo et al. [16] |
| Scenedesmus sp. Stored at 4 °C | After secondary treatment | 57 | 21 | 57 | 23 | 57 | 23 | González-Camejo et al. [16] |
| Total carbon | Inorganic Carbon | Total Organic Carbon | ||||||
| Scenedesmus sp. Fresh microalgae | After secondary treatment | 174 | 107 | 124 | 67 | 50 | 48 | González-Camejo et al. [16] |
| Scenedesmus sp. Lyophilized microalgae | After secondary treatment | 174 | 107 | 124 | 66 | 50 | 48 | González-Camejo et al. [16] |
| Scenedesmus sp. Stored at 4 °C | After secondary treatment | 174 | 107 | 124 | 67 | 50 | 48 | González-Camejo et al. [16] |
| Microalgae | Initial Value (g m−2) | Final Value (g m−2) | Biomass Productivity (g m−2 d−1) | Days | MWW Origin | References |
|---|---|---|---|---|---|---|
| Scenedesmus (Halochlorella) rubescens (western side) | 2 | 74 | 2.5 | 32 | Frechen (Germany) | Shi et al. [46] |
| Scenedesmus (Halochlorella) rubescens (eastern side) | 2 | 54 | 1.6 | 32 | Frechen (Germany) | Shi et al. [46] |
| Scenedesmus sp. | 3 | 60 | 0.81 | 90 | Cordoba (Spain) | González -Camejo et al. [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, D.A.; Melkonian, M. Potential of Porous Substrate Bioreactors for Removal of Pollutants from Wastewater Using Microalgae. Bioengineering 2023, 10, 1173. https://doi.org/10.3390/bioengineering10101173
Carbone DA, Melkonian M. Potential of Porous Substrate Bioreactors for Removal of Pollutants from Wastewater Using Microalgae. Bioengineering. 2023; 10(10):1173. https://doi.org/10.3390/bioengineering10101173
Chicago/Turabian StyleCarbone, Dora Allegra, and Michael Melkonian. 2023. "Potential of Porous Substrate Bioreactors for Removal of Pollutants from Wastewater Using Microalgae" Bioengineering 10, no. 10: 1173. https://doi.org/10.3390/bioengineering10101173
APA StyleCarbone, D. A., & Melkonian, M. (2023). Potential of Porous Substrate Bioreactors for Removal of Pollutants from Wastewater Using Microalgae. Bioengineering, 10(10), 1173. https://doi.org/10.3390/bioengineering10101173






