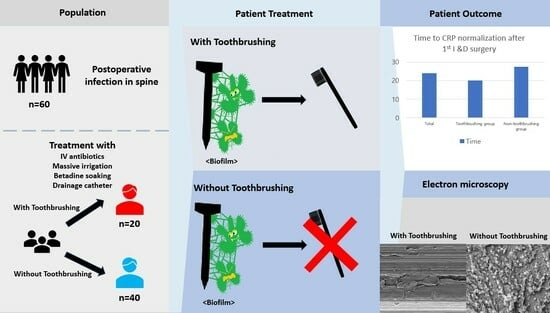
Effectiveness of Toothbrushing Technique for Biofilm Removal and Postoperative Infection Control after Spinal Fusion Surgery: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
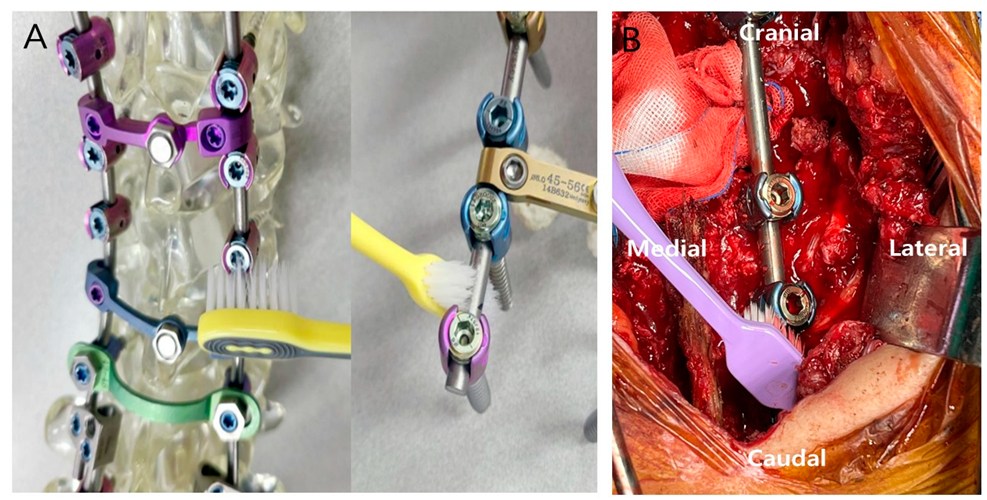
2.2. Preparation and Patient Treatment
2.3. Patient Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes
3.2.1. Toothbrush Group Patient: Successful Treatment
3.2.2. No-Toothbrush Group Patient: Treatment Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, C.; Park, J.-W.; Song, M.G.; Choi, H.-S. Suction Drain Tip Cultures in Predicting a Surgical Site Infection. Asian Spine J. 2023, 17, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Pinchera, B.; Buonomo, A.R.; Schiano Moriello, N.; Scotto, R.; Villari, R.; Gentile, I. Update on the Management of Surgical Site Infections. Antibiotics 2022, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.Y.; Biswas, S.K. Postoperative Spine Infections. Asian Spine J. 2016, 10, 176–183. [Google Scholar] [CrossRef]
- Eun, D.-C.; Suk, K.-S.; Kim, H.-S.; Kwon, J.-W.; Moon, S.-H.; Lee, Y.-H.; Lee, B.-H. Is Vancomycin More Effective than Taurolidine? Comparative Analysis of Their Preventive Effect against Spinal Infection in 1000 Patients with Spinal Fusion. Antibiotics 2022, 11, 1388. [Google Scholar] [CrossRef]
- Nabi, V.; Ayhan, S.; Yuksel, S.; Adhikari, P.; Vila-Casademunt, A.; Pellise, F.; Perez-Grueso, F.S.; Alanay, A.; Obeid, I.; Kleinstueck, F.; et al. The Effect of Discharging Patients with Low Hemoglobin Levels on Hospital Readmission and Quality of Life after Adult Spinal Deformity Surgery. Asian Spine J. 2022, 16, 261–269. [Google Scholar] [CrossRef]
- Tan, T.; Lee, H.; Huang, M.S.; Rutges, J.; Marion, T.E.; Matthew, J.; Fitzgerald, M.; Gonzalvo, A.; Hunn, M.K.; Kwon, B.K.; et al. Prophylactic postoperative measures to minimize surgical site infections in spine surgery: Systematic review and evidence summary. Spine J. 2020, 20, 435–447. [Google Scholar] [CrossRef]
- Spina, N.T.; Aleem, I.S.; Nassr, A.; Lawrence, B.D. Surgical Site Infections in Spine Surgery: Preoperative Prevention Strategies to Minimize Risk. Glob. Spine J. 2018, 8, 31S–36S. [Google Scholar] [CrossRef]
- Maruo, K.; Berven, S.H. Outcome and treatment of postoperative spine surgical site infections: Predictors of treatment success and failure. J. Orthop. Sci. 2014, 19, 398–404. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Fiani, B.; Jarrah, R.; Momin, A.A.; Rasouli, J. Surgical Site Infection Prophylaxis and Wound Management in Spine Surgery. Asian Spine J. 2022, 16, 451–461. [Google Scholar] [CrossRef]
- Lener, S.; Hartmann, S.; Barbagallo, G.M.V.; Certo, F.; Thomé, C.; Tschugg, A. Management of spinal infection: A review of the literature. Acta Neurochir. 2018, 160, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, T.J.; Woelber, E.; Bellabarba, C.; Bransford, R.; Spina, N. Predictors of increased cost and length of stay in the treatment of postoperative spine surgical site infection. Spine J. 2018, 18, 300–306. [Google Scholar] [CrossRef]
- Iida, Y.; Inoue, Y.; Hasegawa, K.; Tsuge, S.; Yokoyama, Y.; Nakamura, K.; Fukano, R.; Takamatsu, R.; Wada, A.; Takahashi, H. Evaluation of antimicrobial prophylaxis against postoperative infection after spine surgery: Limit of the first generation cephem. J. Infect. Chemother. 2016, 22, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Dupré, D.A.; Cheng, B.; Kreft, R.; Nistico, L.; Ehrlich, G.D.; Averick, S.; Altman, D.T. The Presence of Biofilms in Instrumented Spinal Fusions. Genet. Test. Mol. Biomark. 2022, 26, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Lewkonia, P.; DiPaola, C.; Street, J. Incidence and risk of delayed surgical site infection following instrumented lumbar spine fusion. J. Clin. Neurosci. 2016, 23, 76–80. [Google Scholar] [CrossRef]
- Kasliwal, M.K.; Tan, L.A.; Traynelis, V.C. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surg. Neurol. Int. 2013, 4, S392–S403. [Google Scholar] [CrossRef]
- Sung, S.; Kim, E.H.; Kwon, J.-W.; Lee, J.-S.; Lee, S.-B.; Moon, S.-H.; Lee, H.-M.; Jung, I.; Lee, B.H. Invasive dental procedures as risk factors for postoperative spinal infection and the effect of antibiotic prophylaxis. J. Clin. Periodontol. 2021, 48, 1270–1280. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontol. 2000 2021, 86, 32–56. [Google Scholar] [CrossRef]
- Cvikl, B.; Lussi, A. Supragingival Biofilm: Toothpaste and Toothbrushes. Monogr. Oral Sci. 2020, 29, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, M.; Shah, S.; Parikh, D.; Choudhary, P.; Bhaskar, V. The effectiveness of a musical toothbrush for dental plaque removal: A comparative study. J. Indian Soc. Pedod. Prev. Dent. 2012, 30, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chałas, R.; Wójcik-Chęcińska, I.; Woźniak, M.J.; Grzonka, J.; Święszkowski, W.; Kurzydłowski, K.J. Dental plaque as a biofilm—A risk in oral cavity and methods to prevent. Postepy Hig. Med. Dosw. 2015, 69, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lee, H.-M.; Kim, T.-H.; Kim, H.-S.; Moon, E.-S.; Park, J.-O.; Chong, H.-S.; Moon, S.-H. Transpedicular curettage and drainage of infective lumbar spondylodiscitis: Technique and clinical results. Clin. Orthop. Surg. 2012, 4, 200–208. [Google Scholar] [CrossRef]
- Lee, B.H.; Park, J.-O.; Kim, H.-S.; Lee, H.-M.; Cho, B.-W.; Moon, S.-H. Transpedicular curettage and drainage versus combined anterior and posterior surgery in infectious spondylodiscitis. Indian J. Orthop. 2014, 48, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Papadopoulos, D.V.; Vrioni, G.; Sioutis, S.; Sapkas, G.; Benzakour, A.; Benzakour, T.; Angelini, A.; Ruggieri, P.; Mavrogenis, A.F.; et al. Spinal Infections: An Update. Microorganisms 2020, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, D.; Arockiaraj, J.; Amritanand, R.; David, K.S.; Krishnan, V. Role of Biochemical Nutritional Parameters as Predictors of Postoperative Morbidity in Major Spine Surgeries. Asian Spine J. 2021, 15, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.M.; Galivanche, A.R.; Halperin, S.J.; Elaydi, A.; Rubio, D.R.; Grauer, J.N. Postoperative spine surgical site infections: High rate of failure of one-stage irrigation and debridement. Spine J. 2023, 23, 484–491. [Google Scholar] [CrossRef]
- Mederake, M.; Hofmann, U.K.; Benda, S.; Schuster, P.; Fink, B. Diagnostic Value of CRP and Serum WBC Count during Septic Two-Stage Revision of Total Hip Arthroplasties. Antibiotics 2022, 11, 1098. [Google Scholar] [CrossRef]
- Lammers, R.L.; Fourré, M.; Callaham, M.L.; Boone, T. Effect of povidone-iodine and saline soaking on bacterial counts in acute, traumatic, contaminated wounds. Ann. Emerg. Med. 1990, 19, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.J.; Ko, B.G.; Chung, J.W.; Kim, S.H.; Park, S.H.; Lee, M.H.; Yeom, J.S. The prevalence and impact of sarcopenia on degenerative lumbar spinal stenosis. Bone Jt. J. 2016, 98-B, 1093–1098. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.-S.; Suk, S.-I. Incidence of Proximal Adjacent Failure in Adult Lumbar Deformity Correction Based on Proximal Fusion Level. Asian Spine J. 2007, 1, 19–26. [Google Scholar] [CrossRef]
- Kim, J.H.; Ham, C.H.; Kwon, W.-K. Current Knowledge and Future Therapeutic Prospects in Symptomatic Intervertebral Disc Degeneration. Yonsei Med. J. 2022, 63, 199–210. [Google Scholar] [CrossRef]
- Abdou, M.; Kwon, J.-W.; Kim, H.J.; Lee, B.; Choi, Y.S.; Moon, S.-H.; Lee, B.H. Tranexamic Acid and Intraoperative and Postoperative Accumulative Bleeding in Elective Degenerative Spine Surgery. Yonsei Med. J. 2022, 63, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Kwon, J.-W.; Lee, S.-B.; Lee, H.-M.; Moon, S.-H.; Lee, B.H. Risk Factors of Clostridium Difficile Infection After Spinal Surgery: National Health Insurance Database. Sci. Rep. 2020, 10, 4438. [Google Scholar] [CrossRef]
- Oikonomidis, S.; Altenrath, L.; Westermann, L.; Bredow, J.; Eysel, P.; Scheyerer, M.J. Implant-Associated Infection of Long-Segment Spinal Instrumentation: A Retrospective Analysis of 46 Consecutive Patients. Asian Spine J. 2021, 15, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Prinz, V.; Vajkoczy, P. Surgical revision strategies for postoperative spinal implant infections (PSII). J. Spine Surg. 2020, 6, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.M.; Osorio, C.; Berns, E.M.; Masood, U.; Alsoof, D.; McDonald, C.L.; Zhang, A.S.; Younghein, J.A.; Kuris, E.O.; Telfeian, A.; et al. Antibiotic Cement Utilization for the Prophylaxis and Treatment of Infections in Spine Surgery: Basic Science Principles and Rationale for Clinical Use. J. Clin. Med. 2022, 11, 3481. [Google Scholar] [CrossRef]
- Jabbar, R.; Szmyd, B.; Jankowski, J.; Lusa, W.; Pawełczyk, A.; Wysiadecki, G.; Tubbs, R.S.; Iwanaga, J.; Radek, M. Intramedullary Spinal Cord Abscess with Concomitant Spinal Degenerative Diseases: A Case Report and Systematic Literature Review. J. Clin. Med. 2022, 11, 5148. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.P.; Luk, K.D. Complications of Anterior and Posterior Cervical Spine Surgery. Asian Spine J. 2016, 10, 385–400. [Google Scholar] [CrossRef]
- Marsh, P.D.; Bradshaw, D.J. Dental plaque as a biofilm. J. Ind. Microbiol. Biotechnol. 1995, 15, 169–175. [Google Scholar] [CrossRef]
- Jian, H.-J.; Yu, J.; Li, Y.-J.; Unnikrishnan, B.; Huang, Y.-F.; Luo, L.-J.; Ma, D.H.-K.; Harroun, S.G.; Chang, H.-T.; Lin, H.-J.; et al. Highly adhesive carbon quantum dots from biogenic amines for prevention of biofilm formation. Chem. Eng. J. 2020, 386, 123913. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Rosan, B.; Lamont, R.J. Dental plaque formation. Microbes Infect. 2000, 2, 1599–1607. [Google Scholar] [CrossRef]
- Bumpass, D.B.; McCarthy, R.E. Can MRSA Biofilm Infections Be Cleared from Pedicle Screws Intraoperatively? Spine J. 2017, 17, S179–S180. [Google Scholar] [CrossRef]
- Hersh, A.; Young, R.; Pennington, Z.; Ehresman, J.; Ding, A.; Kopparapu, S.; Cottrill, E.; Sciubba, D.M.; Theodore, N. Removal of instrumentation for postoperative spine infection: Systematic review. J. Neurosurg. Spine 2021, 35, 376–388. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental plaque biofilm in oral health and disease. Chin. J. Dent. Res. 2011, 14, 87–94. [Google Scholar]
- Ames, N.J. Evidence to support tooth brushing in critically ill patients. Am. J. Crit. Care 2011, 20, 242–250. [Google Scholar] [CrossRef] [PubMed]
- McCracken, G.I.; Janssen, J.; Swan, M.; Steen, N.; De Jager, M.; Heasman, P.A. Effect of brushing force and time on plaque removal using a powered toothbrush. J. Clin. Periodontol. 2003, 30, 409–413. [Google Scholar] [CrossRef]
- Smakman, F. Effects of Dead Bacterial Cells on Growth, Gene Expression and Evolution of Surrounding Microbes. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2021. [Google Scholar]
- Eick, S. Biofilms; Monographs in Oral Science; Karger: Basel, Switzerland, 2020; Volume 29, pp. 1–11. [Google Scholar] [CrossRef]
- Baker, J.F.; George, M.D. Prevention of Infection in the Perioperative Setting in Patients with Rheumatic Disease Treated with Immunosuppression. Curr. Rheumatol. Rep. 2019, 21, 17. [Google Scholar] [CrossRef] [PubMed]
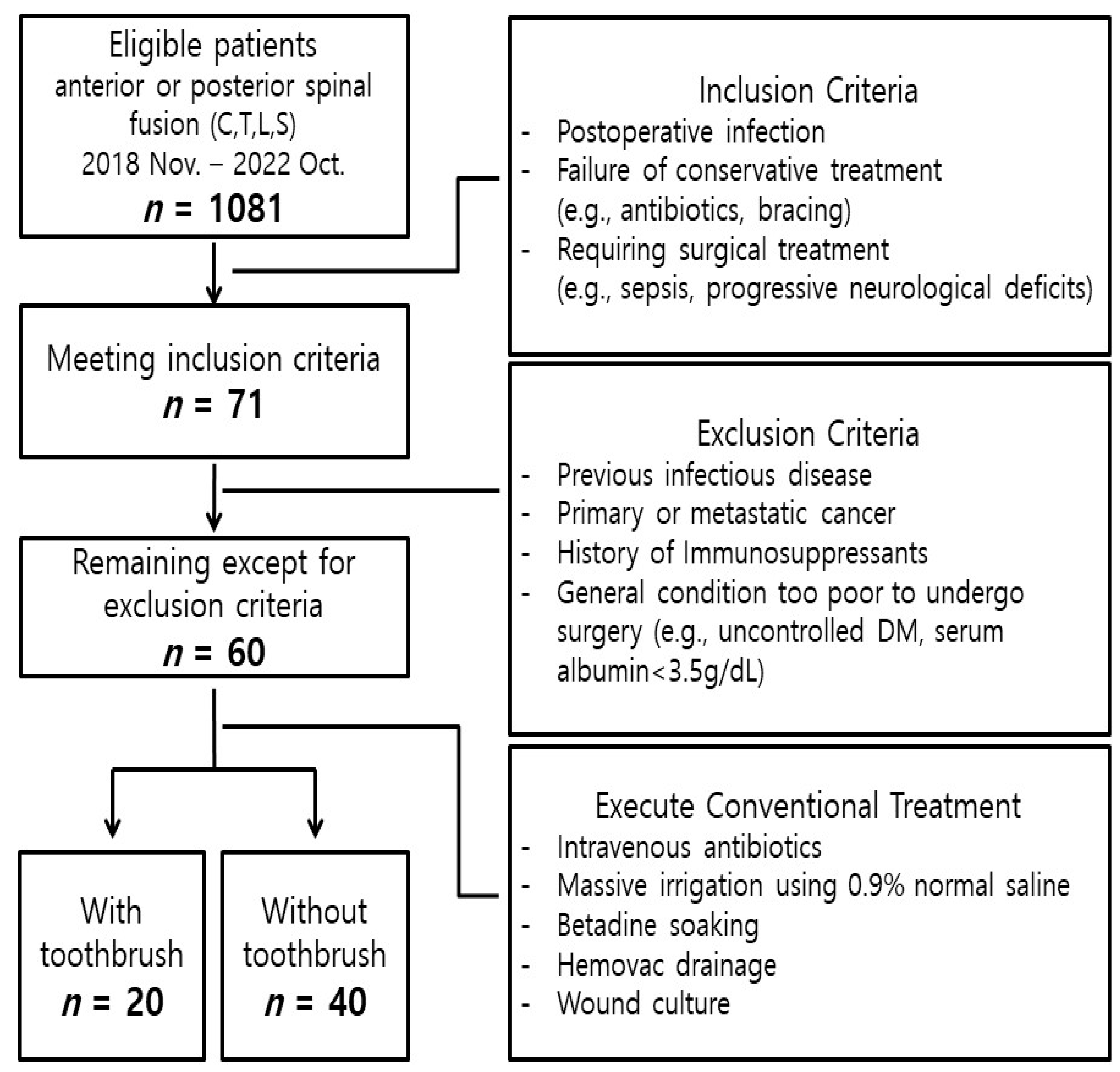
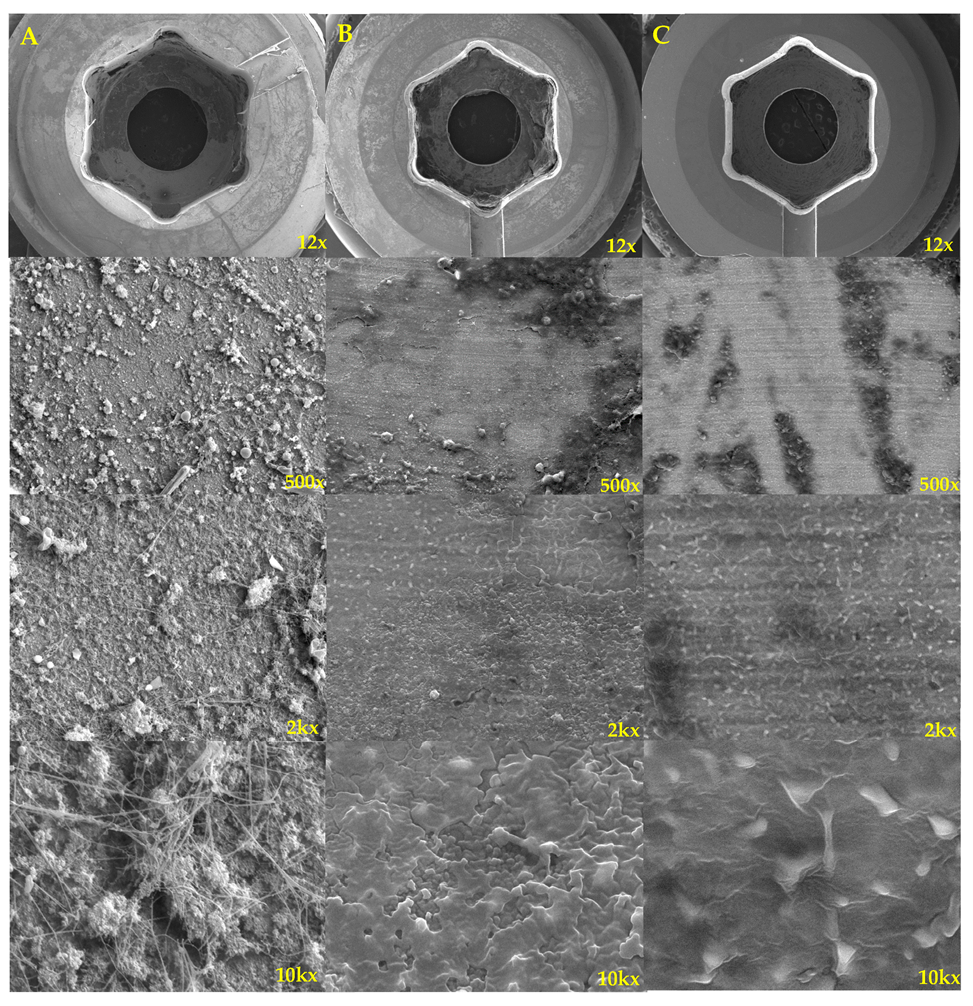

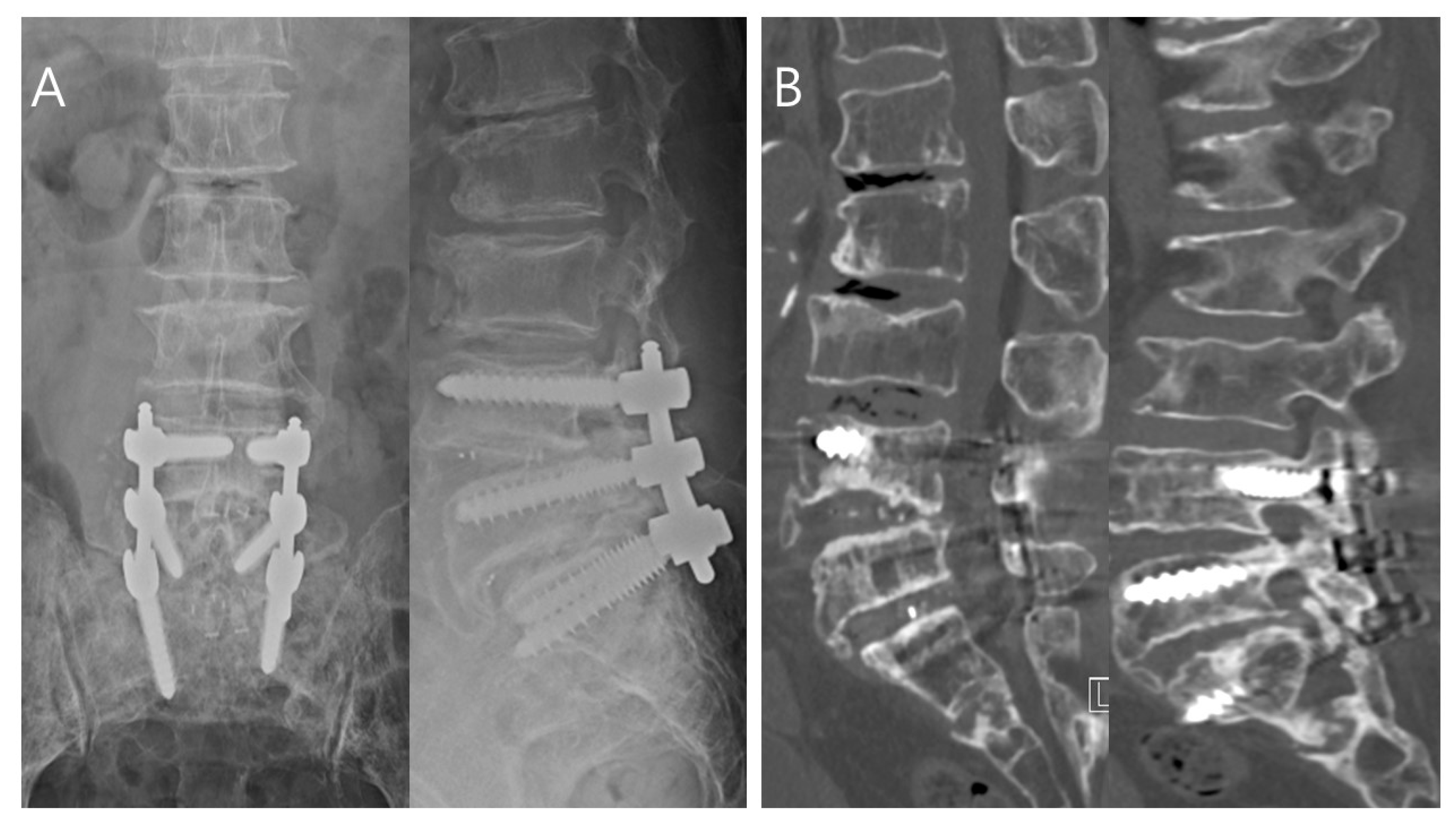

| Characteristics | Total (n = 60) | Toothbrush Group (n = 20) | No-Toothbrush Group (n = 40) | p-Value |
|---|---|---|---|---|
| Age (y) | 69.0 (55.0–89.0) | 66.5 (58.0–89.0) | 71.0 (55.0–82.0) | 0.0461 * |
| Sex | 0.0935 | |||
| Male | 36 (60.00) | 9 (45.00) | 27 (67.50) | |
| Female | 24 (40.00) | 11 (55.00) | 13 (32.50) | |
| Body mass index (kg/m2) | 0.5680 | |||
| <25 | 35 (58.33) | 15 (75.00) | 20 (50.00) | |
| 25–29.9 | 19 (31.67) | 4 (20.00) | 15 (37.50) | |
| ≥30 | 6 (10.00) | 1 (5.00) | 5 (12.50) | |
| Smoking | 0.4714 | |||
| Yes | 10 (16.67) | 5 (25.00) | 5 (12.50) | |
| No | 50 (83.33) | 15 (75.00) | 35 (87.50) | |
| Hypertension | 0.5820 | |||
| Yes | 33 (55.00) | 10 (50.00) | 23 (57.50) | |
| No | 27 (45.00) | 10 (50.00) | 17 (42.50) | |
| Diabetes mellitus | 0.7144 | |||
| Yes | 28 (46.67) | 10 (50.00) | 18 (45.00) | |
| No | 32 (53.33) | 10 (50.00) | 22 (55.00) | |
| Renal disease | 0.0986 | |||
| Yes | 16 (26.67) | 8 (40.00) | 8 (20.00) | |
| No | 44 (73.33) | 12 (60.00) | 32 (80.00) | |
| Pulmonary disease | >0.9999 | |||
| Yes | 9 (15) | 3 (15.00) | 6 (15.00) | |
| No | 51(85) | 17 (85.00) | 34 (85.00) | |
| Surgical site | 0.1750 | |||
| Cervical | 7 (11.67) | 2 (10.00) | 5 (12.50) | |
| Thoracolumbar | 22 (36.67) | 9 (45.00) | 13 (32.50) | |
| Lumbar | 20 (33.33) | 4 (20.00) | 16 (40.00) | |
| Lumbosacral | 11 (18.33) | 5 (25.00) | 6 (15.00) | |
| Number of instrumented fusion levels | 2.00 (1.00–7.00) | 2.00 (1.00–6.00) | 2.00 (1.00–7.00) | 0.7312 |
| Estimated blood loss during initial fusion surgery (mL) | 450 (30–2500) | 490 (100–2500) | 400 (30–2500) | 0.0884 |
| Transfusion (packed RBCs) during initial fusion surgery | 0.0792 | |||
| Yes | 14 (23.33) | 3 (15.00) | 11 (27.50) | |
| No | 46 (76.67) | 17 (85.00) | 29 (72.50) | |
| Pre-1st I&D surgery WBC count (×103 cells/µL) | 7.12 (2.78–18.62) | 6.97 (2.78–18.62) | 8.44 (4.24–17.91) | 0.2141 |
| Pre-1st I&D surgery ANC (cells/µL) | 4217.80 (1278.49–7944.78) | 3980.71 (1765.12–7944.78) | 4324.96 (1278.49–6823.21) | 0.3645 |
| Microorganisms | Total (n) | Toothbrush Group (n) | No-Toothbrush Group (n) |
|---|---|---|---|
| Staphylococcus epidermidis | 15 | 4 | 11 |
| Staphylococcus aureus | 8 | 3 | 5 |
| Methicillin-resistant coagulase-negative Staphylococcus | 4 | 2 | 2 |
| Methicillin-resistant Staphylococcus aureus | 9 | 2 | 7 |
| Pseudomonas aeruginosa | 3 | 2 | 1 |
| Klebsiella pneumonia | 3 | - | 3 |
| Escherichia coli | 5 | 2 | 3 |
| Enterococcus faecalis | 4 | 2 | 2 |
| Candida parapsilosis | 1 | 1 | - |
| Mycobacterium tuberculosis | - | - | - |
| No growth | 8 | 2 | 6 |
| Clinical Outcomes | Total (n = 60) | Toothbrush Group (n = 20) | No-Toothbrush Group (n = 40) | p-Value |
|---|---|---|---|---|
| Postoperative hemovac removal after I&D surgery (POD) | 7.0 (5.0–14.0) | 7.0 (5.0–12.0) | 7.0 (5.0–14.0) | 0.3742 |
| Cumulative hemovac volume after I&D surgery (mL) | 410 (20–2600) | 440 (20–2600) | 350 (80–1400) | 0.1160 |
| Post I&D surgery serum glucose (mg/dL) | 130.8 (57–284) | 146.7 (92–284) | 112.8 (57–198) | 0.7522 |
| Post I&D surgery serum albumin (g/dL) | 2.9 (1.80–4.94) | 3.3 (1.80–4.94) | 2.8 (2.20–4.54) | 0.2021 |
| Time from fusion surgery to diagnosis of SSI (days) | 75.0 (10.0–500.0) | 45.0 (10.0–500.0) | 90.0 (12.0–450.0) | 0.0796 |
| Early SSI (<30 days) | 34 (55.00) | 12 (60.00) | 22 (55.00) | |
| Late SSI (≥30 days) | 26 (45.00) | 8 (40.00) | 18 (45.00) | |
| Time from SSI diagnosis to 1st I&D surgery (days) | 8.0 (1.0–90.0) | 10.0 (2.0–30.0) | 7.5 (1.0–90.0) | 0.4074 |
| Total time to CRP normalization after 1st I &D surgery (days) | 24.0 (10.0–90.0) | 20.0 (10.0–50.0) | 27.5 (14.0–90.0) | 0.0449 * |
| Duration of intravenous antibiotics usage in the hospital perioperative 1st I&D surgery (days) | 25.0 (10.0–90.0) | 20.0 (12.0–58.0) | 26.0 (10.0–90.0) | 0.2082 |
| Duration of oral antibiotics usage after hospital discharge (days) | 15.75 (7.0–28.0) | 16.45 (7.0–28.0) | 15.4 (7.0–28.0) | 0.4871 |
| Need for revision surgery after 1st I&D surgery | 0.0395 * | |||
| Yes | 16 (26.67) | 2 (10.00) | 14 (35.00) | |
| No | 44 (73.33) | 18 (90.00) | 26 (65.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-r.; Kwon, J.-W.; Suk, K.-S.; Kim, H.-S.; Moon, S.-H.; Park, S.-Y.; Moon, S.-E.; Lee, B.-H. Effectiveness of Toothbrushing Technique for Biofilm Removal and Postoperative Infection Control after Spinal Fusion Surgery: A Retrospective Study. Bioengineering 2023, 10, 1143. https://doi.org/10.3390/bioengineering10101143
Choi S-r, Kwon J-W, Suk K-S, Kim H-S, Moon S-H, Park S-Y, Moon S-E, Lee B-H. Effectiveness of Toothbrushing Technique for Biofilm Removal and Postoperative Infection Control after Spinal Fusion Surgery: A Retrospective Study. Bioengineering. 2023; 10(10):1143. https://doi.org/10.3390/bioengineering10101143
Chicago/Turabian StyleChoi, Sung-ryul, Ji-Won Kwon, Kyung-Soo Suk, Hak-Sun Kim, Seong-Hwan Moon, Si-Young Park, Seung-Eon Moon, and Byung-Ho Lee. 2023. "Effectiveness of Toothbrushing Technique for Biofilm Removal and Postoperative Infection Control after Spinal Fusion Surgery: A Retrospective Study" Bioengineering 10, no. 10: 1143. https://doi.org/10.3390/bioengineering10101143
APA StyleChoi, S.-r., Kwon, J.-W., Suk, K.-S., Kim, H.-S., Moon, S.-H., Park, S.-Y., Moon, S.-E., & Lee, B.-H. (2023). Effectiveness of Toothbrushing Technique for Biofilm Removal and Postoperative Infection Control after Spinal Fusion Surgery: A Retrospective Study. Bioengineering, 10(10), 1143. https://doi.org/10.3390/bioengineering10101143