Attenuation of SCI-Induced Hypersensitivity by Intensive Locomotor Training and Recombinant GABAergic Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results
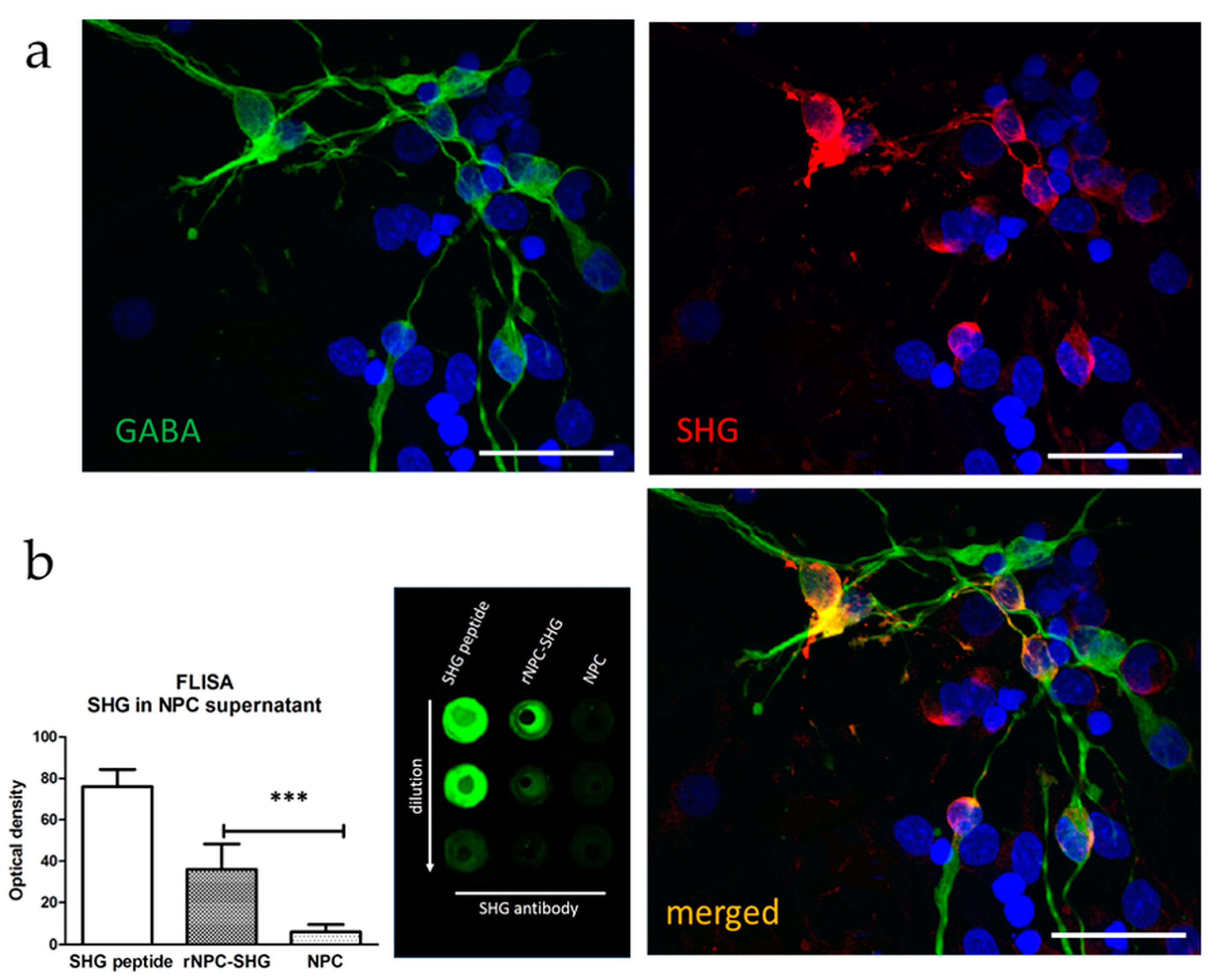
3.1. Characterization of Recombinant GABAergic NPCs
3.2. Behavior
3.2.1. Locomotor Score
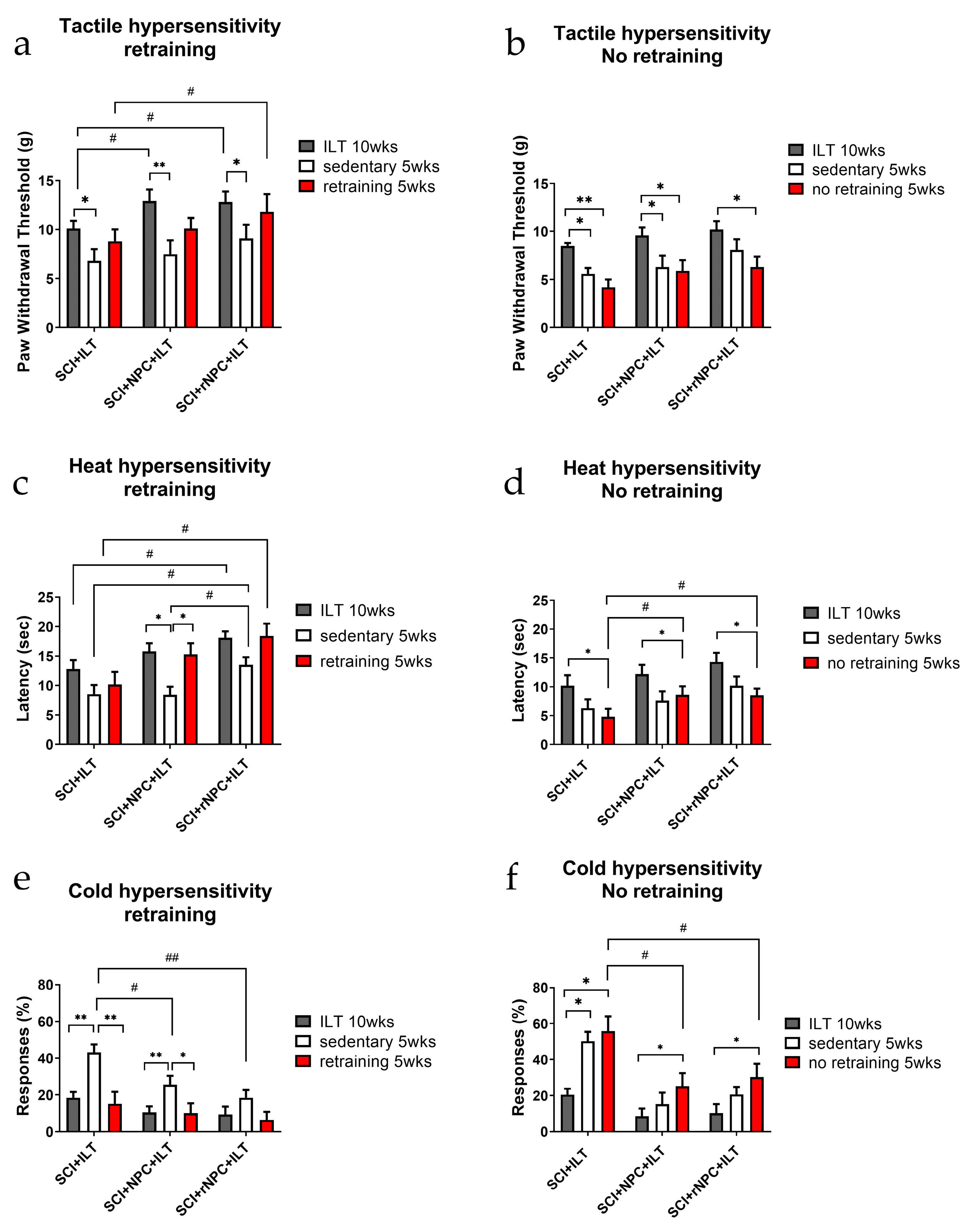
3.2.2. Tactile Hypersensitivity
3.2.3. Heat Hypersensitivity
3.2.4. Cold Hypersensitivity
3.2.5. First Retraining
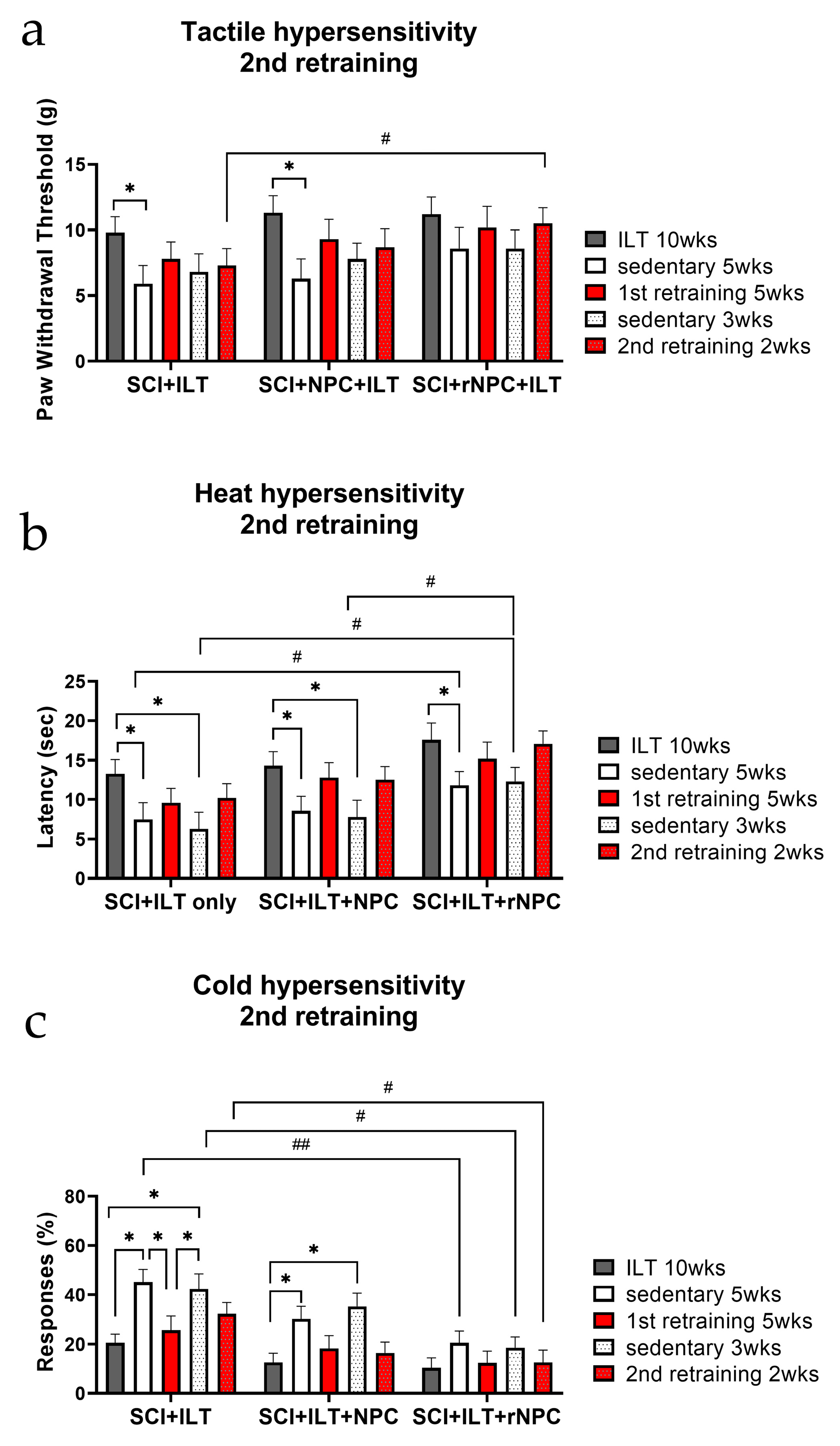
3.2.6. Second Retraining
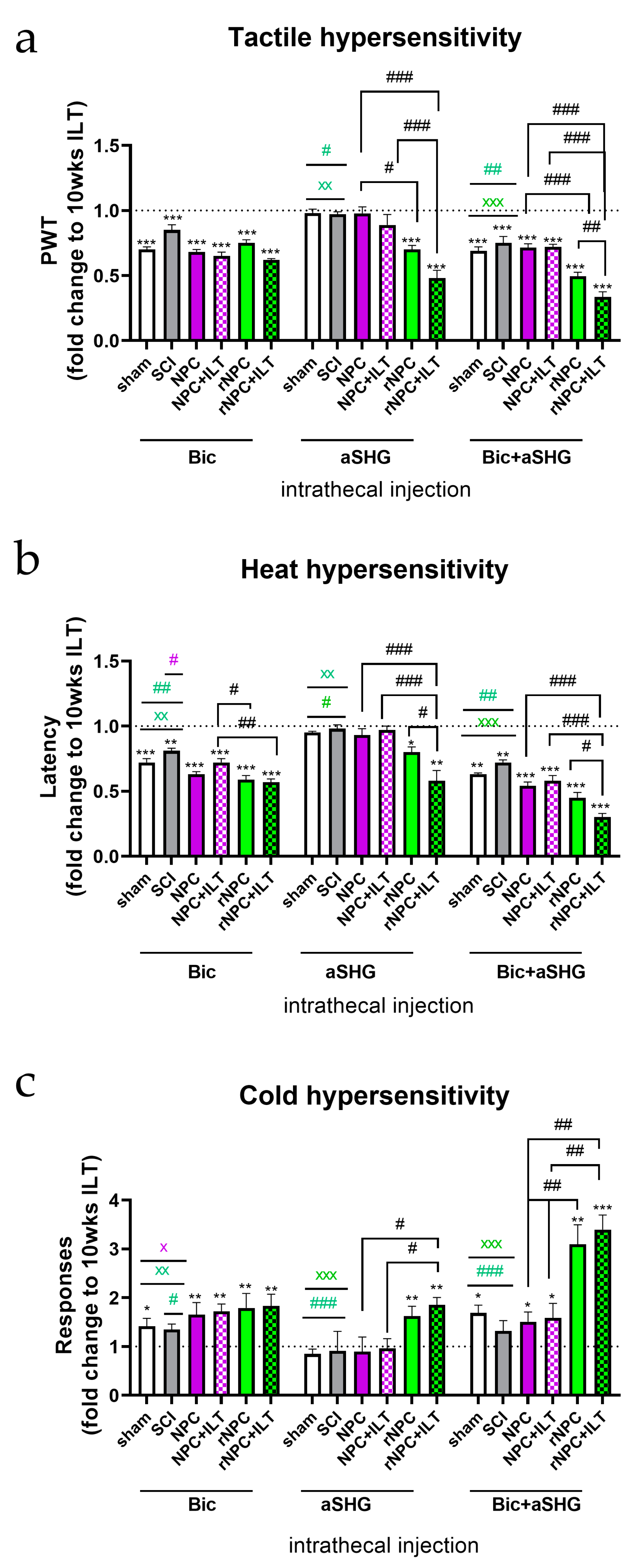
3.3. Intrathecal Injection of Anti-SHG and Bicuculline
3.4. ELISA Evaluation of Cytokines in Spinal Tissue and CSF
3.5. Immunohistochemical and Biochemical Analysis of Spinal Tissue and CSF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Widerstrom-Noga, E.; Felipe-Cuervo, E.; Yezierski, R.P. Chronic pain after spinal injury: Interference with sleep and daily activities. Arch. Phys. Med. Rehabil. 2001, 82, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Almeida, Y.; Martinez-Arizala, A.; Widerstrom-Noga, E.G. Chronicity of pain associated with spinal cord injury: A longitudinal analysis. J. Rehabil. Res. Dev. 2005, 42, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Johannesen, I.L.; Sindrup, S.H.; Bach, F.W.; Jensen, T.S. Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal Cord 2001, 39, 256–262. [Google Scholar] [CrossRef]
- Siddall, P.J.; McClelland, J.M.; Rutkowski, S.B.; Cousins, M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 2003, 103, 249–257. [Google Scholar] [CrossRef]
- Burke, D.; Fullen, B.M.; Stokes, D.; Lennon, O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur. J. Pain 2017, 21, 29–44. [Google Scholar] [CrossRef]
- Widerstrom-Noga, E. Neuropathic pain and spinal cord injury: Phenotypes and pharmacological management. Drugs 2017, 77, 967–984. [Google Scholar] [CrossRef]
- Dvoracsko, S.; Stefanucci, A.; Novellino, E.; Mollica, A. The design of multitarget ligands for chronic and neuropathic pain. Future Med. Chem. 2015, 7, 2469–2483. [Google Scholar] [CrossRef]
- Castro-Lopes, J.M.; Tavares, I.; Coimbra, A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993, 620, 287–291. [Google Scholar] [CrossRef]
- Eaton, M.J.; Plunkett, J.A.; Karmally, S.; Martinez, M.A.; Montanez, K. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J. Chem. Neuroanat. 1998, 16, 57–72. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Tan, H.Y.; Nam, T.S.; Paik, K.S.; Hulsebosch, C.E.; Leem, J.W. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J. Neurotrauma 2006, 23, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, T.; Hama, A.T.; Wang, X.T.; Pappas, G.D.; Sagen, J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience 1997, 76, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Furmanski, O.; Castellanos, D.A.; Daniels, L.A.; Hama, A.T.; Sagen, J. Prolonged nociceptive responses to hind paw formalin injection in rats with a spinal cord injury. Neurosci. Lett. 2008, 439, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Schuler, V.; Luscher, C.; Blanchet, C.; Klix, N.; Sansig, G.; Klebs, K.; Schmutz, M.; Heid, J.; Gentry, C.; Urban, L.; et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 2001, 31, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, S.D.; Homanics, G.E.; Firestone, L.L.; Hammond, D.L. Sensory thresholds and the antinociceptive effects of GABA receptor agonists in mice lacking the beta3 subunit of the GABA(A) receptor. Neuroscience 2000, 95, 795–806. [Google Scholar] [CrossRef]
- Drew, G.M.; Siddall, P.J.; Duggan, A.W. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain 2004, 109, 379–388. [Google Scholar] [CrossRef]
- Moore, K.A.; Kohno, T.; Karchewski, L.A.; Scholz, J.; Baba, H.; Woolf, C.J. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 2002, 22, 6724–6731. [Google Scholar] [CrossRef]
- Zhang, A.L.; Hao, J.X.; Seiger, A.; Xu, X.J.; Wiesenfeld-Hallin, Z.; Grant, G.; Aldskogius, H. Decreased GABA immunoreactivity in spinal cord dorsal horn neurons after transient spinal cord ischemia in the rat. Brain Res. 1994, 656, 187–190. [Google Scholar] [CrossRef]
- Lee, J.W.; Jergova, S.; Furmanski, O.; Gajavelli, S.; Sagen, J. Predifferentiated GABAergic neural precursor transplants for alleviation of dysesthetic central pain following excitotoxic spinal cord injury. Front. Physiol. 2012, 3, 167. [Google Scholar] [CrossRef]
- Jergova, S.; Gajavelli, S.; Varghese, M.S.; Shekane, P.; Sagen, J. Analgesic effect of recombinant GABAergic cells in a model of peripheral neuropathic pain. Cell Transplant. 2016, 25, 629–643. [Google Scholar] [CrossRef]
- Llewellyn-Smith, I.J.; Basbaum, A.I.; Braz, J.M. Long-term, dynamic synaptic reorganization after GABAergic precursor cell transplantation into adult mouse spinal cord. J. Comp. Neurol. 2018, 526, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Etlin, A.; Braz, J.M.; Kuhn, J.A.; Wang, X.; Hamel, K.A.; Llewellyn-Smith, I.J.; Basbaum, A. Functional synaptic integration of forebrain gabaergic precursors into the adult spinal cord. J. Neurosci. 2016, 36, 11634–11645. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Braz, J.M. Cell transplants to treat the "disease" of neuropathic pain and itch. Pain 2016, 157 (Suppl. S1), S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Braz, J.M.; Wang, X.; Guan, Z.; Rubenstein, J.L.; Basbaum, A.I. Transplant-mediated enhancement of spinal cord GABAergic inhibition reverses paclitaxel-induced mechanical and heat hypersensitivity. Pain 2015, 156, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Braz, J.M.; Sharif-Naeini, R.; Vogt, D.; Kriegstein, A.; Alvarez-Buylla, A.; Rubenstein, J.L.; Basbaum, A. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron 2012, 74, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Fandel, T.M.; Trivedi, A.; Nicholas, C.R.; Zhang, H.; Chen, J.; Martinez, A.F.; Noble-Haeusslein, L.J.; Kriegstein, A.R. Transplanted human stem cell-derived interneuron precursors mitigate mouse bladder dysfunction and central neuropathic pain after spinal cord injury. Cell Stem Cell 2016, 19, 544–557. [Google Scholar] [CrossRef]
- Van Gorp, S.; Leerink, M.; Kakinohana, O.; Platoshyn, O.; Santucci, C.; Galik, J.; Joosten, E.A.; Hruska-Plochan, M.; Goldberg, D.; Marsala, S.; et al. Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation. Stem Cell Res. Ther. 2013, 4, 57. [Google Scholar] [CrossRef]
- Hendricks, W.A.; Pak, E.S.; Owensby, J.P.; Menta, K.J.; Glazova, M.; Moretto, J.; Hollis, S.; Brewer, K.L.; Murashov, A.K. Predifferentiated embryonic stem cells prevent chronic pain behaviors and restore sensory function following spinal cord injury in mice. Mol. Med. 2006, 12, 34–46. [Google Scholar] [CrossRef]
- Jergova, S.; Hentall, I.D.; Gajavelli, S.; Varghese, M.S.; Sagen, J. Intraspinal transplantation of GABAergic neural progenitors attenuates neuropathic pain in rats: A pharmacologic and neurophysiological evaluation. Exp. Neurol. 2012, 234, 39–49. [Google Scholar] [CrossRef]
- Jergova, S.; Gajavelli, S.; Pathak, N.; Sagen, J. Recombinant neural progenitor transplants in the spinal dorsal horn alleviate chronic central neuropathic pain. Pain 2016, 157, 977. [Google Scholar] [CrossRef]
- Tashiro, S.; Nishimura, S.; Shinozaki, M.; Takano, M.; Konomi, T.; Tsuji, O.; Nagoshi, N.; Toyama, Y.; Liu, M.; Okano, H.; et al. The amelioration of pain-related behavior in mice with chronic spinal cord injury treated with neural stem/progenitor cell transplantation combined with treadmill training. J. Neurotrauma 2018, 35, 2571. [Google Scholar] [CrossRef] [PubMed]
- Carlton, S.M.; Hargett, G.L. Treatment with the NMDA antagonist memantine attenuates nociceptive responses to mechanical stimulation in neuropathic rats. Neurosci. Lett. 1995, 198, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Davar, G.; Hama, A.; Deykin, A.; Vos, B.; Maciewicz, R. MK-801 blocks the development of thermal hyperalgesia in a rat model of experimental painful neuropathy. Brain Res. 1991, 553, 327–330. [Google Scholar] [CrossRef]
- Eliav, E.; Herzberg, U.; Ruda, M.A.; Bennett, G.J. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. Pain 1999, 83, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Jergova, S.; Gordon, C.E.; Gajavelli, S.; Sagen, J. Experimental gene therapy with serine-histogranin and endomorphin 1 for the treatment of chronic neuropathic pain. Front. Mol. Neurosci. 2017, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279. [Google Scholar] [CrossRef]
- Kressler, J.; Thomas, C.K.; Field-Fote, E.C.; Sanchez, J.; Widerstrom-Noga, E.; Cilien, D.C.; Gant, K.; Ginnety, K.; Gonzalez, H.; Martinez, A.; et al. Understanding therapeutic benefits of overground bionic ambulation: Exploratory case series in persons with chronic, complete spinal cord injury. Arch. Phys. Med. Rehabil. 2014, 95, 1878–1887.e4. [Google Scholar] [CrossRef]
- Gant, K.L.; Nagle, K.G.; Cowan, R.E.; Field-Fote, E.C.; Nash, M.S.; Kressler, J.; Thomas, C.K.; Castellanos, M.; Widerström-Noga, E.; Anderson, K.D. Body System Effects of a Multi-Modal Training Program Targeting Chronic, Motor Complete Thoracic Spinal Cord Injury. J. Neurotrauma 2018, 35, 411–423. [Google Scholar] [CrossRef]
- Hutchinson, K.J.; Gomez-Pinilla, F.; Crowe, M.J.; Ying, Z.; Basso, D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004, 127, 1403–1414. [Google Scholar] [CrossRef]
- Brown, A.K.; Woller, S.A.; Moreno, G.; Grau, J.W.; Hook, M.A. Exercise therapy and recovery after SCI: Evidence that shows early intervention improves recovery of function. Spinal Cord 2011, 49, 623–628. [Google Scholar] [CrossRef]
- Detloff, M.R.; Smith, E.J.; Quiros Molina, D.; Ganzer, P.D.; Houle, J.D. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp. Neurol. 2014, 255, 38–48. [Google Scholar] [CrossRef]
- Sliwinski, C.; Nees, T.A.; Puttagunta, R.; Weidner, N.; Blesch, A. Sensorimotor activity partially ameliorates pain and reduces nociceptive fiber density in the chronically injured spinal cord. J. Neurotrauma 2018, 35, 2222–2238. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, S.J.; Quiros-Molina, D.; Tamashiro-Orrego, A.D.; Houle, J.D.; Detloff, M.R. Exercise-Induced Changes to the Macrophage Response in the Dorsal Root Ganglia Prevent Neuropathic Pain after Spinal Cord Injury. J. Neurotrauma 2019, 36, 877–890. [Google Scholar] [CrossRef]
- Da Silva, A.E.; dos Reis-Neto, E.T.; da Silva, N.P.; Sato, E.I. The effect of acute physical exercise on cytokine levels in patients with systemic lupus erythematosus. Lupus 2013, 22, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Rosety-Rodriguez, M.; Camacho, A.; Rosety, I.; Fornieles, G.; Rosety, M.A.; Diaz, A.J.; Bernardi, M.; Rosety, M.; Ordonez, F.J. Low-grade systemic inflammation and leptin levels were improved by arm cranking exercise in adults with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2014, 95, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Neefkes-Zonneveld, C.R.; Bakkum, A.J.; Bishop, N.C.; van Tulder, M.W.; Janssen, T.W. Effect of long-term physical activity and acute exercise on markers of systemic inflammation in persons with chronic spinal cord injury: A systematic review. Arch. Phys. Med. Rehabil. 2015, 96, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, S.; Arbat-Plana, A.; Lopez-Alvarez, V.M.; Navarro, X. Neuroprotective effects of exercise treatments after injury: The dual role of neurotrophic factors. Curr. Neuropharmacol. 2017, 15, 495–518. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, F.; Teixeira, J.M.; Sluka, K.A.; Santos, A.R.S. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018, 159, 437–450. [Google Scholar] [CrossRef]
- Dugan, E.A.; Jergova, S.; Sagen, J. Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp. Neurol. 2020, 327, 113208. [Google Scholar] [CrossRef]
- Jergova, S.; Dugan, E.; Hernandez, M.; Arthur, A.; Restrepo, M.; Sagen, J. (279) Management of SCI-Induced Chronic Pain in Rats: Intensive Locomotor Training and Recombinant GABAergic Cell Tranplants. J. Pain 2019, 20 (Suppl. S4), S44. [Google Scholar] [CrossRef]
- Schachner, B.D.; Jergova, S.; Sagen, J. (Eds.) Intensive Locomotor Training Provides Sustained Alleviation of Chronic Spinal Cord Injury Associated Neuropathic Pain; Society of Neuroscience: Chicago, IL, USA, 2019. [Google Scholar]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; National Centre for the Replacement, R. Animal research: Reporting in vivo experiments—The ARRIVE guidelines. J. Cereb. Blood Flow Metab. 2011, 31, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.C.; Oatway, M.A.; Weaver, L.C. Chronic pain after clip-compression injury of the rat spinal cord. Exp. Neurol. 2002, 178, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Dugan, E.A.; Sagen, J. An Intensive Locomotor Training Paradigm Improves Neuropathic Pain following Spinal Cord Compression Injury in Rats. J. Neurotrauma 2015, 32, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Dugan, E.A.; Sagen, J. A novel affective-motivational-based Overground System for detecting spinal cord injury-associated thermal and mechanical hypersensitivity in rats. Eur. J. Pain 2018, 22, 1628–1640. [Google Scholar] [CrossRef]
- Hama, A.; Sagen, J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Res. 2007, 1185, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.; Sagen, J. Antinociceptive effect of cannabinoid agonist WIN 55,212-2 in rats with a spinal cord injury. Exp. Neurol. 2007, 204, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.; Sagen, J. Antinociceptive effects of the marine snail peptides conantokin-G and conotoxin MVIIA alone and in combination in rat models of pain. Neuropharmacology 2009, 56, 556–563. [Google Scholar] [CrossRef]
- Hama, A.; Sagen, J. Sustained antinociceptive effect of cannabinoid receptor agonist WIN 55,212-2 over time in rat model of neuropathic spinal cord injury pain. J. Rehabil. Res. Dev. 2009, 46, 135–143. [Google Scholar] [CrossRef]
- Furmanski, O.; Gajavelli, S.; Lee, J.W.; Collado, M.E.; Jergova, S.; Sagen, J. Combined extrinsic and intrinsic manipulations exert complementary neuronal enrichment in embryonic rat neural precursor cultures: An in vitro and in vivo analysis. J. Comp. Neurol. 2009, 515, 56–71. [Google Scholar] [CrossRef]
- Siddall, P.J.; Taylor, D.A.; Cousins, M.J. Pain associated with spinal cord injury. Curr. Opin. Neurol. 1995, 8, 447–450. [Google Scholar] [CrossRef]
- Siddall, P.J.; Taylor, D.A.; Cousins, M.J. Classification of pain following spinal cord injury. Spinal Cord 1997, 35, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Siddall, P.J.; Taylor, D.A.; McClelland, J.M.; Rutkowski, S.B.; Cousins, M.J. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain 1999, 81, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Almeida, Y.; Felix, E.R.; Martinez-Arizala, A.; Widerstrom-Noga, E.G. Pain symptom profiles in persons with spinal cord injury. Pain Med. 2009, 10, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Detloff, M.R.; Fisher, L.C.; McGaughy, V.; Longbrake, E.E.; Popovich, P.G.; Basso, D.M. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008, 212, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Hulsebosch, C.E. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience 2009, 161, 895–903. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Kang, J.; Unabia, G.C.; Hulsebosch, C.E. Spatial and temporal activation of spinal glial cells: Role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp. Neurol. 2012, 234, 362–372. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Garcia-Alias, G.; Navarro, X. Plastic changes in lumbar segments after thoracic spinal cord injuries in adult rats: An integrative view of spinal nociceptive dysfunctions. Restor. Neurol. Neurosci. 2013, 31, 411–430. [Google Scholar] [CrossRef]
- Lee, C.; Won, D.; Cantoria, M.J.; Hamlin, M.; de Leon, R.D. Robotic assistance that encourages the generation of stepping rather than fully assisting movements is best for learning to step in spinally contused rats. J. Neurophysiol. 2011, 105, 2764–2771. [Google Scholar] [CrossRef]
- De Leon, R.D.; Acosta, C.N. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J. Neurotrauma 2006, 23, 1147–1163. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Roy, R.R. Robotic training and spinal cord plasticity. Brain Res. Bull. 2009, 78, 4–12. [Google Scholar] [CrossRef]
- Heng, C.; de Leon, R.D. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp. Neurol. 2009, 216, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharm. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yoon, Y.W.; Na, H.S.; Kim, S.H.; Chung, J.M. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994, 59, 369–376. [Google Scholar]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Rudy, T.A. Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 1976, 17, 1031–1036. [Google Scholar] [CrossRef]
- Reeve, A.J.; Dickenson, A.H.; Kerr, N.C. Spinal effects of bicuculline: Modulation of an allodynia-like state by an A1-receptor agonist, morphine, and an NMDA-receptor antagonist. J. Neurophysiol. 1998, 79, 1494–1507. [Google Scholar] [CrossRef]
- Sorkin, L.S.; Puig, S.; Jones, D.L. Spinal bicuculline produces hypersensitivity of dorsal horn neurons: Effects of excitatory amino acid antagonists. Pain 1998, 77, 181–190. [Google Scholar] [CrossRef]
- Harkema, S.J.; Hillyer, J.; Schmidt-Read, M.; Ardolino, E.; Sisto, S.A.; Behrman, A.L. Locomotor training: As a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 1588–1597. [Google Scholar] [CrossRef]
- Harkema, S.J.; Schmidt-Read, M.; Lorenz, D.J.; Edgerton, V.R.; Behrman, A.L. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Nees, T.A.; Tappe-Theodor, A.; Sliwinski, C.; Motsch, M.; Rupp, R.; Kuner, R.; Weidner, N.; Blesch, A. Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. Pain 2016, 157, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.J.; Herrity, A.N.; Smith, R.R.; Willhite, A.; Harrison, B.J.; Petruska, J.C.; Harkema, S.J.; Hubscher, C.H. Novel multi-system functional gains via task specific training in spinal cord injured male rats. J. Neurotrauma 2014, 31, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Yezierski, R.P. Spinal cord injury pain: Spinal and supraspinal mechanisms. J. Rehabil. Res. Dev. 2009, 46, 95–107. [Google Scholar] [CrossRef]
- Carlton, S.M.; Du, J.; Tan, H.Y.; Nesic, O.; Hargett, G.L.; Bopp, A.C.; Yamani, A.; Lin, Q.; Willis, W.D.; Hulsebosch, C.E. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 2009, 147, 265–276. [Google Scholar] [CrossRef]
- Hulsebosch, C.E. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp. Neurol. 2008, 214, 6–9. [Google Scholar] [CrossRef]
- Kitagawa, J.; Kanda, K.; Sugiura, M.; Tsuboi, Y.; Ogawa, A.; Shimizu, K.; Koyama, N.; Kamo, H.; Watanabe, T.; Ren, K.; et al. Effect of chronic inflammation on dorsal horn nociceptive neurons in aged rats. J. Neurophysiol. 2005, 93, 3594–3604. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002, 82, 981–1011. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Zhai, J.; Xue, J. The roles and applications of neural stem cells in spinal cord injury repair. Front. Bioeng. Biotechnol. 2022, 10, 966866. [Google Scholar] [CrossRef]
- Hao, J.X.; Xu, X.J.; Yu, Y.X.; Seiger, A.; Wiesenfeld-Hallin, Z. Baclofen reverses the hypersensitivity of dorsal horn wide dynamic range neurons to mechanical stimulation after transient spinal cord ischemia; implications for a tonic GABAergic inhibitory control of myelinated fiber input. J. Neurophysiol. 1992, 68, 392–396. [Google Scholar] [CrossRef]
- Hama, A.; Sagen, J. Altered antinociceptive efficacy of tramadol over time in rats with painful peripheral neuropathy. Eur. J. Pharmacol. 2007, 559, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Von Heijne, M.; Hao, J.X.; Sollevi, A.; Xu, X.J. Effects of intrathecal morphine, baclofen, clonidine and R-PIA on the acute allodynia-like behaviours after spinal cord ischaemia in rats. Eur. J. Pain 2001, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, A.B.; Yamakura, T.; Askalany, A.R.; Kohno, T.; Sakimura, K.; Baba, H. Effects of ketamine on acute somatic nociception in wild-type and N-methyl-D-aspartate (NMDA) receptor epsilon1 subunit knockout mice. Neuropharmacology 2006, 50, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Jorum, E.; Warncke, T.; Stubhaug, A. Cold allodynia and hyperalgesia in neuropathic pain: The effect of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine--a double-blind, cross-over comparison with alfentanil and placebo. Pain 2003, 101, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Lin, M.F.; Chen, Y.C.; Hung, C.H.; Tzeng, J.I.; Wang, J.J. Exercise training attenuates postoperative pain and expression of cytokines and N-methyl-D-aspartate receptor subunit 1 in rats. Reg. Anesth. Pain Med. 2013, 38, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Dugan, E.A.; Schachner, B.; Jergova, S.; Sagen, J. Intensive Locomotor Training Provides Sustained Alleviation of Chronic Spinal Cord Injury-Associated Neuropathic Pain: A Two-Year Pre-Clinical Study. J. Neurotrauma 2021, 38, 789–802. [Google Scholar] [CrossRef]
- Lundström, U.; Wahman, K.; Seiger, Å.; Gray, D.B.; Isaksson, G.; Lilja, M. Participation in activities and secondary health complications among persons aging with traumatic spinal cord injury. Spinal Cord 2017, 55, 367–372. [Google Scholar] [CrossRef]
- Simmons, O.L.; Kressler, J.; Nash, M.S. Reference fitness values in the untrained spinal cord injury population. Arch. Phys. Med. Rehabil. 2014, 95, 2272–2278. [Google Scholar] [CrossRef]
- Alcobendas-Maestro, M.; Esclarin-Ruz, A.; Casado-Lopez, R.M.; Munoz-Gonzalez, A.; Perez-Mateos, G.; Gonzalez-Valdizan, E.; Martín, J.L. Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: Randomized controlled trial. Neurorehabilit. Neural Repair 2012, 26, 1058–1063. [Google Scholar] [CrossRef]
- Hornby, T.G.; Reisman, D.S.; Ward, I.G.; Scheets, P.L.; Miller, A.; Haddad, D.; Fox, E.J.; Fritz, N.E.; Hawkins, K.; Henderson, C.E.; et al. Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. J. Neurol. Phys. Ther. 2020, 44, 49–100. [Google Scholar] [CrossRef]
- Van Straaten, M.G.; Cloud, B.A.; Morrow, M.M.; Ludewig, P.M.; Zhao, K.D. Effectiveness of home exercise on pain, function, and strength of manual wheelchair users with spinal cord injury: A high-dose shoulder program with telerehabilitation. Arch. Phys. Med. Rehabil. 2014, 95, 1810–1817.e2. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Kim, H.J.; Kwon, B.S.; Park, J.W.; Lee, H.J.; Yoo, A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jergova, S.; Dugan, E.A.; Sagen, J. Attenuation of SCI-Induced Hypersensitivity by Intensive Locomotor Training and Recombinant GABAergic Cells. Bioengineering 2023, 10, 84. https://doi.org/10.3390/bioengineering10010084
Jergova S, Dugan EA, Sagen J. Attenuation of SCI-Induced Hypersensitivity by Intensive Locomotor Training and Recombinant GABAergic Cells. Bioengineering. 2023; 10(1):84. https://doi.org/10.3390/bioengineering10010084
Chicago/Turabian StyleJergova, Stanislava, Elizabeth A. Dugan, and Jacqueline Sagen. 2023. "Attenuation of SCI-Induced Hypersensitivity by Intensive Locomotor Training and Recombinant GABAergic Cells" Bioengineering 10, no. 1: 84. https://doi.org/10.3390/bioengineering10010084
APA StyleJergova, S., Dugan, E. A., & Sagen, J. (2023). Attenuation of SCI-Induced Hypersensitivity by Intensive Locomotor Training and Recombinant GABAergic Cells. Bioengineering, 10(1), 84. https://doi.org/10.3390/bioengineering10010084





