Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions—Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy for Systematic Review
2.2. Eligibility
2.3. Population
2.4. Intervention (Exposure)
2.5. Comparison
2.6. Outcome
2.7. Study Design
2.8. Inclusion Criteria
2.9. Exclusion Criteria
2.10. Search Strategy for Narrative Review
3. Epidemiology
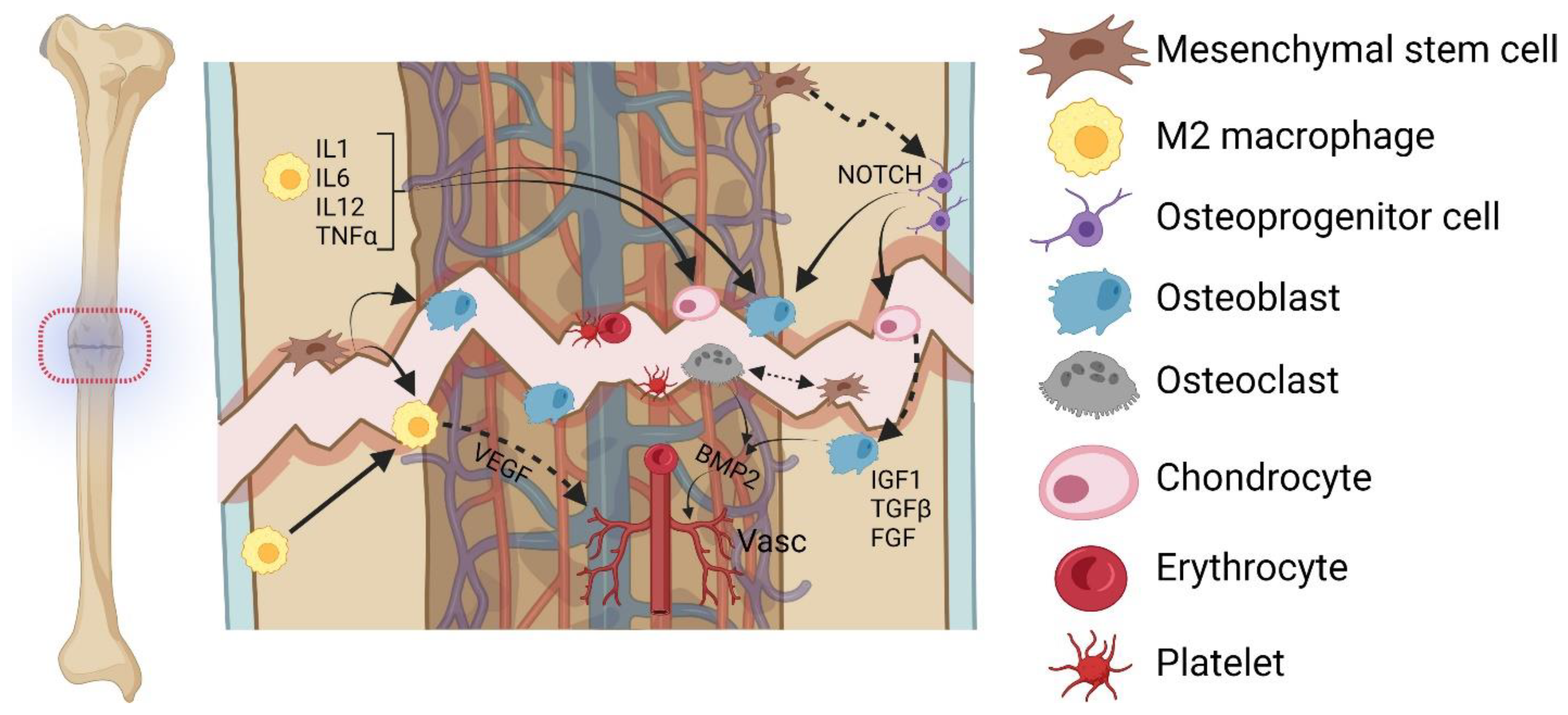
4. Cellular Components Contributing to (Impaired) Bone Healing
4.1. MSCs
4.2. Macrophages
4.3. Osteoprogenitor Cells
4.4. Osteoblasts
4.5. Osteocytes
4.6. Osteoclasts
4.7. Chondrocytes
4.8. Vasculature
5. Risk Factors for Non-Union
6. Therapeutic Options
7. Biomarkers for Non-Union
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghimire, S.; Miramini, S.; Edwards, G.; Rotne, R.; Xu, J.; Ebeling, P.; Zhang, L. The investigation of bone fracture healing under intramembranous and endochondral ossification. Bone Rep. 2021, 14, 100740. [Google Scholar] [CrossRef] [PubMed]
- Borgel, S.; Latimer, B.; McDermott, Y.; Sarig, R.; Pokhojaev, A.; Abulafia, T.; Goder-Goldberger, M.; Barzilai, O.; May, H. Early Upper Paleolithic human foot bones from Manot Cave, Israel. J. Hum. Evol. 2021, 160, 102668. [Google Scholar] [CrossRef] [PubMed]
- Alt, K.W.; Jeunesse, C.; Buitrago-Téllez, C.H.; Wächter, R.; Boës, E.; Pichler, S.L. Evidence for stone age cranial surgery. Nature 1997, 387, 360. [Google Scholar] [CrossRef] [PubMed]
- Riccomi, G.; Fornaciari, G.; Vitiello, A.; Bini, A.; Caramella, D.; Giuffra, V. Trepanation to Treat a Head Wound: A Case of Neurosurgery from 13th-Century Tuscany. World Neurosurg. 2017, 104, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.A.; Fessler, R.G. Paradigm changes in spine surgery: Evolution of minimally invasive techniques. Nat. Rev. Neurol. 2012, 8, 443–450. [Google Scholar] [CrossRef]
- Karthik, K.; Colegate-Stone, T.; Dasgupta, P.; Tavakkolizadeh, A.; Sinha, J. Robotic surgery in trauma and orthopaedics: A systematic review. Bone Jt. J. 2015, 97, 292–299. [Google Scholar] [CrossRef]
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The risk of non-union per fracture: Current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef]
- Buckley, R.E.; Moran, C.G. AO Principles of Fracture Management; Buckley, R.E., Moran, C.G., Apivatthakakul, T., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2018; ISBN 9783132423091. [Google Scholar]
- Özkan, S.; Nolte, P.A.; van den Bekerom, M.P.J.; Bloemers, F.W. Diagnosis and management of long-bone nonunions: A nationwide survey. Eur. J. Trauma Emerg. Surg. 2019, 45, 3–11. [Google Scholar] [CrossRef]
- Panagiotis, M. Classification of non-union. Injury 2005, 36 (Suppl. S4), S30–S37. [Google Scholar] [CrossRef]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45 (Suppl. S2), S3–S7. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Zimmermann, G.; Moghaddam, A. Trauma: Non-Union: New Trends. In European Instructional Lectures; Bentley, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 15–19. ISBN 978-3-642-11831-9. [Google Scholar]
- Walter, N.; Hierl, K.; Brochhausen, C.; Alt, V.; Rupp, M. The epidemiology and direct healthcare costs of aseptic nonunions in Germany—A descriptive report. Bone Jt. Res. 2022, 11, 541–547. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015, 70, 93–101. [Google Scholar] [CrossRef]
- Bishop, J.A.; Palanca, A.A.; Bellino, M.J.; Lowenberg, D.W. Assessment of compromised fracture healing. J. Am. Acad. Orthop. Surg. 2012, 20, 273–282. [Google Scholar] [CrossRef]
- Wildemann, B.; Ignatius, A.; Leung, F.; Taitsman, L.A.; Smith, R.M.; Pesántez, R.; Stoddart, M.J.; Richards, R.G.; Jupiter, J.B. Non-union bone fractures. Nat. Rev. Dis. Prim. 2021, 7, 57. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Edwards, E.R.; de Steiger, R.; Gabbe, B.J. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int. J. Environ. Res. Public Health 2018, 15, 2845. [Google Scholar] [CrossRef]
- Nauth, A.; Lee, M.; Gardner, M.J.; Brinker, M.R.; Warner, S.J.; Tornetta, P.; Leucht, P. Principles of Nonunion Management: State of the Art. J. Orthop. Trauma 2018, 32 (Suppl. S1), S52–S57. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38 (Suppl. S4), S3–S6. [Google Scholar] [CrossRef]
- Tanner, M.C.; Hagelskamp, S.; Vlachopoulos, W.; Miska, M.; Findeisen, S.; Grimm, A.; Schmidmaier, G.; Haubruck, P. Non-Union Treatment Based on the “Diamond Concept” Is a Clinically Effective and Safe Treatment Option in Older Adults. Clin. Interv. Aging 2020, 15, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Kanakaris, N.K.; Giannoudis, P.V. The health economics of the treatment of long-bone non-unions. Injury 2007, 38 (Suppl. S2), S77–S84. [Google Scholar] [CrossRef] [PubMed]
- Thurairajah, K.; Briggs, G.D.; Balogh, Z.J. Stem cell therapy for fracture non-union: The current evidence from human studies. J. Orthop. Surg. 2021, 29, 23094990211036545. [Google Scholar] [CrossRef] [PubMed]
- Antonova, E.; Le, T.K.; Burge, R.; Mershon, J. Tibia shaft fractures: Costly burden of nonunions. BMC Musculoskelet. Disord. 2013, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Sprague, S.; Bhandari, M. An economic evaluation of early versus delayed operative treatment in patients with closed tibial shaft fractures. Arch. Orthop. Trauma Surg. 2002, 122, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Khosla, S. Fracture Healing in the Setting of Endocrine Diseases, Aging, and Cellular Senescence. Endocr. Rev. 2022, 43, 984–1002. [Google Scholar] [CrossRef]
- Lai, S.H.S.; Tang, C.Q.Y.; Chiow, S.M.; Chia, D.S.Y. Renal impairment and time to fracture healing following surgical fixation of distal radius fracture. Eur. J. Orthop. Surg. Traumatol. 2022. [Google Scholar] [CrossRef]
- Rinderknecht, H.; Nussler, A.K.; Steinestel, K.; Histing, T.; Ehnert, S. Smoking Impairs Hematoma Formation and Dysregulates Angiogenesis as the First Steps of Fracture Healing. Bioengineering 2022, 9, 186. [Google Scholar] [CrossRef]
- Tanios, M.; Brickman, B.; Cage, E.; Abbas, K.; Smith, C.; Atallah, M.; Baroi, S.; Lecka-Czernik, B. Diabetes and Impaired Fracture Healing: A Narrative Review of Recent Literature. Curr. Osteoporos. Rep. 2022, 20, 229–239. [Google Scholar] [CrossRef]
- Otsuru, S.; Tamai, K.; Yamazaki, T.; Yoshikawa, H.; Kaneda, Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells 2008, 26, 223–234. [Google Scholar] [CrossRef]
- Wang, C.; Inzana, J.A.; Mirando, A.J.; Ren, Y.; Liu, Z.; Shen, J.; O’Keefe, R.J.; Awad, H.A.; Hilton, M.J. NOTCH signaling in skeletal progenitors is critical for fracture repair. J. Clin. Investig. 2016, 126, 1471–1481. [Google Scholar] [CrossRef]
- Hofmann, A.; Ritz, U.; Hessmann, M.H.; Schmid, C.; Tresch, A.; Rompe, J.D.; Meurer, A.; Rommens, P.M. Cell viability, osteoblast differentiation, and gene expression are altered in human osteoblasts from hypertrophic fracture non-unions. Bone 2008, 42, 894–906. [Google Scholar] [CrossRef]
- Orth, M.; Fritz, T.; Stutz, J.; Scheuer, C.; Ganse, B.; Bullinger, Y.; Lee, J.S.; Murphy, W.L.; Laschke, M.W.; Menger, M.D.; et al. Local Application of Mineral-Coated Microparticles Loaded With VEGF and BMP-2 Induces the Healing of Murine Atrophic Non-Unions. Front. Bioeng. Biotechnol. 2021, 9, 809397. [Google Scholar] [CrossRef]
- Cuthbert, R.J.; Jones, E.; Sanjurjo-Rodríguez, C.; Lotfy, A.; Ganguly, P.; Churchman, S.M.; Castana, P.; Tan, H.B.; McGonagle, D.; Papadimitriou, E.; et al. Regulation of Angiogenesis Discriminates Tissue Resident MSCs from Effective and Defective Osteogenic Environments. J. Clin. Med. 2020, 9, 1628. [Google Scholar] [CrossRef]
- Vallim, F.C.; Guimarães, J.A.M.; Dias, R.B.; Sartore, R.C.; Cavalcanti, A.D.S.; Leal, A.C.; Duarte, M.E.L.; Bonfim, D.C. Atrophic nonunion stromal cells form bone and recreate the bone marrow environment in vivo. OTA Int. 2018, 1, e008. [Google Scholar] [CrossRef]
- El-Jawhari, J.J.; Kleftouris, G.; El-Sherbiny, Y.; Saleeb, H.; West, R.M.; Jones, E.; Giannoudis, P.V. Defective Proliferation and Osteogenic Potential with Altered Immunoregulatory phenotype of Native Bone marrow-Multipotential Stromal Cells in Atrophic Fracture Non-Union. Sci. Rep. 2019, 9, 17340. [Google Scholar] [CrossRef]
- Chen, T.L. Inhibition of growth and differentiation of osteoprogenitors in mouse bone marrow stromal cell cultures by increased donor age and glucocorticoid treatment. Bone 2004, 35, 83–95. [Google Scholar] [CrossRef]
- Shigeno, Y.; Ashton, B.A. Human bone-cell proliferation in vitro decreases with human donor age. J. Bone Jt. Surg. Br. 1995, 77, 139–142. [Google Scholar] [CrossRef]
- Bajada, S.; Marshall, M.J.; Wright, K.T.; Richardson, J.B.; Johnson, W.E.B. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone 2009, 45, 726–735. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells Current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, X.; Liang, S.; Chen, Z.; Zhang, Y.; Qian, A.; Hu, L. The Application of MSCs-Derived Extracellular Vesicles in Bone Disorders: Novel Cell-Free Therapeutic Strategy. Front. Cell Dev. Biol. 2020, 8, 619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Chahla, J.; Safran, M.R.; Krych, A.J.; Saris, D.B.F.; Caplan, A.I.; LaPrade, R.F. International Expert Consensus on a Cell Therapy Communication Tool: DOSES. J. Bone Jt. Surg. Am. 2019, 101, 904–911. [Google Scholar] [CrossRef]
- Wu, A.C.; Raggatt, L.J.; Alexander, K.A.; Pettit, A.R. Unraveling macrophage contributions to bone repair. Bonekey Rep. 2013, 2, 373. [Google Scholar] [CrossRef]
- Grundnes, O.; Reikeraas, O. Effects of macrophage activation on bone healing. J. Orthop. Sci. 2000, 5, 243–247. [Google Scholar] [CrossRef]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J.; Nanchahal, J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1585–1590. [Google Scholar] [CrossRef]
- Löffler, J.; Sass, F.A.; Filter, S.; Rose, A.; Ellinghaus, A.; Duda, G.N.; Dienelt, A. Compromised Bone Healing in Aged Rats Is Associated With Impaired M2 Macrophage Function. Front. Immunol. 2019, 10, 2443. [Google Scholar] [CrossRef]
- Willenborg, S.; Lucas, T.; van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Khan, M.; Mahmood, R.; Mehmood, A.; Khan, S.N.; Riazuddin, S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol. Int. 2012, 36, 747–753. [Google Scholar] [CrossRef]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Horst, K.; Greven, J.; Lüken, H.; Zhi, Q.; Pfeifer, R.; Simon, T.P.; Relja, B.; Marzi, I.; Pape, H.-C.; Hildebrand, F. Trauma Severity and Its Impact on Local Inflammation in Extremity Injury—Insights From a Combined Trauma Model in Pigs. Front. Immunol. 2019, 10, 3028. [Google Scholar] [CrossRef]
- Neunaber, C.; Yesilkaya, P.; Pütz, C.; Krettek, C.; Hildebrand, F. Differentiation of osteoprogenitor cells is affected by trauma-haemorrhage. Injury 2013, 44, 1279–1284. [Google Scholar] [CrossRef]
- Sardesai, N.R.; Gaski, G.E.; Gunderson, Z.J.; Cunningham, C.M.; Slaven, J.; Meagher, A.D.; McKinley, T.O.; Natoli, R.M. Base Deficit ≥ 6 within 24 h of Injury is a risk factor for fracture nonunion in the polytraumatized patient. Injury 2021, 52, 3271–3276. [Google Scholar] [CrossRef]
- Xia, W.; Xie, J.; Cai, Z.; Liu, X.; Wen, J.; Cui, Z.-K.; Zhao, R.; Zhou, X.; Chen, J.; Mao, X.; et al. Damaged brain accelerates bone healing by releasing small extracellular vesicles that target osteoprogenitors. Nat. Commun. 2021, 12, 6043. [Google Scholar] [CrossRef]
- Muschler, G.F.; Nitto, H.; Boehm, C.A.; Easley, K.A. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J. Orthop. Res. 2001, 19, 117–125. [Google Scholar] [CrossRef]
- Hernigou, P.; Beaujean, F. Moelle osseuse des patients présentant une pseudarthrose. Etude des progéniteurs par clonage in vitro. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1997, 83, 33–40. [Google Scholar]
- Wagner, J.M.; Schmidt, S.V.; Dadras, M.; Huber, J.; Wallner, C.; Dittfeld, S.; Becerikli, M.; Jaurich, H.; Reinkemeier, F.; Drysch, M.; et al. Inflammatory processes and elevated osteoclast activity chaperon atrophic non-union establishment in a murine model. J. Transl. Med. 2019, 17, 416. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Menendez, A.; Fong, C.; Babey, M.; Tahimic, C.G.T.; Cheng, Z.; Li, A.; Chang, W.; Bikle, D.D. Osteoblast-Specific Loss of IGF1R Signaling Results in Impaired Endochondral Bone Formation During Fracture Healing. J. Bone Miner. Res. 2015, 30, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.H.V.; Wong, R.M.Y.; Chow, S.K.H.; Li, M.C.; Chim, Y.N.; Li, T.K.; Ho, W.T.; Cheng, J.C.Y.; Cheung, W.H. How much do we know about the role of osteocytes in different phases of fracture healing? A systematic review. J. Orthop. Transl. 2020, 21, 111–121. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Liedert, A.; Ignatius, A. Mechanobiology of bone remodeling and fracture healing in the aged organism. Innov. Surg. Sci. 2016, 1, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, I.N.C.; Cícero, A.M.; Issa, J.P.M.; Feldman, S. Bone fracture healing: Perspectives according to molecular basis. J. Bone Miner. Metab. 2021, 39, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shibata, Y.; Zhu, T.; Zhou, J.; Zhang, J. Osteocytes in bone aging: Advances, challenges, and future perspectives. Ageing Res. Rev. 2022, 77, 101608. [Google Scholar] [CrossRef]
- Farr, J.N.; Fraser, D.G.; Wang, H.; Jaehn, K.; Ogrodnik, M.B.; Weivoda, M.M.; Drake, M.T.; Tchkonia, T.; LeBrasseur, N.K.; Kirkland, J.L.; et al. Identification of Senescent Cells in the Bone Microenvironment. J. Bone Miner. Res. 2016, 31, 1920–1929. [Google Scholar] [CrossRef]
- Schira, J.; Schulte, M.; Döbele, C.; Wallner, C.; Abraham, S.; Daigeler, A.; Kneser, U.; Lehnhardt, M.; Behr, B. Human scaphoid non-unions exhibit increased osteoclast activity compared to adjacent cancellous bone. J. Cell. Mol. Med. 2015, 19, 2842–2850. [Google Scholar] [CrossRef]
- Hou, W.; Ye, C.; Li, W.; Zhang, W.; He, R.; Zheng, Q. Bioengineering application using co-cultured mesenchymal stem cells and preosteoclasts may effectively accelerate fracture healing. Med. Hypotheses 2019, 123, 24–26. [Google Scholar] [CrossRef]
- Kodama, J.; Wilkinson, K.J.; Iwamoto, M.; Otsuru, S.; Enomoto-Iwamoto, M. The role of hypertrophic chondrocytes in regulation of the cartilage-to-bone transition in fracture healing. Bone Rep. 2022, 17, 101616. [Google Scholar] [CrossRef]
- Hu, D.P.; Ferro, F.; Yang, F.; Taylor, A.J.; Chang, W.; Miclau, T.; Marcucio, R.S.; Bahney, C.S. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 2017, 144, 221–234. [Google Scholar] [CrossRef]
- Dimitriou, R.; Carr, I.M.; West, R.M.; Markham, A.F.; Giannoudis, P.V. Genetic predisposition to fracture non-union: A case control study of a preliminary single nucleotide polymorphisms analysis of the BMP pathway. BMC Musculoskelet. Disord. 2011, 12, 44. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. S2), 106–110. [Google Scholar] [CrossRef]
- Menger, M.M.; Laschke, M.W.; Nussler, A.K.; Menger, M.D.; Histing, T. The vascularization paradox of non-union formation. Angiogenesis 2022, 25, 279–290. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Oreffo, R.O.C. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Brownlow, H.C.; Reed, A.; Simpson, A.H.R.W. The vascularity of atrophic non-unions. Injury 2002, 33, 145–150. [Google Scholar] [CrossRef]
- Schwabe, P.; Simon, P.; Kronbach, Z.; Schmidmaier, G.; Wildemann, B. A pilot study investigating the histology and growth factor content of human non-union tissue. Int. Orthop. 2014, 38, 2623–2629. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef]
- Tikhonova, A.N.; Dolgalev, I.; Hu, H.; Sivaraj, K.K.; Hoxha, E.; Cuesta-Domínguez, Á.; Pinho, S.; Akhmetzyanova, I.; Gao, J.; Witkowski, M.; et al. The bone marrow microenvironment at single-cell resolution. Nature 2019, 569, 222–228. [Google Scholar] [CrossRef]
- van Gastel, N.; Stegen, S.; Eelen, G.; Schoors, S.; Carlier, A.; Daniëls, V.W.; Baryawno, N.; Przybylski, D.; Depypere, M.; Stiers, P.-J.; et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature 2020, 579, 111–117. [Google Scholar] [CrossRef]
- Zura, R.; Mehta, S.; Della Rocca, G.J.; Steen, R.G. Biological Risk Factors for Nonunion of Bone Fracture. JBJS Rev. 2016, 4, e5. [Google Scholar] [CrossRef]
- Nowak, J.; Holgersson, M.; Larsson, S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J. Shoulder Elb. Surg. 2004, 13, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Court-Brown, C.M.; McQueen, M.M.; Wakefield, A.E. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J. Bone Jt. Surg. Am. 2004, 86, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, M.R.; Will, E.; McQueen, M.M. Prediction of outcome after humeral diaphyseal fracture. Injury 2010, 41, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-L.; Chang, H.-C.; Lu, K.-H. Risk factors for nonunion in 337 displaced midshaft clavicular fractures treated with Knowles pin fixation. Arch. Orthop. Trauma Surg. 2013, 133, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Court-Brown, C.M.; McQueen, M.M. Nonunions of the proximal humerus: Their prevalence and functional outcome. J. Trauma 2008, 64, 1517–1521. [Google Scholar] [CrossRef]
- Jensen, S.S.; Jensen, N.M.; Gundtoft, P.H.; Kold, S.; Zura, R.; Viberg, B. Risk factors for nonunion following surgically managed, traumatic, diaphyseal fractures: A systematic review and meta-analysis. EFORT Open Rev. 2022, 7, 516–525. [Google Scholar] [CrossRef]
- Moghaddam, A.; Zimmermann, G.; Hammer, K.; Bruckner, T.; Grützner, P.A.; von Recum, J. Cigarette smoking influences the clinical and occupational outcome of patients with tibial shaft fractures. Injury 2011, 42, 1435–1442. [Google Scholar] [CrossRef]
- Westgeest, J.; Weber, D.; Dulai, S.K.; Bergman, J.W.; Buckley, R.; Beaupre, L.A. Factors Associated With Development of Nonunion or Delayed Healing After an Open Long Bone Fracture: A Prospective Cohort Study of 736 Subjects. J. Orthop. Trauma 2016, 30, 149–155. [Google Scholar] [CrossRef]
- Makaram, N.S.; Leow, J.M.; Clement, N.D.; Oliver, W.M.; Ng, Z.H.; Simpson, C.; Keating, J.F. Risk factors associated with delayed and aseptic nonunion following tibial diaphyseal fractures managed with intramedullary nailing. Bone Jt. Open 2021, 2, 227–235. [Google Scholar] [CrossRef]
- Murray, I.R.; Foster, C.J.; Eros, A.; Robinson, C.M. Risk factors for nonunion after nonoperative treatment of displaced midshaft fractures of the clavicle. J. Bone Jt. Surg. Am. 2013, 95, 1153–1158. [Google Scholar] [CrossRef]
- Hernigou, J.; Schuind, F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int. Orthop. 2013, 37, 883–887. [Google Scholar] [CrossRef]
- Goudie, E.B.; Robinson, C.M. Prediction of Nonunion After Nonoperative Treatment of a Proximal Humeral Fracture. J. Bone Jt. Surg. Am. 2021, 103, 668–680. [Google Scholar] [CrossRef]
- Ding, L.; He, Z.; Xiao, H.; Chai, L.; Xue, F. Factors affecting the incidence of aseptic nonunion after surgical fixation of humeral diaphyseal fracture. J. Orthop. Sci. 2014, 19, 973–977. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; MacDonald, D.A.; Matthews, S.J.; Smith, R.M.; Furlong, A.J.; Boer, P.D. Nonunion of the femoral diaphysis: The influence of reaming and non-steroidal anti-inflammatory drugs. J. Bone Jt. Surg. Br. 2000, 82, 655–658. [Google Scholar] [CrossRef]
- Scolaro, J.A.; Schenker, M.L.; Yannascoli, S.; Baldwin, K.; Mehta, S.; Ahn, J. Cigarette smoking increases complications following fracture: A systematic review. J. Bone Jt. Surg. Am. 2014, 96, 674–681. [Google Scholar] [CrossRef]
- Xu, B.; Anderson, D.B.; Park, E.-S.; Chen, L.; Lee, J.H. The influence of smoking and alcohol on bone healing: Systematic review and meta-analysis of non-pathological fractures. EClinicalMedicine 2021, 42, 101179. [Google Scholar] [CrossRef]
- Pearson, R.G.; Clement, R.G.E.; Edwards, K.L.; Scammell, B.E. Do smokers have greater risk of delayed and non-union after fracture, osteotomy and arthrodesis? A systematic review with meta-analysis. BMJ Open 2016, 6, e010303. [Google Scholar] [CrossRef]
- Quan, K.; Xu, Q.; Zhu, M.; Liu, X.; Dai, M. Analysis of Risk Factors for Non-union After Surgery for Limb Fractures: A Case-Control Study of 669 Subjects. Front. Surg. 2021, 8, 754150. [Google Scholar] [CrossRef]
- Thakore, R.V.; Francois, E.L.; Nwosu, S.K.; Attum, B.; Whiting, P.S.; Siuta, M.A.; Benvenuti, M.A.; Smith, A.K.; Shen, M.S.; Mousavi, I.; et al. The Gustilo-Anderson classification system as predictor of nonunion and infection in open tibia fractures. Eur. J. Trauma Emerg. Surg. 2017, 43, 651–656. [Google Scholar] [CrossRef]
- Nowak, J.; Holgersson, M.; Larsson, S. Sequelae from clavicular fractures are common: A prospective study of 222 patients. Acta Orthop. 2005, 76, 496–502. [Google Scholar] [CrossRef]
- Heath, D.; Momtaz, D.; Ghali, A.; Salazar, L.; Gibbons, S.; Hogue, G. Obesity Increases Time to Union in Surgically Treated Pediatric Fracture Patients. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2022, 6, e21.00185. [Google Scholar] [CrossRef] [PubMed]
- Liska, F.; Haller, B.; Voss, A.; Mehl, J.; Imhoff, F.B.; Willinger, L.; Imhoff, A.B. Smoking and obesity influence the risk of nonunion in lateral opening wedge, closing wedge and torsional distal femoral osteotomies. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- van Wunnik, B.P.W.; Weijers, P.H.E.; van Helden, S.H.; Brink, P.R.G.; Poeze, M. Osteoporosis is not a risk factor for the development of nonunion: A cohort nested case-control study. Injury 2011, 42, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.D.; Bennet, S.J.; Aderinto, J.; Keating, J.F. Fixation of intracapsular fractures of the femoral neck in young patients: Risk factors for failure. J. Bone Jt. Surg. Br. 2011, 93, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.K.; Do, T.P.; Critchlow, C.W.; Dent, R.E.; Jick, S.S. Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop. 2012, 83, 653–660. [Google Scholar] [CrossRef]
- Zura, R.; Kaste, S.C.; Heffernan, M.J.; Accousti, W.K.; Gargiulo, D.; Wang, Z.; Steen, R.G. Risk factors for nonunion of bone fracture in pediatric patients: An inception cohort study of 237,033 fractures. Medicine 2018, 97, e11691. [Google Scholar] [CrossRef]
- Fernandez-Arroyabe, N.; García-Meléndez, G.; de Castro-Almeida, A.R.; Escalona-Perez, F.; Pérez-Lara, A.; González-Quevedo, D.; García-Quevedo, D.; Tamimi, I. Non-union and use of proton pump inhibitors in the treatment of femoral and tibial shaft fractures: A nested case-control study. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 1371–1377. [Google Scholar] [CrossRef]
- Foulke, B.A.; Kendal, A.R.; Murray, D.W.; Pandit, H. Fracture healing in the elderly: A review. Maturitas 2016, 92, 49–55. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Blokhuis, T.J.; Pape, H.C.; Giannoudis, P.V. Pharmacological agents and impairment of fracture healing: What is the evidence? Injury 2008, 39, 384–394. [Google Scholar] [CrossRef]
- Baht, G.S.; Vi, L.; Alman, B.A. The Role of the Immune Cells in Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 138–145. [Google Scholar] [CrossRef]
- Muire, P.J.; Mangum, L.H.; Wenke, J.C. Time Course of Immune Response and Immunomodulation During Normal and Delayed Healing of Musculoskeletal Wounds. Front. Immunol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Richardson, J.; Hill, A.M.; Johnston, C.J.C.; McGregor, A.; Norrish, A.R.; Eastwood, D.; Lavy, C.B.D. Fracture healing in HIV-positive populations. J. Bone Jt. Surg. Br. 2008, 90, 988–994. [Google Scholar] [CrossRef]
- Schlundt, C.; Schell, H.; Goodman, S.B.; Vunjak-Novakovic, G.; Duda, G.N.; Schmidt-Bleek, K. Immune modulation as a therapeutic strategy in bone regeneration. J. Exp. Orthop. 2015, 2, 1. [Google Scholar] [CrossRef]
- Kaspiris, A.; Hadjimichael, A.C.; Vasiliadis, E.S.; Papachristou, D.J.; Giannoudis, P.V.; Panagiotopoulos, E.C. Therapeutic Efficacy and Safety of Osteoinductive Factors and Cellular Therapies for Long Bone Fractures and Non-Unions: A Meta-Analysis and Systematic Review. J. Clin. Med. 2022, 11, 3901. [Google Scholar] [CrossRef]
- Piuzzi, N.S.; Mantripragada, V.P.; Sumski, A.; Selvam, S.; Boehm, C.; Muschler, G.F. Bone Marrow-Derived Cellular Therapies in Orthopaedics: Part I: Recommendations for Bone Marrow Aspiration Technique and Safety. JBJS Rev. 2018, 6, e4. [Google Scholar] [CrossRef]
- Miller, M.A.; Ivkovic, A.; Porter, R.; Harris, M.B.; Estok, D.M.; Smith, R.M.; Evans, C.H.; Vrahas, M.S. Autologous bone grafting on steroids: Preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int. Orthop. 2011, 35, 599–605. [Google Scholar] [CrossRef]
- Porter, R.M.; Liu, F.; Pilapil, C.; Betz, O.B.; Vrahas, M.S.; Harris, M.B.; Evans, C.H. Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J. Orthop. Res. 2009, 27, 42–49. [Google Scholar] [CrossRef]
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Jt. Surg. Am. 2005, 87, 1430–1437. [Google Scholar] [CrossRef]
- Brozovich, A.; Sinicrope, B.J.; Bauza, G.; Niclot, F.B.; Lintner, D.; Taraballi, F.; McCulloch, P.C. High Variability of Mesenchymal Stem Cells Obtained via Bone Marrow Aspirate Concentrate Compared With Traditional Bone Marrow Aspiration Technique. Orthop. J. Sports Med. 2021, 9, 23259671211058459. [Google Scholar] [CrossRef]
- Cotter, E.J.; Wang, K.C.; Yanke, A.B.; Chubinskaya, S. Bone Marrow Aspirate Concentrate for Cartilage Defects of the Knee: From Bench to Bedside Evidence. Cartilage 2018, 9, 161–170. [Google Scholar] [CrossRef]
- Harrison, A.; Lin, S.; Pounder, N.; Mikuni-Takagaki, Y. Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics 2016, 70, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.D.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Kilcoyne, R.F. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J. Bone Jt. Surg. Am. 1994, 76, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, T.K.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Roe, L.R. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J. Bone Jt. Surg. Am. 1997, 79, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Busse, J.W.; Bhandari, M.; Einhorn, T.A.; Schemitsch, E.; Heckman, J.D.; Tornetta, P.; Leung, K.-S.; Heels-Ansdell, D.; Makosso-Kallyth, S.; Della Rocca, G.J.; et al. Re-evaluation of low intensity pulsed ultrasound in treatment of tibial fractures (TRUST): Randomized clinical trial. BMJ 2016, 355, i5351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poolman, R.W.; Agoritsas, T.; Siemieniuk, R.A.C.; Harris, I.A.; Schipper, I.B.; Mollon, B.; Smith, M.; Albin, A.; Nador, S.; Sasges, W.; et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: A clinical practice guideline. BMJ 2017, 356, j576. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, T.; Liu, F.; Qu, J.; Chen, Y.; Fan, S.; Chen, H.; Sun, L.; Zhao, C.; Hu, J.; et al. Effect of Low-Intensity Pulsed Ultrasound After Autologous Adipose-Derived Stromal Cell Transplantation for Bone-Tendon Healing in a Rabbit Model. Am. J. Sports Med. 2019, 47, 942–953. [Google Scholar] [CrossRef]
- Elvey, M.H.; Miller, R.; Khor, K.S.; Protopapa, E.; Horwitz, M.D.; Hunter, A.R. The use of low-intensity pulsed ultrasound in hand and wrist nonunions. J. Plast. Surg. Hand Surg. 2020, 54, 101–106. [Google Scholar] [CrossRef]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e1900155. [Google Scholar] [CrossRef]
- Ehnert, S.; Falldorf, K.; Fentz, A.-K.; Ziegler, P.; Schröter, S.; Freude, T.; Ochs, B.G.; Stacke, C.; Ronniger, M.; Sachtleben, J.; et al. Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure—Clinical implication possible. Bone Rep. 2015, 3, 48–56. [Google Scholar] [CrossRef]
- Shi, H.; Xiong, J.; Chen, Y.; Wang, J.; Qiu, X.; Wang, Y.; Qiu, Y. Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: A prospective randomized controlled study. BMC Musculoskelet. Disord. 2013, 14, 35. [Google Scholar] [CrossRef]
- Caliogna, L.; Bina, V.; Brancato, A.M.; Gastaldi, G.; Annunziata, S.; Mosconi, M.; Grassi, F.A.; Benazzo, F.; Pasta, G. The Role of PEMFs on Bone Healing: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 14298. [Google Scholar] [CrossRef]
- Chen, Y.; Menger, M.M.; Braun, B.J.; Schweizer, S.; Linnemann, C.; Falldorf, K.; Ronniger, M.; Wang, H.; Histing, T.; Nussler, A.K.; et al. Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing. Bioengineering 2021, 8, 167. [Google Scholar] [CrossRef]
- Umiatin, U.; Dilogo, I.H.; Sari, P.; Wijaya, S.K. Histological Analysis of Bone Callus in Delayed Union Model Fracture Healing Stimulated with Pulsed Electromagnetic Fields (PEMF). Scientifica 2021, 2021, 4791172. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Qu, M.; Huang, X.; Yin, L.; Liao, Y.; Huang, F.; Ning, P.; Zhong, P.; Zeng, Y. Effect of the Pulsed Electromagnetic Field Treatment in a Rat Model of Senile Osteoporosis In Vivo. Bioelectromagnetics 2022, 43, 438–447. [Google Scholar] [CrossRef]
- Sohn, H.-S.; Oh, J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112466. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Schneider, S.; Unger, M.; van Griensven, M.; Balmayor, E.R. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur. J. Med. Res. 2017, 22, 17. [Google Scholar] [CrossRef]
- Palombella, S.; Lopa, S.; Gianola, S.; Zagra, L.; Moretti, M.; Lovati, A.B. Bone Marrow-Derived Cell Therapies to Heal Long-Bone Nonunions: A Systematic Review and Meta-Analysis—Which Is the Best Available Treatment? Stem Cells Int. 2019, 2019, 3715964. [Google Scholar] [CrossRef]
- Freitas, J.; Santos, S.G.; Gonçalves, R.M.; Teixeira, J.H.; Barbosa, M.A.; Almeida, M.I. Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects. Int. J. Mol. Sci. 2019, 20, 3430. [Google Scholar] [CrossRef]
- Rubin, J.; Rubin, C.; Jacobs, C.R. Molecular pathways mediating mechanical signaling in bone. Gene 2005, 367, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell Dev. Biol. 2021, 9, 770143. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, R.E.; Silva, M.J. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013, 1, 311–322. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Pneumaticos, S.; Giannoudis, P.V. Fracture non-union: Can biomarkers predict outcome? Injury 2013, 44, 1725–1732. [Google Scholar] [CrossRef]
- Zimmermann, G.; Henle, P.; Küsswetter, M.; Moghaddam, A.; Wentzensen, A.; Richter, W.; Weiss, S. TGF-beta1 as a marker of delayed fracture healing. Bone 2005, 36, 779–785. [Google Scholar] [CrossRef]
- Zimmermann, G.; Moghaddam, A.; Reumann, M.; Wangler, B.; Breier, L.; Wentzensen, A.; Henle, P.; Weiss, S. TGF-beta1 als pathophysiologischer Faktor bei der Frakturheilung. Unfallchirurg 2007, 110, 130–136. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Gan, Y.; Dai, K.; Zhao, J.; Huang, M.; Huang, Y.; Zhuang, Y.; Zhang, X. High-Dose TGF-β1 Impairs Mesenchymal Stem Cell-Mediated Bone Regeneration via Bmp2 Inhibition. J. Bone Miner. Res. 2020, 35, 167–180. [Google Scholar] [CrossRef]
- Könnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.-D.; Radbruch, A.; Duda, G.N.; et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014, 64, 155–165. [Google Scholar] [CrossRef]
- Cheng, A.; Vantucci, C.E.; Krishnan, L.; Ruehle, M.A.; Kotanchek, T.; Wood, L.B.; Roy, K.; Guldberg, R.E. Early systemic immune biomarkers predict bone regeneration after trauma. Proc. Natl. Acad. Sci. USA 2021, 118, e2017889118. [Google Scholar] [CrossRef]
- Kirkwood, K.L.; Zhang, L.; Thiyagarajan, R.; Seldeen, K.L.; Troen, B.R. Myeloid-Derived Suppressor Cells at the Intersection of Inflammaging and Bone Fragility. Immunol. Investig. 2018, 47, 844–854. [Google Scholar] [CrossRef]
- Panteli, M.; Vun, J.S.H.; Pountos, I.; Howard, A.J.; Jones, E.; Giannoudis, P.V. Biological and molecular profile of fracture non-union tissue: A systematic review and an update on current insights. J. Cell. Mol. Med. 2022, 26, 601–623. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Yu, Y.; Zhao, Y.; Zhang, D.; Yu, A. Identification of Up-Regulated ANXA3 Resulting in Fracture Non-Union in Patients With T2DM. Front. Endocrinol. 2022, 13, 890941. [Google Scholar] [CrossRef]
- Wu, J.; Liu, L.; Hu, H.; Gao, Z.; Lu, S. Bioinformatic analysis and experimental identification of blood biomarkers for chronic nonunion. J. Orthop. Surg. Res. 2020, 15, 208. [Google Scholar] [CrossRef]
- Burska, A.N.; Giannoudis, P.V.; Tan, B.H.; Ilas, D.; Jones, E.; Ponchel, F. Dynamics of Early Signalling Events during Fracture Healing and Potential Serum Biomarkers of Fracture Non-Union in Humans. J. Clin. Med. 2020, 9, 492. [Google Scholar] [CrossRef]

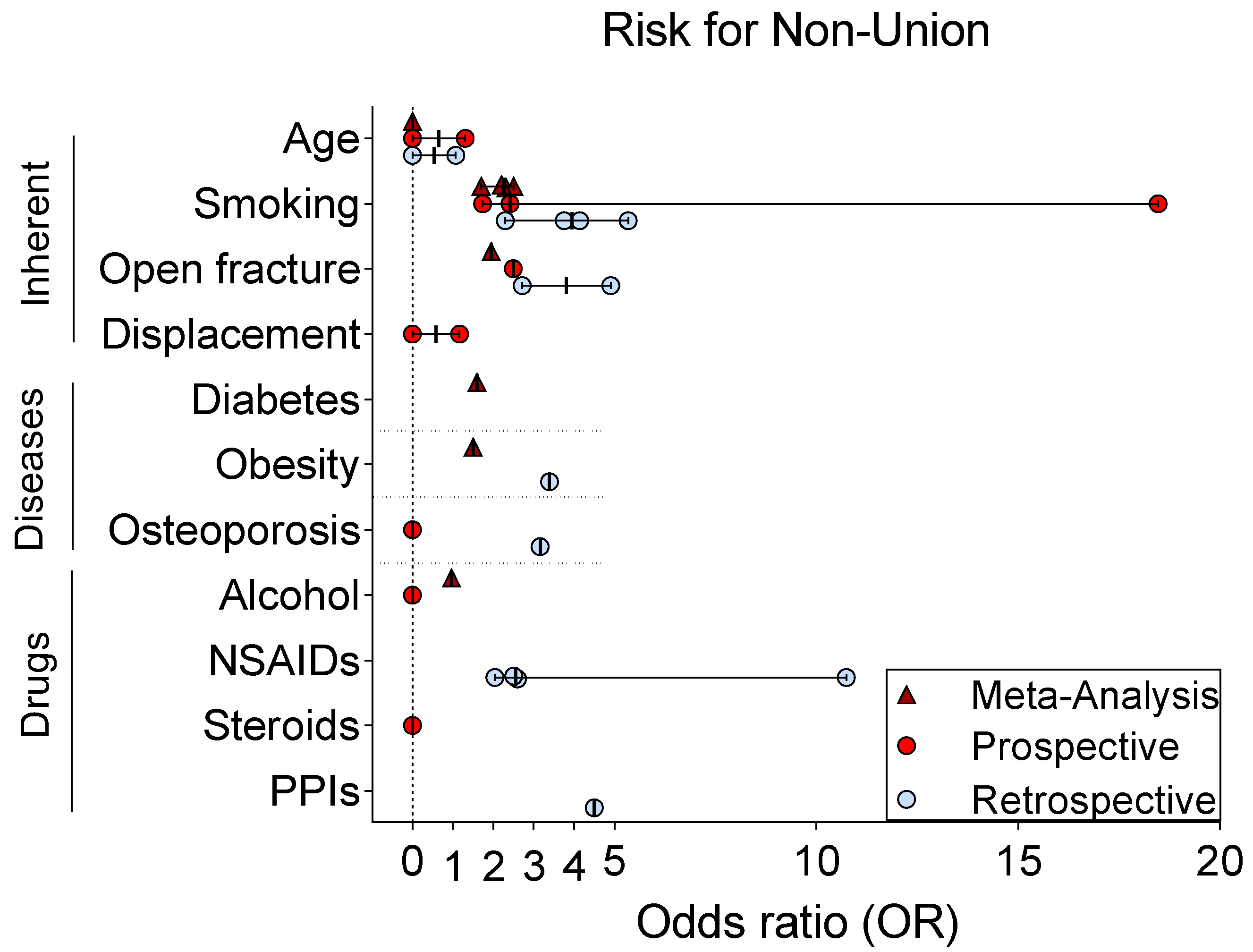
| Factor | Study | Study Design | Bone | Patients (n) | Non-Unions (n) | Follow-Up (yrs) | Result (OR) |
|---|---|---|---|---|---|---|---|
| Age | [83] | Prospective | Clavicle | 245 | 15 | 9–10 | 1.3 |
| [84] | Prospective | Clavicle | 868 | 53 | 0.5 | - | |
| [85] | Prospective | Humerus | 110 | 16 | 1 | neg | |
| [86] | Retrospective | Clavicle | 337 | 19 | ≥0.5 | 1.07 | |
| [87] | Retrospective | Humerus | 1027 | 11 | 1 | neg | |
| [88] | Meta-Analysis | Diaphyseal | 38,465 | 3975 | neg | ||
| Sex | [88] | Meta-Analysis | Diaphyseal | 38,465 | 3975 | neg | |
| Smoking | [89] | Prospective | Tibia | 85 | 9 | 3 | 18.46 |
| [90] | Prospective | Long bone | 736 | 124 | 1 | 1.73 | |
| [91] | Prospective | Tibia | 647 | 41 | 0.5 | 2.417 | |
| [92] | Retrospective | Clavicle | 1196 | 125 | - | 3.76 | |
| [93] | Retrospective | Diaphyseal | 114 | 38 | ≥1 | 4.14 | |
| [94] | Retrospective | Humerus | 2230 | 231 | n.a. | pos | |
| [95] | Retrospective | Humerus | 659 | 24 | 0.75 | 5.34 | |
| [96] | Retrospective (case-control) | Femur | 32 | 32 | n.a. | 2.29 | |
| [97] | Meta-Analysis | All bones | 6356 | 2.32 | |||
| [98] | Meta-Analysis | All bones | 39,920 | 2.5 | |||
| [99] | Meta-Analysis | All bones | 7516 | 2.2 | |||
| [88] | Meta-Analysis | Diaphyseal | 38,465 | 1.7 | |||
| Open fracture | [90] | Prospective | Long bone | 736 | 124 | 1 | 2.49 |
| [100] | Retrospective (case-control) | Limb | 446 | 223 | 0.75 | 2.71 | |
| [101] | Retrospective | Tibia | 486 | 56 | n.a. | 4.91 | |
| [97] | Meta-Analysis | All bones | 6356 | 1.95 | |||
| Displacement | [84] | Prospective | Clavicle | 868 | 53 | 0.5 | neg |
| [102] | Prospective | Clavicle | 222 | 15 | 2 | - | |
| [92] | Prospective | Clavicle | 941 | 125 | - | 1.17 | |
| Diabetes | [88] | Meta-Analysis | Diaphyseal | 38,465 | 3975 | 1.6 | |
| Obesity | [103] | Retrospective (delayed union) | Extremity | 147 | - | - | 3.39 |
| [104] | Retrospective (osteotomy) | Femur | 150 | 7 | 1 | ||
| [88] | Meta-Analysis | Diaphyseal | 38,465 | 3975 | 1.5 | ||
| Osteoporosis | [105] | Prospective (case-control) | All bones | 1498 | 40 | 1 | neg |
| [95] | Retrospective | Humerus | 659 | 24 | pos | ||
| [100] | Retrospective (case-control) | Limb | 446 | 223 | 0.75 | 3.16 | |
| Alcohol | [106] | Prospective | Femur | 112 | 9 | 4.8 (mean) | neg |
| [98] | Meta-Analysis | All bones | 39,920 | 0.97 | |||
| Drugs | |||||||
| NSAIDs | [107] | Retrospective (case-control) | All bones | 2257 | 401 | 1 | 2.6 |
| [96] | Retrospective (case-control) | Femur | 32 | 32 | n.a. | 10.74 | |
| [100] | Retrospective (case-control) | Limb | 446 | 223 | 0.75 | 2.04 | |
| [95] | Retrospective | Humerus | 659 | 24 | 0.75 | 2.51 | |
| Steroids | [91] | Prospective | Tibia | 647 | 41 | 2 | neg |
| [108] | Retrospective (pediatric) | All bones | 237,033 | 2003 | 1 | pos | |
| PPIs | [109] | Retrospective (case-control) | Femur and tibia | 254 | 12 | n.a. | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saul, D.; Menger, M.M.; Ehnert, S.; Nüssler, A.K.; Histing, T.; Laschke, M.W. Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions—Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors. Bioengineering 2023, 10, 85. https://doi.org/10.3390/bioengineering10010085
Saul D, Menger MM, Ehnert S, Nüssler AK, Histing T, Laschke MW. Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions—Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors. Bioengineering. 2023; 10(1):85. https://doi.org/10.3390/bioengineering10010085
Chicago/Turabian StyleSaul, Dominik, Maximilian M. Menger, Sabrina Ehnert, Andreas K. Nüssler, Tina Histing, and Matthias W. Laschke. 2023. "Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions—Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors" Bioengineering 10, no. 1: 85. https://doi.org/10.3390/bioengineering10010085
APA StyleSaul, D., Menger, M. M., Ehnert, S., Nüssler, A. K., Histing, T., & Laschke, M. W. (2023). Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions—Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors. Bioengineering, 10(1), 85. https://doi.org/10.3390/bioengineering10010085