Fracture Healing in Elderly Mice and the Effect of an Additional Severe Blood Loss: A Radiographic and Biomechanical Murine Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
2.2. Age Calculation
2.3. Operation Steps
2.4. Harvesting Procedure
2.5. µCT Analysis
2.6. Three-Point Bending Assay
2.7. Statistical Evaluation
3. Results
3.1. Survival Rate, Physiological Response and Macroscopic Evaluation
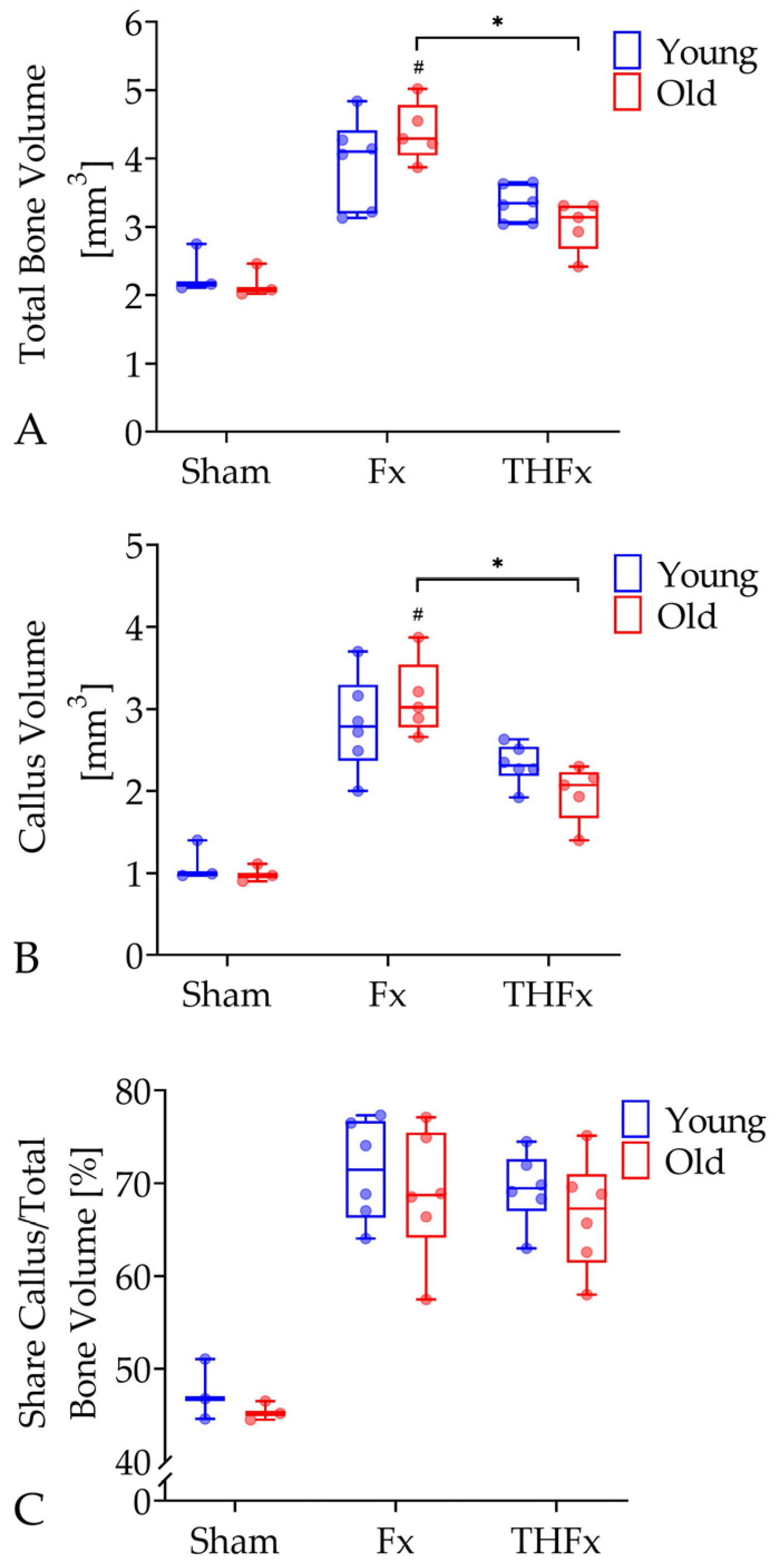
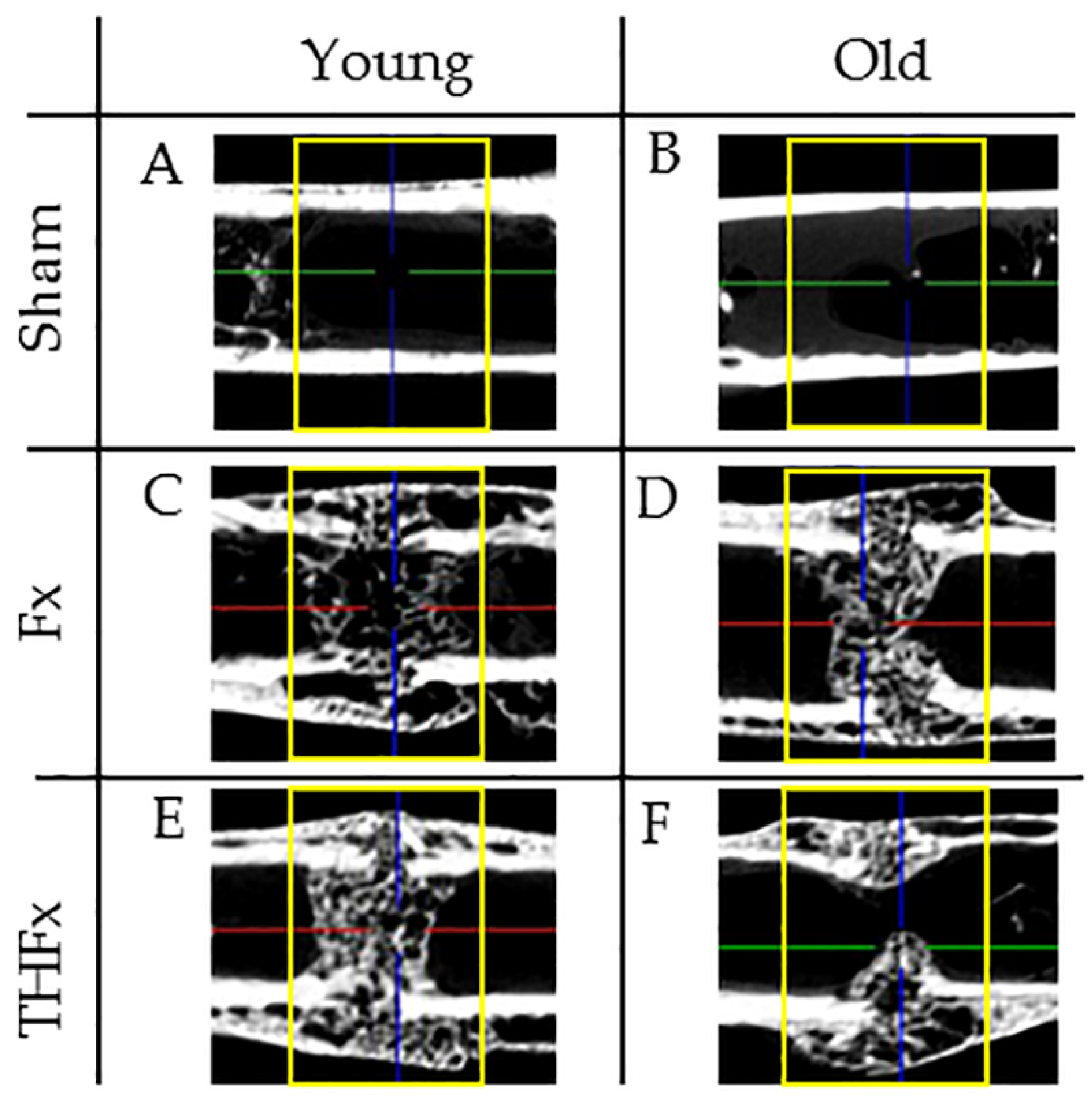
3.2. µCT Evaluation In Vivo after Two Weeks
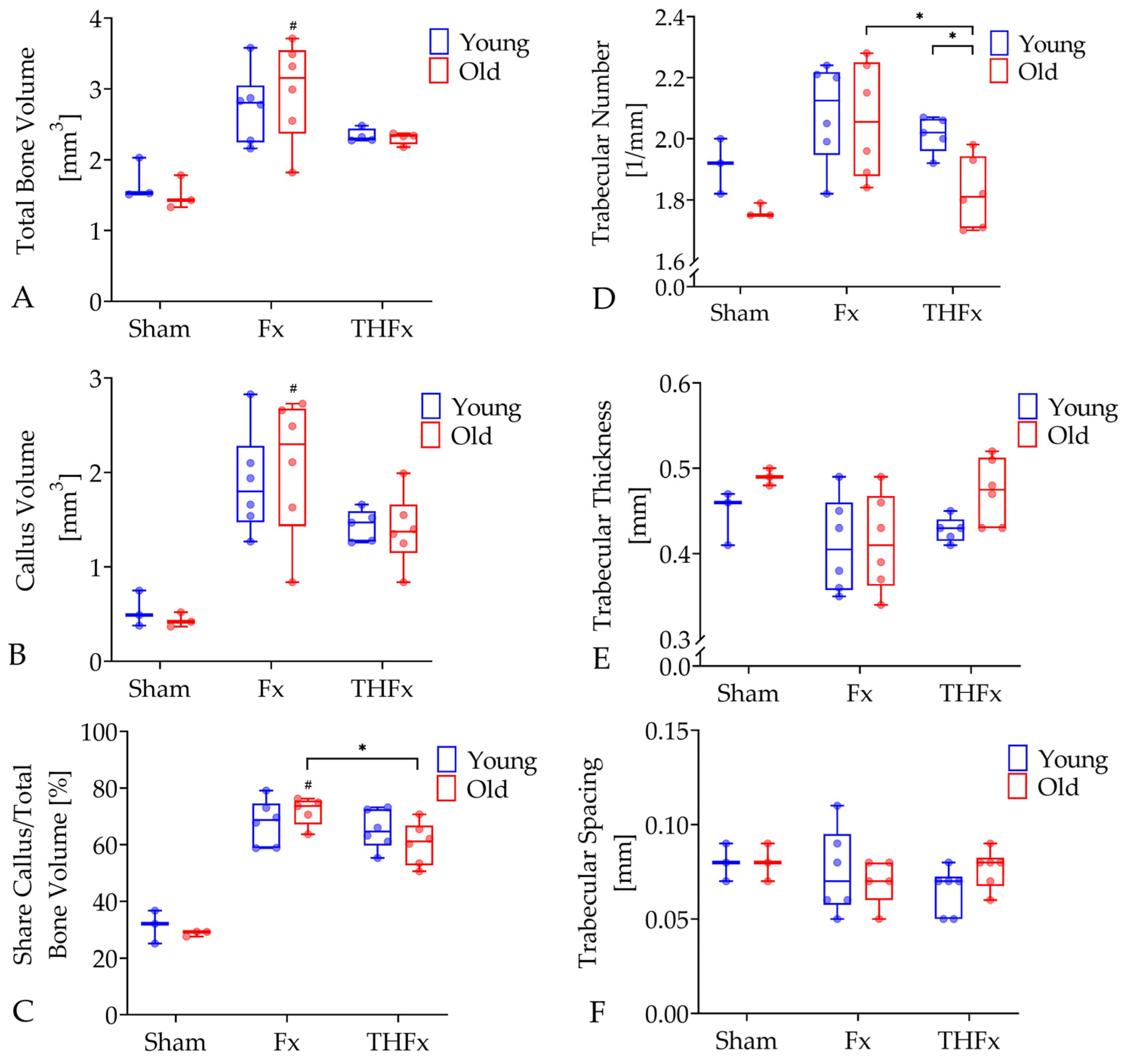
3.3. µCT Evaluation Ex Vivo after Three Weeks
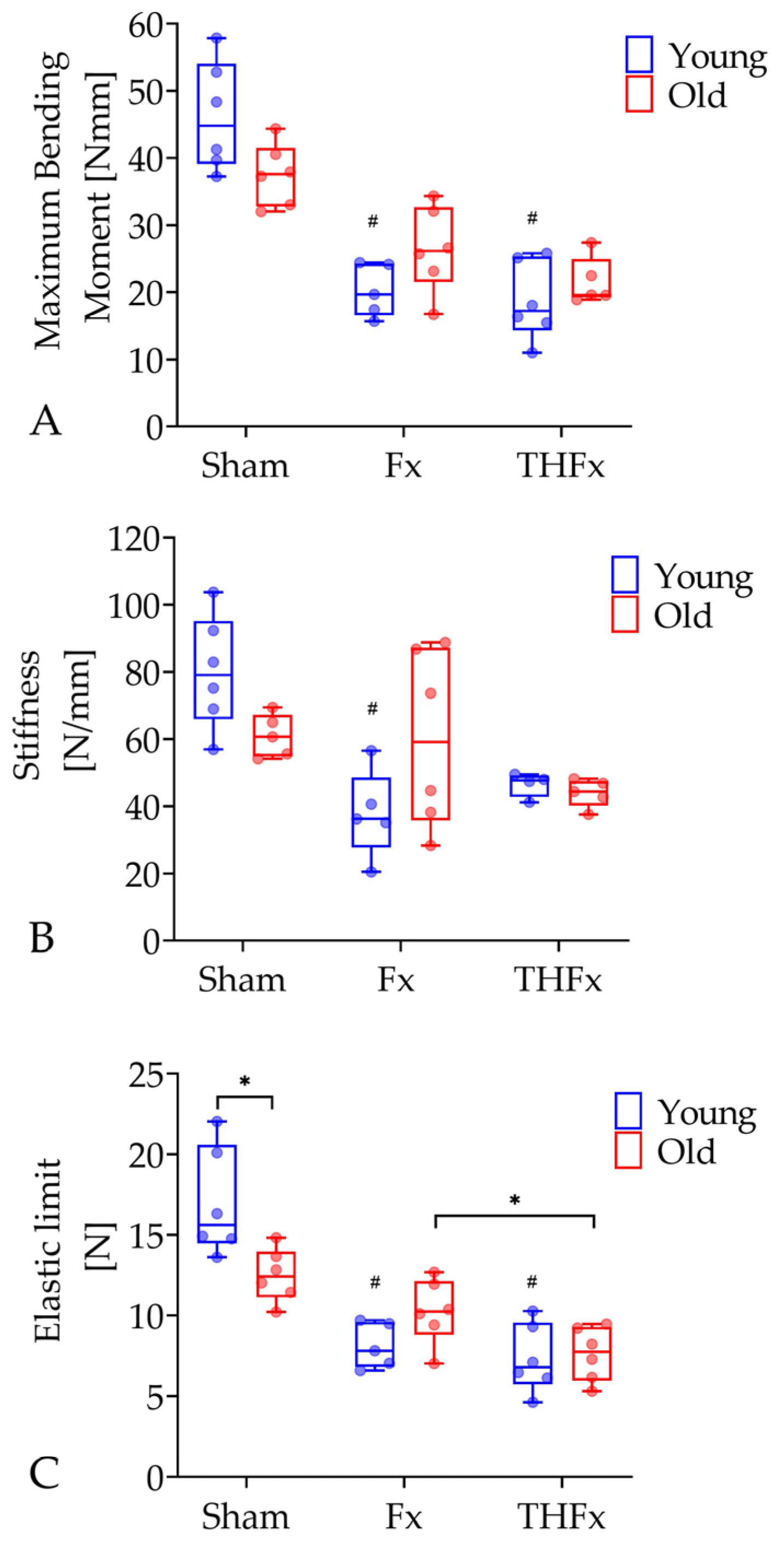
3.4. Biomechanical Evaluation after Three Weeks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Department of Economic and Social Affairs. World Population Ageing 2017 Highlights; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Borgstrom, F.; Karlsson, L.; Ortsater, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Gorter, E.A.; Reinders, C.R.; Krijnen, P.; Appelman-Dijkstra, N.M.; Schipper, I.B. The effect of osteoporosis and its treatment on fracture healing a systematic review of animal and clinical studies. Bone Rep. 2021, 15, 101117. [Google Scholar] [CrossRef] [PubMed]
- Meinberg, E.G.; Clark, D.; Miclau, K.R.; Marcucio, R.; Miclau, T. Fracture repair in the elderly: Clinical and experimental considerations. Injury 2019, 50 (Suppl. S1), S62–S65. [Google Scholar] [CrossRef] [PubMed]
- Aho, A.J. Electron microscopic and histologic studies on fracture repair in old and young rats. Acta Chir. Scand. Suppl. 1966, 357, 162–165. [Google Scholar]
- Bak, B.; Andreassen, T.T. The effect of aging on fracture healing in the rat. Calcif. Tissue Int. 1989, 45, 292–297. [Google Scholar] [CrossRef]
- Hemmatian, H.; Laurent, M.R.; Bakker, A.D.; Vanderschueren, D.; Klein-Nulend, J.; van Lenthe, G.H. Age-related changes in female mouse cortical bone microporosity. Bone 2018, 113, 1–8. [Google Scholar] [CrossRef]
- Histing, T.; Kuntz, S.; Stenger, D.; Scheuer, C.; Garcia, P.; Holstein, J.H.; Klein, M.; Pohlemann, T.; Menger, M.D. Delayed fracture healing in aged senescence-accelerated P6 mice. J. Investig. Surg. 2013, 26, 30–35. [Google Scholar] [CrossRef]
- Lu, C.; Miclau, T.; Hu, D.; Hansen, E.; Tsui, K.; Puttlitz, C.; Marcucio, R.S. Cellular basis for age-related changes in fracture repair. J. Orthop. Res. 2005, 23, 1300–1307. [Google Scholar] [CrossRef]
- Prisby, R.D.; Ramsey, M.W.; Behnke, B.J.; Dominguez, J.M., 2nd; Donato, A.J.; Allen, M.R.; Delp, M.D. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J. Bone Miner. Res. 2007, 22, 1280–1288. [Google Scholar] [CrossRef]
- Sethe, S.; Scutt, A.; Stolzing, A. Aging of mesenchymal stem cells. Ageing Res. Rev. 2006, 5, 91–116. [Google Scholar] [CrossRef]
- Lichte, P.; Kobbe, P.; Pfeifer, R.; Campbell, G.C.; Beckmann, R.; Tohidnezhad, M.; Bergmann, C.; Kadyrov, M.; Fischer, H.; Gluer, C.C.; et al. Impaired Fracture Healing after Hemorrhagic Shock. Mediat. Inflamm. 2015, 2015, 132451. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. Emerging bone healing therapies. J. Orthop. Trauma. 2010, 24 (Suppl. S1), S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.; Hardy, B.M.; Yoshino, O.; Buxton, A.; Quail, A.; Balogh, Z.J. The effect of haemorrhagic shock and resuscitation on fracture healing in a rabbit model: An animal study. Bone Jt. J. 2018, 100-B, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Bumann, M.; Henke, T.; Gerngross, H.; Claes, L.; Augat, P. Influence of haemorrhagic shock on fracture healing. Langenbecks Arch. Surg. 2003, 388, 331–338. [Google Scholar] [CrossRef]
- Bundkirchen, K.; Macke, C.; Angrisani, N.; Schack, L.M.; Noack, S.; Fehr, M.; Krettek, C.; Neunaber, C. Hemorrhagic shock alters fracture callus composition and activates the IL6 and RANKL/OPG pathway in mice. J. Trauma Acute Care Surg. 2018, 85, 359–366. [Google Scholar] [CrossRef]
- Bundkirchen, K.; Macke, C.; Reifenrath, J.; Schack, L.M.; Noack, S.; Relja, B.; Naber, P.; Welke, B.; Fehr, M.; Krettek, C.; et al. Severe Hemorrhagic Shock Leads to a Delayed Fracture Healing and Decreased Bone Callus Strength in a Mouse Model. Clin. Orthop. Relat. Res. 2017, 475, 2783–2794. [Google Scholar] [CrossRef]
- Lucas, T.S.; Bab, I.A.; Lian, J.B.; Stein, G.S.; Jazrawi, L.; Majeska, R.J.; Attar-Namdar, M.; Einhorn, T.A. Stimulation of systemic bone formation induced by experimental blood loss. Clin. Orthop. Relat. Res. 1997, 340, 267–275. [Google Scholar] [CrossRef]
- Moochhala, S.; Wu, J.; Lu, J. Hemorrhagic shock: An overview of animal models. Front. Biosci. 2009, 14, 4631–4639. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.J.; Welch, R.D.; Eastridge, B.J.; Pierce, W.; Zhang, H. The effect of hemorrhagic shock in a caprine tibial fracture model. J. Orthop. Trauma 2002, 16, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, M.W.; Arnoczky, S.P.; DeMaso, C.M.; Ayala, A.; Chaudry, I.H. Depressed osteoblast activity and increased osteocyte necrosis after closed bone fracture and hemorrhagic shock. J. Trauma 1996, 41, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Hooper, N.; Armstrong, T.J. Hemorrhagic Shock. In StatPearls; United Nations: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vanzant, E.L.; Hilton, R.E.; Lopez, C.M.; Zhang, J.; Ungaro, R.F.; Gentile, L.F.; Szpila, B.E.; Maier, R.V.; Cuschieri, J.; Bihorac, A.; et al. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit. Care 2015, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Geifman, N.; Rubin, E. The mouse age phenome knowledgebase and disease-specific inter-species age mapping. PLoS ONE 2013, 8, e81114. [Google Scholar] [CrossRef]
- Leppanen, O.; Sievanen, H.; Jokihaara, J.; Pajamaki, I.; Jarvinen, T.L. Three-point bending of rat femur in the mediolateral direction: Introduction and validation of a novel biomechanical testing protocol. J. Bone Miner. Res. 2006, 21, 1231–1237. [Google Scholar] [CrossRef]
- Dülsner, A.; Hack, R.; Krüger, C.; Pils, M.; Scherer, K.; Schmelting, B.; Schmidt, M.; Weinert, H.; Jourdan, T. Fachinfomation aus dem Ausschuss für Tierschutzbeauftragte und dem Arbeitskreis 4 in der TVT. In Empfehlung zur Blutentnahme bei Versuchstieren, Insbesondere Kleinen Versuchstieren; GV SOLAS: Freiburg, Germany, 2017. [Google Scholar]
- Lieurance, R.; Benjamin, J.B.; Rappaport, W.D. Blood loss and transfusion in patients with isolated femur fractures. J. Orthop. Trauma 1992, 6, 175–179. [Google Scholar] [CrossRef]
- Wertheimer, A.; Olaussen, A.; Perera, S.; Liew, S.; Mitra, B. Fractures of the femur and blood transfusions. Injury 2018, 49, 846–851. [Google Scholar] [CrossRef]
- Marx, G.; Muhl, E.; Zacharowski, K.; Zeuzem, S. Die Intensivmedizin; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Beerekamp, M.S.H.; de Muinck Keizer, R.J.O.; Schep, N.W.L.; Ubbink, D.T.; Panneman, M.J.M.; Goslings, J.C. Epidemiology of extremity fractures in the Netherlands. Injury 2017, 48, 1355–1362. [Google Scholar] [CrossRef]
- Lopas, L.A.; Belkin, N.S.; Mutyaba, P.L.; Gray, C.F.; Hankenson, K.D.; Ahn, J. Fractures in geriatric mice show decreased callus expansion and bone volume. Clin. Orthop. Relat. Res. 2014, 472, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Borgiani, E.; Figge, C.; Kruck, B.; Willie, B.M.; Duda, G.N.; Checa, S. Age-Related Changes in the Mechanical Regulation of Bone Healing Are Explained by Altered Cellular Mechanoresponse. J. Bone Miner. Res. 2019, 34, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Strube, P.; Sentuerk, U.; Riha, T.; Kaspar, K.; Mueller, M.; Kasper, G.; Matziolis, G.; Duda, G.N.; Perka, C. Influence of age and mechanical stability on bone defect healing: Age reverses mechanical effects. Bone 2008, 42, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M. Diagnosis and treatment of definitive diverticular hemorrhage (DDH). Am. J. Gastroenterol. 2018, 113, 1570–1573. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bundkirchen, K.; Ye, W.; Nowak, A.J.; Lienenklaus, S.; Welke, B.; Relja, B.; Neunaber, C. Fracture Healing in Elderly Mice and the Effect of an Additional Severe Blood Loss: A Radiographic and Biomechanical Murine Study. Bioengineering 2023, 10, 70. https://doi.org/10.3390/bioengineering10010070
Bundkirchen K, Ye W, Nowak AJ, Lienenklaus S, Welke B, Relja B, Neunaber C. Fracture Healing in Elderly Mice and the Effect of an Additional Severe Blood Loss: A Radiographic and Biomechanical Murine Study. Bioengineering. 2023; 10(1):70. https://doi.org/10.3390/bioengineering10010070
Chicago/Turabian StyleBundkirchen, Katrin, Weikang Ye, Aleksander J. Nowak, Stefan Lienenklaus, Bastian Welke, Borna Relja, and Claudia Neunaber. 2023. "Fracture Healing in Elderly Mice and the Effect of an Additional Severe Blood Loss: A Radiographic and Biomechanical Murine Study" Bioengineering 10, no. 1: 70. https://doi.org/10.3390/bioengineering10010070
APA StyleBundkirchen, K., Ye, W., Nowak, A. J., Lienenklaus, S., Welke, B., Relja, B., & Neunaber, C. (2023). Fracture Healing in Elderly Mice and the Effect of an Additional Severe Blood Loss: A Radiographic and Biomechanical Murine Study. Bioengineering, 10(1), 70. https://doi.org/10.3390/bioengineering10010070







