About 3D Printability of Thermoplastic Collagen for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Rheology
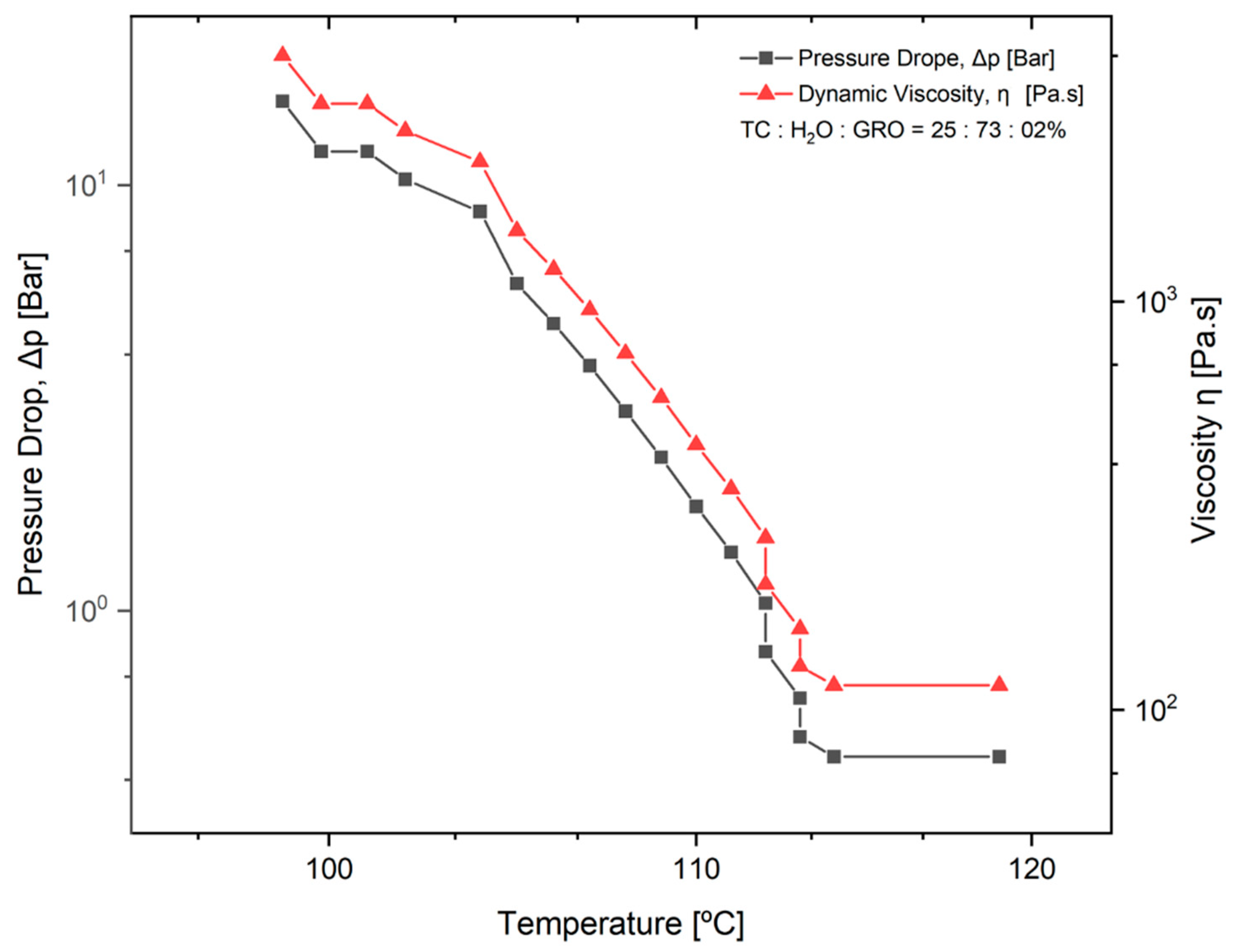
2.2.2. Effect of Dynamic Viscosity on Pressure
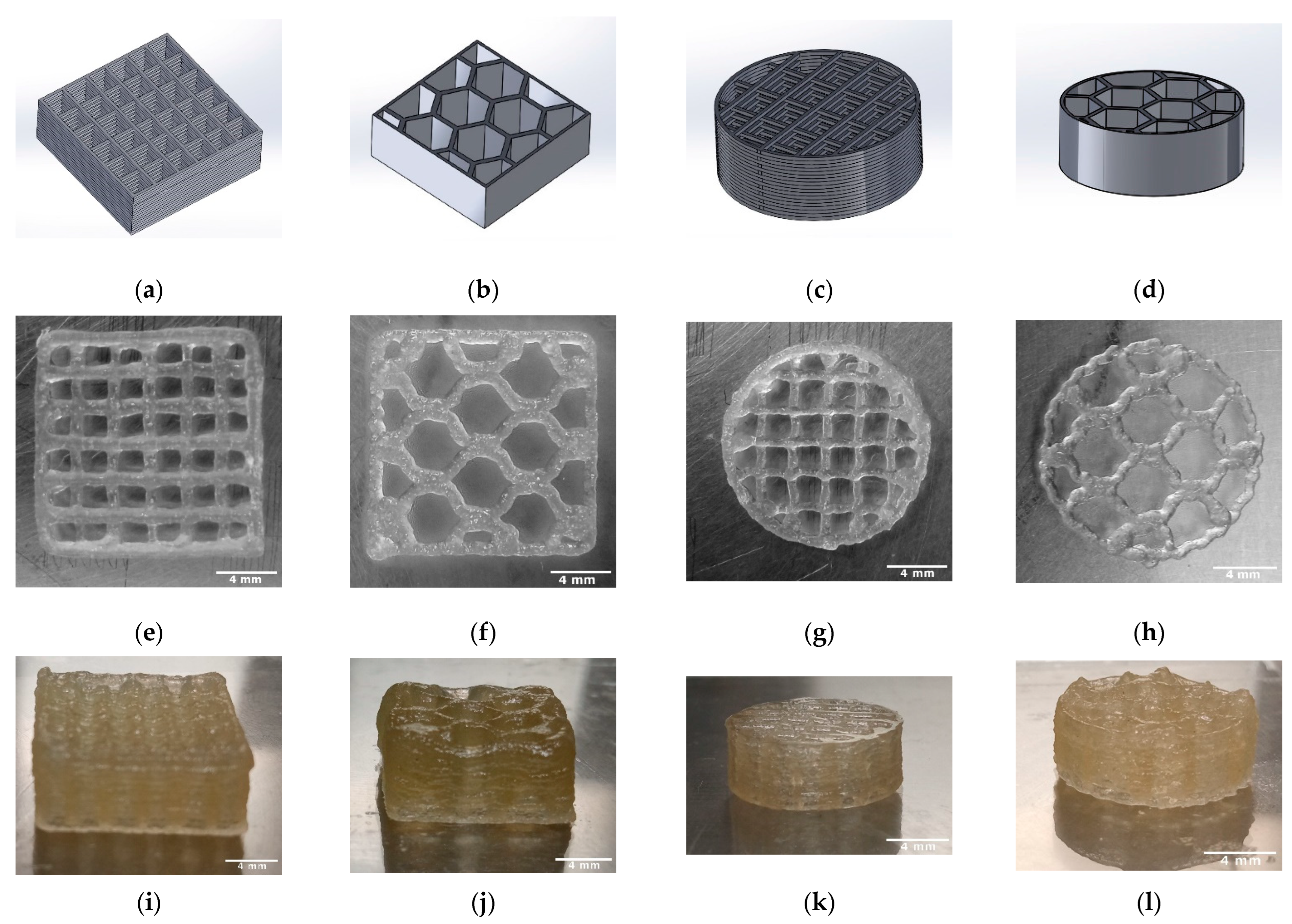
2.3. Manufacturing of the TC Scaffold
2.4. Characterization of the TC Scaffold
2.4.1. Dimensions and Weight
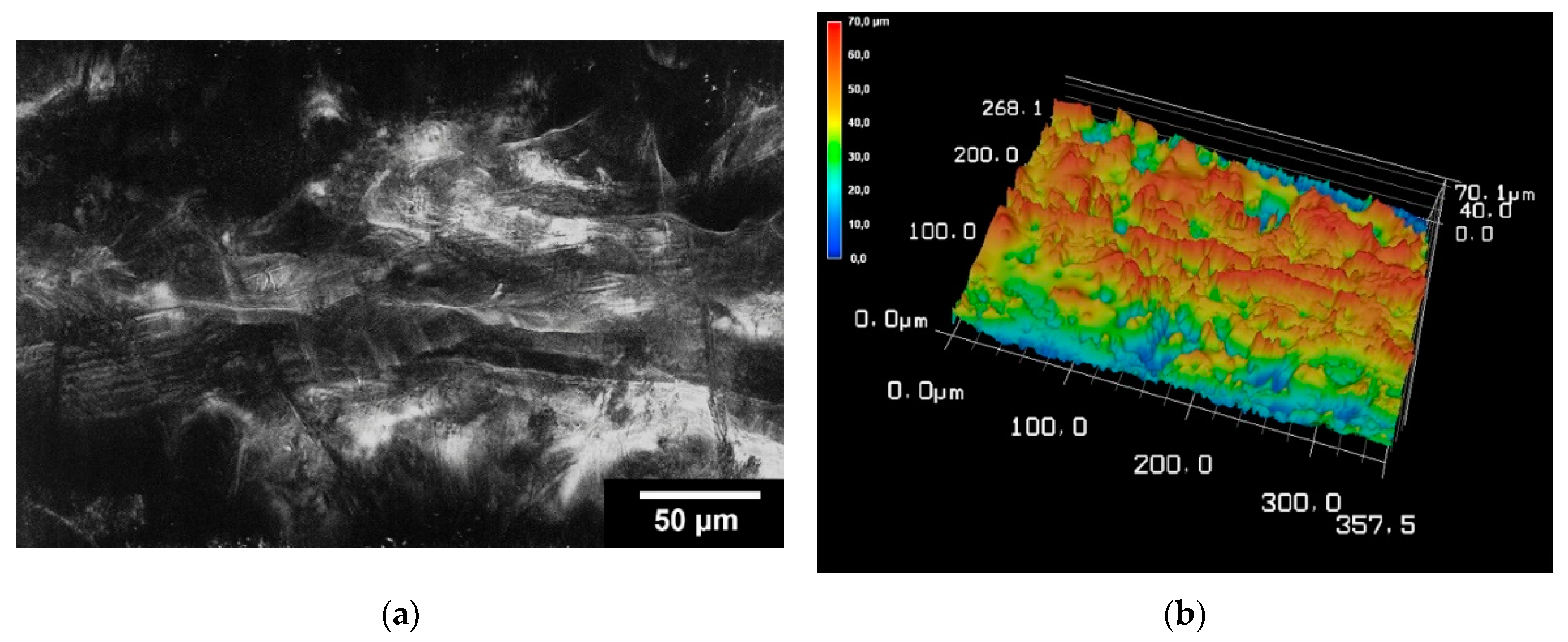
2.4.2. Surface Roughness
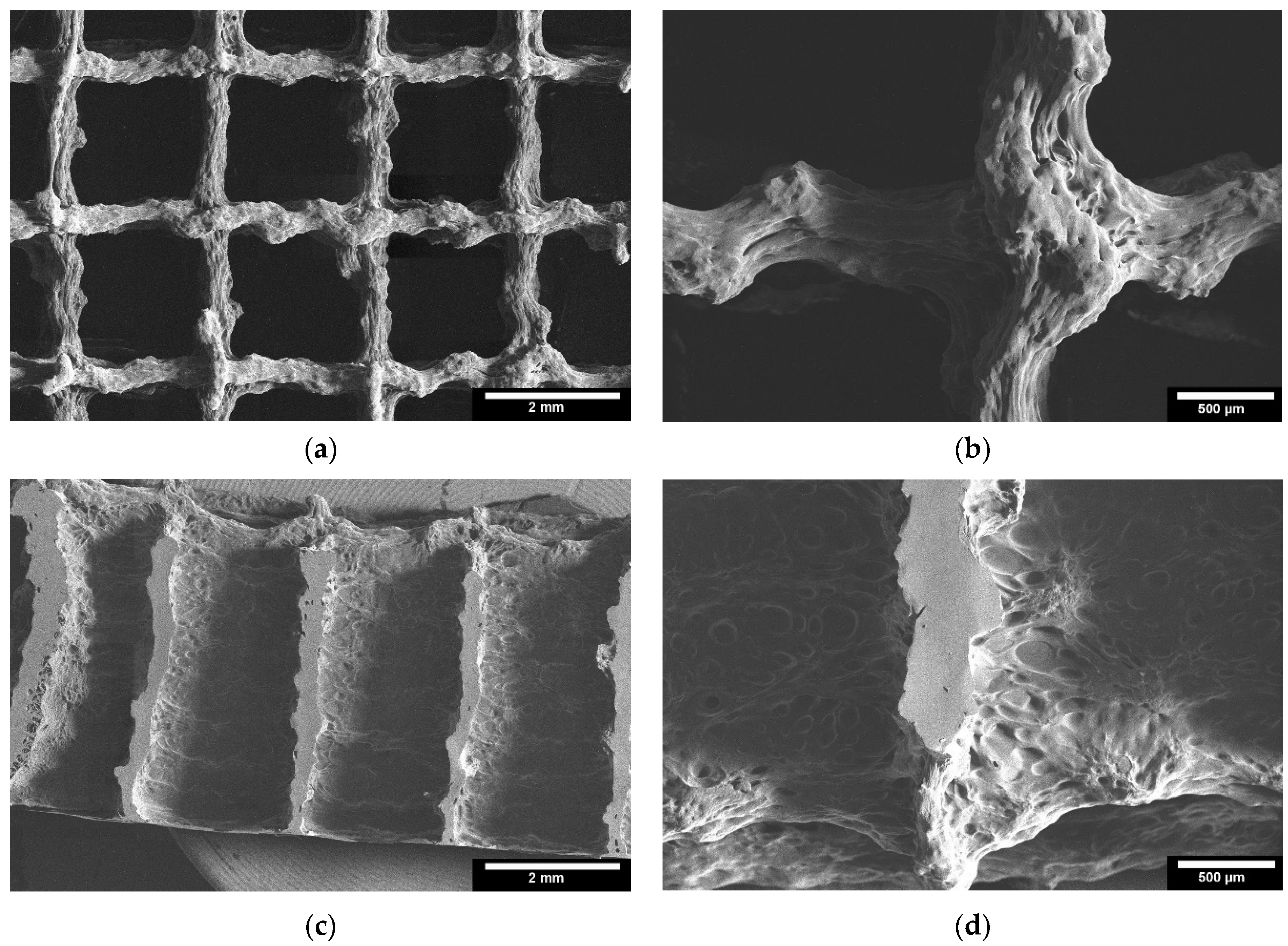
2.4.3. Microstructure by ESEM
2.4.4. Mechanical Testing
2.5. Statistics
3. Results
3.1. Viscosity
3.1.1. Rheology
3.1.2. Effect of Dynamic Viscosity on Pressure While 3D Printing
3.2. Characterization of the TC Scaffold
3.2.1. Dimensions and Weight
3.2.2. Surface Roughness
3.2.3. Microstructure by ESEM
3.2.4. Mechanical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, E.M.; Parvizi, J.; Gehrke, T.; Aiyer, A.; Battenberg, A.; Brown, S.A.; Callaghan, J.J.; Citak, M.; Egol, K.; Garrigues, G.E.; et al. 2018 International Consensus Meeting on Musculoskeletal Infection: Research Priorities from the General Assembly Questions. J. Orthop. Res. 2019, 37, 997–1006. [Google Scholar] [CrossRef]
- Koster, L.A.; Meinardi, J.E.; Kaptein, B.L.; Van der Linden-Van der Zwaag, E.; Nelissen, R.G.H.H. Two-year RSA migration results of symmetrical and asymmetrical tibial components in total knee arthroplasty: A randomized controlled trial. Bone Jt. J. 2021, 103-B, 855–863. [Google Scholar] [CrossRef]
- Eurostat. Record High Old-Age Dependency Ratio in the EU. Available online: https://ec.europa.eu/eurostat/de/web/products-eurostat-news/-/DDN-20180508-1 (accessed on 14 August 2022).
- Eurostat. Old-Age Dependency Ratio Increases across EU Regions. Available online: https://ec.europa.eu/eurostat/de/web/products-eurostat-news/-/edn-20210930-1 (accessed on 12 January 2022).
- Eurostat. Old-Age Dependency Ratio Increasing in the EU. Available online: https://ec.europa.eu/eurostat/de/web/products-eurostat-news/-/ddn-20200713-1 (accessed on 17 November 2021).
- Alonzo, M.; Alvarez Primo, F.; Anil Kumar, S.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Zhang, Y.; Zheng, L.; Zhang, H.; Shi, H.-H.; Zhang, X.-C.; Liu, B. Mineralized self-assembled silk fibroin/cellulose interpenetrating network aerogel for bone tissue engineering. Mater. Sci. Eng. C 2021, 134, 112549. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- He, J.; Hu, X.; Cao, J.; Zhang, Y.; Xiao, J.; Chen, D.; Xiong, C.; Zhang, L. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Carbohydr. Polym. 2021, 253, 117198. [Google Scholar] [CrossRef]
- Sathain, A.; Monvisade, P.; Siriphannon, P. Bioactive alginate/carrageenan/calcium silicate porous scaffolds for bone tissue engineering. Mater. Today Commun. 2021, 26, 102165. [Google Scholar] [CrossRef]
- Gupta, S.; Mukherjee, R.; Jangle, R.K.; Singh, D.; Singh, M.; Bit, A. Fabrication of Hydroxyapatite-Chitosan-Silk Fibroin Based Composite Film as Bone Tissue Regeneration Material. In Advances in Biomedical Engineering and Technology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 437–445. [Google Scholar]
- Klüver, E.; Meyer, M. Preparation, processing, and rheology of thermoplastic collagen. J. Appl. Polym. Sci. 2013, 128, 4201–4211. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Solis, D.M.; Czekanski, A. 3D and 4D additive manufacturing techniques for vascular-like structures—A review. Bioprinting 2022, 25, e00182. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, Á. Fused deposition modelling: Current status, methodology, applications and future prospects. Addit. Manuf. 2021, 47, 102378. [Google Scholar] [CrossRef]
- Klüver, E.; Baltzer, M.; Langer, A.; Meyer, M. Additive Manufacturing with Thermoplastic Collagen. Polymers 2022, 14, 974. [Google Scholar] [CrossRef] [PubMed]
- Duty, C.; Ajinjeru, C.; Kishore, V.; Compton, B.; Hmeidat, N.; Chen, X.; Liu, P.; Hassen, A.A.; Lindahl, J.; Kunc, V. What makes a material printable? A viscoelastic model for extrusion-based 3D printing of polymers. J. Manuf. Process. 2018, 35, 526–537. [Google Scholar] [CrossRef]
- Guo, X.; Yao, Y.; Zhu, P.; Zhou, M.; Zhou, T. Preparation of porous PTFE/C composite foam and its application in gravity-driven oil–water separation. Polym. Int. 2022, 71, 874–883. [Google Scholar] [CrossRef]
- Gerdes, S.; Mostafavi, A.; Ramesh, S.; Memic, A.; Rivero, I.V.; Rao, P.; Tamayol, A. Process–structure–quality relationships of three-dimensional printed poly (caprolactone)-hydroxyapatite scaffolds. Tissue Eng. Part A 2020, 26, 279–291. [Google Scholar] [CrossRef]
- Mishra, A.A.; Momin, A.; Strano, M.; Rane, K. Implementation of viscosity and density models for improved numerical analysis of melt flow dynamics in the nozzle during extrusion-based additive manufacturing. Prog. Addit. Manuf. 2022, 7, 41–54. [Google Scholar] [CrossRef]
- Gopi, S.; Kontopoulou, M. Investigation of thermoplastic melt flow and dimensionless groups in 3D bioplotting. Rheol. Acta 2020, 59, 83–93. [Google Scholar] [CrossRef]
- Tajuddin, M.; Ahmad, Z.; Ismail, H. A review of natural fibers and processing operations for the production of binderless boards. BioResources 2016, 11, 5600–5617. [Google Scholar] [CrossRef]
- Sukindar, N.A.; Ariffin, M.; Baharudin, B.H.T.; Jaafar, C.N.A.; Ismail, M.I.S. Analyzing the effect of nozzle diameter in fused deposition modeling for extruding polylactic acid using open source 3D printing. J. Teknol. 2016, 78, 7–15. [Google Scholar] [CrossRef]
- Weingärtner, L.; Latorre, S.H.; Velten, D.; Bernstein, A.; Schmal, H.; Seidenstuecker, M. The Effect of Collagen-I Coatings of 3D Printed PCL Scaffolds for Bone Replacement on Three Different Cell Types. Appl. Sci. 2021, 11, 11063. [Google Scholar] [CrossRef]
- Huber, F.; Vollmer, D.; Vinke, J.; Riedel, B.; Zankovic, S.; Schmal, H.; Seidenstuecker, M. Influence of 3D Printing Parameters on the Mechanical Stability of PCL Scaffolds and the Proliferation Behavior of Bone Cells. Materials 2022, 15, 2091. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Klarner, M.; Klammert, U.; Grover, L.M.; Patel, S.; Barralet, J.E.; Gbureck, U. 3D powder printing of β-tricalcium phosphate ceramics using different strategies. Adv. Eng. Mater. 2008, 10, B67–B71. [Google Scholar] [CrossRef]
- Goldstein, S.A. The mechanical properties of trabecular bone: Dependence on anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Keaveny, T.M. Cancellous bone. In Handbook of Biomaterial Properties; Black, J., Hastings, G., Eds.; Springer: Boston, MA, USA, 1998; pp. 15–23. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Schilling, P.; Ritschl, L.; Lange, S.; Schmal, H.; Bernstein, A.; Esslinger, S. Inverse 3D Printing with Variations of the Strand Width of the Resulting Scaffolds for Bone Replacement. Materials 2021, 14, 1964. [Google Scholar] [CrossRef]
- Han, Y.; Lian, M.; Wu, Q.; Qiao, Z.; Sun, B.; Dai, K. Effect of Pore Size on Cell Behavior Using Melt Electrowritten Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 495. [Google Scholar] [CrossRef]


| Needle Inner Diameter [µm] | Temperature [°C] | Pressure [bar] | Speed [mm/s] | Needle Offset [mm] | Pre-Flow [s] | Post-Flow [s] | Platform Temperature [°C] |
|---|---|---|---|---|---|---|---|
| 300 | 90 | 0.9 | 40 | 0.3 | 0.09 | −0.01 | 17 |
| Sample | Length [mm] | Height [mm] | Weight [g] | Strand Width [µm] | Pore Size [µm] | Porosity [%] | Nozzle Size [µm] |
|---|---|---|---|---|---|---|---|
| Cuboid-lines | 13.7 ± 0.2 | 5.5 ± 0.03 | 0.37 ± 0.03 | 304 ± 5 | 1923 ± 11 | 25.6 | 300 |
| Cuboid-honeycomb | 14.8 ± 0.5 | 4.4 ± 0.4 | 0.41 ± 0.06 | 428 ± 15 | 2812 ± 47 | 23.4 | 400 |
| Cylinder-lines | 13.8 ± 0.5 | 4.5 ± 0.2 | 0.14 ± 0.007 | 425 ± 13 | 1923 ± 11 | 43.1 | 400 |
| Cylinder-honeycomb | 14.2 ± 0.9 | 4.8 ± 0.3 | 0.24 ± 0.03 | 425 ± 13 | 2812 ± 47 | 36.4 | 400 |
| Sample | Maximum Failure Load [N] | Compressive Strength [MPa] |
|---|---|---|
| Cuboid-lines | 806 ± 43 | 3.6 ± 0.2 |
| Cuboid-honeycomb | 1649 ± 290 | 6.9 ± 1.2 |
| Cylinder-lines | 452 ± 42 | 3 ± 0.3 |
| Cylinder-honeycomb | 1435 ± 172 | 10 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passos, M.; Zankovic, S.; Minas, G.; Klüver, E.; Baltzer, M.; Schmal, H.; Seidenstuecker, M. About 3D Printability of Thermoplastic Collagen for Biomedical Applications. Bioengineering 2022, 9, 780. https://doi.org/10.3390/bioengineering9120780
Passos M, Zankovic S, Minas G, Klüver E, Baltzer M, Schmal H, Seidenstuecker M. About 3D Printability of Thermoplastic Collagen for Biomedical Applications. Bioengineering. 2022; 9(12):780. https://doi.org/10.3390/bioengineering9120780
Chicago/Turabian StylePassos, Marina, Sergej Zankovic, Graça Minas, Enno Klüver, Marit Baltzer, Hagen Schmal, and Michael Seidenstuecker. 2022. "About 3D Printability of Thermoplastic Collagen for Biomedical Applications" Bioengineering 9, no. 12: 780. https://doi.org/10.3390/bioengineering9120780
APA StylePassos, M., Zankovic, S., Minas, G., Klüver, E., Baltzer, M., Schmal, H., & Seidenstuecker, M. (2022). About 3D Printability of Thermoplastic Collagen for Biomedical Applications. Bioengineering, 9(12), 780. https://doi.org/10.3390/bioengineering9120780










