Applications of Extracellular Vesicles in Nervous System Disorders: An Overview of Recent Advances
Abstract
1. Introduction
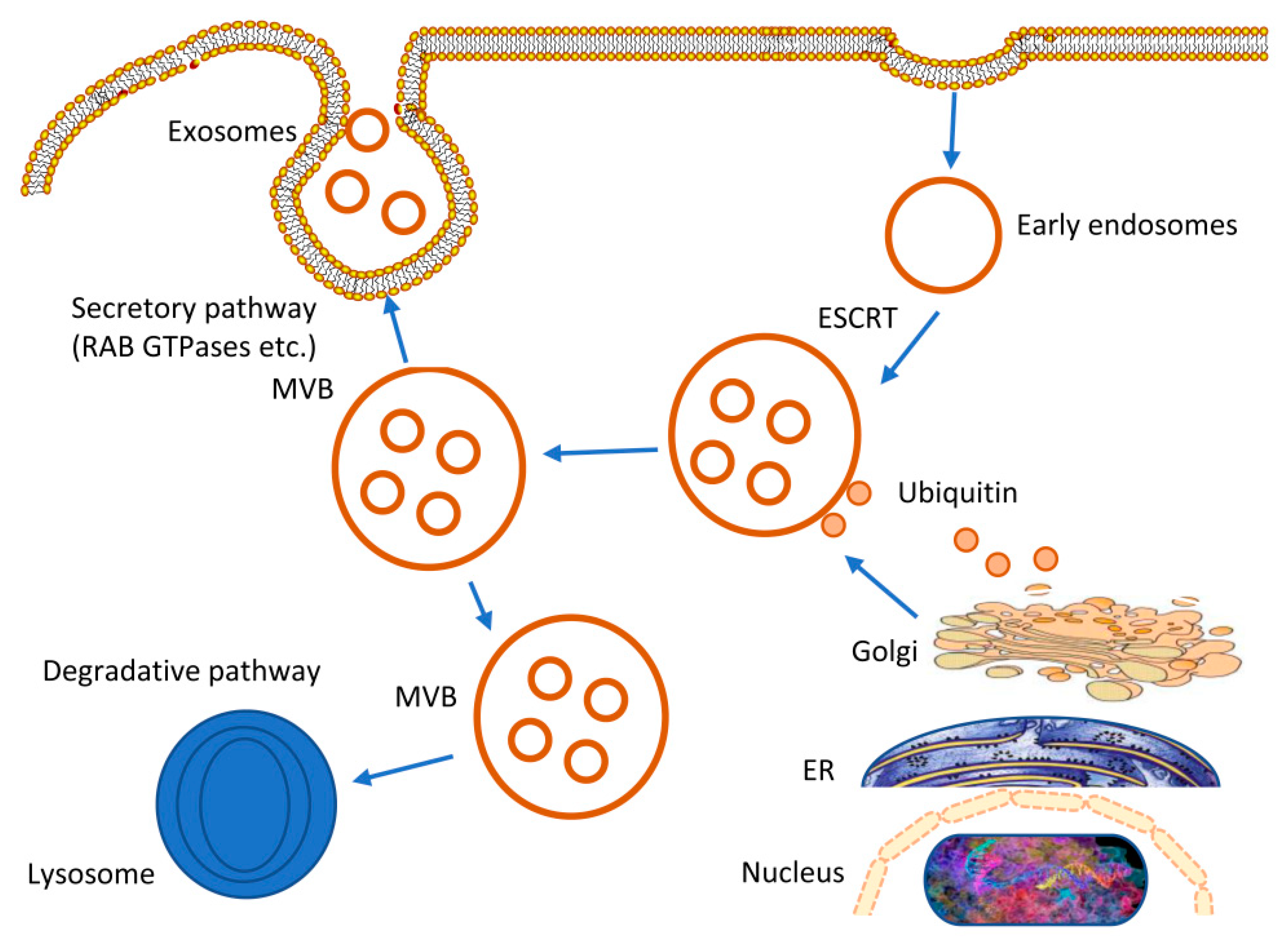
2. Origin and Biogenesis of Exosomes
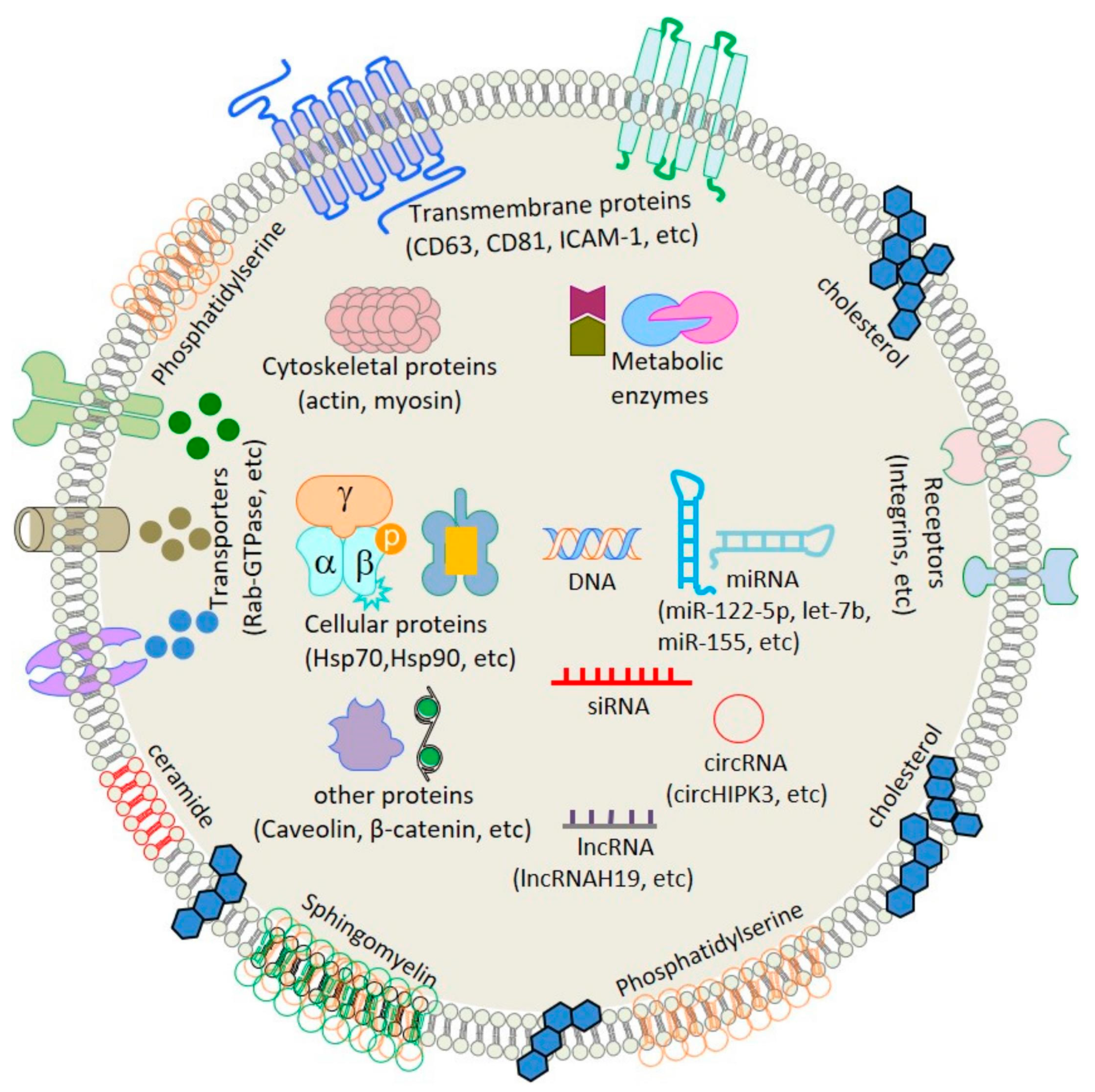
3. Subtypes and Classification of Extracellular Vesicles (Evs)
4. Exosomes’ Neuroprotective Properties
Improving Angiogenic Potential
5. Carrier Characteristics and Engineering Modification Mechanism of Exosomes
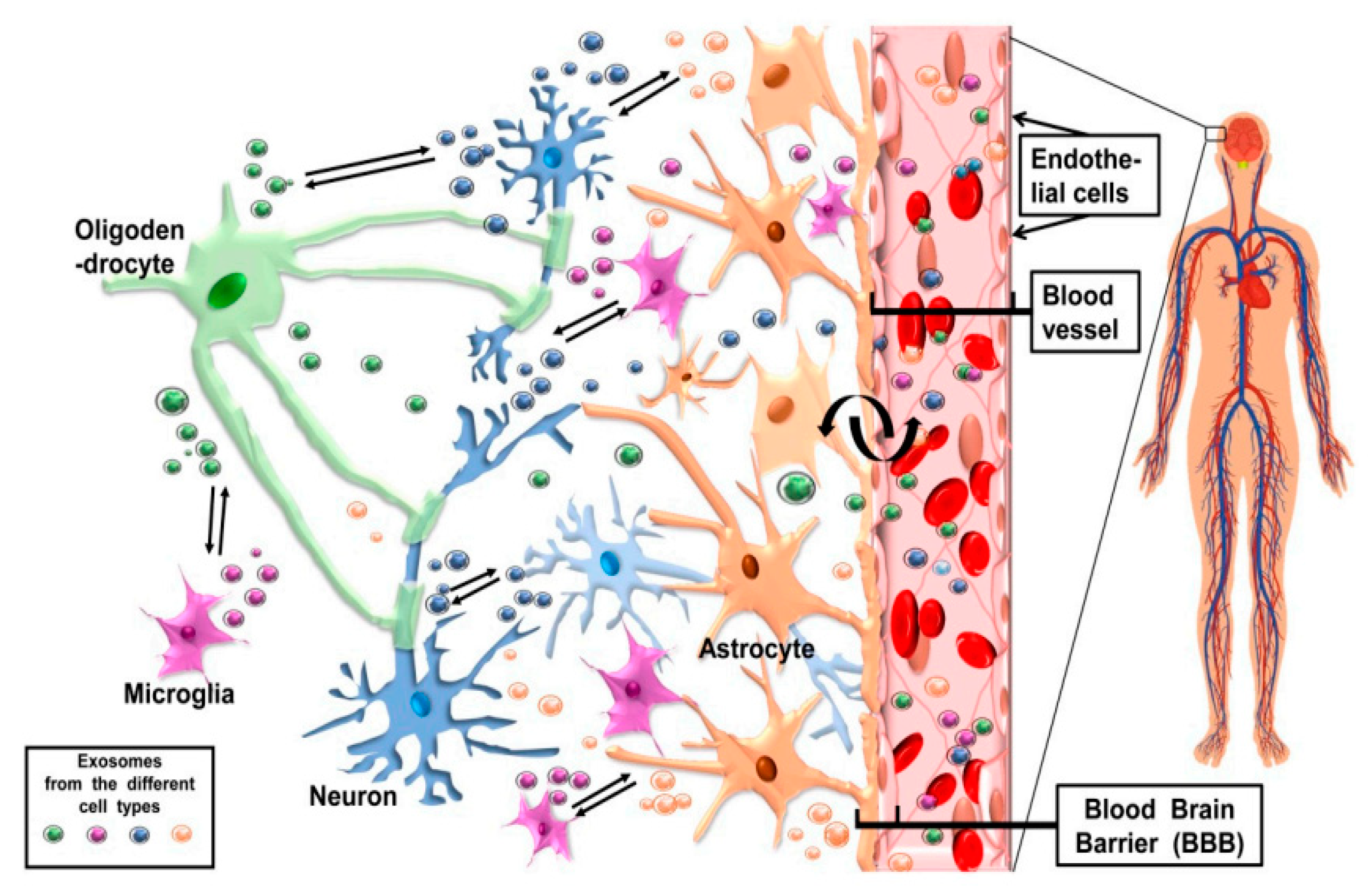
6. CNS Cell-Release Exosomes and Their Potentially Bidirectional Pathway
7. Application of Exosome Vector Therapy in Central Nervous System Diseases
7.1. Exosomes as Drug Carriers
7.2. Exosomes as Protein Carriers
7.3. Exosomes as Nucleic acid Carriers
8. Exosome in the Diagnosis and Treatment of Different CNS Diseases
8.1. Alzheimer’s Disease
8.2. Huntington’s Disease
8.3. Brain Tumor
8.4. Parkinson’s Disease
8.5. Stroke
8.6. Alternate Disorders of the Brain: Viruses and Drug Addiction Issue
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, Y.; Chen, Z.; Zhang, M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J. Transl. Med. 2022, 20, 291. [Google Scholar] [CrossRef] [PubMed]
- Naval, N.; Chandolu, S.; Mirski, M. Organ failure: Central nervous system. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2011; pp. 587–597. [Google Scholar]
- Zhou, X.; Smith, Q.R.; Liu, X. Brain penetrating peptides and peptide–drug conjugates to overcome the blood–brain barrier and target CNS diseases. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1695. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Leuraud, K.; Klokov, D.; Durand, C.; Bernier, M.-O.; Baudin, C. Risk of Developing Non-Cancerous Central Nervous System Diseases Due to Ionizing Radiation Exposure during Adulthood: Systematic Review and Meta-Analyses. Brain Sci. 2022, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Lotze, T.; Muscal, E. Inflammatory diseases of the central nervous system. Neurol. Clin. 2021, 39, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, C.f.; Adem, B.; Silva, M.; Melo, S.A. The biology of cancer exosomes: Insights and new perspectives. Cancer Res. 2017, 77, 6480–6488. [Google Scholar] [CrossRef]
- Wildner, P.; Stasiołek, M.; Matysiak, M. Differential diagnosis of multiple sclerosis and other inflammatory CNS diseases. Mult. Scler. Relat. Disord. 2019, 37, 101452. [Google Scholar] [CrossRef]
- Piguet, F.; de Saint Denis, T.; Audouard, E.; Beccaria, K.; André, A.; Wurtz, G.; Schatz, R.; Alves, S.; Sevin, C.; Zerah, M. The challenge of gene therapy for neurological diseases: Strategies and tools to achieve efficient delivery to the central nervous system. Hum. Gene Ther. 2021, 32, 349–374. [Google Scholar] [CrossRef]
- JSamal, J.; Rebelo, A.L.; Pandit, A. A window into the brain: Tools to assess pre-clinical efficacy of biomaterials-based therapies on central nervous system disorders. Adv. Drug Deliv. Rev. 2019, 148, 68–145. [Google Scholar]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.; Ma, R.; Cheng, G.; Guan, Y.; Chen, X.; Wu, Z.; Chen, T. Anti-Parkinsonian Therapy: Strategies for Crossing the Blood–Brain Barrier and Nano-Biological Effects of Nanomaterials. Nano-Micro Lett. 2022, 14, 105. [Google Scholar] [CrossRef]
- Alzate-Correa, D.; Lawrence, W.R.; Salazar-Puerta, A.; Higuita-Castro, N.; Gallego-Perez, D. Nanotechnology-Driven Cell-Based Therapies in Regenerative Medicine. AAPS J. 2022, 24, 43. [Google Scholar] [CrossRef]
- Khan, M.I.; Batool, F.; Ali, R.; Zahra, Q.U.A.; Wang, W.; Li, S.; Wang, G.; Liu, L.; Khan, S.U.; Mansoor, M.; et al. Tailoring radiotherapies and nanotechnology for targeted treatment of solid tumors. Co-ord. Chem. Rev. 2022, 472, 214757. [Google Scholar] [CrossRef]
- Nichols, E.; Szoeke, C.E.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W.; et al. Asgedom, Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef]
- de Castilla, P.E.M.; Tong, L.; Huang, C.; Sofias, A.M.; Pastorin, G.; Chen, X.; Storm, G.; Schiffelers, R.M.; Wang, J.-W. Extracellular vesicles as a drug delivery system: A systematic review of preclinical studies. Adv. Drug Deliv. Rev. 2021, 175, 113801. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yao, S.; Wan, J.; Tian, Y.; Huang, L.; Wang, S.; Akter, F.; Wu, Y.; Yao, Y.; Zhang, X. BBB-crossing adeno-associated virus vector: An excellent gene delivery tool for CNS disease treatment. J. Control. Release 2021, 333, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Martins, C.; Silveira, M.J.; Moura, R.P.; Pereira, C.L.; Sarmento, B. Advances on erythrocyte-mimicking nanovehicles to overcome barriers in biological microenvironments. Adv. Drug Deliv. Rev. 2020, 170, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Mendanha, D.; de Castro, J.V.; Ferreira, H.; Neves, N. Biomimetic and cell-based nanocarriers—New strategies for brain tumor targeting. J. Control. Release 2021, 337, 482–493. [Google Scholar] [CrossRef]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.-R.; Awan, N.R.; Yar, M. Neurodegenerative diseases and effective drug delivery: A review of challenges and novel therapeutics. J. Control. Release 2020, 330, 1152–1167. [Google Scholar] [CrossRef]
- Nash, A.; Aghlara-Fotovat, S.; Hernandez, A.; Scull, C.; Veiseh, O. Clinical translation of immunomodulatory therapeutics. Adv. Drug Deliv. Rev. 2021, 176, 113896. [Google Scholar] [CrossRef]
- Ke, W.; Afonin, K.A. Exosomes as natural delivery carriers for programmable therapeutic nucleic acid nanoparticles (NANPs). Adv. Drug Deliv. Rev. 2021, 176, 113835. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Khan, M.U.; Gao, Y.; Khan, M.I.; Puswal, S.M.; Zubair, M.; Farwa, R.; Gao, S.; Ali, R.; Hussain, N. Unique therapeutic potentialities of exosomes based nanodrug carriers to target tumor microenvironment in cancer therapy. Opennano 2022, 8, 100091. [Google Scholar] [CrossRef]
- Sun, K.; Zheng, X.; Jin, H.; Yu, F.; Zhao, W. Exosomes as CNS Drug Delivery Tools and Their Applications. Pharmaceutics 2022, 14, 2252. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2022, 4, 69–83. [Google Scholar] [CrossRef]
- Salunkhe, S.; Dheeraj; Basak, M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar]
- Newton, W.C.; Kim, J.W.; Luo, J.Z.Q.; Luo, L. Stem cell-derived exosomes: A novel vector for tissue repair and diabetic therapy. J. Mol. Endocrinol. 2017, 59, R155–R165. [Google Scholar] [CrossRef]
- Anel, A.; Gallego-Lleyda, A.; de Miguel, D.; Naval, J.; Martínez-Lostao, L. Role of exosomes in the regulation of T-cell mediated immune responses and in autoimmune disease. Cells 2019, 8, 154. [Google Scholar] [CrossRef]
- Masaoutis, C.; Mihailidou, C.; Tsourouflis, G.; Theocharis, S. Exosomes in lung cancer diagnosis and treatment. From the translating research into future clinical practice. Biochimie 2018, 151, 27–36. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Humle, N.; Hede, E.; Moos, T.; Burkhart, A.; Thomsen, L.B. The blood-brain barrier studied in vitro across species. PLoS ONE 2021, 16, e0236770. [Google Scholar] [CrossRef]
- Kumar, A.; Zhou, L.; Zhi, K.; Raji, B.; Pernell, S.; Tadrous, E.; Kodidela, S.; Nookala, A.; Kochat, H.; Kumar, S. Challenges in Biomaterial-Based Drug Delivery Approach for the Treatment of Neurodegenerative Diseases: Opportunities for Extracellular Vesicles. Int. J. Mol. Sci. 2020, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Dong, Y.; Hou, Q.; He, Y.; Lai, Y.; Liao, C.; Kawamura, Y.; Li, J.; Zhang, B. Intestinal Microbiota Regulate Certain Meat Quality Parameters in Chicken. Front. Nutr. 2022, 9, 747705. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Hornung, S.; Kruayatidee, A.; Maina, K.N.; del Rosario, I.; Paul, K.C.; Wong, D.Y.; Folle, A.D.; Markovic, D.; Palma, J.-A.; et al. α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol. 2021, 142, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y.; et al. α-Synuclein in blood exosomes imptomatic stage. Alzheimer’s Dement. 2021, 17, 49–60. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef]
- Beckler, M.D.; Higginbotham, J.N.; Franklin, J.L.; Ham, A.-J.; Halvey, P.J.; Imasuen, I.E.; Whitwell, C.; Li, M.; Liebler, D.C.; Coffey, R.J. Proteomic Analysis of Exosomes from Mutant KRAS Colon Cancer Cells Identifies Intercellular Transfer of Mutant KRAS. Mol. Cell. Proteom. 2013, 12, 343–355. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Rincón-Riveros, A.; Lopez, L.; Villegas, V.; Rodriguez, J.A. Regulation of Antitumor Immune Responses by Exosomes Derived from Tumor and Immune Cells. Cancers 2021, 13, 847. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. Exosomes/microvesicles: Mediators of cancer-associated immunosuppressive microenvironments. Semin. Immunopathol. 2011, 33, 441–454. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Huang, Y.; Zhang, H.; Lu, H.; Zheng, J.C. Exosomal miRNAs in central nervous system diseases: Biomarkers, pathological mediators, protective factors and therapeutic agents. Prog. Neurobiol. 2019, 183, 101694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, Y.-Y.; Liu, L.; Qiao, Y.-N.; Geng, H.-R.; Lin, Y.; Xu, W.; Cao, J.; Zhao, J.-Y. Homocysteine Inhibits Pro-Insulin Receptor Cleavage and Causes Insulin Resistance via Protein Cysteine-Homocysteinylation. Cell Rep. 2021, 37, 109821. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, M.; Brooks, R.W.; Boccuzzi, L.; Robbins, P.D.; Ricordi, C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2940–2956. [Google Scholar] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Anjum, K.; Shagufta, B.I.; Abbas, S.Q.; Patel, S.; Khan, I.; Shah, S.A.A.; Akhter, N.; Hassan, S.S. ul Current Status and Future Therapeutic Perspectives of Glioblastoma Multiforme (GBM) Therapy: A Review. Biomed. Pharmacother. 2017, 92, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Di Vizio, D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L.; et al. Large Oncosomes in Human Prostate Cancer Tissues and in the Circulation of Mice with Metastatic Disease. Am. J. Pathol. 2012, 181, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nature 2021, 23, 1240–1254. [Google Scholar] [CrossRef]
- Wang, X.-H.; Xu, S.; Zhou, X.-Y.; Zhao, R.; Lin, Y.; Cao, J.; Zang, W.-D.; Tao, H.; Xu, W.; Li, M.-Q.; et al. Low Chorionic Villous Succinate Accumulation Associates with Recurrent Spontaneous Abortion Risk. Nat. Commun. 2021, 12, 3428. [Google Scholar] [CrossRef]
- Suire, C.N.; Hade, M.D. Extracellular Vesicles in Type 1 Diabetes: A Versatile Tool. Bioengineering 2022, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.M.; Kaiser, J.; Schwab, M.E. Extracellular Vesicles: Multimodal Envoys in Neural Maintenance and Repair. Trends Neurosci. 2018, 41, 360–372. [Google Scholar] [CrossRef]
- Delpech, J.-C.; Herron, S.; Botros, M.B.; Ikezu, T. Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease. Trends Neurosci. 2019, 42, 361–372. [Google Scholar] [CrossRef]
- Van Balkom, B.W.M.; de Jong, O.; Smits, M.; Brummelman, J.; Ouden, K.D.; De Bree, P.M.; Van Eijndhoven, M.A.J.; Pegtel, D.M.; Stoorvogel, W.; Würdinger, T.; et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013, 121, 3997–4006. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Deng, H.; Xiao, S.; Xie, J.; Li, H.; Zhao, X.; Han, D.; Sun, X.; Lou, X.; Ye, C.; et al. Accelerate Gas Diffusion-Weighted MRI for Lung Morphometry with Deep Learning. Eur. Radiol. 2022, 32, 702–713. [Google Scholar] [CrossRef]
- Komaki, M.; Numata, Y.; Morioka, C.; Honda, I.; Tooi, M.; Yokoyama, N.; Ayame, H.; Iwasaki, K.; Taki, A.; Oshima, N.; et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res. Ther. 2017, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Sheng, C.; Zhou, Y. Extracellular vesicles as a novel therapeutic tool for cell-free regenerative medicine in oral rehabilitation. J. Oral Rehabilitation 2019, 47, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Chun-Yuan, C.; Rao, S.-S.; Wang, Z.-X.; Cao, J.; Tan, Y.-J.; Luo, J.; Li, H.-M.; Zhang, W.-S.; Chen, C.-Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar]
- Johnson, T.K.; Zhao, L.; Zhu, D.; Wang, Y.; Xiao, Y.; Oguljahan, B.; Zhao, X.; Kirlin, W.G.; Yin, L.; Chilian, W.M.; et al. Exosomes derived from induced vascular progenitor cells promote angiogenesis in vitro and in an in vivo rat hindlimb ischemia model. Am. J. Physiol. Circ. Physiol. 2019, 317, H765–H776. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zeng, Z.; Song, Y.; Li, L.; Wu, Z.; Zhang, X.; Li, Z.; Ke, X.; Hu, X. YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT. Stem Cell Res. Ther. 2019, 10, 263. [Google Scholar] [CrossRef]
- Zeng, Q.; Bie, B.; Guo, Q.; Yuan, Y.; Han, Q.; Han, X.; Chen, M.; Zhang, X.; Yang, Y.; Liu, M.; et al. Hyperpolarized Xe NMR signal advancement by metal-organic framework entrapment in aqueous solution. Proc. Natl. Acad. Sci. USA 2020, 117, 17558–17563. [Google Scholar] [CrossRef]
- Jan, A.; Rahman, S.; Badierah, R.; Lee, E.; Mattar, E.; Redwan, E.; Choi, I. Expedition into Exosome Biology: A Perspective of Progress from Discovery to Therapeutic Development. Cancers 2021, 13, 1157. [Google Scholar] [CrossRef]
- Bellavia, D.; Raimondi, L.; Costa, V.; De Luca, A.; Carina, V.; Maglio, M.; Fini, M.; Alessandro, R.; Giavaresi, G. Engineered exosomes: A new promise for the management of musculoskeletal diseases. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 1893–1901. [Google Scholar] [CrossRef]
- Yu, X.; Odenthal, M.; Fries, J. Exosomes as miRNA carriers: Formation–function–future. Int. J. Mol. Sci. 2016, 17, 2028. [Google Scholar] [CrossRef]
- Oskouie, M.; Moghaddam, N.A.; Butler, A.; Zamani, P.; Sahebkar, A. Therapeutic use of curcuminjournal of molecular sciencesimed exosomes. J. Cell. Physiol. 2019, 234, 8182–8191. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Hislop, J.N.; Islam, T.; Eleftheriadou, I.; Carpentier, D.; Trabalza, A.; Parkinson, M.; Schiavo, G.; Mazarakis, N.D. Rabies Virus Envelope Glycoprotein Targets Lentiviral Vectors to the Axonal Retrograde Pathway in Motor Neurons. J. Biol. Chem. 2014, 289, 16148–16163. [Google Scholar] [CrossRef]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, M.; Gürsoy-Özdemir, Y.; Kaya, M.; Abriz, A.E.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Marzesco, A.-M.; Janich, P.; Wilsch-Bräuninger, M.; Dubreuil, V.; Langenfeld, K.; Corbeil, D.; Huttner, W.B. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J. Cell Sci. 2005, 118, 2849–2858. [Google Scholar] [CrossRef]
- Bakhti, M.; Winter, C.; Simons, M. Inhibition of Myelin Membrane Sheath Formation by Oligodendrocyte-derived Exosome-like Vesicles. J. Biol. Chem. 2011, 286, 787–796. [Google Scholar] [CrossRef]
- Zou, M.; Yang, Z.; Fan, Y.; Gong, L.; Han, Z.; Ji, L.; Hu, X.; Wu, D. Gut Microbiota on Admission as Predictive Biomarker for Acute Necrotizing Pancreatitis. Front. Immunol. 2022, 13, 988326. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R. Soc. B: Biol. Sci. 2014, 369, 20130516. [Google Scholar] [CrossRef]
- Chivet, M.; Hemming, F.; Pernet-Gallay, K.; Fraboulet, S.; Sadoul, R. Emerging Role of Neuronal Exosomes in the Central Nervous System. Front. Physiol. 2012, 3, 145. [Google Scholar] [CrossRef]
- Liu, C.-A.; Chang, C.-Y.; Hsueh, K.-W.; Su, H.-L.; Chiou, T.-W.; Lin, S.-Z.; Harn, H.-J. Migration/Invasion of Malignant Gliomas and Implications for Therapeutic Treatment. Int. J. Mol. Sci. 2018, 19, 1115. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chung, J.; Lee, K.; Balaj, L.; Min, C.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Lee, H.; Weissleder, R. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 2015, 6, 6999. [Google Scholar] [CrossRef] [PubMed]
- Quek, C.; Hill, A. The role of extracellular vesicles in neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2017, 483, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.; Pace, A.; Caruso Bavisotto, C.; Marzullo, P.; Marino Gammazza, A.; Buscemi, S.; Palumbo Piccionello, A. Heat Shock Proteins in Alzheimer’s Disease: Role and Targeting. Int. J. Mol. Sci. 2018, 19, 2603. [Google Scholar] [CrossRef]
- Gammazza, A.M.; Bavisotto, C.C.; Barone, R.; de Macario, E.C.; Macario, A.J. Alzheimer’s Disease and Molecular Chaperones: Current Knowledge and the Future of Chaperonotherapy. Curr. Pharm. Des. 2016, 22, 4040–4049. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics Across the Blood-Brain Barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef]
- Zhang, H.; Trivedi, A.; Lee, J.-U.; Lohela, M.; Lee, S.M.; Fandel, T.M.; Werb, Z.; Haeusslein, L.J.N. Matrix Metalloproteinase-9 and Stromal Cell-Derived Factor-1 Act Synergistically to Support Migration of Blood-Borne Monocytes into the Injured Spinal Cord. J. Neurosci. 2011, 31, 15894–15903. [Google Scholar] [CrossRef]
- Liu, S.; Yang, B.; Wang, Y.; Tian, J.; Yin, L.; Zheng, W. 2D/3D Multimode Medical Image Registration Based on Normalized Cross-Correlation. Appl. Sci. 2022, 12, 2828. [Google Scholar] [CrossRef]
- Vignon, A.; Salvador-Prince, L.; Lehmann, S.; Perrier, V.; Torrent, J. Deconstructing Alzheimer’s Disease: How to Bridge the Gap between Experimental Models and the Human Pathology? Int. J. Mol. Sci. 2021, 22, 8769. [Google Scholar] [CrossRef]
- Bavisotto, C.C.; Scalia, F.; Gammazza, A.M.; Carlisi, D.; Bucchieri, F.; de Macario, E.C.; Macario, A.J.L.; Cappello, F.; Campanella, C. Extracellular Vesicle-Mediated Cell–Cell Communication in the Nervous System: Focus on Neurological Diseases. Int. J. Mol. Sci. 2019, 20, 434. [Google Scholar] [CrossRef]
- Pulatov, S.S. Efficacy of ipidacrine in the recovery period of ischaemic stroke. World Bull. Public Health 2022, 7, 28–32. [Google Scholar]
- Wu, A.-G.; Yong, Y.-Y.; Pan, Y.-R.; Zhang, L.; Wu, J.-M.; Zhang, Y.; Tang, Y.; Wei, J.; Yu, L.; Law, B.Y.-K.; et al. Targeting Nrf2-Mediated Oxidative Stress Response in Traumatic Brain Injury: Therapeutic Perspectives of Phytochemicals. Oxidative Med. Cell. Longev. 2022, 2022, 1015791. [Google Scholar] [CrossRef]
- Sun, H.; Zhan, M.; Mignani, S.; Shcharbin, D.; Majoral, J.-P.; Rodrigues, J.; Shi, X.; Shen, M. Modulation of Macrophages Using Nanoformulations with Curcumin to Treat Inflammatory Diseases: A Concise Review. Pharmaceutics 2022, 14, 2239. [Google Scholar] [CrossRef]
- Bashyal, S.; Thapa, C.; Lee, S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J. Control. Release 2022, 348, 723–744. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zheng, W.; Yin, L.; Hu, R.; Yang, B. Endoscope image mosaic based on pyramid ORB. Biomed. Signal Process. Control. 2021, 71, 103261. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2017, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, N.; Gu, Z.; Zhao, Y.; Liu, C.; Zhou, T.; Zhang, K.; Zhang, Z.; Liu, J.; Shi, J. Engineered biomimetic drug-delivery systems for ischemic stroke therapy. Med. Drug Discov. 2022, 15, 100129. [Google Scholar] [CrossRef]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369. [Google Scholar] [CrossRef]
- Ingato, D.; Lee, J.U.; Sim, S.J.; Kwon, Y.J. Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. J. Control. Release 2016, 241, 174–185. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Fernández-Calle, R.; Konings, S.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; Martinson, I.; Boza-Serrano, A.; Venero, J.; Nielsen, H. APOE in the bullseye of neurodegenerative diseases: Impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol. Neurodegener. 2022, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, L.; Jiang, Y.; Shi, Y.; Sui, H.; Zhao, L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020, 27, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Podolak, I. A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders. Life 2022, 12, 591. [Google Scholar] [CrossRef]
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y. Embryonic stem cellscetin as a Potential Therapeutic Ageting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv. Sci. 2019, 6, 1801899. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Thakur, A.; Sidu, R.K.; Zou, H.; Alam, K.; Yang, M.; Lee, Y. Inhibition of Glioma Cells’ Proliferation by Doxorubicin-Loaded Exosomes via Microfluidics. Int. J. Nanomed. 2020, 15, 8331–8343. [Google Scholar] [CrossRef]
- Shams ul Hassan, S.; Ishaq, M.; Zhang, W.; Jin, H.-Z. An Overview of the Mechanisms of Marine Fungi-Derived Antiinflammatory and Anti-Tumor Agents and Their Novel Role in Drug Targeting. Curr. Pharm. Des. 2021, 27, 2605–2614. [Google Scholar] [CrossRef]
- Riabinska, A.; Zille, M.; Terzi, M.; Cordell, R.; Nieminen-Kelhä, M.; Klohs, J.; Piña, A. Pigment Epithelium-Derived Factor Improves Paracellular Blood–Brain Barrier Integrity in the Normal and Ischemic Mouse Brain. Cell. Mol. Neurobiol. 2020, 40, 751–764. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Khan, M.I.; Fadahunsi, A.A.; Khan, A.; Gao, S.; Bilal, M.; Li, F. Role of circular RNAs in disease progression and diagnosis of cancers: An overview of recent advanced insights. Int. J. Biol. Macromol. 2022, 220, 973–984. [Google Scholar] [CrossRef]
- Huang, X.; Ding, J.; Li, Y.; Liu, W.; Ji, J.; Wang, H.; Wang, X. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp. Cell Res. 2018, 371, 269–277. [Google Scholar] [CrossRef]
- Haney, M.J.; Zhao, Y.; Jin, Y.S.; Li, S.M.; Bago, J.R.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. J. Neuroimmune Pharmacol. 2019, 15, 487–500. [Google Scholar] [CrossRef]
- Haney, M.; Klyachko, N.; Zhao, Y.; Gupta, R.; Plotnikova, E.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.-Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA-17–92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Hade, M.; Suire, C.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Guy, R.; Offen, D. Promising Opportunities for Treating Neurodegenerative Diseases with Mesenchymal Stem Cell-Derived Exosomes. Biomolecules 2020, 10, 1320. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol. Ther. Nucleic Acids 2017, 7, 278–287. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Wu, J.; Fan, Q.; Zhou, J.; Wu, J.; Liu, S.; Zang, J.; Ye, J.; Xiao, M.; et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnology 2019, 17, 29. [Google Scholar] [CrossRef]
- Shen, H.; Yao, X.; Li, H.; Li, X.; Zhang, T.; Sun, Q.; Ji, C.; Chen, G. Role of Exosomes Derived from miR-133b Modified MSCs in an Experimental Rat Model of Intracerebral Hemorrhage. J. Mol. Neurosci. 2018, 64, 421–430. [Google Scholar] [CrossRef]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’Arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: A new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef]
- Rong, X.; Jiang, L.; Qu, M.; Hassan, S.S.U.; Liu, Z. Enhancing Therapeutic Efficacy of Donepezil by Combined Therapy: A Comprehensive Review. Curr. Pharm. Des. 2021, 27, 332–344. [Google Scholar] [CrossRef]
- Lai, N.-S.; Wu, D.; Liang, T.; Pan, P.; Yuan, G.; Li, X.; Li, H.; Shen, H.; Wang, Z.; Chen, G. Systemic exosomal miR-193b-3p delivery attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage in mice. J. Neuroinflammation 2020, 17, 74. [Google Scholar] [CrossRef]
- Jahangard, Y.; Monfared, H.; Moradi, A.; Zare, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Therapeutic Effects of Transplanted Exosomes Containing miR-29b to a Rat Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 564. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.B.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef]
- Izco, M.; Blesa, J.; Schleef, M.; Schmeer, M.; Porcari, R.; Al-Shawi, R.; Ellmerich, S.; de Toro, M.; Gardiner, C.; Seow, Y.; et al. Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol. Ther. 2019, 27, 2111–2122. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, G.; Zhang, Y.; Jiang, H.; Wang, W.; Zhao, D.; Hong, J.; Yu, H.; Qi, L. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res. Ther. 2019, 10, 381. [Google Scholar] [CrossRef]
- Yu, L.; Gui, S.; Liu, Y.; Qiu, X.; Zhang, G.; Zhang, X.a.; Pan, J.; Fan, J.; Qi, S.; Qiu, B. Exosomes derived from microrna-199a-overexpressing mesenchymal stem cells inhibit glioma progression by down-regulating agap2. Aging 2019, 11, 5300–5318. [Google Scholar] [CrossRef]
- Katakowski, M.; Zheng, X.; Jiang, F.; Rogers, T.; Szalad, A.; Chopp, M. MiR-146b-5p Suppresses EGFR Expression and Reduces In Vitro Migration and Invasion of Glioma. Cancer Investig. 2010, 28, 1024–1030. [Google Scholar] [CrossRef]
- Bronisz, A.; Wang, Y.; Nowicki, M.; Peruzzi, P.; Ansari, K.; Ogawa, D.; Balaj, L.; De Rienzo, G.; Mineo, M.; Nakano, I. Extracellular Vesicles Modulate the Glioblastoma Microenvironment via a Tumor Suppression Signaling Network Directed by miR-1A miR-1 Targeting of GBM Microenvironment via Extracellular Vesicles. Cancer Res. 2014, 74, 738–750. [Google Scholar] [CrossRef]
- Oboudiyat, C.; Glazer, H.; Seifan, A.; Greer, C.; Isaacson, R. Alzheimer’s disease. Semin. Neurol. 2013, 33, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Abduljawad, A.A.; Elawad, M.A.; Elkhalifa, M.E.M.; Ahmed, A.; Hamdoon, A.A.E.; Salim, L.H.M.; Ashraf, M.; Ayaz, M.; Hassan, S.S.U.; Bungau, S. Alzheimer’s Disease as a Major Public Health Concern: Role of Dietary Saponins in Mitigating Neurodegenerative Disorders and Their Underlying Mechanisms. Molecules 2022, 27, 6804. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Creese, B.; Aarsland, D.; Kales, H.; Lyketsos, C.; Sweet, R.; Ballard, C. Psychosis in Alzheimer disease—Mechanisms, genetics and therapeutic opportunities. Nat. Rev. Neurol. 2022, 18, 131–144. [Google Scholar] [CrossRef]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Wang, X.; Rai, A.; Groot, M.; Jin, Y. Exosome-Mediated Small RNA Delivery: A Novel Therapeutic Approach for Inflammatory Lung Responses. Mol. Ther. 2018, 26, 2119–2130. [Google Scholar] [CrossRef]
- Wahlgren, J.; Karlson, T.D.L.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef]
- Kim, T.-O.; Park, K.-J.; Cho, Y.-N.; Jin, H.-M.; Jo, Y.-G.; Kim, H.S.; Ju, J.K.; Shin, H.-J.; Kho, B.-G.; Kee, S.-J.; et al. Altered distribution, activation and increased IL-17 production of mucosal-associated invariant T cells in patients with acute respiratory distress syndrome. Thorax 2022, 77, 865–872. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alshahrani, M.A.; Nahari, M.H.; Hassan, S.S.U.; Jan, M.S.; Ayaz, M.; Ullah, F.; Alshehri, O.M.; Alshehri, M.A.; Rashid, U.; et al. In-Vitro, In-Vivo, Molecular Docking and ADMET Studies of 2-Substituted 3,7-Dihydroxy-4H-chromen-4-one for Oxidative Stress, Inflammation and Alzheimer’s Disease. Metabolites 2022, 12, 1055. [Google Scholar] [CrossRef]
- Karthika, C.; Appu, A.; Akter, R.; Rahman, M.; Tagde, P.; Ashraf, G.; Abdel-Daim, M.; Abid, A.; Bungau, S. Potential innovation against Alzheimer’s disorder: A tricomponent combination of natural antioxidants (vitamin E, quercetin, and basil oil) and the development of its intranasal delivery. Environ. Sci. Pollut. Res. 2022, 29, 10950–10965. [Google Scholar] [CrossRef]
- Ross, C.; Tabrizi, S. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.; Ambrose, C.; Duyao, M.; Myers, R.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.; James, M.; Groot, N. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Zhang, X.; Huang, H.; Tang, S.; Chai, Y.; Xu, Z.; Li, M.; Chen, X.; Liu, J.; et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J. Nanobiotechnology 2022, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhai, Y.; Hao, Y.; Wang, Q.; Han, F.; Zheng, W.; Hong, J.; Cui, L.; Jin, W.; Ma, S. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J. Extracell. Vesicles 2022, 11, e12255. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Reddy, K. Global Burden of Disease Study 2015 provides GPS for global health 2030. Lancet 2016, 388, 1448–1449. [Google Scholar] [CrossRef]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.-E.; El-Baba, M.; Saxena, P.; Ausländer, S.; Tan, K.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef]
- Ganguly, K.; Khanna, P.; Morecraft, R.J.; Lin, D.J. Modulation of neural co-firing to enhance network transmission and improve motor function after stroke. Neuron 2022, 110, 2363–2385. [Google Scholar] [CrossRef]
- Debette, S.; Markus, H.S. Stroke genetics: Discovery, insight into mechanisms, and clinical perspectives. Circ. Res. 2022, 130, 1095–1111. [Google Scholar] [CrossRef]
- Owolabi, M.; Thrift, A.G.; Mahal, A.; Ishida, M.; Martins, S.; Johnson, W.D.; Pandian, J.; Abd-Allah, F.; Yaria, J.; Phan, H.T.; et al. Primary stroke prevention worldwide: Translating evidence into action. Lancet Public Health 2021, 7, e74–e85. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation 2017, 133, e38–e360. [Google Scholar]
- Hamzei Taj, S.; Kho, W.; Riou, A.; Wiedermann, D.; Hoehn, M. MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 2016, 91, 151–165. [Google Scholar] [CrossRef]
- Williams, A.M.; Dennahy, I.S.; Bhatti, U.F.; Halaweish, I.; Xiong, Y.; Chang, P.; Nikolian, V.C.; Chtraklin, K.; Brown, J.; Zhang, Y.; et al. Mesenchymal Stem Cell-Derived Exosomes Provide Neuroprotection and Improve Long-Term Neurologic Outcomes in a Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J. Neurotrauma 2019, 36, 54–60. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.-C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019, 10, 105. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, Y.; Xu, G.; Hua, K.; Liu, D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. 2020, 260, 118403. [Google Scholar] [CrossRef]
- Yuan, R.; Li, Y.; Han, S.; Chen, X.; Chen, J.; He, J.; Gao, H.; Yang, Y.; Yang, S.; Yang, Y. Fe-Curcumin Nanozyme-Mediated Reactive Oxygen Species Scavenging and Anti-Inflammation for Acute Lung Injury. ACS Central Sci. 2021, 8, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Pouletty, P. Drug addictions: Towards socially accepted and medically treatable diseases. Nat. Rev. Drug Discov. 2002, 1, 731–736. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, Y.; Xue, F.; Zheng, Y.; Huang, H.; Wang, W.; Chang, Y.; Yang, H.; Zhang, J. Exosomal DNA Aptamer Targeting α-Synuclein Aggregates Reduced Neuropathological Deficits in a Mouse Parkinson’s Disease Model. Mol. Ther. Nucleic Acids 2019, 17, 726–740. [Google Scholar] [CrossRef]
- Didiot, M.-C.; Hall, L.M.; Coles, A.H.; A Haraszti, R.; Godinho, B.M.; Chase, K.; Sapp, E.; Ly, S.; Alterman, J.F.; Hassler, M.R.; et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol. Ther. 2016, 24, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, S.; Hou, L.; Zhu, D.; Yin, S.; Yang, G.; Wang, Y. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol. Ther. Nucleic Acids 2020, 21, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, B.; Zhang, Z.; Wang, S.; Bai, Y.; Zhang, Y.; Tang, Y.; Du, L.; Xu, L.; Wu, F.; et al. Extracellular Vesicle–Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation 2020, 142, 556–574. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fu, Y.; Cheng, M.; Ma, W.; Zheng, N.; Wang, Y.; Wu, Z. sEVsRVG selectively delivers antiviral siRNA to fetus brain, inhibits ZIKV infection and mitigates ZIKV-induced microcephaly in mouse model. Mol. Ther. 2021, 30, 2078–2091. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Liu, Z.; Zhou, Y.; Chu, D.; Li, X.; Jiang, X.; Hou, D.; Chen, X.; Chen, Y.; et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep. 2015, 5, 17543. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnology 2018, 16, 81. [Google Scholar] [CrossRef]
- Schiffelers, R.; Kooijmans, S.; Vader, P.; Dommelen, V.; Van Solinge, W. Exosome mimetics: A novel class of drug delivery systems. Int. J. Nanomed. 2012, 7, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
| Exosome Origin | Transition | Downstream Route or Molecule | Cells or Models | Downstream | References |
|---|---|---|---|---|---|
| molecule | effect | ||||
| Endothelial cell | miR-214 | ATM | Endothelial cells | − | [57] |
| Adipose-derived MSCs | miR-125a | DLL4 | Endothelial cells | − | [58] |
| MSC from umbilical cord | NO | Wnt4/β-catenin | NAO | + | [59] |
| Bone-marrow MSCs | pSTAT3 | NF-κB | NO | + | [60] |
| Human term PlaMSCs | NA | Angiogenesis-related gene | In vivo murine auricle | + | [61] |
| Ischemic injury model | |||||
| Human UCB | miR-21-3p | NO | Fibroblasts, endothelial cells | NO | [64] |
| Induced vascular progenitor cells | NO | NO | Rat hindlimb ischemia model | NO | [65] |
| Human EPC | NO | NO | H/R induction | NO | [66] |
| Hypoxic-induced MSC | NO | NO | NO | NO | [67] |
| Disease | Therapeutic Molecule | Donor Cell | Modification Strategy | Drug Loading Method | Administration Route | Animal | Targeted Cells | Ref. |
|---|---|---|---|---|---|---|---|---|
| Alzheimer’s disease | BACE1 siRNA | self-derived dendritic cells | Lamp2b-RVG | electroporation | intravenous | Mice | neurons, microglia | [73] |
| Parkinson’s disease | a-Syn siRNA | primary dendritic cells | Lamp2b-RVG | electroporation | intravenous | Mice | unknown | [126] |
| aptamer F5R1 | HEK-293T cells | Lamp2b-RVG | co-incubation | intraperitoneal | Mice | microglia, neurons | [161] | |
| catalase | RAW264.7 | None | sonication orextrusion | intranasal | Mice | neurons andmicroglia | [115] | |
| Huntington’sdisease | hsiRNAHTT | glioblastoma U87cells | co-incubation | None | Unilateral brain infusion | Mice | neurons | [162] |
| Stroke | curcumin | RAW264.7 | None | co-incubation | intravenous | Rats | neurons and endothelium cells | [97] |
| curcumin | mouse embryonic stem cells (MESCs) | None | co-incubation | intranasal | Mice | astrocytes and neurons | [100] | |
| recombinant human NGF mRN | HEK-293T cells | Lamp2b-RVG | transfection | intravenous | Mice | microglia, neurons | [163] | |
| PEDF | stem cells (ADSCs) | None | transfection | intravenous | Rats | unknown | [113] | |
| circ SCMH1 | HEK-293T cells | Lamp2b-RVG | transfection | intravenous | Mice and rhesusmonkeys | microglia, neurons, and astrocytes | [164] | |
| Brain tumor | doxorubicin | brain endothelial cell (bEND.3) | None | co-incubation | intravenous | Zebrafishes | unknown | [108] |
| SPIONs | RAW264.7 | RGE-peptide | electroporation | intravenous | Mice | glioma | [165] | |
| ZIKV infection | ZIKV-specific siRNA | HEK-293T cells | Lamp2b-RVG | electroporation | intravenous | Mice | microglia, neurons | [166] |
| Morphine addiction | Mu (MOR) siRN | HEK-293T cells | Lamp2b-RVG | transfection | intravenous | Mice | neuro2A | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.U.; Khan, M.I.; Khan, M.U.; Khan, N.M.; Bungau, S.; Hassan, S.S.u. Applications of Extracellular Vesicles in Nervous System Disorders: An Overview of Recent Advances. Bioengineering 2023, 10, 51. https://doi.org/10.3390/bioengineering10010051
Khan SU, Khan MI, Khan MU, Khan NM, Bungau S, Hassan SSu. Applications of Extracellular Vesicles in Nervous System Disorders: An Overview of Recent Advances. Bioengineering. 2023; 10(1):51. https://doi.org/10.3390/bioengineering10010051
Chicago/Turabian StyleKhan, Safir Ullah, Muhammad Imran Khan, Munir Ullah Khan, Noor Muhammad Khan, Simona Bungau, and Syed Shams ul Hassan. 2023. "Applications of Extracellular Vesicles in Nervous System Disorders: An Overview of Recent Advances" Bioengineering 10, no. 1: 51. https://doi.org/10.3390/bioengineering10010051
APA StyleKhan, S. U., Khan, M. I., Khan, M. U., Khan, N. M., Bungau, S., & Hassan, S. S. u. (2023). Applications of Extracellular Vesicles in Nervous System Disorders: An Overview of Recent Advances. Bioengineering, 10(1), 51. https://doi.org/10.3390/bioengineering10010051










