Abstract
Acute pancreatitis (AP) is a prevalent clinical condition of the digestive system, with a growing frequency each year. Approximately 20% of patients suffer from severe acute pancreatitis (SAP) with local consequences and multi-organ failure, putting a significant strain on patients’ health insurance. According to reports, the lungs are particularly susceptible to SAP. Acute respiratory distress syndrome, a severe type of acute lung injury (ALI), is the primary cause of mortality among AP patients. Controlling the mortality associated with SAP requires an understanding of the etiology of AP-associated ALI, the discovery of biomarkers for the early detection of ALI, and the identification of potentially effective drug treatments. Exosomes are a class of extracellular vesicles with a diameter of 30–150 nm that are actively released into tissue fluids to mediate biological functions. Exosomes are laden with bioactive cargo, such as lipids, proteins, DNA, and RNA. During the initial stages of AP, acinar cell-derived exosomes suppress forkhead box protein O1 expression, resulting in M1 macrophage polarization. Similarly, macrophage-derived exosomes activate inflammatory pathways within endothelium or epithelial cells, promoting an inflammatory cascade response. On the other hand, a part of exosome cargo performs tissue repair and anti-inflammatory actions and inhibits the cytokine storm during AP. Other reviews have detailed the function of exosomes in the development of AP, chronic pancreatitis, and autoimmune pancreatitis. The discoveries involving exosomes at the intersection of AP and acute lung injury (ALI) are reviewed here. Furthermore, we discuss the therapeutic potential of exosomes in AP and associated ALI. With the continuous improvement of technological tools, the research on exosomes has gradually shifted from basic to clinical applications. Several exosome-specific non-coding RNAs and proteins can be used as novel molecular markers to assist in the diagnosis and prognosis of AP and associated ALI.
1. Introduction
Acute pancreatitis (AP) is a frequent occurring, acute abdominal illness. The majority of patients present with mild AP, which may spontaneously resolve. Nonetheless, approximately 20% of patients report severe AP (SAP) that is fast progressive and aggressive [1,2]. Acute lung injury (ALI) is a life-threatening condition characterized by diffuse interstitial and alveolar edema resulting from the damage of pulmonary microvascular endothelial cells (PMVECs) and alveolar epithelial cells (AECs) [3,4]. ALI and its severe form, acute respiratory distress syndrome (ARDS), are among the most prevalent consequences of SAP and are leading causes of mortality in SAP patients [5].
New insights into the pathogenesis of AP and associated ALI have emerged in recent years. The systemic inflammatory response (SIRS) caused by the abnormal activation of pancreatic enzymes, mitochondrial dysfunction, impaired autophagy, endoplasmic reticulum stress, programmed cell death, intestinal mucosal barrier damage, and bacterial translocation are the initiating factors of multiple organ dysfunction syndromes (MODS) in AP [6]. Key molecules causing pulmonary air–blood barrier disruption and alveolar edema include pancreatic, intestinal, and liver-derived non-coding RNAs (ncRNAs), damage-associated molecular patterns (DAMPs), and pathogen-associated molecular patterns (PAMPs). Cross-signaling between immune cells (such as neutrophils, macrophages, and T cells) and parenchymal cells (such as acinar cells, intestinal epithelial cells, PMVECs, and AECs) is a crucial mechanism for maintaining the AP cytokine storm [7]. However, the molecular network of intercellular communication is intricate and requires immediate clarification. Exosome-related research has expanded quickly in recent years. The basics of exosomes, including the biogenesis, processes of secretion, and cargo they carry, have been steadily uncovered [8,9]. We hypothesize that exosomes may be a key mediator of communication between immune cells and parenchymal cells, mediating the local and systemic inflammatory response during AP.
Extracellular vesicles (EVs), which include various types of vesicles such as exosomes, microvesicles, and apoptotic vesicles ranging from 30 nm to 10 µm in diameter, were first discovered by Erwin Chargaff and Randolph West using high-speed centrifugation; they detected a platelet-free clotting component in plasma [10,11]. Later, Johnstone et al. discovered and suggested EVs as a cellular waste disposal mechanism in the culture supernatant of sheep erythrocytes [12]. Since the 21st century, numerous studies have reported the importance of exosomal cargo and its function as a unique biological device [13,14]. The biogenesis of exosomes involves both production and secretion. The process of exosome production mainly consists of two invaginations of the plasma membrane and the formation of a multivesicular body (MVB) containing intraluminal vesicles (ILVs). The MVBs bind to lysosomes for degradation and fuse again with the cytoplasmic membrane to release ILVs, completing the exosome release process. Exosome stability is a major contributing factor to their widespread acceptance. The first thing to know about exosomes is that they are very tiny and can thus easily pass through both blood vessels and the extracellular matrix. Since they are released by one’s own cells, monocytes and macrophages cannot phagocytose exosomes. Furthermore, exosomes can circulate freely in bodily fluids because CD55 and CD59 on their surface protect them from being altered by opsonin and coagulation factors [15]. As a result of their unique vesicular structure, the contents of exosomes may be kept reasonably stable for up to a few weeks [16]. Kalra et al. [17] showed by proteomic analysis that the protein composition of plasma exosomes is stable for up to 90 days and may still be picked up by target cells and exert biological effects. In addition, it has been observed that plasma exosomal microRNAs (miRNAs) are relatively stable, even when kept in various storage settings [18]. When stored at −20 degrees Celsius for 5 weeks, the overall quantity of exosomal miRNA remained almost the same, with very modest variations in the amounts of specific miRNAs. Thus, when exosomes are picked up by the recipient cells and internalized, they contain cargo that can impact the recipient cell function through triggering intracytoplasmic signaling pathways [19]. Specifically, exosome-derived nucleic acids and proteins are widely involved in physiopathological processes such as the inflammatory response, infection, and immune regulation, affecting the development of AP [20].
This paper sheds light on the AP-mediated cytokine storm by describing the signaling regulatory actions of exosomal proteins and nucleic acids. We will also show that exosomes, which are found in all biological fluids, including blood, bronchoalveolar lavage, urine, and pancreatic fluid, may be used as diagnostic and prognostic indicators for AP. Furthermore, exosomes are stable, non-toxic liposomes that may escape immune detection and be absorbed by recipient cells. With the growth of exosome research and the implementation of new experimental procedures, it is anticipated that exosomes will also be exploited for medication delivery in clinical study.
2. Exosomes and AP-Associated ALI
The molecular mechanisms involved in AP-associated ALI that lead to SIRS and diffuse alveolar damage have been studied in detail. Multiple signaling pathways are engaged during AP. To summarize, the activation of the cytokine storm is caused by the upregulation of extracellular mediators such as DAMPs, histones, and ncRNAs during AP. Intriguingly, emerging research has shown that the pancreas–lung axis [6] and the gut–lung axis [21] may mediate the cytokine storm in AP-associated ALI, and that exosomes may be major carriers of extracellular mediators transported along the signaling axis.
We hypothesize that the pancreas–lung axis is a putative signaling pathway for AP-associated ALI based on the findings of many investigations. Zhu et al. discovered that plasma exosomal miR-216a was considerably elevated in AP patients with ALI compared to AP patients without ALI [22]. Exosomal miR-216a seems to be a particular modulator of inflammation in AP-induced ALI. As shown in animal investigations, miR-216a expression was undetectable in all organs save the pancreas, including the lung, gut, heart, and kidney. It is possible that exosomal miR-216a is pancreas-specific. Moreover, exosomal miR-216a enhanced the permeability of pulmonary microvascular endothelial cells, which was linked with the degree of ALI during AP. Xu et al. discovered that cold-inducible RNA-binding protein (CIRP) may play a crucial role in alveolar macrophage (AM) pyroptosis as well as neutrophil recruitment during AP-associated ALI [23]. The level of CIRP was found to be enhanced in the pancreatic tissue, serum, and lung tissue of AP rats by Xu and his colleagues. Interestingly, immunohistochemical staining revealed that pancreatic islet cells may be the predominant cell type that secretes CIRP, which may be an additional inflammatory mediator secreted by injured pancreatic tissue that induces ALI. In addition, Murao et al. discovered that CIRP may persist extracellularly as exosomes and mediate inflammation during sepsis. Thus, we hypothesized that exosome-loaded inflammatory mediators, such as CIRP and miR-216a, delivered along the pancreas–lung axis mediate the development of AP-associated ALI.
The gut–lung axis is a commonly recognized pathophysiological signal of crosstalk between intestinal and pulmonary diseases. In the case of AP-associated ALI, intestinal damage and its subsequent response has an “amplifier” effect [24]. Firstly, intestinal barrier damage and increased intestinal permeability are prevalent in AP patients and models generated by a variety of causes [25,26]. Secondly, the intestinal barrier is a factor that exacerbates the inflammatory response to SIRS [27]. After intestinal barrier damage, the most direct consequence may be the “second strike” of intestine-derived endotoxins entering the circulation and lungs through the portal vein or mesenteric lymphatic system [28,29]. On the other hand, SIRS may be promoted by exosomes released from the injured gut during AP. Under physiological conditions, intestinal epithelial cells (IECs) or DC-derived exosomes carrying transforming growth factor-β, MHC class I and II complexes and co-stimulatory molecules coordinate the regulation of intestinal immunity and maintain immune homeostasis. However, miRNAs such as miR-122a and miR-29a released from damaged IECs can exacerbate intestinal barrier damage and increase intestinal permeability [30].
Thus, we hypothesize that the pancreas and gut release exosomes containing pro-inflammatory mediators during AP, with the pancreas–lung axis and the gut–lung axis serving as the primary delivery pathways (Figure 1).
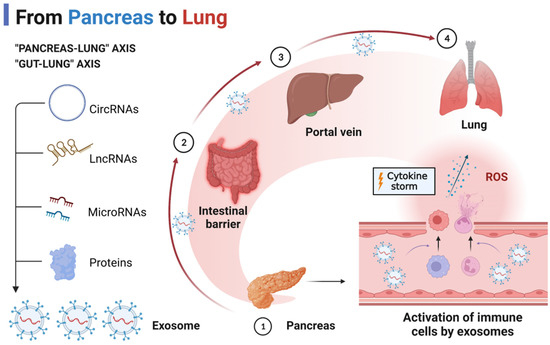
Figure 1.
The “pancreas–lung” axis and “gut–lung” axis in AP-associated ALI.
3. Exosome-Specific ncRNAs
3.1. LncRNAs
LncRNAs, or long non-coding RNAs, are a subset of ncRNAs that are longer than 200 nucleotides and are synthesized in a manner analogous to mRNA. First dismissed as meaningless “transcriptional noise” [31], lncRNAs have now been shown to play essential roles in various biological processes. A growing body of evidence indicates that lncRNAs may interact with RNA, DNA, and proteins. In particular, lncRNAs can regulate gene expression at the epigenetic, transcriptional, and post-transcriptional levels and even directly regulate protein activity [32,33,34,35]. The lncRNAs may also take part in chromatin modification [36]. Many lncRNAs have been implicated in pathophysiological processes such as cancer [37], inflammation [38], hematopoiesis [31], and metabolism [39]; the interest in lncRNAs has grown with the advancement of high-throughput technology.
3.1.1. MALAT1
As one of the first lncRNAs discovered, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) has been linked to the pathophysiology of inflammatory illnesses, metabolic diseases, and cancer [40,41,42]. As discovered by Gu et al. [43], the early inflammatory response of acinar cells may include a “MALAT1-miR-194-yes-associated protein 1 (YAP1)” mutual feedback loop. MiR-194 is a protective microRNA that dampens cerulein-induced inflammation in AR42J cells (pancreatic acinar cells) by targeting the YAP signaling pathway. It is interesting to note that, in vitro, YAP1 and MALAT1 enhance each other, whereas MALAT1 and miR-194 counteract each other [43]. It has also been established that the MALAT1/miR-181a-5p/high-mobility group box 1 (HMGB1) axis plays a role in macrophage polarization during AP [44]. First, the researchers found elevated levels of MALAT1 in EVs taken from the plasma of AP patients compared to those found in healthy participants. MPC-83 cells (mouse pancreatic acinar cells) were encouraged to generate MALAT1-carrying EVs in response to cerulein, providing further evidence that the MALAT1-carrying EVs observed in AP patients’ plasma likely originate from inflamed acinar cells. Mechanistic studies demonstrated that MALAT1 upregulates HMGB1 (a notorious DAMP) expression by competitively binding to miR-181a-5p, stimulating the toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) signaling pathway and promoting M1 macrophage polarization [44]. It is noteworthy that hepatocytes, similar to acinar cells, also emit exosomes containing MALAT1. Risk factors for AP include the presence of non-alcoholic fatty liver disease. The authors hypothesized that hepatocyte injury and the subsequent release of exosomes would foster the progression of AP [45]. Exosome-specific MALAT1 was secreted by damaged hepatocytes, which exacerbated inflammation in acinar cells by downregulating autophagy and upregulating the Hippo–YAP pathway [45].
In addition to AP, MALAT1 expression was elevated in the plasma of sepsis and ARDS patients [46,47]. The expression of MALAT1 was strongly connected with illness severity, organ damage, and death, and its diagnostic performance in patients with sepsis was found to be excellent (AUC = 0.931) by Lu et al. [46]. MALAT1 levels in plasma and peripheral blood mononuclear cells were considerably more significant in ARDS patients compared to the control group. It was also shown that MALAT1 levels in plasma exosomes were higher than in the control group, though this difference did not reach statistical significance [47]. All studies have consistently linked MALAT1 to worse outcomes in ALI/ARDS from a mechanical standpoint. Moreover, the knockdown of MALAT1 expression significantly inhibited the apoptosis of alveolar epithelial cells, disruption of the endothelial barrier, and inflammatory signaling pathways in ALI.
3.1.2. TUG1
LncRNA taurine upregulated gene 1 (TUG1) consists of 7598 nucleotides and was first discovered in the retinal cells of neonatal mice. Retinal development in mice is hampered when TUG1 is silenced [48]. TUG1 expression was markedly downregulated in lung tissue and airway epithelial cells in sepsis-induced-ALI animals. By blocking miR-494, TUG1 protected the airway epithelial cells and reduced lung inflammation [49]. If pulmonary microvascular endothelial cells are under assault, TUG1 may potentially serve as a protective factor. In particular, increasing TUG1 expression suppressed lipopolysaccharide (LPS)-induced apoptosis and inflammation in endothelial cells. Overexpressing miR-34b-5p in rescue tests decreased TUG1’s protective impact on endothelial cells. GRB2-associated binding protein 1, positively correlated with TUG1, was negatively regulated by miR-34b-5p and, similarly, exerted a protective effect [50]. EVs-specific TUG1 is also protective against sepsis-induced ALI. Ma et al. [51] reported that EVs released from endothelial progenitor cells were rich in TUG1, promoting tissue repair, and attenuating vascular injury by inducing macrophage polarization towards the M2 type. Mechanistically, TUG1 upregulates silent information regulator 1 (SIRT1) expression by competitively binding miR-9-5p. The latter is known as an antioxidant and anti-inflammatory deacetylase. In contrast to ALI, TUG1 and exosomal TUG1 expression was stimulated to be upregulated in AR42J cells and was significantly associated with Treg cell differentiation. The overexpression of TUG1 promotes LPS/cerulein-induced apoptosis and inflammation in AR42J cells [52]. According to recent research, lncRNA TUG1 may play opposing roles in AP and ALI. As there are no experimental investigations on the impact of exosomal TUG1 produced by acinar cells on lung parenchymal cells, such as epithelial and endothelial cells, this piqued our curiosity.
3.1.3. Others
Patients with AP or ARDS may potentially benefit from testing for a variety of additional lncRNAs that have been proven to have diagnostic utility. Patients with AP have an elevated plasma plasmacytoma variant translocation gene 1 (PVT1) expression compared to healthy volunteers; this elevated expression is correlated with various clinical characteristics and has a substantial predictive value for mortality (AUC = 0.838) [46]. Similarly, PVT1 can be an independent risk factor for patients with ARDS and can help to assess the severity of ARDS and predict mortality [53]. In addition, exosome-specific PVT1 has been shown to have diagnostic relevance in human disease [54]. As a result, we have become more curious about exosome-specific PVT1 and its function in AP and ALI. Other lncRNAs, such as FBXL19-AS1 [55] and lnc-ITSN1-2 [56], have also been shown to be helpful in the diagnosis of AP and the evaluation of prognosis. It is essential to investigate whether such lncRNAs are present in exosomes and, if so, what role they play in AP and the accompanying ALI.
3.2. MiRNAs
According to the current data, microRNAs (miRNAs) are the primary form of RNA delivered by exosomes. MiRNAs are small ncRNAs that are highly conserved in organisms and are approximately 20–24 nucleotides in length [57]. In recent years, miRNAs have been shown to regulate gene expression at the post-transcriptional level by pairing with mRNA 3′-untranslated regions of target genes [58]. The functions of miRNAs were more than imagined [59,60]. At present, the synthesis process of miRNA in animals is well understood. MiRNA is first transcribed in the nucleus by RNA polymerase II into pri-miRNA, which is about 300–1000 bases in length. Pri-miRNA is cleaved in the nucleus by RNase III-Drosha into precursor miRNA (pre-miRNA), at approximately 70 bases in length. Subsequently, the pre-miRNA is transferred from the nucleus to the cytoplasm by the action of the transporter protein exportin 5 and further cleaved by RNase III-Dicer into a mature double-stranded miRNA (mature miRNA) of approximately 20–24 nt in length [61,62]. The mature double-stranded miRNAs form RNA-induced silencing complexes with Argonaute proteins to degrade target gene mRNAs or play a biological role in translation inhibition [63]. MiRNAs have become a hot research target as regulators of gene expression and biomarker candidates. MiRNAs in exosomes are important in disease development, diagnosis, etc. [64]. The discovery of exosomal miRNAs in AP, a common and dangerous acute abdominal disease, provides new ideas on the pathogenesis and diagnosis of AP and has some reference value.
3.2.1. MiR-155
MiR-155 is a miRNA encoded by a non-coding B cell integration cluster, with two mature forms, miR-155-3p and miR-155-5p. MiR-155 has a crucial regulatory function in the organism’s innate immune and inflammatory response [65,66]. The miR-155 level is often increased in the presence of severe disease [67,68]. The expression of miR-155 in the peripheral blood of AP patients is, however, debatable. Lin et al. discovered that the expression of miR-155 was considerably higher in the serum of AP patients and strongly linked with the Ranson score and APACHE II score [69]. Wang et al. also found a correlation between higher miR-155 levels and the severity of AP patients [70]. Miao et al. found that the level of plasma-derived exosomal miR-155 in AP-associated ALI was significantly higher than in AP without ALI. In comparison, the exosome-specific miR-155 level in the AP without the ALI group was significantly higher than that in healthy volunteers [71]. However, Hu et al. discovered considerably lower miR-155 levels in the blood of AP patients than in healthy participants [72]. The cause for this opposite outcome is, as yet, unclear. More clinical research with large sample sizes is needed to assess miR-155 levels in AP patients.
MiR-155 appears to be consistently highly expressed in the pancreas, gut, lung, and peripheral blood of AP models. Inhibition of miR-155 alleviates intestinal barrier damage [73], immune imbalance [70], and impaired autophagy [74,75] induced by AP. For exosome-specific miR-155, researchers discovered that plasma-derived exosomes were enriched with inflammatory miR-155, whereas ascites-derived exosomes were not [76]. Exosome-specific miR-155 that the liver has activated may reach the alveoli and contribute to the development of ALI. Specifically, exosome-specific miR-155 activates alveolar macrophages and triggers NOD-like receptor protein 3 (NLRP3)-dependent pyroptosis [77]. The inhibition of MiR-155 alleviates ALI by inhibiting macrophage proliferation [78], antagonizing nuclear factor kappa beta (NF-κB) signaling [79], and modulating neutrophil extracellular trap formation [80] in the lungs.
3.2.2. MiR-21
The miR-21 gene is found in the 10th intron region of the transmembrane protein 49 gene on chromosome 17 q23.2. MiR-21, unlike other miRNAs, has an independent promoter region and is regulated by transcription factors, including AP-1, STAT3, and p53 [41]. In research including 164 patients, miR-21-3p levels in AP groups were considerably higher than in the healthy control group [81]. The level of miR-21-3p was considerably more significant in the SAP group compared to the MSAP and MAP groups. In patients with autoimmune pancreatitis, the level of extracellular vesicle (EV)-specific miR-21-5p in the plasma was considerably higher than in patients with chronic pancreatitis and healthy volunteers [82]. Hu and colleagues discovered, similar to miR-155, that AP patients had lower levels of miR-21 than healthy volunteers [72]. Therefore, the level of miR-21 in the circulation of AP patients requires additional investigation.
MiR-21 is said to favor the AP procedure. Stimulation of acinar cells with cerulein and taurolithocholic acid 3-sulfate induced significant changes in miRNA expression profiles, with miR-21-3p being significantly elevated [54]. The mouse AP model induced by cerulein and L-arginine also confirmed the high level of miR-21-3p in the pancreatic tissue [83]. Mechanistically, miR-21-3p exacerbated inflammation and lung injury by activating transient receptor potential signaling pathways in SAP rats [84]. Knockdown of miR-21 decreased AP-associated ALI by reducing trypsinogen activation [85] and inhibiting the release of HMGB1. Intriguingly, miR-21-5p may prevent lung damage. In AEC cells, the overexpression of miR-21-5p inhibited hyperoxia-induced apoptosis [86] and mitochondrial damage [87]. Exosomes generated from mesenchymal stromal cells decreased ischemia/reperfusion-induced ALI in an miR-21-5p-dependent manner [88].
3.2.3. MiR-216a
Extensive research has been conducted on the function of miR-216a in diagnosing AP [89,90,91]. The miR-216a level was elevated in the plasma and mesenteric lymph after induction of AP in rats and mice and was positively correlated with the severity of the disease [92]. In an AP dog model, elevated miR-216a levels were substantially linked with the extent of tissue damage and showed a more dynamic response than amylase and lipase [93]. According to a clinical investigation, the plasma miR-216a level in SAP patients was considerably higher than in mild AP (MAP) and MSAP patients, but there was no difference between MAP and MSAP patients [94]. This indicates that miR-216a may serve as a biomarker for the early detection of SAP. Notably, miR-216a was tightly linked to SAP-induced liver damage. In SAP patients with liver injury, miR-216a levels in the peripheral blood were considerably higher than in SAP patients without liver injury [95]. Furthermore, Zhu et al. examined the differences in plasma-derived exosomal miR-216a levels in patients with AP-associated ALI, patients with AP without ALI, and healthy volunteers. They found that exosome-specific miR-216a levels were significantly higher in patients in the AP-associated ALI group than in the AP without ALI group, which in turn had significantly higher exosome-specific miR-216a levels than in healthy volunteers [22]. These results suggest that miR-216a is present in plasma-derived exosomes and plays a vital role in the process of AP-associated ALI. Mechanistic experiments confirmed the role of miR-216a as a risk factor for PMVEC injury in AP. Overexpression of miR-216a increases endothelial cell permeability by altering the expression of tight junction proteins and is associated with the development of lung injury. However, Fan et al. [96] offered an alternative viewpoint. They discovered that miR-216a overexpression in ALI animals dramatically decreased serum inflammatory factor levels and improved lung permeability. The miR-216a target Janus protein tyrosine kinase 2 dramatically reduced ALI by regulating the NF-κB signaling pathway [96]. Thus, many more experiments are needed to prove the function of miR-216a in AP and associated ALI.
3.2.4. Others
A recent clinical study reported that seven exosome-specific miRNAs might be potential biomarkers for the diagnosis of patients with SAP [97]. Among them, the levels of exosome-specific miR-583 and miR-603 were strongly correlated with the severity of AP [97]. MiR-583 and miR-603 are considered to be tumor suppressors, and their related studies are mainly focused on tumors such as breast cancer [98], hepatocellular carcinoma [99], and colorectal cancer [100]. There are no mechanistic studies on the association of miR-603 with AP or SIRS. Exosome-specific miR-192-5p levels were downregulated in mild AP patients. This result is similar to that of previous studies. Patients with AP with nonalcoholic fatty liver disease had significantly lower circulating miR-192-5p levels than the healthy control group [101]. Furthermore, the level of exosome-specific miR-122-5p was upregulated only in the SAP group but not in the MAP group [97]. Interestingly, miR-122-5p has previously been shown to be associated with lipopolysaccharide (LPS)-induced ALI. Knockdown of miR-122-5p helps to attenuate the damage of PMVECs and AECs during ALI. Thus, miR-122-5p may be the essential medium for SAP-associated ALI [102,103]. This, of course, needs to be confirmed by further studies.
The role of exosome-specific ncRNAs in SIRS is a rapidly developing area of study in AP. Synergistic interactions between neutrophils, macrophages, platelets, endothelial cells, and AECs create the cytokine storm of AP and associated ALI. Several exosome-specific lncRNAs and miRNAs have been shown to have an altered expression in AP and ALI (Table 1), and these genes play essential roles in the AP-induced inflammatory cascade response, AM activation, increased endothelial cell permeability, and alveolar epithelial cell injury by targeting and regulating downstream genes and associated signaling pathways (Figure 2).

Table 1.
Exosome-ncRNAs in AP and ALI.
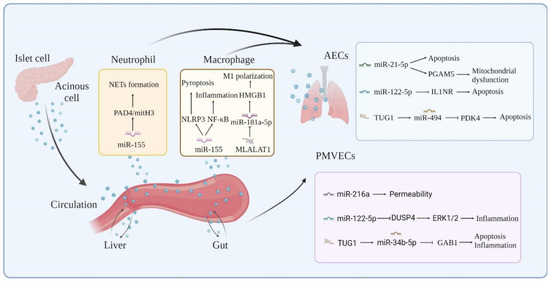
Figure 2.
Molecular mechanisms of exosome-ncRNAs in AP-associated ALI.
4. Exosome-Specific Proteins
Proteins, in addition to ncRNAs, are the most common exosomal cargo. Exosome-specific proteins are mostly found on the vesicle surface and in the endolumen. Membrane transport and fusion proteins, transmembrane proteins, and marker proteins such as CD63 and CD9 are found on the vesicle surface, while heat shock proteins, signal transduction proteins, and backbone proteins are found in the vesicle lumen. Exosome-specific proteins are physicochemically stable, less vulnerable to the extracellular setting, and may play an essential role in disease development [104,105].
4.1. HMGB1
High-mobility group box 1 protein (HMGB1) is a well-known DAMP that received its name from its high mobility in polyacrylamide gel electrophoresis [106,107]. In 2006, Yasuda et al. discovered a link between serum HMGB1 levels and disease severity in SAP patients. This work piqued the attention of academics in investigating the role of HMGB1 in AP [108]. Later, it was shown that serum HMGB1 levels in SAP patients might be an essential predictor of intestinal barrier failure and infection [109], and aid in predicting the risk of AP-related death [110]. Mechanistically, the A box of HMGB1 has an anti-inflammatory effect and inhibits AP-induced pancreatic injury [111]. In contrast, the B box has pro-inflammatory activity and is involved in the inflammatory response and organ damage in AP as a late inflammatory mediator. Specifically, to activate inflammation-related signaling pathways, promote the release of the neutrophil extracellular traps (NETs), induce cell necrosis, and amplify pancreatic inflammation, AP stimulates pancreatic acinar cells, pancreatic macrophages, and Kupffer cells to release HMGB1 rapidly [112,113,114,115]. The AP inflammatory cascade response has a refueling station in the gut. SAP patients are more susceptible to complications such as ALI/ARDS and sepsis because of disruption to the intestinal barrier [6]. HMGB1 has been implicated in SAP-induced intestinal barrier injury via the activation of the TLR4/9/NF-κB signaling pathway, disruption of intestinal flora, and disruption of intestinal tight junctions, according to several research findings [116,117,118]. Similarly, AP-associated ALI is exacerbated by HMGB1, which promotes inflammation by triggering nuclear translocation of NF-κB and inflammasome formation [119,120]. SAP may cause myocardial injury, which is an uncommon yet life-threatening consequence. Myocardial damage from SAP may be reduced if HMGB1-mediated oxidative stress is blocked [121]. Thus, HMGB1 may be a direct inflammatory cytokine that helps begin and sustain the inflammatory cascade response in the pancreas, gut, lungs, liver, and heart during acute pancreatitis.
The mechanism of HMGB1 release has recently piqued the curiosity of scientists. The main ways by which cells produce DAMPs may include PCD, and lysosomal- and exosomal-exocytosis [105]. Exosome-specific HMGB1 became a new favorite among researchers, as anticipated. In addition, in 2006, Liu et al. found that HMGB1, which was identified in the vesicles of Caco-2 cells, was strongly related to inflammation-induced epithelial hyperpermeability [122]. Later, researchers discovered that exosomal HMGB1 produced from the dysbiosis of the intestinal flora might be transported from the gut to the liver, thereby causing hepatic steatosis. This finding implies that exosomal HMGB1 may be a key modulator of the gut–liver axis [123]. The gut–liver axis is a well-known feedback mechanism in the SAP process. During SAP, high circulating levels of HMGB1 are primarily caused by the release of HMGB1 from hepatocytes activated by inflammatory mediators produced from the gut. Li et al. found that the extracellular release of hepatic HMGB1 from mice with sepsis occurs via exosomes and is dependent on the TLR4/caspase-11/GsdmD signaling pathway [124]. Despite the lack of substantial evidence, exosomal HMGB1 may have an inflammatory function in SAP. However, comparable disorders have provided us with valuable clues. We speculate that exosomal HMGB1 has promise for the investigation of SAP mechanisms and clinical diagnosis.
4.2. S100A8/A9
Solubility in 100% ammonium sulfate is a vital feature of the S100 protein family, consisting of calcium-binding proteins with a low molecular weight [125]. Currently, a total of 25 S100 proteins have been identified. S100A8 and S100A9, all of the S100 protein family, are involved in a wide range of cellular signaling processes [126]. Monomeric, homodimeric, and heterodimeric forms of S100A8 and S100A9 function as natural immune mediators by binding to cytoplasmic membrane receptors to sustain and increase the inflammatory response [127]. In addition, research suggests that S100A8/S100A9 may serve as a diagnostic and prognostic marker for COVID-19 [128], inflammatory diseases [129], and human cancers [130].
A clinical study including 246 patients found that plasma S100A8/S100A9 levels were more significant in patients with mild and severe AP compared to healthy volunteers [131]. At the time, researchers discovered no statistically significant difference in plasma S100A8/S100A9 levels between individuals with severe AP and those with mild AP. Interestingly, when exosomes were introduced into biological research, it was discovered that the difference in exosome-specific S100A8/A9 levels from severe AP vs. mild AP was substantial. The study by Waldron et al. was the first to give us some hints [132]. They collected plasma from 12 patients with alcoholic AP and 12 healthy controls for proteomic analysis. They identified 37 differentially expressed proteins, 31 of which were upregulated and 6 of which were downregulated. Interestingly, almost all of the upregulated proteins were associated with cellular compartments, including exosomes and extracellular spaces. Among them, S100A8, a DAMP, previously described, which is closely associated with the development of SAP, was also shown to be significantly up-regulated in the plasma of AP patients [132]. The potential of exosome-specific S100A8 in predicting the severity of SAP was also confirmed in a recent study. Exosome-specific S100A8 and S100A9 isolated from the plasma of SAP patients promoted the activation of the NF-κB signaling pathway and the level of inflammatory mediators in macrophages. Furthermore, the promotion of SIRS by S100A8 and S100A9 depends on the activation mechanism of nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase. In summary, these findings suggest that exosome-specific S100A8 and S100A9 are associated with the intensity of SIRS during AP [133].
Mechanistic experiments further demonstrated that the exosome-specific S100A8/S100A9 protein, which exists as a heterodimer, may induce NF-κB activation and inflammatory factor production via the activation of NADPH oxidase. These data show a link between the severity of AP and the pro-inflammatory activity of exosome-specific S100A8/S100A9. Nesvaderan et al. developed a four-gene signature including S100A8, S100A9, matrix metalloproteinase (MMP)25, and MT-ND4L that predicts severe AP with 4% accuracy [134]. According to their findings, this gene panel might be used to detect severe AP at an early stage. To summarize, exosomal S100A8/S100A9 has a unique potential in the diagnosis of AP, and its relation to disease severity and prognosis should be explored in the future.
On the other hand, local pancreatic injury may progress to systemic inflammation and ALI through the S100A8/A9 protein. S100A8/A9 is involved in the release of NETs and the activation of neutrophils [135]. S100A9 directly impacts trypsinogen activation in pancreatic inflammation via the regulation of neutrophil infiltration. The activation of trypsinogen is well-known as a critical step in in the early stages of AP [136]. As a result, AP inflammation and S100A9 go hand in hand. When activated, intracellular protein complexes known as the NLRP3 inflammasome release IL-1β and IL-18, which are proinflammatory mediators involved in cell inflammation and pyroptosis. S100A9 caused pancreatic injury and inflammation by interacting directly with VNN1 and boosting the enormous production of reactive oxygen species (ROS), which activated NLRP3 inflammasome [137], as demonstrated by Xiang et al. In ALI, S100A8/A9 promoted neutrophil activation, damaged alveolar epithelial cells, and increased excessive inflammatory and immune responses in the airways and lung tissue. The S100A9 blockade significantly reduces the ALI induced by LPS or cercal ligation and puncture [138,139]. S100A8/A9 proteins are abundantly expressed in alveolar macrophages and intestinal epithelial cells after SARS-CoV-2 infection and may be biomarkers connecting respiratory and intestinal damage [140]. This discovery shows that the S100A8/A9 proteins may be essential mediators on the gut–lung axis, a proven bridge to severe AP-associated ALI. Therefore, exosome-specific S100A8/A9 is a crucial mediator of the inflammatory response induced by AP, leading to the development of ALI.
4.3. CIRP
CIRP was the first cold-shock protein discovered in mammals, and it is overexpressed in response to cold shock, oxidative stress, and hypoxia [141,142]. The function of CIRP is inextricably linked to its localization [143]. Intracellular CIRP (iCIRP) protects RNA from degradation while regulating RNA transcription and translation. Multiple biological mechanisms are regulated by iCIRP, which aids cells in responding to a wide range of physiological and pathological stimuli [144]. It turns out, however, that CIRP released from cells plays an essential role in mediating innate immunity and contributing to the inflammatory cascade reaction [142]. According to recent studies, exosome-specific CIRP may play an “amplifier” role in the progression of AP to ALI. Serum CIRP concentrations were found to be an independent predictor of major adverse events in patients with SAP in a clinical study involving 252 patients in 2017. CIRP outperforms other commonly used inflammatory markers such as procalcitonin, white blood cell count, and C-reactive protein in diagnostic performance for SAP [145]. This finding suggests that CIRP is released into the bloodstream during SAP and correlates with its severity, including SIRS, ALI, and MODS. CIRP levels in the pancreas, serum, and lungs of SAP rats were also shown to be considerably elevated by Xu et al. An antagonist of CIRP, C23, dramatically reduced the pancreatic injury and systemic inflammation produced by SAP, as well as the resulting ALI [23]. These data imply that SAP-induced systemic damage is mediated in part by CIRP. In addition, an experiment in vitro showed that CIRP may target toll-like receptor (TLR) 4 of alveolar macrophages (AMs), which activates NF-κB signaling, induces NLRP3 inflammasome-mediated pyroptosis, and amplifies the inflammatory response. CIRP suppresses M2 macrophage polarization by decreasing Ras superfamilies of small G proteins/erythropoietin receptor signaling in addition to triggering pyroptosis in AMs. During ALI/ARDS, the reduction in inflammation is greatly influenced by this inflammatory pathway [146].
Neutrophils release NETs, which are web-like extrusions that may kill microorganisms directly. NETs play a crucial regulatory function in the development of severe inflammatory illnesses [147,148]. SAP-induced MODS is hypothesized to be exacerbated by the excessive formation of NETs [149]. Intriguingly, during SAP, CIRP strongly influences the formation of NETs in the pancreas. The pancreatic injury produced by NETs was significantly decreased when CIRP was targeted [150]. In addition, CIRP targets AEC as well as macrophages and neutrophils. Triggering receptor expressed on myeloid cells-1 (TREM-1) has been shown to have a role in CIRP-induced inflammation in the AEC. The AEC is stimulated to produce high quantities of chemokines and cytokines when CIRP is binding to TREM-1 [151]. Alveoli are particularly vulnerable to the effects of this. The mechanism of CIRP’s release is also of relevance. CIRP is released after a variety of cell deaths, according to earlier research. Lin and his colleagues, on the other hand, confirmed an active release mechanism of CIRP [152]. CIRP concentrations in mouse serum exosomes rose dramatically during sepsis. CIRP is present mainly on the surface of exosomes and promotes inflammation by triggering cytokine production and neutrophil migration. As a result, we anticipate that the injured pancreas in SAP will produce exosome-specific CIRP, which may be capable of targeting the lung more effectively than free extracellular CIRP [78].
4.4. Histones
Histones are basic proteins in eukaryotic cells that combine with DNA to form chromatin and regulate gene expression. Histones may be transferred from the nucleus to the extracellular space in response to cell stimulation, initiating the immunological response and worsening inflammation [153,154]. There are three forms of extracellular histones: (1) Nucleosomes are formed by histones bound to DNA. (2) NETs are composed of histones, DNA, histone granule proteins, and cytoplasmic proteins, among others. (3) Free circulatory histones [155]. Indeed, EVs have been found to carry histones, adding to their widespread availability in normal and pathophysiological conditions. Nair et al. found that LPS encourages macrophages to release histone-carrying microvesicles (EVs 50–1000 nm in diameter) into the extracellular compartment. Histones, detected on the vesicle surface, interacted with TLR4 to induce an inflammatory response [156]. N-terminally cleaved histones H3 and H2A were also discovered in the exosomes [156]. Additionally, histones have been previously detected in many proteomic investigations of EVs [157,158]. Although no experimental investigations have demonstrated a direct link between histones and exosomes in AP, we believe this is simply a matter of time. Since extracellular histones are so prominent in AP pathogenesis, this makes sense.
The presence of extracellular histones, nucleosomes, and NETs in the bodily fluid of AP patients is clinically relevant. CitH3 levels were significantly higher and diagnostically helpful in patients with septic AP compared to healthy volunteers and non-septic AP patients (AUC = 0.93). Correlation analysis revealed a positive correlation between serum CitH3 and prognostic markers such as survival, duration of ICU stay, and sequential organ failure assessment scores (SOFA), suggesting that serum CitH3 may be a prognostic marker in patients with septic AP [159]. On the other hand, histones and nucleosomes in circulation are crucial for the diagnosis and prognosis of SAP patients. The diagnostic significance of plasma nucleosomes in severe AP was verified by a prospective trial of 74 patients (AUC = 0.718), which was more significant than CRP (AUC = 0.673) [160]. Researchers made the intriguing discovery that obesity increases nucleosome release in AP patients. Systemic histone release may be a significant factor to in obesity-induced SAP and its mortality [161]. Furthermore, Liu et al. found that the detection of circulating histones in plasma within 48 h after the onset of abdominal pain onset predicted persistent organ failure and death in AP patients (AUC = 0.92) [162]. They also discovered that the dead immune cells might be the primary source of circulating histones.
However, Keskin and his colleagues reached a different conclusion. They discovered that serum histone levels did not substantially vary between severe and moderate AP and had no clinical significance [163]. They proposed two possible explanations. On the one hand, the sample size was relatively modest. On the other hand, most of the included patients had biliary AP, which differed from previous studies that included a more significant proportion of AP due to alcohol abuse. Consequently, we hypothesize that the release of circulating histones during AP may be linked to the pathophysiology of inflammation. Additional clinical studies must confirm this.
The widespread organ toxicity of extracellular histones is amazing. There is a substantial relationship between circulating histones and SOFA in patients with sepsis, SAP, and severe trauma [164]. Experiments in vivo and in vitro verified the temporal association of histones with multi-organ damage, particularly cardiac and pulmonary injury [164]. The significance of histones in AP initiation was also established mechanistically. Guo et al. discovered that extracellular histones induce dose-dependent calcium oscillations [165]. Histones induce calcium oscillations in acinar tumor cells through activation of plasma membrane TLR9, which is required for trypsin activation and activation of the inflammatory signaling pathway during AP. Extracellular histones are critical mediators of AP-induced organ damage and may have clinical value in the diagnosis and prognosis of AP. Recent research has shown that histones may also be actively produced by live cells in the form of EVs [156], resulting in inflammation and sepsis. However, EV-specific histones have not been proven in AP, and additional research is needed to determine their clinical importance.
Exosome-specific proteins are increasingly being detected in bodily fluids and are linked to AP and associated ALI (Table 2). We hypothesize that exosomes released from the pancreas, gut, and liver, together with the proteins they contain, trigger intracellular signals that govern the receptor cell phenotype and impact the inflammatory cascade response and lung damage during AP. Exosomes transport inflammatory mediators via the pancreas–lung axis and the gut–lung axis to activate AMs, enhance their polarization toward the M1 type, and cause pyroptosis. AMs amplify the inflammatory response while also secreting exosomes, further stimulating PMVECs and AECs and disrupting the pulmonary air–blood barrier (Figure 3).

Table 2.
Exosome-proteins in AP and ALI.
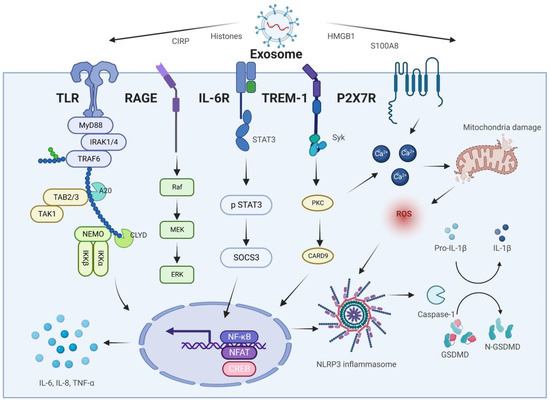
Figure 3.
Molecular mechanisms of exosome-specific proteins in AP-associated ALI.
5. The Therapeutic Potential of Exosomes in AP and Associated ALI
Aa a double-edged sword, exosomes release both anti-inflammatory and pro-inflammatory substances into the cells to which they bind. They regulate the inflammatory cascade response during AP by selectively binding downstream molecules and modulating receptor cell activity. As a result, researchers are considering exosomes as possible therapeutic targets. The first step in the chain of AP is pancreatic acinar cell damage. Recently, in vitro experiments confirmed that exosomes produced by acinar cells were shown to drastically lower intracellular ROS and the inflammatory factor level and ameliorate pathological pancreatic injury [166]. These findings show that injured cells may be able to heal tissue damage but that certain triggering elements are required. Several in vivo and in vitro studies demonstrated emodin’s protective properties against AP-induced pancreatic injury, intestinal barrier dysfunction, and ALI [23,167]. Emodin was discovered to boost the differentiation and anti-inflammatory activity of regulatory T cells by stimulating the release of exosome-specific lncRNA taurine upregulated 1 (TUG1) from pancreatic acinar cells, hence limiting the development of AP [52]. Recent research suggests that the inflamed pancreas may release exosomes into the circulation during SAP. These exosomes have been shown to have a pro-inflammatory action. However, emodin may prevent “bad” exosomes from being secreting by acinar cells. Proteomics was employed to characterize the impact of rhodopsin on the plasma-derived exosome proteome in SAP rats. According to the results of the enrichment study, peroxisome proliferator-activated receptors (PPAR) signaling is the primary mechanism by which emodin influences the exosomal proteome. Mechanistic investigations showed that emodin protected the lungs by preventing exosome-mediated M1 polarization of alveolar macrophages via regulating PPAR signaling [168]. During AP-induced ALI, AECs are critical target cells in the lung, and their destruction directly leads to pulmonary edema and widespread alveolar injury. Evidence suggests that exosomes derived from AECs in ALI help to regulate the immune balance and the inflammatory cascade response [169]. As a natural antioxidant and anti-inflammatory agent, salidroside has been shown to protect against AP and ALI/ARDS caused by AP in mice. Activation of NF-κB, interleukin receptor-associated kinase, and tumor necrosis factor receptor-associated molecule 6 in AMs was inhibited by the salidroside-stimulated release of exosomal miR-146a from AECs and improved ALI in rats [170]. DAMPs and PAMPs activate AMs, an essential innate immune cell population during the outset of AP. They produce significant doses of inflammatory mediators that contribute to lung damage [171], unlike alveolar and alveolar epithelial cells. Similarly, pyroptotic AM-derived pyroptotic bodies increased AEC damage and vascular leakage [172]. Furthermore, the Hippo signaling pathway was activated in AECs by AMs-derived exosomal transfer RNA-derived fragments, which caused AECs ferroptosis and aided in the establishment of ALI [173]. Modulation of AMs-derived exosomes may therefore be a promising method for combating AP and associated ALI.
The therapeutic potential of mesenchymal stem cell (MSC)-derived exosomes cannot be overlooked in exosome-related treatment techniques [174,175,176]. Human umbilical cord mesenchymal stem cells (hucMSC) have been frequently recognized for their capacity for self-renewal and multilineage differentiation, particularly for the bioactive chemicals they contain for tissue repair and the management of inflammation [177,178]. Han et al. discovered that hucMSC-derived exosomes showed a remarkable tissue regeneration potential in rats suffering from traumatic pancreatitis (trauma-induced non-infectious AP) [179]. In particular, hucMSC-Evs injected intravenously could colonize injured pancreatic tissue and inhibit inflammatory response and apoptosis of acinar cells, promoting damaged tissue repair [179]. A less frequent consequence of AP is myocardial injury, which is challenging to treat and has a high death rate [180]. MSC-derived exosomes were found to upregulate vascular hemophilia factor and vascular endothelial growth factor through the activation of the Akt/nuclear factor E2 related factors 2/heme oxygenase 1 signaling pathway, which led to the amelioration of SAP-induced myocardial injury [181]. Unfortunately, the previous research did not characterize the components transported by stem-cell-derived exosomes, i.e., the molecules that exert the protective effects. Xia et al., on the other hand, discovered that adipose-derived MSC-derived exosome increased AM mitochondrial function by transferring mitochondrial components to them, which led to a reduction in pulmonary inflammation in mice [182].
Researchers have discovered that edible plants may generate nanoscale EVs with shapes and components comparable to animal exosomes [183]. Furthermore, plant-derived exosomes are biocompatible and safe to consume, with no side effects or possible toxicity [184,185]. Plant-derived exosomes may penetrate biological barriers to transport lipid-soluble and hydrophilic target molecules to tissues in vivo, increasing the target molecules’ bioavailability or effectiveness of the target molecules [186]. Ginger exosome-like nanoparticles (GELN) have been found to be taken up by lung macrophages and epithelial cells, with a preference for ACE2-positive cells. Furthermore, ginger GELN miRNA has the potential to bind to many locations in the SARS-CoV-2 virus genome and be transported to lung epithelial cells to decrease Nsp12 production and, consequently, lung inflammation [187]. As a result, plant-derived exosomes are potential AP therapeutic agents.
Exosomes maybe a therapeutic target for AP and related organ failures. Using novel medications to control the levels of lipids, proteins, and nucleic acids carried by exosomes, as well as the exosome-mediated drug delivery, offers fantastic potential to decrease the AP-induced cytokine storm. On the other hand, stem-cell-based exosome therapies have been in existence for some time and should be tested in AP clinical trials as soon as is feasible.
6. Exosome-Based Diagnostic Strategy
Compared with cell-free nucleic acids and proteins, exosome-specific nucleic acids and proteins are protected by a lipid bilayer and have better stability in the extracellular environment by avoiding degradation by RNA hydrolases [188,189]. In addition, exosomes are more accessible than solid biopsy samples and are present in almost all biological fluids, such as plasma, urine, saliva, ascites, breast milk, and amniotic fluid. Therefore, exosomes have been established as ideal biomarkers and have been widely used in disease diagnosis, prognosis evaluation, and treatment monitoring. The diagnostic and prognostic value of exosomes in pancreatic diseases such as pancreatic cancer and chronic pancreatitis has been widely confirmed [190,191,192,193].
Peripheral blood-derived exosomal miR-155, miR-216a, miR-21, (miR-603, miR-548ad-5p, miR-122-5p, miR-4477a, miR-192-5p, miR-215-5p, and miR-583), lncRNA PVT1, and MALAT1 [162] were linked with the severity of SAP and may provide new insight into the etiology of SAP and act as biomarkers of SAP. In addition, some AP serum/mesenteric lymph/plasma markers such as FBXL19-AS1 [55] and lnc-ITSN1-2 [56], miR-214-3p [194], miR-27a-5p [195], miR-217-5p [196], miR-193a-5p [197], miR-375 [198], miR-148a [199], miR-138-5p [200], miR-92b [201], miR-10a [201], miR-7 [202], miR-9 [202], miR-141 [203], miR-551b-5p [204], miR-126-5p [205], miR-24 [206], (miR-22-3p, miR-1260b, miR-762, miR-23b, miR-23a, miR-550a-5p, miR-324-5p, miR-484, miR-331-3p, miR-140-3p, and miR-342-3p [207]), miR-127 [208], miR-372 [209], miR-126-5p [210], miR-146 [211], miR-153 [212], miR-320-5p [197], Circ_0000284 [213], and Circ_0073748 [214], also exist in the exosome (Figure 4). These potential exosomal biomarkers also provide an important direction for the diagnosis and prognosis of AP in clinical applications.
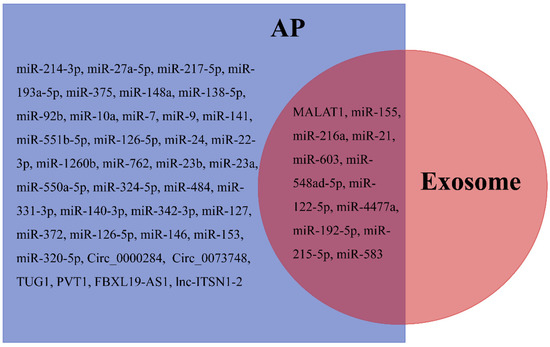
Figure 4.
Differentially expressed non-coding RNAs in plasma/serum/mesenteric lymph/exosomes after AP.
Exosome-specific S100A8 correlates with the inflammatory response and predicts severity in individuals with SAP. Similarly, several free proteins and DNA are elevated in the plasma of patients with SAP, and this has implications for the diagnosis and prognostic assessment of the disease [145,215,216]. Moreover, the above substances such as HMGB1 [217], heat shock protein 70 [110], histones [162], CIRP [145], S100A12 [215], gamma-enolase [218], and mtDNA [216] have also been proven to be essential cargoes loaded by exosomes. Therefore, in the future, two areas of interest will be exploring whether the above exosome-specific cargoes can recognize AP and whether exosome-specific proteins or DNAs have better diagnostic performance than free proteins or DNAs.
In addition to blood samples, many exosomes exist in biological fluids, including urine and pancreatic juice. Urine samples are easy to obtain and non-invasive, which is desirable for both clinicians and patients. Several studies have found that nucleic acids and proteins carried by urine-derived exosomes have potential diagnostic value in pancreatic diseases [219,220,221]. In the case of AP, a 2014 study confirmed that urinary ketone bodies, glucose, plasma choline, and lipid levels were increased in patients’ urine, while levels of urinary hippurate, creatine, and plasma-branched chain amino acids decreased. A biomarker panel of guanine, hippurate, and creatine reliably identified AP with high sensitivity and specificity [222]. Later, a proteomic study confirmed that the peak intensity ratio of urinary β-2 microglobulin to saponin B has a better diagnostic performance in patients with SAP, especially with renal injury and inflammation [223]. In short, urine is also a promising biospecimen for mining AP biomarkers. Exploring the changes in exosomal cargo in urine during AP is urgent.
Pancreatic juice is secreted by pancreatic acinar cells and duct wall cells, an alkaline liquid with a strong digestibility. In terms of accuracy and the characterization that best reflects the pathological mechanisms of AP, pancreatic fluid is second to pancreatic tissue and is a biofluid superior to blood and urine [224]. In 2018, Osteikoetxea et al. found that the detection and characterization of EVs in pancreatic juice are feasible and confirmed that mucin, CFTR, and MDR1 proteins carried by pancreatic juice-derived EVs are potential biomarkers of pancreatic cancer [225]. Later, Nakamura et al. found that miR-21 and miR-15 carried by pancreatic juice-derived exosomes have the potential to diagnose patients with PDAC and CP [226]. The diagnostic value of pancreatic juice examination in pancreas-related diseases is constantly updated. The changes in the exosomal cargo of pancreatic juice in AP patients should be explored as soon as possible.
Bronchoalveolar lavage fluid (BALF), which is in direct contact with lung tissue, is an ideal biologic fluid for the diagnosis of lung diseases [227]. Previous studies have found that exosomes derived from BALF have potential diagnostic value in patients with ALI/ARDS [228], chronic obstructive pulmonary disease [229], nodular pulmonary disease [230], lung cancer [231], lung infections [232], and asthma [233]. Therefore, we speculate that BALF-derived exosomes are a potential direction for diagnosing AP-associated ALI.
Because of their stability, exosome-specific ncRNAs and proteins have been employed as biomarkers in AP and associated ALI research. Exosome isolation from blood, pleural fluid, urine, ascitic fluid, alveolar lavage fluid, and pancreatic fluid is an emerging method of fluid biopsy with broad potential clinical applications, especially for patients with AP who are experiencing multi-organ failure. Exosome-specific ncRNA and protein detection are complicated by several variables, as has been described. Different clinical investigations on the expression of a specific exosomal cargo in the bodily fluids of AP patients may find contradictory results. To further establish the sensitivity and specificity of exosomal cargoes, substantial cohort studies are still required before their use can be advocated for in clinical applications.
7. Conclusions
This paper sought to summarize the function of the exosomal cargo in AP and associated ALI processes. Our hypothetic pancreas–lung axis and gut–lung axis are noteworthy. After AP begins, exosomes may be released by pancreatic acinar cells, macrophages, and intestinal epithelial cells. Exosomes harboring pro-inflammatory mediators such as miR-155, miR-216a, S100A8, and CIRP are delivered through the pancreas–lung axis and gut–lung axis to the circulation and distant lung regions in order to influence the inflammatory cascade response to AP. We identified 45 ncRNAs from serum/plasma/mesenteric lymph that may be useful for the diagnosis of AP based on a comprehensive literature review spanning the last two decades. Eleven of these have been shown to be transported by exosomes in the presence of AP. Several questions have arisen, such as: (1) Does the diagnostic performance of free ncRNAs, and exosome-specific ncRNAs (the same ncRNA) vary in terms of sensitivity and specificity? (2) With so many differentially expressed miRNAs accessible, how can their value be integrated to enhance the performance of AP diagnosis? Certainly, exosome-specific proteins such as S100A8, HMGB1, and CIRP face comparable difficulties. The methods used to isolate and purify exosomes are also of interest. Numerous methods exist for isolating and purifying exosomes, but they are often somewhat involved and have their benefits and drawbacks. Furthermore, many patients find the high cost of exosome extraction unaffordable, limiting its practical use. As a result, it is crucial to establish a reliable, productive, quick, and economical method for isolating exosomes. Furthermore, exosomes have the potential to serve as a future therapy for AP. The initial objective is to identify modulatory medications, such as natural products, that are based on the pro/anti-inflammatory characteristics of the exosomal cargo. The second is loading a part of the medicine onto exosomes to enhance its bioavailability and targeting. The final objective is the source of exosomes. On the one hand, the production of exosomes derived from mesenchymal stem cells has been shown to have therapeutic value. On the other hand, plant-derived exosomes are also considered to be significant prospects.
Exosomes are involved in the development of AP and associated ALI through multiple pathways as participants in the inflammatory and immune response. Several exosome-specific ncRNAs and proteins have been studied in the diagnosis of AP, suggesting the possibility of exosomes as “novel markers”. The unique vesicular structure of exosomes has inspired researchers to develop targeted drugs. However, there is still a long way to go to promote exosomes in clinical applications, and many issues must be addressed, such as the isolation techniques, drug delivery methods, and potential toxicity. The application of exosomes in AP in the future should continue to be expanded upon to overcome these issues.
Author Contributions
Conceptualization, H.C.; Data Curation, Y.L.; Writing-Original Draft Preparation, Q.Y.; Writing-Review and Editing, P.G.; Visualization and Supervision, B.L., X.D. and Z.W.; Project Administration, G.Z.; Funding Acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by National Key R&D Program of China (NO.2019YFE0119300) and the National Natural Science Foundation of China (NO. 82074158 and NO. 82274311).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in the review manuscript.
Conflicts of Interest
The authors have no conflicts of interest to disclose. Figures were created with BioRender software (https://biorender.com, accessed on 30 August 2022).
Abbreviations
| AP | acute pancreatitis |
| SAP | severe acute pancreatitis |
| ALI | acute lung injury |
| PMVECs | pulmonary microvascular endothelial cells |
| AECs | alveolar epithelial cells |
| ARDS | acute respiratory distress syndrome |
| SIRS | systemic inflammatory response |
| MODS | multiple organ dysfunction syndromes |
| NcRNAs | non-coding RNAs |
| DAMPs | damage-associated molecular patterns |
| PAMPs | pathogen-associated molecular patterns |
| MVB | multivesicular body |
| ILVs | intraluminal vesicles |
| MiRNAs | microRNAs |
| CIRP | cold-inducible RNA-binding protein |
| AM | alveolar macrophage |
| IECs | intestinal epithelial cells |
| LncRNAs | long non-coding RNAs |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| YAP1 | yes-associated protein 1 |
| HMGB1 | high-mobility group box 1 |
| TLR4 | toll-like receptor 4 |
| NF-κB | nuclear factor kappa B |
| TUG1 | taurine upregulated gene 1 |
| LPS | lipopolysaccharide |
| SIRT1 | silent information regulator 1 |
| PVT1 | plasmacytoma variant translocation gene 1 |
| NLRP3 | NOD-like receptor protein 3 |
| JAK2 | Janus protein tyrosine kinase 2 |
| NETs | neutrophil extracellular traps |
| TLR | toll-like receptor |
| NAPDH | nicotinamide adenine dinucleotide phosphate |
| MMP | matrix metalloproteinase |
| ROS | reactive oxygen species |
| TREM-1 | triggering receptor expressed on myeloid cells-1 |
| SOFA | sequential organ failure assessment scores |
| PPAR | peroxisome proliferator-activated receptor |
| MSC | mesenchymal stem cell |
| GELN | ginger exosome-like nanoparticles |
| BALF | bronchoalveolar lavage fluid |
References
- Leppäniemi, A.; Tolonen, M.; Tarasconi, A.; Lohse, H.A.S.; Gamberini, E.; Kirkpatrick, A.W.; Ball, C.G.; Parry, N.; Sartelli, M.; Wolbrink, D.R.J.; et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 2019, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Stephanie, C.; Nauzer, F.; Steven, J.H.; Abdel-Aziz, S.; et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, J.; Chen, H.; Wang, C.; Qiu, Y.; Liu, Y.; Wan, J.; Guo, H. Effects and mechanisms of alveolar type II epithelial cell apoptosis in severe pancreati-tis-induced acute lung injury. Exp. Ther. Med. 2014, 7, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Vrolyk, V.; Singh, B. Animal models to study the role of pulmonary intravascular macrophages in spontaneous and induced acute pancreatitis. Cell Tissue Res. 2020, 380, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Luiken, I.; Eisenmann, S.; Garbe, J.; Sternby, H.; Verdonk, R.C.; Dimova, A.; Ignatavicius, P.; Ilzarbe, L.; Koiva, P.; Penttilä, A.K.; et al. Pleuropulmonary pathologies in the early phase of acute pancreatitis correlate with disease severity. PLoS ONE 2022, 17, e0263739. [Google Scholar] [CrossRef]
- Ge, P.; Luo, Y.; Okoye, C.S.; Chen, H.; Liu, J.; Zhang, G.; Xu, C.; Chen, H. Intestinal barrier damage, systemic inflammatory response syndrome, and acute lung injury: A troublesome trio for acute pancreatitis. Biomed. Pharmacother. 2020, 132, 110770. [Google Scholar] [CrossRef]
- Liu, D.; Wen, L.; Wang, Z.; Hai, Y.; Yang, D.; Zhang, Y.; Bai, M.; Song, B.; Wang, Y. The Mechanism of Lung and Intestinal Injury in Acute Pancreatitis: A Review. Front. Med. 2022, 9, 904078. [Google Scholar] [CrossRef]
- Suire, C.N.; Hade, M.D. Extracellular Vesicles in Type 1 Diabetes: A Versatile Tool. Bioengineering 2022, 9, 105. [Google Scholar] [CrossRef]
- Hade, M.; Suire, C.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Chargaff, E.; West, R. The Biological Significance of the Thromboplastic Protein of Blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Webbera, J.; Johnsons, A. Platelet participation in blood coagulation aspects of hemostasis. Am. J. Pathol. 1970, 60, 19–42. [Google Scholar]
- Fan, Y.; Li, Z.; He, Y. Exosomes in the Pathogenesis, Progression, and Treatment of Osteoarthritis. Bioengineering 2022, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lei, B.; Zhang, E.; Gong, P.; Gu, J.; He, L.; Han, L.; Yuan, Z. Targeted Therapy for Inflammatory Diseases with Mesenchymal Stem Cells and Their Derived Exosomes: From Basic to Clinics. Int. J. Nanomed. 2022, 17, 1757–1781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Im, H.; Lee, K.; Weissleder, R.; Lee, H.; Castro, C.M. Novel nanosensing technologies for exosome detection and profiling. Lab Chip 2017, 17, 2892–2898. [Google Scholar] [CrossRef]
- Kalra, H.; Adda, C.G.; Liem, M.; Ang, C.-S.; Mechler, A.; Simpson, R.J.; Hulett, M.D.; Mathivanan, S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013, 13, 3354–3364. [Google Scholar] [CrossRef]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in Plasma Exosome is Stable under Different Storage Conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef]
- AzamAnsari, M.; Thiruvengadam, M.; Venkidasamy, B.; Alomary, M.N.; Salawi, A.; Chung, I.-M.; Shariati, M.A.; Rebezo, M. Exosome-based nanomedicine for cancer treatment by tar-geting inflammatory pathways: Current status and future perspectives. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Jia, Y.-C.; Ding, Y.-X.; Mei, W.-T.; Wang, Y.-T.; Zheng, Z.; Qu, Y.-X.; Liang, K.; Li, J.; Cao, F.; Li, F. Extracellular vesicles and pancreatitis: Mechanisms, status and perspectives. Int. J. Biol. Sci. 2021, 17, 549–561. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Li, F.; Luo, Y.; Ge, P.; Zhang, Y.; Wen, H.; Yang, Q.; Ma, S.; Chen, H. The gut-lung axis in severe acute Pancreatitis-associated lung injury: The protection by the gut microbiota through short-chain fatty acids. Pharmacol. Res. 2022, 182, 106321. [Google Scholar] [CrossRef]
- Zhu, H.; Song, Y.; Kong, X.; Du, Y. Expression of microrNA-216A in patients with acute pancreatitis complicated with lung injury and its effect on endothelial cell permeability. J. Chin. Pancreas Dis. 2020, 20, 4. [Google Scholar]
- Xu, Q.; Wang, M.; Guo, H.; Liu, H.; Zhang, G.; Xu, C.; Chen, H. Emodin Alleviates Severe Acute Pancreatitis-Associated Acute Lung Injury by Inhibiting the Cold-Inducible RNA-Binding Protein (CIRP)-Mediated Activation of the NLRP3/IL-1β/CXCL1 Signaling. Front. Pharmacol. 2021, 12, 655372. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.Y.; Jia, T.X.; Zhang, M. Intestinal bacterial overgrowth in the early stage of severe acute pancreatitis is associated with acute respiratory distress syndrome. World J. Gastroenterol. 2021, 27, 1643–1654. [Google Scholar] [CrossRef]
- Jabłońska, B.; Mrowiec, S. Nutritional Support in Patients with Severe Acute Pancreatitis-Current Standards. Nutrients 2021, 13, 1498. [Google Scholar] [CrossRef]
- Kanthasamy, K.A.; Akshintala, V.S.; Singh, V.K. Nutritional Management of Acute Pancreatitis. Gastroenterol. Clin. N. Am. 2021, 50, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sonika, U.; Moka, P.; Sharma, B.; Sachdev, V.; Mishra, S.K.; Upadhyay, A.D.; Saraya, A. Association of endotoxaemia & gut permeability with complications of acute pancre-atitis: Secondary analysis of data. Indian J. Med. Res. 2019, 149, 763–770. [Google Scholar]
- Hu, X.; Gong, L.; Zhou, R.; Han, Z.; Ji, L.; Zhang, Y.; Zhang, S.; Wu, D. Variations in Gut Microbiome are Associated with Prognosis of Hypertriglyceridem-ia-Associated Acute Pancreatitis. Biomolecules 2021, 11, 695. [Google Scholar] [CrossRef]
- Tang, Y.; Kong, J.; Zhou, B.; Wang, X.; Liu, X.; Wang, Y.; Zhu, S. Mesenteric Lymph Duct Ligation Alleviates Acute Lung Injury Caused by Severe Acute Pancreatitis Through Inhibition of High Mobility Group Box 1-Induced Inflammation in Rats. Am. J. Dig. Dis. 2021, 66, 4344–4353. [Google Scholar] [CrossRef]
- Park, E.J.; Shimaoka, M.; Kiyono, H. Functional Flexibility of Exosomes and MicroRNAs of Intestinal Epithelial Cells in Affecting Inflammation. Front. Mol. Biosci. 2022, 9, 854487. [Google Scholar] [CrossRef]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.-J.; Ji, Y.-X.; Zhang, P.; Deng, K.-Q.; Gong, J.; Ren, S.; Wang, X.; Chen, I.; Wang, H.; et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat. Med. 2016, 22, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Engreitz, J.M.; Haines, J.E.; Perez, E.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Dykes, I.M.; Emanueli, C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, D.; Li, G.; Ming, X.; Tu, Y.F.; Tian, J.; Lu, H.; Yu, B. Long Non Coding RNA-UCA1 Contributes to Cardiomyocyte Apoptosis by Suppression of p27 Expression. Cell. Physiol. Biochem. 2015, 35, 1986–1998. [Google Scholar] [CrossRef]
- Senmatsu, S.; Hirota, K. Roles of lncRNA transcription as a novel regulator of chromosomal function. Genes Genet. Syst. 2020, 95, 213–223. [Google Scholar] [CrossRef]
- Luo, Y.; Ge, P.; Wang, M.; Chen, H.; Liu, J.; Wei, T.; Jiang, Y.; Qu, J.; Chen, H. Research progress of DLX6-AS1 in human cancers. Hum. Cell 2021, 34, 1642–1652. [Google Scholar] [CrossRef]
- Zaki, A.; Ali, M.S.; Hadda, V.; Ali, S.M.; Chopra, A.; Fatima, T. Long non-coding RNA (lncRNA): A potential therapeutic target in acute lung injury. Genes Dis. 2021, 9, 1258–1268. [Google Scholar] [CrossRef]
- Yao, Z.; Yang, Y.; Sun, M.; He, Y.; Liao, L.; Chen, K.; Li, B. New insights into the interplay between long non-coding RNAs and RNA-binding proteins in cancer. Cancer Commun. 2022, 42, 117–140. [Google Scholar] [CrossRef]
- Lai, X.; Zhong, J.; Zhang, A.; Zhang, B.; Zhu, T.; Liao, R. Focus on long non-coding RNA MALAT1: Insights into acute and chronic lung diseases. Front. Genet. 2022, 13, 2704. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Taheri, M.; Arefian, N. Regulatory Role of Non-Coding RNAs on Immune Responses During Sepsis. Front. Immunol. 2021, 12, 798713. [Google Scholar] [CrossRef]
- Su, K.; Wang, N.; Shao, Q.; Liu, H.; Zhao, B.; Ma, S. The role of a ceRNA regulatory network based on lncRNA MALAT1 site in cancer progression. Biomed. Pharmacother. 2021, 137, 111389. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, J.; Xu, D.; Lu, Y. Reciprocal Feedback Loop of the MALAT1-MicroRNA-194-YAP1 Pathway Regulates Progression of Acute Pancreatitis. Med. Sci. Monit. 2019, 25, 6894–6904. [Google Scholar] [CrossRef]
- Liu, J.; Niu, Z.; Zhang, R.; Peng, Z.; Wang, L.; Liu, Z.; Gao, Y.; Pei, H.; Pan, L. MALAT1 shuttled by extracellular vesicles promotes M1 polarization of macrophages to induce acute pancreatitis via miR-181a-5p/HMGB1 axis. J. Cell. Mol. Med. 2021, 25, 9241–9254. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yao, W.; Liu, K.; Wang, G.; Chen, B.; Wang, Z.; Hou, S. Nonalcoholic Fatty Liver Hepatocyte-Derived lncRNA MALAT1 Aggravates Pancreatic Cell Inflammation via the Inhibition of Autophagy by Upregulating YAP. Comput. Intell. Neurosci. 2022, 2022, 2930960. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, Y. Long non-coding RNA plasmacytoma variant translocation 1 correlates with higher inflammation, multiple organ injury and mortality risk in acute pancreatitis, especially in severe acute pancreatitis. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101870. [Google Scholar] [CrossRef]
- Liu, W.; Geng, F.; Yu, L. Long non-coding RNA MALAT1/microRNA 125a axis presents excellent value in discriminating sepsis patients and exhibits positive association with general disease severity, organ injury, inflammation level, and mortality in sepsis patients. J. Clin. Lab. Anal. 2020, 34, e23222. [Google Scholar] [CrossRef]
- Guo, C.; Qi, Y.; Qu, J.; Gai, L.; Shi, Y.; Yuan, C. Pathophysiological Functions of the lncRNA TUGCurr. Pharm. Des. 2020, 26, 688–700. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, L.; Zhang, H.; Chen, P. Up-regulation of TUG1 can regulate miR-494/PDK4 axis to inhibit LPS-induced acute lung injury caused by sepsis. Am. J. Transl. Res. 2021, 13, 12375–12385. [Google Scholar]
- Qiu, N.; Xu, X.; He, Y. LncRNA TUG1 alleviates sepsis-induced acute lung injury by targeting miR-34b-5p/GABBMC. Pulm. Med. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, W.; Cui, B.; Gao, J.; Liu, Q.; Yao, M.; Ning, H.; Xing, L. Functional delivery of lncRNA TUG1 by endothelial progenitor cells derived extracellular vesicles confers anti-inflammatory macrophage polarization in sepsis via impairing miR-9-5p-targeted SIRT1 inhibition. Cell Death Dis. 2021, 12, 1056. [Google Scholar] [CrossRef]
- Wen, X.; He, B.; Tang, X.; Wang, B.; Chen, Z. Emodin inhibits the progression of acute pancreatitis via regulation of lncRNA TUG1 and exosomal lncRNA TUGMol. Med. Rep. 2021, 24, 785. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, H.; Gui, F. Long noncoding plasmacytoma variant translocation 1 facilitates the surveillance of acute respiratory distress syndrome and mortality prediction in sepsis. Biomark. Med. 2021, 15, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, J.; Guo, M.; Bai, R.; Lei, G.; Sun, H.; Tong, S.; He, K.; He, L. Aberrant expressions of MIAT and PVT1 in serum exosomes of schizophrenia patients. Schizophr. Res. 2021, 240, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Gan, G.-F.; Niu, Y.; Tong, S.-J. Analysis of associations of FBXL19-AS1 with occurrence, development and prognosis of acute pancreatitis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12763–12769. [Google Scholar] [PubMed]
- Li, J.; Bu, X.; Chen, X.; Xiong, P.; Chen, Z.; Yu, L. Predictive value of long non-coding RNA intersectin 1-2 for occurrence and in-hospital mortality of severe acute pancreatitis. J. Clin. Lab. Anal. 2019, 34, e23170. [Google Scholar] [CrossRef] [PubMed]
- Maiese, A.; Scatena, A.; Costantino, A.; Chiti, E.; Occhipinti, C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Expression of MicroRNAs in Sepsis-Related Organ Dysfunction: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9354. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from Repression to Activation: MicroRNAs Can Up-Regulate Translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, T.F.; Fabian, M.R. Mechanistic Insights into MicroRNA-Mediated Gene Silencing. Cold Spring Harb. Perspect. Biol. 2019, 11, a032771. [Google Scholar] [CrossRef]
- Lopez, J.G.; Brieño-Enriquez, M.A.; DEL Mazo, J. MicroRNA biogenesis and variability. Biomol. Concepts 2013, 4, 367–380. [Google Scholar] [CrossRef]
- Zlotorynski, E. Insights into the kinetics of microRNA biogenesis and turnover. Nat. Rev. Mol. Cell Biol. 2019, 20, 511. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Dawson, O.; Piccinini, A.M. miR-155-3p: Processing by-product or rising star in immunity and cancer? Open Biol. 2022, 12, 220070. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Spehlmann, M.E.; Vucur, M.; Benz, F.; Luedde, M.; Cardenas, D.V.; Roy, S.; Loosen, S.; Hippe, H.-J.; Frey, N.; et al. miR-155 Predicts Long-Term Mortality in Critically Ill Patients Younger than 65 Years. Mediat. Inflamm. 2019, 2019, 6714080. [Google Scholar] [CrossRef]
- Obradovic, D.; Rommel, K.; Blazek, S.; Klingel, K.; Gutberlet, M.; Lücke, C.; Büttner, P.; Thiele, H.; Adams, V.; Lurz, P.; et al. The potential role of plasma miR-155 and miR-206 as circulatory biomarkers in inflammatory cardiomyopathy. ESC Hear. Fail. 2021, 8, 1850–1860. [Google Scholar] [CrossRef]
- Lin, S.; Chen, X.; Wang, X. Expression and clinical significance of Mir-155 in peripheral blood of patients with acute pan-creatitis. World Chin. Dig. J. 2015, 23, 3935–3939. [Google Scholar] [CrossRef]
- Wang, D.; Tang, M.; Zong, P.; Liu, H.; Zhang, T.; Liu, Y.; Zhao, Y. MiRNA-155 Regulates the Th17/Treg Ratio by Targeting SOCS1 in Severe Acute Pancreatitis. Front. Physiol. 2018, 9, 686. [Google Scholar] [CrossRef]
- Miao, B.; Wang, J.; Zhong, J.; Pan, E.; Lin, S. Exosomal Mir-155 affects severe acute pancreatitis lung injury by regulating SOCS. Chin. Foreign Med. 2021, 40, 23–27. [Google Scholar]
- Hu, L.; Han, D.; Yu, D.; Ao, D.; Yang, Z. Circulating Blood miR-155 and miR-21 Promote the Development of Acute Pancreatitis and Can Be Used to Assess the Risk Stratification of Pancreatitis. J. Health Eng. 2021, 2021, 2064162. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Wang, R.L.; Xie, H.; Jin, W.; Yu, K.L. Overexpressed miRNA-155 dysregulates intestinal epithelial apical junctional complex in severe acute pancreatitis. World J. Gastroenterol. 2013, 19, 8282–8291. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Yang, X.; Ren, Y.; Li, X.; Zhu, Y.; Haddock, A.N.; Ji, B.; Xia, L.; Lu, N. Inhibition of miR-155 reduces impaired autophagy and improves prognosis in an experimental pancreatitis mouse model. Cell Death Dis. 2019, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chu, J.; Sun, H.; Zhao, D.; Ma, B.; Xue, D.; Zhang, W.; Li, Z. MiR-155 aggravates impaired autophagy of pancreatic acinar cells through targeting Rictor. Acta Biochim. Biophys. Sin. 2020, 52, 192–199. [Google Scholar] [CrossRef]
- Jiménez-Alesanco, A.; Marcuello, M.; Pastor-Jiménez, M.; López-Puerto, L.; Bonjoch, L.; Gironella, M.; Carrascal, M.; Abian, J.; de-Madaria, E.; Closa, D. Acute pancreatitis promotes the generation of two different ex-osome populations. Sci. Rep. 2019, 9, 19887. [Google Scholar] [CrossRef]
- Wu, X.-B.; Sun, H.-Y.; Luo, Z.-L.; Cheng, L.; Duan, X.-M.; Ren, J.-D. Plasma-derived exosomes contribute to pancreatitis-associated lung injury by triggering NLRP3-dependent pyroptosis in alveolar macrophages. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165685. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol. Ther. 2019, 27, 1758–1771. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, H.; Shen, M.; Li, C.; Liu, C.; Zhu, H.; Zhang, W. MicroRNA-155-5p modulates the progression of acute respiratory distress syndrome by targeting interleukin receptors. Bioengineered 2022, 13, 11732–11741. [Google Scholar] [CrossRef]
- Hawez, A.; Taha, D.; Algaber, A.; Madhi, R.; Rahman, M.; Thorlacius, H. MiR-155 regulates neutrophil extracellular trap formation and lung injury in abdominal sepsis. J. Leukoc. Biol. 2021, 111, 391–400. [Google Scholar] [CrossRef]
- Wu, K.; Wang, L.; Fu, G.; Zheng, Y. Expression and clinical significance of microRNA-21-3p and microRNA-551-5p in patients with acute pancreatitis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 463–467. [Google Scholar]
- Nakamaru, K.; Tomiyama, T.; Kobayashi, S.; Ikemune, M.; Tsukuda, S.; Ito, T.; Tanaka, T.; Yamaguchi, T.; Ando, Y.; Ikeur, T.; et al. Extracellular vesicles microRNA analysis in type 1 autoimmune pan-creatitis: Increased expression of microRNA. Pancreatology 2020, 20, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.K.; Sarver, A.E.; Yuan, Z.; George, J.; Barlass, U.; Cheema, H.; Sareen, A.; Banerjee, S.; Dudeja, V.; Dawra, R.; et al. Comprehensive analysis of microRNA signature of mouse pancreatic acini: Over-expression of miR-21-3p in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G974–G980. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, L.; Wei, X.; Liu, B.; Zhao, J.; Xie, P.; Yang, B.; Wang, L. MiR-21-3p aggravates injury in rats with acute hemorrhagic necrotizing pancreatitis by activating TRP signaling pathway. Biomed. Pharmacother. 2018, 107, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, J.; Gao, W.; Sun, Y.; Liu, X.; Lv, Z.; Li, L.; Xue, D. The lncRNA TCONS_00021785/miR-21-5p/Trim33 axis regulates VMP1-mediated zymophagy, reduces the activation of trypsinogen, and promotes acinar cell recovery. Cell Death Discov. 2022, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chen, M.; Ji, H.; Liu, G.; Mei, H.; Li, K.; Chen, T. miR-21-5p regulates type II alveolar epithelial cell apoptosis in hyperoxic acute lung injury. Mol. Med. Rep. 2018, 17, 5796–5804. [Google Scholar] [CrossRef]
- Liu, G.; Qian, M.; Chen, M.; Chen, T.; Qin, S. miR-21-5p Suppresses Mitophagy to Alleviate Hyperoxia-Induced Acute Lung Injury by Directly Targeting PGAM5. BioMed Res. Int. 2020, 2020, 4807254. [Google Scholar] [CrossRef]
- Li, J.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76. [Google Scholar] [CrossRef]
- Kong, X.; Du, Y.; Li, L.; Liu, J.; Wang, G.; Zhu, J.; Man, X.; Gong, Y.; Xiao, L.; Zheng, Y.; et al. Plasma miR-216a as a potential marker of pancreatic injury in a rat model of acute pancreatitis. World J. Gastroenterol. 2010, 16, 4599–4604. [Google Scholar] [CrossRef]
- Endo, K.; Weng, H.; Kito, N.; Fukushima, Y.; Iwai, N. miR-216a and miR-216b as markers for acute phased pancreatic injury. Biomed. Res. 2013, 34, 179–188. [Google Scholar] [CrossRef]
- Goodwin, D.; Rosenzweig, B.; Zhang, J.; Xu, L.; Stewart, S.; Thompson, K.; Rouse, R. Evaluation of miR-216a and miR-217 as potential biomarkers of acute pan-creatic injury in rats and mice. Biomarkers 2014, 19, 517–529. [Google Scholar] [CrossRef]
- Blenkiron, C.; Askelund, K.J.; Shanbhag, S.T.; Chakraborty, M.; Petrov, M.; Delahunt, B.; Windsor, J.A.; Phillips, A.R. MicroRNAs in mesenteric lymph and plasma during acute pancreatitis. Ann. Surg. 2014, 260, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, H.; Choi, H.; Lee, S.; Lee, S.; Lee, J.; Cho, E.; Han, H.; Seok, J.; Son, W. Evaluation of Circulating MicroRNA Biomarkers in the Acute Pancreatic Injury Dog Model. Int. J. Mol. Sci. 2018, 19, 3048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Deng, L.; Chen, W.; Shi, N.; Jin, T.; Lin, Z.; Ma, Y.; Jiang, K.; Yang, X.; Xia, Q. Circulating microRNA 216 as a Marker for the Early Identification of Severe Acute Pancreatitis. Am. J. Med. Sci 2017, 353, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, C.; Yang, L.; Zhao, G.; Liu, H.; Li, J.; Chen, P.; Cui, K. Significance of microRNA 216a, 324–325p and 29a expression in peripheral blood in patients with acute pancreatitis and their correlation with liver injury. Zhonghua Yi Xue Za Zhi 2020, 100, 2126–2131. [Google Scholar]
- Kong, F.; Sun, Y.; Song, W.; Zhou, Y.; Zhu, S. MiR-216a alleviates LPS-induced acute lung injury via regulating JAK2/STAT3 and NF-κB signaling. Hum. Cell 2019, 33, 67–78. [Google Scholar] [CrossRef]
- Qu, Y.; Ding, Y.; Lu, J.; Jia, Y.; Bian, C.; Guo, Y.; Zheng, Z.; Mei, W.; Cao, F.; Li, F. Identification of key microRNAs in exosomes derived from patients with the severe acute pancreatitis. Asian J. Surg. 2022; in press. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Li, L.; Chen, Z.; Wang, N.; Zhu, M.; Mi, H.; Xiong, Y.; Guo, G.; Gu, Y. Circular RNA circ_IRAK3 contributes to tumor growth through upregulating KIF2A via adsorbing miR-603 in breast cancer. Cancer Cell Int. 2022, 22, 81. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.; Zheng, C.; Zhang, Q.; Zhang, G.; Chen, K.; Zhan, Q.; An, F. Mechanistic Investigation on the Regulation of FABP1 by the IL-6/miR-603 Signaling in the Pathogenesis of Hepatocellular Carcinoma. Biomed. Res. Int. 2021, 2021, 8579658. [Google Scholar] [CrossRef]
- Gao, X.; Yin, J.; Yao, Y. hsa_circ_0001955 Promotes Colorectal Cancer Progression by Regulating miR-583/FGF21 Axis. J. Oncol. 2022, 2022, 4288474. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, Y. Dysregulation of miR-192-5p in acute pancreatitis patients with nonalcoholic fatty liver and its functional role in acute pancreatitis progression. Biosci. Rep. 2020, 40, BSR20194345. [Google Scholar] [CrossRef]
- Lu, Z.; Feng, H.; Shen, X.; He, R.; Meng, H.; Lin, W.; Geng, Q. MiR-122-5p protects against acute lung injury via regulation of DUSP4/ERK signaling in pulmonary microvascular endothelial cells. Life Sci. 2020, 256, 117851. [Google Scholar] [CrossRef]
- Li, J.; Zeng, X.; Wang, W. miR-122-5p downregulation attenuates lipopolysaccharide-induced acute lung injury by targeting, I.L.1.R.N. Exp. Ther. Med. 2021, 22, 1278. [Google Scholar] [CrossRef] [PubMed]
- Caserta, S.; Ghezzi, P. Release of redox enzymes and micro-RNAs in extracellular vesicles, during infection and inflammation. Free Radic. Biol. Med. 2021, 169, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Block, H.; Rossaint, J.; Zarbock, A. The Fatal Circle of NETs and NET-Associated DAMPs Contributing to Organ Dys-function. Cells 2022, 11, 1919. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhao, L.; Yang, Z.; Shang, J.-J.; Wang, C.-Y.; Shen, M.-Z.; Jiang, S.; Li, T.; Di, W.-C.; Chen, Y.; et al. Targeting HMGB1 for the treatment of sepsis and sepsis-induced organ injury. Acta Pharmacol. Sin. 2021, 43, 520–528. [Google Scholar] [CrossRef]
- Yasuda, T.; Ueda, T.; Takeyama, Y.; Shinzeki, M.; Sawa, H.; Nakajima, T.; Ajiki, T.; Fujino, Y.; Suzuki, Y.; Kuroda, Y. Significant Increase of Serum High-Mobility Group Box Chromosomal Protein 1 Levels in Patients With Severe Acute Pancreatitis. Pancreas 2006, 33, 359–363. [Google Scholar] [CrossRef]
- Xu, G.-F.; Guo, M.; Tian, Z.-Q.; Wu, G.-Z.; Zou, X.-P.; Zhang, W.-J. Increased of serum high-mobility group box chromosomal protein 1 correlated with intestinal mucosal barrier injury in patients with severe acute pancreatitis. World J. Emerg. Surg. 2014, 9, 61. [Google Scholar] [CrossRef]
- Arriaga-Pizano, L.; Boscó-Gárate, I.; Martínez-Ordaz, J.L.; Wong-Baeza, I.; Gutiérrez-Mendoza, M.; Sánchez-Fernandez, P.; Macías, C.I.R.L.; Isibasi, A.; Pelaez-Luna, M.; Cérbulo-Vázquez, A.; et al. High Serum Levels of High-Mobility Group Box 1 (HMGB1) and Low Levels of Heat Shock Protein 70 (Hsp70) are Associated with Poor Prognosis in Patients with Acute Pancreatitis. Arch. Med. Res. 2018, 49, 504–511. [Google Scholar] [CrossRef]
- Yuan, H.; Jin, X.; Sun, J.; Li, F.; Feng, Q.; Zhang, C.; Cao, Y.; Wang, Y. Protective Effect of HMGB1 A Box on Organ Injury of Acute Pancreatitis in Mice. Pancreas 2009, 38, 143–148. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Dui, D.; Ren, H.; Liu, J. High mobility group box 1 induces the activation of the Janus kinase 2 and signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway in pancreatic acinar cells in rats, while AG490 and rapamycin inhibit their activation. Bosn. J. Basic Med. Sci. 2016, 16, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.; Jiang, Y.; Li, Z.; Xue, D.; Zhang, W. Association between high mobility group box-1 protein expression and cell death in acute pancreatitis. Mol. Med. Rep. 2017, 15, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Tsubota, M.; Ishikura, H.; Sekiguchi, F.; Terada, Y.; Tsujiuchi, T.; Liu, K.; Nishibori, M.; Kawabata, A. Macrophage-derived HMGB1 as a Pain Mediator in the Early Stage of Acute Pancreatitis in Mice: Targeting RAGE and CXCL12/CXCR4 Axis. J. Neuroimmune Pharmacol. 2017, 12, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, Z.; Wang, H.; Zhao, Y.; Gao, X.; Zang, B. High-mobility group box protein-1 induces acute pancreatitis through ac-tivation of neutrophil extracellular trap and subsequent production of IL-1β. Life Sci. 2021, 286, 119231. [Google Scholar] [CrossRef]
- Luan, Z.-G.; Zhang, X.-J.; Yin, X.-H.; Ma, X.-C.; Zhang, H.; Zhang, C.; Guo, R.-X. Downregulation of HMGB1 protects against the development of acute lung injury after severe acute pancreatitis. Immunobiology 2013, 218, 1261–1270. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, H.-X.; Bai, C.; Zhou, X.-Y. Blockade of high-mobility group box 1 attenuates intestinal mucosal barrier dysfunction in experimental acute pancreatitis. Sci. Rep. 2017, 7, 6799. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, D.; Han, W.; Guo, C. High-mobility group box-1 inhibition stabilizes intestinal permeability through tight junctions in experimental acute necrotizing pancreatitis. Agents Actions 2019, 68, 677–689. [Google Scholar] [CrossRef]
- Kang, R.; Zhang, Q.; Hou, W.; Yan, Z.; Chen, R.; Bonaroti, J.; Bansal, P.; Billiar, T.R.; Tsung, A.; Wang, Q.; et al. Intracellular Hmgb1 Inhibits Inflammatory Nucleosome Release and Limits Acute Pancreatitis in Mice. Gastroenterology 2014, 146, 1097–1107.e8. [Google Scholar] [CrossRef]
- Zhu, C.-J.; Yang, W.-G.; Li, D.-J.; Song, Y.-D.; Chen, S.-Y.; Wang, Q.-F.; Liu, Y.-N.; Zhang, Y.; Cheng, B.; Wu, Z.-W.; et al. Calycosin attenuates severe acute pancreatitis-associated acute lung injury by curtailing high mobility group box 1—Induced inflammation. World J. Gastroenterol. 2021, 27, 7669–7686. [Google Scholar] [CrossRef]
- Wen, Y.; Sun, H.Y.; Tan, Z.; Liu, R.H.; Huang, S.Q.; Chen, G.Y.; Qi, H.; Tang, L.J. Abdominal paracentesis drainage ameliorates myocardial injury in severe experimental pan-creatitis rats through suppressing oxidative stress. World J. Gastroenterol. 2020, 26, 35–54. [Google Scholar] [CrossRef]
- Liu, S.; Stolz, D.B.; Sappington, P.L.; Macias, C.A.; Killeen, M.E.; Tenhunen, J.J.; Delude, R.L.; Fink, M.P. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am. J. Physiol. Physiol. 2006, 290, C990–C999. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, H.; Bai, Y.; Zhi, F. Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem. Biophys. Res. Commun. 2019, 509, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, M.; Loughran, P.A.; Yang, M.; Lin, M.; Yang, C.; Gao, W.; Jin, S.; Li, S.; Scott, M.J.; et al. LPS Induces Active HMGB1 Release From Hepatocytes Into Exosomes Through the Co-ordinated Activities of TLR4 and Caspase-11/GSDMD Signaling. Front. Immunol. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ali, S.A. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef]
- Kerkhoff, C.; Klempt, M.; Sorg, C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9). Biochim. Biophys. Acta 1998, 1448, 200–211. [Google Scholar] [CrossRef]
- Schiopu, A.; Cotoi, O.S. S100A8 and S100A9: DAMPs at the Crossroads between Innate Immunity, Traditional Risk Factors, and Cardiovascular Disease. Mediat. Inflamm. 2013, 2013, 828354. [Google Scholar] [CrossRef]
- Norman, G.L.; Navaz, S.A.; Kanthi, Y.; Albesa, R.; Mahler, M.; Knight, J.S.; Zuo, Y. Circulating Calprotectin as a Predictive and Severity Biomarker in Patients with COVID-19. Diagnostics 2022, 12, 1324. [Google Scholar] [CrossRef]
- Mortensen, J.H.; Sinkeviciute, D.; Manon-Jensen, T.; Domislović, V.; McCall, K.; Thudium, C.S.; Brinar, M.; Önnerfjord, P.; Goodyear, C.S.; Krznarić, Ž.; et al. A specific calprotectin neo-epitope (CPa9-HNE) in serum from in-flammatory bowel disease patients is associated with neutrophil activity and endoscopic severity. J. Crohns Colitis 2022, 16, 1447–1460. [Google Scholar] [CrossRef]
- Liao, W.-C.; Huang, B.-S.; Yu, Y.-H.; Yang, H.-H.; Chen, P.-R.; Huang, C.-C.; Huang, H.-Y.; Wu, M.-S.; Chow, L.-P. Galectin-3 and S100A9: Novel Diabetogenic Factors Mediating Pancreatic Cancer–Associated Diabetes. Diabetes Care 2019, 42, 1752–1759. [Google Scholar] [CrossRef]
- Farkas, G., Jr.; Tiszlavicz, Z.; Takács, T.; Szabolcs, A.; Farkas, G.; Somogyvári, F.; Mándi, Y. Analysis of plasma levels and polymorphisms of S100A8/9 and S100A12 in patients with acute pancreatitis. Pancreas 2014, 43, 485–487. [Google Scholar] [CrossRef]
- Waldron, R.T.; Lugea, A.; Gulla, A.; Pandol, S.J. Proteomic Identification of Novel Plasma Biomarkers and Pathobiologic Pathways in Alcoholic Acute Pancreatitis. Front. Physiol. 2018, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, M.; Areny-Balagueró, A.; De-Madaria, E.; Cárdenas-Jaén, K.; García-Rayado, G.; Rivera, R.; Mateos, R.M.M.; Pascual-Moreno, I.; Gironella, M.; Abian, J.; et al. Inflammatory capacity of exosomes released in the early stages of acute pancreatitis predicts the severity of the disease. J. Pathol. 2021, 256, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Nesvaderani, M.; Dhillon, B.K.; Chew, T.; Tang, B.; Baghela, A.; Hancock, R.; Eslick, G.; Cox, M. Gene Expression Profiling: Identification of Novel Pathways and Potential Bi-omarkers in Severe Acute Pancreatitis. J. Am. Coll. Surg. 2022, 234, 803–815. [Google Scholar] [CrossRef]
- Sprenkeler, E.G.G.; Zandstra, J.; van Kleef, N.D.; Goetschalckx, I.; Verstegen, B.; Aarts, C.E.M.; Janssen, H.; Tool, A.T.J.; van Mierlo, G.; van Bruggen, R.; et al. S100A8/A9 Is a Marker for the Release of Neutrophil Extracellular Traps and Induces Neutrophil Activation. Cells 2022, 11, 236. [Google Scholar] [CrossRef]
- Schnekenburger, J.; Schick, V.; Krüger, B.; Manitz, M.P.; Sorg, C.; Nacken, W.; Kerkhoff, C.; Kahlert, A.; Mayerle, J.; Domschke, W.; et al. The calcium binding protein S100A9 is essential for pancreatic leukocyte infil-tration and induces disruption of cell-cell contacts. J. Cell Physiol. 2008, 216, 558–567. [Google Scholar] [CrossRef]
- Xiang, H.; Guo, F.; Tao, X.; Zhou, Q.; Xia, S.; Deng, D.; Li, L.; Shang, D. Pancreatic ductal deletion of S100A9 alleviates acute pancreatitis by targeting VNN1-mediated ROS release to inhibit NLRP3 activation. Theranostics 2021, 11, 4467–4482. [Google Scholar] [CrossRef]
- Ding, Z.; Du, F.; Averitt, V.R.G.; Jakobsson, G.; Rönnow, C.-F.; Rahman, M.; Schiopu, A.; Thorlacius, H. Targeting S100A9 Reduces Neutrophil Recruitment, Inflammation and Lung Damage in Abdominal Sepsis. Int. J. Mol. Sci. 2021, 22, 12923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lu, R.; Chen, J.; Xie, M.; Zhao, X.; Kong, L. S100A9 blockade prevents lipopolysaccharide-induced lung injury via sup-pressing the NLRP3 pathway. Respir. Res. 2021, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Niemi, M.S.; Alexiou, A.; Batiha, G.E.-S. Calprotectin: The link between acute lung injury and gastrointestinal injury in Covid-19: Ban or boon. Curr. Protein Pept. Sci. 2022, 23, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Aziz, M.; Wang, P. Damage-Associated Molecular Patterns As Double-Edged Swords in Sepsis. Antioxid. Redox Signal. 2021, 35, 1308–1323. [Google Scholar] [CrossRef]
- Aziz, M.; Brenner, M.; Wang, P. Extracellular CIRP (eCIRP) and inflammation. J. Leukoc. Biol. 2019, 106, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Tong, L.; Tang, L.; Wu, S. The role of cold-inducible RNA binding protein in cell stress response. Int. J. Cancer 2017, 141, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Peng, J.; Yuan, M.; Kong, B.; Huang, H. Cold-inducible RNA-binding protein (CIRP) in inflammatory diseases: Molecular insights of its associated signalling pathways. Scand. J. Immunol. 2020, 93, e12949. [Google Scholar] [CrossRef]
- Gong, J.-D.; Qi, X.-F.; Zhang, Y.; Li, H.-L. Increased admission serum cold-inducible RNA-binding protein concentration is associated with prognosis of severe acute pancreatitis. Clin. Chim. Acta 2017, 471, 135–142. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Li, C.; Xu, Y.; Wang, X.; Wu, D.; Gao, Z.; Qian, H.; You, Z.; Zhang, Z.; et al. Extracellular CIRP-Impaired Rab26 Restrains EPOR-Mediated Macrophage Polarization in Acute Lung Injury. Front. Immunol. 2021, 12, 768435. [Google Scholar] [CrossRef] [PubMed]
- Herre, M.; Cedervall, J.; Mackman, N.; Olsson, A.K. Neutrophil extracellular traps in the pathology of cancer and other in-flammatory diseases. Physiol. Rev. 2022, 103, 277–312. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef]
- Wan, J.; Ren, Y.; Yang, X.; Li, X.; Xia, L.; Lu, N. The Role of Neutrophils and Neutrophil Extracellular Traps in Acute Pancreatitis. Front. Cell Dev. Biol. 2021, 8, 565758. [Google Scholar] [CrossRef]
- Linders, J.; Madhi, R.; Rahman, M.; Mörgelin, M.; Regner, S.; Brenner, M.; Wang, P.; Thorlacius, H. Extracellular cold-inducible RNA-binding protein regulates neutrophil extracellular trap formation and tissue damage in acute pancreatitis. Lab. Investig. 2020, 100, 1618–1630. [Google Scholar] [CrossRef]
- Tan, C.; Gurien, S.D.; Royster, W.; Aziz, M.; Wang, P. Extracellular CIRP Induces Inflammation in Alveolar Type II Cells via TREM-1. Front. Cell Dev. Biol. 2020, 8, 579157. [Google Scholar] [CrossRef]
- Murao, A.; Tan, C.; Jha, A.; Wang, P.; Aziz, M. Exosome-Mediated eCIRP Release From Macrophages to Induce Inflammation in Sepsis. Front. Pharmacol. 2021, 12, 791648. [Google Scholar] [CrossRef] [PubMed]
- Szatmary, P.; Huang, W.; Criddle, D.; Tepikin, A.; Sutton, R. Biology, role and therapeutic potential of circulating histones in acute in-flammatory disorders. J. Cell Mol. Med. 2018, 22, 4617–4629. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Giglio, R.V.; Henry, B.M.; Lippi, G.; Ciaccio, M.; Plebani, M.; Mannello, F. What is the impact of circulating histones in COVID-19: A systematic review. Clin. Chem. Lab. Med. 2022, 60, 1506–1517. [Google Scholar] [CrossRef]
- Li, Y.; Wan, D.; Luo, X.; Song, T.; Wang, Y.; Yu, Q.; Jiang, L.; Liao, R.; Zhao, W.; Su, B. Circulating Histones in Sepsis: Potential Outcome Predictors and Therapeutic Targets. Front. Immunol. 2021, 12, 650184. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Mazza, D.; Brambilla, F.; Gorzanelli, A.; Agresti, A.; Bianchi, M.E. LPS-Challenged Macrophages Release Microvesicles Coated With Histones. Front. Immunol. 2018, 9, 1463. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2018, 47, D516–D519. [Google Scholar] [CrossRef]
- Pan, B.; Li, Y.; Liu, Y.; Wang, W.; Huang, G.; Ouyang, Y. Circulating CitH3 Is a Reliable Diagnostic and Prognostic Biomarker of Septic Patients in Acute Pancreatitis. Front. Immunol. 2021, 12, 766391. [Google Scholar] [CrossRef]
- Penttilä, A.K.; Rouhiainen, A.; Kylänpää, L.; Mustonen, H.; Puolakkainen, P.; Rauvala, H.; Repo, H. Circulating nucleosomes as predictive markers of severe acute pancreatitis. J. Intensive Care 2016, 4, 14. [Google Scholar] [CrossRef]
- Pérez, S.; Finamor, I.; Martí-Andrés, P.; Pereda, J.; Campos, A.; Domingues, R.; Haj, F.; Sabater, L.; De-Madaria, E.; Sastre, J. Role of obesity in the release of extracellular nucleosomes in acute pancreatitis: A clinical and experimental study. Int. J. Obes. 2018, 43, 158–168. [Google Scholar] [CrossRef]
- Liu, T.; Huang, W.; Szatmary, P.; Abrams, S.T.; Alhamdi, Y.; Lin, Z.; Greenhalf, W.; Wang, G.; Sutton, R.; Toh, C.H. Accuracy of circulating histones in predicting persistent organ failure and mortality in patients with acute pancreatitis. Br. J. Surg. 2017, 104, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Biberci Keskin, E.; İnce, A.T.; Sümbül Gültepe, B.; Köker, İ.H.; Şentürk, H. The relationship between serum histon levels and the severity of acute pancreatitis. Turk. J. Gastroenterol. 2019, 30, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Abrams, S.T.; Alhamdi, Y.; Toh, J.; Yu, W.; Wang, G.; Toh, C.-H. Circulating Histones Are Major Mediators of Multiple Organ Dysfunction Syndrome in Acute Critical Illnesses. Crit. Care Med. 2019, 47, e677–e684. [Google Scholar] [CrossRef]
- Guo, H.Y.; Cui, Z.J. Extracellular Histones Activate Plasma Membrane Toll-Like Receptor 9 to Trigger Calcium Oscillations in Rat Pancreatic Acinar Tumor Cell AR4-2J. Cells 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, F.; Ding, Y.; Lu, J.; Liu, S.; Li, F. Acinar Cells Derived Exosomes Alleviate the Severity of Acute Pancreatitis. Discov. Med. 2021, 31, 95–105. [Google Scholar] [PubMed]
- Zhou, Q.; Xiang, H.; Liu, H.; Qi, B.; Shi, X.; Guo, W.; Zou, J.; Wan, X.; Wu, W.; Wang, Z.; et al. Emodin Alleviates Intestinal Barrier Dysfunction by Inhibiting Apoptosis and Regulating the Immune Response in Severe Acute Pancreatitis. Pancreas 2021, 50, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Yao, J.; Wu, X.; Li, J.; Li, G.; Tang, W.; Liu, J.; Wan, M. Emodin attenuates severe acute pancreatitis-associated acute lung injury by suppressing pancreatic exosome-mediated alveolar macrophage activation. Acta Pharm. Sin. B 2021, 12, 3986–4003. [Google Scholar] [CrossRef]
- Feng, Z.; Zhou, J.; Liu, Y.; Xia, R.; Li, Q.; Yan, L.; Chen, Q.; Chen, X.; Jiang, Y.; Chao, G.; et al. Epithelium- and endothelium-derived exosomes regulate the alveolar macrophages by targeting RGS1 mediated calcium signaling-dependent immune response. Cell Death Differ. 2021, 28, 2238–2256. [Google Scholar] [CrossRef]
- Zheng, L.; Su, J.; Zhang, Z.; Jiang, L.; Wei, J.; Xu, X.; Lv, S. Salidroside regulates inflammatory pathway of alveolar macrophages by influencing the se-cretion of miRNA-146a exosomes by lung epithelial cells. Sci. Rep. 2020, 10, 20750. [Google Scholar] [CrossRef]
- Ye, C.; Li, H.; Bao, M.; Zhuo, R.; Jiang, G.; Wang, W. Alveolar macrophage—Derived exosomes modulate severity and outcome of acute lung injury. Aging 2020, 12, 6120–6128. [Google Scholar] [CrossRef]
- Qin, X.; Zhou, Y.; Jia, C.; Chao, Z.; Qin, H.; Liang, J.; Liu, X.; Liu, Z.; Sun, T.; Yuan, Y.; et al. Caspase-1-mediated extracellular vesicles derived from pyroptotic alveolar macrophages promote inflammation in acute lung injury. Int. J. Biol. Sci. 2022, 18, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, L.; Li, H.; Ren, W.; Zhuo, R.; Feng, C.; He, Y.; Hu, Y.; Ye, C. Alveolar macrophage-derived exosomal tRF-22-8BWS7K092 activates Hippo signaling pathway to induce ferroptosis in acute lung injury. Int. Immunopharmacol. 2022, 107, 108690. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cao, X.; Qin, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Novel Cell-Free Therapy for Sepsis. Front. Immunol. 2020, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.R.; Jong, M.K.; Davies, J.E. Concise review: The challenges and opportunities of employing mesenchymal stromal cells in the treatment of acute pancreatitis. Biotechnol. Adv. 2019, 42, 107338. [Google Scholar] [CrossRef]
- Pu, Q.; Xiu, G.; Sun, J.; Liu, P.; Ling, B. Progress on the effect of mesenchymal stem cell derived exosomes on multiple organ dysfunction in sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 757–760. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Li, X.; Su, X.; Xiao, H.; Yang, J. Hypoxic Preconditioning of Human Umbilical Cord Mesenchymal Stem Cells Is an Effective Strategy for Treating Acute Lung Injury. Stem Cells Dev. 2021, 30, 128–134. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Shahzad, Z.; Tash, E.A.; Janjua, O.S.; Khan, M.I.; Zafar, M.S. Human Umbilical Cord Mesenchymal Stem Cells: Current Literature and Role in Periodontal Regeneration. Cells 2022, 11, 1168. [Google Scholar] [CrossRef]
- Han, L.; Zhao, Z.; Chen, X.; Yang, K.; Tan, Z.; Huang, Z.; Zhou, L.; Dai, R. Human umbilical cord mesenchymal stem cells-derived exosomes for treating traumatic pan-creatitis in rats. Stem Cell Res. Ther. 2022, 13, 221. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Ge, P.; Guo, H.; Li, L.; Zhang, G.; Xu, C.; Chen, H. Comprehensive Mechanism, Novel Markers and Multidisciplinary Treatment of Severe Acute Pan-creatitis-Associated Cardiac Injury—A Narrative Review. J. Inflamm. Res. 2021, 14, 3145–3169. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Huang, W.; Li, C.; Luo, H.; Xue, Z.; Xiao, Y.; Wu, Q.; Chen, C. Exosomes from human induced pluripotent stem cells derived mesenchymal stem cells improved myocardial injury caused by severe acute pancreatitis through activating Akt/Nrf2/HO-1 axis. Cell Cycle 2022, 21, 1578–1589. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, C.; Lv, N.; Liang, Z.; Ma, T.; Cheng, H.; Xia, Y.; Shi, L. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics 2022, 12, 2928–2947. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Q.; Kim, K.-H. Emergence of Edible Plant-Derived Nanovesicles as Functional Food Components and Nanocarriers for Therapeutics Delivery: Potentials in Human Health and Disease. Cells 2022, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, L.; Zhang, Y.; Lu, R. Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Pharmaceutics 2022, 14, 822. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Zhu, G.; Zeng, L.; Xu, S.; Cheng, H.; Ouyang, Z.; Chen, J.; Pathak, J.L.; Wu, L.; et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front. Immunol. 2022, 13, 896745. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Ma, Z.-J.; Kang, X.-W. Current status and outlook of advances in exosome isolation. Anal. Bioanal. Chem. 2022, 414, 7123–7141. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.S.; Khan, A.; Bellio, M.A.; Willis, M.L.; Mahung, C.; Ma, X.; Baldwin, X.; Williams, B.M.; Baron, T.H.; Coleman, L.G.; et al. Characterization of extracellular vesicle miRNA identified in peripheral blood of chronic pancreatitis patients. Mol. Cell. Biochem. 2021, 476, 4331–4341. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, J.; Ye, N.; Li, F.; Zhan, H.; Chen, S.; Xu, J. Plasma-Derived Exosome MiR-19b Acts as a Diagnostic Marker for Pancreatic Cancer. Front. Oncol. 2021, 11, 739111. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, H.; Yu, Q.; Huang, H.; Fervers, B.; Chen, Z.-S.; Lu, L. A Circulating Exosome RNA Signature Is a Potential Diagnostic Marker for Pancreatic Cancer, a Systematic Study. Cancers 2021, 13, 2565. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Gao, X.; Yuan, Y.; Zhao, J.; Zhou, S.; Wang, H.; Wang, L.; Xu, G.; Li, X.; et al. Plasma-Derived Exosomal ALIX as a Novel Biomarker for Diagnosis and Classification of Pancreatic Cancer. Front. Oncol. 2021, 11, 628346. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zang, B.; Gong, X.; Ren, J.; Wang, R. MiR-214-3p exacerbates kidney damages and inflammation induced by hyperlipidemic pancreatitis complicated with acute renal injury. Life Sci. 2019, 241, 117118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, S.; Wang, F. MicroRNA MiR-27a-5p Alleviates the Cerulein-Induced Cell Apoptosis and Inflammatory Injury of AR42J Cells by Targeting Traf3 in Acute Pancreatitis. Inflammation 2020, 43, 1988–1998. [Google Scholar] [CrossRef]
- Erdos, Z.; Barnum, J.E.; Wang, E.; DeMaula, C.; Dey, P.M.; Forest, T.; Bailey, W.J.; Glaab, W.E. Evaluation of the Relative Performance of Pancreas-Specific MicroRNAs in Rat Plasma as Biomarkers of Pancreas Injury. Toxicol. Sci. 2019, 173, 5–18. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, M.; Li, X.; Pan, N.; Bian, X.; Wu, W. Protective Effect of miR-193a-5p and miR-320-5p on Caerulein-Induced Injury in AR42J Cells. Am. J. Dig. Dis. 2021, 66, 4333–4343. [Google Scholar] [CrossRef]
- Usborne, A.L.; Smith, A.T.; Engle, S.K.; Watson, D.E.; Sullivan, J.M.; Walgren, J.L. Biomarkers of exocrine pancreatic injury in 2 rat acute pancreatitis models. Toxicol. Pathol. 2014, 42, 195–203. [Google Scholar] [CrossRef]
- Miao, B.; Qi, W.; Zhang, S.; Wang, H.; Wang, C.; Hu, L.; Huang, G.; Li, S.; Wang, H. miR-148a suppresses autophagy by down-regulation of IL-6/STAT3 signaling in ceru-lein-induced acute pancreatitis. Pancreatology 2019, 19, 557–565. [Google Scholar] [CrossRef]
- Song, G.; Zhou, J.; Song, R.; Liu, D.; Yu, W.; Xie, W.; Ma, Z.; Gong, J.; Meng, H.; Yang, T.; et al. Long noncoding RNA H19 regulates the therapeutic efficacy of mesenchymal stem cells in rats with severe acute pancreatitis by sponging miR-138-5p and miR-141-3p. Stem Cell Res. Ther. 2020, 11, 420. [Google Scholar] [CrossRef]
- Liu, P.; Xia, L.; Zhang, W.-L.; Ke, H.-J.; Su, T.; Deng, L.-B.; Chen, Y.-X.; Lv, N.-H. Identification of serum microRNAs as diagnostic and prognostic biomarkers for acute pancreatitis. Pancreatology 2014, 14, 159–166. [Google Scholar] [CrossRef]
- Lu, P.; Wang, F.; Wu, J.; Wang, C.; Yan, J.; Li, Z.-L.; Song, J.-X.; Wang, J.-J. Elevated Serum miR-7, miR-9, miR-122, and miR-141 Are Noninvasive Biomarkers of Acute Pancreatitis. Dis. Markers 2017, 2017, 7293459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Huang, L.; Zhu, S.; Li, X.; Li, Z.; Yu, C.; Yu, X. Regulation of autophagy by systemic admission of microRNA-141 to target HMGB1 in l-arginine-induced acute pancreatitis in vivo. Pancreatology 2016, 16, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, L.; Han, W. Elevated Level of miR-551b-5p is Associated With Inflammation and Disease Progression in Patients With Severe Acute Pancreatitis. Ther. Apher. Dial. 2018, 22, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Kuśnierz-Cabala, B.; Nowak, E.; Sporek, M.; Kowalik, A.; Kuźniewski, M.; Enguita, F.J.; Stępień, E. Serum levels of unique miR-551-5p and endothelial-specific miR-126a-5p allow discrimination of patients in the early phase of acute pancreatitis. Pancreatology 2015, 15, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wang, H.; Xue, D.; Zhang, W. Screening and validation of differentially expressed extracellular miRNAs in acute pan-creatitis. Mol. Med. Rep. 2017, 16, 6412–6418. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.G.; Kang, X.; Zhan, L.B.; Kang, L.M.; Fan, Z.W.; Bai, L.Z. Circulating miRNAs as biomarkers for severe acute pancreatitis associated with acute lung injury. World J. Gastroenterol. 2017, 23, 7440–7449. [Google Scholar] [CrossRef]
- Shi, N.; Deng, L.; Chen, W.; Zhang, X.; Luo, R.; Jin, T.; Ma, Y.; Du, C.; Lin, Z.; Jiang, K.; et al. Is MicroRNA-127 a Novel Biomarker for Acute Pancreatitis with Lung Injury? Dis. Markers 2017, 2017, 1204295. [Google Scholar] [CrossRef]
- Shan, Y.; Kong, W.; Zhu, A.; Zhang, J.; Ying, R.; Zhu, W. Increased levels of miR-372 correlate with disease progression in patients with hyper-lipidemic acute pancreatitis. Exp. Ther. Med. 2020, 19, 3845–3850. [Google Scholar]
- Chen, Y.J.; Lin, T.L.; Cai, Z.; Yan, C.H.; Gou, S.R.; Zhuang, Y.D. Assessment of acute pancreatitis severity via determination of serum levels of hsa-miR-126-5p and IL-6. Exp. Ther. Med. 2021, 21, 26. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, Y.F.; Li, N. Circulating microRNA-146a and microRNA-146b exhibit potential to serve as markers for acute pancreatitis management and prognosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12770–12780. [Google Scholar]
- Dai, J.; Jiang, M.; Hu, Y.; Xiao, J.; Hu, B.; Xu, J.; Han, X.; Shen, S.; Li, B.; Wu, Z.; et al. Dysregulated SREBP1c/miR-153 signaling induced by hypertriglyceridemia worsens acute pancreatitis and delays tissue repair. JCI Insight 2021, 6, e138584. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, W.; Lu, J.; Zhang, S.; Xiang, X.; Wang, X.; Tang, G. Circ_0000284 Promoted Acute Pancreatitis Progression through the Regulation of miR-10a-5p/Wnt/β-Catenin Pathway. Chem. Biodivers. 2022, 19, e202101006. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Pan, L.; Yang, L.; Niu, Z.; Wang, L.; Gao, Y.; Liu, J.; Liu, Z.; Pei, H. Interfering hsa_circ_0073748 alleviates caerulein-induced ductal cell injury in acute pancreatitis by inhibiting miR-132-3p/TRAF3/NF-κB pathway. Cell Cycle 2022, 21, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, Y.; Sun, W.W.; Chen, W.W.; Ma, L.; Yang, Z.T.; Huang, J.; Chen, E.Z.; Fei, J.; Mao, E.Q. Effect of S100A12 and soluble receptor for advanced glycation end products on the occurrence of severe acute pancreatitis. J. Dig. Dis. 2016, 17, 475–482. [Google Scholar] [CrossRef]
- Wu, L.; Xu, W.; Wang, F.; Lv, T.; Yin, Z.; Song, Y. Plasma mtDNA Analysis Aids in Predicting Pancreatic Necrosis in Acute Pancreatitis Patients: A Pilot Study. Am. J. Dig. Dis. 2018, 63, 2975–2982. [Google Scholar] [CrossRef]
- Li, N.; Wang, B.; Cai, S.; Liu, P. The Role of Serum High Mobility Group Box 1 and Interleukin-6 Levels in Acute Pancreatitis: A Meta-Analysis. J. Cell. Biochem. 2017, 119, 616–624. [Google Scholar] [CrossRef]
- Owusu, L.; Xu, C.; Chen, H.; Liu, G.; Zhang, G.; Zhang, J.; Tang, Z.; Sun, Z.; Yi, X. Gamma-enolase predicts lung damage in severe acute pancreatitis-induced acute lung injury. Histochem. J. 2018, 49, 347–356. [Google Scholar] [CrossRef]
- Moutinho-Ribeiro, P.; Macedo, G.; Melo, S.A. Pancreatic Cancer Diagnosis and Management: Has the Time Come to Prick the Bubble? Front. Endocrinol. 2019, 9, 779. [Google Scholar] [CrossRef]
- Yoshizawa, N.; Sugimoto, K.; Tameda, M.; Inagaki, Y.; Ikejiri, M.; Inoue, H.; Usui, M.; Ito, M.; Takei, Y. miR-3940-5p/miR-8069 ratio in urine exosomes is a novel diagnostic biomarker for pancreatic ductal adenocarcinoma. Oncol. Lett. 2020, 19, 2677–2684. [Google Scholar] [CrossRef]
- Yang, J.; Xu, R.; Wang, C.; Qiu, J.; Ren, B.; You, L. Early screening and diagnosis strategies of pancreatic cancer: A comprehensive review. Cancer Commun. 2021, 41, 1257–1274. [Google Scholar] [CrossRef]
- Villaseñor, A.; Kinross, J.M.; Li, J.V.; Penney, N.; Barton, R.H.; Nicholson, J.K.; Darzi, A.; Barbas, C.; Holmes, E. 1H NMR Global Metabolic Phenotyping of Acute Pancreatitis in the Emergency Unit. J. Proteome Res. 2014, 13, 5362–5375. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Liao, H.-Y.; Huang, W.-H.; Lin, S.-Y.; Tsai, T.-Y.; Yang, C.-Y.; Tsai, F.-J.; Chen, C.-J. Early prediction of severe acute pancreatitis by urinary β-2 microglobulin/saposin B peak ratios on MALDI-TOF. Clin. Chim. Acta 2015, 440, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pallagi, P.; Hegyi, P.; Rakonczay, Z., Jr. The Physiology and Pathophysiology of Pancreatic Ductal Secretion: The Back-ground for Clinicians. Pancreas 2015, 44, 1211–1233. [Google Scholar] [CrossRef] [PubMed]
- Osteikoetxea, X.; Benke, M.; Rodriguez, M.; Pálóczi, K.; Sódar, B.W.; Szvicsek, Z.; Szabó-Taylor, K.; Vukman, K.V.; Kittel, Á.; Wiener, Z.; et al. Detection and proteomic characterization of extracellular vesicles in human pancreatic juice. Biochem. Biophys. Res. Commun. 2018, 499, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Sadakari, Y.; Ohtsuka, T.; Okayama, T.; Nakashima, Y.; Gotoh, Y.; Saeki, K.; Mori, Y.; Nakata, K.; Miyasaka, Y.; et al. Pancreatic Juice Exosomal MicroRNAs as Biomarkers for Detection of Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 2104–2111. [Google Scholar] [CrossRef]
- Ge, P.; Zhang, J.; Zhang, G.; Chen, H. Research progress on application of metabolomics in acute lung injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 1266–1271. [Google Scholar]
- Papadopoulos, S.; Kazepidou, E.; Antonelou, M.H.; Leondaritis, G.; Tsapinou, A.; Koulouras, V.P.; Avgeropoulos, A.; Nakos, G.; Lekka, M.E. Secretory Phospholipase A(2)-IIA Protein and mRNA Pools in Extra-cellular Vesicles of Bronchoalveolar Lavage Fluid from Patients with Early Acute Respiratory Distress Syndrome: A New Perception in the Dissemination of Inflammation. Pharmaceuticals 2020, 13, 415. [Google Scholar] [CrossRef]
- Kaur, G.; Maremanda, K.P.; Campos, M.; Chand, H.S.; Li, F.; Hirani, N.; Haseeb, M.A.; Li, D.; Rahman, I. Distinct Exosomal miRNA Profiles from BALF and Lung Tissue of COPD and IPF Patients. Int. J. Mol. Sci. 2021, 22, 11830. [Google Scholar] [CrossRef]
- Kishore, A.; Navratilova, Z.; Kolek, V.; Novosadova, E.; Čépe, K.; Du Bois, R.M.; Petrek, M. Expression analysis of extracellular microRNA in bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Respirology 2018, 23, 1166–1172. [Google Scholar] [CrossRef]
- Domagala-Kulawik, J. The relevance of bronchoalveolar lavage fluid analysis for lung cancer patients. Expert Rev. Respir. Med. 2019, 14, 329–337. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, M.; Hao, X.; Lou, B.; Liu, J.; She, J. Altered exosomal microRNA profiles in bronchoalveolar lavage fluid can mediate metabolism in patients with Acinetobacter baumannii ventilator-associated pneumonia. Ann. Transl. Med. 2020, 8, 1561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, Y.-P.; Geng, X.-R.; Zhao, M.; Ma, S.-B.; Yang, Y.-H.; Deng, Z.-H.; Luo, L.-M.; Pan, X.-Q. Expression Level of MiRNA-126 in Serum Exosomes of Allergic Asthma Patients and Lung Tissues of Asthmatic Mice. Curr. Drug Metab. 2019, 20, 799–803. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).