Flood-Pulse Variability and Climate Change Effects Increase Uncertainty in Fish Yields: Revisiting Narratives of Declining Fish Catches in India’s Ganga River
Abstract
:1. Introduction
2. Materials and Methods
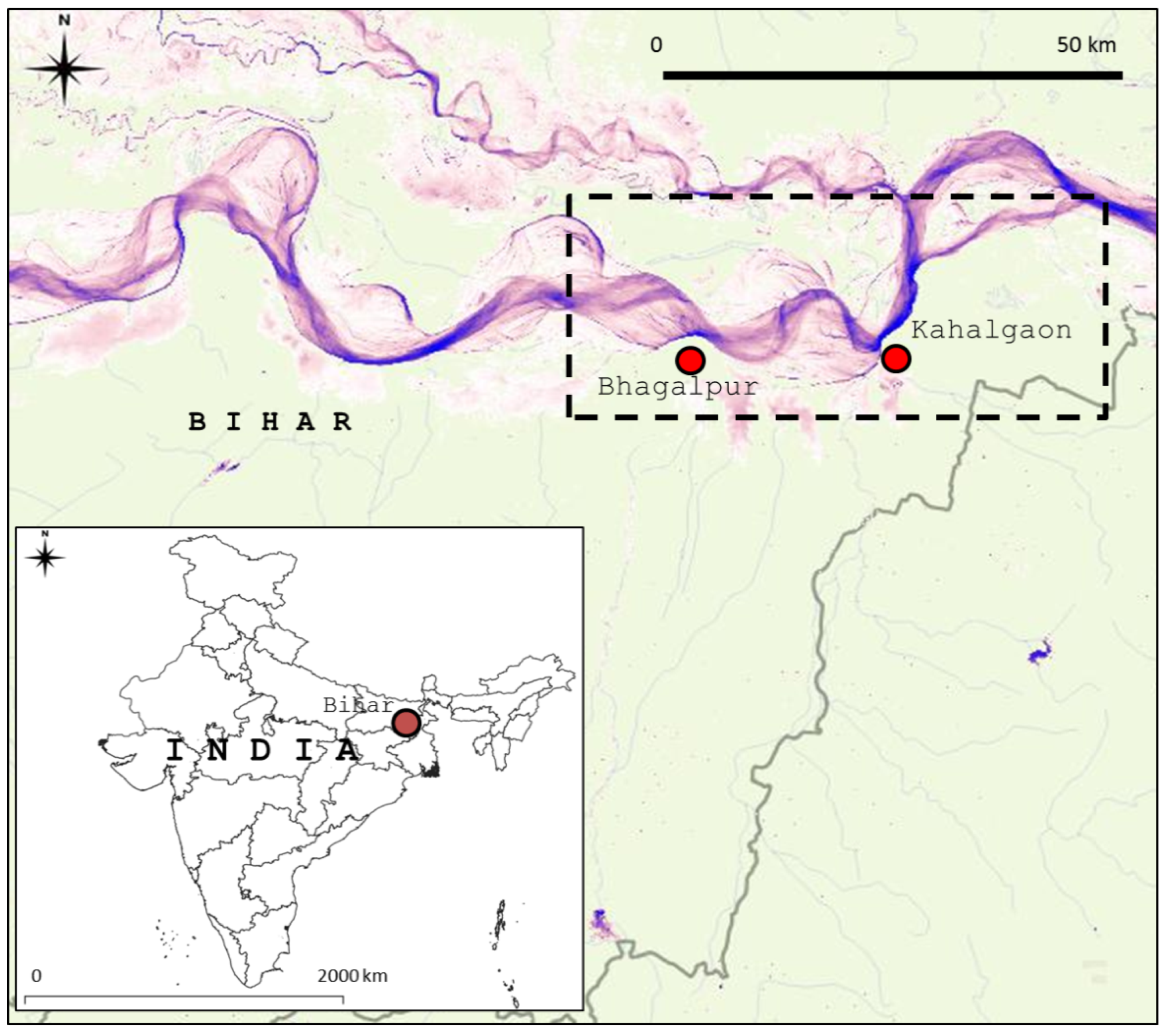
2.1. Study Area
2.2. Data Collection
2.2.1. Interviews and Group Discussions with Fishers and Key Informants
2.2.2. Past Fish Yields and Landings Data
2.2.3. Fish Catch–Effort Data from Landing Sites and Market Surveys
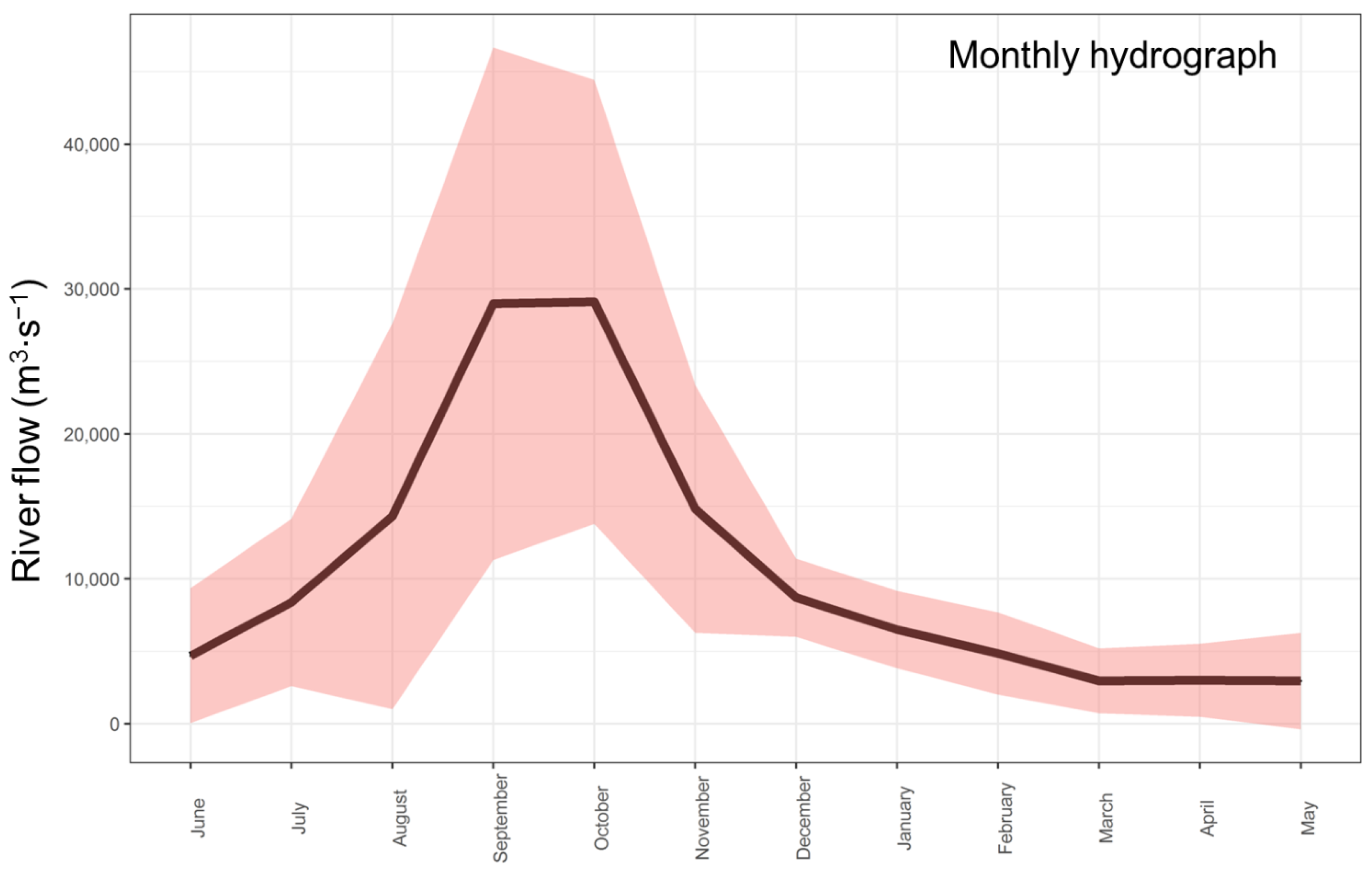
2.2.4. River Flow Data
2.2.5. Discharge Estimation from Active River Channel Width
2.2.6. Rainfall Data
2.2.7. Climatic Indices
2.3. Data Analysis
2.3.1. Long-Term Changes and Trends in Fish Catch Composition (1950s Onwards)
2.3.2. Long-Term Hydrological Trends
2.3.3. Seasonal Trends in Fish Yields
2.3.4. Responses of Aggregate Fish Yields to Hydroclimatic Variability and Fishing Effort
2.3.5. Interpreting the Relationship between Yield and Effort in Terms of Fishing Behaviour
3. Results
3.1. Fishers’ Perceptions of Fish Decline
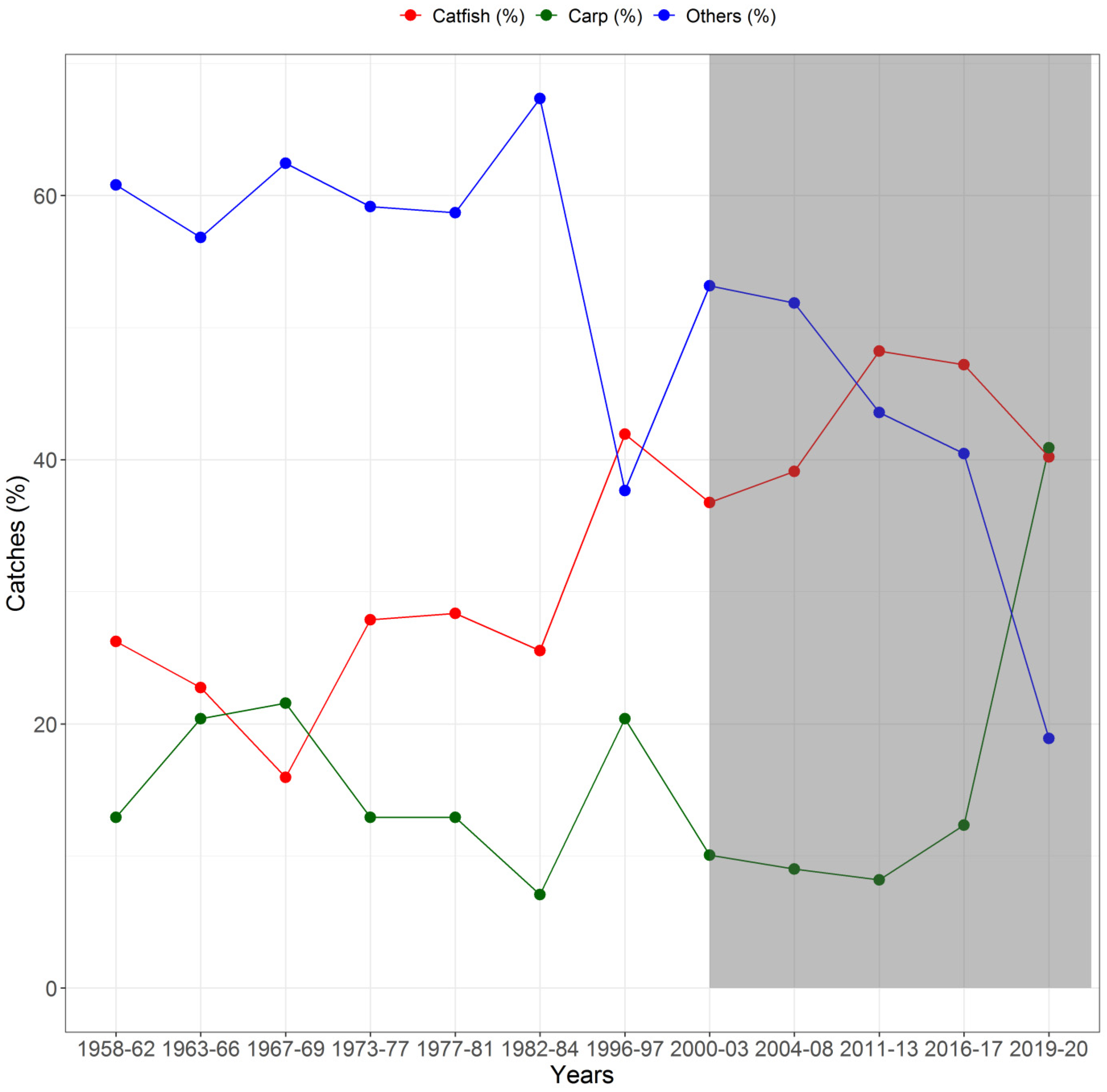
3.2. Historical Trends in Fish Catches and Local Rainfall at Bhagalpur
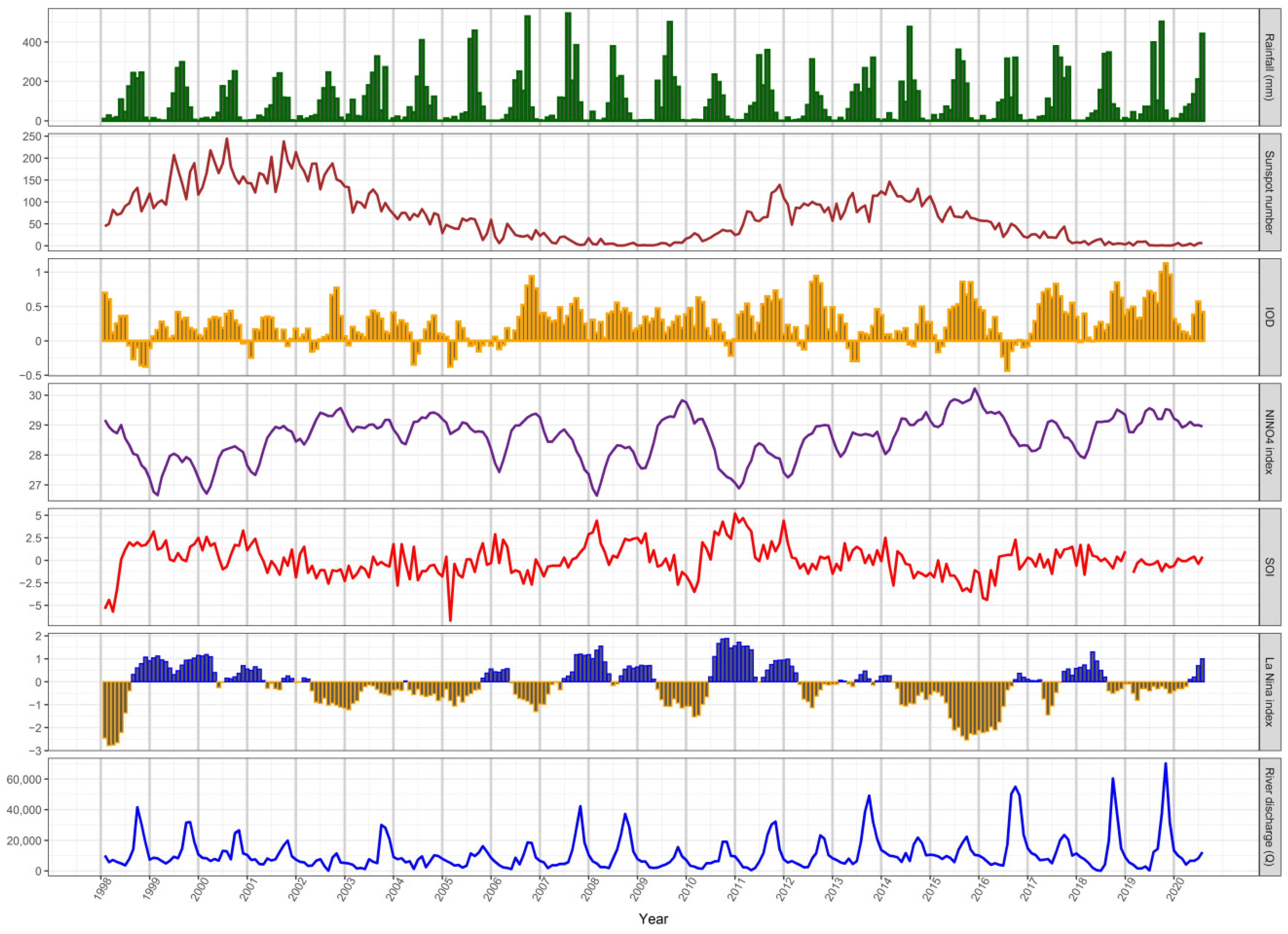
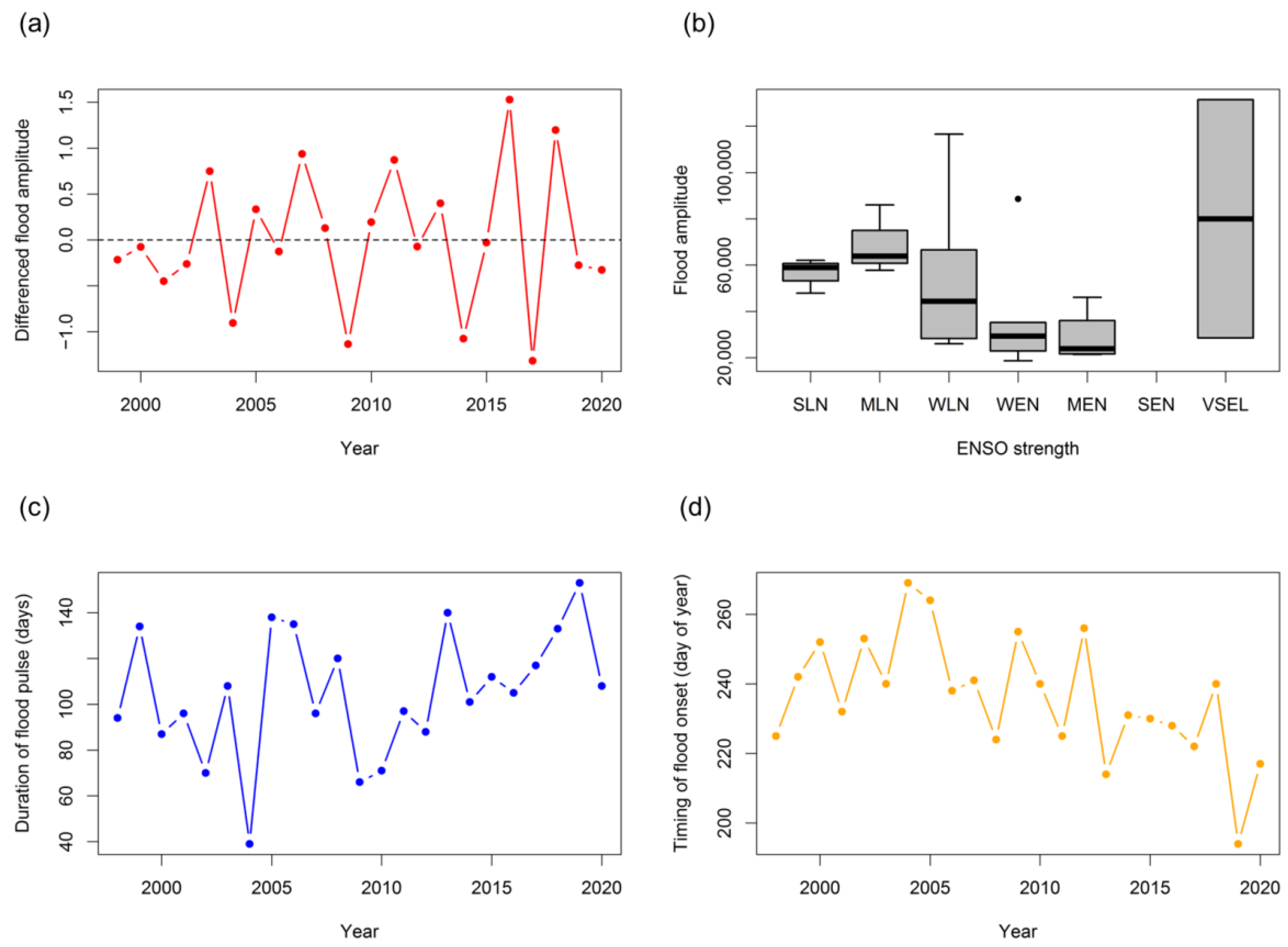
3.3. Long-Term Hydroclimatic Trends
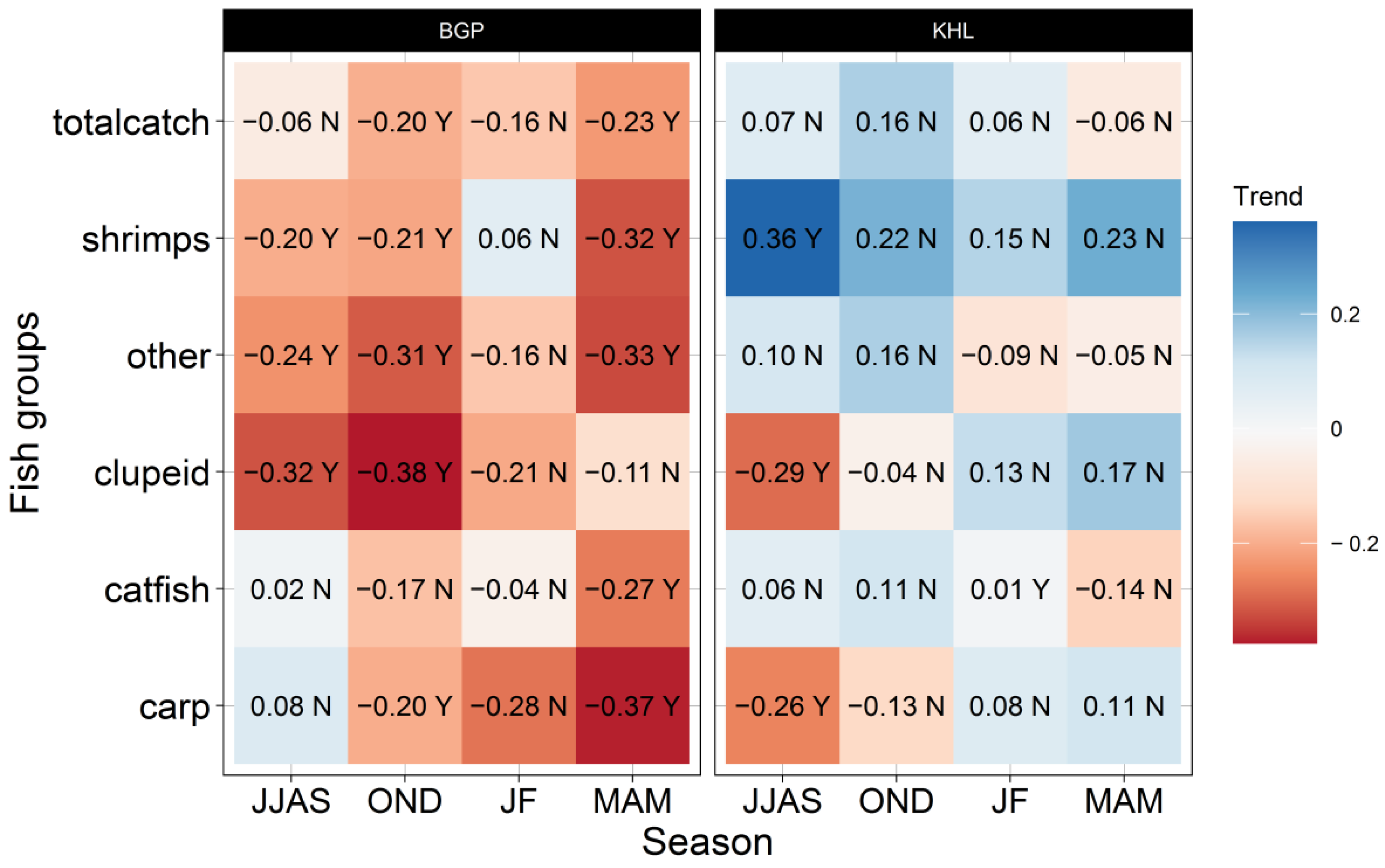
3.4. Long-Term Seasonal Trends in Fish Group Yields
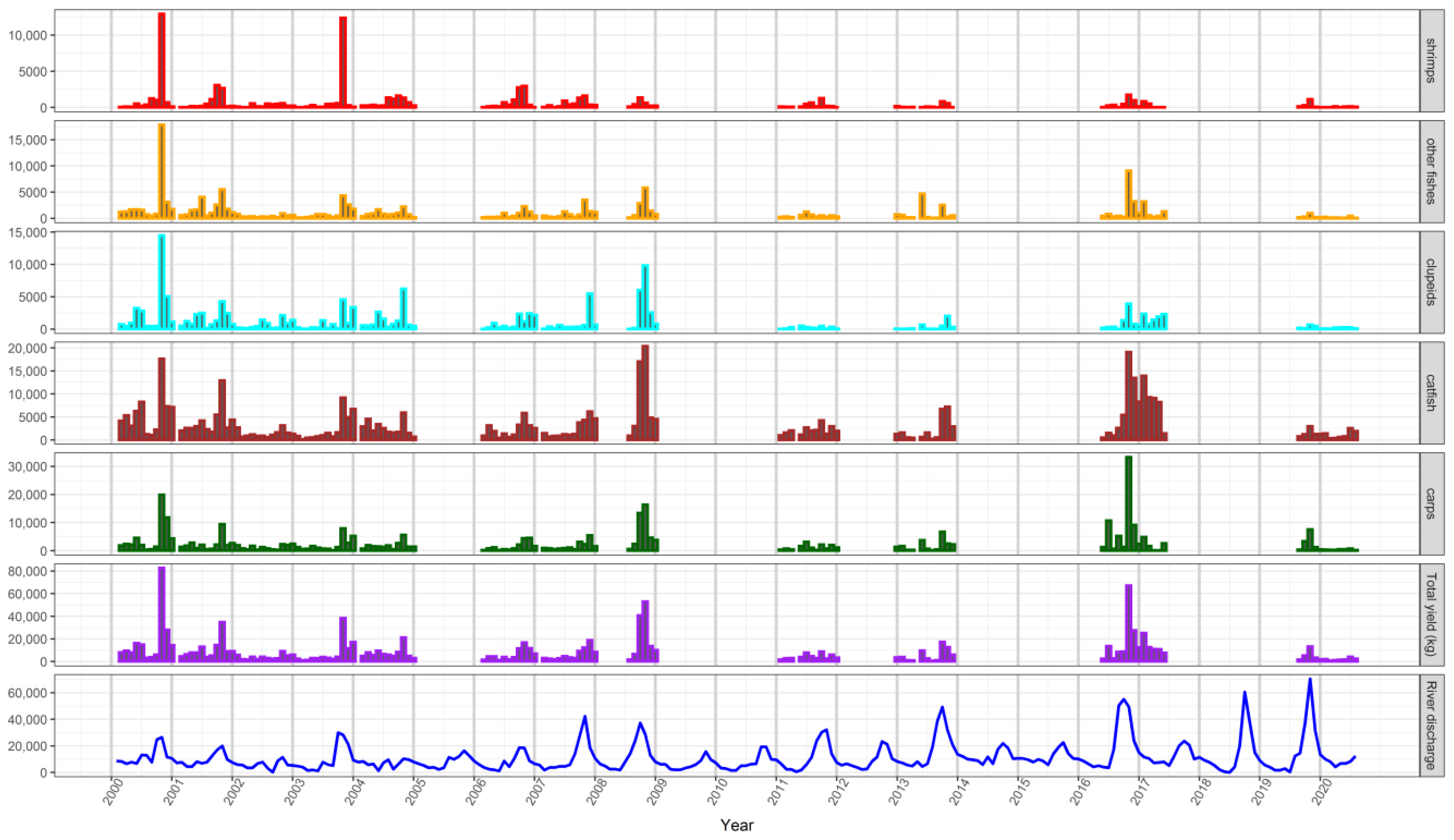
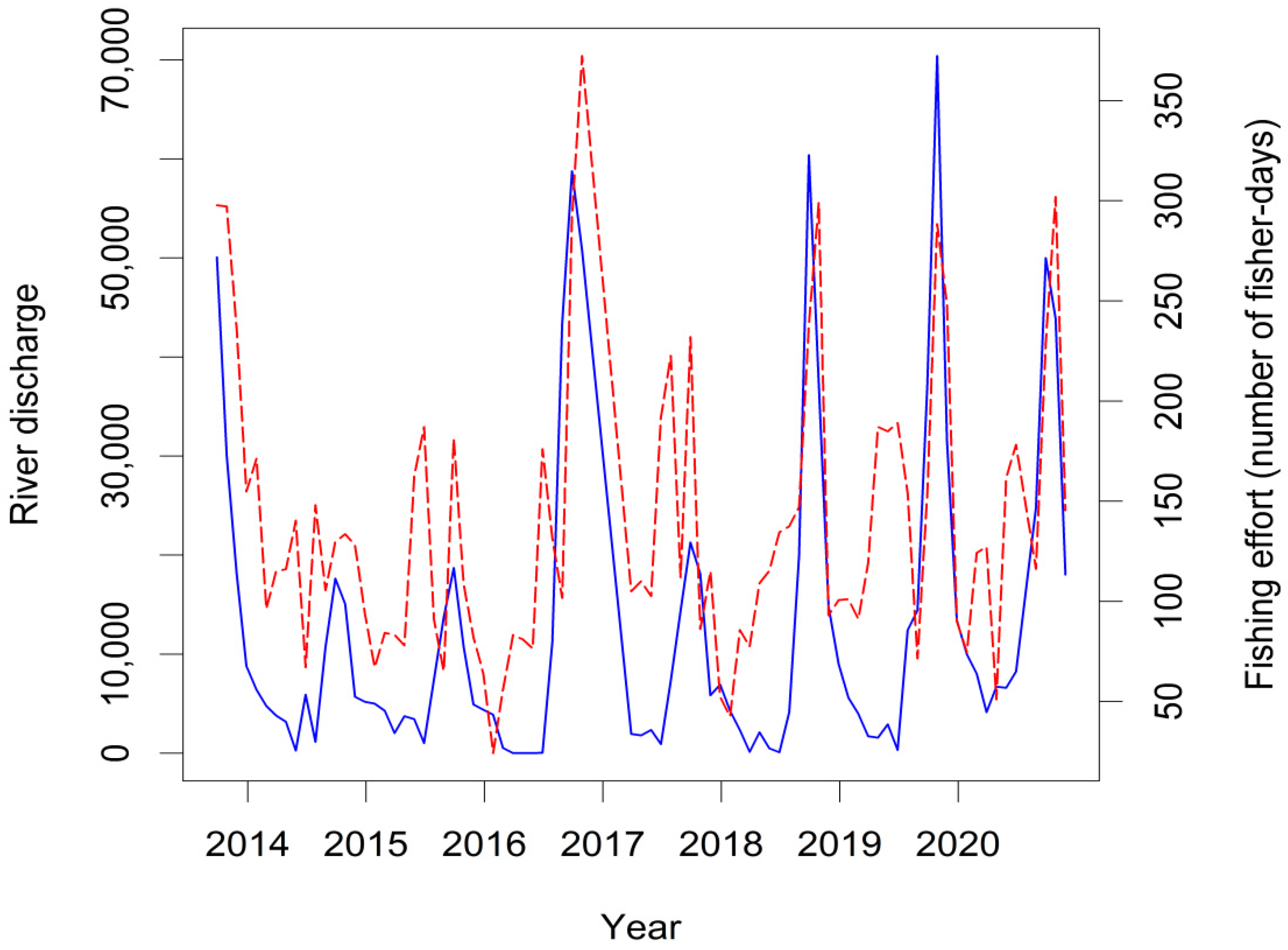
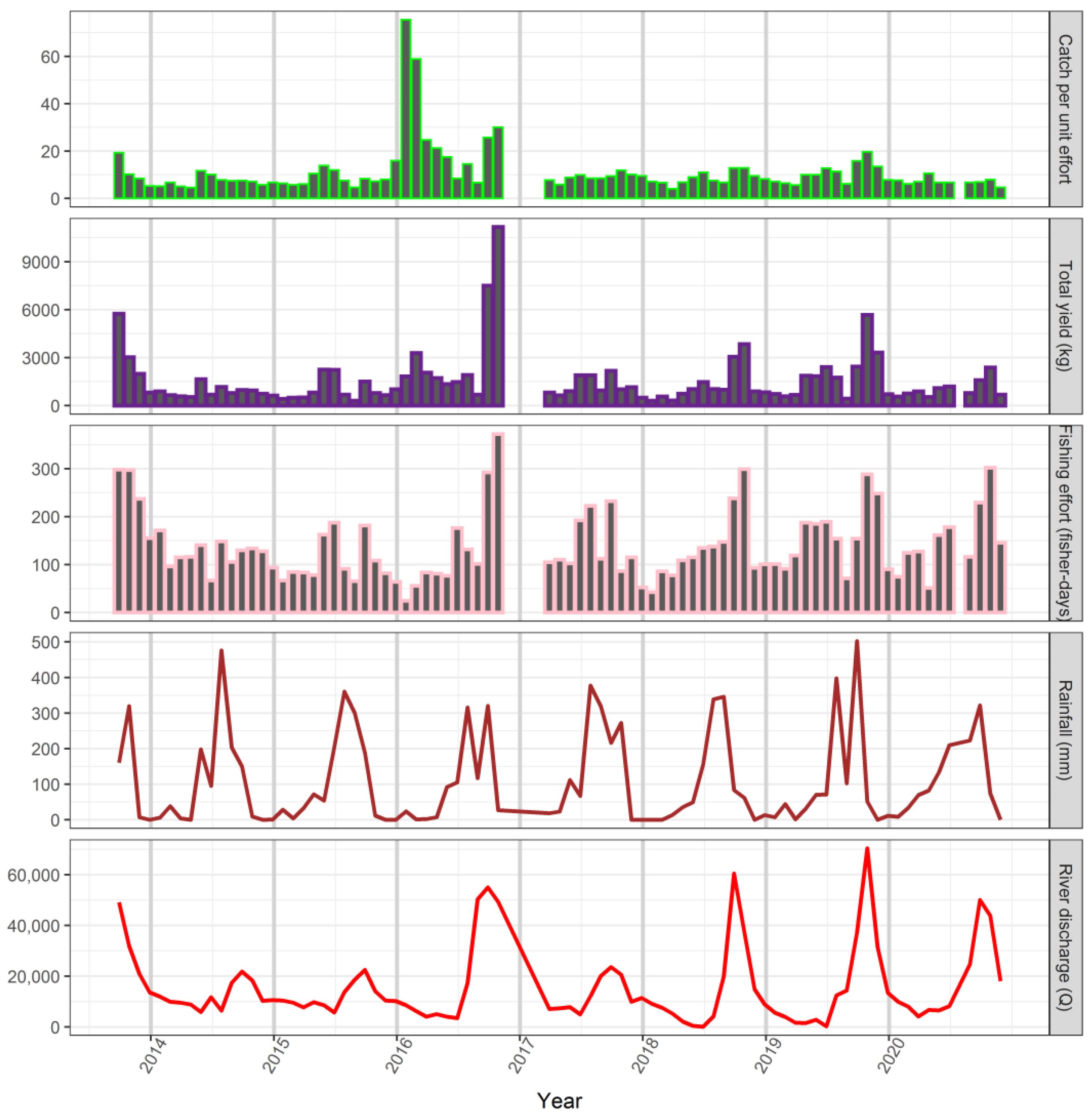
3.5. Responses of Fish Yields to Environmental Variability and Fishing Effort
3.6. Fishing Behaviour and Hyperstability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, L.E.D.; Khoa, S.N.; Lorenzen, K. Livelihood functions of inland fisheries: Policy implications in developing countries. Water Policy 2005, 7, 359–383. [Google Scholar] [CrossRef]
- Lynch, A.J.; Cooke, S.J.; Deines, A.M.; Bower, S.D.; Bunnell, D.B.; Cowx, I.G.; Nguyen, V.M.; Nohner, J.; Phouthavong, K.; Riley, B.; et al. The social, economic, and environmental importance of inland fish and fisheries. Environ. Rev. 2016, 24, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Funge-Smith, S.; Bennett, A. A fresh look at inland fisheries and their role in food security and livelihoods. Fish Fish. 2019, 20, 1176–1195. [Google Scholar] [CrossRef] [Green Version]
- Humphries, P.; King, A.J.; Koehn, J.D. Fish, flows and flood plains: Links between freshwater fishes and their environment in the Murray-Darling River system, Australia. Environ. Biol. Fishes 1999, 56, 129–151. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef] [Green Version]
- Agostinho, A.A.; Gomes, L.C.; Veríssimo, S.; Okada, E.K. Flood regime, dam regulation and fish in the Upper Paraná River: Effects on assemblage attributes, reproduction and recruitment. Rev. Fish Biol. Fish. 2004, 14, 11–19. [Google Scholar] [CrossRef]
- Dudgeon, D. River Rehabilitation for Conservation of Fish Biodiversity in Monsoonal Asia. Ecol. Soc. 2005, 10, 15. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Humphries, P.; Richardson, A.; Wilson, G.; Ellison, T. River regulation and recruitment in a protracted-spawning riverine fish. Ecol. Appl. 2013, 23, 208–225. [Google Scholar] [CrossRef] [Green Version]
- Masood, M.; Yeh, P.J.-F.; Hanasaki, N.; Takeuchi, K. Model study of the impacts of future climate change on the hydrology of Ganges–Brahmaputra–Meghna basin. Hydrol. Earth Syst. Sci. 2015, 19, 747–770. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, P.G.; Barbour, E.; Futter, M.N.; Sarkar, S.; Rodda, H.; Caesar, J.; Butterfield, D.; Jin, L.; Sinha, R.; Nicholls, R.; et al. Impacts of climate change and so-cio-economic scenarios on flow and water quality of the Ganges, Brahmaputra and Meghna (GBM) river systems: Low flow and flood statistics. Environ. Sci. Process. Impacts 2015, 17, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Anand, J.; Gosain, A.K.; Khosa, R.; Srinivasan, R. Regional scale hydrologic modeling for prediction of water balance, analysis of trends in streamflow and variations in streamflow: The case study of the Ganga River basin. J. Hydrol. Reg. Stud. 2018, 16, 32–53. [Google Scholar] [CrossRef]
- Welcomme, R.L. Inland Fisheries: Ecology and Management; Blackwell Science for Food and Agriculture Organization of the United Nations: Ames, IN, USA, 2002. [Google Scholar]
- Welcomme, R.L.; Cowx, I.G.; Coates, D.; Béné, C.; Funge-Smith, S.; Halls, A.; Lorenzen, K. Inland capture fisheries. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2881–2896. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, G. The flood pulse and growth of floodplain fish in Bangladesh. Fish. Manag. Ecol. 2003, 10, 241–247. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Winemiller, K.O.; Lewis, W.M., Jr.; Taphorn Baechle, D.C. The freshwater habitats, fishes, and fisheries of the Orinoco River basin. Aquat. Ecosyst. Health Manag. 2007, 10, 140–152. [Google Scholar] [CrossRef]
- Dey, S.; Choudhary, S.K.; Dey, S.; Deshpande, K.; Kelkar, N. Identifying potential causes of fish declines through local ecological knowledge of fishers in the Ganga River, eastern Bihar, India. Fish. Manag. Ecol. 2020, 27, 140–154. [Google Scholar] [CrossRef]
- Bayazit, M. Nonstationarity of hydrological records and recent trends in trend analysis: A state-of-the-art review. Environ. Process. 2015, 2, 527–542. [Google Scholar] [CrossRef]
- Krishnaswamy, J.; Vaidyanathan, S.; Rajagopalan, B.; Bonell, M.; Sankaran, M.; Bhalla, R.S.; Badiger, S. Non-stationary and non-linear influence of ENSO and Indian Ocean Dipole on the variability of Indian monsoon rainfall and extreme rain events. Clim. Dyn. 2015, 45, 175–184. [Google Scholar] [CrossRef]
- Humphries, P.; Winemiller, K.O. Historical Impacts on River Fauna, Shifting Baselines, and Challenges for Restoration. BioScience 2009, 59, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Bladon, A.J.; Mohammed, E.Y.; Ali, L.; Milner-Gulland, E.J. Developing a frame of reference for fisheries management and conservation interventions. Fish. Res. 2018, 208, 296–308. [Google Scholar] [CrossRef]
- Ngor, P.B.; McCann, K.S.; Grenouillet, G.; So, N.; McMeans, B.C.; Fraser, E.; Lek, S. Evidence of indiscriminate fishing effects in one of the world’s largest inland fisheries. Sci. Rep. 2018, 8, 8947. [Google Scholar] [CrossRef] [Green Version]
- De Graaf, G.; Bartley, D.; Jorgensen, J.; Marmulla, G. The scale of inland fisheries, can we do better? Alternative approaches for assessment. Fish. Manag. Ecol. 2015, 22, 64–70. [Google Scholar] [CrossRef]
- Myers, B.J.E.; Lynch, A.J.; Bunnell, D.B.; Chu, C.; Falke, J.A.; Kovach, R.P.; Krabbenhoft, T.J.; Kwak, T.; Paukert, C.P. Global synthesis of the documented and projected effects of climate change on inland fishes. Rev. Fish Biol. Fish. 2017, 27, 339–361. [Google Scholar] [CrossRef]
- Keyl, F.; Wolff, M. Environmental variability and fisheries: What can models do? Rev. Fish Biol. Fish. 2008, 18, 273–299. [Google Scholar] [CrossRef]
- Gamble, R.J.; Link, J.S. Analyzing the tradeoffs among ecological and fishing effects on an example fish community: A multispecies (fisheries) production model. Ecol. Model. 2009, 220, 2570–2582. [Google Scholar] [CrossRef]
- Ahmed, K.K.U.; Ahmed, S.U.; Haldar, G.C.; Hossain, M.R.A.; Ahmed, T. Primary Production and Fish Yield Estimation in the Meghna River System, Bangladesh. Asian Fish. Sci. 2005, 18, 95. [Google Scholar] [CrossRef]
- Hilborn, R.; Quinn, T.P.; Schindler, D.E.; Rogers, D.E. Biocomplexity and fisheries sustainability. Proc. Natl. Acad. Sci. USA 2003, 100, 6564–6568. [Google Scholar] [CrossRef] [Green Version]
- Baran, E. Fish migration triggers in the Lower Mekong Basin and other tropical freshwater systems. MRC Tech. Pap. 2006, 14, 55. [Google Scholar]
- Das, M.K.; Sharma, A.P.; Sahu, S.K.; Srivastava, P.K.; Rej, A. Impacts and vulnerability of inland fisheries to climate change in the Ganga River system in India. Aquat. Ecosyst. Health Manag. 2013, 16, 415–424. [Google Scholar] [CrossRef]
- Sarkar, U.K.; Naskar, M.; Roy, K.; Sudeeshan, D.; Srivastava, P.; Gupta, S.; Bose, A.K.; Verma, V.K.; Das Sarkar, S.; Karnatak, G.; et al. Benchmarking pre-spawning fitness, climate preferendum of some catfishes from river Ganga and its proposed utility in climate research. Environ. Monit. Assess. 2017, 189, 491. [Google Scholar] [CrossRef]
- Sarkar, U.K.; Roy, K.; Naskar, M.; Srivastava, P.K.; Bose, A.K.; Verma, V.K.; Gupta, S.; Nandy, S.K.; Das Sarkar, S.; Karnatak, G.; et al. Minnows may be more reproductively resilient to climatic variability than anticipated: Synthesis from a reproductive vulnerability assessment of Gangetic pool barbs (Puntius sophore). Ecol. Indic. 2019, 105, 727–736. [Google Scholar] [CrossRef]
- Jul-Larsen, E. (Ed.) Management, Co-Management, or no Management? Major Dilemmas in Southern African Freshwater Fisheries; Food & Agriculture Organization: Rome, Italy, 2003. [Google Scholar]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Humphries, P.; Keckeis, H.; Finlayson, B. The River Wave Concept: Integrating River Ecosystem Models. BioScience 2014, 64, 870–882. [Google Scholar] [CrossRef]
- Tockner, K.; Malard, F.; Ward, J.V. An extension of the flood pulse concept. Hydrol. Process. 2000, 14, 2861–2883. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River Res. Appl. 2006, 22, 123–147. [Google Scholar] [CrossRef]
- Baran, E.; van Zalinge, N.; Ngor, P.B. Floods, floodplains, and fish production in the Mekong Basin: Present and past trends. In Proceedings of the second Asian wetlands symposium, Penang, Malaysia, 27–30 August 2001; Ahyaudi, A., Ed.; Penerbit Universiti Sains Malaysia: Pulau Pinang, Malaysia, 2001; pp. 920–932. [Google Scholar]
- Graham, R.; Harris, J.H. Floodplain Inundation and Fish Dynamics in the Murray-Darling Basin: Current Concepts and Future Research: A Scoping Study; CRC for Freshwater Ecology: Canberra, Australia, 2005; 56p. [Google Scholar]
- Cloern, J.E. Habitat connectivity and ecosystem productivity: Implications from a simple model. Am. Nat. 2007, 169, E21–E33. [Google Scholar] [CrossRef]
- Lyon, J.; Stuart, I.; Ramsey, D.; O’Mahony, J. The effect of water level on lateral movements of fish between river and off-channel habitats and implications for management. Mar. Freshw. Res. 2010, 61, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Rolls, R.J.; Growns, I.O.; Khan, T.A.; Wilson, G.G.; Ellison, T.L.; Prior, A.; Waring, C.C. Fish recruitment in rivers with modified discharge depends on the interacting effects of flow and thermal regimes. Freshw. Biol. 2013, 58, 1804–1819. [Google Scholar] [CrossRef]
- Qasim, S.Z.; Qayyum, A. Spawning frequencies and breeding seasons of some freshwater fishes with special reference to those occurring in the plains of northern India. Indian J. Fish. 1962, 8, 24–43. [Google Scholar]
- King, A.J.; Humphries, P.; Lake, P.S. Fish recruitment on floodplains: The roles of patterns of flooding and life history characteristics. Can. J. Fish. Aquat. Sci. 2003, 60, 773–786. [Google Scholar] [CrossRef]
- Payne, A.I.; Sinha, R.; Singh, H.R.; Huq, S. A review of the Ganges Basin: Its fish and fisheries. In Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries, Phnom Penh, Cambodia, 11–14 February 2003; Welcomme, R.L., Peter, T., Eds.; FAO: Bangkok, Thailand, 2004; Volume 1, pp. 229–250. [Google Scholar]
- Vilizzi, L. Abundance trends in floodplain fish larvae: The role of annual flow characteristics in the absence of overbank flooding. Fundam. Appl. Limnol. Arch. Furhydrobiologie 2012, 181, 215–227. [Google Scholar] [CrossRef]
- Das, M.K.; Srivastava, P.K.; Rej, A.; Mandal, L.; Sharma, A.P. A framework for assessing vulnerability of inland fisheries to impacts of climate variability in India. Mitig. Adapt. Strat. Glob. Chang. 2016, 21, 279–296. [Google Scholar] [CrossRef]
- Walton, S.E.; Nunn, A.D.; Probst, W.N.; Bolland, J.D.; Acreman, M.; Cowx, I.G. Do fish go with the flow? The effects of periodic and episodic flow pulses on 0+ fish biomass in a constrained lowland river. Ecohydrology 2017, 10, e1777. [Google Scholar] [CrossRef]
- Bernhardt, J.R.; O’Connor, M.I.; Sunday, J.M.; Gonzalez, A. Life in fluctuating environments. Philos. Trans. R. Soc. B 2020, 375, 20190454. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Khan, M.A.; Jha, B.C. (Eds.) Ecology, Fisheries and Fish-Stock Assessment of Indian Rivers; Bulletin No. 90; CIFRI: Barrackpore, India, 1999; 325p. [Google Scholar]
- Sinha, M.; Khan, M.A. Impact of environmental aberrations on fisheries of the Ganga (Ganges) River. Aquat. Ecosyst. Health Manag. 2001, 4, 493–504. [Google Scholar] [CrossRef]
- Halls, A.S.; Welcomme, R.L. Dynamics of river fish populations in response to hydrological conditions: A simulation study. River Res. Appl. 2004, 20, 985–1000. [Google Scholar] [CrossRef]
- Halls, A.S.; Welcomme, R.L.; Burn, R.W. The relationship between multi-species catch and effort: Among fishery comparisons. Fish. Res. 2006, 77, 78–83. [Google Scholar] [CrossRef]
- Vass, K.K.; Mondal, S.K.; Samanta, S.; Suresh, V.R.; Katiha, P.K. The environment and fishery status of the River Ganges. Aquat. Ecosyst. Health Manag. 2010, 13, 385–394. [Google Scholar] [CrossRef]
- Castello, L.; McGrath, D.G.; Beck, P.S. Resource sustainability in small-scale fisheries in the Lower Amazon floodplains. Fish. Res. 2011, 110, 356–364. [Google Scholar] [CrossRef]
- FAO and WorldFish Center. Small-Scale Capture Fisheries: A Global Overview with Emphasis on Developing Countries; WorldFish: Penang, Malaysia, 2008. [Google Scholar]
- Bartley, D.M.; De Graaf, G.; Valbo-Jørgensen, J.; Marmulla, G. Inland capture fisheries: Status and data issues. Fish. Manag. Ecol. 2015, 22, 71–77. [Google Scholar] [CrossRef]
- Baran, E.; Myschowoda, C. Have fish catches been declining in the Mekong River Basin? In Moderns Myths of the Mekong; Kummu, M., Keskinen, M., Varis, O., Eds.; Water and Development Pubs: Helsinki, Finland, 2008; pp. 55–64. [Google Scholar]
- Campbell, I.C. Perceptions, data, and river management: Lessons from the Mekong River. Water Resour. Res. 2007, 43. [Google Scholar] [CrossRef] [Green Version]
- Government of India. Manual on Fishery Statistics. Section B—Inland Fishery; Ministry of Statistics and Program Implementation, Central Statistics Office, Govt. of India: New Delhi, India, 2021. [Google Scholar]
- Sarkar, U.K.; Roy, K.; Karnatak, G.; Naskar, M.; Puthiyottil, M.; Baksi, S.; Lianthuamluaia, L.; Kumari, S.; Ghosh, B.D.; Das, B.K. Reproductive environment of the decreasing Indian river shad in Asian inland waters: Disentangling the climate change and indiscriminative fishing threats. Environ. Sci. Pollut. Res. 2021, 28, 30207–30218. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.L.; Rittger, K.; Brodzik, M.J.; Racoviteanu, A.; Barrett, A.P.; Khalsa, S.-J.S.; Raup, B.; Hill, A.; Khan, A.; Wilson, A.M.; et al. Runoff from glacier ice and seasonal snow in High Asia: Separating melt water sources in river flow. Reg. Environ. Chang. 2019, 19, 1249–1261. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, U.K.; Pathak, A.K.; Sinha, R.K.; Sivakumar, K.; Pandian, A.K.; Pandey, A.; Dubey, V.K.; Lakra, W.S. Freshwater fish biodiversity in the River Ganga (India): Changing pattern, threats and conservation perspectives. Rev. Fish Biol. Fish. 2012, 22, 251–272. [Google Scholar] [CrossRef]
- Das, M.K.; Samanta, S.; Saha, P.K. Riverine Health and Impact on Fisheries in India; Policy Paper No. 01; Central Inland Fisheries Research Institute: Barrackpore, Kolkata, 2007; p. 120. [Google Scholar]
- Jhingran, A.G.; Ghosh, K.K. The fisheries of the Ganga River System in the context of Indian aquaculture. Aquaculture 1978, 14, 141–162. [Google Scholar] [CrossRef]
- Talwar, P.K.; Jhingran, A.G. Inland Fishes of India and Adjacent Countries; Oxford and IBH Publishing Co.: New Delhi, India; Bombay, India; Calcutta, India, 1991; Volume 1–2, 1158p. [Google Scholar]
- Ray, P. Ecological Imbalance of the Ganga River System: Its Impact on Aquaculture; Daya Books: Delhi, India, 1998; pp. 198–226. [Google Scholar]
- Vass, K.K.; Das, M.K.; Srivastava, P.K.; Dey, S. Assessing the impact of climate change on inland fisheries in River Ganga and its plains in India. Aquat. Ecosyst. Health Manag. 2009, 12, 138–151. [Google Scholar] [CrossRef]
- Vass, K.K.; Tyagi, R.K.; Singh, H.P.; Pathak, V. Ecology, changes in fisheries, and energy estimates in the middle stretch of the River Ganges. Aquat. Ecosyst. Health Manag. 2010, 13, 374–384. [Google Scholar] [CrossRef]
- Das, B.K.; Sarkar, U.K.; Roy, K. Global Climate Change and Inland Open Water Fisheries in India: Impact and Adaptations; Climate Change and Agriculture in India: Impact and Adaptation; Central Inland Fisheries Research Institute: Barrackpore, West Bengal, India, 2019; pp. 79–95. [Google Scholar]
- CIFRI. Inland Fisheries and Climate Change: Vulnerability and Adaptation Options; ICAR-CIFRI Special Publication, Policy Paper No. 2015-1611; ICAR-CIFRI: Barrackpore, India, 2015; ISSN 0970-616X. [Google Scholar]
- Datta Munshi, J.; Bilgrami, K.S.; Saha, M.P.; Johar, M.A. Survey of fish-spawn collection grounds in the river Ganges. Environ. Conserv. 1979, 6, 153–157. [Google Scholar] [CrossRef]
- Rahman, M.; Rahaman, M.M. Impacts of Farakka barrage on hydrological flow of Ganges river and environment in Bangladesh. Sustain. Water Resour. Manag. 2018, 4, 767–780. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Dey, S.; Kelkar, N. Locating fisheries and livelihood issues in river biodiversity conservation: Insights from long-term engagement with fisheries in the Vikramshila Gangetic Dolphin Sanctuary riverscape, Bihar, India. In Proceedings of the IUCN Symposium on Riverine Biodiversity, Patna, India, 4–6 April 2014. [Google Scholar]
- Kelkar, N.; Dey, S. On Temperaments, Communities, and Conflicts in the River Fisheries of Bihar, Amidst Rigidly Persistent Caste and Class Discrimination; Samudra Newsletter; International Collective in Support of Fishworkers (ICSF): Chennai, India, 2016; Volume 75, pp. 72–75. [Google Scholar]
- Kelkar, N. The resource of tradition: Changing identities and conservation conflicts in Gangetic fisheries. In Conservation from the Margins; Srinivasan, U., Velho, N., Eds.; Orient BlackSwan: Hyderabad, India, 2018; pp. 9–32. [Google Scholar]
- Montaña, C.G.; Choudhary, S.K.; Dey, S.; Winemiller, K.O. Compositional trends of fisheries in the River Ganges, India. Fish. Manag. Ecol. 2011, 18, 282–296. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Smith, B.D.; Dey, S.; Dey, S.; Prakash, S. Conservation and biomonitoring in the Vikramshila Gangetic Dolphin Sanctuary, Bihar, India. Oryx 2006, 40, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.P.; Katiha, P.K.; Sahoo, A.K.; Chandra, G. State of Inland Fisheries and Inland Fisher Community of India; FAO Bay of Bengal Project Report; CIFRI: Barrackpore, West Bengal, India, 2013; 21p. [Google Scholar]
- Narayanan, S. Inland Fisheries, Food Security, and Poverty Eradication: A Case Study of Bihar and West Bengal; International Collective in Support of Fishworkers (ICSF) Occasional Paper; International Collective in Support of Fishworkers: Chennai, India, 2016; 59+vi p. [Google Scholar]
- India Meteorological Department (IMD). Ministry of Earth Sciences, Gov. of India. Available online: https://www.imd.gov.in (accessed on 15 October 2021).
- Singh, M.; Singh, I.B.; Müller, G. Sediment characteristics and transportation dynamics of the Ganga River. Geomorphology 2007, 86, 144–175. [Google Scholar] [CrossRef]
- Hooke, J. River meander behaviour and instability: A framework for analysis. Trans. Inst. Br. Geogr. 2003, 28, 238–253. [Google Scholar] [CrossRef]
- Mirza, M.M.Q. Hydrological changes in the Ganges system in Bangladesh in the post-Farakka period. Hydrol. Sci. J. 1997, 42, 613–631. [Google Scholar] [CrossRef]
- Adel, M.M. Effect on Water Resources from Upstream Water Diversion in the Ganges Basin. J. Environ. Qual. 2001, 30, 356–368. [Google Scholar] [CrossRef]
- Kawser, M.A.; Samad, M.A. Political history of Farakka barrage and its effects on environment in Bangladesh. Bandung 2016, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish STOCK Assessment, Part I: Manua. FAO Fisheries Technical Paper 306. 1998. Available online: http://www.fao.org/docrep/w5449e/w5449e00.htm (accessed on 20 May 2018).
- Lorenzen, K.; Almeida, O.; Arthur, R.; Garaway, C.; Khoa, S.N. Aggregated yield and fishing effort in multispecies fisheries: An empirical analysis. Can. J. Fish. Aquat. Sci. 2006, 63, 1334–1343. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, K.; Cowx, I.G.; Entsua-Mensah, R.E.M.; Lester, N.P.; Koehn, J.D.; Randall, R.G.; So, N.; Bonar, S.A.; Bunnell, D.B.; Venturelli, P.; et al. Stock assessment in in-land fisheries: A foundation for sustainable use and conservation. Rev. Fish Biol. Fish. 2016, 26, 405–440. [Google Scholar] [CrossRef]
- Welcomme, R.L. Data requirements for inland fisheries management. In New Approaches for the Improvement of Inland Capture Fishery Statistics in the Mekong Basin; FAO: Rome, Italy; Mekong River Commission: Vientiane, Laos; Govt. of Thailand: Bangkok, Thailand, 2003; Available online: http://www.fao.org/docrep/005/ad070e/ad070e09.htm#bm9 (accessed on 27 July 2018).
- Bishop, J. Standardizing fishery-dependent catch and effort data in complex fisheries with technology change. Rev. Fish Biol. Fish. 2006, 16, 21–38. [Google Scholar] [CrossRef]
- Maunder, M.N.; Sibert, J.R.; Fonteneau, A.; Hampton, J.; Kleiber, P.; Harley, S.J. Interpreting catch per unit effort data to assess the status of individual stocks and communities. Ices J. Mar. Sci. 2006, 63, 1373–1385. [Google Scholar] [CrossRef]
- Maunder, M.N.; Punt, A.E. A review of integrated analysis in fisheries stock assessment. Fish. Res. 2013, 142, 61–74. [Google Scholar] [CrossRef]
- Pope, K.L.; Willis, D.W. Seasonal influences on freshwater fisheries sampling data. Rev. Fish. Sci. 1996, 4, 57–73. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.F.; Moreno-Báez, M.; Giron-Nava, A.; Corominas, J.; Erisman, B.; Ezcurra, E.; Aburto-Oropeza, O. A spatial method to calculate small-scale fisheries effort in data poor scenarios. PLoS ONE 2017, 12, e0174064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakenridge, G.R.; Kettner, A.; Syvitski, J.; Overeem, I.; De Groeve, T.; Cohen, S.; Nghiem, S.V. Experimental Satel-Lite-Based River Discharge Measurements: Technical Summary (River Watch Version 3.4). 2017. Available online: http://floodobservatory.colorado.edu/satellitegaugingsites/technical.html (accessed on 31 December 2020).
- Chow, V.T. Open-Channel Hydraulics; McGraw-Hill: New York, NY, USA, 1959; 680p. [Google Scholar]
- Williams, G.P. Bank-full discharge of rivers. Water Resour. Res. 1978, 14, 1141–1154. [Google Scholar] [CrossRef]
- Arcement, G.J.; Schneider, V.R. Guide for Selecting Manning’s Roughness Coefficients for Natural Channels and Floodplains; Water-Supply Paper 23; United States Geological Survey: Reston, VA, USA, 1989; 39p. [Google Scholar]
- Dai, A.; Qian, T.; Trenberth, K.E.; Milliman, J.D. Changes in Continental Freshwater Discharge from 1948 to 2004. J. Clim. 2009, 22, 2773–2792. [Google Scholar] [CrossRef]
- Pavelsky, T.M. Using width-based rating curves from spatially discontinuous satellite imagery to monitor river discharge. Hydrol. Process. 2014, 28, 3035–3040. [Google Scholar] [CrossRef]
- Sichangi, A.W.; Wang, L.; Hu, Z. Estimation of River Discharge Solely from Remote-Sensing Derived Data: An Initial Study Over the Yangtze River. Remote Sens. 2018, 10, 1385. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, D.; O’Loughlin, F.; Trigg, M.A.; Miller, Z.F.; Pavelsky, T.M.; Bates, P.D. Development of the Global Width Database for Large Rivers. Water Resour. Res. 2014, 50, 3467–3480. [Google Scholar] [CrossRef]
- Pekel, J.-F.; Cottam, A.; Gorelick, N.; Belward, A.S. High-resolution mapping of global surface water and its long-term changes. Nature 2016, 540, 418–422. [Google Scholar] [CrossRef]
- Ward, P.J.; Beets, W.; Bouwer, L.M.; Aerts, J.C.J.H.; Renssen, H. Sensitivity of river discharge to ENSO. Geophys. Res. Lett. 2010, 37, L12402. [Google Scholar] [CrossRef] [Green Version]
- Pervez, M.S.; Henebry, G.M. Spatial and seasonal responses of precipitation in the Ganges and Brahmaputra river basins to ENSO and Indian Ocean dipole modes: Implications for flooding and drought. Nat. Hazards Earth Syst. Sci. 2015, 15, 147–162. [Google Scholar] [CrossRef] [Green Version]
- Pokhrel, S.; Chaudhari, H.S.; Saha, S.K.; Dhakate, A.; Yadav, R.K.; Salunke, K.; Mahapatra, S.; Rao, S.A. ENSO, IOD and Indian Summer Monsoon in NCEP climate forecast system. Clim. Dyn. 2012, 39, 2143–2165. [Google Scholar] [CrossRef]
- Mishra, V.; Aadhar, S.; Asoka, A.; Pai, S.; Kumar, R. On the frequency of the 2015 monsoon season drought in the Indo-Gangetic Plain. Geophys. Res. Lett. 2016, 43, 12102–12112. [Google Scholar] [CrossRef]
- Ashok, K.; Saji, N.H. On the impacts of ENSO and Indian Ocean dipole events on sub-regional Indian summer monsoon rainfall. Nat. Hazards 2007, 42, 273–285. [Google Scholar] [CrossRef]
- Roy, I.; Collins, M. On identifying the role of sun and the El Niño southern oscillation on Indian summer monsoon rainfall. Atmos. Sci. Lett. 2015, 16, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Hassan, D.; Iqbal, A.; Hassan, S.A.; Abbas, S.; Ansari, M.R. Sunspots and ENSO relationship using Markov method. J. Atmos. Sol. Terr. Phys. 2016, 137, 53–57. [Google Scholar] [CrossRef]
- Yue, S.; Pilon, P.; Cavadias, G. Power of the Mann–Kendall and Spearman’s rho tests for detecting monotonic trends in hydrological series. J. Hydrol. 2002, 259, 254–271. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org (accessed on 31 October 2021).
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall/CRC Press: London, UK, 1989; 532p. [Google Scholar]
- Harrell, F.E. Regression Modelling Strategies, with Applications to Linear Models, Logistic and Ordinal Regres-Sion, and Survival Analysis, 2nd ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Theodosiou, M. Forecasting monthly and quarterly time series using STL decomposition. Int. J. Forecast. 2011, 27, 1178–1195. [Google Scholar] [CrossRef]
- Erisman, B.E.; Allen, L.G.; Claisse, J.T.; Pondella, D.J.; Miller, E.F.; Murray, J.H. The illusion of plenty: Hyperstability masks collapses in two recreational fisheries that target fish spawning aggregations. Can. J. Fish. Aquat. Sci. 2011, 68, 1705–1716. [Google Scholar] [CrossRef]
- Walters, C. Folly and fantasy in the analysis of spatial catch rate data. Can. J. Fish. Aquat. Sci. 2003, 60, 1433–1436. [Google Scholar] [CrossRef]
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty; Chap-Man and Hall: New York, NY, USA, 1992. [Google Scholar]
- Halley, J.M.; Stergiou, K.I. The implications of increasing variability of fish landings. Fish Fish. 2005, 6, 266–276. [Google Scholar] [CrossRef]
- Friend, R.M.; Arthur, R.I. Overplaying Overfishing: A Cautionary Tale from the Mekong. Soc. Nat. Resour. 2012, 25, 285–301. [Google Scholar] [CrossRef]
- Pauly, D. From growth to Malthusian overfishing: Stages of fisheries resources misuse. Tradit. Mar. Re-Source Manag. Knowl. Inf. Bull. 1994, 7, 7–14. [Google Scholar]
- Finkbeiner, E.M.; Bennett, N.J.; Frawley, T.H.; Mason, J.G.; Briscoe, D.K.; Brooks, C.M.; Ng, C.A.; Ourens, R.; Seto, K.; Switzer Swanson, S.; et al. Reconstructing overfishing: Moving beyond Malthus for effective and equitable solutions. Fish Fish. 2017, 18, 1180–1191. [Google Scholar] [CrossRef]
- Melnychuk, M.C.; Peterson, E.; Elliott, M.; Hilborn, R. Fisheries management impacts on target species status. Proc. Natl. Acad. Sci. USA 2017, 114, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelton, A.O.; Mangel, M. Fluctuations of fish populations and the magnifying effects of fishing. Proc. Natl. Acad. Sci. USA 2011, 108, 7075–7080. [Google Scholar] [CrossRef] [Green Version]
- Santoso, A.; Mcphaden, M.J.; Cai, W. The defining characteristics of ENSO extremes and the strong 2015/2016 El Niño. Rev. Geophys. 2017, 55, 1079–1129. [Google Scholar] [CrossRef]
- Chea, R.; Pool, T.K.; Chevalier, M.; Ngor, P.; So, N.; Winemiller, K.O.; Lek, S.; Grenouillet, G. Impact of seasonal hydrological variation on tropical fish assemblages: Abrupt shift following an extreme flood event. Ecosphere 2020, 11, e03303. [Google Scholar] [CrossRef]
- Kuparinen, A.; Keith, D.M.; Hutchings, J.A. Increased environmentally driven recruitment variability decreases resilience to fishing and increases uncertainty of recovery. ICES J. Mar. Sci. 2014, 71, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Yaduvanshi, A.; Ranade, A. Effect of global temperature changes on rainfall fluctuations over river basins across Eastern Indo-Gangeticplains. Aquat. Procedia 2015, 4, 721–729. [Google Scholar] [CrossRef]
- Bera, S. Trend Analysis of Rainfall in Ganga Basin, India during 1901–2000. Am. J. Clim. Chang. 2017, 6, 116–131. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, K.; Aggarwal, P.K.; Mequanint, F.; Shirsath, P.B.; Stirling, C.M.; Khatri-Chhetri, A.; Rahut, D.B. Climate Variability and Change in Bihar, India: Challenges and Opportunities for Sustainable Crop Production. Sustainability 2017, 9, 1998. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Aadhar, S.; Stone, D.; Mishra, V. Increase in extreme precipitation events under anthropogenic warming in India. Weather. Clim. Extrems 2018, 20, 45–53. [Google Scholar] [CrossRef]
- Subash, N.; Singh, S.S.; Priya, N. Variability of rainfall and effective onset and length of the monsoon season over a sub-humid climatic environment. Atmos. Res. 2011, 99, 479–487. [Google Scholar] [CrossRef]
- Ajayamohan, R.S.; Merryfield, W.J.; Kharin, V.V. Increasing Trend of Synoptic Activity and Its Relationship with Extreme Rain Events over Central India. J. Clim. 2010, 23, 1004–1013. [Google Scholar] [CrossRef]
- Pathak, A.; Ghosh, S.; Kumar, P. Precipitation Recycling in the Indian Subcontinent during Summer Monsoon. J. Hydrometeorol. 2014, 15, 2050–2066. [Google Scholar] [CrossRef]
- Goswami, B.N.; Venugopal, V.; Sengupta, D.; Madhusoodanan, M.S.; Xavier, P.K. Increasing Trend of Extreme Rain Events Over India in a Warming Environment. Science 2006, 314, 1442–1445. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Chakraborty, A.; Karmakar, N.; Moulds, S.; Mijic, A.; Buytaert, W. Effects of winter and summer-time irrigation over Gangetic Plain on the mean and intra-seasonal variability of Indian summer monsoon. Clim. Dyn. 2019, 53, 3147–3166. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Hsu, N.C.; Lau, K.-M.; Kafatos, M. Aerosol and rainfall variability over the Indian monsoon region: Distributions, trends and coupling. Ann. Geophys. 2009, 27, 3691–3703. [Google Scholar] [CrossRef]
- Pandey, S.K.; Vinoj, V.; Landu, K.; Babu, S.S. Declining pre-monsoon dust loading over South Asia: Signature of a changing regional climate. Sci. Rep. 2017, 7, 16062. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, C.M.; Gupta, A.; Roy, A.; Dalal, P.; Chauhan, P. Geospatial analysis of September, 2019 floods in the lower gangetic plains of Bihar using multi-temporal satellites and river gauge data. Geomat. Nat. Hazards Risk 2020, 12, 84–102. [Google Scholar] [CrossRef]
- Anderson, C.N.; Hsieh, C.H.; Sandin, S.A.; Hewitt, R.; Hollowed, A.; Beddington, J.; May, R.M.; Sugihara, G. Why fishing magnifies fluctuations in fish abundance. Nature 2008, 452, 835–859. [Google Scholar] [CrossRef] [PubMed]
- Siderius, C.; Hellegers, P.J.G.J.; Mishra, A.; van Ierland, E.C.; Kabat, P. Sensitivity of the agroecosystem in the Ganges basin to inter-annual rainfall variability and associated changes in land use. Int. J. Clim. 2014, 34, 3066–3077. [Google Scholar] [CrossRef] [Green Version]
- Caddy, J.F.; Gulland, J.A. Historical patterns of fish stocks. Mar. Policy 1983, 7, 267–278. [Google Scholar] [CrossRef]
- Lehodey, P.; Alheit, J.; Barange, M.; Baumgartner, T.; Beaugrand, G.; Drinkwater, K.; Fromentin, J.M.; Hare, S.R.; Ottersen, G.; Perry, R.I.; et al. Climate variability, fish, and fisheries. J. Clim. 2006, 19, 5009–5030. [Google Scholar] [CrossRef]
- Pinaya, W.H.D.; Lobon-Cervia, F.J.; Pita, P.; De Souza, R.B.; Freire, J.; Isaac, V.J. Multispecies Fisheries in the Lower Amazon River and Its Relationship with the Regional and Global Climate Variability. PLoS ONE 2016, 11, e0157050. [Google Scholar] [CrossRef]
- Mallen-Cooper, M.; Stuart, I.G. Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system. River Res. Appl. 2003, 19, 697–719. [Google Scholar] [CrossRef]
- Castello, L.; Isaac, V.J.; Thapa, R. Flood pulse effects on multispecies fishery yields in the Lower Amazon. R. Soc. Open Sci. 2015, 2, 150299. [Google Scholar] [CrossRef] [Green Version]
- Alós, J.; Campos-Candela, A.; Arlinghaus, R. A modelling approach to evaluate the impact of fish spatial behavioural types on fisheries stock assessment. ICES J. Mar. Sci. 2019, 76, 489–500. [Google Scholar] [CrossRef]
- Prince, J.; Hilborn, R. Concentration profiles and invertebrate fisheries management. Can. Spec. Publ. Fish. Aquat. Sci. 1998, 125, 187–196. [Google Scholar]
- Rose, G.A.; Kulka, D.W. Hyperaggregation of fish and fisheries: How catch-per-unit-effort increased as the northern cod (Gadus morhua) declined. Can. J. Fish. Aquat. Sci. 1999, 56, 118–127. [Google Scholar] [CrossRef]
- Salas, S.; Gaertner, D. The behavioural dynamics of fishers: Management implications. Fish Fish. 2004, 5, 153–167. [Google Scholar] [CrossRef] [Green Version]
- van Poorten, B.T.; Walters, C.J.; Ward, H.G. Predicting changes in the catchability coefficient through effort sorting as less skilled fishers exit the fishery during stock declines. Fish. Res. 2016, 183, 379–384. [Google Scholar] [CrossRef]
- Kelkar, N. River fisheries of the Gangetic basin, India: A primer. Dams Rivers People Newsl. 2004, 13, 1–40. [Google Scholar]
- Bennett, E.; Neiland, A.; Anang, E.; Bannerman, P.; Rahman, A.A.; Huq, S.; Bhuiyan, S.; Day, M.; Clerveaux, W. Towards a Better Understanding of Conflict Management in Tropical Fisheries: Evidence from Ghana, Bangladesh and the Caribbean; CEMARE Working Paper 159; University of Portsmouth: Portsmouth, UK, 2001; 22p. [Google Scholar]
- McAllister, M.K.; Starr, P.J.; Restrepo, V.R.; Kirkwood, G.P. Formulating quantitative methods to evaluate fishery-management systems: What fishery processes should be modelled and what trade-offs should be made? ICES J. Mar. Sci. 1999, 56, 900–916. [Google Scholar] [CrossRef]
- Allan, J.D.; Abell, R.; Hogan, Z.E.; Revenga, C.; Taylor, B.W.; Welcomme, R.L.; Winemiller, K. Overfishing of Inland Waters. BioScience 2005, 55, 1041–1051. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, D. Asian river fishes in the Anthropocene: Threats and conservation challenges in an era of rapid environmental change. J. Fish Biol. 2011, 79, 1487–1524. [Google Scholar] [CrossRef]
- Carson, S.; Shackell, N.; Mills Flemming, J. Local overfishing may be avoided by examining parameters of a spatio-temporal model. PLoS ONE 2017, 12, e0184427. [Google Scholar] [CrossRef] [Green Version]
- Kelkar, N.; Krishnaswamy, J. Restoring the Ganga for its fauna and fisheries. Ch. 2. In Nature without Borders; Madhusudan, M.D., Rangarajan, M., Shahabuddin, G., Eds.; Orient BlackSwan: Hyderabad, India, 2014; pp. 58–80. [Google Scholar]
- Thoms, M.C. Floodplain–river ecosystems: Lateral connections and the implications of human interference. Geomorphology 2003, 56, 335–349. [Google Scholar] [CrossRef]
- Badjeck, M.-C.; Allison, E.H.; Halls, A.S.; Dulvy, N.K. Impacts of climate variability and change on fishery-based livelihoods. Mar. Policy 2010, 34, 375–383. [Google Scholar] [CrossRef]
- Arthington, A.H.; Naiman, R.J.; Mcclain, M.E.; Nilsson, C. Preserving the biodiversity and ecological services of rivers: New challenges and research opportunities. Freshw. Biol. 2009, 55, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.M.; Lynch, A.J.; Young, N.; Cowx, I.G.; Beard, T.D., Jr.; Taylor, W.W.; Cooke, S.J. To manage inland fisheries is to manage at the social-ecological watershed scale. J. Environ. Manag. 2016, 181, 312–325. [Google Scholar] [CrossRef] [PubMed]
| Group | JJAS Rainfall | OND Rainfall | JFMAM Rainfall |
|---|---|---|---|
| Catfish% | 51.80–0.02.JJAS, R2 = 0.10, p = 0.30 NS | 17.37 + 0.16.OND, R2 = 0.18, p = 0.16 NS | 24.21 + 0.068.JFMAM, R2 = 0.19, p = 0.16 NS |
| Carp% | −13.48 + 0.03.JJAS, R2 = 0.32, p = 0.05 * | 30– 0.14.OND, R2 = 0.18, p = 0.17 NS | 1.62 + 0.10.JFMAM, R2 = 0.54, p = 0.006 ** |
| Others% | 61.68–0.019.JJAS, R2 = 0.02, p = 0.65 NS | 52.63–0.017.OND, R2 = 0.001, p = 0.9 NS | 74.17–0.17.JFMAM, R2 = 0.685, p = 0.0008 *** |
| Fish Group | Intercept | Effect Size Estimates, p-Values | Regression Fit and Test Statistics |
|---|---|---|---|
| carp | 1106 (879.3) NS | River flow: 0.168 (0.029) *** Rainfall: −7.22 (2.52) **, p = 0.004 IOD: −2538 (1135) *, p = 0.027 Month: 183.3 (101.8) ^, p = 0.07 Trend: −9.06 (8.44) NS, p = 0.28 | R2 = 0.316, F = 11.63, df = 5, 126 |
| catfish | 32,350 (13,960) * | River flow: 0.15 (0.025) *** NINO4 index: −1039 (491.5) *, p = 0.36 Rainfall: −6.18 (2.52) *, p = 0.016 Trend: −9.77 (8.05) NS, p = 0.23 | R2 = 0.27, F = 11.63, df = 5, 126 |
| clupeid | 1611.02 (329.90) *** | River flow: 0.05 (0.01) *** Rainfall: −2.30 (1.28) ^, p = 0.07 Trend: −13.95 (4.24) ** | R2 = 0.14, F = 7.14, df = 3, 128 |
| other | 1511.14 (334.59) *** | River flow: 0.066 (0.01) *** Rainfall: −2.75 (1.30) *, p = 0.036 Trend: −14.10 (4.30) ** | R2 = 0.18, F = 9.57, df = 3, 128 |
| shrimp | 811.91 (271.56) ** | River flow: 0.045 (0.01) *** Trend: −11.61 (3.62) ** | R2 = 0.136, F = 10.17, df = 2, 129 |
| total catch | 8578.95 (1906.16) *** | River flow: 0.49 (0.07) *** Rainfall: −19.11 (7.40) * Trend: −64.285 (24.53) ** | R2 = 0.25, F = 14.47, df = 3, 128 |
| Fish Group | Intercept | Effect Size Estimates | TSLM Statistics |
|---|---|---|---|
| Response: Fish yields | |||
| carp | 571.7 (195.3) ** | River flow: −0.037 (0.009) *** Fishing effort: −0.07 (1.035) NS, p = 0.51 Flow × Effort: 0.00016 (0.00004) *** La Nina index: −306.7 (56.35) *** IOD: −679.4 (151.7) *** Trend: 4.31 (1.90) *, p = 0.03 | R2 = 0.43, F = 10.29, df = 6, 82 |
| catfish | 450.8 (204.7) ** | River flow: −0.05 (0.01) *** Fishing effort: −0.84 (1.17) NS, p = 0.47 Flow × Effort: 0.0006 (0.00009) *** Trend: −1.03 (1.86) NS, p = 0.58 | R2 = 0.86, F = 132.7, df = 4, 84 |
| clupeid | 264.05 (100.01) ** | Fishing effort: 1.31 (0.306) *** IOD: −183.02 (74.57) *, p = 0.016 Sunspot number: −2.62 (0.77) ** Trend: −3.84 (1.205) ** | R2 = 0.31, F = 9.48, df = 4, 84 |
| other | −147.64 (59.74) * | Fishing effort: 1.89 (0.297) *** Rainfall: 0.31 (0.17) ^ Trend: 0.795 (0.82) NS, p = 0.336 | R2 = 0.38, F = 17.31, df = 3, 85 |
| shrimp | −3.9 (6.56) NS | Rainfall: 0.09 (0.02) *** IOD: −23.38 (9.82) *, p = 0.02 Trend: 0.30 (0.116) * | R2 = 0.25, F = 9.28, df = 3, 85 |
| total catch | 1151 (463.3) * | River flow: −0.11 (0.02) *** Fishing effort: 0.95 (2.4) NS, p = 0.69 Flow × Effort: 0.0006 (0.00009) *** La Nina index: −198.7 (120.1) ^, p = 0.10 Trend: 0.61 (4.21) NS, p = 0.885 | R2 = 0.74, F = 47.24, df = 5, 83 |
| Response: Catch per unit effort (fisher days) | |||
| carp | 4.54 (2.31) ^ | La Nina index: −4.75 (1.01) *** Month: −0.47 (0.23) *, p = 0.045 IOD: −6.62 (2.89) *, p = 0.024 Trend: 0.07 (0.03) ^, p = 0.056 | R2 = 0.28, F = 8.09, df = 4, 84 |
| catfish | 393.7 (281.32) NS | River flow: 0.06 (0.005) *** Sunspot number: −5.79 (2.62) *, p = 0.03 Trend: −8.48 (4.24) *, p = 0.049 | R2 = 0.64, F = 50.59, df = 3, 85 |
| clupeid | 2.14 (0.44) *** | IOD: −0.615 (0.376) ^, p = 0.10 Sunspot number: −0.01 (0.004) ** Trend: −0.016 (0.006) ** | R2 = 0.124, F = 4.016, df = 3, 85 |
| other | 0.42 (0.29) NS | La Nina Index: −0.26 (0.14) ^, p = 0.06 Rainfall: 0.002 (0.0009) ** Trend: 0.795 (0.82) NS, p = 0.08 | R2 = 0.13, F = 4.22, df = 3.85 |
| shrimp | −0.02 (0.05) NS | Rainfall: 0.0005 (0.0002) ** IOD: −0.14 (0.081) *, p = 0.097 Trend: 0.002 (0.0009) *, p = 0.04 | R2 = 0.156, F = 5.22, df = 3, 85 |
| total catch | 8 (3.03) ** | River flow: 0.0002 (0.00008) ** La Nina index: −4.12 (1.22) ** Month: −0.72 (0.33) *, p = 0.03 Trend: 0.05 (0.04) NS, p = 0.21 | R2 = 0.175, F = 4.46, df = 4, 84 |
| Response: Overall fishing effort | |||
| Number of fisher days | 185.1 (24.88) *** | River flow: 0.0029 (0.00043) *** Sunspot number: −0.57 (0.21) ** La Nina index: 15.26 (7.55) *, p = 0.046 Trend: −1.19 (0.36) ** | R2 = 0.40, F = 13.97, df = 4, 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelkar, N.; Arthur, R.; Dey, S.; Krishnaswamy, J. Flood-Pulse Variability and Climate Change Effects Increase Uncertainty in Fish Yields: Revisiting Narratives of Declining Fish Catches in India’s Ganga River. Hydrology 2022, 9, 53. https://doi.org/10.3390/hydrology9040053
Kelkar N, Arthur R, Dey S, Krishnaswamy J. Flood-Pulse Variability and Climate Change Effects Increase Uncertainty in Fish Yields: Revisiting Narratives of Declining Fish Catches in India’s Ganga River. Hydrology. 2022; 9(4):53. https://doi.org/10.3390/hydrology9040053
Chicago/Turabian StyleKelkar, Nachiket, Rohan Arthur, Subhasis Dey, and Jagdish Krishnaswamy. 2022. "Flood-Pulse Variability and Climate Change Effects Increase Uncertainty in Fish Yields: Revisiting Narratives of Declining Fish Catches in India’s Ganga River" Hydrology 9, no. 4: 53. https://doi.org/10.3390/hydrology9040053
APA StyleKelkar, N., Arthur, R., Dey, S., & Krishnaswamy, J. (2022). Flood-Pulse Variability and Climate Change Effects Increase Uncertainty in Fish Yields: Revisiting Narratives of Declining Fish Catches in India’s Ganga River. Hydrology, 9(4), 53. https://doi.org/10.3390/hydrology9040053






