Removal of Arsenic in Groundwater Using Fe(III) Oxyhydroxide Coated Sand: A Case Study in Mekong Delta, Vietnam
Abstract
:1. Introduction
2. Materials and Methods
2.1. FeOOH Coated Sand Preparation
2.2. Batch Study
- →
- qt: the amount of adsorbed metal ions of the adsorbent (μg/g),
- →
- C0: the initial concentration of As(III) in the solution (μg/L)
- →
- Ct: the equilibrium concentration of As(III) in the solution (μg/L)
- →
- V: the volume of the medium (L),
- →
- m: the amount of the adsorbent used in the adsorption process (g).
2.2.1. Adsorption Kinetic Test
- →
- qe: the amounts of As(III) adsorbed at equilibrium, μg/g
- →
- qt: the amounts of As(III) adsorbed at time t, μg/g
- →
- k1 is the first-order rate constant of adsorption, g/μg·h
- →
- k2 is the second-order kinetic rate constant, g/μg·h
- →
- t: retention time, hour
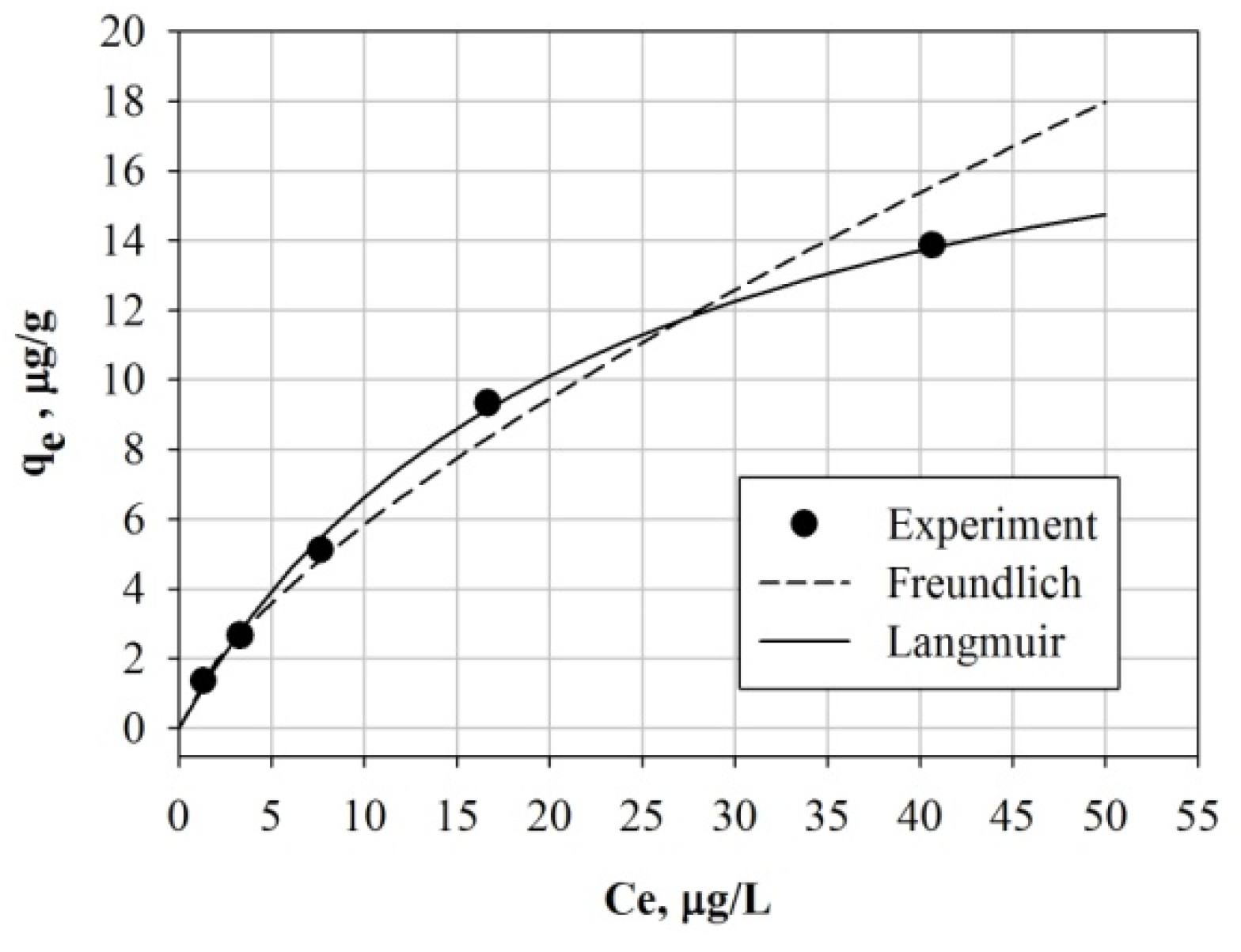
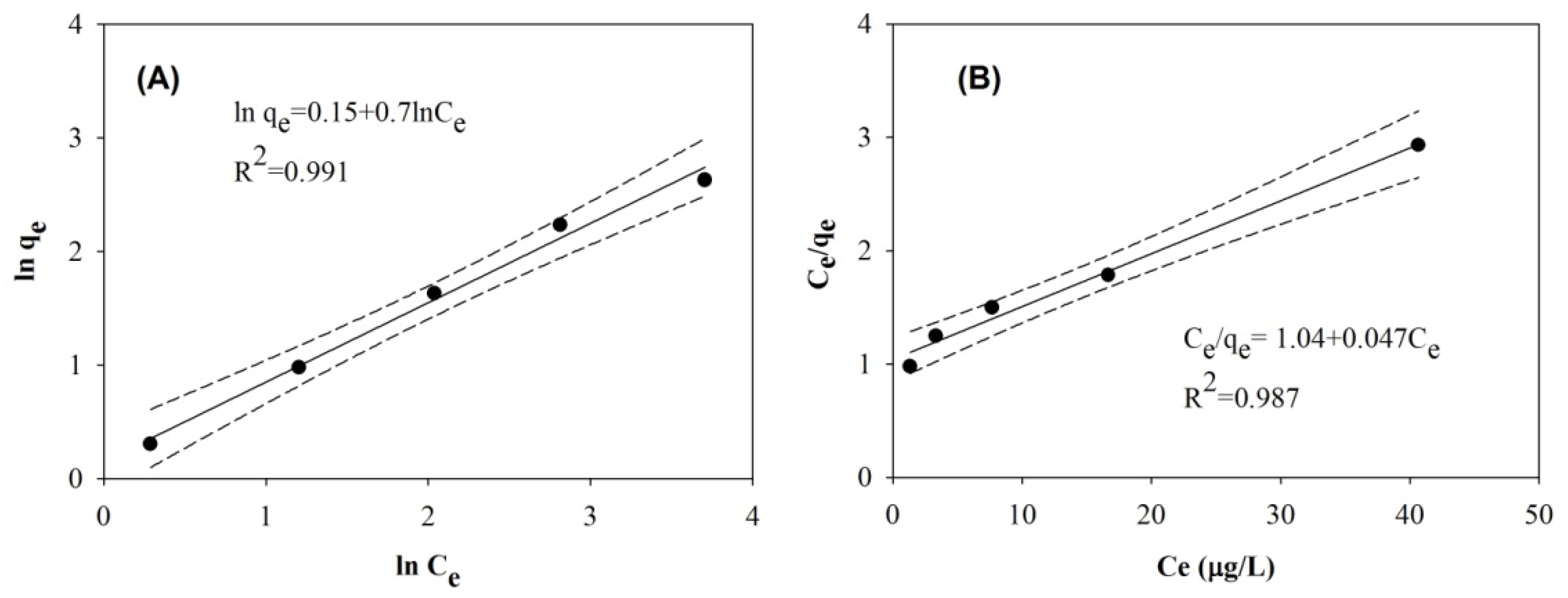
2.2.2. Adsorption Isotherm Study
- →
- qe: As(III) concentration adsorbed on the FeOOH coated sand (μg/g)
- →
- Ce: concentration of As(III) remaining in the solution at equilibrium (μg/L)
- →
- qm: the maximum attainable sorbent capacity, μg/g
- →
- KL: the Langmuir constant, L/μg
- →
- KF: Freundlich adsorption cofficient
- →
- n: a dimensionless parameter of Freundlich adsorption intensity
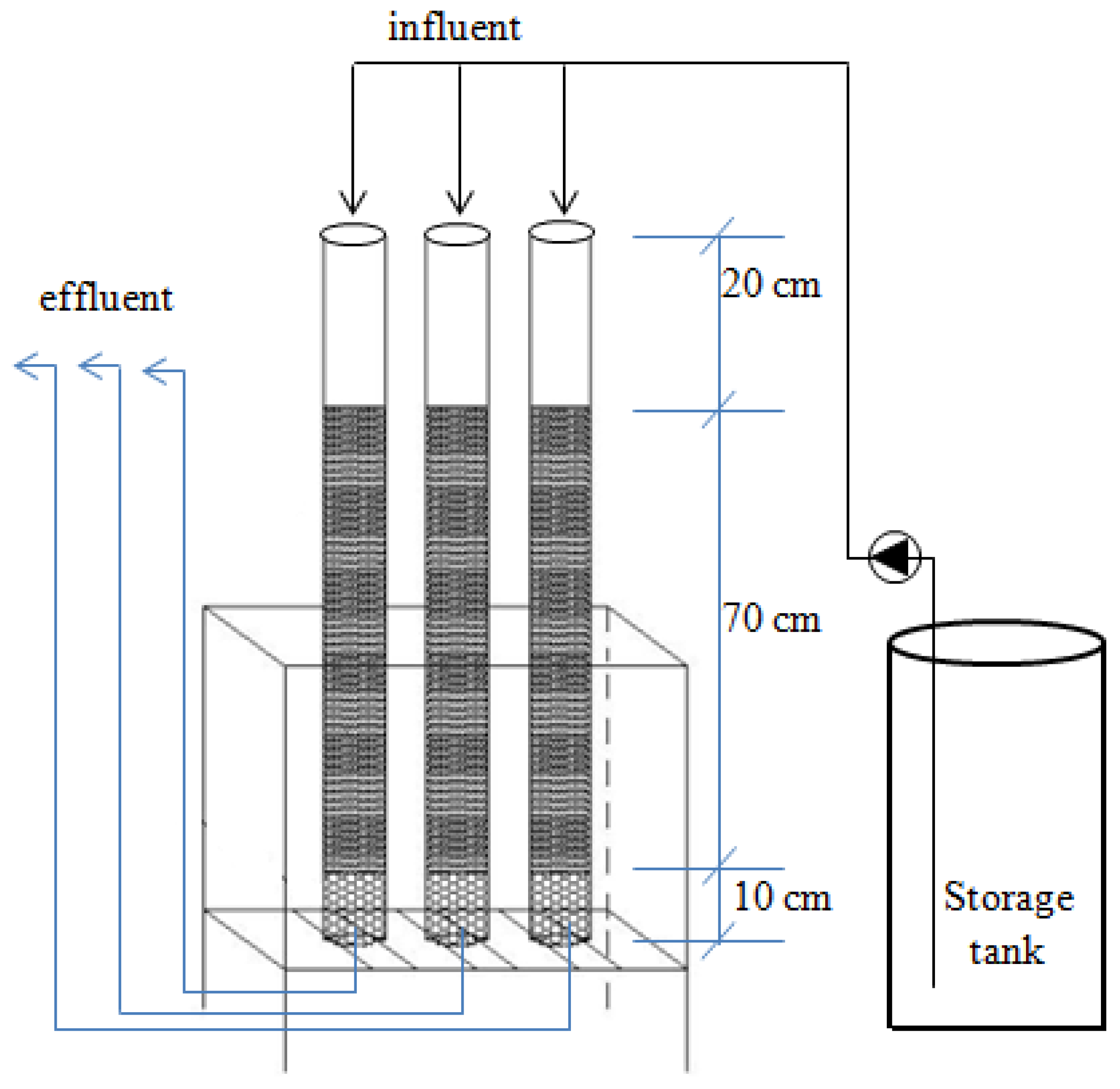
2.3. Column Study
- →
- R: removal efficiency (%)
- →
- Cin: the influent concentration of arsenic in the solution (μg/L)
- →
- Ceff: the effluent concentration of arsenic in the solution (μg/L)
2.4. Analytical Methods and Data Analysis
3. Results and Discussion
3.1. Effects of Contact Time
3.2. Adsorption Kinetics
3.3. Adsorption Isotherm
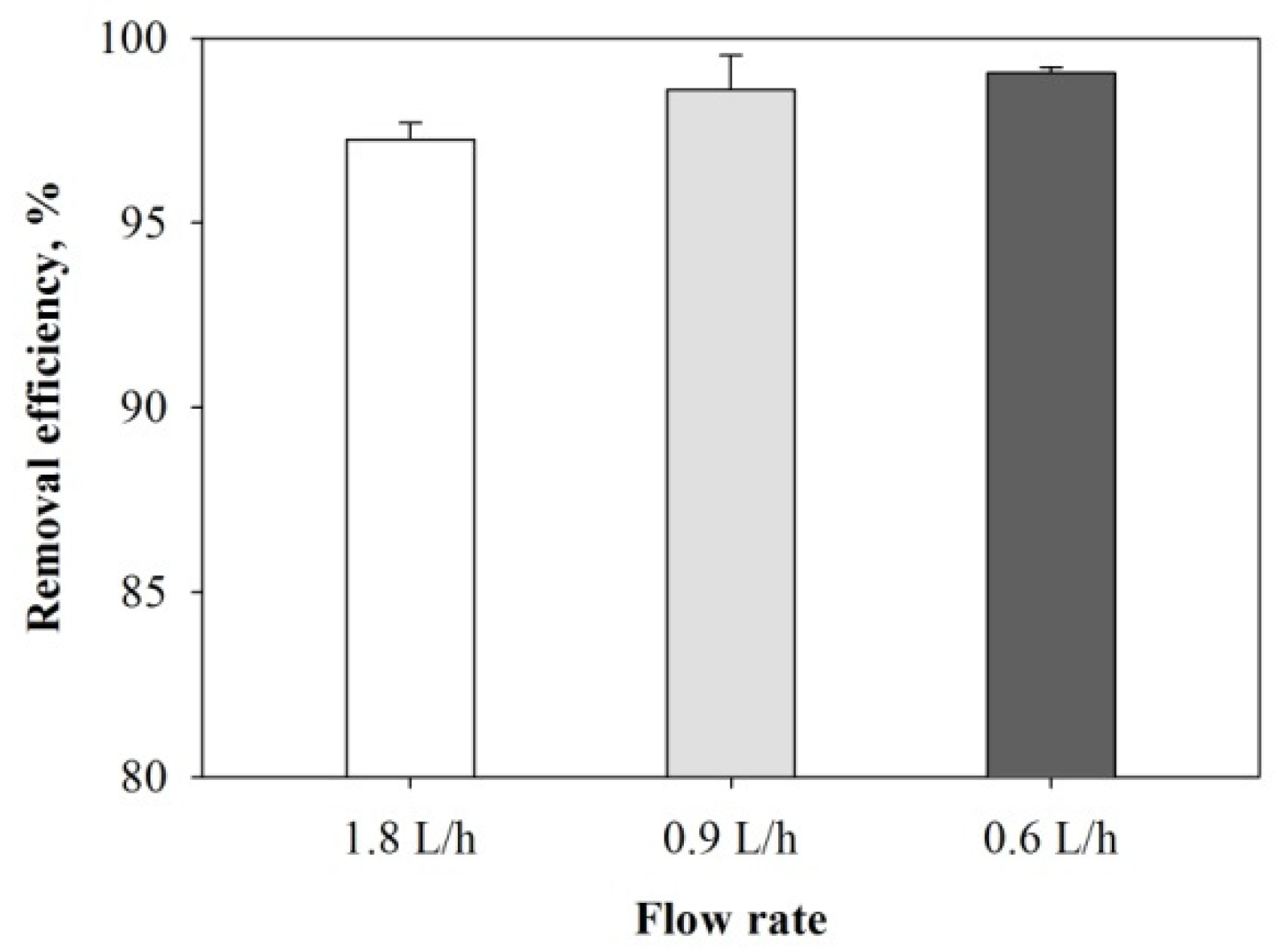
3.4. Performance of Filter Column
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, M.; Stengel, C.; Trang, P.; Hungviet, P.; Sampson, M.L.; Leng, M.; Samreth, S.; Fredericks, D. Magnitude of arsenic pollution in the Mekong and Red River Deltas—Cambodia and Vietnam. Sci. Total Environ. 2007, 372, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Tran, K.T.; Vo, M. Research on arsenic pollution in groundwater source in An Phu district, An Giang. J. Sci. Cantho Univ. 2010, 17, 118–123. [Google Scholar]
- Le Hoang, V.; Nguyen, H.C.; Huynh, L.T.; Phan, T.T. Treatment of arsenic contaminated groundwater at household scale. J. Sci. Cantho Univ. 2013, 25, 36–43. [Google Scholar]
- Erban, L.E.; Gorelick, S.M.; Zebker, H.A.; Fendorf, S. Release of arsenic to deep groundwater in the Mekong Delta, Vietnam, linked to pumping-induced land subsidence. Proc. Natl. Acad. Sci. USA 2013, 110, 13751–13756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.; Li, Z.; Chen, B.; Liang, H.; Zhang, X.; Xu, R.; Li, Z.; Dai, H.; Wei, C.; Liu, S. Comparison of sand-based water filters for point-of-use arsenic removal in China. Chemosphere 2017, 168, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Le, S.C.; Mai, T.N.; Nguyen, X.H.; Nguyen, T.H.; Đang, N.T.; Nguyen, T.G.; Tran, Đ.Q.; Nguyen, T.H.H. Evaluation of the ability to treat heavy metals in water using materials made from sludge from iron processing mines. VNU J. Sci.-Earth Environ. Sci. 2016, 2, 7. [Google Scholar]
- Hsu, J.-C.; Lin, C.-J.; Liao, C.-H.; Chen, S.-T. Removal of As(V) and As(III) by reclaimed iron-oxide coated sands. J. Hazard. Mater. 2008, 153, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hristovski, K.D.; Markovski, J. Engineering metal (hydr)oxide sorbents for removal of arsenate and similar weak-acid oxyanion contaminants: A critical review with emphasis on factors governing sorption processes. Sci. Total Environ. 2017, 598, 258–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, B.; Dangol, B.; Ngai, T.K.K.; Hug, S.J. Kanchan arsenic filters in the lowlands of Nepal: Mode of operation, arsenic removal, and future improvements. Environ. Geochem. Health 2020, 43, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Krok, B.; Mohammadian, S.; Noll, H.M.; Surau, C.; Markwort, S.; Fritzsche, A.; Nachev, M.; Sures, B.; Meckenstock, R.U. Remediation of zinc-contaminated groundwater by iron oxide in situ adsorption barriers—From lab to the field. Sci. Total Environ. 2021, 807, 151066. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.L.; Herman, J.S.; Hornberger, G.M.; DeJesús, T.H. Effect of Solution Ionic Strength and Iron Coatings on Mineral Grains on the Sorption of Bacterial Cells to Quartz Sand. Appl. Environ. Microbiol. 1994, 60, 3300–3306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoppert, A.A.; Loginova, I.V.; Rogozhnikov, D.A.; Karimov, K.A.; Chaikin, L.I. Increased As Adsorption on Maghemite-Containing Red Mud Prepared by the Alkali Fusion-Leaching Method. Minerals 2019, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Jeon, E.-K.; Ryu, S.; Park, S.-W.; Wang, L.; Tsang, D.; Baek, K. Enhanced adsorption of arsenic onto alum sludge modified by calcination. J. Clean. Prod. 2018, 176, 54–62. [Google Scholar] [CrossRef]
- Joshi, A.; Chaudhuri, M. Removal of Arsenic from Ground Water by Iron Oxide-Coated Sand. J. Environ. Eng. 1996, 122, 769–771. [Google Scholar] [CrossRef]


| Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 |
| 0.69 | 5.37 | 0.19 | 0.84 | 5.37 | 0.97 |
| Freundlich | Langmuir | ||||
|---|---|---|---|---|---|
| n | KF | R | qm | KL | R |
| 1.43 | 1.16 | 0.995 | 21.3 | 0.045 | 0.993 |
| Parameter | Unit | Input | Output of Differenet Flow Rates (n = 5) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 mL/min | 15 mL/min | 30 mL/min | |||||||
| Mean | STD | Mean | STD | Mean | STD | Mean | STD | ||
| pH | - | 5.1 | 0.1 | 7.5 | 0.2 | 7.3 | 0.2 | 7.4 | 0.3 |
| EC | µS/cm | 1967 | 15 | 1863 | 21 | 1855 | 16 | 1858 | 29 |
| Turbidity | NTU | 1.35 | 0.13 | 2.9 | 1.56 | 3.0 | 1.68 | 3.4 | 1.93 |
| Total Fe | mg/L | 0.122 | 0.0006 | 0.11 | 0.01 | 0.14 | 0.02 | 0.12 | 0.02 |
| As(III) | µg/L | 81 | 0 | 1.1 | 0.62 | 1.3 | 0.95 | 1.73 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, L.; Thanh, N.T.; Toan, P.V.; Minh, H.V.T.; Kumar, P. Removal of Arsenic in Groundwater Using Fe(III) Oxyhydroxide Coated Sand: A Case Study in Mekong Delta, Vietnam. Hydrology 2022, 9, 15. https://doi.org/10.3390/hydrology9010015
Kim L, Thanh NT, Toan PV, Minh HVT, Kumar P. Removal of Arsenic in Groundwater Using Fe(III) Oxyhydroxide Coated Sand: A Case Study in Mekong Delta, Vietnam. Hydrology. 2022; 9(1):15. https://doi.org/10.3390/hydrology9010015
Chicago/Turabian StyleKim, Lavane, Nguyen Truong Thanh, Pham Van Toan, Huynh Vuong Thu Minh, and Pankaj Kumar. 2022. "Removal of Arsenic in Groundwater Using Fe(III) Oxyhydroxide Coated Sand: A Case Study in Mekong Delta, Vietnam" Hydrology 9, no. 1: 15. https://doi.org/10.3390/hydrology9010015
APA StyleKim, L., Thanh, N. T., Toan, P. V., Minh, H. V. T., & Kumar, P. (2022). Removal of Arsenic in Groundwater Using Fe(III) Oxyhydroxide Coated Sand: A Case Study in Mekong Delta, Vietnam. Hydrology, 9(1), 15. https://doi.org/10.3390/hydrology9010015








