An Assessment of Woody Plant Water Source Studies from across the Globe: What Do We Know after 30 Years of Research and Where Do We Go from Here?
Abstract
1. Introduction
- (1)
- Evaluate the spatiotemporal dynamics of global research on woody species water sources; and
- (2)
- Set new research priorities in the study of woody species water sources.
2. Materials and Methods
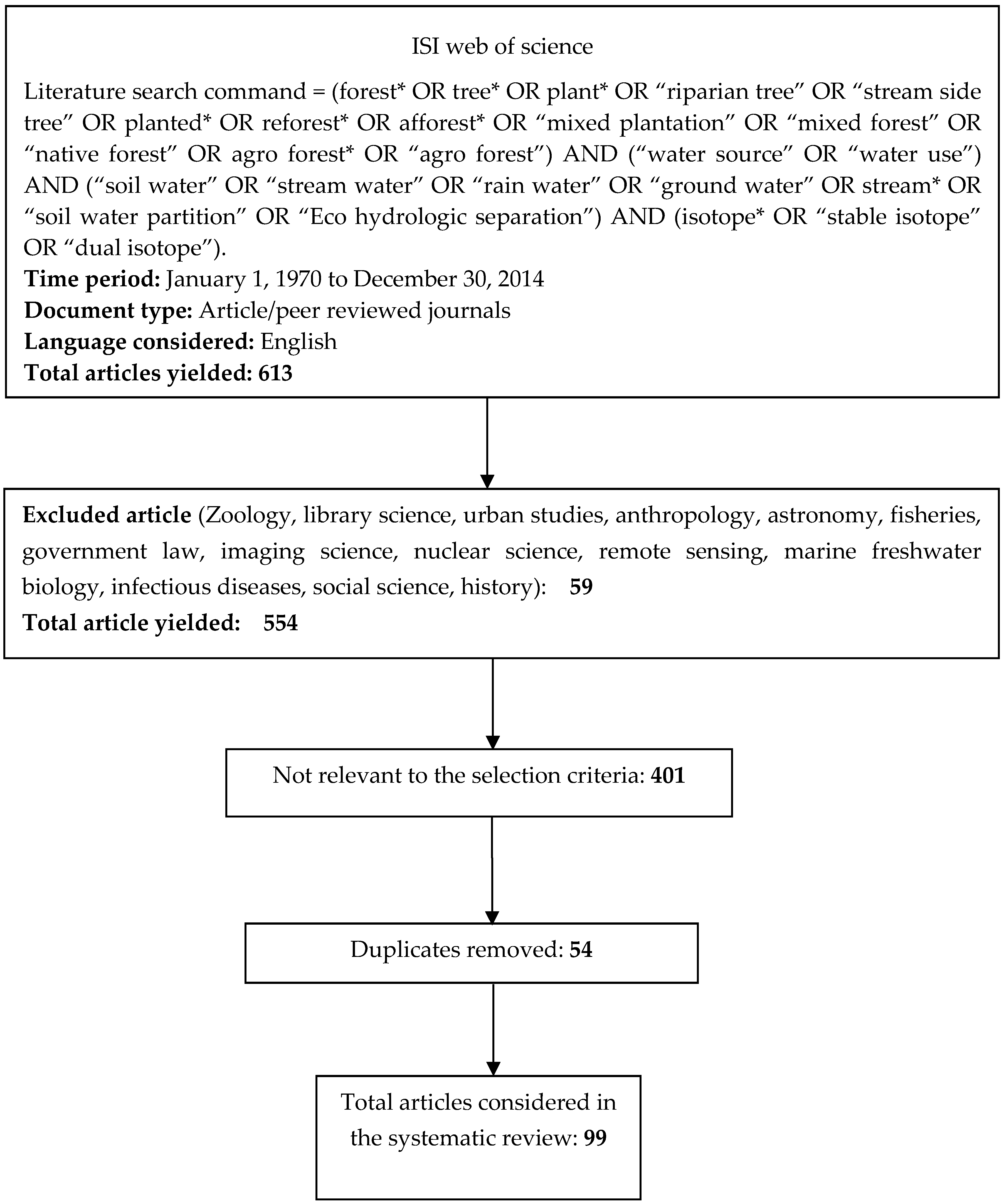
2.1. Literature Search and Article Inclusion Criteria
- The research was undertaken in forest areas;
- The research focused on woody species (trees and shrubs) only;
- The water source was measured without any treatment, such as measuring isotopes before and after pruning or harvesting;
- The article was written in English; and
- The article had a clear description of the investigated species.
2.2. Data Extraction, Interpretation, and Analysis
3. Results
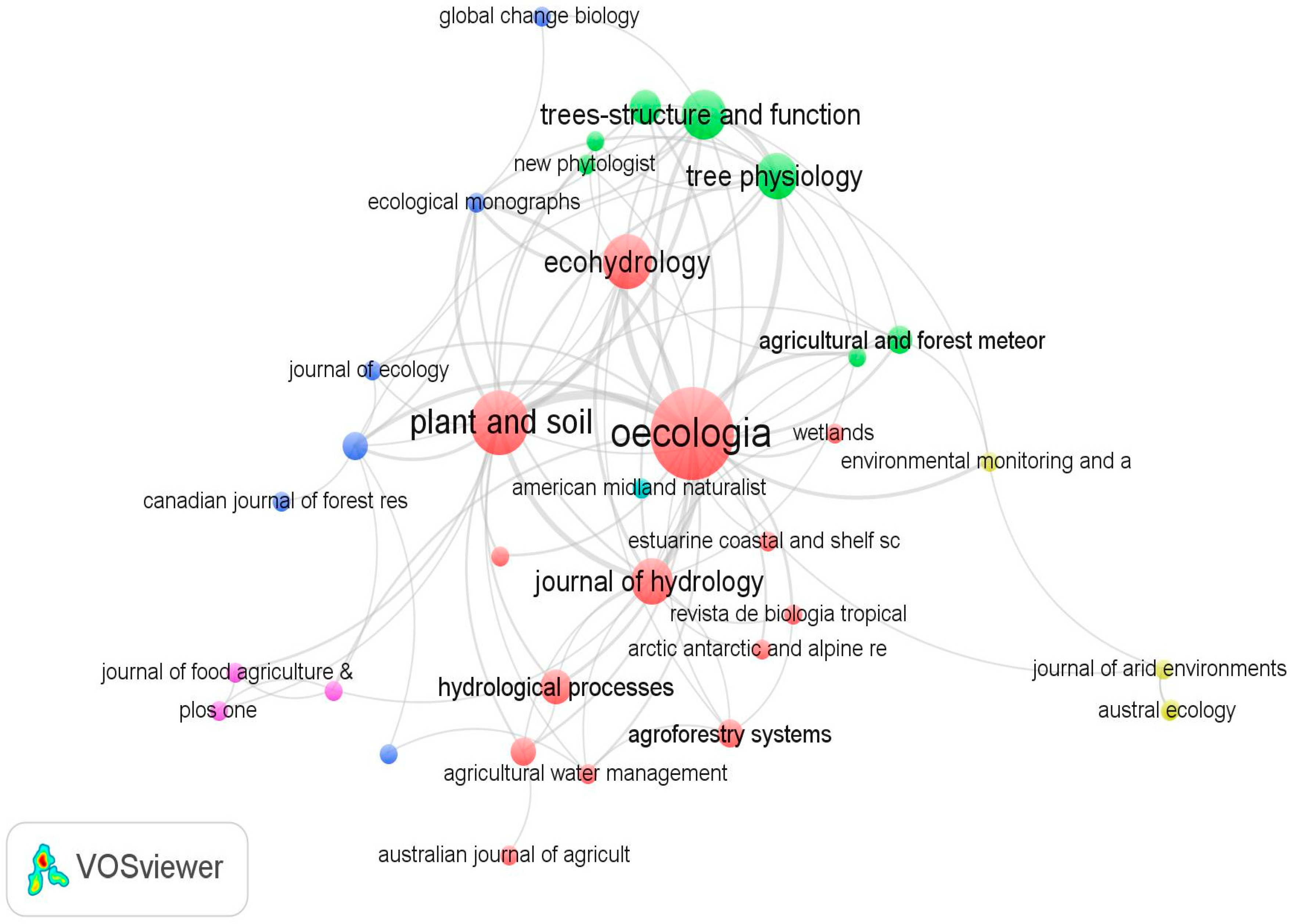
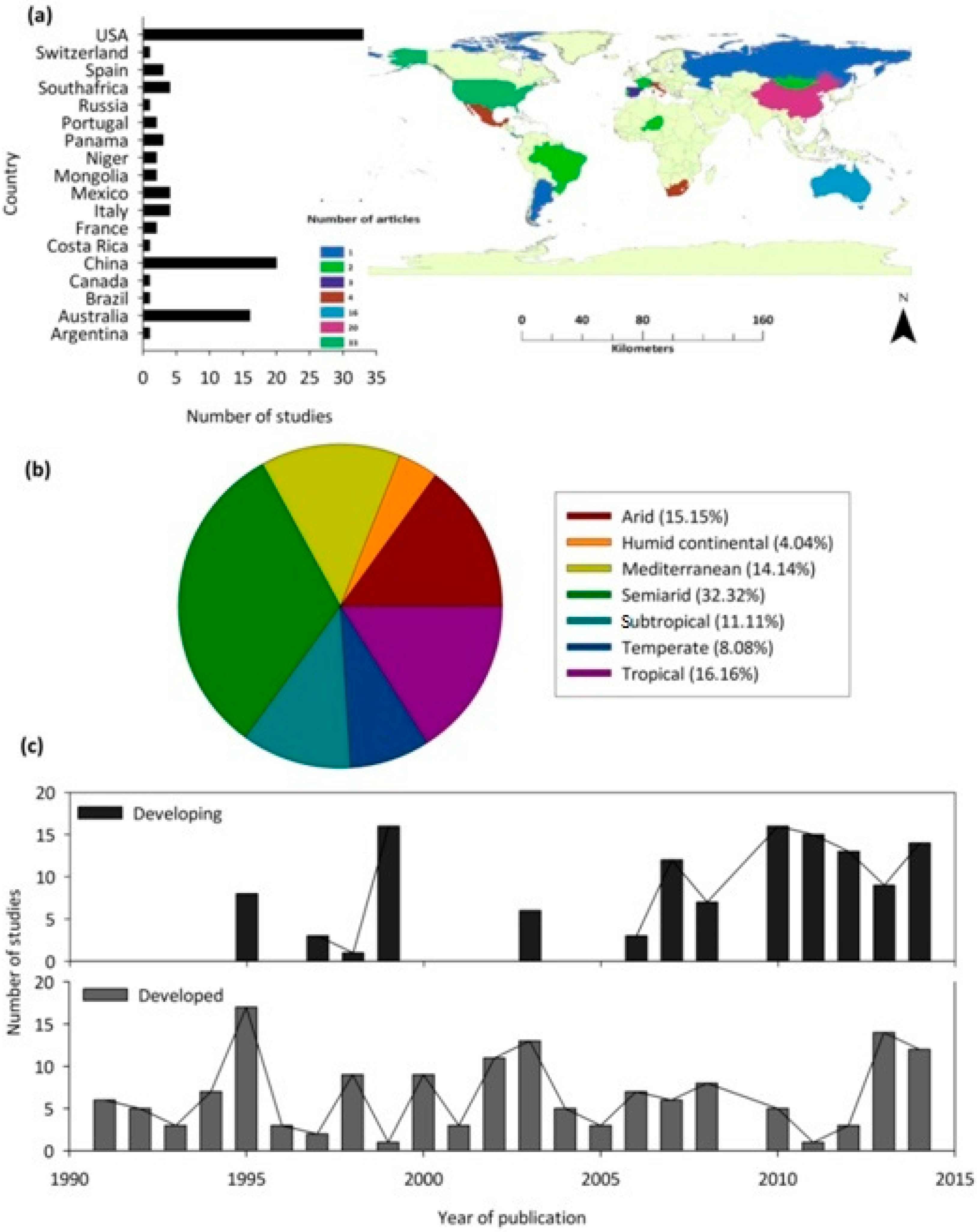
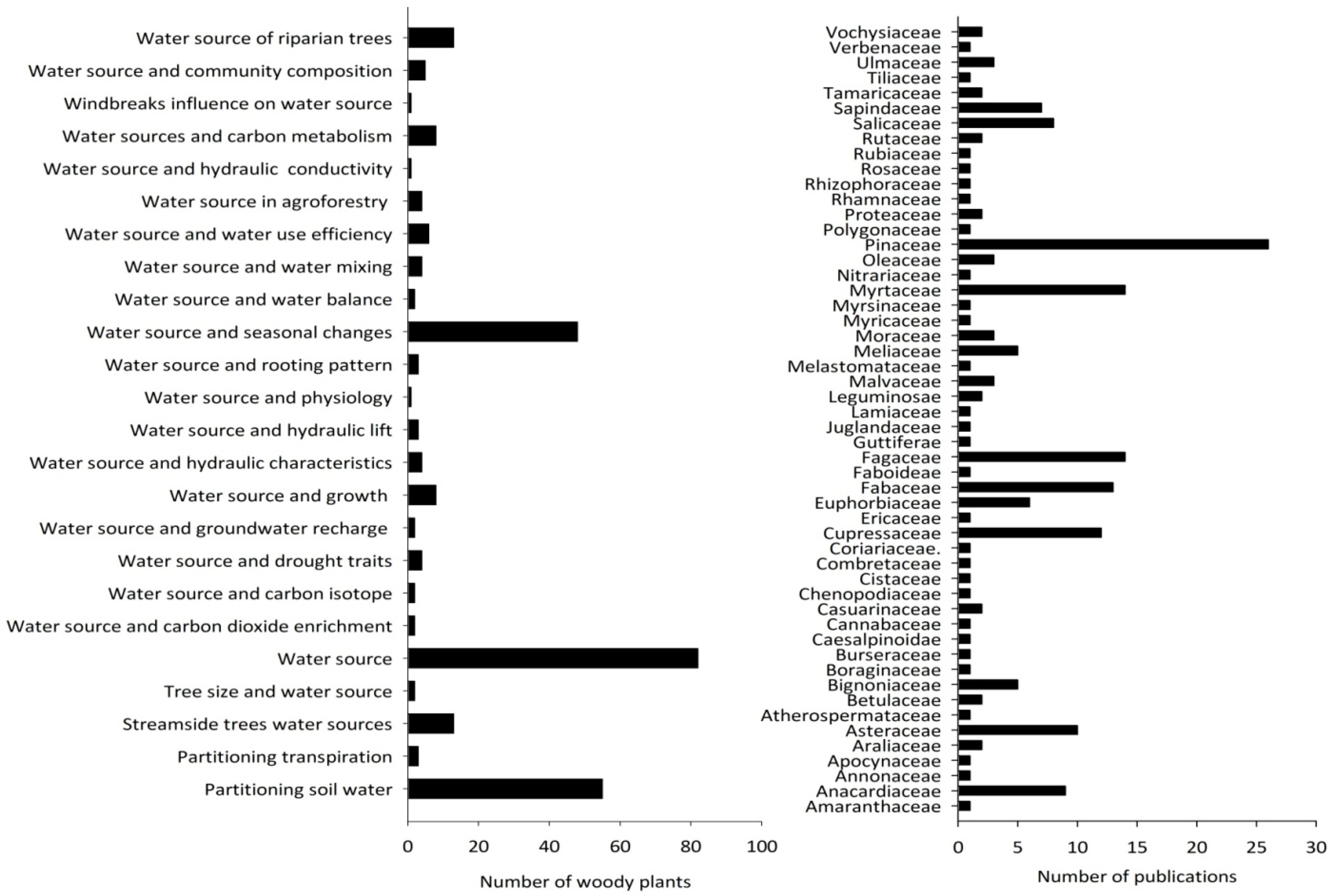
3.1. Publication Trends and Geographical Distribution of Research on Woody Plant Water Source
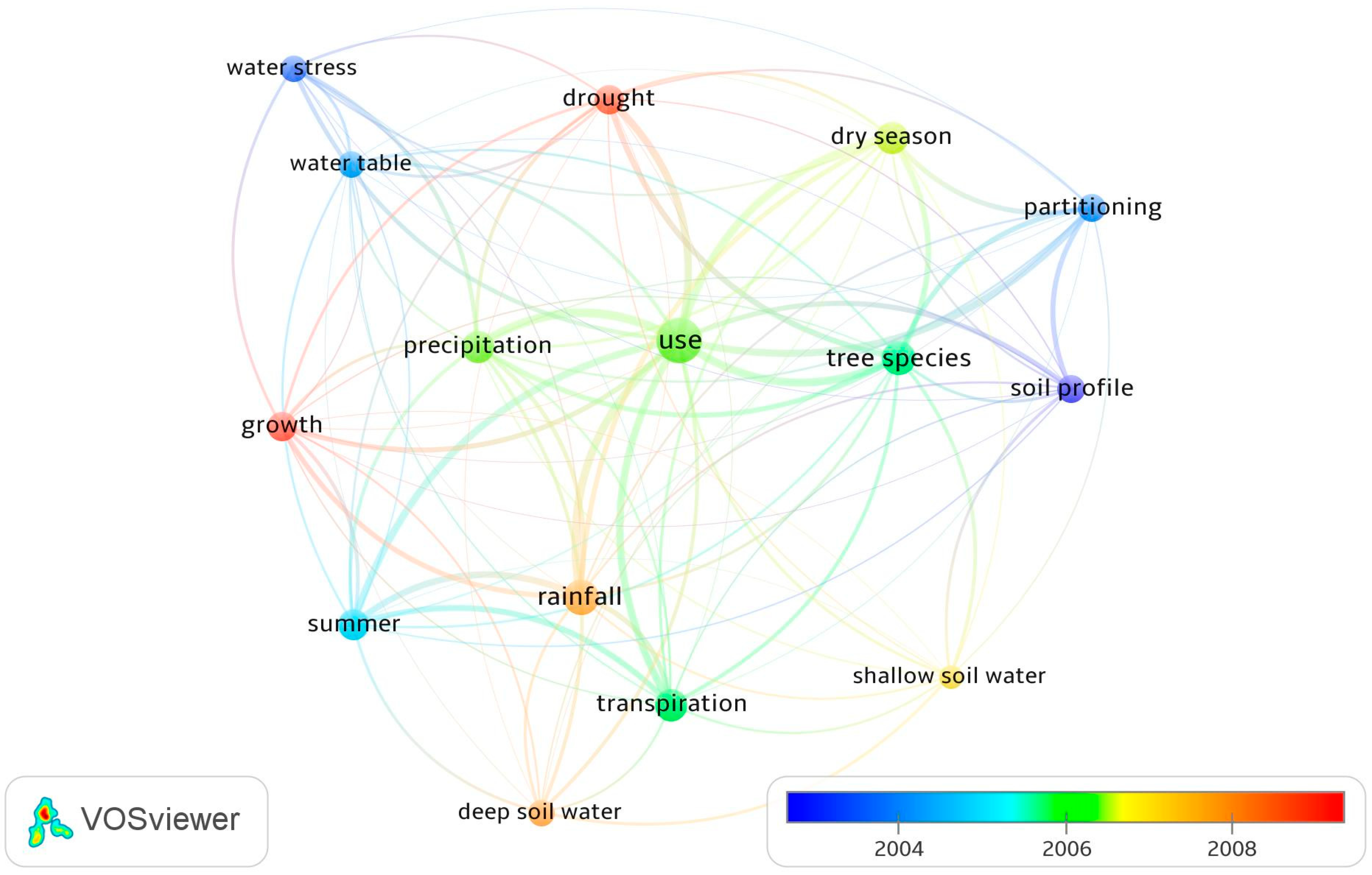
3.2. Research Focus on Woody Plant Species
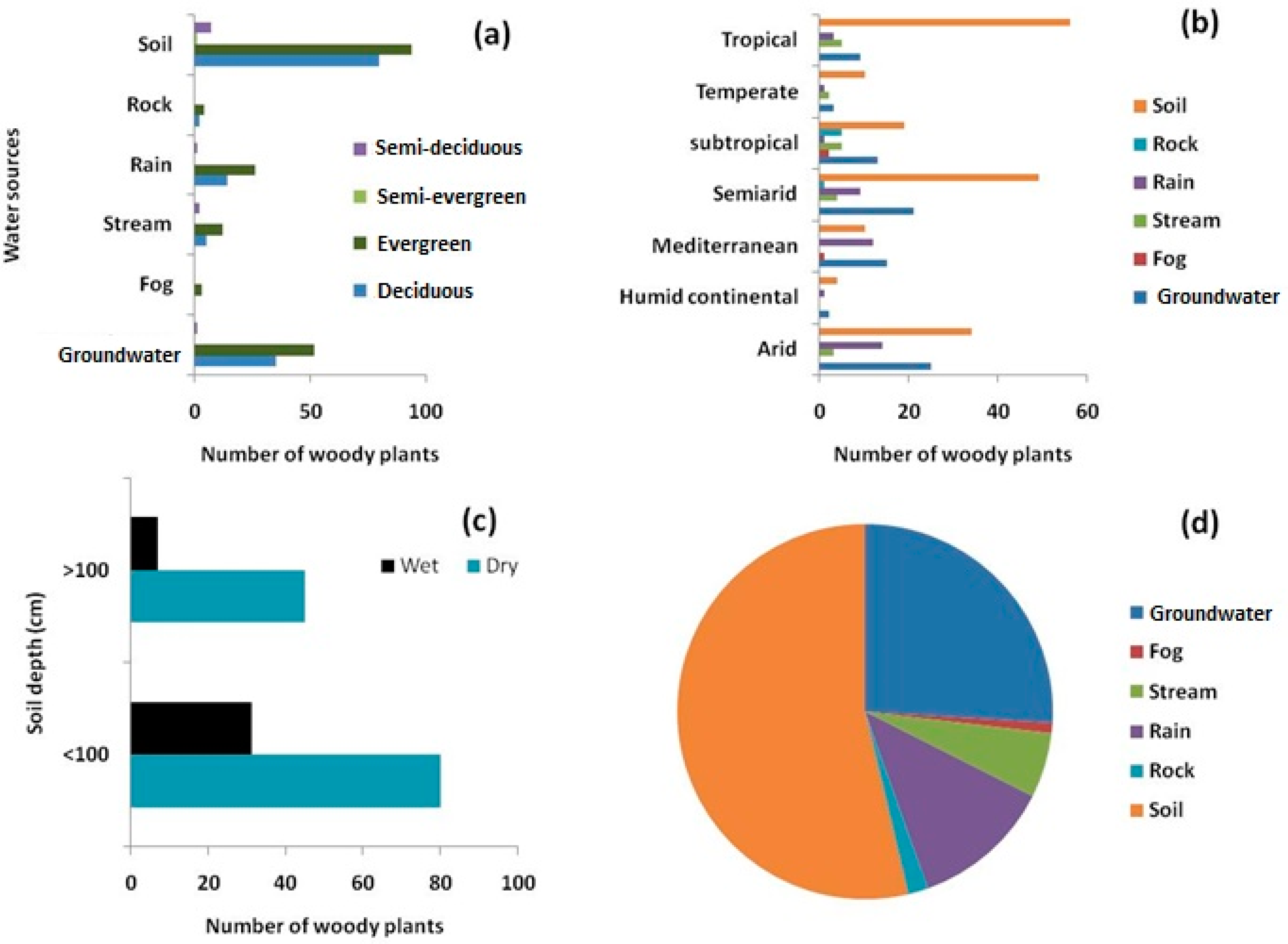
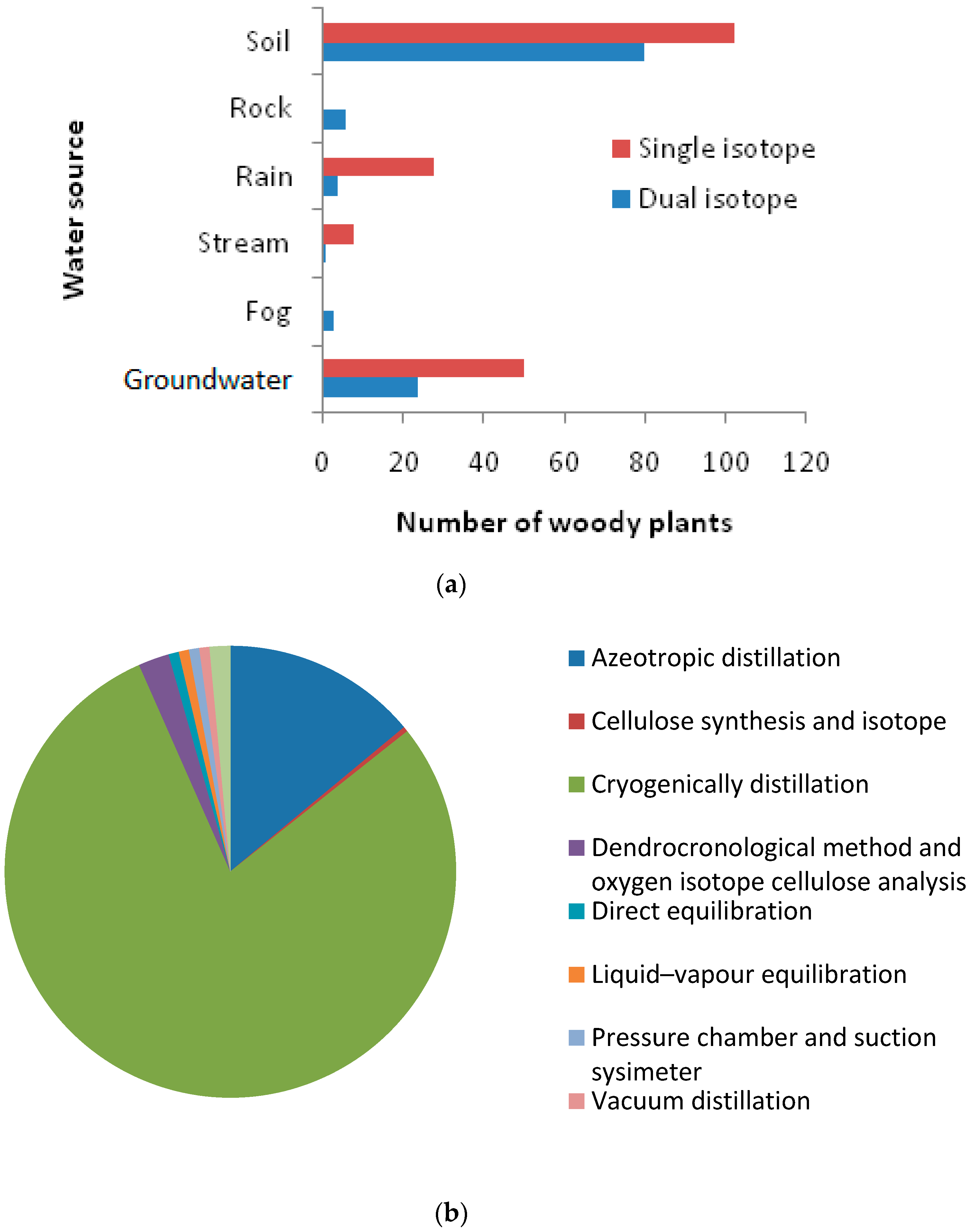
3.3. Woody Plant Water Sources and Their Variation due to Season, Climate, Leaf Phenology, and Method of Measurement
4. Discussion
4.1. Geographic Biases in Woody Species Water Source Research
4.2. Environmental Significance and Factors Influencing Plant Water Sources Study
4.3. Research Gaps and New Research Priorities
4.3.1. The Need for High-Frequency Sampling and Dual Isotope Approaches
4.3.2. Do Species with High Mortality Rates Source Their Water Mainly from Surface Layers?
4.3.3. During Droughts, Do Large and Small-Sized Trees Source Their Water from the Same or Different Soil Depths?
4.3.4. What Is the Relationship between Growth and the Depth at Which Species/Individuals Uptake Water?
4.3.5. Is the Assumption that Water-Stable Isotope Fractionation Does Not Occur During Water Uptake by Roots and Sap Transfer within the Tree Universally True?
5. Concluding Remarks and Recommendations
- There is a critical need to develop a single standard method to study plant water sources. This will lead to greater comparability of results from studies conducted across vegetation types and regions;
- Catchment-scale hydrological models should consider plant water sources for better predictions of stream flows; and
- Further research is required to better understand how plant water uptake strategies differ across vegetation types and climatic conditions, and how this understanding can be applied to the design of reforestation systems and understanding the likely impacts of climate change.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gazis, C.; Feng, X. A stable isotope study of soil water: Evidence for mixing and preferential flow paths. Geoderma 2004, 119, 97–111. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Ma, J.Y.; Sun, W.; Sun, J.H.; Duan, Z.H. A preliminary study of water use strategy of desert plants in Dunhuang, China. J. Arid Land 2015, 7, 73–81. [Google Scholar] [CrossRef]
- Noy-Meir, I. Desert Ecosystems: Environment and Producers. Annu. Ecol. Syst. 1973, 4, 25–51. [Google Scholar] [CrossRef]
- Webb, W.L.; Lauenroth, W.K.; Szarek, S.R.; Kinerson, R.S. Primary production and abiotic controls in forests, grasslands, and desert ecosystems of the United States. Ecology 1983, 64, 134–151. [Google Scholar] [CrossRef]
- Saleska, S.R.; Didan, K.; Da Rocha, H.R.; Huete, A.R.; Huete, A.R.; Huete, A. Amazon Forests Green-Up During 2005 Drought. Science 2007, 318, 612. [Google Scholar] [CrossRef]
- Anderson, L.O.; Malhi, Y.; Aragão, L.E.O.C.; Ladle, R.; Arai, E.; Barbier, N.; Phillips, O. Remote sensing detection of droughts in Amazonian forest canopies. New Phytol. 2010, 187, 733–750. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T., Qin, D., Plattner, G., Tignor, M., Allen, S., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Malhi, Y.; Aragao, L.E.O.C.; Galbraith, D.; Huntingford, C.; Fisher, R.; Zelazowski, P.; Sitch, S.; McSweeney, C.; Mei, P. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl. Acad. Sci. USA 2009, 106, 20610–20615. [Google Scholar] [CrossRef]
- Sternberg, L.D.S.L.; Swart, P.K. Utilization of Freshwater and Ocean Water by Coastal Plants of Southern Florida. Ecology 1987, 68, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- James, M.L.; Zedler, J.B. Dynamics of Wetland and Upland Subshrubs at the Salt Marsh-Coastal Sage Scrub Ecotone. Am. Midl. Nat. 2000, 143, 298–311. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Dawson, T.E. Water uptake by plants: Perspectives from stable isotope composition. Plantcell 1992, 15, 1073–1082. [Google Scholar] [CrossRef]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- Sternberg, L.D.S.L.; Yakir, D. The use of stable isotopes to study ecosystem gas exchange. Oecologia 2000, 123, 297–311. [Google Scholar]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Ann. Rev. Ecol. Sys. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Lin, G.; Sternberg, L.D.S.L. Hydrogen Isotopic Fractionation by Plant Roots during Water Uptake in Coastal Wetland Plants. In Stable Isotopes and Plant Carbon-water Relations; Elsevier BV: Amsterdam, The Netherlands, 1993; pp. 497–510. [Google Scholar]
- Brunel, J.-P.; Kennett-Smith, A.K.; Walker, G.R. Field validation of isotopic procedures for determining sources of water used by plants in a semi-arid environment. J. Hydrol. 1995, 167, 351–368. [Google Scholar] [CrossRef]
- Schwinning, S.; Starr, B.I.; Ehleringer, J.R. Dominant cold desert plants do not partition warm season precipitation by event size. Oecologia 2003, 136, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Querejeta, J.I.; Estrada-Medina, H.; Allen, M.F.; Jiménez-Osornio, J.J.; Ruenes, R. Utilization of bedrock water by Brosimum alicastrum trees growing on shallow soil atop limestone in a dry tropical climate. Plant Soil 2006, 287, 187–197. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Estrada-Medina, H.; Allen, M.F.; Jiménez-Osornio, J.J. Water source partitioning among trees growing on shallow karst soils in a seasonally dry tropical climate. Oecologia 2007, 152, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Asbjornsen, H.; Mora, G.; Helmers, M.J. Variation in water uptake dynamics among contrasting agricultural and native plant communities in the Midwestern U.S. Agric. Ecosyst. 2007, 121, 343–356. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Allen, M.F.; Santiago, L.S. Water relations of evergreen and drought-deciduous trees along a seasonally dry tropical forest chronosequence. Oecologia 2010, 164, 881–890. [Google Scholar] [CrossRef]
- Nie, Y.P.; Chen, H.S.; Wang, K.L.; Tan, W.; Deng, P.Y.; Yang, J. Seasonal water use patterns of woody species growing on the continuous dolostone outcrops and nearby thin soils in subtropical China. Plant Soil 2011, 341, 399–412. [Google Scholar] [CrossRef]
- Jackson, P.C.; Bustamante, M.; Franco, A.; Caldas, L.; Igler, E.; Causin, F.; Meinzer, F.C.; Goldstein, G.; Rundel, P.W. Partitioning of soil water among tree species in a Brazilian Cerrado ecosystem. Tree Physiol. 1999, 19, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Armas, C.; Pugnaire, F.I. And Hydraulic lift promotes selective root foraging in nutrient-rich soil patches. Funct. Plant Boil. 2012, 39, 804. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Phillips, S.L.; Schuster, W.S.F.; Sandquist, D.R. Differential utilization of summer rains by desert plants. Oecologia 1991, 88, 430–434. [Google Scholar] [CrossRef]
- Fazey, I.; Fischer, J.; Lindenmayer, D.B. Who does all the research in conservation biology? Biodiv. Conser. 2005, 14, 917–934. [Google Scholar] [CrossRef]
- Williams, D.G.; Ehleringer, J.R. Intra- and inter-specific variation for summer precipitation use in Pinyon–Juniper woodlands. Ecol. Monog. 2000, 70, 517–537. [Google Scholar]
- Dawson, T.E.; Ehleringer, J.R. Streamside trees that do not use streamside water. Nature 1991, 350, 335–337. [Google Scholar] [CrossRef]
- Meinzer, F.; Goldstein, G.; Holbrook, N.M.; Cavelier, J.; Wright, S.J.; Andrade, J.L. Partitioning of soil water among canopy trees in a seasonally dry tropical forest. Oecologia 1999, 121, 293. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E. Fog in the California redwood forest: Ecosystem inputs and use by plants. Oecologia 1998, 117, 476–485. [Google Scholar] [CrossRef]
- Mensforth, L.J.; Thorburn, P.J.; Tyerman, S.D.; Walker, G.R. Sources of water used by riparian Eucalyptus camaldulensis overlying highly saline groundwater. Oecologia 1994, 100, 21–28. [Google Scholar] [CrossRef]
- Dawson, T.E.; Pate, J.S. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia 1996, 107, 13–20. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Ehleringer, J.R.; Marshall, J.D. Differential uptake of summer precipitation and groundwater among co-occurring trees and shrubs in the southwestern United States. Plant Cell Environ. 1992, 15, 831–836. [Google Scholar] [CrossRef]
- Ehleringer, J.; Phillips, S. Ecophysiological factors contributing to the distributions of several Quercus species in the intermountain west. Ann. For. Sci. 1996, 53, 291–302. [Google Scholar] [CrossRef]
- Burgess, S.S.; A Adams, M.; Turner, N.C.; Ward, B. Characterisation of hydrogen isotope profiles in an agroforestry system: Implications for tracing water sources of trees. Agric. Water Manag. 2000, 45, 229–241. [Google Scholar] [CrossRef]
- Guojun, L.; Jinling, L.; Ximing, Z. Preliminary study of water sources for maintenance and water utilization strategies of Haloxylon ammodendron in the arid desert area of northwestern China. Peer. J. 2016. [Google Scholar] [CrossRef][Green Version]
- Engel, M.; Frentress, J.; Penna, D.; Andreoli, A.; Hecher, P.; van Meerveld, I.; Comiti, F. Ecohydrological dynamics of Alder trees in riparian zones: A tracer-based comparison study of two rivers in the Eastern Italian Alps. In Proceedings of the Workshop on Isotope-Based Studies of Water Partitioning and Plant-Soil Interactions in Forested and Agricultural Environments Villa Montepaldi, San Casciano in Val di Pesa, Florence, Italy, 27–29 September 2017. [Google Scholar]
- Barbeta, A.; Mejía-Chang, M.; Ogaya, R.; Voltas, J.; Dawson, T.E.; Peñuelas, J. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Glob. Chang. Biol. 2015, 21, 1213–1225. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Tohver, I.M.; Ray, D.; Moutinho, P.; Cardinot, G. Mortality of large trees and lianas following experimental drought in an amazon forest. Ecology 2007, 88, 2259–2269. [Google Scholar] [CrossRef]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger trees suffer most during drought in forests worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef]
- Lorimer, C.G.; Dahir, S.E.; Nordheim, E.V. Tree mortality rates and longevity in mature and old-growth hemlock-hardwood forests. J. Ecol. 2001, 89, 960–971. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fulé, P.Z.; E Harmon, M.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread Increase of Tree Mortality Rates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef]
- Peng, C.; Ma, Z.; Lei, X.; Zhu, Q.; Chen, H.; Wang, W.; Liu, S.; Li, W.; Fang, X.; Zhou, X. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Chang. 2011, 1, 467–471. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.; He, F. Half-century evidence from western Canada shows forest dynamics are primarily driven by competition followed by climate. Proc. Natl. Acad. Sci. USA 2015, 112, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.L.; Hart, S.C. Hydraulic lift: A potentially important ecosystem process. Trends Ecol. Evol. 1998, 13, 232–235. [Google Scholar] [CrossRef]
- Mueller, R.C.; Scudder, C.M.; Porter, M.E.; Trotter, R.T.; Gehring, C.A.; Whitham, T.G. Differential tree mortality in response to severe drought: Evidence for long-term vegetation shifts. J. Ecol. 2005, 93, 1085–1093. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; McGarvey, J.C.; Muller-Landau, H.C.; Park, J.Y.; Gonzalez-Akre, E.B.; Herrmann, V.; Bennett, A.C.; So, C.V.; Bourg, N.; Thompson, J.R.; et al. Size-related scaling of tree form and function in a mixed-age forest. Funct. Ecol. 2015, 29, 1587–1602. [Google Scholar] [CrossRef]
- Romero-Saltos, H.; Sternberg, L.D.S.L.; Moreira, M.Z.; Nepstad, D.C.; Sternberg, L.D.S.L. Rainfall exclusion in an eastern Amazonian forest alters soil water movement and depth of water uptake. Am. J. Bot. 2005, 92, 443–455. [Google Scholar] [CrossRef]
- Meißner, M.; Kohler, M.; Schwendenmann, L.; Holscher, D. Partitioning of soil water among canopy trees during a soil desiccation period in a temperate mixed forest. Biogeoscience 2012, 9, 3465–3474. [Google Scholar] [CrossRef]
- Brooks, R.; Barnard, R.; Coulombe, R.; McDonnell, J.J. Ecohydrologic separation of water between trees and streams in a Mediterranean climate. Nat. Geosci. 2010, 3, 100–104. [Google Scholar] [CrossRef]
- Ellsworth, P.Z.; Williams, D.G. Hydrogen isotope fractionation during water uptake by woody xerophytes. Plant Soil 2007, 291, 93–107. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Cernusak, L.A.; Liu, X.; Xiao, H.; Zhou, M.; Zhang, S. Significant difference in hydrogen isotope composition between xylem and tissue water in Populus euphratica. Plant Cell Environ. 2016, 39, 1848–1857. [Google Scholar] [CrossRef]
- McPherson, G.R.; Weltzin, J.F. Spatial and temporal soil moisture resource partitioning by trees and grasses in a temperate savanna, Arizona, USA. Oecologia 1997, 112, 156–164. [Google Scholar]
- Stratton, L.C.; Meinzer, F.; Goldstein, G. Temporal and spatial partitioning of water resources among eight woody species in a Hawaiian dry forest. Oecologia 2000, 124, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, J.; Comas, C.; Voltas, J.; Ferrio, J.P. Dynamics of competition over water in a mixed oak-pine Mediterranean forest: Spatio-temporal and physiological components. Forest Ecol. Mang. 2016, 382, 214–224. [Google Scholar] [CrossRef]
- Evaristo, J.; McDonnell, J.J.; Clemens, J. Plant source water apportionment using stable isotopes: A comparison of simple linear, two-compartment mixing model approaches. Hydrol. Proc. 2017, 31, 3750–3758. [Google Scholar] [CrossRef]
- Geris, J.; Tetzlaff, D.; McDonnell, J.; Anderson, J.; Paton, G.; Soulsby, C. Ecohydrological separation in wet, low energy northern environments? A preliminary assessment using different soil water extraction techniques. Hydrol. Proc. 2015, 29, 5139–5152. [Google Scholar] [CrossRef]
- Gómez, P.M.; Aguilera, M.; Pemán, J.; Pelegrín, E.G.; Ferrio, P.J. Uncoupling between soil and xylem water isotopic composition: how to discriminate mobile and tightly-bound water? Geophy. Res. Abs. 2014, 16, EGU2014-1340. [Google Scholar]
- Martín-Gómez, P.; Serrano, L.; Ferrio, J.P. Short-term dynamics of evaporative enrichment of xylem water in woody stems: implications for ecohydrology. Tree Phy. 2016. [Google Scholar] [CrossRef]
- Sohel, M.S.I. Spatial and Temporal Variation of Sources of Water Across Multiple Tropical Rainforest Trees. Ph.D. Thesis, The University of Queensland, Queensland, Australia, 2018. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohel, M.S.I.; Salam, M.A.; Herbohn, J. An Assessment of Woody Plant Water Source Studies from across the Globe: What Do We Know after 30 Years of Research and Where Do We Go from Here? Hydrology 2019, 6, 40. https://doi.org/10.3390/hydrology6020040
Sohel MSI, Salam MA, Herbohn J. An Assessment of Woody Plant Water Source Studies from across the Globe: What Do We Know after 30 Years of Research and Where Do We Go from Here? Hydrology. 2019; 6(2):40. https://doi.org/10.3390/hydrology6020040
Chicago/Turabian StyleSohel, Md. Shawkat I., Mohammed Abdus Salam, and John Herbohn. 2019. "An Assessment of Woody Plant Water Source Studies from across the Globe: What Do We Know after 30 Years of Research and Where Do We Go from Here?" Hydrology 6, no. 2: 40. https://doi.org/10.3390/hydrology6020040
APA StyleSohel, M. S. I., Salam, M. A., & Herbohn, J. (2019). An Assessment of Woody Plant Water Source Studies from across the Globe: What Do We Know after 30 Years of Research and Where Do We Go from Here? Hydrology, 6(2), 40. https://doi.org/10.3390/hydrology6020040




