Unlocking the Potential of Microbially Induced Calcium Carbonate Precipitation (MICP) for Hydrological Applications: A Review of Opportunities, Challenges, and Environmental Considerations
Abstract
1. Introduction
2. Bioremediation via MICP
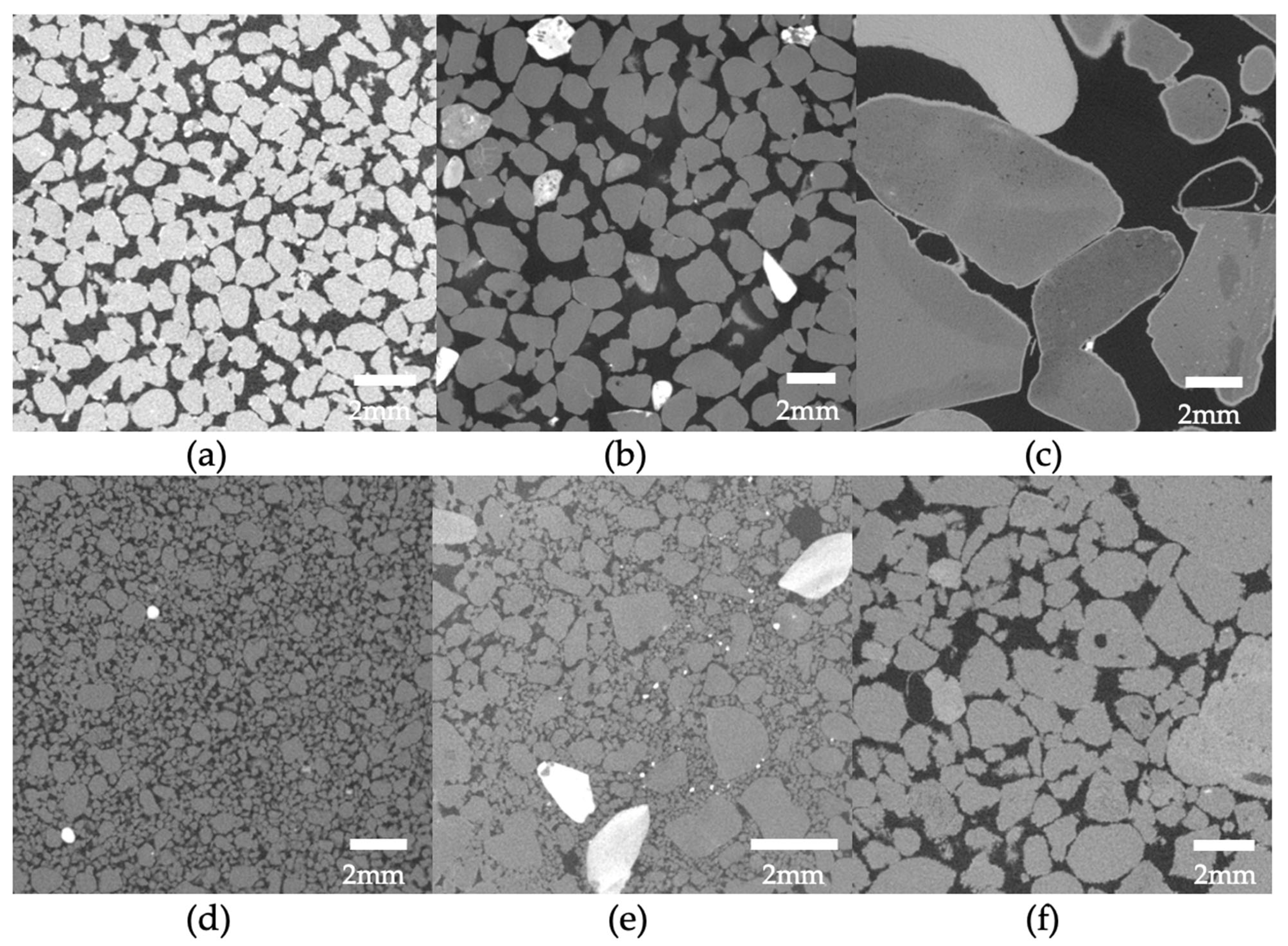
3. Mechanical and Hydraulic Properties Alteration
- (i)
- contact-cementing, in which the carbonate crystals precipitate on and around the contacts between the grains (see schematic in Figure 3a);
- (ii)
- grain coating, in which cement forms a uniform film around the grains (see schematic in Figure 3b); and
- (iii)
- matrix-supporting, in which the precipitate is identified within the granular network (see schematic in Figure 3c).
3.1. Strength Enchancement
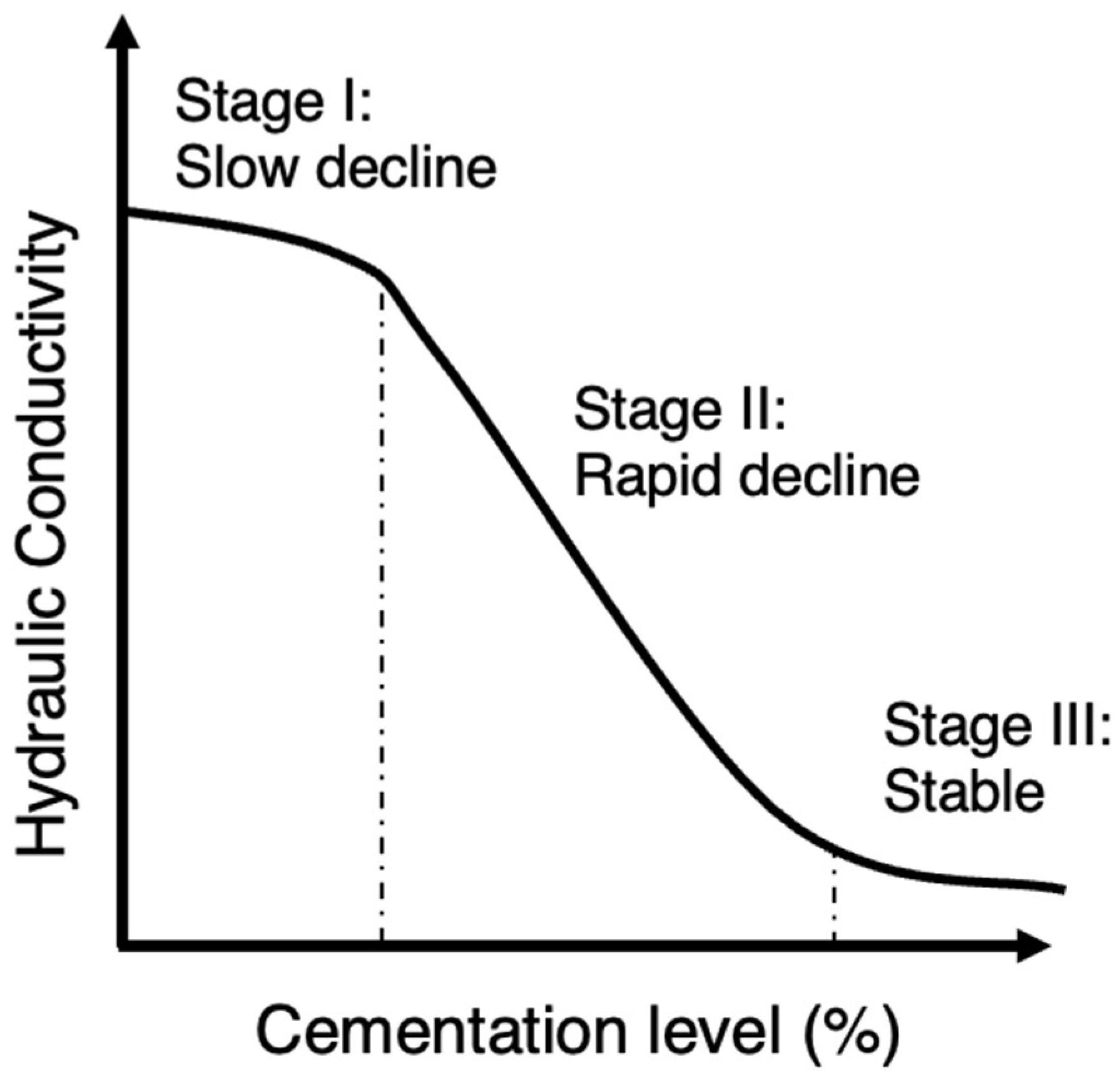
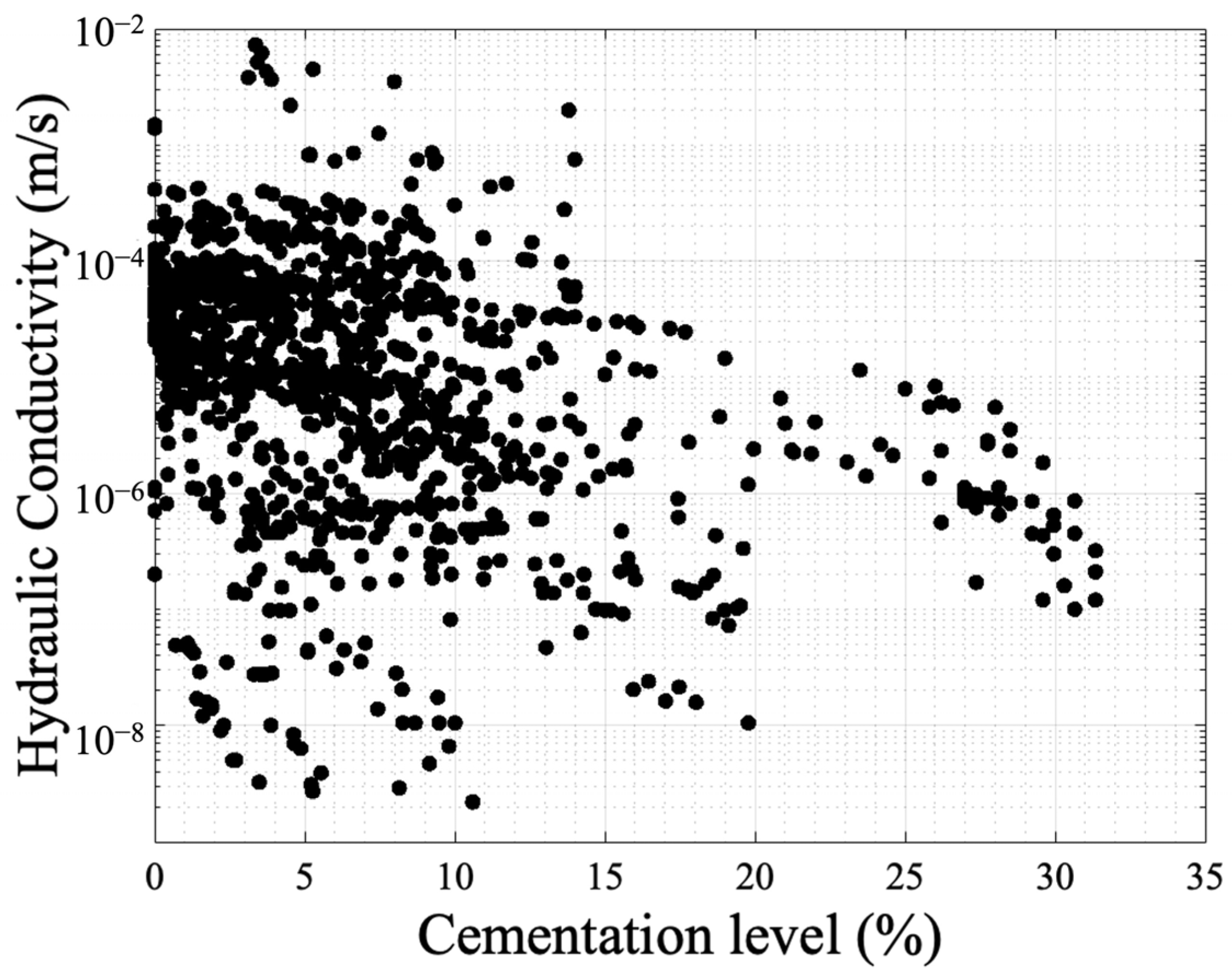
3.2. Hydraulic Conductivity
4. Applications of MICP to the Fields of Hydrology and Water Resources
4.1. Groundwater and Soil Remediation
- (a)
- Pump-and-treat schemes: extraction of groundwater through a well, surface treatment to remove contaminants, and injection of treated water back to the aquifer;
- (b)
- Permeable reactive barriers (PRBs) which are proffered in the presence of plumes: generation of subsurface walls which react when in contact with contaminants and, therefore, immobilize the contaminant by the processes described earlier (adsorption, precipitation, and microbial degradation);
- (c)
- In situ bioremediation: direct injection of bacteria and chemicals via a well into the contaminated region;
- (d)
- Remediation train: effective remediation often involves a combination of these techniques, known as a “remediation train”, to achieve the best results.
4.2. Applications of MICP Related to Hydraulic Conductivity Reduction
- (i)
- The efficiency of the process could vary considerably in dynamic environments where water levels, wave action, and tidal fluctuations constantly change;
- (ii)
- The metabolic activity of bacterial strains might be affected by variations in environmental conditions such as salinity, temperature, and nutrient availability, which can have an impact on the performance of MICP overall;
- (iii)
- The mechanical and hydraulic properties of the precipitating carbonate and the porous medium could be affected by the mechanical stresses from wave action and other adverse environmental conditions;
- (iv)
- The scaling-up challenge also is of great importance. The uniform distribution of bacteria, nutrients, and chemicals over larger areas, especially in heterogeneous coastal environments, may be difficult to achieve and monitor effectively.
4.3. Applications of MICP Related to Erosion Control (Gaining Cohesion)
4.4. Applications of MICP Related to Studies Involving Artificially Generated Porous Media
5. Further Challenges and Environmental Considerations of the Application of MICP in Hydrological Applications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially Induced Cementation to Control Sand Response to Undrained Shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Kawano, J.; Shimobayashi, N.; Kitamura, M.; Shinoda, K.; Aikawa, N. Formation Process of Calcium Carbonate from Highly Supersaturated Solution. J. Cryst. Growth 2002, 237–239, 419–423. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial Carbonate Precipitation in Construction Materials: A Review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hora, R.N.; Ahenkorah, I.; Beecham, S.; Karim, M.R.; Iqbal, A. State-of-the-Art Review of Microbial-Induced Calcite Precipitation and Its Sustainability in Engineering Applications. Sustainability 2020, 12, 6281. [Google Scholar] [CrossRef]
- Gollapudi, U.K.; Knutson, C.L.; Bang, S.S.; Islam, M. A New Method for Controlling Leaching through Permeable Channels. Chemosphere 1995, 30, 695–705. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological Precipitation of CaCO3. Soil. Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Dissertation, Murdoch University, Perth, WA, Australia, 2004. [Google Scholar]
- van Paassen, L. Biogrout: Ground Improvement by Microbially Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Medici, G.; West, L.J. Reply to Discussion on ‘Review of Groundwater Flow and Contaminant Transport Modelling Approaches for the Sherwood Sandstone Aquifer, UK; Insights from Analogous Successions Worldwide’ by Medici and West (QJEGH, 55, Qjegh2021-176). Q. J. Eng. Geol. Hydrogeol. 2022, 56, qjegh2022-097. [Google Scholar] [CrossRef]
- Burley, S.D. Patterns of Diagenesis in the Sherwood Sandstone Group (Triassic), United Kingdom. Clay Min. 1984, 19, 403–440. [Google Scholar] [CrossRef]
- Bella, G.; Barbero, M.; Barpi, F.; Borri-Brunetto, M.; Peila, D. An Innovative Bio-Engineering Retaining Structure for Supporting Unstable Soil. J. Rock. Mech. Geotech. Eng. 2017, 9, 247–259. [Google Scholar] [CrossRef][Green Version]
- Konstantinou, C.; Biscontin, G. Soil Enhancement via Microbially Induced Calcite Precipitation. In Proceedings of the 10th International Symposium on Geotechnical Aspects of Underground Construction in Soft Ground, Cambridge, UK, 27–29 June 2022; Taylor & Francis: Cambridge, UK, 2021; pp. 765–772. [Google Scholar]
- Jiang, N.-J.; Soga, K. The Applicability of Microbially Induced Calcite Precipitation (MICP) for Internal Erosion Control in Gravel–Sand Mixtures. Géotechnique 2017, 67, 42–55. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Soga, K.; Kuo, M. Microbially Induced Carbonate Precipitation for Seepage-Induced Internal Erosion Control in Sand–Clay Mixtures. J. Geotech. Geoenviron. Eng. 2017, 143, 04016100. [Google Scholar] [CrossRef]
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Erşan, Y.Ç.; Hernandez-Sanabria, E.; Boon, N.; De Belie, N. Enhanced Crack Closure Performance of Microbial Mortar through Nitrate Reduction. Cem. Concr. Compos. 2016, 70, 159–170. [Google Scholar] [CrossRef]
- Hata, T.; Saracho, A.C.; Haigh, S.K.; Yoneda, J.; Yamamoto, K. Microbial-Induced Carbonate Precipitation Applicability with the Methane Hydrate-Bearing Layer Microbe. J. Nat. Gas. Sci. Eng. 2020, 81, 103490. [Google Scholar] [CrossRef]
- Yu, X.; Rong, H. Seawater Based MICP Cements Two/One-Phase Cemented Sand Blocks. Appl. Ocean. Res. 2022, 118, 102972. [Google Scholar] [CrossRef]
- Cui, M.J.; Zheng, J.J.; Chu, J.; Wu, C.C.; Lai, H.J. Bio-Mediated Calcium Carbonate Precipitation and Its Effect on the Shear Behaviour of Calcareous Sand. Acta Geotech. 2021, 16, 1377–1389. [Google Scholar] [CrossRef]
- Lin, W.; Gao, Y.; Lin, W.; Zhuo, Z.; Wu, W.; Cheng, X. Seawater-Based Bio-Cementation of Natural Sea Sand via Microbially Induced Carbonate Precipitation. Environ. Technol. Innov. 2023, 29, 103010. [Google Scholar] [CrossRef]
- Wang, Y.; Konstantinou, C.; Tang, S.; Chen, H. Applications of Microbial-Induced Carbonate Precipitation: A State-of-the-Art Review. Biogeotechnics 2023, 1, 100008. [Google Scholar] [CrossRef]
- Jiang, N.J.; Liu, R.; Du, Y.J.; Bi, Y.Z. Microbial Induced Carbonate Precipitation for Immobilizing Pb Contaminants: Toxic Effects on Bacterial Activity and Immobilization Efficiency. Sci. Total Environ. 2019, 672, 722–731. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization Based Remediation of As(III) Contaminated Soil by Sporosarcina Ginsengisoli. J. Hazard. Mater. 2012, 201–202, 178–184. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, S.; Roh, Y. Effect of Divalent Cations (Cu, Zn, Pb, Cd, and Sr) on Microbially Induced Calcium Carbonate Precipitation and Mineralogical Properties. Front. Microbiol. 2021, 12, 646748. [Google Scholar] [CrossRef]
- He, J.; Chen, X.; Zhang, Q.; Achal, V. More Effective Immobilization of Divalent Lead than Hexavalent Chromium through Carbonate Mineralization by Staphylococcus Epidermidis HJ2. Int. Biodeterior. Biodegrad. 2019, 140, 67–71. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.; Guo, H. Heavy Metal Removal by Biomineralization of Urease Producing Bacteria Isolated from Soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Peng, D.; Qiao, S.; Luo, Y.; Ma, H.; Zhang, L.; Hou, S.; Wu, B.; Xu, H. Performance of Microbial Induced Carbonate Precipitation for Immobilizing Cd in Water and Soil. J. Hazard. Mater. 2020, 400, 123116. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Niu, Q.; Cheng, L.; Jiang, J.; Yu, Y.Y.; Chu, J.; Achal, V.; You, T. Ca-Mediated Alleviation of Cd2+ Induced Toxicity and Improved Cd2+ Biomineralization by Sporosarcina Pasteurii. Sci. Total Environ. 2021, 787, 147627. [Google Scholar] [CrossRef]
- Rajasekar, A.; Wilkinson, S.; Moy, C.K.S. MICP as a Potential Sustainable Technique to Treat or Entrap Contaminants in the Natural Environment: A Review. Environ. Sci. Ecotechnol. 2021, 6, 100096. [Google Scholar] [CrossRef]
- Torres-Aravena, Á.E.; Duarte-Nass, C.; Azócar, L.; Mella-Herrera, R.; Rivas, M.; Jeison, D. Can Microbially Induced Calcite Precipitation (MICP) through a Ureolytic Pathway Be Successfully Applied for Removing Heavy Metals from Wastewaters? Crystals 2018, 8, 438. [Google Scholar] [CrossRef]
- Qian, X.; Fang, C.; Huang, M.; Achal, V. Characterization of Fungal-Mediated Carbonate Precipitation in the Biomineralization of Chromate and Lead from an Aqueous Solution and Soil. J. Clean. Prod. 2017, 164, 198–208. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Kamala-Kannan, S.; Selvankumar, T.; Srinivasan, P.; Kim, H. In-Vitro Bio-Mineralization of Arsenic and Lead from Aqueous Solution and Soil by Wood Rot Fungus, Trichoderma Sp. Ecotoxicol. Environ. Saf. 2019, 174, 699–705. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Xu, R.; Qin, H.; Liu, H.; Zhao, K. Heavy Metal Bioremediation Using Microbially Induced Carbonate Precipitation: Key Factors and Enhancement Strategies. Front. Microbiol. 2023, 14, 1116970. [Google Scholar] [CrossRef]
- Li, M.; Cheng, H.; Guo, X.; Yang, Z. Biomineralization of Carbonate by Terrabacter Tumescens for Heavy Metal Removal and Biogrouting Applications. J. Environ. Eng. 2015, 142, C4015005. [Google Scholar] [CrossRef]
- Fujita, Y.; Taylor, J.L.; Wendt, L.M.; Reed, D.W.; Smith, R.W. Evaluating the Potential of Native Ureolytic Microbes to Remediate a 90Sr Contaminated Environment. Environ. Sci. Technol. 2010, 44, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- Lauchnor, E.G.; Schultz, L.N.; Bugni, S.; Mitchell, A.C.; Cunningham, A.B.; Gerlach, R. Bacterially Induced Calcium Carbonate Precipitation and Strontium Coprecipitation in a Porous Media Flow System. Environ. Sci. Technol. 2013, 47, 1557–1564. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Jiang, X.; Zhao, D.; Liu, X.; Zhou, J.; He, Z.; Zheng, C.; Pan, X. Study on Soil Physical Structure after the Bioremediation of Pb Pollution Using Microbial-Induced Carbonate Precipitation Methodology. J. Hazard. Mater. 2021, 411, 125103. [Google Scholar] [CrossRef] [PubMed]
- Mwandira, W.; Nakashima, K.; Kawasaki, S. Bioremediation of Lead-Contaminated Mine Waste by Pararhodobacter Sp. Based on the Microbially Induced Calcium Carbonate Precipitation Technique and Its Effects on Strength of Coarse and Fine Grained Sand. Ecol. Eng. 2017, 109, 57–64. [Google Scholar] [CrossRef]
- Chen, X.; Achal, V. Biostimulation of Carbonate Precipitation Process in Soil for Copper Immobilization. J. Hazard. Mater. 2019, 368, 705–713. [Google Scholar] [CrossRef]
- Kang, C.H.; Kwon, Y.J.; So, J.S. Bioremediation of Heavy Metals by Using Bacterial Mixtures. Ecol. Eng. 2016, 89, 64–69. [Google Scholar] [CrossRef]
- Qiao, S.; Zeng, G.; Wang, X.; Dai, C.; Sheng, M.; Chen, Q.; Xu, F.; Xu, H. Multiple Heavy Metals Immobilization Based on Microbially Induced Carbonate Precipitation by Ureolytic Bacteria and the Precipitation Patterns Exploration. Chemosphere 2021, 274, 129661. [Google Scholar] [CrossRef]
- Mugwar, A.J.; Harbottle, M.J. Toxicity Effects on Metal Sequestration by Microbially-Induced Carbonate Precipitation. J. Hazard. Mater. 2016, 314, 237–248. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.; Horn, E.J.; Randall, D.G. Copper Mine Tailings Valorization Using Microbial Induced Calcium Carbonate Precipitation. J. Environ. Manag. 2021, 298, 113440. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, C.; Wang, Y.; Biscontin, G.; Soga, K. The Role of Bacterial Urease Activity on the Uniformity of Carbonate Precipitation Profiles of Bio-Treated Coarse Sand Specimens. Sci. Rep. 2021, 11, 6161. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, Z.; Du, Y.; Lyu, Q.; Yang, Z.; Liu, Y.; Yan, Z. Microbiologically Induced Calcite Precipitation Technology for Mineralizing Lead and Cadmium in Landfill Leachate. J. Environ. Manag. 2021, 296, 113199. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Xue, Q.; Chen, Z.; Zhou, Y.; Poon, C.S. Bacterial-Induced Mineralization (BIM) for Soil Solidification and Heavy Metal Stabilization: A Critical Review. Sci. Total Environ. 2020, 746, 140967. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, Q.; Song, W.; Zhang, D.; Ahati, J.; Pan, X.; Al-Misned, F.A.; Mortuza, M.G. Calcifying Cyanobacterium (Nostoc Calcicola) Reactor as a Promising Way to Remove Cadmium from Water. Ecol. Eng. 2015, 81, 107–114. [Google Scholar] [CrossRef]
- Gadd, G.M. Bioremedial Potential of Microbial Mechanisms of Metal Mobilization and Immobilization. Curr. Opin. Biotechnol. 2000, 11, 271–279. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K. Effect of Chemical Treatment Used in MICP on Engineering Properties of Cemented Soils. Geotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors Affecting Efficiency of Microbially Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2012, 138, 992–1001. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Mujah, D. Influence of Key Environmental Conditions on Microbially Induced Cementation for Soil Stabilization. J. Geotech. Geoenviron. Eng. 2017, 143, 04016083. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-Mediated Soil Improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Mortensen, B.M.; Haber, M.J.; Dejong, J.T.; Caslake, L.F.; Nelson, D.C. Effects of Environmental Factors on Microbial Induced Calcium Carbonate Precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Rebata-Landa, V. Microbial Activity in Sediments: Effects on Soil Behavior; Georgia Institute of Technology: Atlanta, GA, USA, 2007. [Google Scholar]
- Lin, H.; Suleiman, M.T.; Brown, D.G.; Kavazanjian, E. Mechanical Behavior of Sands Treated by Microbially Induced Carbonate Precipitation. J. Geotech. Geoenviron. Eng. 2015, 142, 04015066. [Google Scholar] [CrossRef]
- Montoya, B.M.; DeJong, J.T. Stress-Strain Behavior of Sands Cemented by Microbially Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2015, 141, 04015019. [Google Scholar] [CrossRef]
- Gao, Y.; Hang, L.; He, J.; Chu, J. Mechanical Behaviour of Biocemented Sands at Various Treatment Levels and Relative Densities. Acta Geotech. 2019, 14, 697–707. [Google Scholar] [CrossRef]
- Feng, K.; Montoya, B.M. Influence of Confinement and Cementation Level on the Behavior of Microbial-Induced Calcite Precipitated Sands under Monotonic Drained Loading. J. Geotech. Geoenviron. Eng. 2015, 142, 04015057. [Google Scholar] [CrossRef]
- Cui, M.J.; Zheng, J.J.; Zhang, R.J.; Lai, H.J.; Zhang, J. Influence of Cementation Level on the Strength Behaviour of Bio-Cemented Sand. Acta Geotech. 2017, 12, 971–986. [Google Scholar] [CrossRef]
- Konstantinou, C.; Biscontin, G.; Logothetis, F. Tensile Strength of Artificially Cemented Sandstone Generated via Microbially Induced Carbonate Precipitation. Materials 2021, 14, 4735. [Google Scholar] [CrossRef]
- Xiao, Y.; He, X.; Evans, T.M.; Stuedlein, A.W.; Liu, H. Unconfined Compressive and Splitting Tensile Strength of Basalt Fiber-Reinforced Biocemented Sand. J. Geotech. Geoenviron. Eng. 2019, 145, 04019048. [Google Scholar] [CrossRef]
- Pakbaz, M.S.; Kolahi, A.; Ghezelbash, G. Assessment of Microbial Induced Calcite Precipitation (MICP) in Fine Sand Using Native Microbes under Both Aerobic and Anaerobic Conditions. KSCE J. Civ. Eng. 2022, 26, 1051–1065. [Google Scholar] [CrossRef]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiol. J. 2017, 34, 524–537. [Google Scholar] [CrossRef]
- Pan, X.; Chu, J.; Yang, Y.; Cheng, L. A New Biogrouting Method for Fine to Coarse Sand. Acta Geotech. 2019, 15, 1–16. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Bio-Cementation of Sandy Soil Using Microbially Induced Carbonate Precipitation for Marine Environments. Geotechnique 2014, 64, 1010–1013. [Google Scholar] [CrossRef]
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. A Microfluidic Chip and Its Use in Characterising the Particle-Scale Behaviour of Microbial-Induced Calcium Carbonate Precipitation (MICP). Geotechnique 2019, 69, 1086–1094. [Google Scholar] [CrossRef]
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. Microscale Visualization of Microbial-Induced Calcium Carbonate Precipitation Processes. J. Geotech. Geoenviron. Eng. 2019, 145, 04019045. [Google Scholar] [CrossRef]
- Wang, Y. Microbial-Induced Calcium Carbonate Precipitation: From Micro to Macro Scale. Ph.D. Dissertation, University of Cambridge, Cambridge, UK, 2018. [Google Scholar]
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. Effects of Bacterial Density on Growth Rate and Characteristics of Microbial-Induced CaCO3 Precipitates: A Particle-Scale Experimental Study. ASCE J. Geotech. Geoenviron. Eng. 2021, 147, 04021036. [Google Scholar] [CrossRef]
- Wang, Y.; Konstantinou, C.; Soga, K.; Biscontin, G.; Kabla, A.J. Use of Microfluidic Experiments to Optimize MICP Treatment Protocols for Effective Strength Enhancement of MICP-Treated Sandy Soils. Acta Geotech. 2022, 17, 3817–3838. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Microscale Investigations of Temperature-Dependent Microbially Induced Carbonate Precipitation (MICP) in the Temperature Range 4–50 °C. Acta Geotech. 2023, 18, 2239–2261. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Konstantinou, C. Strength Behavior of Temperature-Dependent Microbially Induced Carbonate Precipitation (MICP)-Treated Soil. J. Geotech. Geoenviron. Eng. 2023. [Google Scholar]
- Wang, Y.; Wang, Y.; Konstantinou, C. Effects of Environmental Temperature on the Effectiveness of Microbially Induced Carbonate Precipitation. ESS Open Archive. 2022. [CrossRef]
- Konstantinou, C.; Wang, Y.; Biscontin, G. A Systematic Study on the Influence of Grain Characteristics on Hydraulic and Mechanical Performance of MICP-Treated Porous Media. Transp. Porous Media 2023, 147, 305–330. [Google Scholar] [CrossRef]
- Terzis, D.; Laloui, L. 3-D Micro-Architecture and Mechanical Response of Soil Cemented via Microbial-Induced Calcite Precipitation. Sci. Rep. 2018, 8, 1416. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Asce, M.; Stuedlein, A.W.; Asce, M.; Ran, J.; Evans, T.M.; Asce, A.M.; Cheng, L.; Liu, H.; Paassen, L.A.V.; et al. Effect of Particle Shape on Strength and Stiffness of Biocemented Glass Beads. J. Geotech. Geoenviron. Eng. 2019, 145, 06019016. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R.; Shahin, M.A. Cementation of Sand Soil by Microbially Induced Calcite Precipitation at Various Degrees of Saturation. Can. Geotech. J. 2013, 50, 81–90. [Google Scholar] [CrossRef]
- Dawoud, O. The Applicability of Microbially Induced Calcite Precipitation (MICP) for Soil Treatment. Ph.D. Dissertation, University of Cambridge, Cambridge, UK, 2015. [Google Scholar]
- van Paassen, L.A.; Ghose, R.; van der Linden, T.J.M.; van der Star, W.R.L.; van Loosdrecht, M.C.M. Quantifying Biomediated Ground Improvement by Ureolysis: Large-Scale Biogrout Experiment. J. Geotech. Geoenviron. Eng. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Konstantinou, C. Hydraulic Fracturing of Artificially Generated Soft Sandstones. Ph.D. Dissertation, University of Cambridge, Cambridge, UK, 2021. [Google Scholar]
- Yasuhara, H.; Hayashi, K.; Okamura, M. Evolution in mechanical and hydraulic properties of calcite-cemented sand mediated by biocatalyst. In Geo-Frontiers 2011: Advances in Geotechnical Engineering; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 3984–3992. [Google Scholar]
- Martinez, B.C.; DeJong, J.T.; Ginn, T.R.; Montoya, B.M.; Barkouki, T.H.; Hunt, C.; Tanyu, B.; Major, D. Experimental Optimization of Microbial-Induced Carbonate Precipitation for Soil Improvement. J. Geotech. Geoenviron. Eng. 2013, 139, 587–598. [Google Scholar] [CrossRef]
- Zamani, A.; Montoya, B.; Gabr, M.A. Investigating the Challenges of in Situ Delivery of MICP in Fine Grain Sands and Silty Sand. Can. Geotech. J. 2019, 56, 1889–1900. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J. Experimental Study on the Permeability of Microbial-Solidified Calcareous Sand Based on MICP. Appl. Sci. 2022, 12, 11447. [Google Scholar] [CrossRef]
- Choi, S.G.; Hoang, T.; Park, S.S. Undrained Behavior of Microbially Induced Calcite Precipitated Sand with Polyvinyl Alcohol Fiber. Appl. Sci. 2019, 9, 1214. [Google Scholar] [CrossRef]
- Choi, S.-G.; Wu, S.; Chu, J. Biocementation for Sand Using an Eggshell as Calcium Source. J. Geotech. Geoenviron. Eng. 2016, 142, 06016010. [Google Scholar] [CrossRef]
- Gomez, M.G.; Anderson, C.M.; Dejong, J.T.; Nelson, D.C.; Lau, X.H. Stimulating In-Situ Soil Bacteria for Bio-Cementation of Sands. In Proceedings of the Geo-Congress, Atlanta, GA, USA, 23–26 February 2014; pp. 1674–1682. [Google Scholar]
- Dawoud, O. Modification of Hydraulic Conductivity of Sandy Soil Using Seawater and Alkaline Solutions. IOP Conf. Ser. Mater. Sci. Eng. 2020, 800, 012011. [Google Scholar] [CrossRef]
- Dawoud, O.; Chen, C.Y.; Soga, K. Microbial induced calcite precipitation for geotechnical and environmental applications. In New Frontiers in Geotechnical Engineering; American Society of Civil Engineers: Reston, VA, USA, 2014; pp. 11–18. [Google Scholar]
- Song, C.; Song, C.; Chen, Y.; Wang, J. Plugging High-Permeability Zones of Oil Reservoirs by Microbially Mediated Calcium Carbonate Precipitation. ACS Omega 2020, 5, 14376–14383. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.G.; Chang, I.; Lee, M.; Lee, J.H.; Han, J.T.; Kwon, T.H. Review on Geotechnical Engineering Properties of Sands Treated by Microbially Induced Calcium Carbonate Precipitation (MICP) and Biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Eryürük, K. Effect of Cell Density on Decrease in Hydraulic Conductivity by Microbial Calcite Precipitation. AMB Express 2022, 12, 104. [Google Scholar] [CrossRef]
- Duo, L.; Kan-liang, T.; Hui-li, Z.; Yu-yao, W.; Kang-yi, N.; Shi-can, Z. Experimental Investigation of Solidifying Desert Aeolian Sand Using Microbially Induced Calcite Precipitation. Constr. Build. Mater. 2018, 172, 251–262. [Google Scholar] [CrossRef]
- Kadhim, F.J.; Zheng, J.-J. Influences of Calcium Sources and Type of Sand on Microbial Induced Carbonate Precipitation. Int. J. Adv. Eng. Technol. 2017, 10, 20–29. [Google Scholar]
- Akoğuz, H.; Çelik, S.; Bariş, Ö. The Effects of Different Sources of Calcium in Improvement of Soils by Microbially Induced Calcite Precipitation (MICP). Sigma J. Eng. Nat. Sci. 2019, 37, 953–965. [Google Scholar]
- Dekuyer, A.; Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Calcium Carbonate Induced Precipitation for Soil Improvement by Urea Hydrolysing Bacteria. In Proceedings of the Advances in Civil, Environmental, and Materials Research (ACEM’ 12), Seoul, South Korea, 26–29 August 2012. [Google Scholar]
- Rowshanbakht, K.; Khamehchiyan, M.; Sajedi, R.H.; Nikudel, M.R. Effect of Injected Bacterial Suspension Volume and Relative Density on Carbonate Precipitation Resulting from Microbial Treatment. Ecol. Eng. 2016, 89, 49–55. [Google Scholar] [CrossRef]
- Tian, K.; Wu, Y.; Zhang, H.; Li, D.; Nie, K.; Zhang, S. Increasing Wind Erosion Resistance of Aeolian Sandy Soil by Microbially Induced Calcium Carbonate Precipitation. Land Degrad. Dev. 2018, 29, 4271–4281. [Google Scholar] [CrossRef]
- Song, C.; Elsworth, D.; Zhi, S.; Wang, C. The Influence of Particle Morphology on Microbially Induced CaCO3 Clogging in Granular Media. Mar. Georesources Geotechnol. 2021, 39, 74–81. [Google Scholar] [CrossRef]
- Panda, M.N.; Lake, L.W. A Physical Model of Cementation and Its Effects on Single-Phase Permeability. Am. Assoc. Pet. Geol. Bull. 1995, 79, 431–443. [Google Scholar] [CrossRef]
- Lin, H.; Suleiman, M.T.; Brown, D.G. Investigation of Pore-Scale CaCO3 Distributions and Their Effects on Stiffness and Permeability of Sands Treated by Microbially Induced Carbonate Precipitation (MICP). Soils Found. 2020, 60, 944–961. [Google Scholar] [CrossRef]
- Song, C.; Elsworth, D.; Jia, Y.; Lin, J. Permeable Rock Matrix Sealed with Microbially-Induced Calcium Carbonate Precipitation: Evolutions of Mechanical Behaviors and Associated Microstructure. Eng. Geol. 2022, 304, 106697. [Google Scholar] [CrossRef]
- Wang, X.; Nackenhorst, U. A Coupled Bio-Chemo-Hydraulic Model to Predict Porosity and Permeability Reduction during Microbially Induced Calcite Precipitation. Adv. Water Resour. 2020, 139, 103563. [Google Scholar] [CrossRef]
- Montoya, B.M.; Safavizadeh, S.; Gabr, M.A. Enhancement of Coal Ash Compressibility Parameters Using Microbial-Induced Carbonate Precipitation. J. Geotech. Geoenviron. Eng. 2019, 145, 04019018. [Google Scholar] [CrossRef]
- Phang, I.R.K.; Wong, K.S.; Chan, Y.S.; Lau, S.Y. Effect of Microbial-Induced Calcite Precipitation towards Strength and Permeability of Peat. Bull. Eng. Geol. Environ. 2022, 81, 314. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, Z.; Fan, C.; Shen, W.; Wang, Q.; Liu, P. Comparative Mechanical Behaviors of Four Fiber-Reinforced Sand Cemented by Microbially Induced Carbonate Precipitation. Bull. Eng. Geol. Environ. 2020, 79, 3075–3086. [Google Scholar] [CrossRef]
- Tian, K.; Wang, X.; Zhang, S.; Zhang, H.; Zhang, F.; Yang, A. Effect of Reactant Injection Rate on Solidifying Aeolian Sand via Microbially Induced Calcite Precipitation. J. Mater. Civ. Eng. 2020, 32, 04020291. [Google Scholar] [CrossRef]
- Yu, X.; He, Z.; Li, X. Bio-Cement-Modified Construction Materials and Their Performances. Environ. Sci. Pollut. Res. 2022, 29, 11219–11231. [Google Scholar] [CrossRef]
- Sharma, M.; Satyam, N.; Reddy, K.R. Hybrid Bacteria Mediated Cemented Sand: Microcharacterization, Permeability, Strength, Shear Wave Velocity, Stress-Strain, and Durability. Int. J. Damage Mech. 2021, 30, 618–645. [Google Scholar] [CrossRef]
- Yang, B.; Li, H.; Li, H.; Ge, N.; Ma, G.; Zhang, H.; Zhang, X.; Zhuang, L. Experimental Investigation on the Mechanical and Hydraulic Properties of Urease Stabilized Fine Sand for Fully Permeable Pavement. Int. J. Transp. Sci. Technol. 2022, 11, 60–71. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, J.; Xiao, Y.; Liu, H.; Cheng, L. Seepage Control in Sand Using Bioslurry. Constr. Build. Mater. 2019, 212, 342–349. [Google Scholar] [CrossRef]
- Fang, X.; Yang, Y.; Chen, Z.; Liu, H.; Xiao, Y.; Shen, C. Influence of Fiber Content and Length on Engineering Properties of MICP-Treated Coral Sand. Geomicrobiol. J. 2020, 37, 582–594. [Google Scholar] [CrossRef]
- Choi, S.G.; Chu, J.; Brown, R.C.; Wang, K.; Wen, Z. Sustainable Biocement Production via Microbially Induced Calcium Carbonate Precipitation: Use of Limestone and Acetic Acid Derived from Pyrolysis of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2017, 5, 5183–5190. [Google Scholar] [CrossRef]
- Ma, G.; He, X.; Jiang, X.; Liu, H.; Chu, J.; Xiao, Y. Strength and Permeability of Bentonite-Assisted Biocemented Coarse Sand. Can. Geotech. J. 2021, 58, 969–981. [Google Scholar] [CrossRef]
- Wen, K.; Li, Y.; Liu, S.; Bu, C.; Li, L. Development of an Improved Immersing Method to Enhance Microbial Induced Calcite Precipitation Treated Sandy Soil through Multiple Treatments in Low Cementation Media Concentration. Geotech. Geol. Eng. 2019, 37, 1015–1027. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Factors Affecting Improvement in Engineering Properties of Residual Soil through Microbial-Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2014, 140, 04014006. [Google Scholar] [CrossRef]
- Safavizadeh, S.; Montoya, B.M.; Gabr, M.A. Effect of Microbial Induced Calcium Carbonate Precipitation on Compressibility and Hydraulic Conductivity of Fly Ash. In Proceedings of the IFCEE, Orlando, FL, USA, 5–10 March 2018; pp. 69–79. [Google Scholar]
- Rajasekar, A.; Moy, C.K.S.; Wilkinson, S.; Sekar, R. Microbially Induced Calcite Precipitation Performance of Multiple Landfill Indigenous Bacteria Compared to a Commercially Available Bacteria in Porous Media. PLoS ONE 2021, 16, e0254676. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Fang, H.; Guo, C.; Zhang, B.; Wang, F. Bentonite-Assisted Microbial-Induced Carbonate Precipitation for Coarse Soil Improvement. Bull. Eng. Geol. Environ. 2021, 80, 5623–5632. [Google Scholar] [CrossRef]
- Jawad, F.; Zheng, J.-J. Improving Poorly Graded Fine Sand with Microbial Induced Calcite Precipitation. Br. J. Appl. Sci. Technol. 2016, 17, 1–9. [Google Scholar] [CrossRef]
- Montoya, B.M.; Do, J.; Gabr, M.M. Erodibility of Microbial Induced Carbonate Precipitation-Stabilized Sand under Submerged Impinging Jet. In Proceedings of the IFCEE, Orlando, FL, USA, 5–10 March 2018; pp. 19–28. [Google Scholar]
- Niu, J.G.; Liang, S.H.; Gong, X.; Feng, D.L.; Luo, Q.Z.; Dai, J. Experimental Study on the Effect of Grouting Interval on Microbial Induced Calcium Carbonate Precipitation. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012071. [Google Scholar] [CrossRef]
- Gong, X.; Niu, J.; Liang, S.; Feng, D.; Luo, Q. Environmental Effect of Grouting Batches on Microbial-Induced Calcite Precipitation. Ekoloji Derg. 2019, 28, 929–936. [Google Scholar]
- Choi, S.G.; Wang, K.; Chu, J. Properties of Biocemented, Fiber Reinforced Sand. Constr. Build. Mater. 2016, 120, 623–629. [Google Scholar] [CrossRef]
- Yasuhara, H.; Neupane, D.; Hayashi, K.; Okamura, M. Experiments and Predictions of Physical Properties of Sand Cemented by Enzymatically-Induced Carbonate Precipitation. Soils Found. 2012, 52, 539–549. [Google Scholar] [CrossRef]
- Sidik, W.S.; Canakci, H.; Kilic, I.H.; Celik, F. Applicability of Biocementation for Organic Soil and Its Effect on Permeability. Geomech. Eng. 2014, 7, 649–663. [Google Scholar] [CrossRef]
- Zamani, A.; Asce, S.M.; Montoya, B.M.; Asce, M. Shearing and Hydraulic Behavior of MICP Treated Silty Sand. In Proceedings of the Geotechnical Frontiers 2017, Orlando, FL, USA, 12–15 March 2017; pp. 290–299. [Google Scholar]
- Stabnikov, V.; Jian, C.; Ivanov, V.; Li, Y. Halotolerant, Alkaliphilic Urease-Producing Bacteria from Different Climate Zones and Their Application for Biocementation of Sand. World J. Microbiol. Biotechnol. 2013, 29, 1453–1460. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Naik, S.N.; Khare, S.K. Harnessing the Bio-Mineralization Ability of Urease Producing Serratia Marcescens and Enterobacter Cloacae EMB19 for Remediation of Heavy Metal Cadmium (II). J. Environ. Manag. 2018, 215, 143–152. [Google Scholar] [CrossRef]
- Maity, J.P.; Chen, G.S.; Huang, Y.H.; Sun, A.C.; Chen, C.Y. Ecofriendly Heavy Metal Stabilization: Microbial Induced Mineral Precipitation (MIMP) and Biomineralization for Heavy Metals within the Contaminated Soil by Indigenous Bacteria. Geomicrobiol. J. 2019, 36, 612–623. [Google Scholar] [CrossRef]
- Jalilvand, N.; Akhgar, A.; Alikhani, H.A.; Rahmani, H.A.; Rejali, F. Removal of Heavy Metals Zinc, Lead, and Cadmium by Biomineralization of Urease-Producing Bacteria Isolated from Iranian Mine Calcareous Soils. J. Soil Sci. Plant Nutr. 2020, 20, 206–219. [Google Scholar] [CrossRef]
- Kang, C.H.; Oh, S.J.; Shin, Y.J.; Han, S.H.; Nam, I.H.; So, J.S. Bioremediation of Lead by Ureolytic Bacteria Isolated from Soil at Abandoned Metal Mines in South Korea. Ecol. Eng. 2015, 74, 402–407. [Google Scholar] [CrossRef]
- Kang, C.H.; Han, S.H.; Shin, Y.; Oh, S.J.; So, J.S. Bioremediation of Cd by Microbially Induced Calcite Precipitation. Appl. Biochem. Biotechnol. 2014, 172, 2907–2915. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Remediation of Copper-Contaminated Soil by Kocuria Flava CR1, Based on Microbially Induced Calcite Precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Yang, J.; Pan, X.; Zhao, C.; Mou, S.; Achal, V.; Al-Misned, F.A.; Mortuza, M.G.; Gadd, G.M. Bioimmobilization of Heavy Metals in Acidic Copper Mine Tailings Soil. Geomicrobiol. J. 2016, 33, 261–266. [Google Scholar] [CrossRef]
- Phillips, A.J.; Cunningham, A.B.; Gerlach, R.; Hiebert, R.; Hwang, C.; Lomans, B.P.; Westrich, J.; Mantilla, C.; Kirksey, J.; Esposito, R.; et al. Fracture Sealing with Microbially-Induced Calcium Carbonate Precipitation: A Field Study. Environ. Sci. Technol. 2016, 50, 4111–4117. [Google Scholar] [CrossRef]
- Cunningham, A.B.; Phillips, A.J.; Troyer, E.; Lauchnor, E.; Hiebert, R.; Gerlach, R.; Spangler, L. Wellbore Leakage Mitigation Using Engineered Biomineralization. Energy Procedia 2014, 63, 4612–4619. [Google Scholar] [CrossRef]
- Tobler, D.J.; Minto, J.M.; El Mountassir, G.; Lunn, R.J.; Phoenix, V.R. Microscale Analysis of Fractured Rock Sealed With Microbially Induced CaCO3 Precipitation: Influence on Hydraulic and Mechanical Performance. Water Resour. Res. 2018, 54, 8295–8308. [Google Scholar] [CrossRef]
- Hataf, N.; Baharifard, A. Reducing Soil Permeability Using Microbial Induced Carbonate Precipitation (MICP) Method: A Case Study of Shiraz Landfill Soil. Geomicrobiol. J. 2020, 37, 147–158. [Google Scholar] [CrossRef]
- Stabnikov, V.; Ivanov, V.; Chu, J. Sealing of Sand Using Spraying and Percolating Biogrouts for the Construction of Model Aquaculture Pond in Arid Desert. Int. Aquat. Res. 2016, 8, 207–216. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Stabnikov, V.; Li, B. Microbial Method for Construction of an Aquaculture Pond in Sand. Geotechnique 2013, 63, 871–875. [Google Scholar] [CrossRef]
- Cuthbert, M.O.; McMillan, L.A.; Handley-Sidhu, S.; Riley, M.S.; Tobler, D.J.; Phoenix, V.R. A Field and Modeling Study of Fractured Rock Permeability Reduction Using Microbially Induced Calcite Precipitation. Environ. Sci. Technol. 2013, 47, 13637–13643. [Google Scholar] [CrossRef]
- Liu, B.; Tang, C.S.; Pan, X.H.; Zhu, C.; Cheng, Y.J.; Xu, J.J.; Shi, B. Potential Drought Mitigation Through Microbial Induced Calcite Precipitation-MICP. Water Resour. Res. 2021, 57, e2020WR029434. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, X.; Chu, J.; He, J. Microbially Induced Calcite Precipitation for Seepage Control in Sandy Soil. Geomicrobiol. J. 2019, 36, 366–375. [Google Scholar] [CrossRef]
- Laabidi, E.; Bouhlila, R. A New Technique of Seawater Intrusion Control: Development of Geochemical Cutoff Wall. Environ. Sci. Pollut. Res. 2021, 28, 41794–41806. [Google Scholar] [CrossRef]
- Abdoulhalik, A.; Ahmed, A.; Hamill, G.A. A New Physical Barrier System for Seawater Intrusion Control. J. Hydrol. 2017, 549, 416–427. [Google Scholar] [CrossRef]
- Wang, Y.N.; Li, S.K.; Li, Z.Y.; Garg, A. Exploring the Application of the MICP Technique for the Suppression of Erosion in Granite Residual Soil in Shantou Using a Rainfall Erosion Simulator. Acta Geotech. 2023, 18, 3273–3285. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R.; Wang, H.; Xia, J. Surface Rainfall Erosion Resistance and Freeze-Thaw Durability of Bio-Cemented and Polymer-Modified Loess Slopes. J. Environ. Manag. 2022, 301, 113883. [Google Scholar] [CrossRef]
- Liu, S.; Du, K.; Wen, K.; Armwood-Gordon, C.; Li, L. Influence of Rainfall-Induced Erosion on the Stability of Sandy Slopes Treated by MICP. Adv. Civ. Eng. 2022, 2022, 5105206. [Google Scholar] [CrossRef]
- Kou, H.; Wu, C.Z.; Ni, P.P.; Jang, B.A. Assessment of Erosion Resistance of Biocemented Sandy Slope Subjected to Wave Actions. Appl. Ocean. Res. 2020, 105, 102401. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Stuedlein, A.W.; Evans, T.M.; Xiao, Y. Strength, Stiffness, and Microstructure Characteristics of Biocemented Calcareous Sand. Can. Geotech. J. 2019, 56, 1502–1513. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Jin, Y.; Li, Q.; Xu, J. Application of Microbially Induced Calcium Carbonate Precipitation (MICP) in Sand Embankments for Scouring/Erosion Control. Mar. Georesources Geotechnol. 2021, 39, 1459–1471. [Google Scholar] [CrossRef]
- Shahin, M.A.; Jamieson, K.; Cheng, L. Microbial-Induced Carbonate Precipitation for Coastal Erosion Mitigation of Sandy Slopes. Geotech. Lett. 2020, 10, 211–215. [Google Scholar] [CrossRef]
- Chek, A.; Crowley, R.; Ellis, T.N.; Durnin, M.; Wingender, B. Evaluation of Factors Affecting Erodibility Improvement for MICP-Treated Beach Sand. J. Geotech. Geoenviron. Eng. 2021, 147, 04021001. [Google Scholar] [CrossRef]
- Salifu, E.; MacLachlan, E.; Iyer, K.R.; Knapp, C.W.; Tarantino, A. Application of Microbially Induced Calcite Precipitation in Erosion Mitigation and Stabilisation of Sandy Soil Foreshore Slopes: A Preliminary Investigation. Eng. Geol. 2016, 201, 96–105. [Google Scholar] [CrossRef]
- Konstantinou, C.; Stoianov, I. A Comparative Study of Statistical and Machine Learning Methods to Infer Causes of Pipe Breaks in Water Supply Networks. Urban Water J. 2020, 17, 534–548. [Google Scholar] [CrossRef]
- Konstantinou, C.; Biscontin, G.; Jiang, N.-J.; Soga, K. Application of Microbially Induced Carbonate Precipitation (MICP) to Form Bio-Cemented Artificial Sandstone. J. Rock Mech. Geotech. Eng. 2021, 13, 579–592. [Google Scholar] [CrossRef]
- Gago, P.A.; Konstantinou, C.; Biscontin, G.; King, P. Stress Inhomogeneity Effect on Fluid-Induced Fracture Behavior into Weakly Consolidated Granular Systems. Phys. Rev. E 2020, 102, 040901. [Google Scholar] [CrossRef]
- Gago, P.A.; Raeini, A.Q.; King, P. A Spatially Resolved Fluid-Solid Interaction Model for Dense Granular Packs/Soft-Sand. Adv. Water Resour. 2020, 136, 103454. [Google Scholar] [CrossRef]
- Konstantinou, C.; Kandasami, R.K.; Wilkes, C.; Biscontin, G. Fluid Injection Under Differential Confinement. Transp. Porous Media 2021, 139, 627–650. [Google Scholar] [CrossRef]
- Germanovich, L.N.; Hurt, R.S.; Huang, H. Hydraulic Fracturing in Saturated Cohesionless Materials. In Proceedings of the AGU Fall Meeting, San Francisco, CA, USA, 9–14 December 2007. [Google Scholar]
- Golovin, E.; Jasarevic, H.; Chudnovsky, A.; Dudley, J.W.; Wong, G.K. Observation and Characterization of Hydraulic Fracture in Cohesionless Sand. In Proceedings of the 44th US Rock Mechanics Symposium and 5th US-Canada Rock Mechanics Symposium, Salt Lake City, UT, USA, 27–30 June 2010. [Google Scholar]
- Jasarevic, H.; Golovin, E.; Chudnovsky, A.; Dudley, J.W.; Wong, G.K. Observation and Modeling of Hydraulic Fracture Initiation in Cohesionless Sand. In Proceedings of the 44th US Rock Mechanics Symposium and 5th US-Canada Rock Mechanics Symposium, Salt Lake City, UT, USA, 27–30 June 2010. [Google Scholar]
- Kandasami, R.; Konstantinou, C.; Biscontin, G. Development of a Fracture Capture Simulator to Quantify the Instability Evolution in Porous Medium. arXiv 2023, arXiv:2304.00207. [Google Scholar] [CrossRef]
- Choi, S.G.; Hoang, T.; Alleman, E.J.; Chu, J. Splitting Tensile Strength of Fiber-Reinforced and Biocemented Sand. J. Mater. Civ. Eng. 2019, 31, 06019007. [Google Scholar] [CrossRef]
- Gago, P.A.; Konstantinou, C.; Biscontin, G.; King, P. A Numerical Characterisation of Unconfined Strength of Weakly Consolidated Granular Packs and Its Effect on Fluid-Driven Fracture Behaviour. Rock Mech. Rock Eng. 2022, 55, 4565–4575. [Google Scholar] [CrossRef]
- Konstantinou, C.; Biscontin, G. Experimental Investigation of the Effects of Porosity, Hydraulic Conductivity, Strength, and Flow Rate on Fluid Flow in Weakly Cemented Bio-Treated Sands. Hydrology 2022, 9, 190. [Google Scholar] [CrossRef]
- Konstantinou, C.; Kandasami, R.K.; Biscontin, G.; Papanastasiou, P. Fluid Injection through Artificially Reconstituted Bio-Cemented Sands. Geomech. Energy Environ. 2023, 34, 100466. [Google Scholar] [CrossRef]
- Konstantinou, C.; Biscontin, G.; Papanastasiou, P. Interpretation of Fluid Injection Experiments in Poorly Consolidated Sands. In Proceedings of the 56th U.S. Rock Mechanics/Geomechanics Symposium, Santa Fe, NM, USA, 26–29 June 2022. [Google Scholar]
- Cheng, L.; Cord-Ruwisch, R. Upscaling Effects of Soil Improvement by Microbially Induced Calcite Precipitation by Surface Percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef]
- Saneiyan, S.; Ntarlagiannis, D.; Ohan, J.; Lee, J.; Colwell, F.; Burns, S. Induced Polarization as a Monitoring Tool for In-Situ Microbial Induced Carbonate Precipitation (MICP) Processes. Ecol. Eng. 2019, 127, 36–47. [Google Scholar] [CrossRef]
- Harran, R.; Terzis, D.; Laloui, L. Mechanics, Modeling, and Upscaling of Biocemented Soils: A Review of Breakthroughs and Challenges. Int. J. Geomech. 2023, 23, 03123004. [Google Scholar] [CrossRef]
- De Jong, J.T.; Martinez, B.C.; Mortensen, B.M.; Nelson, D.C.; Waller, J.T.; Weil, M.H.; Ginn, T.R.; Weathers, T.; Barkouki, T.; Fujita, Y.; et al. Upscaling of Bio-Mediated Soil Improvement. In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering, Alexandria, Egypt, 5–9 October 2009. [Google Scholar]
- Zhang, K.; Tang, C.S.; Jiang, N.J.; Pan, X.H.; Liu, B.; Wang, Y.J.; Shi, B. Microbial-induced Carbonate Precipitation (MICP) Technology: A Review on the Fundamentals and Engineering Applications. Environ. Earth Sci. 2023, 82, 229. [Google Scholar] [CrossRef]
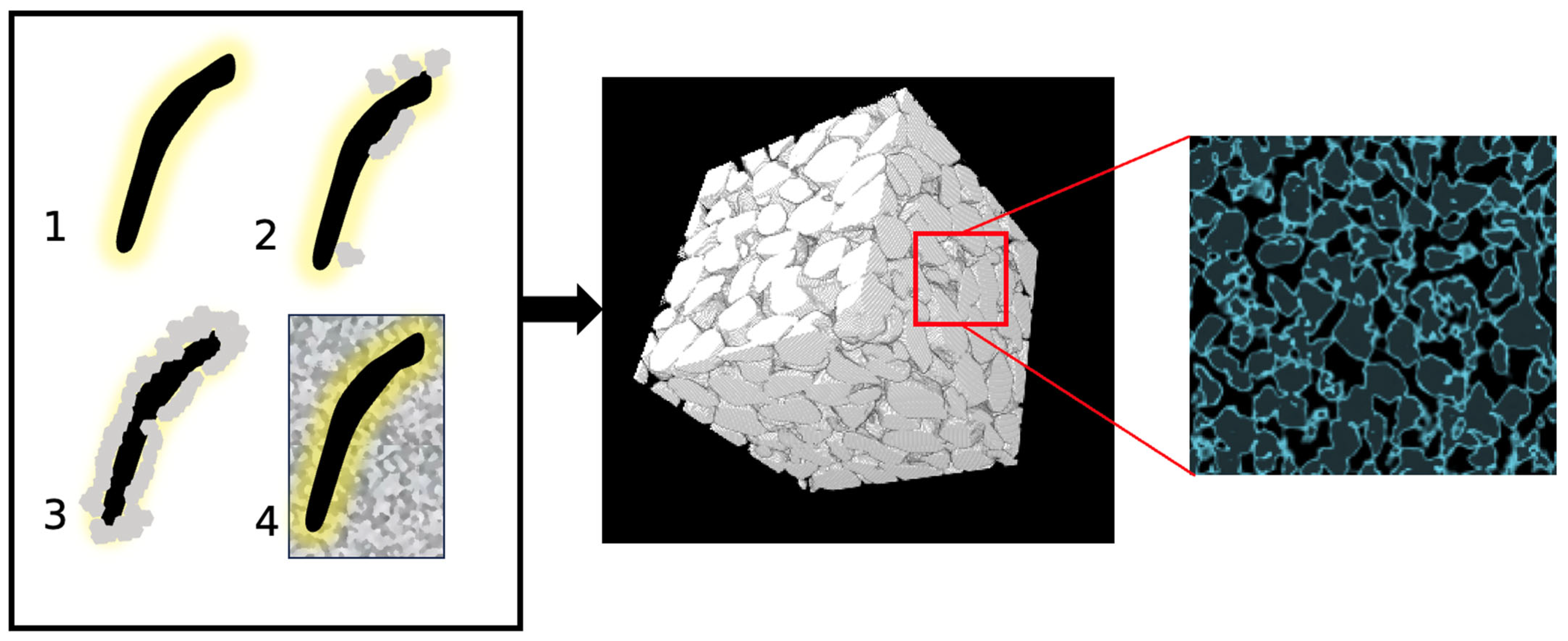

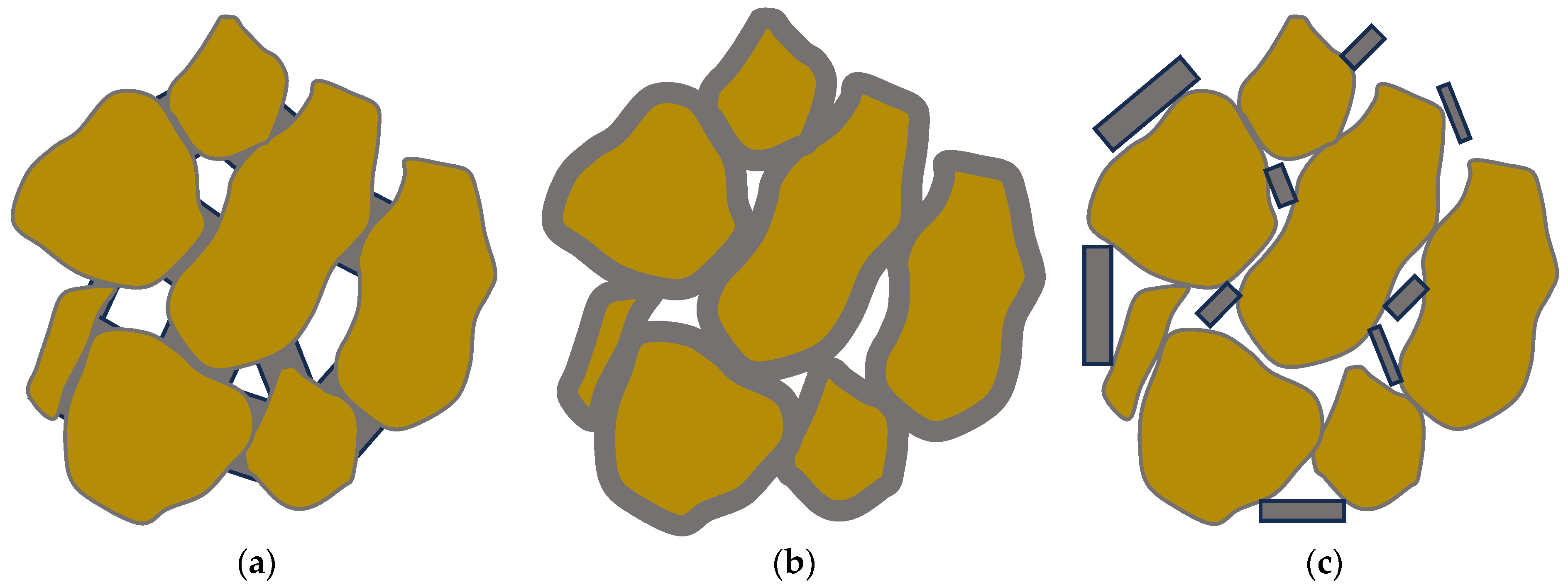
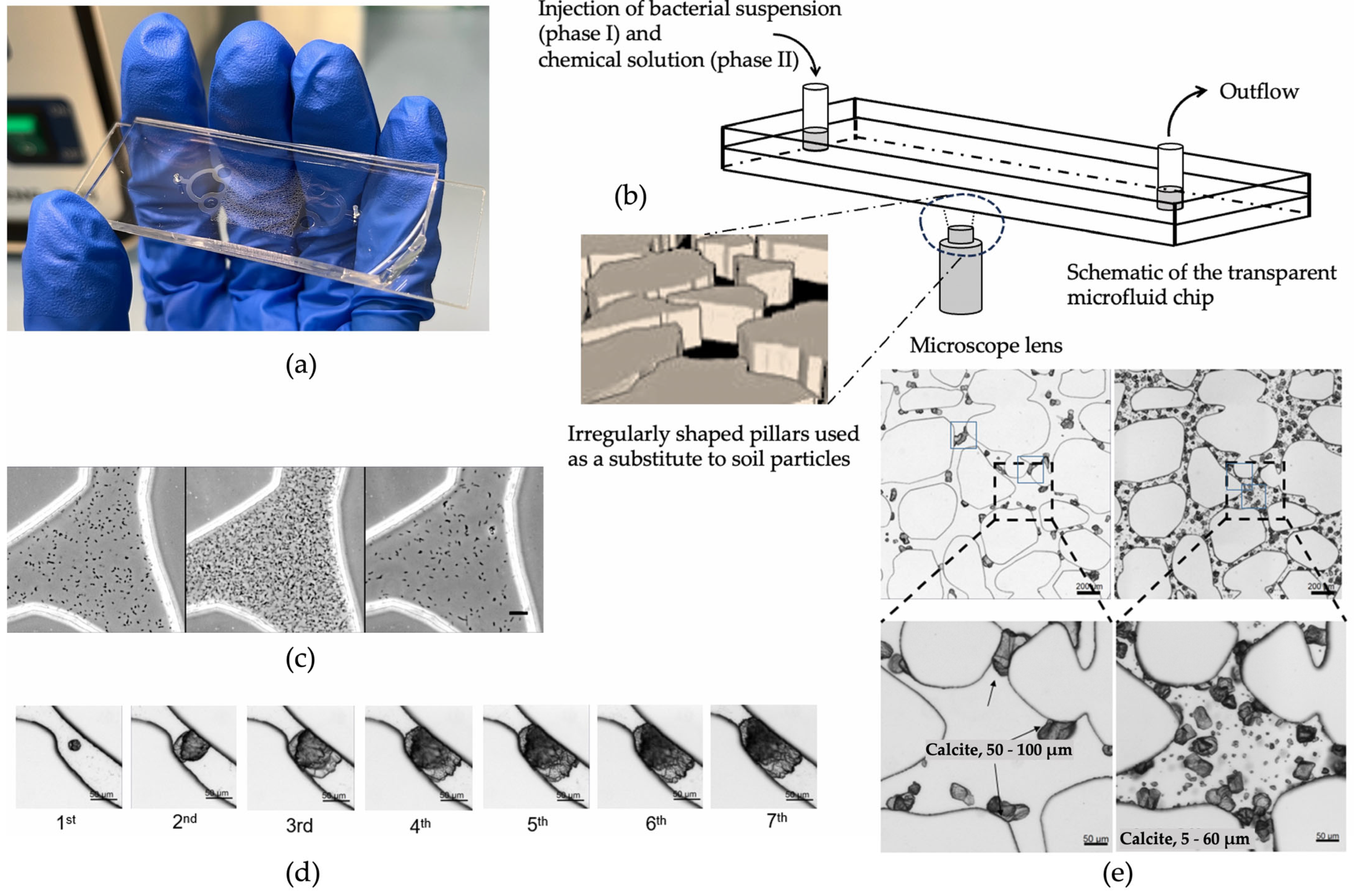
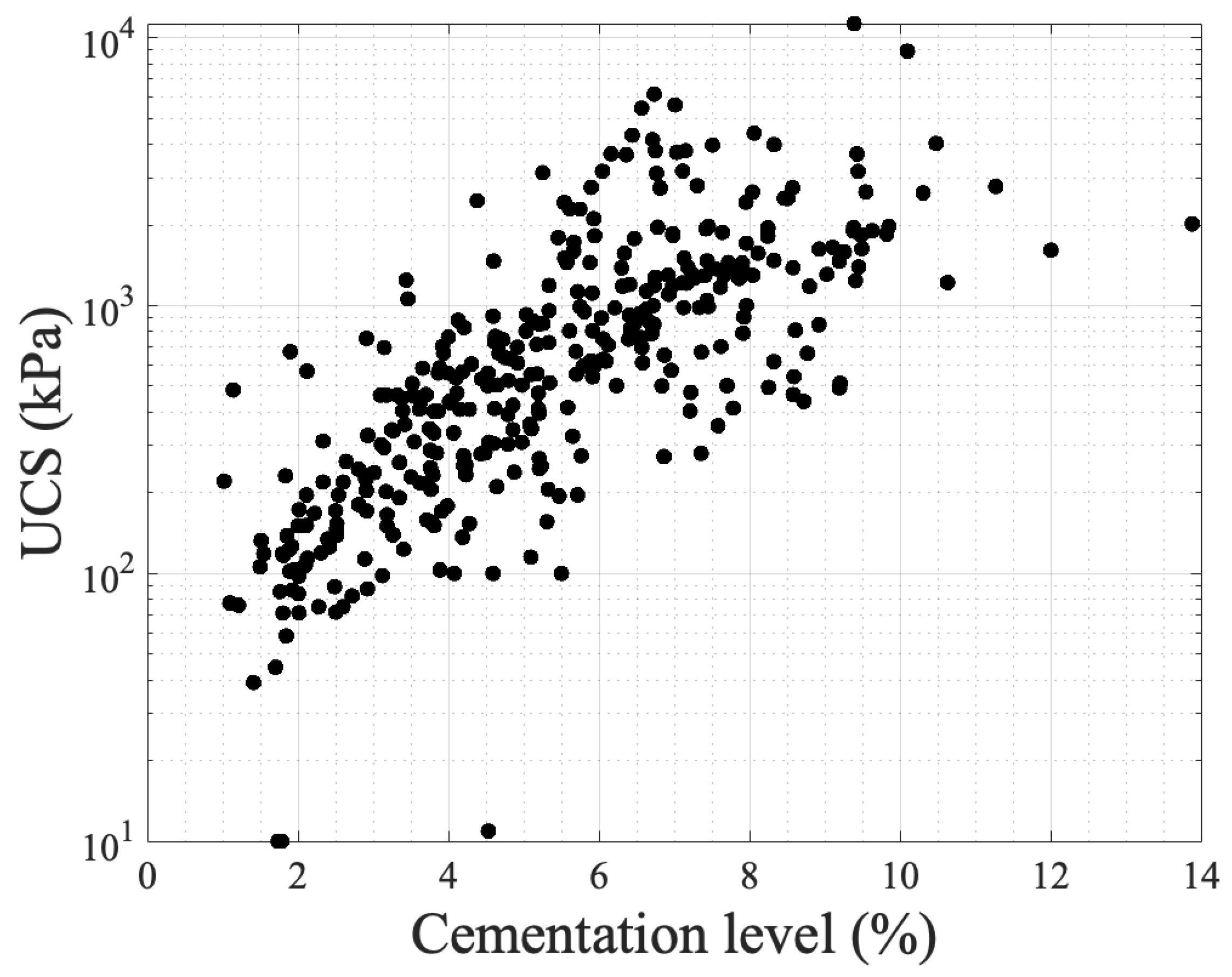
| Authors | Contaminant(s) | Type of Environment |
|---|---|---|
| [131] | Cd2+ | Liquid environment |
| [24] | Pb2+ | Liquid environment |
| [132] | Cr6+, Cu2+, Zn2+ | Contaminated soil (Changhua County in Taiwan) |
| [133] | Zn2+, Ld2+, Cd2+ | Liquid environment |
| [28] | Ni2+, Cu2+, Pb2+, Co2+, Zn2+ | Liquid environment |
| [134] | Ld2+ | Liquid environment |
| [135] | Cd2+ | Liquid environment |
| [25] | As3+ | Farmland soil |
| [136] | Cu2+ | Liquid environment |
| [137] | Cu2+, Ld2+, Cd2+ | Contaminated mine tailing soils |
| [45] | Cu2+ | Mine tailing soils (Greenfields copper mine project in Columbia) |
| [29] | Cd2+ | Soil and liquid environment |
| [43] | Cu2+, Zn2+, Ni2+, Cd2+ | Soil |
| [47] | Ld2+, Cd2+ | Pyrite mine sites synthetic landfill leachate |
| [38] | 90Sr | Two-dimensional porous media reactors |
| References | Application |
|---|---|
| [138] | Sealing subsurface fractures in the near-wellbore environment. |
| [139] | Reduction of near-wellbore permeability to reduce CO2-related corrosion and lower the risk of unwanted migration of CO2 or other fluids. |
| [140] | Seal fluid pathways in subsurface ground, for example, to secure waste storage repositories/hydraulic barriers. |
| [141] | Generation of a landfill barrier to inhibit contaminant transport underground. |
| [142,143] | Construction of an aquaculture pond in sand and/or in arid desert. |
| [144] | Prevention of contaminant transport within the porous medium. |
| [113] | Seepage control in sand using bioslurry applicable to many infrastructure projects, such as reservoirs, earth dams, tunnels, and other underground constructions. |
| [15,16] | Seepage control to mitigate erosion in existing dams. |
| [112] | Permeability preservation for strength enhancement for fully permeable pavement. |
| [120] | Landfill control/groundwater contamination prevention. |
| [94] | Generation of an impermeable layer in the soil. |
| [92,104] | Natural fracture sealing/permeable rock sealing/plugging in high-permeability zones. |
| [145] | Drought mitigation by water evaporation suppression. |
| [146] | Irrigation channels and water reservoirs construction in sandy soil ground. |
| References | Application |
|---|---|
| [86] | Artificial islands generation. |
| [96] | MICP for areas near river applications (different calcium sources). |
| [22,67] | Biocementation in seawater and marine environment. |
| [123] | Prevention of surface erosion. |
| [95,100,109] | Dust control and wind erosion control 1. |
| [149,150,151] | Rainfall-induced erosion control. |
| [152] | Erosion resistance of biocemented sandy slope subjected to wave actions. |
| [153,154,155,156] | Coastal line protection. |
| References | Application |
|---|---|
| [10,72,153,159,167] | Generation of artificial rocks of controlled properties. |
| [14,61,76,78,82] | Assessment of artificial rock mechanical and hydraulic properties. |
| [168] | Numerical simulations of artificial rock properties and fluid flow numerical experiments. |
| [169] | Fluid flow experiments in porous media for hydrological applications. |
| [170,171] | Fluid flow experiments in porous media for reservoir engineering applications. |
| Sustainable Development Goals | MICP Application |
|---|---|
| SDG 14: Life below water | Water pollution—heavy metals removal. Water pollution—seawater intrusion control. |
| SDG 15: Life on land | Solid waste disposal—contaminated soil remediation. Erosion—rainfall erosion control. Erosion—surface erosion control in water environments on land. Erosion—coastline erosion control. Sand stability—artificial lands generation. |
| SDG 6: Clean water and sanitation | Water resource conservation—leakage mitigation. Water resource conservation—construction of ponds for water collection. Drought mitigation—water evaporation suppression. Groundwater recharge—MAR control via MICP. |
| SDG 13: Climate action | Global warming control—carbon capture and storage. Global warming control—natural fracture sealing/permeable rock sealing/plugging high permeability zones. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinou, C.; Wang, Y. Unlocking the Potential of Microbially Induced Calcium Carbonate Precipitation (MICP) for Hydrological Applications: A Review of Opportunities, Challenges, and Environmental Considerations. Hydrology 2023, 10, 178. https://doi.org/10.3390/hydrology10090178
Konstantinou C, Wang Y. Unlocking the Potential of Microbially Induced Calcium Carbonate Precipitation (MICP) for Hydrological Applications: A Review of Opportunities, Challenges, and Environmental Considerations. Hydrology. 2023; 10(9):178. https://doi.org/10.3390/hydrology10090178
Chicago/Turabian StyleKonstantinou, Charalampos, and Yuze Wang. 2023. "Unlocking the Potential of Microbially Induced Calcium Carbonate Precipitation (MICP) for Hydrological Applications: A Review of Opportunities, Challenges, and Environmental Considerations" Hydrology 10, no. 9: 178. https://doi.org/10.3390/hydrology10090178
APA StyleKonstantinou, C., & Wang, Y. (2023). Unlocking the Potential of Microbially Induced Calcium Carbonate Precipitation (MICP) for Hydrological Applications: A Review of Opportunities, Challenges, and Environmental Considerations. Hydrology, 10(9), 178. https://doi.org/10.3390/hydrology10090178







