Hydrogeochemistry, Geothermometry, and Sourcing of High Dissolved Boron, Tungsten, and Chlorine Concentrations in the Trans-Himalayan Hotsprings of Ladakh, India
Abstract
1. Introduction
2. Field Sites and Geology
3. Methodology
3.1. In-Situ Physicochemical Measurements and Water Sample Collection
3.2. Trace and Major Elements Analysis
3.3. Ions Analysis
4. Results
Aqueous Geochemistry
5. Discussion
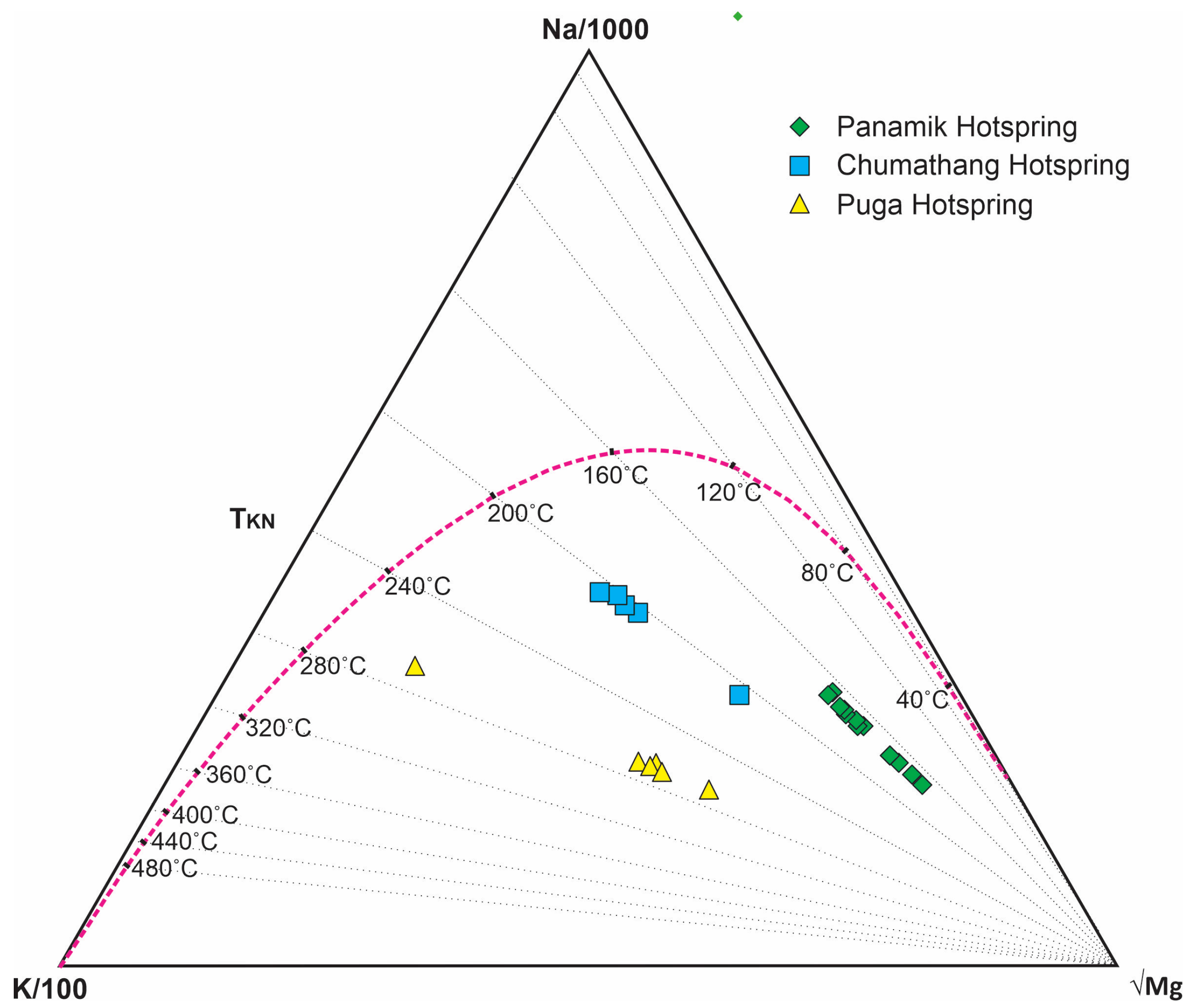
5.1. Reservoir Temperature, Magmatic Water Input, and Rock-Water Interactions
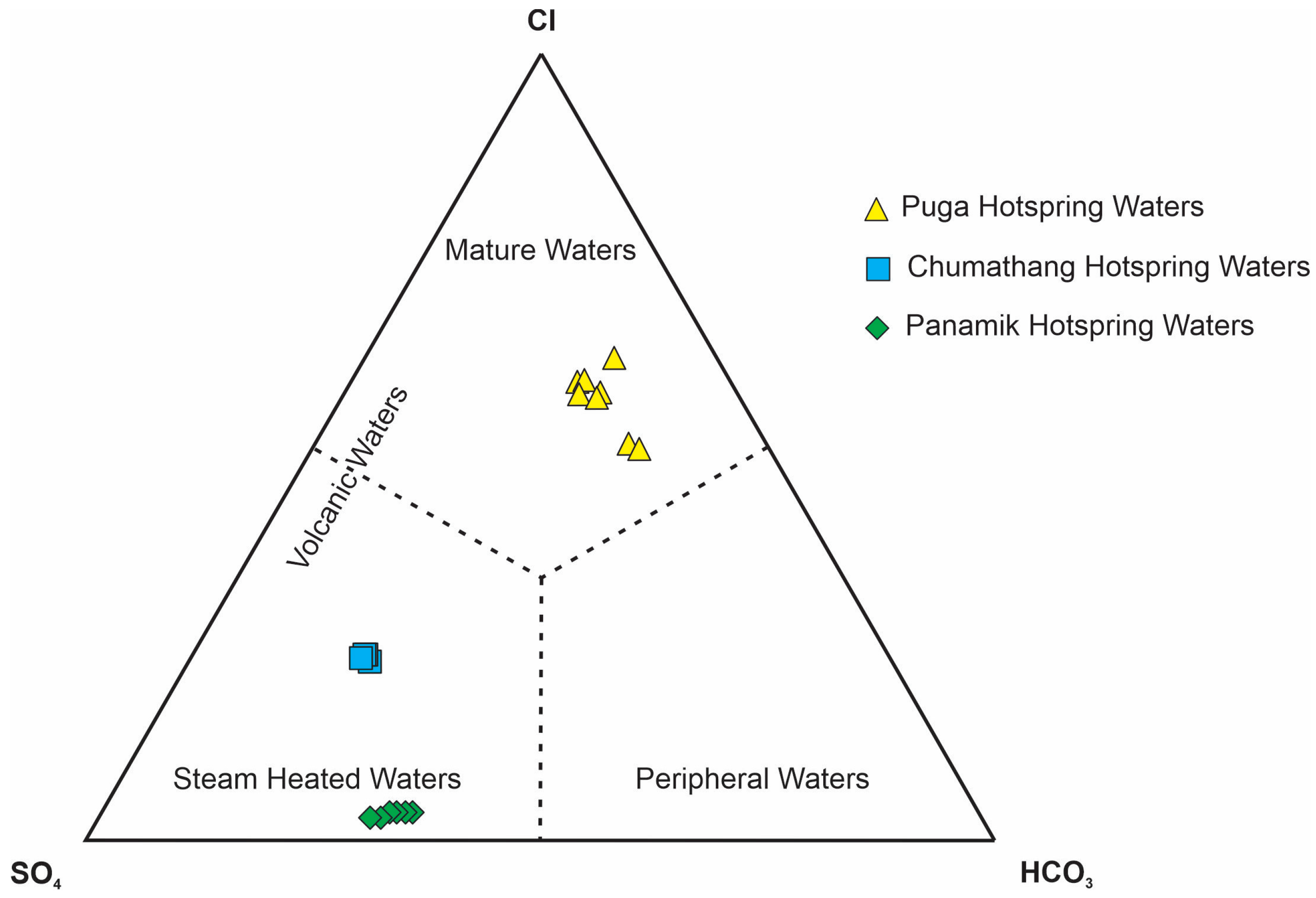
5.2. Sources of Major Anions
5.3. Sources of Major Cations
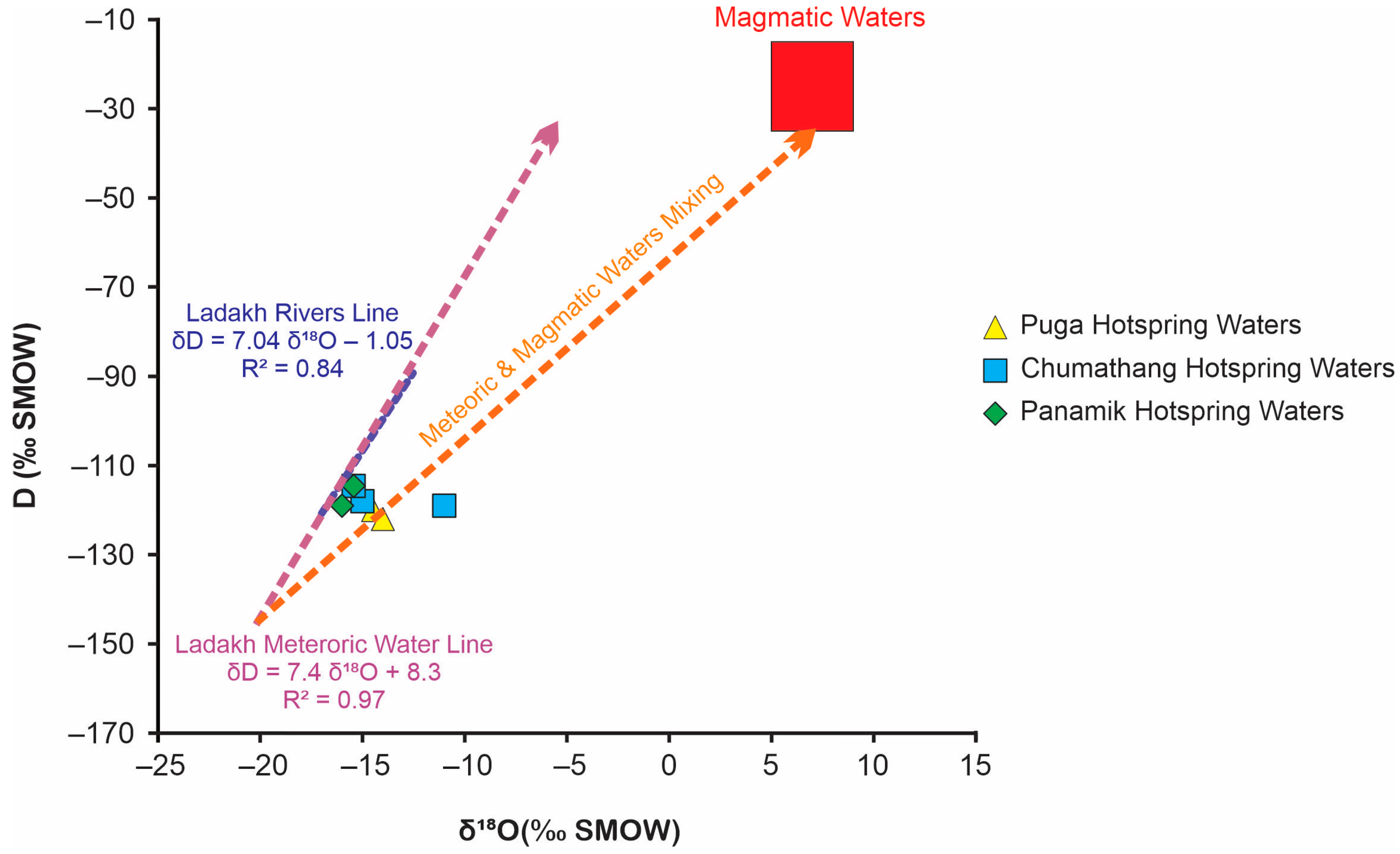
5.4. Meteoric Versus Magmatic Water Sourcing through δD–δ18O Plot
5.5. Phase Separation
5.6. Sourcing of Elevated B and W Concentrations in the Hydrothermal Waters of Ladakh
5.6.1. Trace Elements
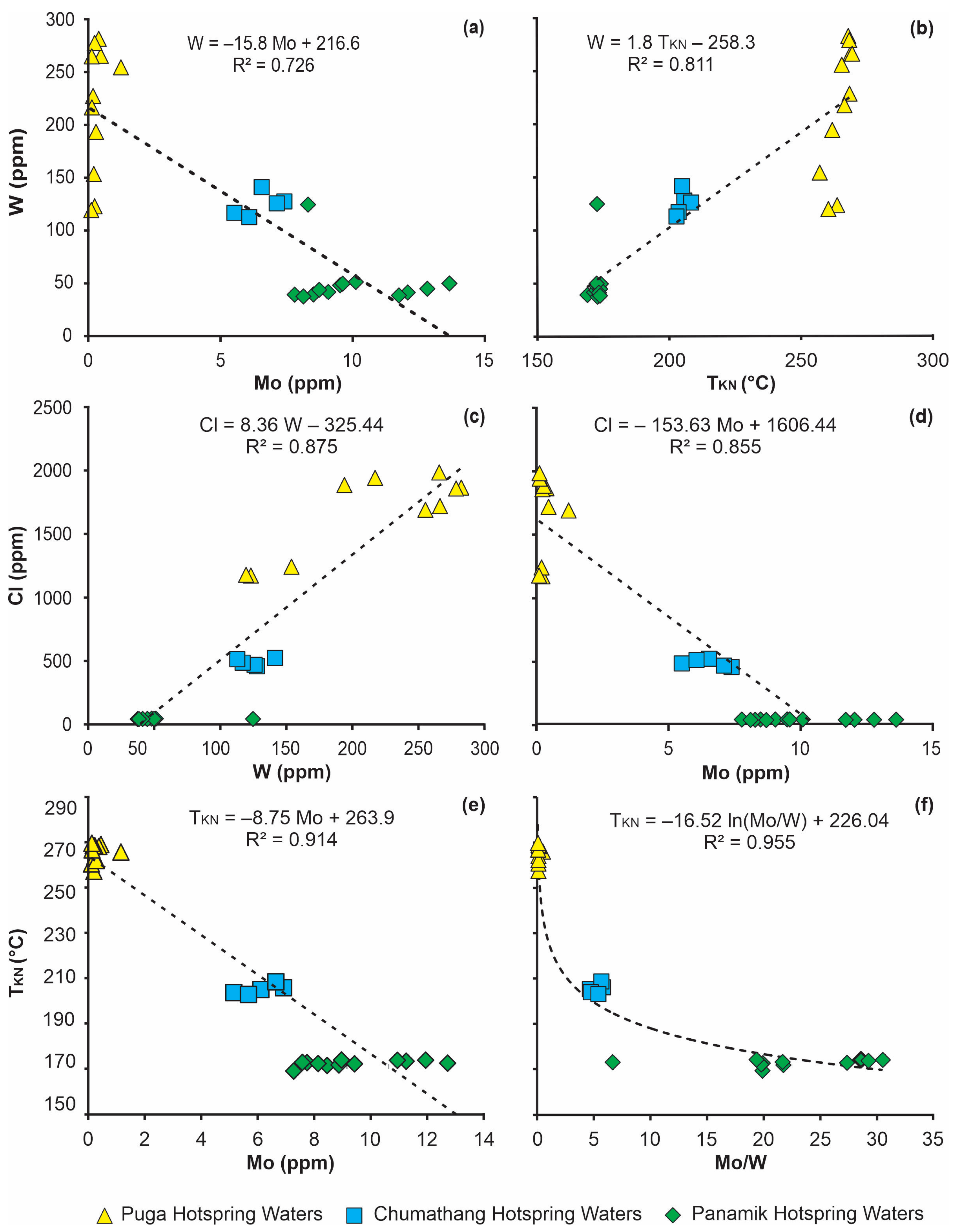
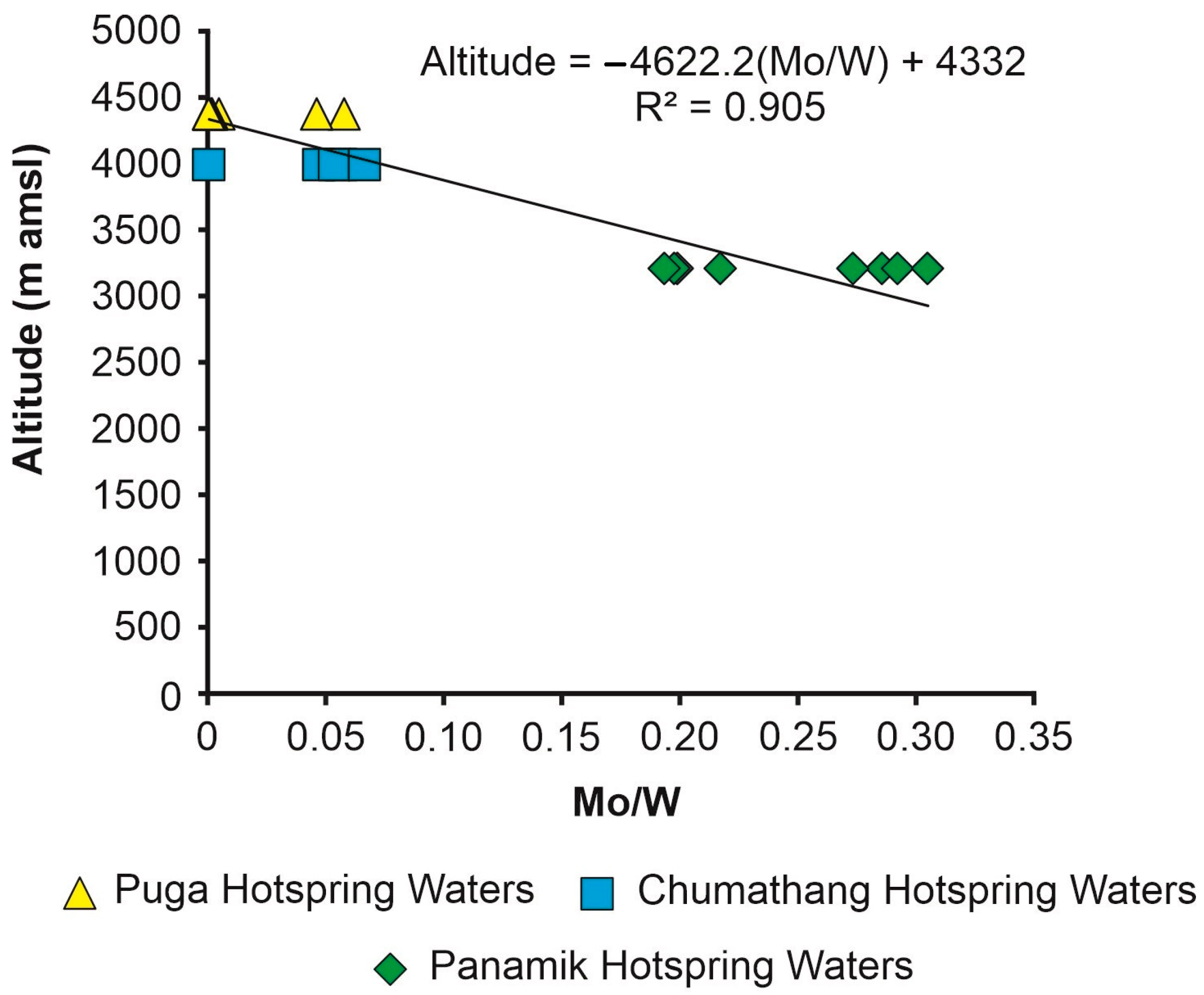
Sources of Mo and W
Source of B
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Sloot, H.A.; Hoede, D.; Wijkstra, J.; Duinker, J.C.; Nolting, R.F. Anionic species of V, As, Se, Mo, Sb, Te and W in the Scheldt and Rhine estuaries and the Southern Bight (North Sea). Estuar. Coast. Shelf Sci. 1985, 21, 633–651. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Lyons, W.B.; Graham, E.Y.; Welch, K.A. Oxyanion concentrations in eastern Sierra Nevada rivers–3. Boron, molybdenum, vanadium, and tungsten. Aquat. Geochem. 2000, 6, 19–46. [Google Scholar] [CrossRef]
- Mohajerin, T.J.; Helz, G.R.; Johannesson, K.H. Tungsten–molybdenum fractionation in estuarine environments. Geochim. et Cosmochim. Acta 2016, 177, 105–119. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Tang, J. Conservative behavior of arsenic and other oxyanion-forming trace elements in an oxic groundwater flow system. J. Hydrol. 2009, 378, 13–28. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Luo, L. Tungsten from typical magmatic hydrothermal systems in China and its environmental transport. Sci. Total Environ. 2019, 657, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, Q.; Wu, G.; Guo, W.; She, W.; Yan, W. Boron geochemistry of the geothermal waters from two typical hydrothermal systems in Southern Tibet (China): Daggyai and Quzhuomu. Geothermics 2019, 82, 190–202. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Q.; Luo, L.; He, T. Environmental impacts of geothermal waters with extremely high boron concentrations: Insight from a case study in Tibet, China. J. Volcanol. Geotherm. Res. 2020, 397, 106887. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, H.; Zhang, Y.; Wei, H.; Dong, T. Boron geochemistry from some typical Tibetan hydrothermal systems: Origin and isotopic fractionation. Appl. Geochem. 2015, 63, 436–445. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, C.; Liu, X.; Yang, X.; Zhou, Y.; Horn, I.; Weyer, S.; Holtz, F. Significant boron isotopic fractionation in the magmatic evolution of Himalayan leucogranite recorded in multiple generations of tourmaline. Chem. Geol. 2021, 571, 120194. [Google Scholar] [CrossRef]
- Stefánsson, A.; Arnórsson, S. The geochemistry of As, Mo, Sb, and W in natural geothermal waters, Iceland. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005; pp. 24–29. [Google Scholar]
- Arnórsson, S.; Óskarsson, N. Molybdenum and tungsten in volcanic rocks and in surface and <100 °C ground waters in Iceland. Geochim. Cosmochim. Acta 2007, 71, 284–304. [Google Scholar] [CrossRef]
- McCleskey, R.B.; Nordstrom, D.K.; Susong, D.D.; Ball, J.W.; Taylor, H.E. Source and fate of inorganic solutes in the Gibbon River, Yellowstone National Park, Wyoming, USA. II. Trace element chemistry. J. Volcanol. Geotherm. Res. 2010, 196, 139–155. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Kubo, S.; Tanaka, M.; Senda, R.; Iizuka, T.; Tanimizu, M.; Takahashi, Y. Stable isotope fractionation of tungsten during adsorption on Fe and Mn (oxyhydr) oxides. Geochim. Cosmochim. Acta 2017, 204, 52–67. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Takahashi, Y.; Marcus, M.A.; Uruga, T.; Tanida, H.; Terada, Y.; Usui, A. Tungsten species in natural ferromanganese oxides related to its different behavior from molybdenum in oxic ocean. Geochim. Cosmochim. Acta 2013, 106, 364–378. [Google Scholar] [CrossRef]
- Havig, J.R.; Kuether, J.E.; Gangidine, A.J.; Schroeder, S.; Hamilton, T.L. Hot Spring Microbial Community Elemental Composition: Hot Spring and Soil Inputs, and the Transition from Biocumulus to Siliceous Sinter. Astrobiology 2021, 21, 1526–1546. [Google Scholar] [CrossRef] [PubMed]
- Iveson, A.A.; Webster, J.D.; Rowe, M.C.; Neill, O.K. Fluid-melt trace-element partitioning behaviour between evolved melts and aqueous fluids: Experimental constraints on the magmatic-hydrothermal transport of metals. Chem. Geol. 2019, 516, 18–41. [Google Scholar] [CrossRef]
- Honegger, K.; Dietrich, V.; Frank, W.; Gansser, A.; Thöni, M.; Trommsdorff, V. Magmatism and metamorphism in the Ladakh Himalayas (the Indus-Tsangpo suture zone). Earth Planet. Sci. Lett. 1982, 60, 253–292. [Google Scholar] [CrossRef]
- Thussu, J.L. Geothermal Energy Resources of India; Geological Survey of India: Kolkata, India, 2002.
- Craig, J.; Absar, A.; Bhat, G.; Cadel, G.; Hafiz, M.; Hakhoo, N.; Kashkari, R.; Moore, J.; Ricchiuto, T.E.; Thurow, J.; et al. Hot springs and the geothermal energy potential of Jammu & Kashmir State, N.W. Himalaya, India. Earth-Sci. Rev. 2013, 126, 156–177. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Rai, S.K.; Bartarya, S.K.; Gupta, A.K.; Negi, M. Stable isotopes (δ13CDIC, δD, δ18O) and geochemical characteristics of geothermal springs of Ladakh and Himachal (India): Evidence for CO2 discharge in northwest Himalaya. Geothermics 2016, 64, 314–330. [Google Scholar] [CrossRef]
- Shankar, R.; Padhi, R.N.; Arora, C.L.; Thussu, J.L.; Dua, K.J.S. Geothermal exploration of the Puga and Chumathang geothermal fields, Ladakh, India. In Proceedings of the 2nd United Nations Symposium on the Development and Use of Geothermal Resources, San Francisco, CA, USA, 20–29 May 1975; pp. 245–258. [Google Scholar]
- Das, P.; Maya, K.; Padmalal, D. Hydrogeochemistry of the Indian thermal springs: Current status. Earth-Sci. Rev. 2022, 224, 103890. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Singh, A.K.; Phartiyal, B.; Sharma, A. Hydrogeochemical characteristics of the Indus river water system. Chem. Ecol. 2021, 37, 780–808. [Google Scholar] [CrossRef]
- Hautman, D.P.; Munch, D.J. Method 300.1 Determination of Inorganic Anions in Drinking Water by Ion Chromatography; US Environmental Protection Agency: Cincinnati, OH, USA, 1997.
- Giggenbach, W. Graphical techniques for the evaluation of water/rock equilibration conditions by use of Na, K, Mg and Ca contents of discharge waters. In Proceedings of 8th New Zealand Geothermal Workshop, 21–23 January 1986; pp. 37–44. Available online: https://www.geothermal-energy.org/pdf/IGAstandard/NZGW/1986/Giggenbach.pdf (accessed on 22 March 2023).
- Giggenbach, W.F. Geothermal solute equilibria. derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, K.; Laskar, A.; Singh, S.K.; Mehta, P. Oxygen, deuterium, and strontium isotope characteristics of the Indus River water system. Geomorphology 2017, 284, 5–16. [Google Scholar] [CrossRef]
- Lone, S.A.; Jeelani, G.; Deshpande, R.D.; Mukherjee, A. Stable isotope (δ18O and δD) dynamics of precipitation in a high altitude Himalayan cold desert and its surroundings in Indus river basin, Ladakh. Atmos. Res. 2019, 221, 46–57. [Google Scholar] [CrossRef]
- Giggenbach, W.F.; Soto, R.C. Isotopic and chemical composition of water and steam discharges from volcanic-magmatic-hydrothermal systems of the Guanacaste Geothermal Province, Costa Rica. Appl. Geochem. 1992, 7, 309–332. [Google Scholar] [CrossRef]
- Saxena, V.; D’Amore, F. Aquifer chemistry of the Puga and Chumatang high temperature geothermal systems in India. J. Volcanol. Geotherm. Res. 1984, 21, 333–346. [Google Scholar] [CrossRef]
- Absar, A.; Kumar, V.; Bajpai, I.; Sinha, A.; Kapoor, A. Reservoir modeling of Puga geothermal system, Ladakh, Jammu and Kashmir. Geol. Surv. India Spec. Publ. 1996, 45, 69–74. [Google Scholar]
- Azeez, K.A.; Harinarayana, T. Magnetotelluric evidence of potential geothermal resource in Puga, Ladakh, NW Himalaya. Curr. Sci. 2007, 93, 323–329. [Google Scholar]
- Bernard, R.; Taran, Y.; Pennisi, M.; Tello, E.; Ramirez, A. Chloride and Boron behavior in fluids of Los Humeros geothermal field (Mexico): A model based on the existence of deep acid brine. Appl. Geochem. 2011, 26, 2064–2073. [Google Scholar] [CrossRef]
- Reyes, A.; Trompetter, W. Hydrothermal water–rock interaction and the redistribution of Li, B and Cl in the Taupo Volcanic Zone, New Zealand. Chem. Geol. 2012, 314, 96–112. [Google Scholar] [CrossRef]
- Palmer, M.; Sturchio, N. The boron isotope systematics of the Yellowstone National Park (Wyoming) hydrothermal system: A reconnaissance. Geochim. Cosmochim. Acta 1990, 54, 2811–2815. [Google Scholar] [CrossRef]
- Kishida, K.; Sohrin, Y.; Okamura, K.; Ishibashi, J.-i. Tungsten enriched in submarine hydrothermal fluids. Earth Planet. Sci. Lett. 2004, 222, 819–827. [Google Scholar] [CrossRef]
- Smith, D.J.; Jenkin, G.; Naden, J.; Boyce, A.; Petterson, M.; Toba, T.; Darling, W.; Taylor, H.; Millar, I. Anomalous alkaline sulphate fluids produced in a magmatic hydrothermal system—Savo, Solomon Islands. Chem. Geol. 2010, 275, 35–49. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; McCleskey, R.B.; Ball, J.W. Sulfur geochemistry of hydrothermal waters in Yellowstone National Park: IV Acid–sulfate waters. Appl. Geochem. 2009, 24, 191–207. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Liu, W. O, H, and Sr isotope evidences of mixing processes in two geothermal fluid reservoirs at Yangbajing, Tibet, China. Environ. Earth Sci. 2009, 59, 1589–1597. [Google Scholar] [CrossRef]
- Guo, L.; Wang, G.; Sheng, Y.; Sun, X.; Shi, Z.; Xu, Q.; Mu, W. Temperature governs the distribution of hot spring microbial community in three hydrothermal fields, Eastern Tibetan Plateau Geothermal Belt, Western China. Sci. Total Environ. 2020, 720, 137574. [Google Scholar] [CrossRef]
- Aiuppa, A.; Baker, D.; Webster, J. Halogens in volcanic systems. Chem. Geol. 2009, 263, 1–18. [Google Scholar] [CrossRef]
- Webster, J.D.; Vetere, F.; Botcharnikov, R.E.; Goldoff, B.; McBirney, A.; Doherty, A.L. Experimental and modeled chlorine solubilities in aluminosilicate melts at 1 to 7000 bars and 700 to 1250 °C: Applications to magmas of Augustine Volcano, Alaska. Am. Mineral. 2015, 100, 522–535. [Google Scholar] [CrossRef]
- Lakhan, N.; Singh, A.K.; Singh, B.; Premi, K.; Oinam, G. Evolution of Late Cretaceous to Palaeogene basalt–andesite–dacite–rhyolite volcanic suites along the northern margin of the Ladakh magmatic arc, NW Himalaya, India. J. Earth Syst. Sci. 2020, 129, 1–23. [Google Scholar] [CrossRef]
- Harinarayana, T.; Abdul Azeez, K.K.; Murthy, D.N.; Veeraswamy, K.; Eknath Rao, S.P.; Manoj, C.; Naganjaneyulu, K. Exploration of geothermal structure in Puga geothermal field, Ladakh Himalayas, India by magnetotelluric studies. J. Appl. Geophys. 2006, 58, 280–295. [Google Scholar] [CrossRef]
- Bos, A. Hydrothermal Element Distributions at High Temperatures: An Experimental Study on the Partitioning of Major and Trace Elements between Phlogopite, Haplogranitic Melt and Vapour; Faculteit Aardwetenschappen: Utrecht, The Netherlands, 1990. [Google Scholar]
- Orville, P.M. Alkali ion exchange between vapor and feldspar phases. Am. J. Sci. 1963, 261, 201–237. [Google Scholar] [CrossRef]
- Ahmad, T.; Thakur, V.C.; Islam, R.; Mukherjee, P.K. Geochemistry and geodynamic implications of magmatic rocks from the Trans-Himalayan arc. Geochem. J. 1998, 32, 383–404. [Google Scholar] [CrossRef]
- Lakhan, N.; Singh, A.K.; Akhtar, S.; Singh, B.P. Geochemical characteristics and petrogenesis of magmatic rocks of the Shyok suture zone, NW Ladakh Himalaya, India. Arab. J. Geosci. 2022, 15, 1–23. [Google Scholar] [CrossRef]
- Giggenbach, W.; Gonfiantini, R.; Jangi, B.; Truesdell, A. Isotopic and chemical composition of Parbati valley geothermal discharges, north-west Himalaya, India. Geothermics 1983, 12, 199–222. [Google Scholar] [CrossRef]
- Li, J.; Lowenstein, T.K.; Blackburn, I.R. Responses of evaporite mineralogy to inflow water sources and climate during the past 100 ky in Death Valley, California. Geol. Soc. Am. Bull. 1997, 109, 1361–1371. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Schotterer, U.; Fröhlich, K.; Gäggeler, H.; Sandjordj, S.; Stichler, W. Isotope records from Mongolian and Alpine ice cores as climate indicators. In Climatic Change at High Elevation Sites; Springer: Berlin/Heidelberg, Germany, 1997; pp. 287–298. [Google Scholar]
- Angert, A.; Lee, J.-E.; Yakir, D. Seasonal variations in the isotopic composition of near-surface water vapour in the eastern Mediterranean. Tellus B Chem. Phys. Meteorol. 2008, 60, 674–684. [Google Scholar] [CrossRef]
- Dansgaard, W.; Clausen, H.B.; Gundestrup, N.; Johnsen, S.J.; Rygner, C. Dating and Climatic Interpretation of Two Deep Greenland Ice Cores. In Greenland Ice Core: Geophysics, Geochemistry, and the Environment; American Geophysical Union: Washington, DC, USA, 1985; pp. 71–76. [Google Scholar] [CrossRef]
- Zhou, S.; Nakawo, M.; Hashimoto, S.; Sakai, A. The effect of refreezing on the isotopic composition of melting snowpack. Hydrol. Process. 2008, 22, 873–882. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Günther, D.; Audétat, A.; Ulrich, T.; Frischknecht, R. Metal fractionation between magmatic brine and vapor, determined by microanalysis of fluid inclusions. Geology 1999, 27, 755. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Heinrich, C.A. 100th Anniversary Special Paper: Vapor Transport of Metals and the Formation of Magmatic-Hydrothermal Ore Deposits. Econ. Geol. 2005, 100, 1287–1312. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Koutsospyros, A.; Braida, W.; Christodoulatos, C.; Dermatas, D.; Strigul, N. A review of tungsten: From environmental obscurity to scrutiny. J. Hazard. Mater. 2006, 136, 1–19. [Google Scholar] [CrossRef]
- Mohajerin, T.J.; Helz, G.R.; White, C.D.; Johannesson, K.H. Tungsten speciation in sulfidic waters: Determination of thiotungstate formation constants and modeling their distribution in natural waters. Geochim. Cosmochim. Acta 2014, 144, 157–172. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 1–64. [Google Scholar] [CrossRef]
- Candela, P.A. Controls on ore metal ratios in granite-related ore systems: An experimental and computational approach. Earth Environ. Sci. Trans. R. Soc. Edinb. 1992, 83, 317–326. [Google Scholar]
- Candela, P.A.; Bouton, S.L. The influence of oxygen fugacity on tungsten and molybdenum partitioning between silicate melts and ilmenite. Econ. Geol. 1990, 85, 633–640. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.-F.; Ma, D.; Pan, J. The role of hydrothermal alteration in tungsten mineralization at the Dahutang tungsten deposit, South China. Ore Geol. Rev. 2018, 95, 1008–1027. [Google Scholar] [CrossRef]
- Wang, X.-S.; Williams-Jones, A.E.; Hu, R.-Z.; Shang, L.-B.; Bi, X.-W. The role of fluorine in granite-related hydrothermal tungsten ore genesis: Results of experiments and modeling. Geochim. Cosmochim. Acta 2021, 292, 170–187. [Google Scholar] [CrossRef]
- Manning, D.A.C.; Henderson, P. The behaviour of tungsten in granitic melt-vapour systems. Contrib. Mineral. Petrol. 1984, 86, 286–293. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, C.; Wang, Y. The relative solubilities of wolframite and scheelite in hydrothermal fluids: Insights from thermodynamic modeling. Chem. Geol. 2021, 584, 120488. [Google Scholar] [CrossRef]
- Steller, L.H.; Nakamura, E.; Ota, T.; Sakaguchi, C.; Sharma, M.; Van Kranendonk, M.J. Boron isotopes in the Puga geothermal system, India, and their implications for the habitat of early life. Astrobiology 2019, 19, 1459–1473. [Google Scholar] [CrossRef]
- Cinti, D.; Pizzino, L.; Voltattorni, N.; Quattrocchi, F.; Walia, V. Geochemistry of thermal waters along fault segments in the Beas and Parvati valleys (north-west Himalaya, Himachal Pradesh) and in the Sohna town (Haryana), India. Geochem. J. 2009, 43, 65–76. [Google Scholar] [CrossRef]
- Valentino, G.M.; Stanzione, D. Source processes of the thermal waters from the Phlegraean Fields (Naples, Italy) by means of the study of selected minor and trace elements distribution. Chem. Geol. 2003, 194, 245–274. [Google Scholar] [CrossRef]
- Aggarwal, J.K.; Palmer, M.R.; Bullen, T.D.; Arnórsson, S.; Ragnarsdóttir, K.V. The boron isotope systematics of Icelandic geothermal waters: 1. Meteoric water charged systems. Geochim. Cosmochim. Acta 2000, 64, 579–585. [Google Scholar] [CrossRef]
- Millot, R.; Hegan, A.; Négrel, P. Geothermal waters from the Taupo Volcanic Zone, New Zealand: Li, B and Sr isotopes characterization. Appl. Geochem. 2012, 27, 677–688. [Google Scholar] [CrossRef]
- Battistel, M.; Hurwitz, S.; Evans, W.C.; Barbieri, M. The chemistry and isotopic composition of waters in the low-enthalpy geothermal system of Cimino-Vico Volcanic District, Italy. J. Volcanol. Geotherm. Res. 2016, 328, 222–229. [Google Scholar] [CrossRef]
- Chaussidon, M.; Albarède, F. Secular boron isotope variations in the continental crust: An ion microprobe study. Earth Planet. Sci. Lett. 1992, 108, 229–241. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, X.; Lu, Y.; Zhang, Z.; Chen, S.; Sun, C.; Hou, Q.; Wang, Y.; Li, S. Geochemistry and boron isotope compositions of tourmalines from the granite-greisen-quartz vein system in Dayishan pluton, Southern China: Implications for potential mineralization. Am. Mineral. 2022, 107, 495–508. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Jiang, S.-Y.; Palmer, M.R. Chemical and boron isotopic compositions of tourmaline from the Nyalam leucogranites, South Tibetan Himalaya: Implication for their formation from B-rich melt to hydrothermal fluids. Chem. Geol. 2015, 419, 102–113. [Google Scholar] [CrossRef]
- Gou, G.-N.; Wang, Q.; Wyman, D.A.; Xia, X.-P.; Wei, G.-J.; Guo, H.-F. In situ boron isotopic analyses of tourmalines from Neogene magmatic rocks in the northern and southern margins of Tibet: Evidence for melting of continental crust and sediment recycling. Solid Earth Sci. 2017, 2, 43–54. [Google Scholar] [CrossRef]
- Hu, G.; Zeng, L.; Gao, L.-E.; Liu, Q.; Chen, H.; Guo, Y. Diverse magma sources for the Himalayan leucogranites: Evidence from B-Sr-Nd isotopes. Lithos 2018, 314–315, 88–99. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, W.; Wang, G.; Liu, Z.; Lai, Y.; Huang, J.; Yan, G.; Zhang, Q. Chemical and boron isotopic composition of tourmaline from the Conadong leucogranite-pegmatite system in South Tibet. Lithos 2019, 326–327, 529–539. [Google Scholar] [CrossRef]
- Marschall, H.R.; Jiang, S.-Y. Tourmaline Isotopes: No Element Left Behind. Elements 2011, 7, 313–319. [Google Scholar] [CrossRef]
- van Hinsberg, V.J.; Henry, D.J.; Marschall, H.R. Tourmaline: An ideal indicator of its host environment. Can. Mineral. 2011, 49, 1–16. [Google Scholar] [CrossRef]





| Sample ID | pH | Temp | Mo | W | B | Na | K | Ca | Mg | Cl | SO4 | HCO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | μg/L (ppb) | mg/L (ppm) | ||||||||||
| PUGA-1.1 | 6.92 | 58.00 | 1.22 | 255.28 | 62.07 | 437.32 | 64.79 | 8.82 | 0.81 | 1690.48 | 455.54 | 817 ᵃ |
| PUGA-1.2 | 10.24 | 36.80 | 0.46 | 266.30 | 378.03 | 396.60 | 60.93 | 7.75 | 0.74 | 1719.34 | 431.78 | |
| PUGA-1.3 | n.a. | 38.40 | 0.38 | 282.43 | 194.91 | 457.46 | 69.59 | 7.86 | 0.88 | 1865.25 | 484.45 | |
| PUGA-1.4 | 8.46 | 57.80 | 0.23 | 278.53 | 114.32 | 463.99 | 70.86 | 2.91 | 0.06 | 1858.02 | 545.72 | |
| PUGA-2.1 | 5.73 | 63.40 | 0.17 | 228.11 | 123.99 | 448.39 | 68.52 | 10.24 | 1.02 | n.a. | n.a. | |
| PUGA-2.2 | 6.83 | 58.10 | 0.23 | 123.14 | 59.90 | 318.00 | 46.11 | 9.30 | 0.81 | 1171.56 | 311.75 | |
| PUGA-2.3 | 7.90 | 45.40 | 0.19 | 153.88 | 108.84 | 364.61 | 49.10 | 9.64 | 0.92 | 1241.15 | 350.78 | |
| PUGA-2.4 | 8.63 | 38.50 | 0.10 | 119.50 | 72.28 | 329.60 | 46.04 | 9.11 | 0.88 | 1177.54 | 317.56 | |
| PUGA-2.5 | 9.68 | 7.00 | 0.28 | 193.97 | 679.62 | 476.46 | 67.71 | 9.06 | 1.30 | 1885.59 | 330.73 | |
| PUGA-3 | 6.29 | 64.90 | 0.12 | 217.19 | 452.17 | 463.93 | 69.42 | 10.28 | 1.52 | 1942.04 | 504.06 | |
| PUGA-4 | 5.60 | 79.20 | 0.12 | 265.73 | 145.85 | 488.52 | 75.51 | 8.08 | 0.92 | 1984.26 | 550.11 | |
| CHTNG-1 | 8.40 | 64.60 | 7.39 | 127.81 | 17.81 | 275.51 | 19.42 | 1.58 | 0.04 | 458.27 | 1054.29 | 365 ᵃ |
| CHTNG-2 | 8.20 | 68.50 | 6.53 | 141.37 | 47.18 | 306.35 | 21.35 | 1.80 | 0.07 | 523.80 | 1177.89 | |
| CHTNG-3 | 7.14 | 84.10 | 7.10 | 125.95 | 99.39 | 261.30 | 19.09 | 2.17 | 0.20 | 469.63 | 1084.36 | |
| CHTNG-4 | 7.91 | 84.30 | 5.50 | 116.92 | 68.54 | 283.45 | 19.38 | 1.62 | 0.05 | 486.58 | 1099.82 | |
| CHTNG-5 | 7.95 | 82.80 | 6.06 | 112.85 | 115.40 | 290.28 | 19.62 | 1.39 | 0.07 | 514.03 | 1141.02 | |
| PAN-1.1 | 9.12 | 70.60 | 8.48 | 39.33 | n.a. | n.a. | n.a. | n.a. | n.a. | 44.94 | 479.01 | 237 ᵃ |
| PAN-1.2 | 10.62 | 70.70 | 8.28 | 124.77 | 0.67 | 134.73 | 5.78 | 11.97 | 0.24 | 42.55 | 470.09 | |
| PAN-1.3 | 9.56 | 70.70 | 7.77 | 39.13 | 0.94 | 160.29 | 6.48 | 11.54 | 0.10 | 44.21 | 483.96 | |
| PAN-1.4 | 10.11 | 69.00 | 9.04 | 41.66 | 4.31 | 132.80 | 5.59 | 11.42 | 0.10 | 44.80 | 488.00 | |
| PAN-1.5 | 10.36 | 68.00 | 8.70 | 43.74 | 1.49 | 122.08 | 5.21 | 11.68 | 0.17 | 39.91 | 435.66 | |
| PAN-1.6 | 10.75 | 65.20 | 10.08 | 51.22 | 2.06 | 140.73 | 5.99 | 10.99 | 0.08 | 45.19 | 498.52 | |
| PAN-1.7 | 10.70 | 63.00 | 9.49 | 48.03 | 3.74 | 134.37 | 5.68 | 10.22 | 0.10 | 45.38 | 493.16 | |
| PAN-1.8 | 9.93 | 67.70 | 8.10 | 37.45 | 0.67 | 131.42 | 5.65 | 10.32 | 0.08 | 41.56 | 463.34 | |
| PAN-1.9 | 8.80 | 66.90 | 9.59 | 49.57 | 0.76 | 139.01 | 6.08 | 10.74 | 0.18 | 45.35 | 487.90 | |
| PAN-5.1 | 11.20 | 65.50 | 12.78 | 44.75 | 0.92 | 136.02 | 5.94 | 12.46 | 0.10 | 42.84 | 470.66 | |
| PAN-5.2 | 10.52 | 67.60 | 13.61 | 49.82 | 0.55 | 135.17 | 5.78 | 12.92 | 0.08 | 43.30 | 547.66 | |
| PAN-5.3 | 7.96 | 72.30 | 12.04 | 41.21 | 0.60 | 137.42 | 5.96 | 13.09 | 0.09 | 42.78 | 467.00 | |
| PAN-5.4 | 8.22 | 74.40 | 11.71 | 38.40 | 0.55 | 137.29 | 5.99 | 11.88 | 0.16 | 42.05 | 460.05 | |
| Shyok River | 10.25 | 17.50 | 1.08 | <0.000 | 0.21 | 15.33 | 4.22 | 22.98 | 11.20 | n.a. | n.a. | n.a. |
| Ladakh Rivers Average b | 7.62 | n.a. | n.a. | n.a. | n.a. | 5.13 | 1.96 | 21.57 | 4.30 | 3.00 | 14.30 | 82.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, A.H.; Singh, V.K.; Kumar, P.; Sharma, M.; Sharma, A.; Patnaik, S.; Gundiga, G.P.; Rahi, I.C.; Ansari, M.A.; Ramanathan, A. Hydrogeochemistry, Geothermometry, and Sourcing of High Dissolved Boron, Tungsten, and Chlorine Concentrations in the Trans-Himalayan Hotsprings of Ladakh, India. Hydrology 2023, 10, 118. https://doi.org/10.3390/hydrology10060118
Ansari AH, Singh VK, Kumar P, Sharma M, Sharma A, Patnaik S, Gundiga GP, Rahi IC, Ansari MA, Ramanathan A. Hydrogeochemistry, Geothermometry, and Sourcing of High Dissolved Boron, Tungsten, and Chlorine Concentrations in the Trans-Himalayan Hotsprings of Ladakh, India. Hydrology. 2023; 10(6):118. https://doi.org/10.3390/hydrology10060118
Chicago/Turabian StyleAnsari, Arif H., Veeru Kant Singh, Pankaj Kumar, Mukund Sharma, Anupam Sharma, Satyakam Patnaik, Gurumurthy P. Gundiga, Ishwar Chandra Rahi, Mohammad Arif Ansari, and AL Ramanathan. 2023. "Hydrogeochemistry, Geothermometry, and Sourcing of High Dissolved Boron, Tungsten, and Chlorine Concentrations in the Trans-Himalayan Hotsprings of Ladakh, India" Hydrology 10, no. 6: 118. https://doi.org/10.3390/hydrology10060118
APA StyleAnsari, A. H., Singh, V. K., Kumar, P., Sharma, M., Sharma, A., Patnaik, S., Gundiga, G. P., Rahi, I. C., Ansari, M. A., & Ramanathan, A. (2023). Hydrogeochemistry, Geothermometry, and Sourcing of High Dissolved Boron, Tungsten, and Chlorine Concentrations in the Trans-Himalayan Hotsprings of Ladakh, India. Hydrology, 10(6), 118. https://doi.org/10.3390/hydrology10060118









