Techno-Economic Analysis of Biogas Upgrading via CO2 Methanation for Sustainable Biomethane Production
Abstract
1. Introduction
2. Methodology
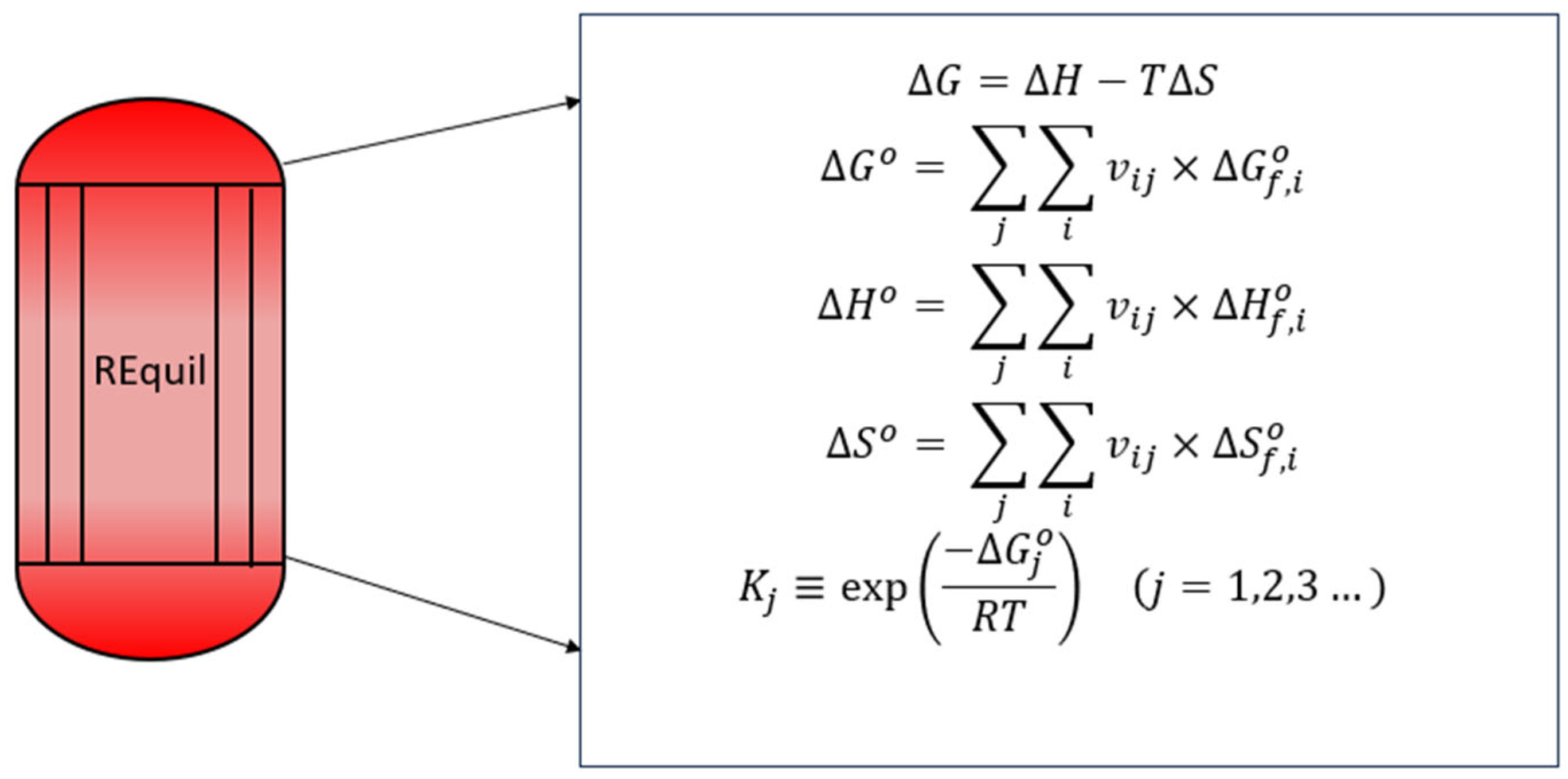
2.1. Equilibrium Studies Investigation
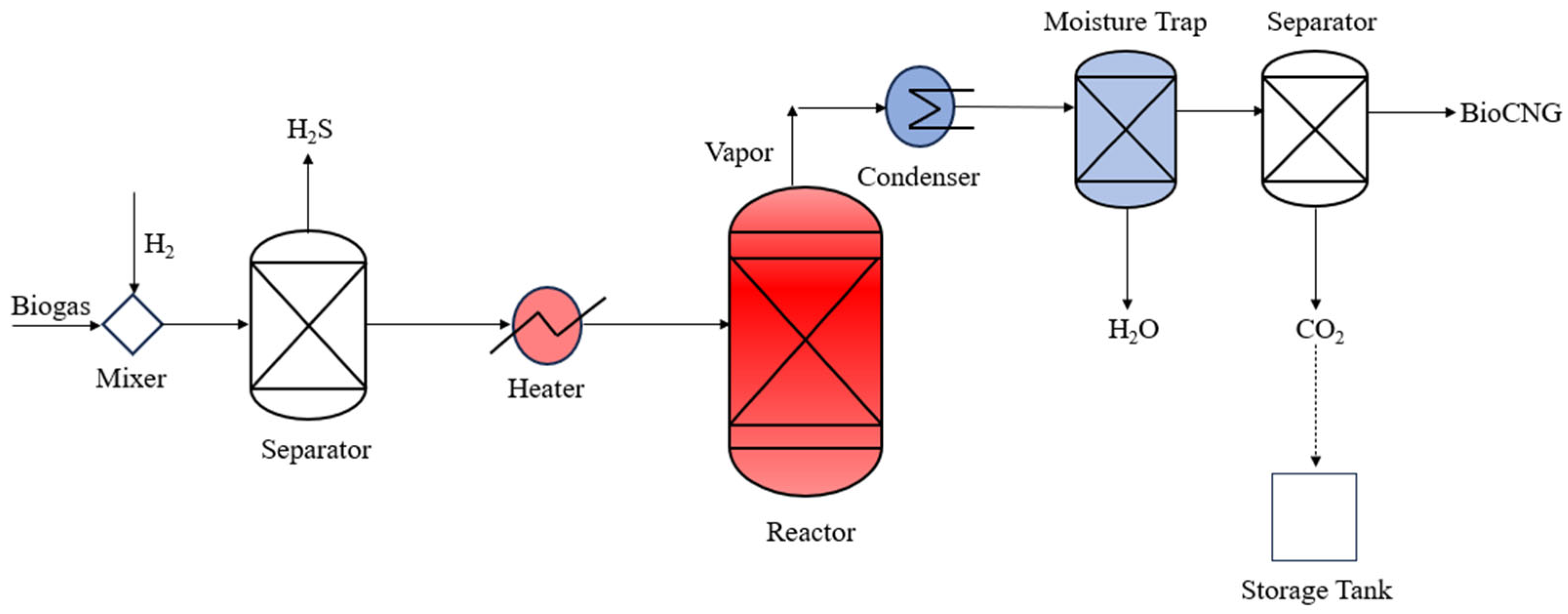
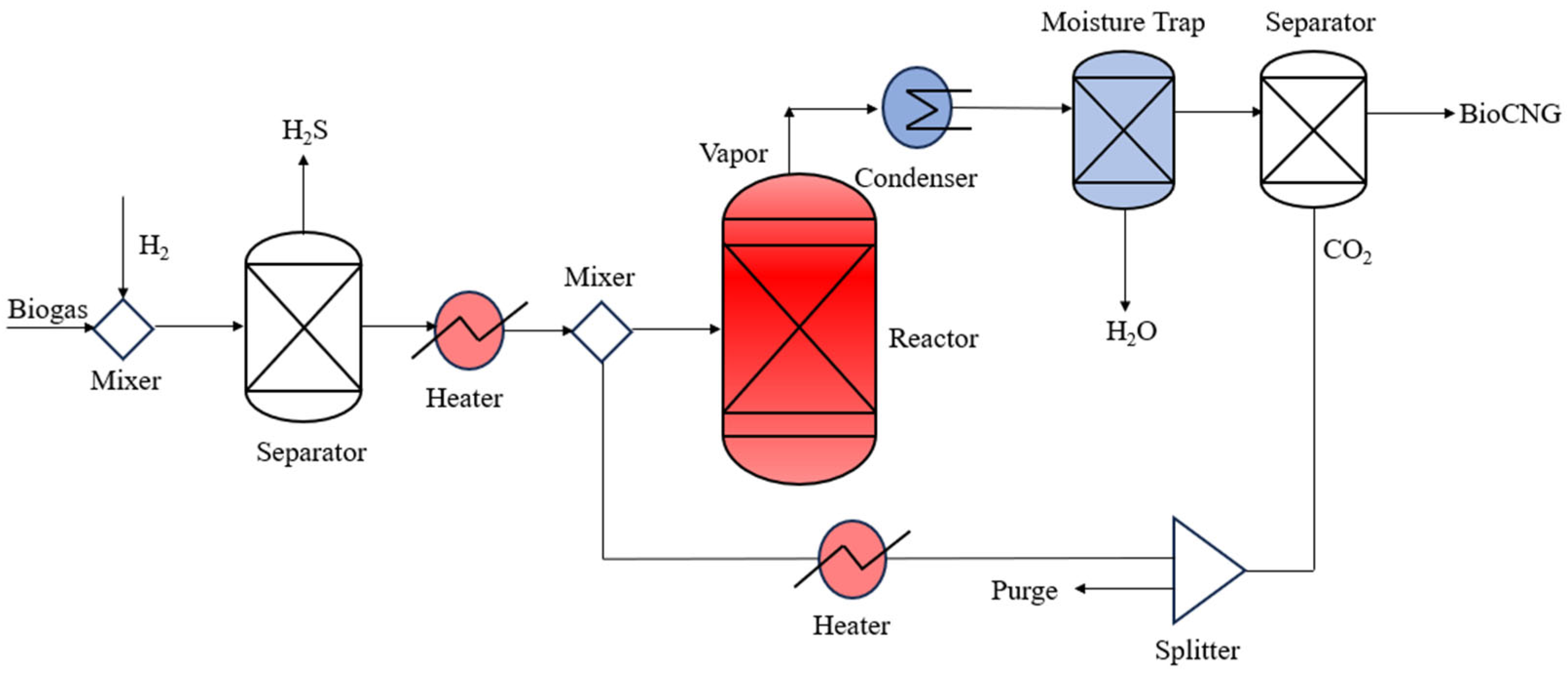
2.2. Development of Flowsheet
2.2.1. Non-Recycling Approach
2.2.2. Recycling Approach
2.3. Techno-Economic Analysis
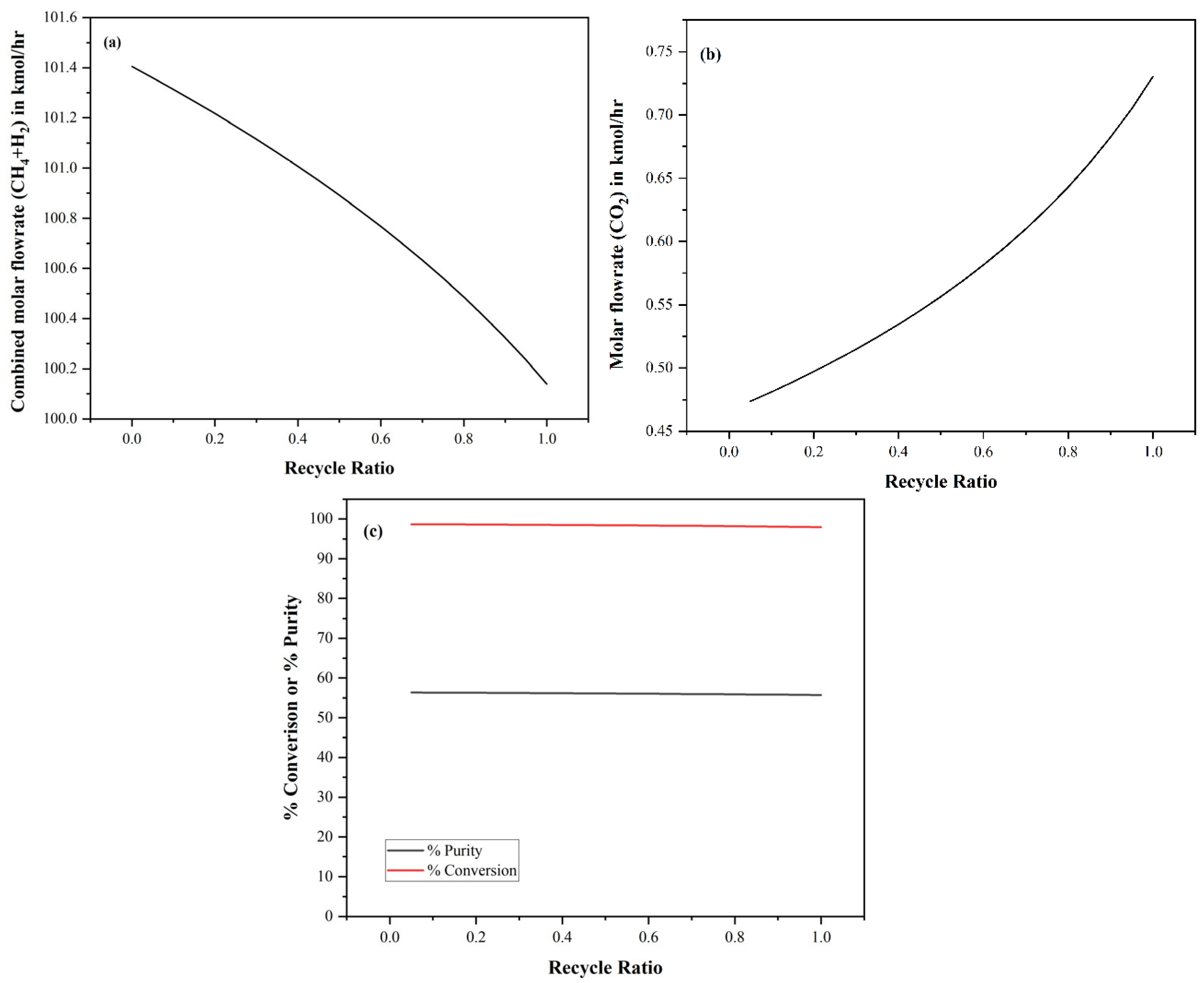
3. Results and Discussion
3.1. Influence of Temperature on Gibbs Free Energy
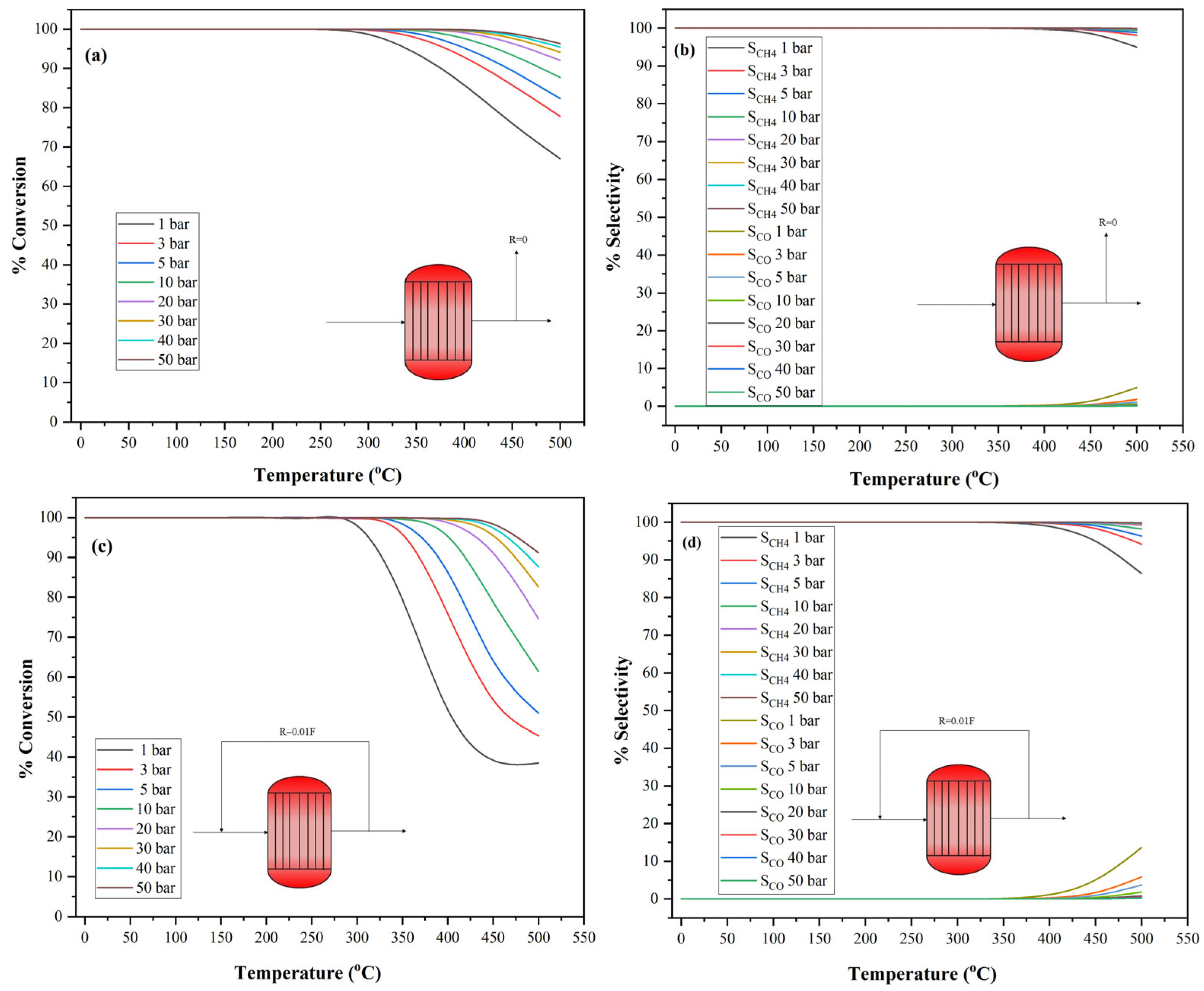
3.2. Influence of Temperature and Pressure
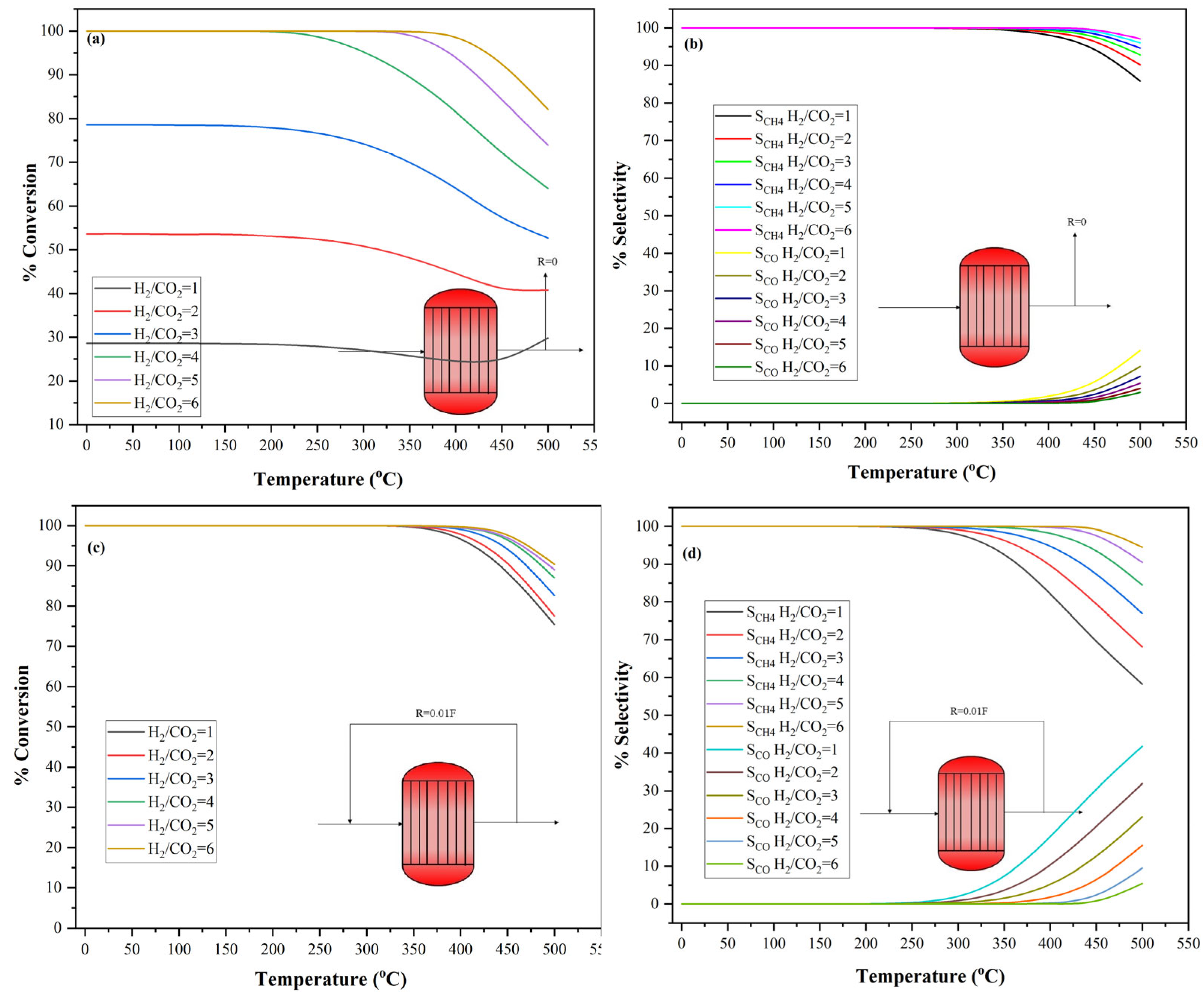
3.3. Influence of H2/CO2 Ratio
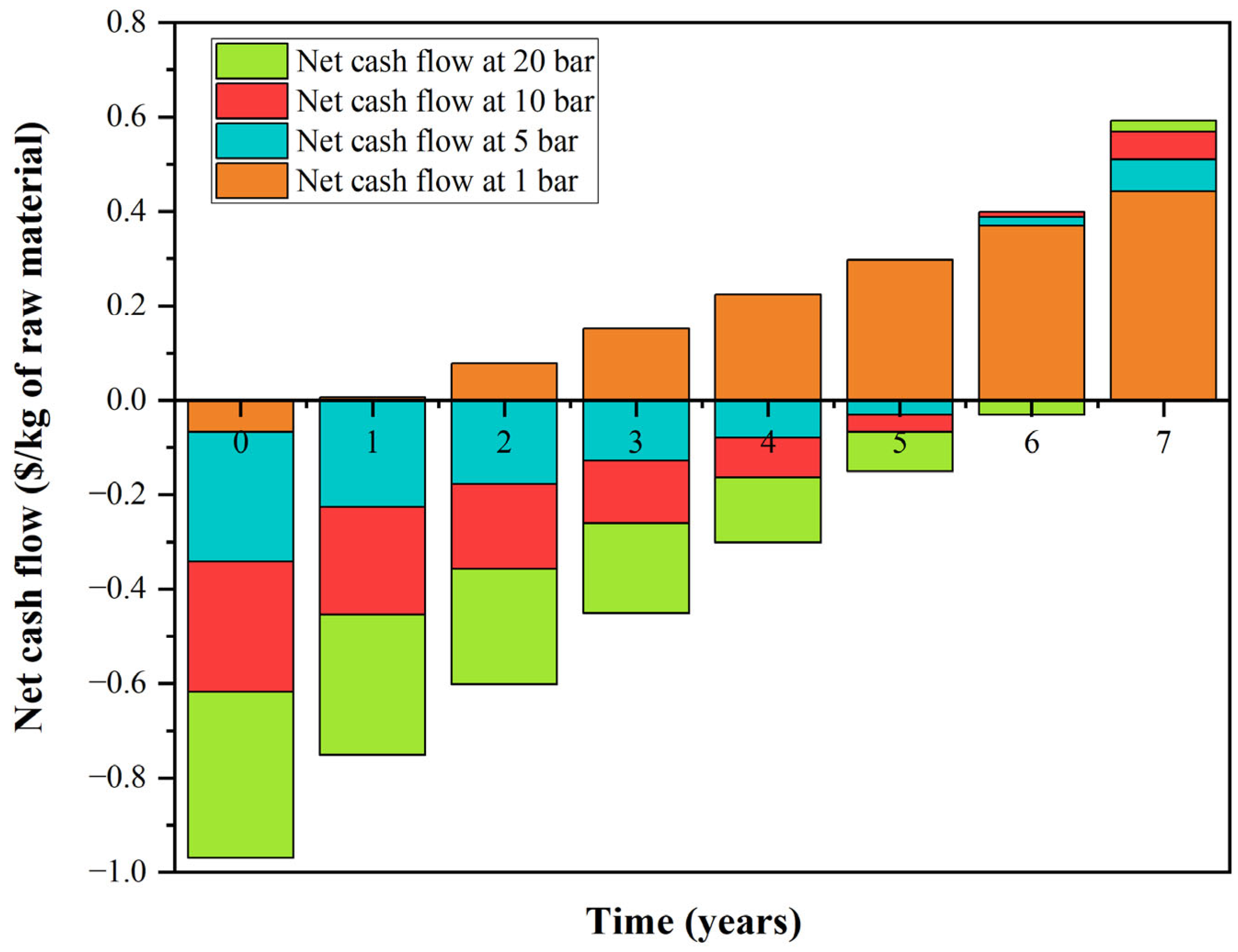
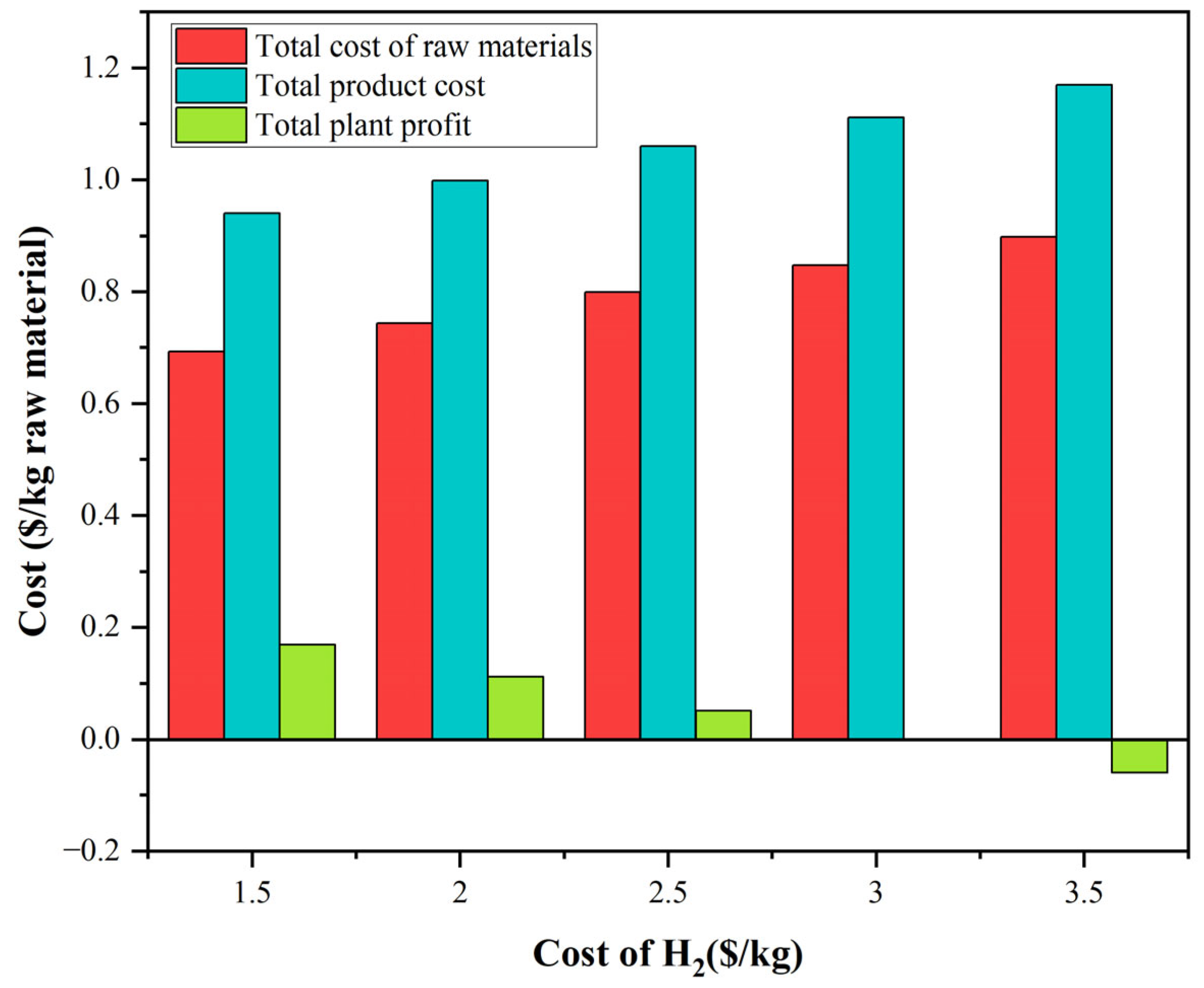
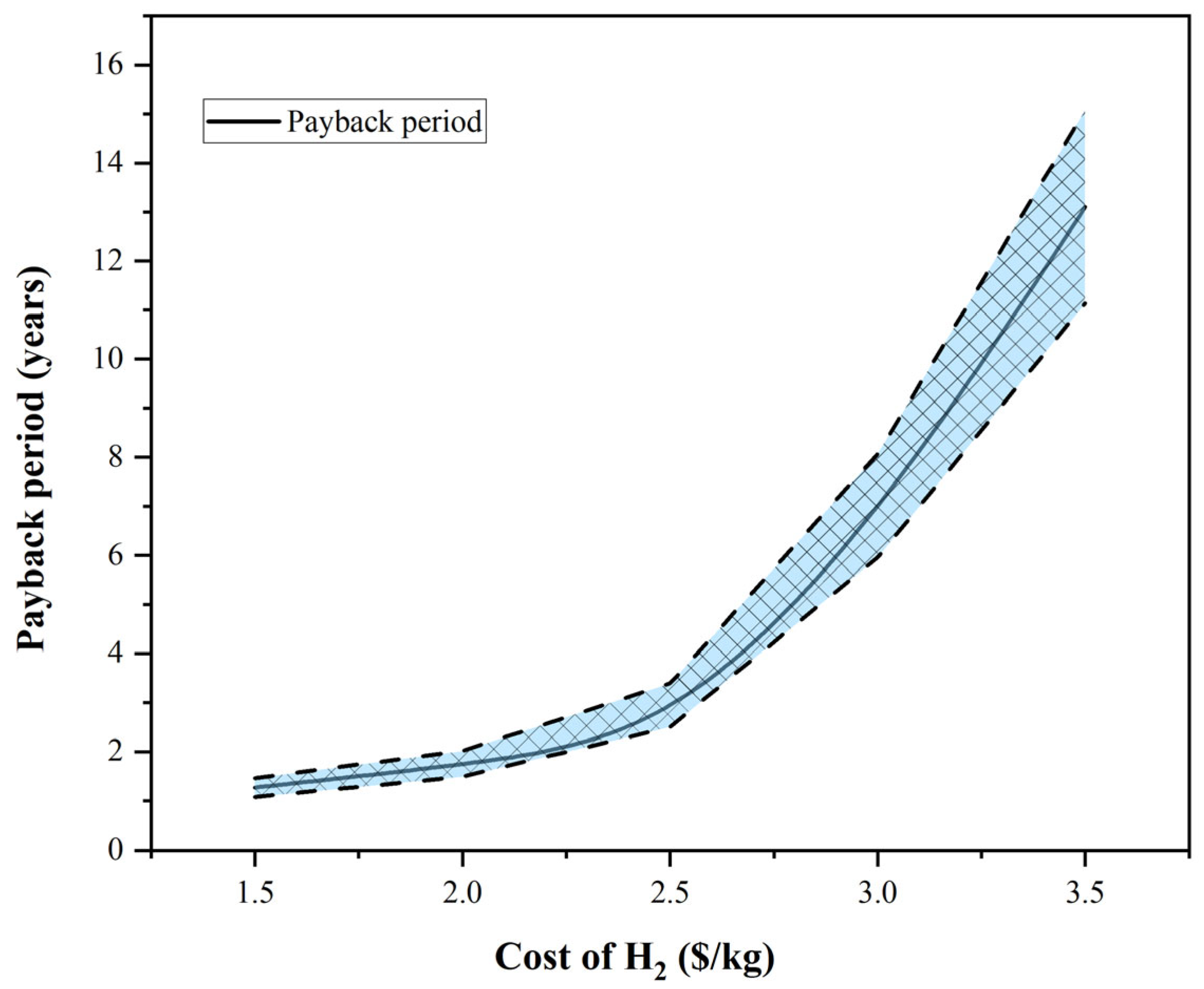
3.4. Techno-Economics Discussion
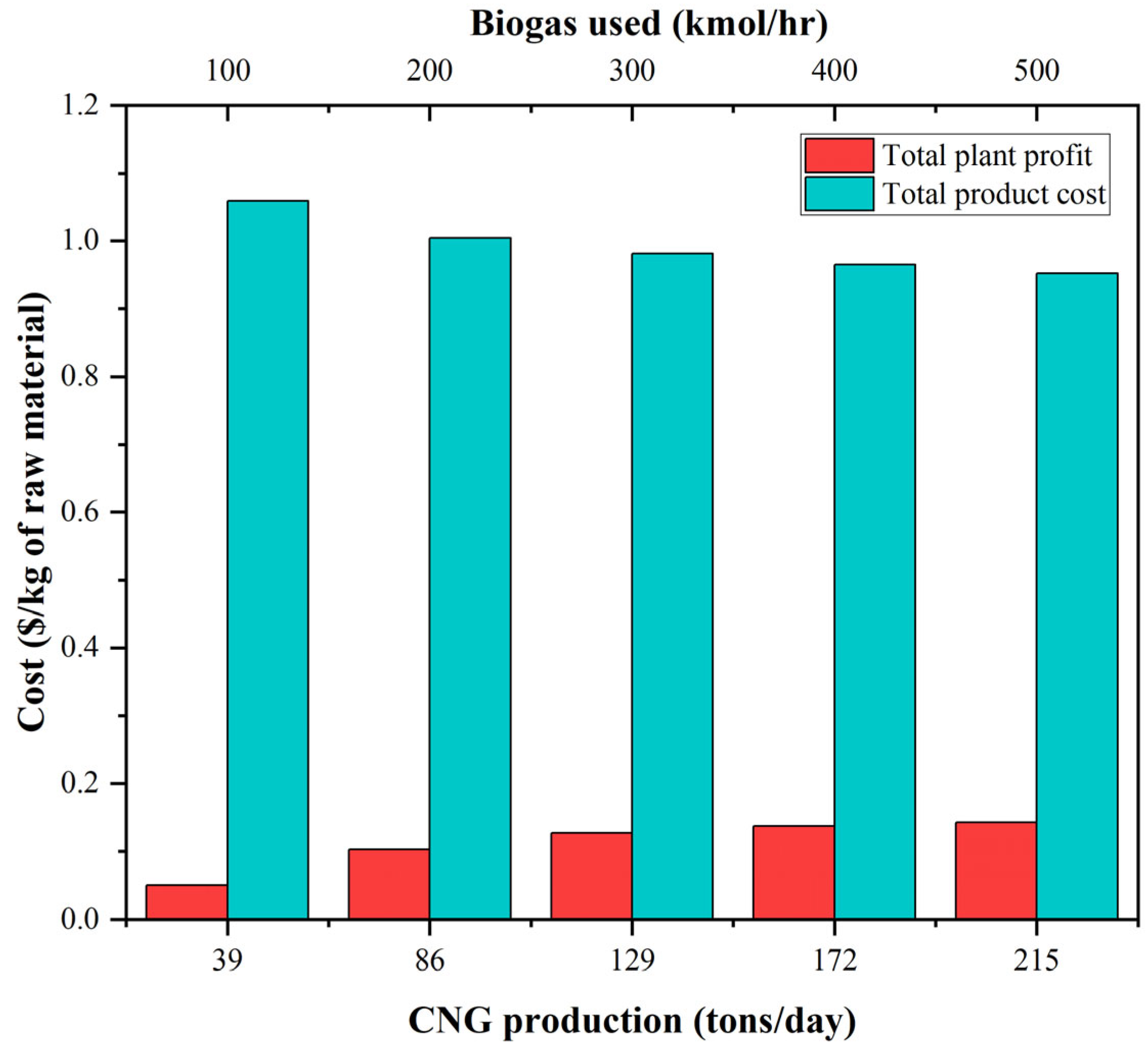
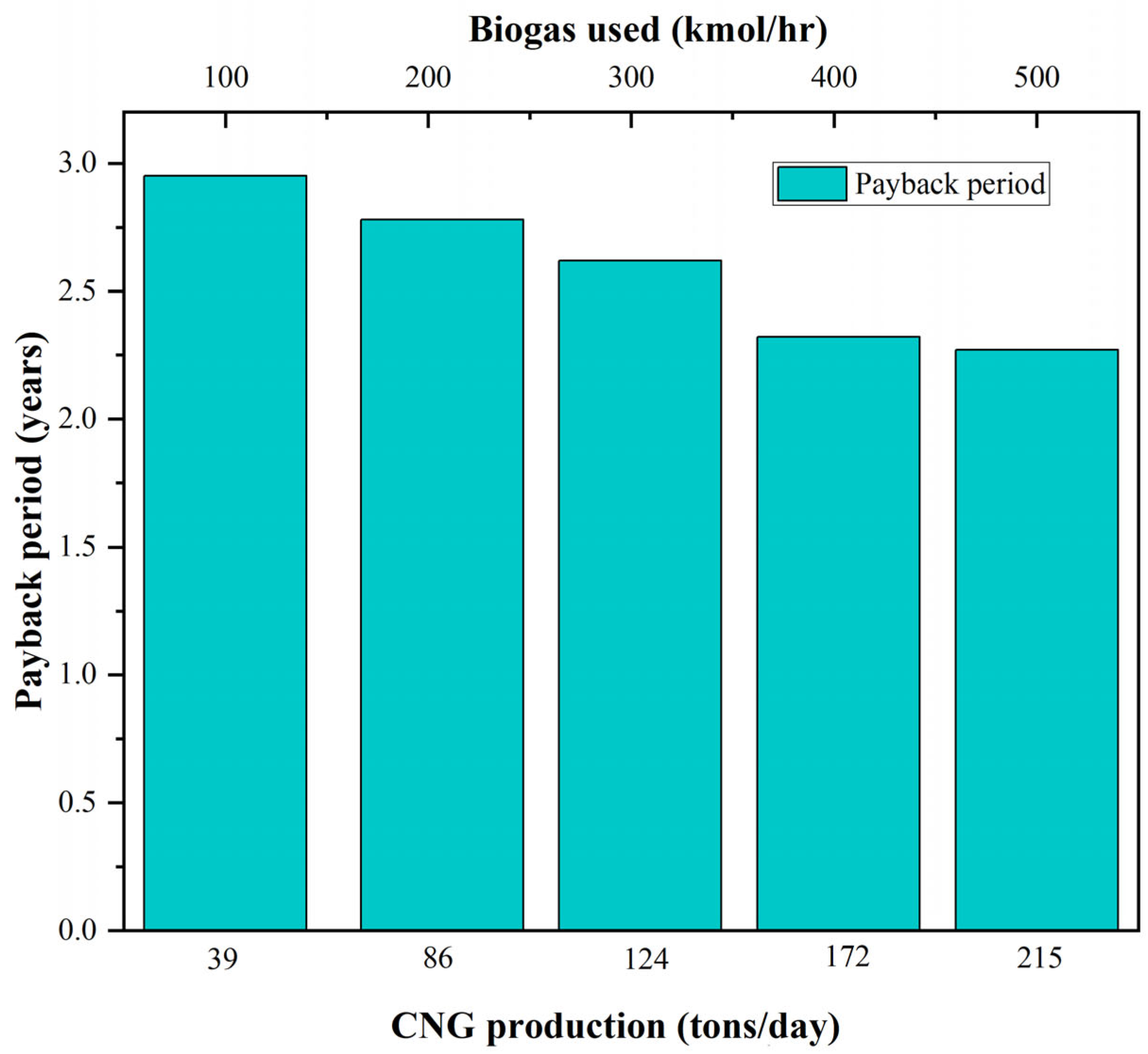
3.5. Energy Integration and Economics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bio-CNG | Biomethane |
| Bio-H-CNG | Biogenic hydrogen-enriched compressed natural gas |
| CCUS | Carbon capture, Utilization and Storage |
| CNG | Compressed Natural Gas |
| DC | Direct Costs |
| FCI | Fixed Capital Investment |
| f.o.b. | Free-On-Board |
| GHG | Greenhouse gas |
| GHSV | Gas Hourly Space Velocity |
| GW | Giga Watt |
| IC | Indirect Costs |
| KPI | Key Performance Indicator |
| MJ | Mega Joule |
| RWGS | Reverse Water Gas Shift |
| SMR | Steam Methane Reforming |
| TCI | Total Capital Investment |
| TPC | Total Product Cost |
| TPP | Total Plant Profit |
| TPS | Total Product Sales |
| WCI | Working Capital Investment |
References
- Jiang, Z.; Xiao, T.; Kuznetsov, V.L.; Edwards, P.P. Turning carbon dioxide into fuel. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3343–3364. [Google Scholar] [CrossRef] [PubMed]
- World Meteorological Organization Report. Available online: https://wmo.int/media/news/record-carbon-emissions-highlight-urgency-of-global-greenhouse-gas-watch#:~:text=Totalcarbondioxide(CO2)emissions,totheGlobalCarbonBudget (accessed on 18 June 2025).
- IEA Greenhouse Gas Emissions Data. Available online: https://www.iea.org/data-and-statistics/data-tools/greenhouse-gas-emissions-from-energy-data-explorer (accessed on 18 June 2025).
- IRENA Renewable Energy Statistics. 2024. Available online: https://www.irena.org/Publications/2024/Mar/Renewable-capacity-statistics-2024 (accessed on 21 June 2025).
- IEA Bioenergy. Available online: https://www.iea.org/energy-system/renewables/bioenergy (accessed on 22 June 2025).
- Ravindranath, D.; Shroff, S.; Rao, N. Diffusion of renewable energy technologies: Case studies of enabling frameworks in developing countries. In Technology Transfer Perspectives Series; Haselip, J., Nygaard, I., Hansen, U., Ackom, E., Eds.; UNEP Risø Centre: Copenhagen, Denmark, 2011; pp. 129–145. [Google Scholar]
- Arora, D.S.; Busche, S.; Cowlin, S.; Engelmeier, T.; Jaritz, J.; Milbrandt, A.; Wang, S. Indian Renewable Energy Status Report: Background Report for DIREC 2010; No. NREL/TP-6A20-48948; National Renewable Energy Lab (NREL): Golden, CO, USA, 2010; Chapter 6; pp. 66–81. [Google Scholar]
- Nevzorova, T.; Kutcherov, V. Barriers to the wider implementation of biogas as a source of energy: A state-of-the-art review. Energy Strat. Rev. 2019, 26, 100414. [Google Scholar] [CrossRef]
- Surendra, K.C.; Tanaka, D.; Hashimoto, A.G.; Khanal, S.K. Biogas as a sustainable energy source for developing countries: Opportunities and challenges. Renew. Sustain. Energy Rev. 2014, 31, 846–859. [Google Scholar] [CrossRef]
- Singhal, S.; Agarwal, S.; Arora, S.; Sharma, P.; Singhal, N. Upgrading techniques for transformation of biogas to bio-CNG: A review. Int. J. Energy Res. 2017, 41, 1657–1669. [Google Scholar] [CrossRef]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and perspectives in converting biogas to transportation fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Matheri, A.N.; Sethunya, V.L.; Belaid, M.; Muzenda, E. Analysis of the biogas productivity from dry anaerobic digestion of organic fraction of municipal solid waste. Renew. Sustain. Energy Rev. 2018, 81, 2328–2334. [Google Scholar] [CrossRef]
- Barbera, E.; Menegon, S.; Banzato, D.; Alpaos, C.D.; Bertucco, A. From biogas to biomethane: A process simulation-based techno-economic comparison of different upgrading technologies in the Italian context. Renew. Energy 2019, 135, 663–673. [Google Scholar] [CrossRef]
- IEA. An Introduction to Biogas and Biomethane, in Outlook for Biogas and Biomethane: Prospects for Organic Growth. Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth/an-introduction-to-biogas-and-biomethane (accessed on 22 June 2025).
- Prajapati, B.K.; Anand, A.; Gautam, S.; Singh, P. Production of hydrogen and methane rich gas by stepped pyrolysis of biomass and its utilization in IC engines. Clean Technol. Environ. Policy 2022, 24, 1375–1388. [Google Scholar] [CrossRef]
- Agrawal, D.; Mahajan, N.; Singh, S.A.; Sreedhar, I. Green hydrogen production pathways for sustainable future with net zero emissions. Fuel 2024, 359, 130131. [Google Scholar] [CrossRef]
- Santhosh, J.; Sarkar, O.; Mohan, S.V. Bioresource Technology Green Hydrogen-Compressed natural gas (bio-H-CNG) production from food waste: Organic load influence on hydrogen and methane fusion. Bioresour. Technol. 2021, 340, 125643. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A. International Journal of Hydrogen Energy Recent advances of biogas reforming for hydrogen production: Methods, purification, utility and techno-economics analysis. Int. J. Hydrogen Energy 2024, 76, 108–140. [Google Scholar] [CrossRef]
- IEA Global Hydrogen Review. Available online: https://www.iea.org/reports/global-hydrogen-review-2022 (accessed on 22 June 2025).
- Chae, M.J.; Kim, J.H.; Moon, B.; Park, S.; Lee, Y.S. The present condition and outlook for hydrogen-natural gas blending technology. Korean J. Chem. Eng. 2022, 39, 251–262. [Google Scholar] [CrossRef]
- Omar, M.; Seyed, A.; Hoseyni, M.; Cordiner, J. Fuelling the Future with Safe Hydrogen Transportation Through Natural Gas Pipelines: A Quantitative Risk Assessment Approach Centre for Chemical Process Safety as Low as Reasonably Practicable. Trans. Indian Natl. Acad. Eng. 2024, 9, 763–781. [Google Scholar]
- Sanz-Monreal, P.; Gil, J.; Sanz-Pérez, A. Techno-economic assessment of a plant for the upgrading of MSW biogas to synthetic natural gas by thermocatalytic methanation. J. Environ. Chem. Eng. 2025, 13, 112345. [Google Scholar] [CrossRef]
- Gagliano, A.; Nocera, F.; Bruno, M.; Cardillo, G. Development of an equilibrium-based model of gasification of biomass by Aspen Plus. Energy Procedia 2017, 111, 1010–1019. [Google Scholar] [CrossRef]
- Leonzio, G. Process analysis of biological Sabatier reaction for bio-methane production. Chem. Eng. J. 2016, 290, 490–498. [Google Scholar] [CrossRef]
- Calbry-muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas composition from agricultural sources and organic fraction of municipal solid waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- Javier, Ó.; Bermúdez, F.; Giraldo, L.; Sierra, R.; Juan, R.; Moreno, C. Removal of hydrogen sulfide from biogas by adsorption and photocatalysis: A review. Environ. Chem. Lett. 2023, 21, 1059–1073. [Google Scholar]
- Al-Samadi, M.; Ibrahim, A.; Abdullah, B.; El-Naas, M.H. Iron-based sorbents for H2S removal from biogas: A review. Arab. J. Chem. 2024, 17, 105821. [Google Scholar]
- Zhang, Y.; Kawasaki, Y.; Oshita, K.; Takaoka, M.; Minami, D.; Inoue, G.; Tanaka, T. Economic assessment of biogas purification systems for removal of both H2S and siloxane from biogas. Renew. Energy 2021, 168, 119–130. [Google Scholar] [CrossRef]
- Rönsch, S.; Köchermann, J.; Schneider, J.; Matthischke, S. Global Reaction Kinetics of CO and CO2 Methanation for Dynamic Process Modeling. Chem. Eng. Technol. 2016, 39, 208–218. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kaydouh, M.N.; Ahmed, H.; Ibrahim, A.A.; Alotibi, M.F.; Osman, A.I.; El Hassan, N. Sr Promoted Ni/W-Zr Catalysts for Highly Efficient CO2 Methanation: Unveiling the Role of Surface Basicity. Langmuir 2023, 39, 17723–17732. [Google Scholar] [CrossRef] [PubMed]
- Vrijburg, W.L.; Moioli, E.; Chen, W.; Zhang, M.; Terlingen, B.J.P.; Zijlstra, B.; Filot, I.A.W.; Züttel, A.; Pidko, E.A.; Hensen, E.J.M. Efficient Base-Metal NiMn/TiO2 Catalyst for CO2 Methanation. ACS Catal. 2019, 9, 7823–7839. [Google Scholar] [CrossRef]
- Sreedhar, I.; Varun, Y.; Singh, S.A.; Venugopal, A.; Reddy, B.M. Developmental trends in CO2 methanation using various catalysts. Catal. Sci. Technol. 2019, 9, 4478–4504. [Google Scholar] [CrossRef]
- Chitturi, H.S.; Lavanya, Y.; Varun, Y.; Ramesh, A.; Pamu, S.H.; Sreedhar, I.; Singh, S.A. Unraveling the CO2 methanation and capture ability of NiO@metal oxides. J. Mater. Chem. A 2025, 10, 7422–7444. [Google Scholar] [CrossRef]
- Dannesboe, S.; Hansen, J.B.; Johannsen, J.R. Catalytic methanation of CO2 in biogas: Experimental results from a reactor at full scale. React. Chem. Eng. 2020, 5, 683–692. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, H.; Ro, Y.; Kim, J.; Chung, W.; Chang, S. Development of Pilot-Scale CO2 Methanation Using Pellet-Type Catalysts for CO2 Recycling in Sewage Treatment Plants and Its Validation through Computational Fluid Dynamics (CFD) Modeling. Catalysts 2021, 11, 1005. [Google Scholar] [CrossRef]
- Mastroianni, L.; Medina, A.; De Domenico, A.; Eränen, K.; Di Serio, M.; Murzin, D.; Russo, V.; Salmi, T. DLP 3D Printing of Alumina Catalyst Architectures: Design, Kinetics and Modelling of Structure Effects on Catalyst Performance. Chem. Eng. J. 2024, 501, 157691. [Google Scholar] [CrossRef]
- Kanuri, S.; Vinodkumar, J.D.; Datta, S.P.; Chakraborty, C.; Roy, S.; Singh, S.A.; Dinda, S. Methanol synthesis from CO2 via hydrogenation route: Thermodynamics and process development with techno-economic feasibility analysis. Korean J. Chem. Eng. 2022, 40, 810–823. [Google Scholar] [CrossRef]
- Bown, R.M.; Joyce, M.; Zhang, Q.; Reina, T.R.; Duyar, M.S. Identifying Commercial Opportunities for the Reverse Water Gas Shift Reaction. Energy Technol. 2021, 9, 28–31. [Google Scholar] [CrossRef]
- Kakoee, A.; Gharehghani, A. Carbon oxides methanation in equilibrium: A thermodynamic approach. Int. J. Hydrogen Energy 2020, 45, 29993–30008. [Google Scholar] [CrossRef]
- Swapnesh, A.; Srivastava, V.C.; Mall, I.D. Comparative Study on Thermodynamic Analysis of CO2 Utilization Reactions. Chem. Eng. Technol. 2014, 37, 1765–1777. [Google Scholar] [CrossRef]
- Varandas, B.; Oliveira, M.; Andrade, C.; Borges, A. Thermodynamic Equilibrium Analysis of CO2 Methanation through Equilibrium Constants: A Comparative Simulation Study. Physchem 2024, 4, 258–271. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Santos, M.F.; Bresciani, A.E.; Ferreira, N.L.; Bassani, G.S.; Alves, R.M.B. Carbon dioxide conversion via reverse water-gas shift reaction: Reactor design. J. Environ. Manag. 2023, 345, 118822. [Google Scholar] [CrossRef]
- Yeo, C.E.; Seo, M.; Kim, D.; Jeong, C.; Shin, H.S.; Kim, S. Optimization of operating conditions for CO2 methanation process using design of experiments. Energies 2021, 14, 8414. [Google Scholar] [CrossRef]
- Bimetallic, C.C.N.T.; Tropsch, F.; Akbarzadeh, O.; Alshahateet, S.F.; Asmawati, N.; Zabidi, M.; Moosavi, S.; Chowdhury, Z.Z.; Sagadevan, S.; Temperature, E.O. Syngas Space Velocity and Catalyst Stability of Co-Mn/CNT Bimetallic Catalyst on Fischer Tropsch Synthesis Performance. Catalysts 2021, 11, 846. [Google Scholar]
- Adelung, S.; Dietrich, R. Impact of the reverse water-gas shift operating conditions on the Power-to-Liquid fuel production cost. Fuel 2022, 317, 123440. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.; Siddique, Z. Hydrogen as an alternative fuel: A comprehensive review of challenges and opportunities in production, storage, and transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Mar, J.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Braga, L.B.; Silveira, J.L.; Da Silva, M.E.; Tuna, C.E.; Machin, E.B.; Pedroso, D.T. Hydrogen production by biogas steam reforming: A technical, economic and ecological analysis. Renew. Sustain. Energy Rev. 2013, 28, 166–173. [Google Scholar] [CrossRef]
- Mansilha, C.; Barbosa-Póvoa, A.; Tarelho, L.; Fonseca, A. A comprehensive review of green hydrogen production technologies: Current status, challenges, research trends and future directions. Renew. Sustain. Energy Rev. 2026, 225, 116119. [Google Scholar] [CrossRef]
- Venizelou, V.; Poullikkas, A. The effect of carbon price towards green hydrogen power generation. Renew. Sustain. Energy Rev. 2025, 211, 115254. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, W.; Ngo, H.H.; Chen, Z.; Wei, C.; Bui, X.T.; Tung, T.V.; Zhang, H. Ways to assess hydrogen production via life cycle analysis. Sci. Total Environ. 2025, 977, 179355. [Google Scholar] [CrossRef] [PubMed]
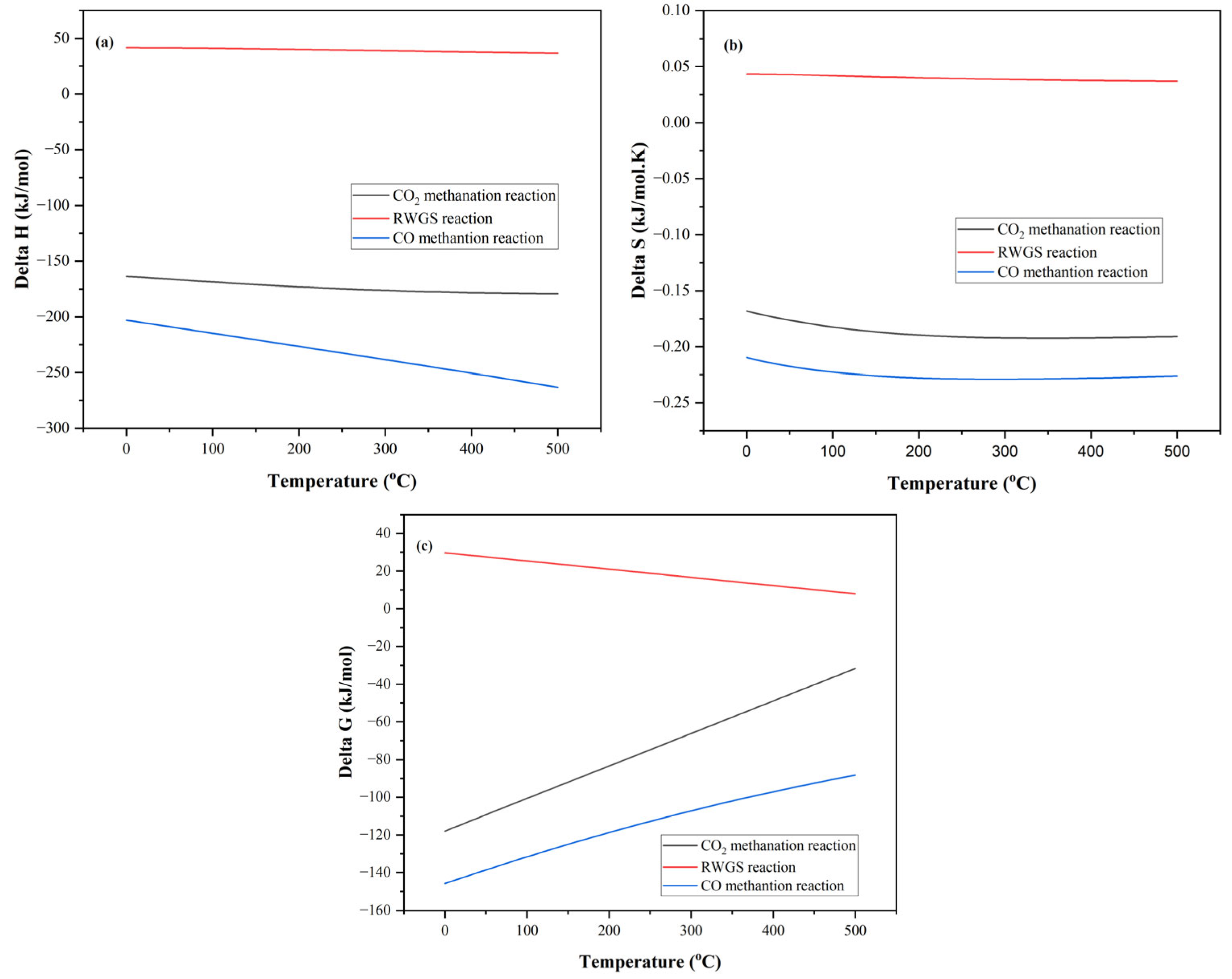
| Reaction Name | Reaction Stoichiometry | ∆H0298 | Reaction No. |
|---|---|---|---|
| CO2 Methanation | CO2 + 4 H2 ↔ CH4 + 2 H2O | −165 kJ/mol | 1 |
| Reverse Water Gas Shift | CO2 + H2 ↔ CO + H2O | 41.5 kJ/mol | 2 |
| CO Methanation | CO + 3 H2 ↔ CH4+ H2O | −206.1 kJ/mol | 3 |
| Component | Composition |
|---|---|
| Methane | 50 w/w% |
| Carbon Dioxide | 35 w/w% |
| Nitrogen | 8 w/w% |
| Hydrogen | 5 w/w% |
| Water | 1 w/w% |
| Hydrogen Sulphide | 0.9 w/w% |
| Oxygen | 0.1 w/w% |
| Hydrogen Type | Cost (USD/kg) | Emissions (kg CO2/kg H2) | Energy Requirement (MJ/kg) | Source of Hydrogen |
|---|---|---|---|---|
| Green H2 | 2.28–7.39 | 0 | 192 | Electrolysis |
| Black H2 | 1.2–2 | 20 | 236 | Gasification (no CCUS) |
| Grey H2 | 0.67–1.31 | 8.5 | 167 | Steam methane reforming (SMR) |
| Blue H2 | 0.99–2.05 | 2 | 169/223 | SMR + CCUS, Gasification + CCUS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, D.; Singh, S.A. Techno-Economic Analysis of Biogas Upgrading via CO2 Methanation for Sustainable Biomethane Production. ChemEngineering 2025, 9, 114. https://doi.org/10.3390/chemengineering9050114
Agrawal D, Singh SA. Techno-Economic Analysis of Biogas Upgrading via CO2 Methanation for Sustainable Biomethane Production. ChemEngineering. 2025; 9(5):114. https://doi.org/10.3390/chemengineering9050114
Chicago/Turabian StyleAgrawal, Diya, and Satyapaul A. Singh. 2025. "Techno-Economic Analysis of Biogas Upgrading via CO2 Methanation for Sustainable Biomethane Production" ChemEngineering 9, no. 5: 114. https://doi.org/10.3390/chemengineering9050114
APA StyleAgrawal, D., & Singh, S. A. (2025). Techno-Economic Analysis of Biogas Upgrading via CO2 Methanation for Sustainable Biomethane Production. ChemEngineering, 9(5), 114. https://doi.org/10.3390/chemengineering9050114






