Petroleum Emulsion Stability and Separation Strategies: A Comprehensive Review
Abstract
1. Introduction
2. Formation and Classification of Petroleum Emulsions
2.1. Emulsion Formation
2.2. Types of Petroleum Emulsions
- water-in-oil (W/O) emulsions;
- oil-in-water (O/W) emulsions;
- multiple emulsions, where both types coexist in more complex structures.
3. Stability of Petroleum Emulsions
3.1. Crude Oil Composition and Interfacial Components
3.1.1. Role of Asphaltenes
3.1.2. Role of Resins
3.1.3. Influence of Acidic Compounds
3.1.4. Effect of Wax Crystals
3.1.5. Solid Particulates and Inorganic Species
3.2. Operational and Environmental Factors
3.2.1. Temperature Effects
3.2.2. Aqueous Phase pH
3.2.3. Droplet Size and Distribution
3.2.4. Effect of Mixing Time and Intensity
3.2.5. Demulsifier Concentration
3.3. Summary of Synergistic Effects on Emulsion Stability
4. Emulsion Destabilization Processes
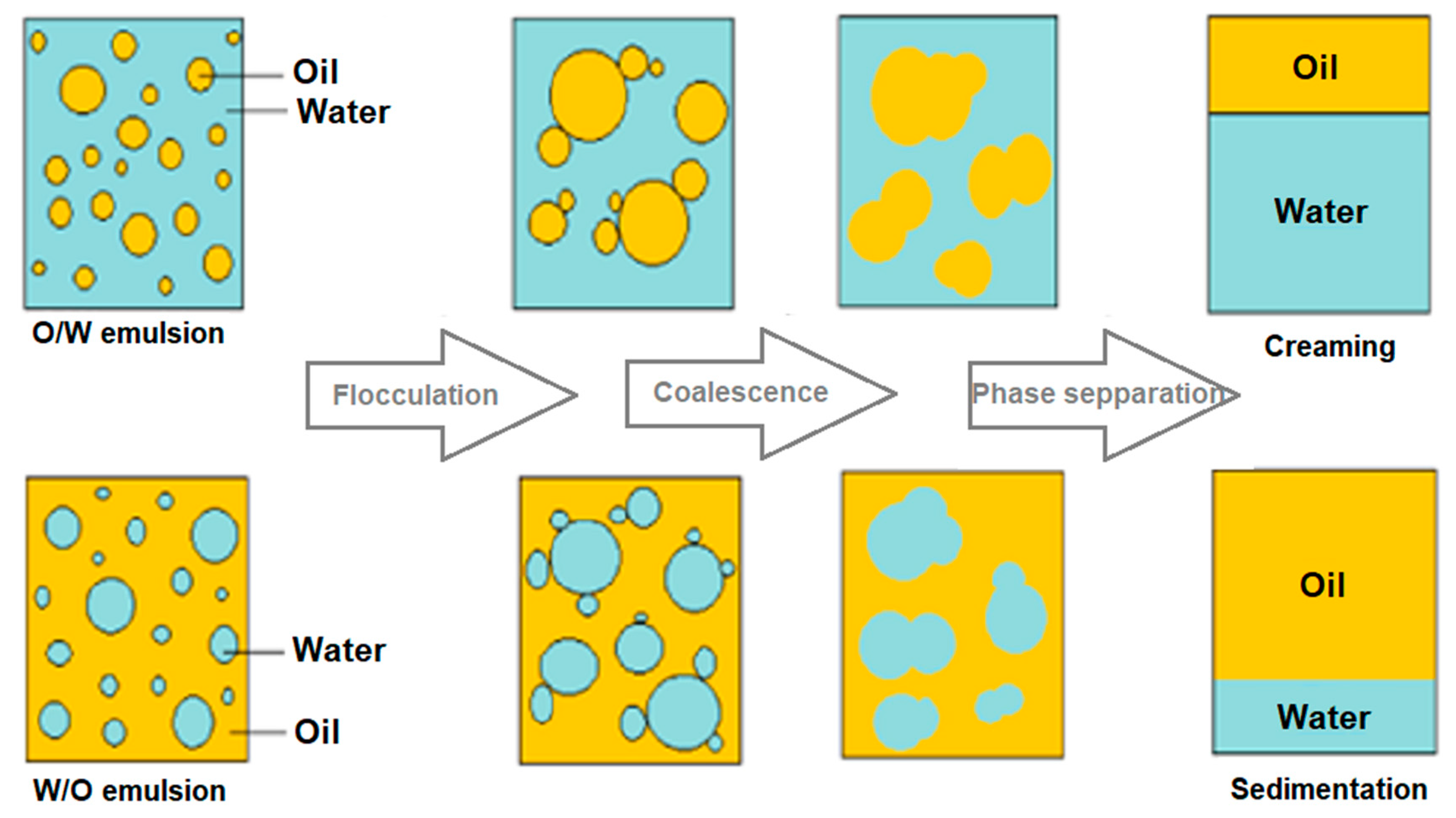
4.1. Flocculation and Coalescence
4.2. Ostwald Ripening
4.3. Creaming
4.4. Sedimentation
5. Demulsification Methods of Oil–Water Emulsions
5.1. Chemical Demulsification
| Demulsifier Class | Representative Compounds | Dominant Mechanism | Key Advantages | Limitations | Typical Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Anionic | Sodium salts of fatty acids (RCOONa), alkyl sulfonates, alkylnaphthalene sulfonates | Anionic headgroups neutralise positive droplet charges in W/O emulsions; reduce electrostatic repulsion to enable coalescence | Low cost; simple synthesis; effective in low–moderate salinity | High dosage (>100 mg/L); poor high-salinity performance; reduced efficiency in O/W | Moderate efficiency; >100 mg/L often required | [168,169] |
| Cationic | Quaternary ammonium salts, polyether–polyquaternium (PPA) | Neutralisation of negative charges on O/W droplets; promotes coalescence and possible hydrogen bonding | High O/W efficiency; PPA dehydration 80.6%; some antimicrobial effect | Limited W/O activity; potential aquatic toxicity | 80–90% separation at 50–100 mg/L | [170,171] |
| Ionic Liquids | Glucose-based GC@DA, pyridinium ILs, halide and non-halide variants | Strong interfacial adsorption via electrostatics, π–π stacking, and hydrogen bonding; displace asphaltenes | Tunable amphiphilicity; high activity at low concentration; low volatility | High synthesis cost; limited field-scale validation | >99% removal at ~15 mg/L | [172,173] |
| Non-Ionic | PO–EO Copolymers: EO–PO–EO and PO–EO–PO (linear, branched, star) | Amphiphilic molecules replace interfacial species and weaken films; steric hindrance prevents re-adsorption | Commercially dominant; EO/PO ratio adjustable; branched forms most effective | High PO ratio reduces performance; less effective for certain high-viscosity oils | High EO type: 20–50 mg/L; high PO type: >100 mg/L | [174,175] |
| PDMS Copolymers: EO–PDMS–EO, PO–PDMS–PO | PDMS backbone with EO/PO ends adsorbs at interface, displacing film components | Effective across crude types; hydrophobic PDMS enhances film disruption | Long PDMS chains may stabilise emulsions; higher cost than PO–EO | 30–80 mg/L | [176] | |
| EC Polymer: Ethyl cellulose with β-glucose backbone | Amphiphilicity enables penetration and rupture of asphaltene films | Biodegradable; tunable hydrophilic–lipophilic balance; rapid rupture | Limited data in high-salinity or high-viscosity systems | Rupture in ~20 s at ~50 mg/L | [177,178] | |
| Dendrimers: Polyamide dendrimers, CHPAMAM | Branched macromolecules penetrate and disrupt films; terminal groups bridge droplets | Strong interfacial activity; functionalisation possible | Slower diffusion at high conc.; complex synthesis | 20–40 mg/L typical | [179] | |
| Magnetic | Fe3O4–EC (M-EC), NH2-MNPs, M-mANP | Magnetic nanoparticles adsorb at droplet interface; bridging via surface functional groups; removed by magnetic field | Ultrafast (>98% in 2 min); potential for reuse | High cost; incomplete recovery; synthesis complexity | 99.7% at optimal loading | [180,181] |
5.2. Physical Demulsification
5.2.1. Thermal Demulsification
5.2.2. Mechanical Demulsification
5.2.3. Electrical Demulsification

5.2.4. Membrane Demulsification
5.2.5. Ultrasonic Demulsification
5.3. Biological Demulsification
5.4. Hybrid and Integrated Demulsification Strategies
6. Environmental and Sustainability Considerations
7. Smart and Bio-Based Emulsifiers: Relevance to EOR
7.1. Stimuli-Responsive Surfactants
7.2. Bio-Based Emulsifiers
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| APGs | alkyl polyglucosides |
| CHPAMAM | N-hexadecanoyl hyperbranched poly (amido-amine) |
| CMC | Critical Micelle Concentration |
| DC | Direct Current |
| DPDA | N-dodecylpropane-1,3-diamine |
| EOR | Enhanced Oil Recovery |
| EO | Ethylene Oxide |
| FBRM | Focused Beam Reflectance Measurement |
| GC@DA | Glucose-based dodecylamine ionic liquid demulsifier |
| HLB | Hydrophilic–Lipophilic Balance |
| IFT | Interfacial Tension |
| ILs | Ionic Liquids |
| LCST | Lower Critical Solution Temperature |
| M-EC | Magnetic Ethyl Cellulose |
| M-mANP | Magnetic nano-modified carboxylated polyether demulsifier |
| MNPs | Magnetic Nanoparticles |
| O/W | Oil-in-Water |
| O/W/O | Oil-in-Water-in-Oil |
| PDMS | Polydimethylsiloxane |
| PNVCL | Poly(N-vinyl caprolactam) |
| PO | Propylene Oxide |
| PVDF | Polyvinylidene Fluoride |
| STM | Scanning Tunneling Microscopy |
| W/O | Water-in-Oil |
| W/O/W | Water-in-Oil-in-Water |
References
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Houston, TX, USA, 2010. [Google Scholar]
- Mousavichoubeh, M.; Shariaty-Niassar, M.; Ghadiri, M. The effect of interfacial tension on secondary drop formation in electro-coalescence of water droplets in oil. Chem. Eng. Sci. 2011, 66, 5330–5337. [Google Scholar] [CrossRef]
- Evdokimov, I.N.; Losev, A.P. Microwave treatment of crude oil emulsions: Effects of water content. J. Pet. Sci. Eng. 2014, 115, 24–30. [Google Scholar] [CrossRef]
- Pereira, J.; Velasquez, I.; Blanco, R.; Sanchez, M.; Pernalete, C.; Canelón, C. Crude oil desalting process. In Advances in Petrochemicals; InTech Open: London, UK, 2015; pp. 1–11. [Google Scholar]
- Ahmadi, S.; Khormali, A. Development of an RSM-based predictive model for evaluation of corrosion efficiency of ATMP in one molar HCl for carbon steel samples. Pet. Sci. Technol. 2023, 42, 4537–4555. [Google Scholar] [CrossRef]
- Khormali, A.; Ahmadi, S. Synergistic effect between oleic imidazoline and 2-mercaptobenzimidazole for increasing the corrosion inhibition performance in carbon steel samples. Iran. J. Chem. Chem. Eng. 2023, 42, 321–336. [Google Scholar]
- Khormali, A.; Ahmadi, S.; Kazemzadeh, Y.; Karami, A. Evaluating the Efficacy of Binary Benzimidazole Derivatives as Corrosion Inhibitors for Carbon Steel Using Multi-Modal Analysis and Optimization Techniques. Results Eng. 2025, 26, 104671. [Google Scholar] [CrossRef]
- Paczuski, M. Physicochemistry of Petroleum Dispersions in Refining Technology; InTech Open: London, UK, 2024. [Google Scholar]
- Ahmadi, S.; Khormali, A.; Kazemzadeh, Y.; Razmjooie, A. Enhancing Dehydration/Desalting Efficiency of Crude Oil Emulsions through Experimental and Computational Insights. Results Eng. 2024, 24, 103094. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, S.; Ye, C.; Wang, M.; Cheng, H.; Wen, H.; Meng, X. Treatment of waste filature oil/water emulsion by combined demulsification and reverse osmosis. Sep. Purif. Technol. 2008, 63, 264–268. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Thring, R.W.; Hu, X.; Song, X. Oil recovery from refinery oily sludge via ultrasound and freeze/thaw. J. Hazard. Mater. 2012, 203, 195–203. [Google Scholar] [CrossRef]
- Wang, S.; Xu, X.; Yang, J.; Gao, J. Effect of the carboxymethyl chitosan on removal of nickel and vanadium from crude oil in the presence of microwave irradiation. Fuel Process. Technol. 2011, 92, 486–492. [Google Scholar] [CrossRef]
- Rashid, Z.; Wilfred, C.D.; Gnanasundaram, N.; Arunagiri, A.; Murugesan, T. A comprehensive review on the recent advances on the petroleum asphaltene aggregation. J. Pet. Sci. Eng. 2019, 176, 249–268. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Liu, Y.; Yin, J. Droplet size distribution in annular flow affected by the breakup and coalescence processes. Int. J. Heat Mass Transf. 2023, 215, 124539. [Google Scholar] [CrossRef]
- Sousa, A.M.; Matos, H.A.; Pereira, M.J. Properties of crude oil-in-water and water-in-crude oil emulsions: A critical review. Ind. Eng. Chem. Res. 2021, 61, 1–20. [Google Scholar] [CrossRef]
- Grace, R. Commercial Emulsion Breaking; ACS Publications: Columbus, OH, USA, 1992. [Google Scholar]
- Ho, T.M.; Razzaghi, A.; Ramachandran, A.; Mikkonen, K.S. Emulsion characterization via microfluidic devices: A review on interfacial tension and stability to coalescence. Adv. Colloid Interface Sci. 2022, 299, 102541. [Google Scholar] [CrossRef]
- Raya, S.A.; Mohd Saaid, I.; Abbas Ahmed, A.; Abubakar Umar, A. A critical review of development and demulsification mechanisms of crude oil emulsion in the petroleum industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1711–1728. [Google Scholar] [CrossRef]
- Saad, M.; Kamil, M.; Abdurahman, N.; Yunus, R.M.; Awad, O.I. An overview of recent advances in state-of-the-art techniques in the demulsification of crude oil emulsions. Processes 2019, 7, 470. [Google Scholar] [CrossRef]
- Al-Otaibi, M.; Elkamel, A.; Al-Sahhaf, T.; Ahmed, A. Experimental investigation of crude oil desalting and dehydration. Chem. Eng. Commun. 2003, 190, 65–82. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Tade, M.O.; Ali, H.A.; Alao, K.T. Demulsifier: An important agent in breaking crude oil emulsions. Chem. Eng. Technol. 2022, 45, 1707–1720. [Google Scholar] [CrossRef]
- Wong, S.; Lim, J.; Dol, S. Crude oil emulsion: A review on formation, classification and stability of water-in-oil emulsions. J. Pet. Sci. Eng. 2015, 135, 498–504. [Google Scholar] [CrossRef]
- Shen, L.; Ai, G.; Liu, H.; Zhu, L.; Lai, L.; Yan, X.; Yu, W.; Mi, Y. Synthesis and demulsification performance of a novel low-temperature demulsifier based on trimethyl citrate. J. Hazard. Mater. 2024, 472, 134543. [Google Scholar] [CrossRef]
- Ahmadi, S.; Khormali, A.; Kazemzadeh, Y. A Critical Review of the Phenomenon of Inhibiting Asphaltene Precipitation in the Petroleum Industry. Processes 2025, 13, 212. [Google Scholar] [CrossRef]
- Fingas, M.; Fieldhouse, B. Studies of the formation process of water-in-oil emulsions. Mar. Pollut. Bull. 2003, 47, 369–396. [Google Scholar] [CrossRef] [PubMed]
- Fingas, M.; Fieldhouse, B. Formation of water-in-oil emulsions and application to oil spill modelling. J. Hazard. Mater. 2004, 107, 37–50. [Google Scholar] [CrossRef]
- Roodbari, N.H.; Badiei, A.; Soleimani, E.; Khaniani, Y. Tweens demulsification effects on heavy crude oil/water emulsion. Arab. J. Chem. 2016, 9, S806–S811. [Google Scholar] [CrossRef]
- de Oliveira, C.B.; Souza, W.; Santana, C.; Santana, C.; Dariva, C.; Franceschi, E.; Guarnieri, R.; Fortuny, M.; Santos, A. Rheological properties of water-in-Brazilian crude oil emulsions: Effect of water content, salinity, and pH. Energy Fuels 2018, 32, 8880–8890. [Google Scholar] [CrossRef]
- Fridjonsson, E.O.; Graham, B.F.; Akhfash, M.; May, E.F.; Johns, M.L. Optimized droplet sizing of water-in-crude oil emulsions using nuclear magnetic resonance. Energy Fuels 2014, 28, 1756–1764. [Google Scholar] [CrossRef]
- Kokal, S. Crude-oil emulsions: A state-of-the-art review. SPE Prod. Facil. 2005, 20, 5–13. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The formation, stabilization and separation of oil–water emulsions: A review. Processes 2022, 10, 738. [Google Scholar] [CrossRef]
- Kumar, S.; Mahto, V. Emulsification of Indian heavy crude oil in water for its efficient transportation through offshore pipelines. Chem. Eng. Res. Des. 2016, 115, 34–43. [Google Scholar] [CrossRef]
- Genot, C.; Kabri, T.-H.; Meynier, A. Stabilization of omega-3 oils and enriched foods using emulsifiers. In Food Enrichment with Omega-3 Fatty Acids; Woodhead Publishing: Sawston, UK, 2013; pp. 150–193. [Google Scholar]
- Schmidts, T.; Dobler, D.; Guldan, A.-C.; Paulus, N.; Runkel, F. Multiple W/O/W emulsions—Using the required HLB for emulsifier evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 48–54. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Webster, T.J.; Kia, P.; Kalantari, K.; Misran, M.; Rasouli, E.; Maghareh Esfahan, Z.; Shameli, K. Nanoemulsions Based Therapeutic Strategies: Enhancing Targeted Drug Delivery against Breast Cancer Cells. Int. J. Nanomed. 2025, 20, 6133–6162. [Google Scholar] [CrossRef]
- Zhi, Z.; Liu, R.; Wang, W.; Dewettinck, K.; Van Bockstaele, F. Recent progress in oil-in-water-in-oil (O/W/O) double emulsions. Crit. Rev. Food Sci. Nutr. 2023, 63, 6196–6207. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Park, J.H.; Shin, S.i.; Oh, S.G. O/W/O multiple emulsions via one-step emulsification process. J. Dispers. Sci. Technol. 2004, 25, 53–62. [Google Scholar] [CrossRef]
- Salager, J.-L.; Marquez, R.; Delgado-Linares, J.G.; Rondon, M.; Forgiarini, A. Fundamental basis for action of a chemical demulsifier revisited after 30 years: HLDN as the primary criterion for water-in-crude oil emulsion breaking. Energy Fuels 2022, 36, 711–730. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, R.; Pu, W.; Liu, R.; Fang, S. Coalescence and separation of surfactant-stabilized water-in-oil emulsion via membrane coalescer functionalized by demulsifier. J. Clean. Prod. 2022, 330, 129945. [Google Scholar] [CrossRef]
- Grenoble, Z.; Trabelsi, S. Mechanisms, performance optimization and new developments in demulsification processes for oil and gas applications. Adv. Colloid Interface Sci. 2018, 260, 32–45. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Zhang, X.; Xiao, J.; An, S.; Luan, Z.; Liu, F.; Zhang, B. Dynamic demulsification of oil-in-water emulsions with electrocoalescence: Diameter distribution of oil droplets. Sep. Purif. Technol. 2021, 254, 117631. [Google Scholar] [CrossRef]
- Hussain, K.A.; Chen, C.; Haggerty, R.; Schubert, M.; Li, Y. Fundamental mechanisms and factors associated with nanoparticle-assisted enhanced oil recovery. Ind. Eng. Chem. Res. 2022, 61, 17715–17734. [Google Scholar] [CrossRef]
- Liu, M.; Hu, Z.; Peng, S.; Chen, J.; Qiao, P. Rapid demulsification and dehydration characteristics of Pickering emulsions under the action of an electric field. Pet. Sci. Technol. 2025, 43, 2789–2805. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A comprehensive review on emulsions and emulsion stability in chemical and energy industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef]
- Song, F.; Zhou, J.; Jia, Z.; He, L.; Sui, H.; Li, X. Interfacial behaviors of ionic liquids in petroleum Production: A review. J. Mol. Liq. 2023, 382, 121864. [Google Scholar] [CrossRef]
- Khormali, A. Asphaltene precipitation and inhibition in carbonate reservoirs. Pet. Sci. Technol. 2017, 35, 515–521. [Google Scholar] [CrossRef]
- Khormali, A. Effect of water cut on the performance of an asphaltene inhibitor package: Experimental and modeling analysis. Pet. Sci. Technol. 2022, 40, 2890–2906. [Google Scholar] [CrossRef]
- Khormali, A.; Sharifov, A.R.; Torba, D.I. Experimental and modeling analysis of asphaltene precipitation in the near wellbore region of oil wells. Pet. Sci. Technol. 2018, 36, 1030–1036. [Google Scholar] [CrossRef]
- Liu, J.; Cui, X.; Huang, J.; Xie, L.; Tan, X.; Liu, Q.; Zeng, H. Understanding the stabilization mechanism of bitumen-coated fine solids in organic media from non-aqueous extraction of oil sands. Fuel 2019, 242, 255–264. [Google Scholar] [CrossRef]
- Lan, T.; Zeng, H.; Tang, T. Molecular dynamics study on the mechanism of graphene oxide to destabilize oil/water emulsion. J. Phys. Chem. C 2019, 123, 22989–22999. [Google Scholar] [CrossRef]
- Jahani, S.; Akbari, A.; Kazemzadeh, Y.; Ahmadi, S.; Khormali, A. Impact of Asphaltene on Water-in-Oil Emulsion Stability. Int. J. Chem. Eng. 2025, 2025, 1654239. [Google Scholar] [CrossRef]
- Ayala, M.; Vazquez-Duhalt, R. Enzymatic catalysis on petroleum products. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 151, pp. 67–111. [Google Scholar]
- Chacón-Patiño, M.L.; Rowland, S.M.; Rodgers, R.P. Advances in asphaltene petroleomics. Part 2: Selective separation method that reveals fractions enriched in island and archipelago structural motifs by mass spectrometry. Energy Fuels 2018, 32, 314–328. [Google Scholar] [CrossRef]
- Schuler, B.; Meyer, G.; Peña, D.; Mullins, O.C.; Gross, L. Unraveling the molecular structures of asphaltenes by atomic force microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, J.; Bian, R.; Cheng, J.; Sui, H.; He, L. Novel polyether for efficient demulsification of interfacially active asphaltene-stabilized water-in-oil emulsions. Energy Fuels 2020, 34, 3591–3600. [Google Scholar] [CrossRef]
- Yang, F.; Tchoukov, P.; Pensini, E.; Dabros, T.; Czarnecki, J.; Masliyah, J.; Xu, Z. Asphaltene subfractions responsible for stabilizing water-in-crude oil emulsions. Part 1: Interfacial behaviors. Energy Fuels 2014, 28, 6897–6904. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, X.; Cheng, L.; Zhang, H.; Tang, J.; Chen, H.; Fan, Q.; Ouyang, X. Effect of Asphaltenes on the Stability of Water in Crude Oil Emulsions. Materials 2025, 18, 630. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, X.; Liang, C.; Xu, Y.; Jia, Y. Effect of asphaltenes structure on interfacial properties: A dissipative particle dynamics study. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131849. [Google Scholar] [CrossRef]
- Mahmoudi Alemi, F.; Mohammadi, S. Experimental Study on water-in-oil emulsion stability induced by asphaltene colloids in heavy oil. ACS Omega 2025, 10, 14327–14342. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Yang, F.; Shi, L.; Yao, B.; Sun, G. Influences of asphaltene subfractions with different polarities on hydrate growth at water/oil interface. Fuel 2022, 330, 125546. [Google Scholar] [CrossRef]
- Spiecker, P.M.; Kilpatrick, P.K. Interfacial rheology of petroleum asphaltenes at the oil−water interface. Langmuir 2004, 20, 4022–4032. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, L.; Xie, L.; Lu, X.; Liu, Q.; He, J.; Mantilla, C.A.; Van den Berg, F.G.; Zeng, H. Surface interaction of water-in-oil emulsion droplets with interfacially active asphaltenes. Langmuir 2017, 33, 1265–1274. [Google Scholar] [CrossRef]
- Gray, M.R.; Yarranton, H.W.; Chacon-Patino, M.L.; Rodgers, R.P.; Bouyssiere, B.; Giusti, P. Distributed properties of asphaltene nanoaggregates in crude oils: A review. Energy Fuels 2021, 35, 18078–18103. [Google Scholar] [CrossRef]
- Spiecker, P.M.; Gawrys, K.L.; Kilpatrick, P.K. Aggregation and solubility behavior of asphaltenes and their subfractions. J. Colloid Interface Sci. 2003, 267, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Saniei, D.; Behroozi, M.H.; Mahani, H.; Ayatollahi, S. The Effect of Dynamic Interaction between Crude Oil and Low-Salinity Water on Asphaltene Instability: A Pore-Scale Perspective. Sci. Iran. 2025, in press. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Li, X.; Sui, H.; He, L. Mechanisms on the stability and instability of water-in-oil emulsion stabilized by interfacially active asphaltenes: Role of hydrogen bonding reconstructing. Fuel 2021, 297, 120763. [Google Scholar] [CrossRef]
- Tang, L.; Wang, T.; Xu, Y.; He, X.; Yan, A.; Zhang, Z.; Li, Y.; Chen, G. Research and application progress of crude oil demulsification technology. Processes 2024, 12, 2292. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Pu, W.; Yang, X.; Varfolomeev, M.; Zou, B.; He, M.; Gou, R. A universal route to deciphering the internal mechanism of crude oil self–emulsification. J. Mol. Liq. 2023, 383, 122165. [Google Scholar] [CrossRef]
- Petroni, M.H.O.; Corona, R.R.; Sad, C.M.; Ramos, R.; Castro, J.M.; Franco, L.G.; da Silva, M.; Elias, M.Z.; Castro, E.V. Role of asphaltenes and resins at the interface of petroleum emulsions (W/O): A literature review. Geoenergy Sci. Eng. 2024, 239, 212932. [Google Scholar] [CrossRef]
- Khadim, M.A.; Sarbar, M.A. Role of asphaltene and resin in oil field emulsions. J. Pet. Sci. Eng. 1999, 23, 213–221. [Google Scholar] [CrossRef]
- Cheshkova, T.V.; Sergun, V.P.; Kovalenko, E.Y.; Gerasimova, N.N.; Sagachenko, T.A.; Min, R.S. Resins and asphaltenes of light and heavy oils: Their composition and structure. Energy Fuels 2019, 33, 7971–7982. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Yang, F.; Sun, G.; You, J.; Cui, K. Synergetic effect of resins and asphaltenes on water/oil interfacial properties and emulsion stability. Fuel 2019, 252, 581–588. [Google Scholar] [CrossRef]
- Tian, Y.; Qi, Y.; Chen, S.; Qiao, Z.; Han, H.; Chen, Z.; Wang, H.; Zhang, Y.; Chen, H.; Wang, L. Hydrogen bond recombination regulated by strongly electronegative functional groups in demulsifiers for efficient separation of oil–water emulsions. J. Hazard. Mater. 2024, 461, 132525. [Google Scholar] [CrossRef]
- Yang, X.; Verruto, V.J.; Kilpatrick, P.K. Dynamic asphaltene−resin exchange at the oil/water interface: Time-dependent W/O emulsion stability for asphaltene/resin model oils. Energy Fuels 2007, 21, 1343–1349. [Google Scholar] [CrossRef]
- Cao, C.; Gu, S.; Song, Z.; Xie, Z.; Chang, X.; Shen, P. The viscosifying behavior of W/O emulsion and its underlying mechanisms: Considering the interfacial adsorption of heavy components. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127794. [Google Scholar] [CrossRef]
- Dong, Z.-x.; Wang, J.; Liu, G.; Lin, M.-q.; Li, M.-y. Experimental study on asphaltene precipitation induced by CO2 flooding. Pet. Sci. 2014, 11, 174–180. [Google Scholar] [CrossRef]
- Yudina, N.; Nebogina, N.; Prozorova, I. Composition of the resin-asphaltene components in the interfacial layers of water-in-oil emulsions. Pet. Chem. 2021, 61, 568–575. [Google Scholar] [CrossRef]
- Khormali, A.; Sharifov, A.R.; Torba, D.I. The control of asphaltene precipitation in oil wells. Pet. Sci. Technol. 2018, 36, 443–449. [Google Scholar] [CrossRef]
- Goual, L.; Horváth-Szabó, G.; Masliyah, J.H.; Xu, Z. Adsorption of bituminous components at oil/water interfaces investigated by quartz crystal microbalance: Implications to the stability of water-in-oil emulsions. Langmuir 2005, 21, 8278–8289. [Google Scholar] [CrossRef]
- Mousavi, M.; Abdollahi, T.; Pahlavan, F.; Fini, E.H. The influence of asphaltene-resin molecular interactions on the colloidal stability of crude oil. Fuel 2016, 183, 262–271. [Google Scholar] [CrossRef]
- Facanali, R.; Porto, N.d.A.; Crucello, J.; Carvalho, R.M.; Vaz, B.G.; Hantao, L.W. Naphthenic Acids: Formation, Role in Emulsion Stability, and Recent Advances in Mass Spectrometry-Based Analytical Methods. J. Anal. Methods Chem. 2021, 2021, 6078084. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, M.; Abdouss, M. Essential role of structure, architecture, and intermolecular interactions of asphaltene molecules on properties (self-association and surface activity). Heliyon 2022, 8, e12170. [Google Scholar] [CrossRef]
- Wu, X. Investigating the stability mechanism of water-in-diluted bitumen emulsions through isolation and characterization of the stabilizing materials at the interface. Energy Fuels 2003, 17, 179–190. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Z.; Xi, C.; Liu, Y. Exploration of the natural waxes-tuned crystallization behavior, droplet shape and rheology properties of O/W emulsions. J. Colloid Interface Sci. 2021, 587, 417–428. [Google Scholar] [CrossRef]
- Freitas, G.B.; Duncke, A.C.; Barbato, C.N.; de Oliveira, M.C.; Pinto, J.C.; Nele, M. Influence of wax chemical structure on W/O emulsion rheology and stability. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 45–56. [Google Scholar] [CrossRef]
- Acevedo, S.c.; Guzmán, K.; Labrador, H.; Carrier, H.; Bouyssiere, B.; Lobinski, R. Trapping of metallic porphyrins by asphaltene aggregates: A size exclusion microchromatography with high-resolution inductively coupled plasma mass spectrometric detection study. Energy Fuels 2012, 26, 4968–4977. [Google Scholar] [CrossRef]
- Taylor, S.E. Thermal destabilisation of bitumen-in-water emulsions–A spinning drop tensiometry study. Fuel 2011, 90, 3028–3039. [Google Scholar] [CrossRef]
- Hannisdal, A.; Ese, M.-H.; Hemmingsen, P.V.; Sjöblom, J. Particle-stabilized emulsions: Effect of heavy crude oil components pre-adsorbed onto stabilizing solids. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 45–58. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Lu, X.; Shi, C.; Tang, T.; Wang, X.; Huang, Q.; Zeng, H. Adsorption kinetics of asphaltenes at oil/water interface: Effects of concentration and temperature. Fuel 2018, 212, 387–394. [Google Scholar] [CrossRef]
- Bourrel, M.; Salager, J.; Schechter, R.; Wade, W. A correlation for phase behavior of nonionic surfactants. J. Colloid Interface Sci. 1980, 75, 451–461. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Miller, C.A.; Raney, O.G.; Poindexter, M.K.; Nguyen, D.T.; Hera, J. Separation of produced emulsions from surfactant enhanced oil recovery processes. Energy Fuels 2011, 25, 555–561. [Google Scholar] [CrossRef]
- Fortuny, M.; Oliveira, C.B.; Melo, R.L.; Nele, M.; Coutinho, R.C.; Santos, A.F. Effect of salinity, temperature, water content, and pH on the microwave demulsification of crude oil emulsions. Energy Fuels 2007, 21, 1358–1364. [Google Scholar] [CrossRef]
- Aman, Z.M.; Haber, A.; Ling, N.N.; Thornton, A.; Johns, M.L.; May, E.F. Effect of brine salinity on the stability of hydrate-in-oil dispersions and water-in-oil emulsions. Energy Fuels 2015, 29, 7948–7955. [Google Scholar] [CrossRef]
- Maaref, S.; Ayatollahi, S. The effect of brine salinity on water-in-oil emulsion stability through droplet size distribution analysis: A case study. J. Dispers. Sci. Technol. 2018, 39, 721–733. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Ismail, I.; Rezvani, H.; Sharifi, M.; Riazi, M. Experimental investigation of stability of water in oil emulsions at reservoir conditions: Effect of ion type, ion concentration, and system pressure. Fuel 2019, 243, 15–27. [Google Scholar] [CrossRef]
- Miadonye, A.; Amadu, M. Theoretical interpretation of pH and salinity effect on oil-in-water emulsion stability based on interfacial chemistry and implications for produced water demulsification. Processes 2023, 11, 2470. [Google Scholar] [CrossRef]
- Dalmazzone, C.; Noïk, C.; Argillier, J.-F. Impact of chemical enhanced oil recovery on the separation of diluted heavy oil emulsions. Energy Fuels 2012, 26, 3462–3469. [Google Scholar] [CrossRef]
- Chen, C.-M.; Lu, C.-H.; Chang, C.-H.; Yang, Y.-M.; Maa, J.-R. Influence of pH on the stability of oil-in-water emulsions stabilized by a splittable surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2000, 170, 173–179. [Google Scholar] [CrossRef]
- Silva, I.; Borges, B.; Blanco, R.; Rondón, M.; Salager, J.-L.; Pereira, J.C. Breaking of water-in-crude oil emulsions. 5. Effect of acid-alkaline additives on the performance of chemical demulsifiers. Energy Fuels 2014, 28, 3587–3593. [Google Scholar] [CrossRef]
- Binks, B.P.; Murakami, R.; Armes, S.P.; Fujii, S. Effects of pH and salt concentration on oil-in-water emulsions stabilized solely by nanocomposite microgel particles. Langmuir 2006, 22, 2050–2057. [Google Scholar] [CrossRef]
- Azim, A.A.; Abdel-Raouf, M.-S.; Abdel-Raheim, A.-R.; Maysour, N.-S. Sugar-based ethoxylated amine surfactants as demulsifiers for crude oil emulsions: 2-demulsification of different types of crudes. Braz. J. Pet. Gas 2010, 4, 155–165. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion formation, stability, and rheology. In Emulsion Formation and Stability; Wiley: Hoboken, NJ, USA, 2013; pp. 1–75. [Google Scholar]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef] [PubMed]
- Onaizi, S.A. Effect of oil/water ratio on rheological behavior, droplet size, zeta potential, long-term stability, and acid-induced demulsification of crude oil/water nanoemulsions. J. Pet. Sci. Eng. 2022, 209, 109857. [Google Scholar] [CrossRef]
- Dong, B.; Qin, Z.; Wang, Y.; Zhang, J.; Xu, Z.; Liu, A.; Guo, X. Investigating the rheology and stability of heavy crude oil-in-water emulsions using APG08 emulsifiers. ACS Omega 2022, 7, 37736–37747. [Google Scholar] [CrossRef] [PubMed]
- Alao, K.T.; Alara, O.R.; Abdurahman, N.H. Trending approaches on demulsification of crude oil in the petroleum industry. Appl. Petrochem. Res. 2021, 11, 281–293. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. An experimental study of stability of oil–water emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Pu, W.; He, M.; Yang, X.; Liu, R.; Shen, C. Experimental study on the key influencing factors of phase inversion and stability of heavy oil emulsion: Asphaltene, resin and petroleum acid. Fuel 2022, 311, 122631. [Google Scholar] [CrossRef]
- van Dijke, K.; Kobayashi, I.; Schroën, K.; Uemura, K.; Nakajima, M.; Boom, R. Effect of viscosities of dispersed and continuous phases in microchannel oil-in-water emulsification. Microfluid. Nanofluidics 2010, 9, 77–85. [Google Scholar] [CrossRef]
- Boxall, J.A.; Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Droplet size scaling of water-in-oil emulsions under turbulent flow. Langmuir 2012, 28, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Mao, L.; Lu, Y.; Ma, P.; Gao, Y.; Miao, S. Interface and Bulk Phase Engineering in Water-in-Oil High Internal Phase Emulsion: A Clean-Label Strategy for Stabilization and Application. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70247. [Google Scholar] [CrossRef]
- Abdulredha, M.M.; Hussain, S.A.; Abdullah, L.C.; Hong, T.L. Water-in-oil emulsion stability and demulsification via surface-active compounds: A review. J. Pet. Sci. Eng. 2022, 209, 109848. [Google Scholar] [CrossRef]
- Razi, M.; Rahimpour, M.R.; Jahanmiri, A.; Azad, F. Effect of a different formulation of demulsifiers on the efficiency of chemical demulsification of heavy crude oil. J. Chem. Eng. Data 2011, 56, 2936–2945. [Google Scholar] [CrossRef]
- Kang, W.; Yin, X.; Yang, H.; Zhao, Y.; Huang, Z.; Hou, X.; Sarsenbekuly, B.; Zhu, Z.; Wang, P.; Zhang, X. Demulsification performance, behavior and mechanism of different demulsifiers on the light crude oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 197–204. [Google Scholar] [CrossRef]
- Tajikmansori, A.; Dehaghani, A.H.S.; Sadeghnejad, S.; Haghighi, M. New insights into effect of the electrostatic properties on the interfacial behavior of asphaltene and resin: An experimental study of molecular structure. J. Mol. Liq. 2023, 377, 121526. [Google Scholar] [CrossRef]
- Lu, R.; Lai, L.; Zhang, H. Stabilization mechanism of emulsion gels of crude oil with low asphaltene, resin, and wax contents. J. Mol. Liq. 2025, 417, 126496. [Google Scholar] [CrossRef]
- Abdel-Raouf, M.E.-S. Factors affecting the stability of crude oil emulsions. In Crude Oil Emulsions—Composition Stability and Characterization; InTech Open: London, UK, 2012; pp. 183–204. [Google Scholar]
- Alvarado, V.; Wang, X.; Moradi, M. Stability proxies for water-in-oil emulsions and implications in aqueous-based enhanced oil recovery. Energies 2011, 4, 1058–1086. [Google Scholar] [CrossRef]
- Kilpatrick, P.K. Water-in-crude oil emulsion stabilization: Review and unanswered questions. Energy Fuels 2012, 26, 4017–4026. [Google Scholar] [CrossRef]
- Yonguep, E.; Kapiamba, K.F.; Kabamba, K.J.; Chowdhury, M.R. Formation, stabilization and chemical demulsification of crude oil-inwater emulsions: A review. Pet. Res. 2022, 7, 459–472. [Google Scholar]
- Saraiva, S.V.; Fontes, D.O.L.; Bonetti, D.; Fileti, A.M.F.; da Silva, F.v.V. Effect of Flow Conditions on the Dynamics of Chemical Demulsification in Highly Viscous Emulsion Flow. Ind. Eng. Chem. Res. 2025, 64, 12323–12336. [Google Scholar] [CrossRef]
- Sjöblom, J.; Øye, G.; Glomm, W.R.; Hannisdal, A.; Knag, M.; Brandal, Ø.; Ese, M.-H.; Hemmingsen, P.V.; Havre, T.E.; Oschmann, H.-J. Modern characterization techniques for crude oils, their emulsions, and functionalized surfaces. In Emulsions and emulsion stability; CRC Press: Boca Raton, FL, USA, 2005; pp. 435–496. [Google Scholar]
- Boxall, J.A.; Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Measurement and calibration of droplet size distributions in water-in-oil emulsions by particle video microscope and a focused beam reflectance method. Ind. Eng. Chem. Res. 2010, 49, 1412–1418. [Google Scholar] [CrossRef]
- Schümann, H.; Khatibi, M.; Tutkun, M.; Pettersen, B.H.; Yang, Z.; Nydal, O.J. Droplet size measurements in oil–water dispersions: A comparison study using FBRM and PVM. J. Dispers. Sci. Technol. 2015, 36, 1432–1443. [Google Scholar] [CrossRef]
- Angardi, V.; Ettehadi, A.; Yücel, Ö. Critical review of emulsion stability and characterization techniques in oil processing. J. Energy Resour. Technol. 2022, 144, 040801. [Google Scholar] [CrossRef]
- Santos, T.P.; Cejas, C.M.; Cunha, R.L. Microfluidics as a tool to assess and induce emulsion destabilization. Soft Matter 2022, 18, 698–710. [Google Scholar] [CrossRef]
- Bourrel, M.; Passade-Boupat, N. Crude oil surface active species: Consequences for enhanced oil recovery and emulsion stability. Energy Fuels 2017, 32, 2642–2652. [Google Scholar] [CrossRef]
- Kumar, K.; Nikolov, A.; Wasan, D. Mechanisms of stabilization of water-in-crude oil emulsions. Ind. Eng. Chem. Res. 2001, 40, 3009–3014. [Google Scholar] [CrossRef]
- Sjöblom, J.; Urdahl, O.; Høiland, H.; Christy, A.; Johansen, E. Water-in-crude oil emulsions. Formation, characterization, and destabilization. In Surfactants and Macromolecules: Self-Assembly at Interfaces and in Bulk; Springer: Berlin/Heidelberg, Germany, 2008; pp. 131–139. [Google Scholar]
- Shilliday, E.R.; Ling, N.N.; Fridjonsson, E.O.; Graham, B.F.; Johns, M.L. Destabilization of water-in-crude oil emulsions using naphthenic acids. J. Dispers. Sci. Technol. 2024, 45, 1955–1971. [Google Scholar] [CrossRef]
- Wu, T.; Firoozabadi, A. Surfactant-enhanced spontaneous emulsification near the crude oil–water interface. Langmuir 2021, 37, 4736–4743. [Google Scholar] [CrossRef]
- L’estimé, M.; Schindler, M.; Shahidzadeh, N.; Bonn, D. Droplet size distribution in emulsions. Langmuir 2023, 40, 275–281. [Google Scholar] [CrossRef]
- Fuhrmann, P.L.; Sala, G.; Stieger, M.; Scholten, E. Clustering of oil droplets in o/w emulsions: Controlling cluster size and interaction strength. Food Res. Int. 2019, 122, 537–547. [Google Scholar] [CrossRef]
- Plasencia, J.; Pettersen, B.; Nydal, O.J. Pipe flow of water-in-crude oil emulsions: Effective viscosity, inversion point and droplet size distribution. J. Pet. Sci. Eng. 2013, 101, 35–43. [Google Scholar] [CrossRef]
- Kamp, J.; Villwock, J.; Kraume, M. Drop coalescence in technical liquid/liquid applications: A review on experimental techniques and modeling approaches. Rev. Chem. Eng. 2017, 33, 1–47. [Google Scholar] [CrossRef]
- Rekvig, L.; Frenkel, D. Molecular simulations of droplet coalescence in oil/water/surfactant systems. J. Chem. Phys. 2007, 127, 134701. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J. Colloids and Interfaces with Surfactants and Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Leal-Calderon, F.; Bibette, J.; Schmitt, V. Stability of concentrated emulsions. In Emulsion Science: Basic Principles; Springer: Berlin/Heidelberg, Germany, 2007; pp. 143–172. [Google Scholar]
- Taylor, P. Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar] [CrossRef]
- Taylor, P. Ostwald ripening in emulsions: Estimation of solution thermodynamics of the disperse phase. Adv. Colloid Interface Sci. 2003, 106, 261–285. [Google Scholar] [CrossRef]
- Urbina-Villalba, G. An algorithm for emulsion stability simulations: Account of flocculation, coalescence, surfactant adsorption and the process of Ostwald ripening. Int. J. Mol. Sci. 2009, 10, 761–804. [Google Scholar] [CrossRef]
- Yao, J.H.; Laradji, M. Dynamics of Ostwald ripening in the presence of surfactants. Phys. Rev. E 1993, 47, 2695. [Google Scholar] [CrossRef]
- Davis, S.; Round, H.; Purewal, T. Ostwald ripening and the stability of emulsion systems: An explanation for the effect of an added third component. J. Colloid Interface Sci. 1981, 80, 508–511. [Google Scholar] [CrossRef]
- Tadros, T.F. Encyclopedia of Colloid and Interface Science; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chanamai, R.; McClements, D.J. Dependence of creaming and rheology of monodisperse oil-in-water emulsions on droplet size and concentration. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 79–86. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; He, Y.; Xu, X.; Zhao, X. Oil density and viscosity affect emulsion stability and destabilization mechanism. J. Food Eng. 2024, 366, 111864. [Google Scholar] [CrossRef]
- Simões, A.; Veiga, F.; Vitorino, C. Progressing towards the sustainable development of cream formulations. Pharmaceutics 2020, 12, 647. [Google Scholar] [CrossRef]
- Deb, T.K.; Lebaz, N.; Ozdemir, M.S.; Govoreanu, R.; Mhamdi, A.; Sin, G.r.; Sheibat-Othman, N. Monitoring and modeling of creaming in oil-in-water emulsions. Ind. Eng. Chem. Res. 2022, 61, 4638–4647. [Google Scholar] [CrossRef]
- Pal, R. Modeling of sedimentation and creaming in suspensions and Pickering emulsions. Fluids 2019, 4, 186. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion science and technology: A general introduction. Emuls. Sci. Technol. 2009, 1, 1–55. [Google Scholar]
- Tadros, T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Colloid Interface Sci. 2004, 108, 227–258. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T. Creaming/Sedimentation of Emulsions and Its Prevention. In Emulsions; De Gruyter: Berlin, Germany, 2016; pp. 95–112. [Google Scholar]
- Fiocco, R.J.; Lewis, A. Oil spill dispersants. Pure Appl. Chem. 1999, 71, 27–42. [Google Scholar] [CrossRef]
- Ahmadi, S.; Khormali, A. Optimization of the corrosion inhibition performance of 2-mercaptobenzothiazole for carbon steel in HCl media using response surface methodology. Fuel 2024, 357, 129783. [Google Scholar] [CrossRef]
- Ahmadi, S.; Khormali, A.; Razmjooie, A. Experimental investigation on separation of water in crude oil emulsions using an oil-soluble demulsifier. Iran. J. Chem. Chem. Eng. 2023, 42, 2332–2343. [Google Scholar]
- Hassanshahi, N.; Hu, G.; Li, J. Application of ionic liquids for chemical demulsification: A review. Molecules 2020, 25, 4915. [Google Scholar] [CrossRef]
- Langevin, D. Coalescence in foams and emulsions: Similarities and differences. Curr. Opin. Colloid Interface Sci. 2019, 44, 23–31. [Google Scholar] [CrossRef]
- Azizi, K.; Nikazar, M. Characterization of chemical demulsification of oil in water emulsion: Comparison between a kinetics model and laboratory experiments. Pet. Sci. Technol. 2015, 33, 8–14. [Google Scholar] [CrossRef]
- Hjartnes, T.N.; Sørland, G.H.; Simon, S.; Sjöblom, J. Demulsification of crude oil emulsions tracked by pulsed field gradient (PFG) nuclear magnetic resonance (NMR). Part I: Chemical demulsification. Ind. Eng. Chem. Res. 2019, 58, 2310–2323. [Google Scholar] [CrossRef]
- Ahmadi, S.; Khormali, A.; Khoutoriansky, F.M. Optimization of the demulsification of water-in-heavy crude oil emulsions using response surface methodology. Fuel 2022, 323, 124270. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Zhang, X.; Sui, H.; He, L.; Wang, S. A novel oxygen-containing demulsifier for efficient breaking of water-in-oil emulsions. Chem. Eng. J. 2020, 385, 123826. [Google Scholar] [CrossRef]
- Shah Buddin, M.M.H.; Ahmad, A.L.; Abd Khalil, A.T.; Puasa, S.W. A review of demulsification technique and mechanism for emulsion liquid membrane applications. J. Dispers. Sci. Technol. 2022, 43, 910–927. [Google Scholar] [CrossRef]
- Koreh, P.; Lashkarbolooki, M.; Peyravi, M.; Jahanshahi, M. Interfacial performance of cationic, anionic and non-ionic surfactants; effect of different characteristics of crude oil. J. Pet. Sci. Eng. 2022, 218, 110960. [Google Scholar] [CrossRef]
- Miranda-Olvera, A.D.; Domínguez-Esquivel, J.-M.; Martinez, J. Hydrophilic–Lipophilic Balance (HLB) Correlation Method for the Selection of Ionic Liquid Surfactant Modifiers of the Viscosity and Emulsion Stability of Heavy Oils. Langmuir 2025, 41, 8753–8765. [Google Scholar] [CrossRef]
- Shehzad, F.; Hussein, I.A.; Kamal, M.S.; Ahmad, W.; Sultan, A.S.; Nasser, M.S. Polymeric surfactants and emerging alternatives used in the demulsification of produced water: A review. Polym. Rev. 2018, 58, 63–101. [Google Scholar] [CrossRef]
- Jafarirad, S. Innovative amphiphilic cellulose nanobiostructures: Physicochemical, spectroscopic, morphological, and hydrophilic/lipophilic properties. J. Dispers. Sci. Technol. 2017, 38, 1187–1195. [Google Scholar] [CrossRef]
- Fan, Y.; Simon, S.; Sjöblom, J. Chemical destabilization of crude oil emulsions: Effect of nonionic surfactants as emulsion inhibitors. Energy Fuels 2009, 23, 4575–4583. [Google Scholar] [CrossRef]
- Pullanchery, S.; Kulik, S.; Rehl, B.; Hassanali, A.; Roke, S. Charge transfer across C–H⋅⋅⋅ O hydrogen bonds stabilizes oil droplets in water. Science 2021, 374, 1366–1370. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, L.; Chao, M.; Jia, X.; Liu, C.; Shi, L. Synthesis and study of a new type of nonanionic demulsifier for chemical flooding emulsion demulsification. ACS Omega 2021, 6, 17709–17719. [Google Scholar] [CrossRef]
- Poli, E.; Jong, K.H.; Hassanali, A. Charge transfer as a ubiquitous mechanism in determining the negative charge at hydrophobic interfaces. Nat. Commun. 2020, 11, 901. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Q.; Li, X.; He, X. Novel polyether-polyquaternium copolymer as an effective reverse demulsifier for O/W emulsions: Demulsification performance and mechanism. Fuel 2020, 263, 116770. [Google Scholar] [CrossRef]
- Shen, L.; Li, H.; Jia, J.; Nan, J.; Wang, X.; Si, Y.; Chen, L.; Zhao, Y.; Mi, Y. A novel glucose-based ionic liquid as an effective demulsifier for treating oily wastewater: Performance and mechanism. Sep. Purif. Technol. 2024, 351, 128151. [Google Scholar] [CrossRef]
- Li, X.; Kersten, S.R.A.; Schuur, B. Efficiency and Mechanism of Demulsification of Oil-in-Water Emulsions Using Ionic Liquids. Energy Fuels 2016, 30, 7622–7628. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; El-Din, M.G.; Kemppi, T. Stabilization mechanism and chemical demulsification of water-in-oil and oil-in-water emulsions in petroleum industry: A review. Fuel 2021, 286, 119390. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Z.; Yuan, S. Understanding the chemical demulsification mechanism of oil/water emulsion by polyether polymers. Ind. Eng. Chem. Res. 2024, 63, 12680–12687. [Google Scholar] [CrossRef]
- Le Follotec, A.; Pezron, I.; Noik, C.; Dalmazzone, C.; Metlas-Komunjer, L. Triblock copolymers as destabilizers of water-in-crude oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 365, 162–170. [Google Scholar] [CrossRef]
- Yang, F.; Tchoukov, P.; Qiao, P.; Ma, X.; Pensini, E.; Dabros, T.; Czarnecki, J.; Xu, Z. Studying demulsification mechanisms of water-in-crude oil emulsions using a modified thin liquid film technique. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 215–223. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Ren, S. Molecular dynamics simulation of self-aggregation of asphaltenes at an oil/water interface: Formation and destruction of the asphaltene protective film. Energy Fuels 2015, 29, 1233–1242. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, P.; Zhang, X.; Guo, Y.; Fang, W. Cryogenic efficient phase separation of oil–water emulsions with amphiphilic hyperbranched poly (amido-amine). J. Mater. Chem. A 2023, 11, 14145–14158. [Google Scholar] [CrossRef]
- Wang, Q.; Puerto, M.C.; Warudkar, S.; Buehler, J.; Biswal, S.L. Recyclable amine-functionalized magnetic nanoparticles for efficient demulsification of crude oil-in-water emulsions. Environ. Sci. Water Res. Technol. 2018, 4, 1553–1563. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; He, L.; Sui, H.; Li, X. Nano-modification of carboxylated polyether for enhanced room temperature demulsification of oil-water emulsions: Synthesis, performance and mechanisms. J. Hazard. Mater. 2022, 439, 129654. [Google Scholar] [CrossRef]
- Lv, X.; Song, Z.; Yu, J.; Su, Y.; Zhao, X.; Sun, J.; Mao, Y.; Wang, W. Study on the demulsification of refinery oily sludge enhanced by microwave irradiation. Fuel 2020, 279, 118417. [Google Scholar] [CrossRef]
- Mowea, W.S.; Ibrahim, R.I.; Oudah, M.K. Electromagnetic heating for the separation of water-oil emulsion. Pet. Chem. 2024, 64, 53–61. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Cerón-Camacho, R.; Chávez, B.; Vallejo, A.A.; Villanueva-Negrete, D.; Castellanos, J.; Karamath, J.; Reyes, J.; Aburto, J. Demulsification of heavy crude oil-in-water emulsions: A comparative study between microwave and thermal heating. Fuel 2013, 113, 407–414. [Google Scholar] [CrossRef]
- Deng, Y.; Dai, M.; Wu, Y.; Peng, C. Emulsion system, demulsification and membrane technology in oil–water emulsion separation: A comprehensive review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1254–1278. [Google Scholar] [CrossRef]
- Alshehri, A.K.; Ricardez-Sandoval, L.A.; Elkamel, A. Designing and Testing a Chemical Demulsifier Dosage Controller in a Crude Oil Desalting Plant: An Artificial Intelligence-Based Network Approach. Chem. Eng. Technol. 2010, 33, 973–982. [Google Scholar] [CrossRef]
- Luo, H.; Wen, J.; Wang, Z.; Lu, Y.; You, C.; He, Z.; Jia, Y. Modeling of droplet diameter of water-in-oil crude oil emulsion by characterization of crude oil physical properties. Phys. Fluids 2025, 37, 073110. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Hao, T.; Ren, L.; Liu, Y. A collaborative emulsification system capable of forming stable small droplets of oil-in-water emulsions for enhancing heavy oil recovery. J. Mol. Liq. 2022, 355, 118970. [Google Scholar] [CrossRef]
- Krebs, T.; Schroën, C.; Boom, R. Separation kinetics of an oil-in-water emulsion under enhanced gravity. Chem. Eng. Sci. 2012, 71, 118–125. [Google Scholar] [CrossRef]
- Hadi, A.A.; Ali, A.A. Chemical demulsification techniques in oil refineries: A review. Mater. Today Proc. 2022, 53, 58–64. [Google Scholar] [CrossRef]
- Hao, M.; Bai, Z.; Wang, H.; Liu, W. Removal of oil from electric desalting wastewater using centrifugal contactors. J. Pet. Sci. Eng. 2013, 111, 37–41. [Google Scholar] [CrossRef]
- Erfani, H.; Madhu, N.R.; Khodayari, S.; Qureshi, M.A.; Swetanshu; Singh, P.; Jadoun, S. Separation and removal of oil from water/wastewater in the oil industry: A review. Environ. Technol. Rev. 2024, 13, 325–343. [Google Scholar] [CrossRef]
- Udourioh, G.A.; Ezeh, C.C.; Solomon, M.M.; Achugasim, O.; Umoren, S.A. Synthesis and Characterization of Biobased Demulsifier from Coconut Oil for the Treatment of Water-in-Crude Oil Emulsion. Langmuir 2025, 41, 19939–19952. [Google Scholar] [CrossRef]
- Nikkhah, M.; Tohidian, T.; Rahimpour, M.R.; Jahanmiri, A. Efficient demulsification of water-in-oil emulsion by a novel nano-titania modified chemical demulsifier. Chem. Eng. Res. Des. 2015, 94, 164–172. [Google Scholar] [CrossRef]
- Taleghani, S.T.; Jahromi, A.F.; Elektorowicz, M. Electro-demulsification of water-in-oil suspensions enhanced with implementing various additives. Chemosphere 2019, 233, 157–163. [Google Scholar] [CrossRef]
- Zhang, H.; Bukosky, S.C.; Ristenpart, W.D. Low-voltage electrical demulsification of oily wastewater. Ind. Eng. Chem. Res. 2018, 57, 8341–8347. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, T.; Gong, H.; Zhang, X. Review of the dynamics of coalescence and demulsification by high-voltage pulsed electric fields. Int. J. Chem. Eng. 2016, 2016, 2492453. [Google Scholar] [CrossRef]
- Li, N.; Pang, Y.; Sun, Z.; Wang, Z.; Sun, X.; Tang, T.; Li, B.; Li, W.; Zeng, H. Probing the coalescence mechanism of water droplet and Oil/Water interface in demulsification process under DC electric field. Sep. Purif. Technol. 2023, 326, 124798. [Google Scholar] [CrossRef]
- Wang, K.; Bi, X.; Xiao, P.; Jiang, M.; Tian, R.; Zhao, P.; Fang, W.; Liu, B. Polar component networks transformation regulated by bidirectional pulsed electric field for rapid demulsification of O/W emulsion. J. Water Process Eng. 2025, 71, 107300. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, H.; Zhang, Y.; Li, X.; Xu, M.; Song, M.; Ru, G.; Jiang, X.; Zhu, X.; Han, D. Efficient electro-demulsification of O/W emulsions and simultaneous oil removal enabled by a multiscale porous biocarbon electrode. Chem. Eng. J. 2024, 481, 148655. [Google Scholar] [CrossRef]
- Ivanov, I.B.; Danov, K.D.; Kralchevsky, P.A. Flocculation and coalescence of micron-size emulsion droplets. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 161–182. [Google Scholar] [CrossRef]
- Mousavi, S.; Ghadiri, M.; Buckley, M. Electro-coalescence of water drops in oils under pulsatile electric fields. Chem. Eng. Sci. 2014, 120, 130–142. [Google Scholar] [CrossRef]
- Zhang, L.; He, L.; Ghadiri, M.; Hassanpour, A. Effect of surfactants on the deformation and break-up of an aqueous drop in oils under high electric field strengths. J. Pet. Sci. Eng. 2015, 125, 38–47. [Google Scholar] [CrossRef]
- Mhatre, S.; Thaokar, R. Electrocoalescence in non-uniform electric fields: An experimental study. Chem. Eng. Process. Process Intensif. 2015, 96, 28–38. [Google Scholar] [CrossRef]
- Wang, B.-B.; Wang, X.-D.; Wang, T.-H.; Lu, G.; Yan, W.-M. Electro-coalescence of two charged droplets under constant and pulsed DC electric fields. Int. J. Heat Mass Transf. 2016, 98, 10–16. [Google Scholar] [CrossRef]
- Muto, A.; Hiraguchi, Y.; Kinugawa, K.; Matsumoto, T.; Mizoguchi, Y.; Tokumoto, H. Effects of organic solvent and ionic strength on continuous demulsification using an alternating electric field. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 228–233. [Google Scholar] [CrossRef]
- Mohammadian, E.; Taju Ariffin, T.S.; Azdarpour, A.; Hamidi, H.; Yusof, S.; Sabet, M.; Yahya, E. Demulsification of light malaysian crude oil emulsions using an electric field method. Ind. Eng. Chem. Res. 2018, 57, 13247–13256. [Google Scholar] [CrossRef]
- Huang, S.; He, X.; Chen, J.; Wang, X.; Zhang, J.; Dong, J.; Zhang, B. Study on the Performance of an Electric-Field-Enhanced Oil–Water Separator in Treating Heavy Oil with High Water Cut. J. Mar. Sci. Eng. 2022, 10, 1516. [Google Scholar] [CrossRef]
- Shalaby, M.S.; Sołowski, G.; Abbas, W. Recent aspects in membrane separation for oil/water emulsion. Adv. Mater. Interfaces 2021, 8, 2100448. [Google Scholar] [CrossRef]
- Baig, N.; Salhi, B.; Sajid, M.; Aljundi, I.H. Recent progress in microfiltration/ultrafiltration membranes for separation of oil and water emulsions. Chem. Rec. 2022, 22, e202100320. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, W.; Li, S.; Zhong, Q.; Liu, F.; Zheng, J.; Wang, J. The effect of membrane surface charges on demulsification and fouling resistance during emulsion separation. J. Membr. Sci. 2018, 563, 126–133. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, N.; Cao, Y.; Lin, X.; Xu, L.; Zhang, W.; Wei, Y.; Feng, L. Fabrication of silica nanospheres coated membranes: Towards the effective separation of oil-in-water emulsion in extremely acidic and concentrated salty environments. Sci. Rep. 2016, 6, 32540. [Google Scholar] [CrossRef]
- Singh, A.K. Polydimethylsiloxane based sustainable hydrophobic/oleophilic coatings for oil/water separation: A review. Clean. Mater. 2022, 6, 100136. [Google Scholar]
- Zhu, Y.; Ding, Y.; Wang, J.; Lin, H.; Liu, F.; Tang, C.Y. Efficient oil recovery from emulsions through PDMS decorated nanofibrous membranes via aggregation-release demulsification. Sep. Purif. Technol. 2024, 343, 126934. [Google Scholar] [CrossRef]
- Cai, Y.; Shi, S.Q.; Fang, Z.; Li, J. Design, development, and outlook of superwettability membranes in oil/water emulsions separation. Adv. Mater. Interfaces 2021, 8, 2100799. [Google Scholar] [CrossRef]
- Hou, C.; Liu, W.; Du, L.; Li, Y.; Zhou, J.; Lin, S.; Qiao, S.; Zhu, Y. Superhydrophobic membrane from double co-crystallization for high-performance separation of water-in-oil emulsion. J. Membr. Sci. 2023, 679, 121702. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Chen, J.; Dong, L.; Tian, Y.; Cui, Z.; Li, J.; He, B.; Yan, F. Demulsifier-inspired superhydrophilic/underwater superoleophobic membrane modified with polyoxypropylene polyoxyethylene block polymer for enhanced oil/water separation properties. Molecules 2023, 28, 1282. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.; Zhu, S.; Lin, G.; Wang, M.; Wang, C. Research on ultrasonic demulsification characteristics and parameter optimization of condensate oil emulsion. Chem. Eng. Process. Process Intensif. 2025, 209, 110185. [Google Scholar] [CrossRef]
- Check, G.R.; Mowla, D. Theoretical and experimental investigation of desalting and dehydration of crude oil by assistance of ultrasonic irradiation. Ultrason. Sonochem. 2013, 20, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Check, G.R. Two-stage ultrasonic irradiation for dehydration and desalting of crude oil: A novel method. Chem. Eng. Process. Process Intensif. 2014, 81, 72–78. [Google Scholar] [CrossRef]
- Antes, F.G.; Diehl, L.O.; Pereira, J.S.; Guimarães, R.C.; Guarnieri, R.A.; Ferreira, B.M.; Dressler, V.L.; Flores, E.M. Feasibility of low frequency ultrasound for water removal from crude oil emulsions. Ultrason. Sonochem. 2015, 25, 70–75. [Google Scholar] [CrossRef]
- Xie, W.; Li, R.; Lu, X. Pulsed ultrasound assisted dehydration of waste oil. Ultrason. Sonochem. 2015, 26, 136–141. [Google Scholar] [CrossRef]
- Antes, F.G.; Diehl, L.O.; Pereira, J.S.; Guimarães, R.C.; Guarnieri, R.A.; Ferreira, B.M.; Flores, E.M. Effect of ultrasonic frequency on separation of water from heavy crude oil emulsion using ultrasonic baths. Ultrason. Sonochem. 2017, 35, 541–546. [Google Scholar] [CrossRef]
- Pedrotti, M.F.; Enders, M.S.; Pereira, L.S.; Mesko, M.F.; Flores, E.M.; Bizzi, C.A. Intensification of ultrasonic-assisted crude oil demulsification based on acoustic field distribution data. Ultrason. Sonochem. 2018, 40, 53–59. [Google Scholar] [CrossRef]
- Diaz Velazquez, H.; Guzmán-Lucero, D.; Martínez-Palou, R. Microwave-assisted demulsification for oilfield applications: A critical review. J. Dispers. Sci. Technol. 2023, 44, 1884–1899. [Google Scholar] [CrossRef]
- Huang, X.; Peng, K.; Feng, Y.; Liu, J.; Lu, L. Separation and characterization of effective demulsifying substances from surface of Alcaligenes sp. S-XJ-1 and its application in water-in-kerosene emulsion. Bioresour. Technol. 2013, 139, 257–264. [Google Scholar] [CrossRef]
- Huang, X.; Xiong, Y.; Yin, W.; Lu, L.; Liu, J.; Peng, K. Demulsification of a new magnetically responsive bacterial demulsifier for water-in-oil emulsions. Energy Fuels 2016, 30, 5190–5197. [Google Scholar] [CrossRef]
- Coutinho, J.O.P.A.; Silva, M.P.S.; Moraes, P.M.; Monteiro, A.S.; Barcelos, J.C.C.; Siqueira, E.P.; Santos, V.L. Demulsifying properties of extracellular products and cells of Pseudomonas aeruginosa MSJ isolated from petroleum-contaminated soil. Bioresour. Technol. 2013, 128, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Dhandhi, Y.; Chaudhari, R.K.; Naiya, T.K. Development in separation of oilfield emulsion toward green technology–A comprehensive review. Sep. Sci. Technol. 2022, 57, 1642–1668. [Google Scholar] [CrossRef]
- Baloyi, J.; Mafunda, A. Novel Methods and Environment-Friendly Techniques for the Remediation of Environmental Petroleum Pollutants. In Environmental Hydrocarbon Pollution and Zero Waste Approach Towards a Sustainable Waste Management; Springer: Berlin/Heidelberg, Germany, 2025; pp. 143–174. [Google Scholar]
- Heeres, A.S.; Picone, C.S.; van der Wielen, L.A.; Cunha, R.L.; Cuellar, M.C. Microbial advanced biofuels production: Overcoming emulsification challenges for large-scale operation. Trends Biotechnol. 2014, 32, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, H.; Khodapanah, E. Comprehensive review of hybrid chemical enhanced oil recovery methods: Synergistic mechanisms, applications, and insights into chemical-based water alternating gas techniques. J. Pet. Explor. Prod. Technol. 2025, 15, 98. [Google Scholar] [CrossRef]
- Issaka, S.A. Review on the fundamental aspects of petroleum oil emulsions and techniques of demulsification. J. Pet. Environ. Biotechnol. 2015, 6, 1000214. [Google Scholar] [CrossRef]
- Yi, M.; Huang, J.; Wang, L. Research on crude oil demulsification using the combined method of ultrasound and chemical demulsifier. J. Chem. 2017, 2017, 9147926. [Google Scholar] [CrossRef]
- Hashem, H.; Kikhavani, T.; Moradkhani, M. Experimental study and machine learning modeling of water removal efficiency from crude oil using demulsifier. Sci. Rep. 2024, 14, 9187. [Google Scholar] [CrossRef]
- Sáez-Martínez, F.J.; Lefebvre, G.; Hernández, J.J.; Clark, J.H. Drivers of sustainable cleaner production and sustainable energy options. J. Clean. Prod. 2016, 138, 1–7. [Google Scholar] [CrossRef]
- Abed, S.; Abdurahman, N.; Yunus, R.; Abdulbari, H.; Akbari, S. Oil emulsions and the different recent demulsification techniques in the petroleum industry—A review. In Proceedings of the 1st ProSES Symposium 2019, Kuantan, Pahang, Malaysia, 4 September 2019; p. 012060. [Google Scholar]
- Wei, X.; Zhang, S.; Han, Y.; Wolfe, F.A. Treatment of petrochemical wastewater and produced water from oil and gas. Water Environ. Res. 2019, 91, 1025–1033. [Google Scholar] [CrossRef]
- Zolfaghari, A.; Gehman, J.; Alessi, D.S. Cost analysis of wastewater production from conventional and unconventional oil and gas wells. Fuel 2022, 323, 124222. [Google Scholar] [CrossRef]
- Okafor, I.; Adewumi, C.N.; Jakada, K.; Nzerem, P.; Oche, O.E.; Danbauchi, S. Preparation and Characterization of Different Bio-Based Demulsifier from Corncob for Crude Oil Emulsion Management. Pet. Coal 2024, 66, 720–730. [Google Scholar]
- Abdullah, M.M.; Al-Lohedan, H.A. Novel bio-based amphiphilic ionic liquids for the efficient demulsification of heavy crude oil emulsions. Molecules 2021, 26, 6119. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, A.D.L.M.; de Silva Junior, C.J.G.; de Amorim, J.D.P.; Durval, I.J.B.; de Santana Costa, A.F.; Sarubbo, L.A. Oily wastewater treatment: Methods, challenges, and trends. Processes 2022, 10, 743. [Google Scholar] [CrossRef]
- Fallon, M.; Halligan, S.; Pezzoli, R.; Geever, L.; Higginbotham, C. Synthesis and characterisation of novel temperature and pH sensitive physically cross-linked poly (N-vinylcaprolactam-co-itaconic acid) hydrogels for drug delivery. Gels 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Romero Vega, G.; Gallo Stampino, P. Bio-Based surfactants and biosurfactants: An overview and main characteristics. Molecules 2025, 30, 863. [Google Scholar] [CrossRef]
- Ahsaei, Z.; Parsaei, R.; Kalantariasl, A.; Abolmaali, S.S.; Tamaddon, A.M. Slow release of surfactant by smart thermosensitive polymer-functionalized mesoporous silica for enhanced oil recovery: Synthesis and characterization. J. Mol. Liq. 2024, 414, 126216. [Google Scholar] [CrossRef]
- Xu, L.; Huang, T.; Alotaibi, M.; Boqmi, A. Novel Thermal Stimuli-Responsive Surfactants to Reduce Operation Costs for Chemical Flooding. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 22–25 April 2024; p. D021S012R001. [Google Scholar]
- Ambagaspitiya, T.D.; Garza, D.J.C.; Skelton, E.; Kubacki, E.; Knight, A.; Bergmeier, S.C.; Cimatu, K.L.A. Using the pH sensitivity of switchable surfactants to understand the role of the alkyl tail conformation and hydrogen bonding at a molecular level in elucidating emulsion stability. J. Colloid Interface Sci. 2025, 678, 164–175. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. The use of surfactants in enhanced oil recovery: A review of recent advances. Energy Rep. 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Chowdhury, S.; Shrivastava, S.; Kakati, A.; Sangwai, J.S. Comprehensive review on the role of surfactants in the chemical enhanced oil recovery process. Ind. Eng. Chem. Res. 2022, 61, 21–64. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Saponins and microbial biosurfactants: Potential raw materials for the formulation of cosmetics. Biotechnol. Prog. 2018, 34, 1482–1493. [Google Scholar] [CrossRef]
- Barbosa, F.G.; Ribeaux, D.R.; Rocha, T.; Costa, R.A.; Guzman, R.R.; Marcelino, P.R.; Lacerda, T.M.; da Silva, S.S. Biosurfactants: Sustainable and versatile molecules. J. Braz. Chem. Soc. 2022, 33, 870–893. [Google Scholar] [CrossRef]
- Bhadani, A.; Kafle, A.; Ogura, T.; Akamatsu, M.; Sakai, K.; Sakai, H.; Abe, M. Current perspective of sustainable surfactants based on renewable building blocks. Curr. Opin. Colloid Interface Sci. 2020, 45, 124–135. [Google Scholar] [CrossRef]
- Stubbs, S.; Yousaf, S.; Khan, I. A review on the synthesis of bio-based surfactants using green chemistry principles. DARU J. Pharm. Sci. 2022, 30, 407–426. [Google Scholar] [CrossRef]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-derived saponins: A review of their surfactant properties and applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Lokesh, K.; West, C.; Kuylenstierna, J.; Fan, J.; Budarin, V.; Priecel, P.; Lopez-Sanchez, J.; Clark, J. Environmental impact assessment of wheat straw based alkyl polyglucosides produced using novel chemical approaches. Green Chem. 2017, 19, 4380–4395. [Google Scholar] [CrossRef]
- Guilbot, J.; Kerverdo, S.; Milius, A.; Escola, R.; Pomrehn, F. Life cycle assessment of surfactants: The case of an alkyl polyglucoside used as a self emulsifier in cosmetics. Green Chem. 2013, 15, 3337–3354. [Google Scholar] [CrossRef]
- Masyithah, Z.; Swasono, A.; Sianturi, P.; Leanon, R.; Wirawan, W.; Riyadi, R. Modeling and optimization of alkyl polyglucoside surfactants from fatty alcohol by response surface methodology. ARPN J. Eng. Appl. Sci. 2020, 15, 1312–1318. [Google Scholar]
- Wisetkomolmat, J.; Suppakittpaisarn, P.; Sommano, S.R. Detergent plants of Northern Thailand: Potential sources of natural saponins. Resources 2019, 8, 10. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, Y.; Preda, F.; Clacens, J.-M.; Shi, H.; Wang, L.; Feng, X.; De Campo, F. Preparation of bio-based surfactants from glycerol and dodecanol by direct etherification. Green Chem. 2015, 17, 882–892. [Google Scholar] [CrossRef]
- Shintaro, I.; Hiroshi, H.; Shun, S.; Hideki, S.; Masahiko, A.; Dai, K.; Keiji, S. Synthesis and interfacial properties of monoacyl glyceric acids as a new class of green surfactants. J. Oleo Sci. 2012, 61, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Aziz, A.A.; Aroua, M. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
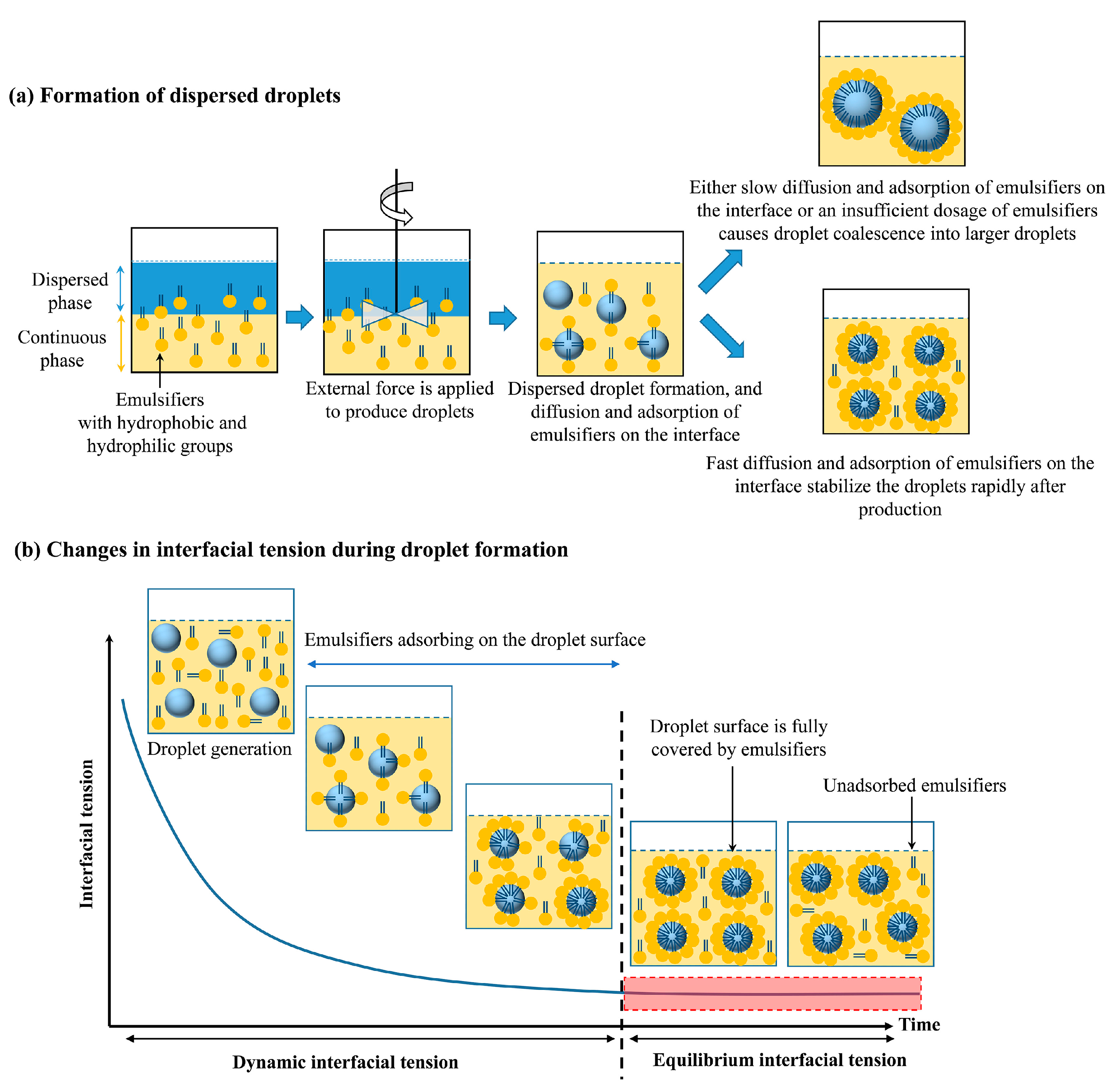
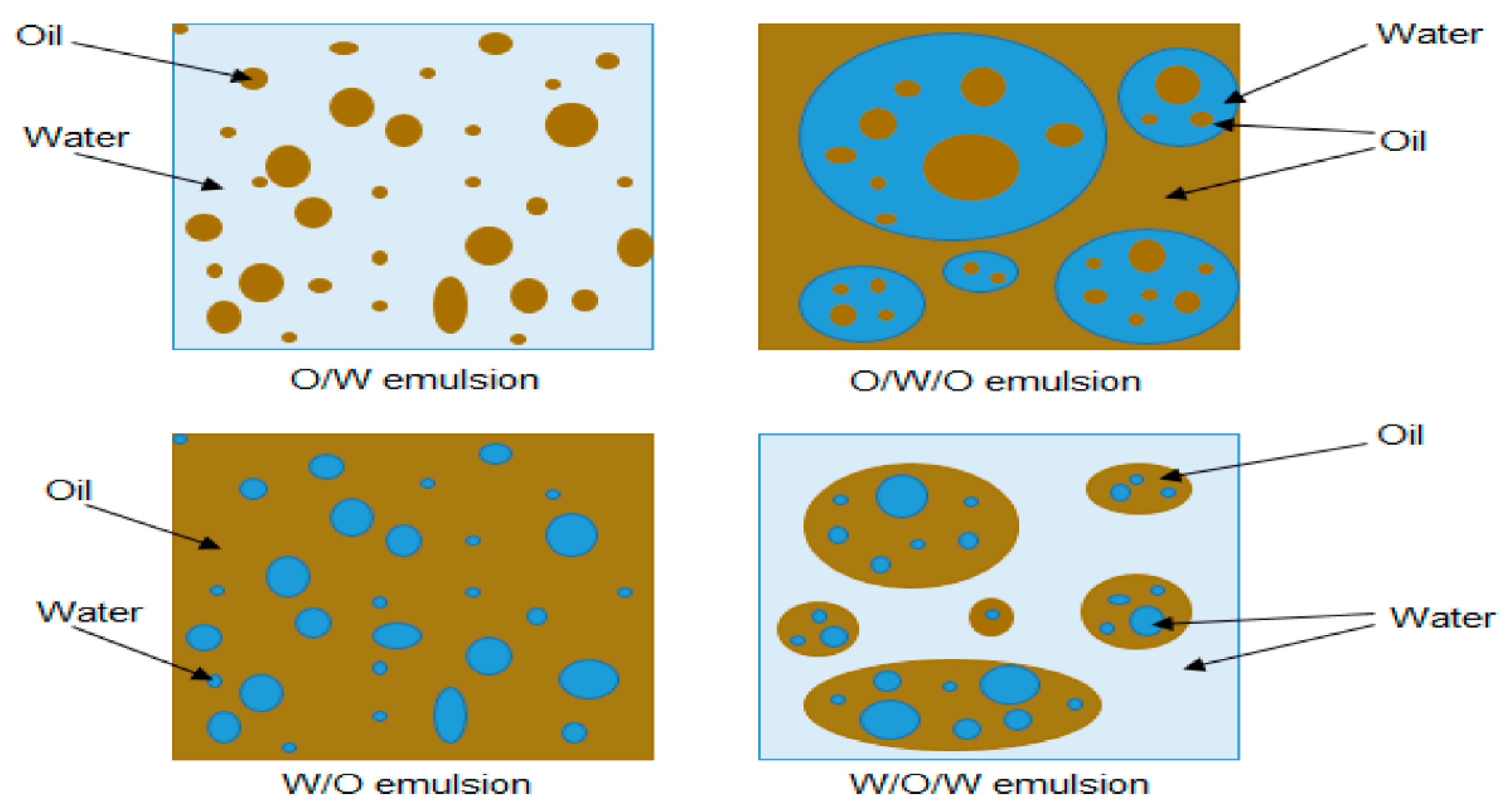

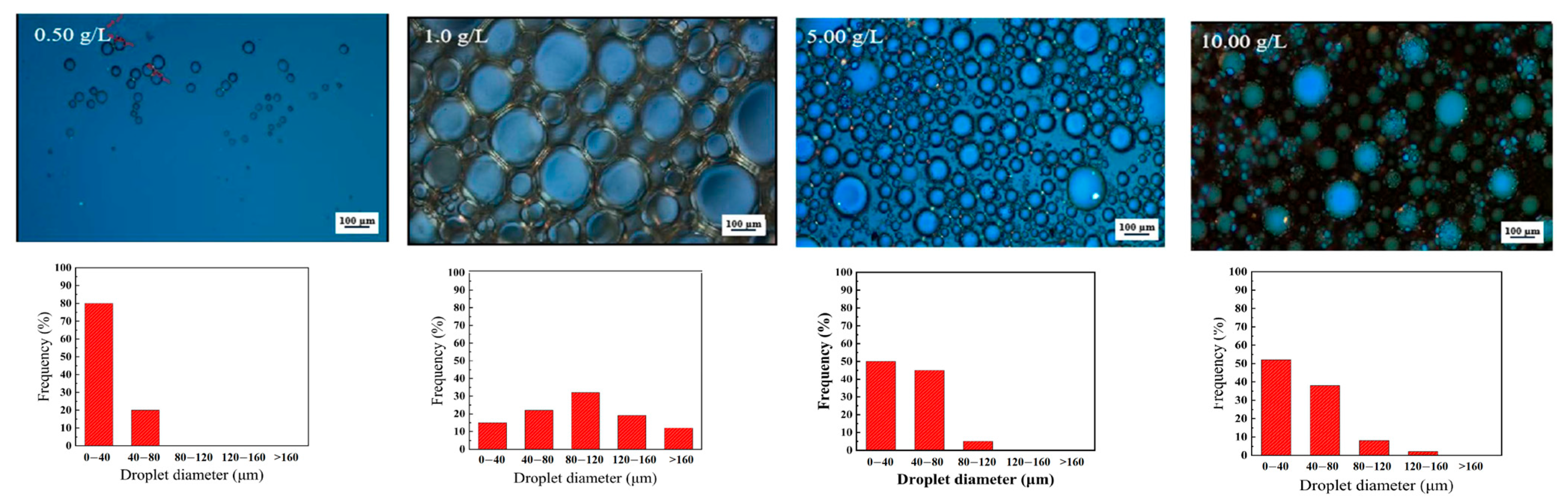
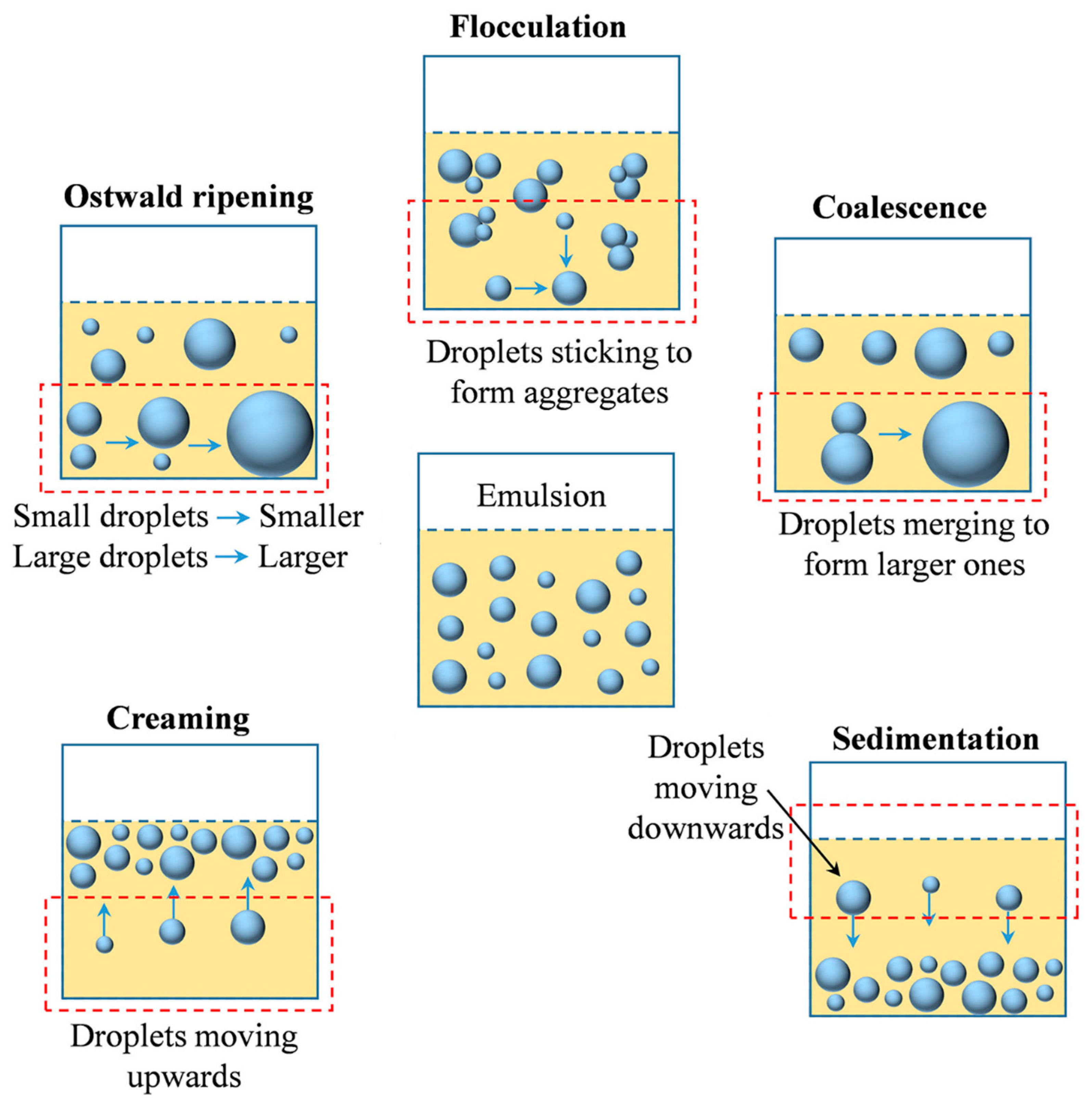
| Factor Category | Sub-Factor | Mechanism/Effect on Stability | Key Considerations/Implications |
|---|---|---|---|
| Crude Oil Composition | Asphaltenes | Adsorb at interface; form rigid films; H-bonding and π-π stacking | Highly polar asphaltenes enhance stability; aggregation affects film strength |
| Resins | Rapid adsorption; enhance asphaltene interfacial films; π-π stacking | Improve emulsion viscosity and resistance to coalescence | |
| Acidic Compounds | Ionize at interface; interact with asphaltenes; H-bonding | Low concentrations strengthen films; excessive conc. reduces stability | |
| Wax Crystals | Form network at low temp; steric hindrance | Increase viscosity and prevent droplet coalescence | |
| Solid Particulates | Clay, silica, metal compounds interact with polar groups | Provide mechanical and steric reinforcement | |
| Operational/ Environmental Factors | Temperature | Reduces viscosity; affects droplet collisions; influences interfacial adsorption | Higher temperature generally decreases emulsion stability by weakening interfacial films |
| Aqueous Phase pH | Modulates droplet charge, surfactant ionization, and film rigidity | Acidic favors W/O emulsions; alkaline favors O/W; optimal demulsification near neutral pH | |
| Droplet Size and Distribution | Smaller droplets increase surface area and viscosity; narrow distribution enhances stability | Directly affects coalescence rate and rheology | |
| Mixing Time and Intensity | Determines droplet size and kinetic stability | Excessive mixing can destabilize films or cause phase inversion | |
| Emulsifier/Demulsifier Concentration | Emulsifiers strengthen films; demulsifiers displace stabilizers to promote coalescence | Optimal demulsifier dosing essential to avoid over-stabilization or secondary emulsification |
| Method | Mechanism | Advantages | Limitations | Industrial Application | |
|---|---|---|---|---|---|
| Chemical | Surfactants/displacers alter interfacial films, promote coalescence | High efficiency, cost-effective, widely applicable | High dosage, environmental concerns, secondary pollution | Industry standard | |
| Physical | Thermal | Heating increases droplet collisions, reduces viscosity | Simple, effective with asphaltene emulsions | High energy demand, volatilization losses | Used in combination with chemicals |
| Mechanical | Gravity settling, centrifugation, separators | Low cost, simple operation | Limited for small droplets, equipment-intensive | Separators, desalters | |
| Electrical | Electric fields polarize droplets, promote chain coalescence | Clean, low chemical use, scalable | Sensitive to emulsion properties, electrode wear | Widely used in desalters | |
| Membrane | Pore filtration ruptures droplets, coalescence on surface | High efficiency, low energy | Fouling, maintenance cost | Emerging field, wastewater treatment | |
| Ultrasonic | Acoustic waves induce droplet aggregation | Fast, pollution-free, versatile | High equipment cost, scalability issues | Research and pilot plants | |
| Biological | Biosurfactants/biodemulsifiers displace stabilizers | Eco-friendly, biodegradable, low energy | Slow production, variability, scalability issues | Potential for green processing | |
| Hybrid | Integration of two or more methods | Combines strengths, reduces limitations | Complex optimization, higher costs | Future industrial adoption | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, S.; Khormali, A. Petroleum Emulsion Stability and Separation Strategies: A Comprehensive Review. ChemEngineering 2025, 9, 113. https://doi.org/10.3390/chemengineering9050113
Ahmadi S, Khormali A. Petroleum Emulsion Stability and Separation Strategies: A Comprehensive Review. ChemEngineering. 2025; 9(5):113. https://doi.org/10.3390/chemengineering9050113
Chicago/Turabian StyleAhmadi, Soroush, and Azizollah Khormali. 2025. "Petroleum Emulsion Stability and Separation Strategies: A Comprehensive Review" ChemEngineering 9, no. 5: 113. https://doi.org/10.3390/chemengineering9050113
APA StyleAhmadi, S., & Khormali, A. (2025). Petroleum Emulsion Stability and Separation Strategies: A Comprehensive Review. ChemEngineering, 9(5), 113. https://doi.org/10.3390/chemengineering9050113






