Coffee Drying as a Catalytic Gas–Solid Dehydration Analogy: A Desiccant-Assisted Theoretical Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Initial Conditions
2.2. Desiccant Selection and Adsorption Properties
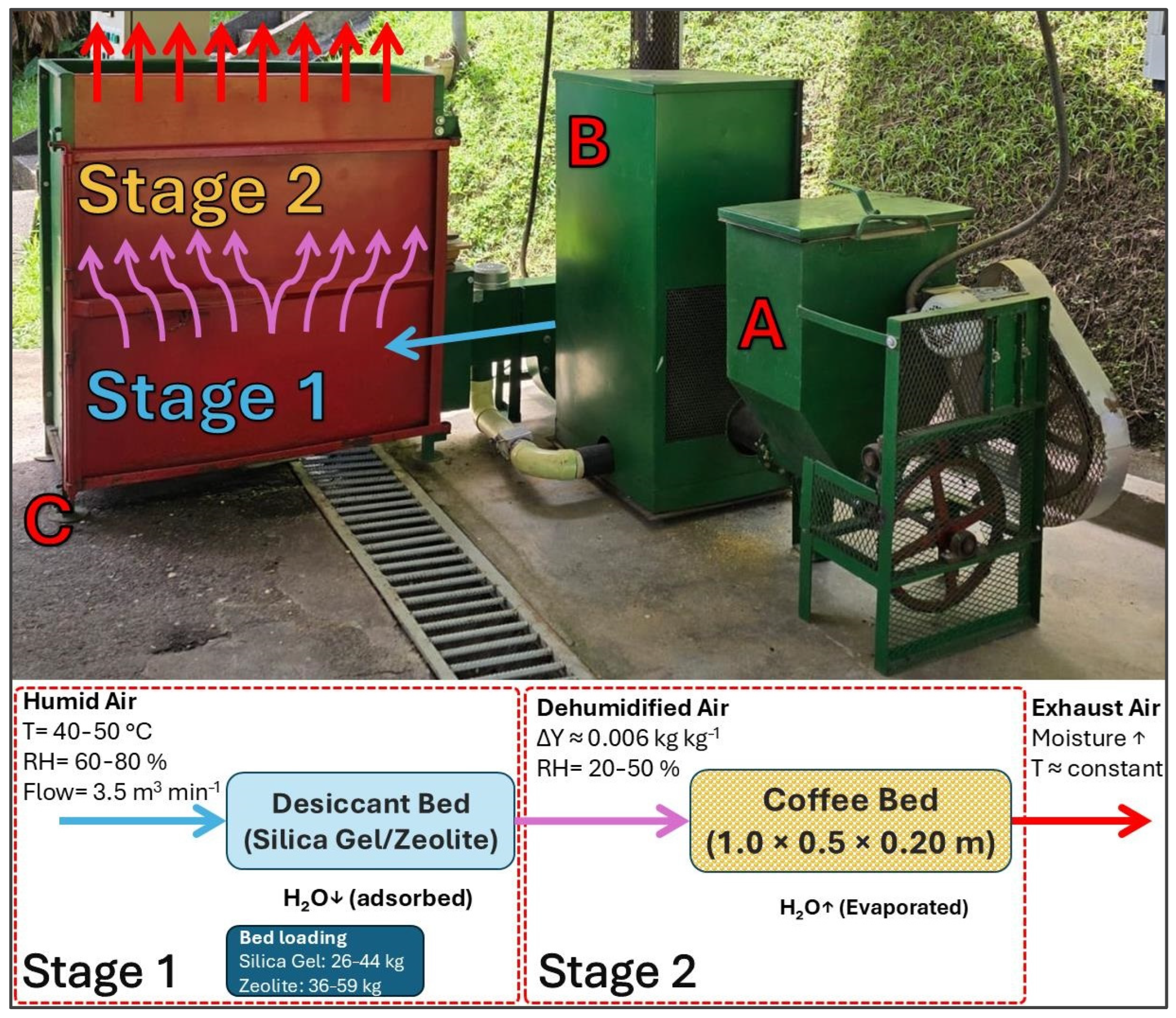
2.3. Reactor Configuration
2.4. Simulation Parameters and Boundary Conditions
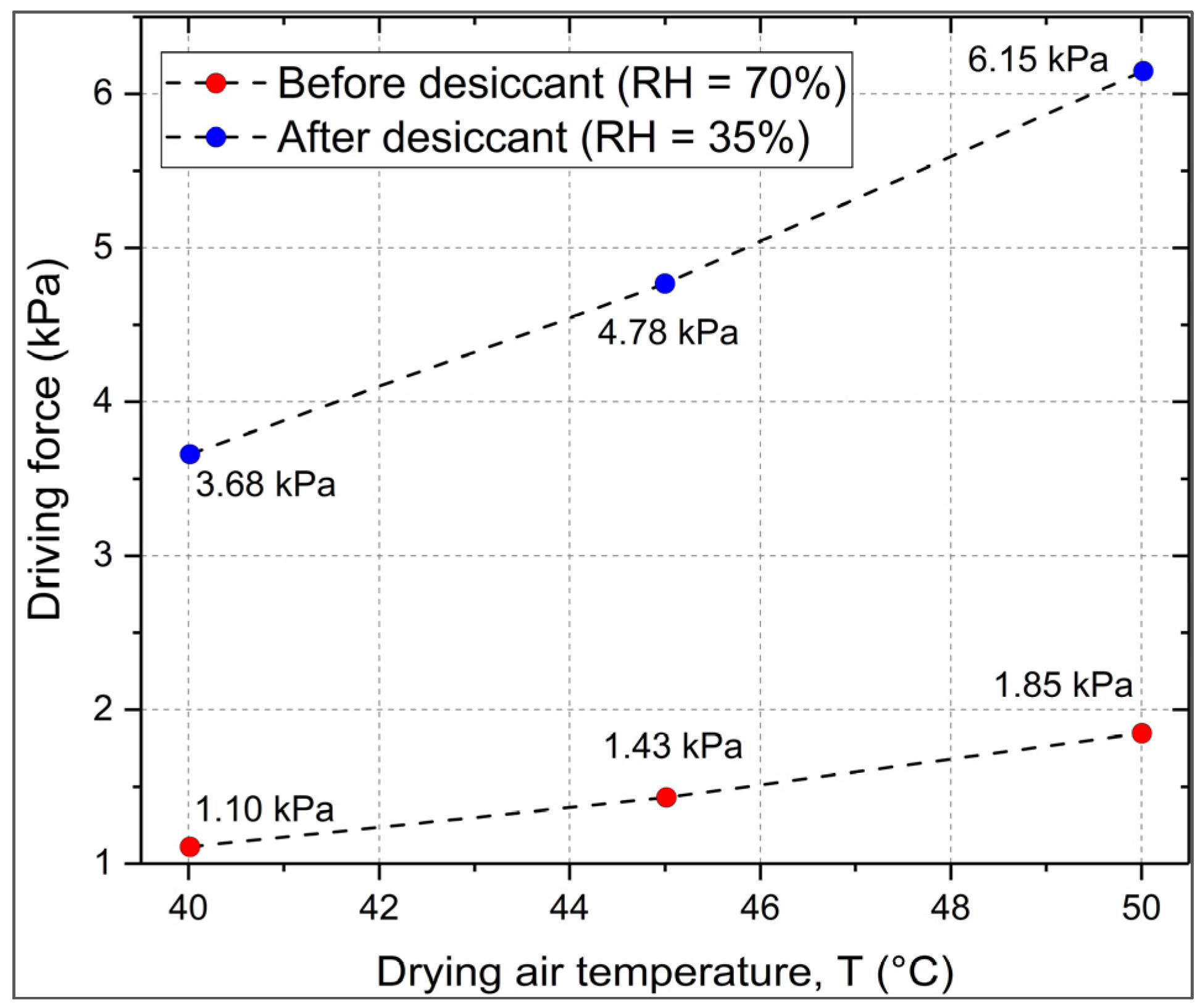
2.5. Coupling and Driving Force Enhancement
2.6. Energy and Economic Considerations
2.7. Numerical Implementation
2.8. Reaction Engineering Analogy
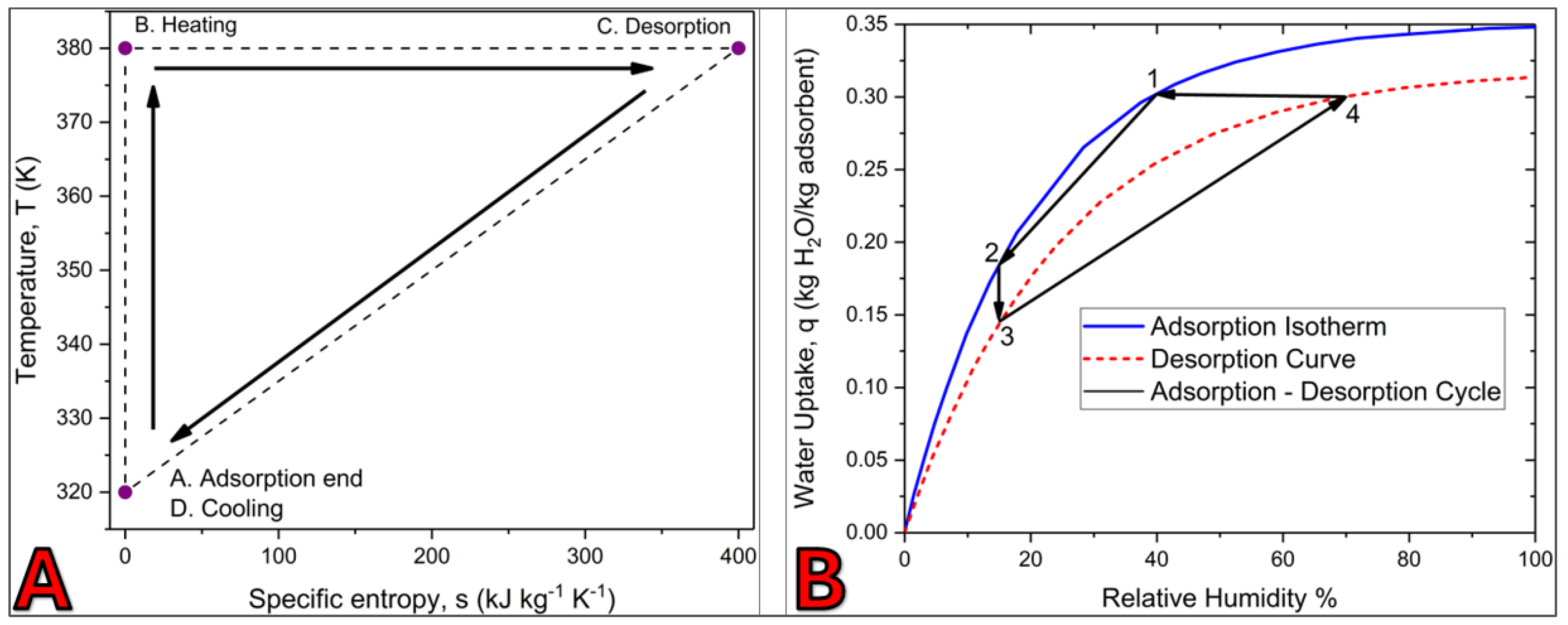
2.9. Thermodynamic Cycle for Desiccant Regeneration
- Adsorption (A→B):
- 2.
- Heating (B→C)
- 3.
- Desorption (C→D):
- 4.
- Cooling (D→A):
3. Results and Discussion
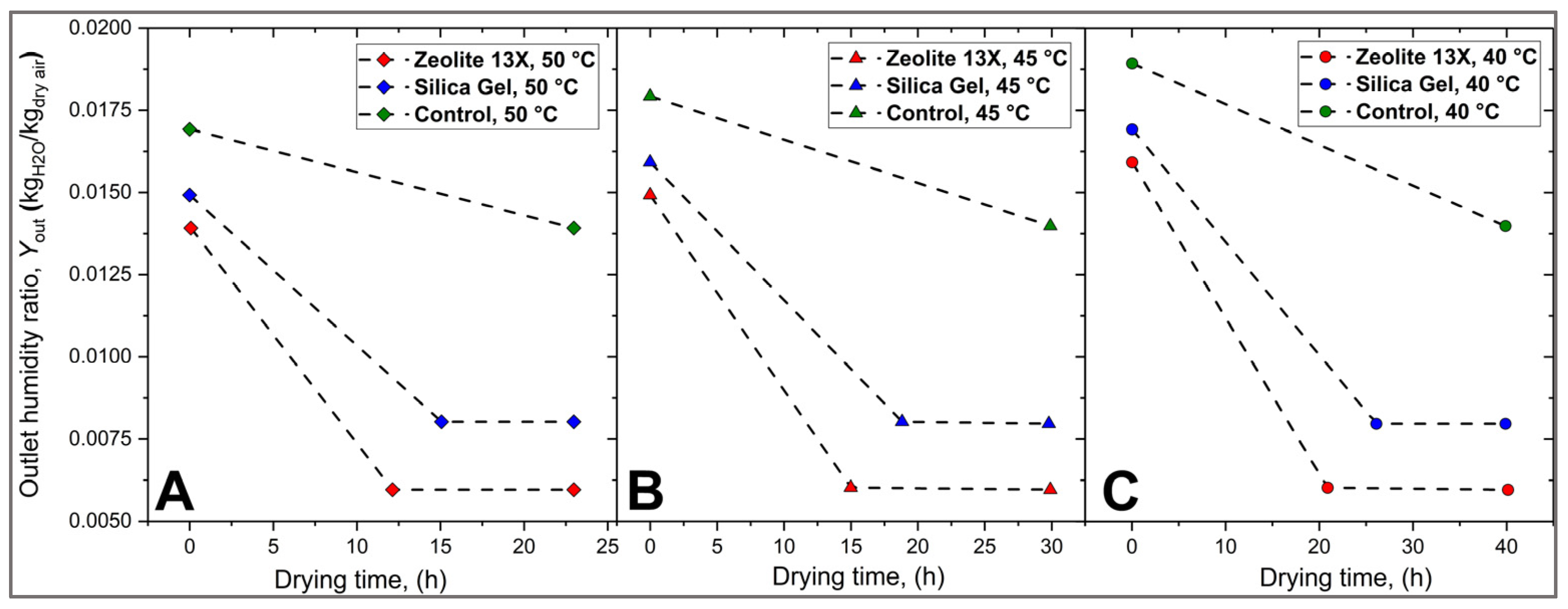
3.1. Drying Kinetics Under Desiccant-Assisted and Control Conditions
3.2. Comparative Performance of Silica Gel and Zeolite 13X
3.3. Energy Balance, Regeneration Cost, and Feasibility Window
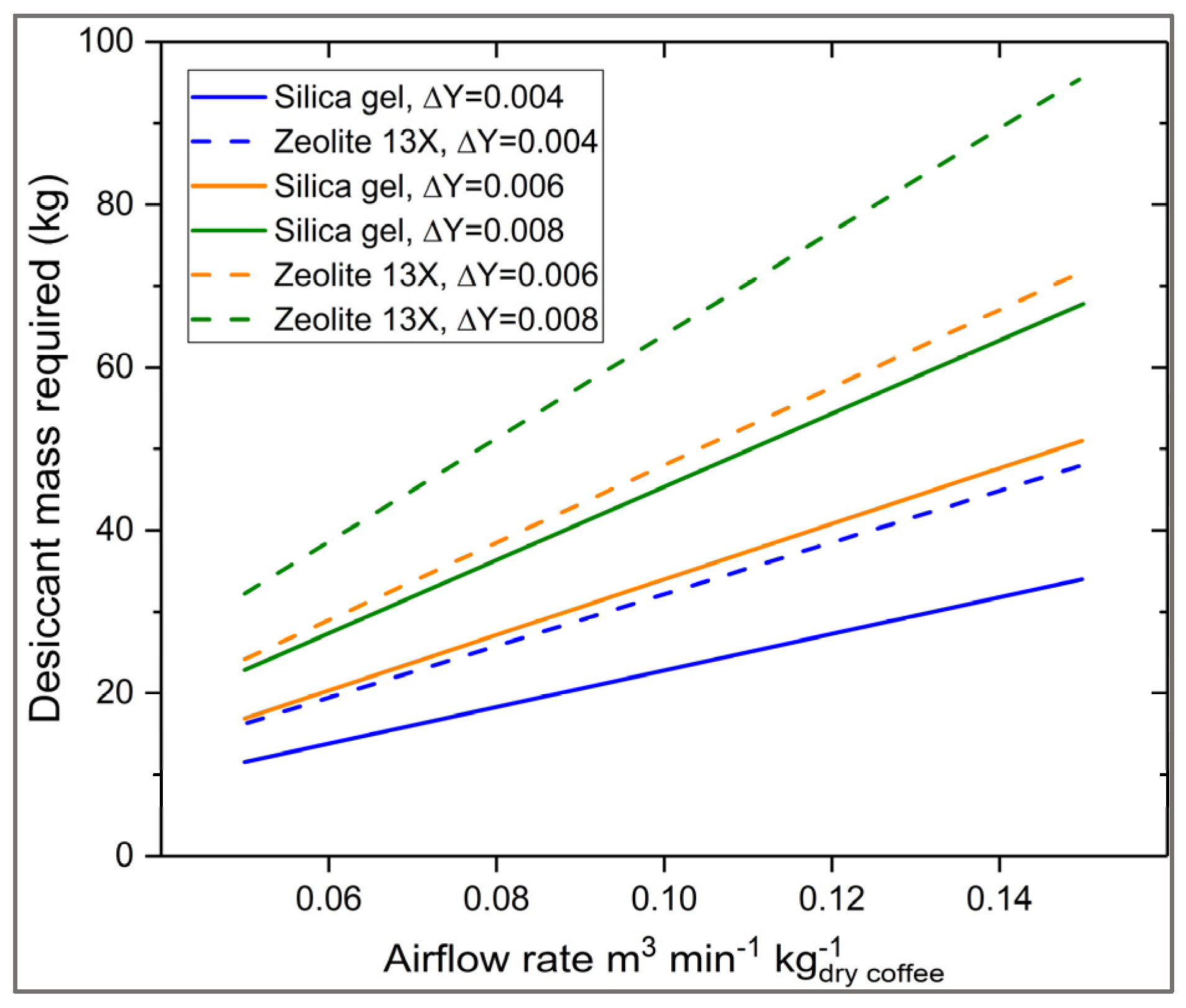
3.4. Sensitivity Analysis and Scaling Guidelines
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worku, M.; Astatkie, T.; Boeckx, P. Effect of Growing Conditions and Postharvest Processing on Arabica Coffee Bean Physical Quality Features and Defects. Heliyon 2022, 8, e09201. [Google Scholar] [CrossRef] [PubMed]
- Borém, F.M.; Andrade, E.T. de Processing and Drying of Coffee. In Drying and Roasting of Cocoa and Coffee; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-1-315-11310-4. [Google Scholar]
- Poltronieri, P.; Rossi, F. Challenges in Specialty Coffee Processing and Quality Assurance. Challenges 2016, 7, 19. [Google Scholar] [CrossRef]
- Restrepo Salazar, I.C.; Peñuela Mesa, G.A. Influence of Temperature, Relative Humidity, and Storage Time Conditions on Ochratoxin a Production by Aspergillus Niger Fungi in Dry Parchment Coffee. Food Addit. Contam. Part A 2025, 42, 491–502. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N.; Taniwaki, M.H. Coffee, Mycotoxins and Climate Change. Food Res. Int. 2014, 61, 1–15. [Google Scholar] [CrossRef]
- Adhikari, M.; Isaac, E.L.; Paterson, R.R.M.; Maslin, M.A. A Review of Potential Impacts of Climate Change on Coffee Cultivation and Mycotoxigenic Fungi. Microorganisms 2020, 8, 1625. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Hu, G.; Hong, D.; Guo, T.; Li, J.; Li, Z.; Qiu, M. Review on Factors Affecting Coffee Volatiles: From Seed to Cup. J. Sci. Food Agric. 2022, 102, 1341–1352. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Chu, Z.; Zhao, J.; Tan, L. Effect of Different Drying Techniques on Bioactive Components, Fatty Acid Composition, and Volatile Profile of Robusta Coffee Beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef]
- Kulapichitr, F.; Borompichaichartkul, C.; Suppavorasatit, I.; Cadwallader, K.R. Impact of Drying Process on Chemical Composition and Key Aroma Components of Arabica Coffee. Food Chem. 2019, 291, 49–58. [Google Scholar] [CrossRef]
- Duque-Dussán, E.; Sanz-Uribe, J.R.; Banout, J. Design and Evaluation of a Hybrid Solar Dryer for Postharvesting Processing of Parchment Coffee. Renew. Energy 2023, 215, 118961. [Google Scholar] [CrossRef]
- Duque-Dussán, E.; Ramírez-Gómez, C.A.; Guerrero-Aguirre, Á.; Rojas-Botina, W.F.; Sanz-Uribe, J.R. Evaluation of Modular Polycarbonate Solar Dryers for Coffee: Technical Performance and Economic Feasibility. J. Food Process Eng. 2025, 48, e70165. [Google Scholar] [CrossRef]
- de Sousa e Silva, J.; Moreli, A.P.; Donzeles, S.M.L.; Soares, S.F.; Vitor, D.G. Harvesting, Drying and Storage of Coffee. In Quality Determinants in Coffee Production; Louzada Pereira, L., Rizzo Moreira, T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–64. ISBN 978-3-030-54437-9. [Google Scholar]
- Meja, E.M.; Dubbe, S.K.; Bekele, A.; Wolde, K.F.; Adaramola, M.S. Investigating the Performance and Optimization of Solar Coffee Drying Technologies—A Systematic Review. J. Food Process. Preserv. 2025, 2025, 7907660. [Google Scholar] [CrossRef]
- Zziwa, A.; Sempiira, J.E.; Kizito, S.S.; Kabenge, I.; Soddo, P. Accelerating Coffee Drying with Innovation: Performance Evaluation of a Sensor-Controlled Hybrid Solar-Biomass Powered Dryer for Coffee Drying in Uganda. Sustain. Energy Technol. Assess. 2025, 82, 104507. [Google Scholar] [CrossRef]
- Manrique, R.; Vásquez, D.; Chejne, F.; Pinzón, A. Energy Analysis of a Proposed Hybrid Solar–Biomass Coffee Bean Drying System. Energy 2020, 202, 117720. [Google Scholar] [CrossRef]
- da Costa, F.O.; Alvarenga, T.F.; de Mesquita, T.V.C.; Petri Júnior, I. Hybrid Drying of Pulped Arabica Coffee Cherry Beans (Coffea arabica L. Cv. Catuai) Using a Hexagonal Microwave Dryer Designed by Numerical Simulations. J. Food Process Eng. 2021, 44, e13666. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, G.-H.; Lee, K.-T.; Gunarathne, D.S.; Hoang, A.T. Synergetic Strategy: Upgrading Fuel Properties of Waste Starch Composite Fuels by Blending Torrefied Spent Coffee Grounds. J. Taiwan Inst. Chem. Eng. 2024, 105820. [Google Scholar] [CrossRef]
- Rocha, T.d.A.F.; Ferreira, M.D.C.; Freire, J.T. Processing Spent Coffee Ground Powders for Renewable Energy Generation: Mechanical Dewatering and Thermal Drying. Process Saf. Environ. Prot. 2021, 146, 300–311. [Google Scholar] [CrossRef]
- Lee, K.-T.; Tsai, J.-Y.; Hoang, A.T.; Chen, W.-H.; Gunarathne, D.S.; Tran, K.-Q.; Selvarajoo, A.; Goodarzi, V. Energy-Saving Drying Strategy of Spent Coffee Grounds for Co-Firing Fuel by Adding Biochar for Carbon Sequestration to Approach Net Zero. Fuel 2022, 326, 124984. [Google Scholar] [CrossRef]
- Villagran, E.; Espitia, J.J.; Velázquez, F.A.; Rodriguez, J. Solar Dryers: Technical Insights and Bibliometric Trends in Energy Technologies. AgriEngineering 2024, 6, 4041–4063. [Google Scholar] [CrossRef]
- John, M.K.; Bandaru, R.; Zachariah, T.J. Comparative Review with a Field Survey on Conventional and Advanced Solar Dryers in Agricultural Processing. J. Therm. Anal. Calorim. 2025, 150, 10267–10298. [Google Scholar] [CrossRef]
- Nilnont, W.; Thepa, S.; Janjai, S.; Kasayapanand, N.; Thamrongmas, C.; Bala, B.K. Finite Element Simulation for Coffee (Coffea arabica) Drying. Food Bioprod. Process. 2012, 90, 341–350. [Google Scholar] [CrossRef]
- Oliveros, N.O.; Hernández, J.A.; Sierra-Espinosa, F.Z.; Guardián-Tapia, R.; Pliego-Solórzano, R. Experimental Study of Dynamic Porosity and Its Effects on Simulation of the Coffee Beans Roasting. J. Food Eng. 2017, 199, 100–112. [Google Scholar] [CrossRef]
- Burmester, K.; Fehr, H.; Eggers, R. A Comprehensive Study on Thermophysical Material Properties for an Innovative Coffee Drying Process. Dry. Technol. 2011, 29, 1562–1570. [Google Scholar] [CrossRef]
- Abdelgaied, M.; Saber, M.A.; Bassuoni, M.M.; Khaira, A.M. Adsorption Air Conditioning: A Comprehensive Review in Desiccant Materials, System Progress, and Recent Studies on Different Configurations of Hybrid Solid Desiccant Air Conditioning Systems. Envron. Sci. Pollut. Res. 2023, 30, 28344–28372. [Google Scholar] [CrossRef]
- Djaeni, M.; A’yuni, D.Q.; Alhanif, M.; Hii, C.L.; Kumoro, A.C. Air Dehumidification with Advance Adsorptive Materials for Food Drying: A Critical Assessment for Future Prospective. Dry. Technol. 2021, 39, 1648–1666. [Google Scholar] [CrossRef]
- Kumar, S.; Salins, S.S.; Kuroor, U.K.; D’Souza, S.W.; Bose, A. Impact of Dry Rice Grain Packing Density and Time on the Performance of Two Stage Dehumidifier. Sci. Rep. 2025, 15, 16908. [Google Scholar] [CrossRef]
- Chai, S.; Kong, X.; Mercangöz, M. State Estimation for Industrial Desiccant Air Dryers Using Hybrid Mechanistic and Machine Learning Models. Comput. Ind. 2025, 168, 104274. [Google Scholar] [CrossRef]
- Jeong, Y.; Ansari, J.R.; Sadeghi, K.; Seo, J. Applicability of Polypropylene/Polyethylene Glycol/Molecular Sieve Composites as Desiccant Pharmaceutical Packaging Materials. Food Packag. Shelf Life 2024, 42, 101266. [Google Scholar] [CrossRef]
- Anokye-Bempah, L.; Han, J.; Kornbluth, K.; Ristenpart, W.; Donis-González, I.R. The Use of Desiccants for Proper Moisture Preservation in Green Coffee during Storage and Transportation. J. Agric. Food Res. 2023, 11, 100478. [Google Scholar] [CrossRef]
- Sanjay; Mishra, S.; Alam, T. Experimental Investigation on Storage of Food Grains Using Cow Dung-Based Desiccant as Preservative. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 12018–12039. [Google Scholar] [CrossRef]
- Zeeshan, M.; Tufail, I.; Khan, S.; Khan, I.; Ayuob, S.; Mohamed, A.; Chauhdary, S.T. Novel Design and Performance Evaluation of an Indirectly Forced Convection Desiccant Integrated Solar Dryer for Drying Tomatoes in Pakistan. Heliyon 2024, 10, e29284. [Google Scholar] [CrossRef]
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A Review on Antimicrobial Packaging for Extending the Shelf Life of Food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Meshcheryakov, E.P.; Reshetnikov, S.I.; Sandu, M.P.; Knyazev, A.S.; Kurzina, I.A. Efficient Adsorbent-Desiccant Based on Aluminium Oxide. Appl. Sci. 2021, 11, 2457. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M.; Agung Susantyoko, R. Zeolites and Superporous Hydrogels-Based Hybrid Composites as Solid Desiccants to Capture Water Vapors from Humid Air. Microporous Mesoporous Mater. 2022, 342, 112116. [Google Scholar] [CrossRef]
- Hraiech, I.; Zallama, B.; Belkhiria, S.; Zili-Ghedira, L.; Maatki, C.; Hassen, W.; Hadrich, B.; Kolsi, L. Experimental Characterization of Silica Gel Adsorption and Desorption Isotherms under Varying Temperature and Relative Humidity in a Fixed Bed Reactor. Sci. Rep. 2025, 15, 29041. [Google Scholar] [CrossRef]
- Majumder, P.; Sinha, A.; Gupta, R.; Sablani, S.S. Drying of Selected Major Spices: Characteristics and Influencing Parameters, Drying Technologies, Quality Retention and Energy Saving, and Mathematical Models. Food Bioprocess. Technol. 2021, 14, 1028–1054. [Google Scholar] [CrossRef]
- Hua, L.; Wang, R. An Exergy Analysis and Parameter Optimization of Solid Desiccant Heat Pumps Recovering the Condensation Heat for Desiccant Regeneration and Heat Transfer Enhancement. Energy 2022, 238, 121811. [Google Scholar] [CrossRef]
- Gorai, V.K.; Singh, S.K.; Jani, D.B. A Comprehensive Review on Solid Desiccant-Assisted Novel Dehumidification and Its Advanced Regeneration Methods. J. Therm. Anal. Calorim. 2024, 149, 8979–9000. [Google Scholar] [CrossRef]
- Zeng, Y.; Woods, J.; Cui, S. The Energy Saving Potential of Thermo-Responsive Desiccants for Air Dehumidification. Energy Convers. Manag. 2021, 244, 114520. [Google Scholar] [CrossRef]
- Andrade, P.S.; Duarte, C.R.; Barrozo, M.A.S. An Innovative Dryer for Arabica Coffee (Coffea arabica L.) Drying: Investigating Heat and Mass Transfer. Dry. Technol. 2024, 42, 1065–1076. [Google Scholar] [CrossRef]
- Collazos-Escobar, G.A.; Gutiérrez-Guzmán, N.; Váquiro, H.A.; García-Pérez, J.V.; Cárcel, J.A. Analysis of Machine Learning Algorithms for the Computer Simulation of Moisture Sorption Isotherms of Coffee Beans. Food Bioprocess. Technol. 2025, 18, 5419–5430. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, M. Water Vapor Adsorption on Desiccant Materials for Rotary Desiccant Air Conditioning Systems. Processes 2023, 11, 2166. [Google Scholar] [CrossRef]
- Almasarani, A.; Ahmad, I.K.; El-Amin, M.F.; Brahimi, T. Experimental Investigations and Modeling of Atmospheric Water Generation Using a Desiccant Material. Energies 2022, 15, 6834. [Google Scholar] [CrossRef]
- ICC-122-12-e; National Quality Standards. International Coffee Organization (ICO): London, UK, 2018.
- Montilla-Pérez, J.; Arcila-Pulgarín, J.; Aristizábal-Loaiza, M.; Montoya-Restrepo, E.C.; Puerta-Quintero, G.I.; Oliveros-Tascón, C.E.; Cadena-Gómez, G. Caracterización de Algunas Propiedades Físicas y Factores de Conversión Del Café Durante El Proceso de Beneficio Húmedo Tradicional. Cenicafé 2008, 59, 120–142. [Google Scholar]
- Moroney, K.M.; O’Connell, K.; Meikle-Janney, P.; O’Brien, S.B.G.; Walker, G.M.; Lee, W.T. Analysing Extraction Uniformity from Porous Coffee Beds Using Mathematical Modelling and Computational Fluid Dynamics Approaches. PLoS ONE 2019, 14, e0219906. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Johnston, R.; Navarini, L.; Liverani, F.S.; Ellero, M. Exploring the Link between Coffee Matrix Microstructure and Flow Properties Using Combined X-Ray Microtomography and Smoothed Particle Hydrodynamics Simulations. Sci. Rep. 2023, 13, 16374. [Google Scholar] [CrossRef] [PubMed]
- Duque-Dussán, E.; Sanz-Uribe, J.R.; Dussán-Lubert, C.; Banout, J. Thermophysical Properties of Parchment Coffee: New Colombian Varieties. J. Food Process Eng. 2023, 46, e14300. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Theoretical Basis for the Potential Theory Adsorption Isotherms. In The Dubinin-Radushkevich and Dubinin-Astakhov Equations; ACS Publications: Washington, DC, USA, 2002. [Google Scholar]
- Golubyatnikov, O.; Akulinin, E. Application of the Dubinin–Radushkevich–Astakhov Equation to Calculate Gases Isotherms on Zeolite Adsorbents (on Example of H2, CO2, CO, CH4, N2 Adsorption on 13X and 5A). Sep. Sci. Technol. 2022, 57, 2871–2884. [Google Scholar] [CrossRef]
- Oh, H.-T.; Lim, S.-J.; Kim, J.H.; Lee, C.-H. Adsorption Equilibria of Water Vapor on an Alumina/Zeolite 13X Composite and Silica Gel. J. Chem. Eng. Data 2017, 62, 804–811. [Google Scholar] [CrossRef]
- Savelli, F.; Socci, L.; Rocchetti, A.; Lippi, M.; Talluri, L. Modelling of a Desiccant-Coated Heat and Mass Exchanger for Airflow Dehumidification in HVAC Systems. Sustain. Energy Technol. Assess. 2025, 82, 104484. [Google Scholar] [CrossRef]
- Van Kampen, J.; Boon, J.; Van Sint Annaland, M. Steam Adsorption on Molecular Sieve 3A for Sorption Enhanced Reaction Processes. Adsorption 2021, 27, 577–589. [Google Scholar] [CrossRef]
- Dayananda, M.A. A Direct Derivation of Fick’s Law from Continuity Equation for Interdiffusion in Multicomponent Systems. Scr. Mater. 2022, 210, 114430. [Google Scholar] [CrossRef]
- Strong, C.; Carrier, Y.; Handan Tezel, F. Experimental Optimization of Operating Conditions for an Open Bulk-Scale Silica Gel/Water Vapour Adsorption Energy Storage System. Appl. Energy 2022, 312, 118533. [Google Scholar] [CrossRef]
- Duque-Dussán, E.; Banout, J. Improving the Drying Performance of Parchment Coffee Due to the Newly Redesigned Drying Chamber. J. Food Process Eng. 2022, 45. [Google Scholar] [CrossRef]
- Duque-Dussán, E.; Villada-Dussán, A.; Roubík, H.; Banout, J. Modeling of Forced and Natural Convection Drying Process of a Coffee Seed. J. ASABE 2022, 65, 1061–1070. [Google Scholar] [CrossRef]
- Coradi, P.C.; Martens, S.; Rodrigues, H.E.; Leal, A.F.; Costa, D.R.d.; Saath, R.; Borém, F.M. Development and Validation of a Heated Drying Air Diffusion System to Optimize Rotary Dryers and Final Coffee Quality. PLoS ONE 2021, 16, e0251312. [Google Scholar] [CrossRef]
- Fabbri, A.; Cevoli, C.; Alessandrini, L.; Romani, S. Numerical Modeling of Heat and Mass Transfer during Coffee Roasting Process. J. Food Eng. 2011, 105, 264–269. [Google Scholar] [CrossRef]
- Chilev, C.; Dicko, M.; Langlois, P.; Lamari, F. Modelling of Single-Gas Adsorption Isotherms. Metals 2022, 12, 1698. [Google Scholar] [CrossRef]
- Ahsan, S.; Ayub, A.; Meeroff, D.; Jahandar Lashaki, M. A Comprehensive Comparison of Zeolite-5A Molecular Sieves and Amine-Grafted SBA-15 Silica for Cyclic Adsorption-Desorption of Carbon Dioxide in Enclosed Environments. Chem. Eng. J. 2022, 437, 135139. [Google Scholar] [CrossRef]
- Cavalcanti-Mata, M.E.; Duarte, M.E.; Tolentino, M.; Mendes, F.A.; Batista, L.; De Lima, J.M.; Lúcio, A.; Nascimento, A.P.; Almeida, R.D.; Lisboa, H.M. Drying Kinetics of Industrial Pineapple Waste: Effective Diffusivity and Thermodynamic Properties Resulting from New Mathematical Models Derived from the Fick Equation. Processes 2024, 12, 1198. [Google Scholar] [CrossRef]
- Jakkaew, P.; Yingchutrakul, Y.; Aunsri, N. A Data-Driven Approach to Improve Coffee Drying: Combining Environmental Sensors and Chemical Analysis. PLoS ONE 2024, 19, e0296526. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, W.; Ozaki, A.; Lee, H. Influence of the Moisture Driving Force of Moisture Adsorption and Desorption on Indoor Hygrothermal Environment and Building Thermal Load. Energy Build. 2021, 253, 111501. [Google Scholar] [CrossRef]
- Fauzi, M.B.; Kosasih, E.A.; Dzaky, M.I.; Prabowo, A.T. Effects of Drying Temperature and Specific Humidity on Drying Rate Constant and Activation Energy of Robusta Coffee. AIP Conf. Proc. 2024, 3090, 060011. [Google Scholar] [CrossRef]
- Cam, I.B.; Basunal Gulmez, H.; Eroglu, E.; Topuz, A. Strawberry Drying: Development of a Closed-Cycle Modified Atmosphere Drying System for Food Products and the Performance Evaluation of a Case Study. Dry. Technol. 2018, 36, 1460–1473. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.; Guan, J. Regeneration Behavior of Solid Desiccants with Microwave Drying. J. Therm. Anal. Calorim. 2024, 149, 10927–10940. [Google Scholar] [CrossRef]
- Misha, S.; Mat, S.; Ruslan, M.H.; Sopian, K. Review of Solid/Liquid Desiccant in the Drying Applications and Its Regeneration Methods. Renew. Sustain. Energy Rev. 2012, 16, 4686–4707. [Google Scholar] [CrossRef]
- Faraldo, F.; Byrne, P. A Review of Energy-Efficient Technologies and Decarbonating Solutions for Process Heat in the Food Industry. Energies 2024, 17, 3051. [Google Scholar] [CrossRef]
- Méndez Rodríguez, C.; Salazar Benítez, J.; Rengifo Rodas, C.F.; Corrales, J.C.; Figueroa Casas, A. A Multidisciplinary Approach Integrating Emergy Analysis and Process Modeling for Agricultural Systems Sustainable Management—Coffee Farm Validation. Sustainability 2022, 14, 8931. [Google Scholar] [CrossRef]
- Garcia-Freites, S.; Röder, M.; Thornley, P. Environmental Trade-Offs Associated with Bioenergy from Agri-Residues in Sub-Tropical Regions: A Case Study of the Colombian Coffee Sector. Biomass Bioenergy 2020, 140, 105581. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Luo, J.; Yang, H. Improving Air Conditioning Efficiency Using Solar-Assisted Liquid Desiccant Systems: Experimental and Simulation Analysis of Two Desiccants. Renew. Energy 2025, 253, 123572. [Google Scholar] [CrossRef]
- Rashidi, M.; Arabhosseini, A.; Samimi-Akhijahani, H.; Kermani, A.M. Acceleration the Drying Process of Oleaster (Elaeagnus angustifolia L.) Using Reflectors and Desiccant System in a Solar Drying System. Renew. Energy 2021, 171, 526–541. [Google Scholar] [CrossRef]
- Metrane, A.; Delhali, A.; Ouikhalfan, M.; Assen, A.H.; Belmabkhout, Y. Water Vapor Adsorption by Porous Materials: From Chemistry to Practical Applications. J. Chem. Eng. Data 2022, 67, 1617–1653. [Google Scholar] [CrossRef]
- Kim, H.; Hong, S.K.; Kang, J.G.; Moon, S.-W.; Kim, G.; Yoon, S.; Wei, D.; Moon, H.; Kim, B.S. Exploration Adsorption Characteristics of Zeolite 13X Depending on Humidity and Flow Rate in Sorption Thermal Energy Storage Applications. Int. J. Heat. Mass. Transf. 2024, 221, 125049. [Google Scholar] [CrossRef]
- Yang, K.; Su, B.; Shi, L.; Wang, H.; Cui, Q. Adsorption Mechanism and Regeneration Performance of 13X for H2 S and SO2. Energy Fuels 2018, 32, 12742–12749. [Google Scholar] [CrossRef]
- Liu, L.; Kubota, M.; Li, J.; Kimura, H.; Bai, Y.; Wu, R.; Deng, L.; Huang, H.; Kobayashi, N. Comparative Study on the Water Uptake Kinetics and Dehumidification Performance of Silica Gel and Aluminophosphate Zeolites Coatings. Energy 2022, 242, 122957. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Alfaifi, B.; Elamin, W.; Lateef, M.A. Advancements in Coffee Manufacturing: From Dehydration Techniques to Quality Control. Food Eng. Rev. 2024, 16, 513–539. [Google Scholar] [CrossRef]
- Abreu, D.J.M.d.; Lorenço, M.S.; Machado, G.G.L.; Silva, J.M.; de Azevedo, E.C.; Carvalho, E.E.N. Influence of Drying Methods on the Post-Harvest Quality of Coffee: Effects on Physicochemical, Sensory, and Microbiological Composition. Foods 2025, 14, 1463. [Google Scholar] [CrossRef] [PubMed]
- Kazama, E.H.; da Silva, R.P.; de Tavares, T.O.; Correa, L.N.; de Lima Estevam, F.N.; de Nicolau, F.E.A.; Maldonado Júnior, W. Methodology for Selective Coffee Harvesting in Management Zones of Yield and Maturation. Precis. Agric. 2021, 22, 711–733. [Google Scholar] [CrossRef]
- Ronchi, C.P.; DaMatta, F.M. Chapter Thirteen—Managing the Coffee Crop for Flowering Synchronisation and Fruit Maturation: Agronomic and Physiological Issues. In Advances in Botanical Research; Damatta, F.M., Ramalho, J.C., Eds.; Coffee—A Glimpse into the Future; Academic Press: Cambridge, MA, USA, 2025; Volume 114, pp. 421–454. [Google Scholar]
- Xu, Z.; Aili, T.; Yang, J.; Liu, Y.; Yue, H. Grape Drying Promote Agentia: Food Safety Assessment of a Broad-Spectrum Use Drying Agent for Dried Fruits. Ecotoxicol. Environ. Saf. 2025, 298, 118204. [Google Scholar] [CrossRef]
- Patel, G.; Prudhvi, P.V.V.P.; Patra, A.; Pathak, S.S.; Sonawane, A.D.; Shirkole, S.S. Different Parameters Affecting the Efficiency of Dryers. In Drying Technology in Food Processing; Jafari, S.M., Malekjani, N., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 705–742. ISBN 978-0-12-819895-7. [Google Scholar]
- Majumder, P.; Deb, B.; Gupta, R. Design and Development of Solar Assisted Fluidized Bed Dryer Integrated with Liquid Desiccant Dehumidifier: Theoretical Analysis and Experimental Investigation. Energy Convers. Manag. 2022, 270, 116281. [Google Scholar] [CrossRef]
- He, F.; Yang, W.; Huang, S.; Wang, X.; Yan, B.; Zhao, X. Moisture Adsorption Performance Investigation on Double Layer Multi-Stage Desiccant Packed Bed under Different Conditions. Energy 2025, 324, 135994. [Google Scholar] [CrossRef]
- Behede, B.; Chakrabarti, S.; Wankhede, U.; Thakare, H. Review on Nanoporous Inorganic Desiccant Materials in the Context of Application in Rotary Dehumidifiers. Mater. Today Proc. 2022, 57, 2174–2179. [Google Scholar] [CrossRef]
- Amani, M.; Bahrami, M. Greenhouse Dehumidification by Zeolite-Based Desiccant Coated Heat Exchanger. Appl. Therm. Eng. 2021, 183, 116178. [Google Scholar] [CrossRef]
- Baruah, N.; Prasanna Kumar, G.V.; Khobragade, C.B. Desiccant Dehumidification: A Potential Method for Different Drying and Cooling Applications. Russ. J. Phys. Chem. B 2022, 16, 1151–1163. [Google Scholar] [CrossRef]
- Suvana, S.; Singh, V.K. Sustainable Green Energy for Enhanced Agricultural Productivity. In Modern Technology for Sustainable Agriculture; Kumar, A., Singh, V.K., Singh, Y., Singh, S.K., Kumar, P., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 151–168. ISBN 978-3-031-88396-5. [Google Scholar]
- Shimpy; Kumar, M.; Kumar, A. Designs, Performance and Economic Feasibility of Domestic Solar Dryers. Food Eng. Rev. 2023, 15, 156–186. [Google Scholar] [CrossRef]


| Inlet Air Temp (°C) | Control | Silica Gel | Zeolite 13X | |||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | k (h−1) | Time (h) | Reduction (%) | k (h−1) | Time (h) | Reduction (%) | k (h−1) | |
| 40 | 40 | 0.032 | 26 | 35% | 0.048 | 21 | 47% | 0.056 |
| 45 | 30 | 0.041 | 19 | 36.7% | 0.062 | 15 | 50% | 0.071 |
| 50 | 23 | 0.050 | 15 | 34.8% | 0.073 | 12 | 46% | 0.082 |
| Parameter | Silica Gel A | Zeolite 13X |
|---|---|---|
| Typical loading per 70 kg batch 1 | 26–44 kg | 36–59 kg |
| Breakthrough time 2 | 6–8 h | 9–12 h |
| Minimum outlet humidity ratio (Yout,min) 3 | ~0.008 kg H2O·kg−1 dry air | ~0.006 kg H2O·kg−1 dry air |
| Relative drying time reduction 4 | 35–37% | 46–50% |
| Regeneration temperature 3 | ~110–130 °C | ~250–300 °C |
| Regeneration energy demand | Low–moderate | High |
| Material cost (relative) | Low | Moderate–high |
| Fuel Source | Control (Fuel, Cost) | Silica Gel Savings (Fuel, Cost) | Silica Gel Net Cost | Zeolite Savings (Fuel, Cost) | Zeolite Net Cost |
|---|---|---|---|---|---|
| Coffee husk (LHV 17 MJ·kg−1, 0.05 USD·kg−1) | 5.2 kg (0.26 USD) | 1.6–1.8 kg (0.08–0.09 USD) | 0.17–0.18 USD | 0.5–0.8 kg (0.02–0.04 USD) | 0.22–0.24 USD |
| Diesel (LHV 43 MJ·kg−1, 1.10 USD·L−1) | 0.65 L (0.72 USD) | 0.20–0.25 L (0.22–0.27 USD) | 0.45–0.50 USD | 0.06–0.10 L (0.07–0.11 USD) | 0.61–0.65 USD |
| LPG (LHV 46 MJ·kg−1, 0.90 USD·kg−1) | 0.63 kg (0.57 USD) | 0.19–0.24 kg (0.17–0.22 USD) | 0.35–0.40 USD | 0.06–0.10 kg (0.05–0.09 USD) | 0.52–0.55 USD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duque-Dussán, E. Coffee Drying as a Catalytic Gas–Solid Dehydration Analogy: A Desiccant-Assisted Theoretical Framework. ChemEngineering 2025, 9, 112. https://doi.org/10.3390/chemengineering9050112
Duque-Dussán E. Coffee Drying as a Catalytic Gas–Solid Dehydration Analogy: A Desiccant-Assisted Theoretical Framework. ChemEngineering. 2025; 9(5):112. https://doi.org/10.3390/chemengineering9050112
Chicago/Turabian StyleDuque-Dussán, Eduardo. 2025. "Coffee Drying as a Catalytic Gas–Solid Dehydration Analogy: A Desiccant-Assisted Theoretical Framework" ChemEngineering 9, no. 5: 112. https://doi.org/10.3390/chemengineering9050112
APA StyleDuque-Dussán, E. (2025). Coffee Drying as a Catalytic Gas–Solid Dehydration Analogy: A Desiccant-Assisted Theoretical Framework. ChemEngineering, 9(5), 112. https://doi.org/10.3390/chemengineering9050112






