Techno-Economic Analysis of Hybrid Adsorption–Membrane Separation Processes for Direct Air Capture
Abstract
1. Introduction
2. Process and Modeling
2.1. Process Modeling
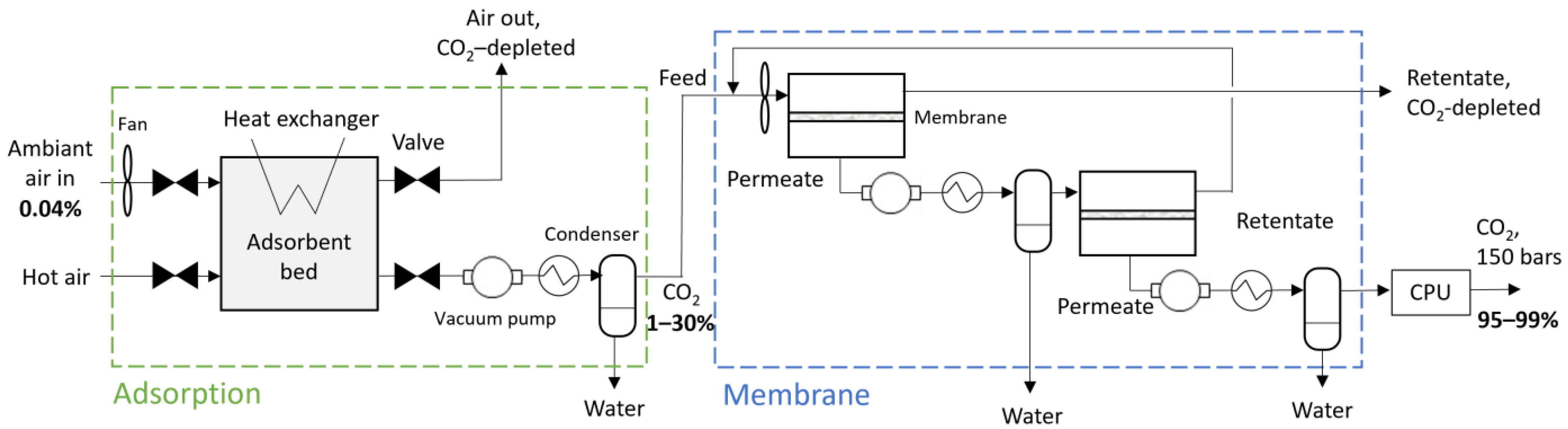
2.1.1. Hybrid DAC Overview
2.1.2. Adsorption Step Modeling
- CO2 and H2O are the only adsorbed substances. N2, O2, and Ar adsorption loadings are neglected in the study [34].
- A 1-D spatial dimension model with axial dispersion is considered.
- The gas mass transfer of components to adsorbent is expressed using the linear driving force (LDF) model.
- Adsorption: Air is pushed by the fan into the bed, where the CO2 and H2O are adsorbed. This step ends when the adsorption criterion is satisfied. The adsorption criterion corresponds to the ratio of the outlet CO2 concentration to the inlet CO2 concentration and is equal to . So, the step ends when the outlet CO2 concentration reaches 50%.
- Evacuation: Air inlet flow stops, and the vacuum pump is turned on until the bed pressure is close to the vacuum pump pressure.
- Repressurization: Air enters the bed until the pressure reaches ambient pressure before starting another cycle.
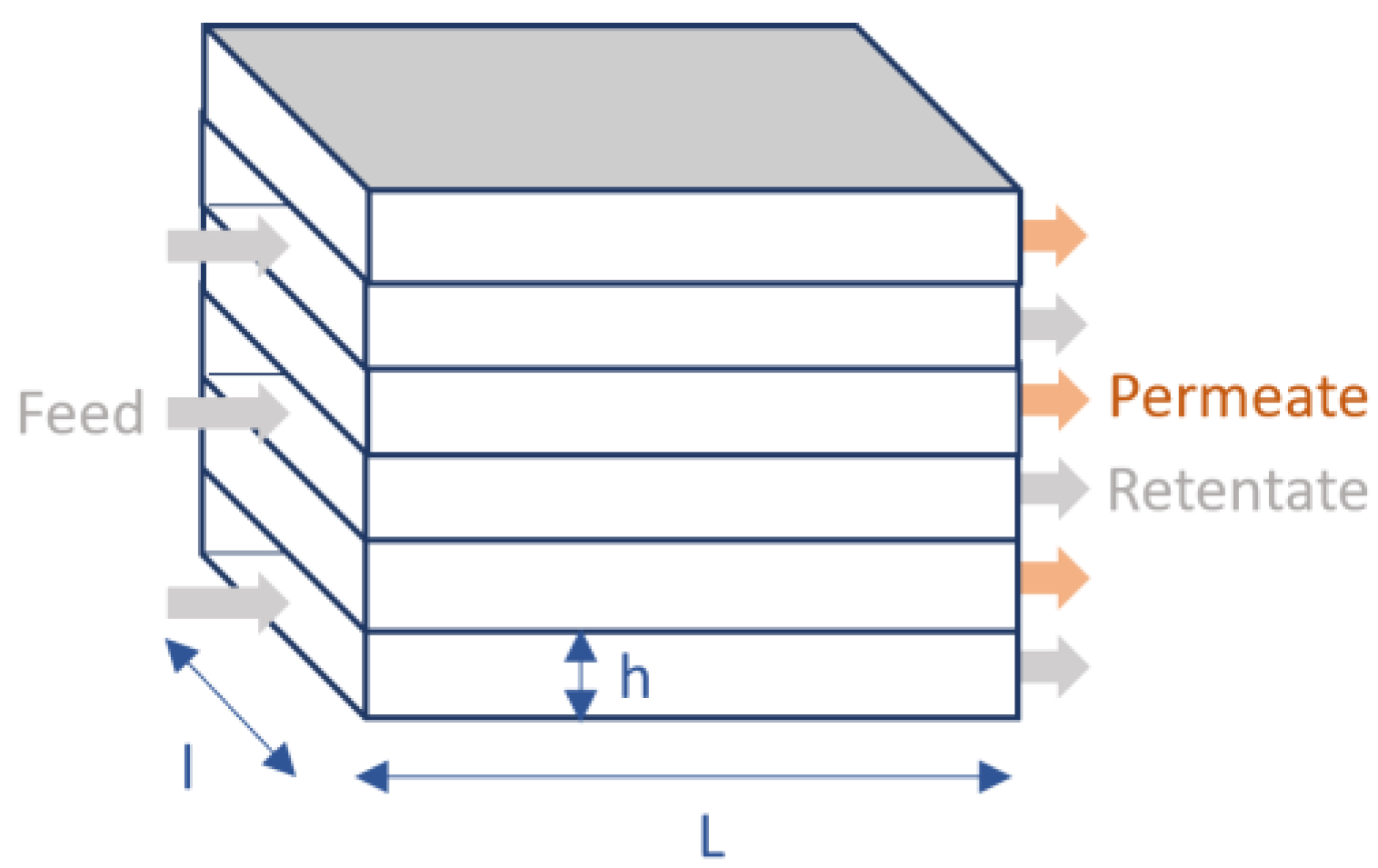
2.1.3. Membrane Step Modeling
2.2. Techno-Economic Methodology
2.2.1. Cases and Key Performance Indicators
- The thermal heat requirement for adsorption regeneration is provided by indirect heating (heat exchanger) and by direct heating (hot air). The heat requirement includes the heat of desorption, the heat capacity of the amine sorbent and support for a monolithic design, the heat capacity of the bed enclosure and indirect heating system, and other heat requirements (adsorbed CO2 and H2O heat capacity, respectively, and gas capacity). Hot air is partially pre-heated by the bed outlet flowrate, considering a 10 °C pinch. These requirements are assessed using Aspen Adsorption V14.
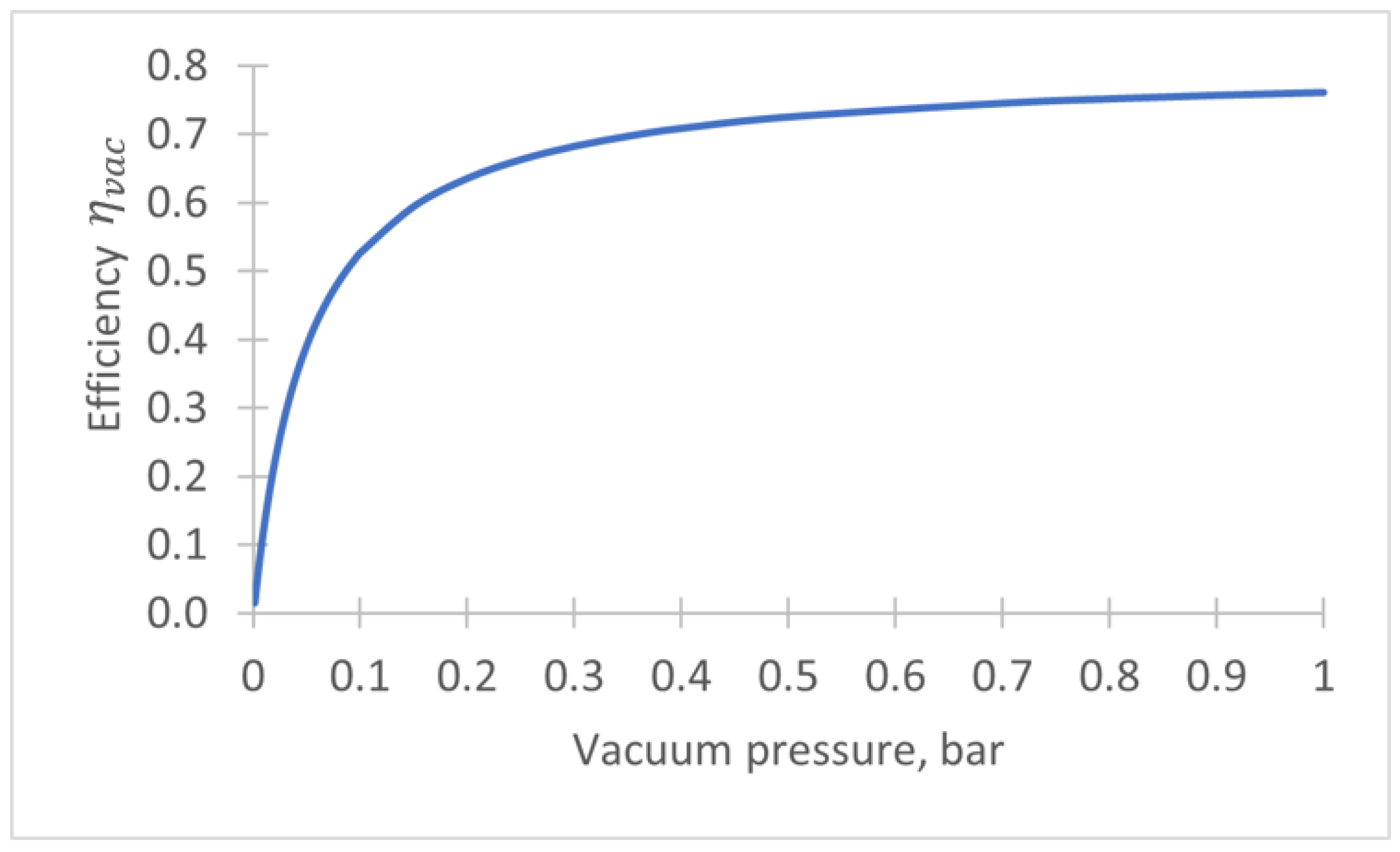
- During the VTSA step and membrane step, vacuum () is provided by a vacuum pump. The vacuum work per tonne of CO2 (MWh/tCO2) is given by:
- Air/gas is pushed through the adsorption bed or membrane module by a fan to compensate for the pressure drop across the adsorption bed or the membrane module in the upstream side (i.e., retentate). The pressure drop in a monolith is calculated using the Hagen–Poiseuille equation for a squared channel [61]:
2.2.2. Cost Analysis
3. Results
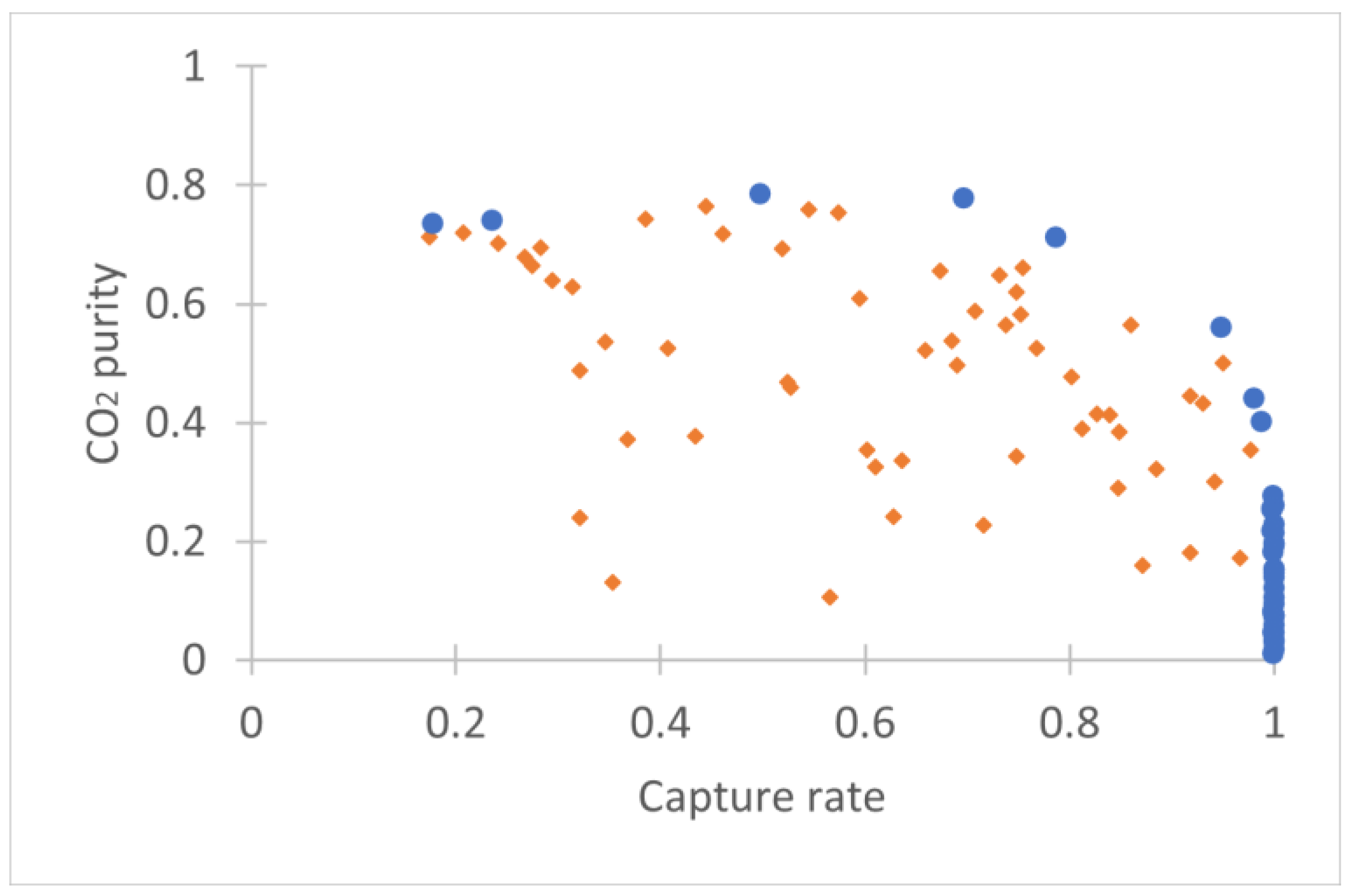
3.1. Adsorption Pre-Concentration Step
3.2. Membrane Step
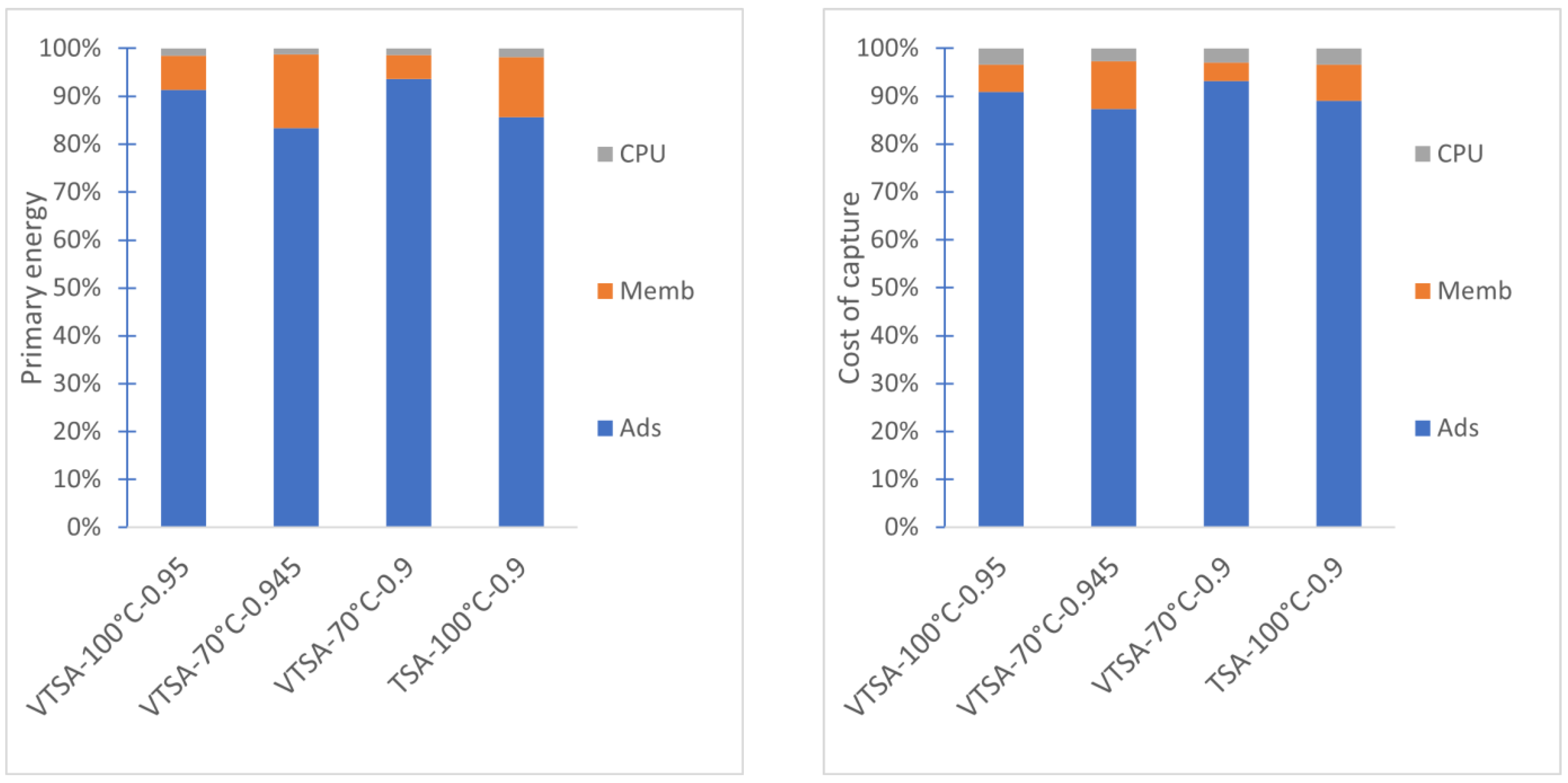
3.3. Complete Hybrid DAC Process
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APEA | Aspen process economic analyzer |
| CAPEX | Capital expenditure |
| CDR | Carbon dioxide removal |
| CPU | Compression and purification unit |
| DAC | Direct air capture |
| GAB | Guggenheim–Anderson–de Boer |
| KPI | Key performance indicator |
| LDF | Linear driving force |
| MRC | Membrane replacement cost |
| OPEX | Operational expenditure |
| TCR | Total capital requirement |
| TFC | Total field cost |
| TPC | Total plant cost |
| TSA | Temperature swing adsorption |
| VTSA | Vacuum thermal swing adsorption |
Appendix A. Compression and Purification Unit (CPU)
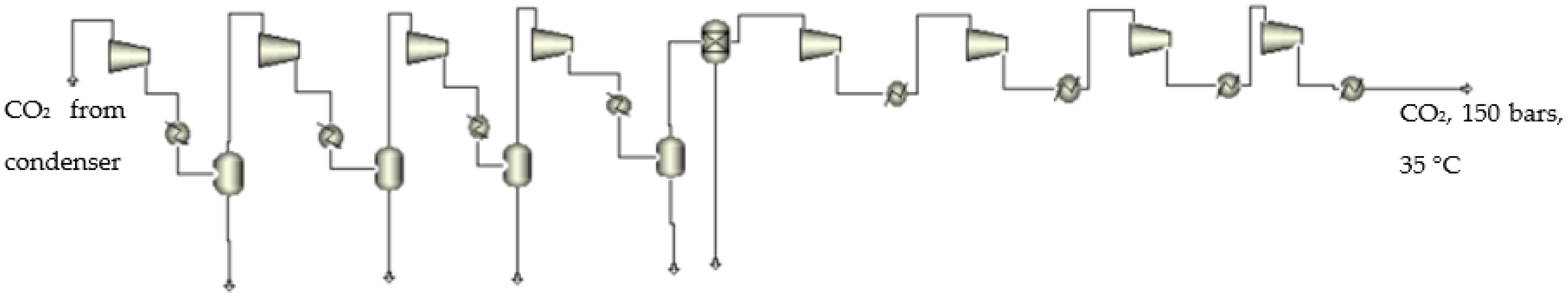
| Stage | Discharge Pressure, Bar | Pressure Ratio |
|---|---|---|
| 1 | 3 | 3 |
| 2 | 7.5 | 2.5 |
| 3 | 17.25 | 2.3 |
| 4 | 30.0 | 1.74 |
| 5 | 48 | 1.6 |
| 6 | 74.3 | 1.55 |
| 7 | 110 | 1.48 |
| 8 | 150 | 1.36 |
Appendix B. Economic Estimation Additional Information
Appendix B.1. Economic Methodology of Hybrid DAC
| Capital Cost | |
|---|---|
| Component capital costs of adsorber, fans, and vacuum pumps at unit scale | APEA estimation |
| Component capital costs of membrane surface at unit scale | 50 €/m2 |
| Component capital costs of membrane frame at unit scale, with being the membrane surface in m2 | € |
| Component capital costs of membrane, membrane frame, fan, and vacuum pump at plant capacity scale (100 ktCO2/yr) | 10–15% learning rate on above cost |
| Component capital of condensers and CPU at plant capacity scale (100 ktCO2/yr) | APEA estimation |
| Total field cost (TFC: total direct cost + total indirect cost) | Sum of above at scale |
| Other costs (power supply, water treatment and conditioning components, steam integration, temporary installations, transport, etc.) | 20% of TFC |
| Engineering, procurement, and construction | 9% of TFC |
| Risks and contingencies | 30% of sum of above |
| Total Plant Cost (TPC) | Sum of above |
| Spare parts | 0.5% of TPC |
| Start-up | 2% of TPC |
| Operator costs (other studies, enquiry, land purchase, site access, permits, etc.) | 7% of TPC |
| Insurance | 0.5% of TPC |
| Local taxes | 0.5% of TPC |
| Interim interest | 17.5% of TPC |
| Total Capital Requirement (TCR) | Sum of above from TPC |
| O&M Cost | |
| Operational labor cost | 10 jobs/100 ktCO2/yr; 60 k€/(jobs.yr) |
| Annual maintenance cost | 3% of TPC |
| Other Assumptions | |
| Cost basis | €2022, Rotterdam, The Netherlands |
| Load factor | 90% |
| Discount rate | 8% |
| Economic lifetime | 20 years |
| Electricity cost | 70 €/MWh |
| Heat cost | 10 €/GJ |
| Lewatit cost | 15.6 €/kg |
| Monolith support cost | 2 €/kg |
| Lewatit/monolith lifetime | 2 years |
| Membrane replacement cost (MRC), per m2 of membrane | 6.25 €/(m2.yr) |
| H2O cost | 0.5 €/tH2O |
Appendix B.2. APEA Mapping and Material Assumptions
| Adsorber | Model: Adsorber—Dual-vessel temperature swing adsorber. Shell material: SS316. Jacket material: CS. Mutualization: Two adsorbers then a learning rate (LR) to plant capacity. |
| Fan | Model: Propeller fan. Material: CS. Mutualization: One fan for one adsorber then LR (adsorption), or fan at 25,400 m3/h then LR (membrane). |
| Vacuum pump | Model: Mechanical oil-sealed vacuum pump. Material: SS. Mutualization: Maximum vacuum pump vacuum flow (1150 m3/h) then LR. |
| Condenser/Exchanger CPU | Model: TEMA shell and tube exchanger BEM. Material: 316 L. Mutualization: Estimated directly at plant capacity. |
| Boiler | Model: Packaged boiler unit. Material: CS. Mutualization: Estimated directly at plant capacity. |
| Flash vessel condenser/CPU | Model: Vertical process vessel. Material: SS304. Mutualization: Estimated directly at plant capacity. |
| Compressors CPU | Model: Centrifugal compressor—horizontal. Material: SS316. Mutualization: Estimated directly at plant capacity. |
| Flash vessels CPU | Model: Vertical process vessel. Material: SS316. Mutualization: Estimated directly at plant capacity. |
| Other components | Other components are neglected in the capital cost. |
Appendix B.3. Interim Interest
| Year | Capital Investment Distribution,% |
|---|---|
| −4.5 | 5 |
| −3.5 | 15 |
| −2.5 | 30 |
| −1.5 | 30 |
| −0.5 | 20 |
Appendix B.4. Deconstruction
References
- European Commission. Communication de la Commisssion: Des Cycles du Carbone Durables; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Energy Transitions Commission Carbon. Capture, Utilisation & Storage in the Energy Transition: Vital but Limited; Energy Transitions Commission Carbon: London, UK, 2022. [Google Scholar]
- International Energy Agency. Direct Air Capture: A Key Technology for Net Zero; OECD: Paris, France, 2022. [Google Scholar]
- De Temmerman, G.; de Rochette, F. Reports—The CDR Series: Direct Air Capture; Zenon Research: Paris, France, 2023. [Google Scholar]
- Bloch, C.; Kahsar, R.; Newcomb, J.; Wohl, G.; Hanson, E. Scoping the Potential Need for Direct Air Capture; Rocky Mountain Institute: Boulder, CO, USA, 2022. [Google Scholar]
- Lackner, K.; Ziock, H.-J.; Grimes, P. Carbon Dioxide Extraction from Air: Is It An Option? Los Alamos National Lab (LANL): Los Alamos, NM, USA,, 1 February 1999. [Google Scholar]
- Bisotti, F.; Hoff, K.A.; Mathisen, A.; Hovland, J. Direct Air Capture (DAC) Deployment: A Review of the Industrial Deployment. Chem. Eng. Sci. 2024, 283, 119416. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- Valentine, J.; Zoelle, A.; Homsy, S.; Mantripragada, H.; Kilstofte, A.; Sturdivan, M.; Steutermann, M.; Fout, T. Direct Air Capture Case Studies: Solvent System; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA; Albany, OR, USA, 2022.
- Valentine, J.; Zoelle, A.; Homsy, S.; Mantripragada, H.; Woods, M.; Roy, N.; Kilstofte, A.; Sturdivan, M.; Steutermann, M.; Fout, T. Direct Air Capture Case Studies: Sorbent System; NETL: Pittsburgh, PA, USA, 2022.
- Sievert, K.; Schmidt, T.S.; Steffen, B. Considering Technology Characteristics to Project Future Costs of Direct Air Capture. Joule 2024, 8, 979–999. [Google Scholar] [CrossRef]
- de Joannis, P.; Castel, C.; Kanniche, M.; Favre, E.; Authier, O. Direct Air Capture by Monoethanolamine Absorption with Heat Pump Enhancements. Ind. Eng. Chem. Res. 2025, 64, 2208–2225. [Google Scholar] [CrossRef]
- Kiani, A.; Lejeune, M.; Li, C.; Patel, J.; Feron, P. Liquefied Synthetic Methane from Ambient CO2 and Renewable H2—A Technoeconomic Study. J. Nat. Gas Sci. Eng. 2021, 94, 104079. [Google Scholar] [CrossRef]
- Deutz, S.; Bardow, A. Life-Cycle Assessment of an Industrial Direct Air Capture Process Based on Temperature–Vacuum Swing Adsorption. Nat. Energy 2021, 6, 203–213. [Google Scholar] [CrossRef]
- Gebald, C. Development of Amine-Functionalized Adsorbent for Carbon Dioxide Capture from Atmospheric Air. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2014. [Google Scholar]
- Wurzbacher, J.A. Development of a Temperature-Vacuum Swing Process for CO2 Capture from Ambient Air. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2015. [Google Scholar]
- Climeworks. The Reality of Deploying Direct Air Capture in the Field. Available online: https://climeworks.com/news/the-reality-of-deploying-direct-air-capture-in-the-field (accessed on 15 January 2025).
- Chuah, C.Y.; Ho, Y.L.; Syed, A.M.H.; Thivyalakshmi, K.G.K.; Yang, E.; Johari, K.; Yang, Y.; Poon, W.C. Applicability of Adsorbents in Direct Air Capture (DAC): Recent Progress and Future Perspectives. Ind. Eng. Chem. Res. 2025, 64, 4117–4147. [Google Scholar] [CrossRef]
- Low, M.-Y.; Barton, L.; Pini, R.; Petit, C. Analytical Review of the Current State of Knowledge of Adsorption Materials and Processes for Direct Air Capture. Chem. Eng. Res. Des. 2022, 189, 745–767. [Google Scholar] [CrossRef]
- Yu, Q.; Delgado, J.d.l.P.; Veneman, R.; Brilman, D.W.F. Stability of a Benzyl Amine Based CO2 Capture Adsorbent in View of Regeneration Strategies. Ind. Eng. Chem. Res. 2017, 56, 3259–3269. [Google Scholar] [CrossRef] [PubMed]
- Sonnleitner, E.; Schöny, G.; Hofbauer, H. Assessment of Zeolite 13X and Lewatit® VP OC 1065 for Application in a Continuous Temperature Swing Adsorption Process for Biogas Upgrading. Biomass Convers. Biorefin. 2018, 8, 379–395. [Google Scholar] [CrossRef]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane Processes for Direct Carbon Dioxide Capture from Air: Possibilities and Limitations. Front. Chem. Eng. 2021, 3, 668867. [Google Scholar] [CrossRef]
- Chevrel, C.; de Joannis, P.; Castel, C.; Authier, O. Modeling of CO2 Capture by Electro-Swing Reactive Adsorption from Low-Concentration Streams. Clean Technol. 2025, 7, 18. [Google Scholar] [CrossRef]
- Voskian, S.; Hatton, T.A. Faradaic Electro-Swing Reactive Adsorption for CO2 Capture. Energy Environ. Sci. 2019, 12, 3530–3547. [Google Scholar] [CrossRef]
- Holmes, G.; Keith, D. An Air-Liquid Contactor for Large-Scale Capture of CO2 from Air. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2012, 370, 4380–4403. [Google Scholar] [CrossRef]
- Kiani, A.; Jiang, K.; Feron, P. Techno-Economic Assessment for CO2 Capture from Air Using a Conventional Liquid-Based Absorption Process. Front. Energy Res. 2020, 8, 92. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R.; Kunitake, T. A New Strategy for Membrane-Based Direct Air Capture. Polym. J. 2021, 53, 111–119. [Google Scholar] [CrossRef]
- Huang, S.; Dakhchoune, M.; Luo, W.; Oveisi, E.; He, G.; Rezaei, M.; Zhao, J.; Alexander, D.T.L.; Züttel, A.; Strano, M.S.; et al. Single-Layer Graphene Membranes by Crack-Free Transfer for Gas Mixture Separation. Nat. Commun. 2018, 9, 2632. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Han, Y.; Ho, W.S.W. Feasibility of Membrane-Adsorption Hybrid Process for Direct Air Capture. In AIChE Annual Meeting; Hyatt Regency Orlando: Orlando, FL, USA, 2023. [Google Scholar]
- Chimani, F.M.; Bhandari, A.A.; Wallmüller, A.; Schöny, G.; Müller, S.; Fuchs, J. Evaluation of CO2/H2O Co-Adsorption Models for the Anion Exchange Resin Lewatit VPOC 1065 under Direct Air Capture Conditions Using a Novel Lab Setup. Separations 2024, 11, 160. [Google Scholar] [CrossRef]
- Schellevis, M. CO2 Capture from Air: A Process Engineering Approach. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2023. [Google Scholar]
- Young, J.; García-Díez, E.; Garcia, S.; van der Spek, M. The Impact of Binary Water–CO2 Isotherm Models on the Optimal Performance of Sorbent-Based Direct Air Capture Processes. Energy Environ. Sci. 2021, 14, 5377–5394. [Google Scholar] [CrossRef]
- Schellevis, H.M.; Van Schagen, T.N.; Brilman, D.W.F. Process Optimization of a Fixed Bed Reactor System for Direct Air Capture. Int. J. Greenh. Gas Control 2021, 110, 103431. [Google Scholar] [CrossRef]
- Low, M.-Y.A.; Danaci, D.; Azzan, H.; Woodward, R.T.; Petit, C. Measurement of Physicochemical Properties and CO2, N2, Ar, O2, and H2O Unary Adsorption Isotherms of Purolite A110 and Lewatit VP OC 1065 for Application in Direct Air Capture. J. Chem. Eng. Data 2023, 68, 3499–3511. [Google Scholar] [CrossRef]
- Veneman, R.; Frigka, N.; Zhao, W.; Li, Z.; Kersten, S.; Brilman, W. Adsorption of H2O and CO2 on Supported Amine Sorbents. Int. J. Greenh. Gas Control 2015, 41, 268–275. [Google Scholar] [CrossRef]
- Bos, M.J.; Kreuger, T.; Kersten, S.R.A.; Brilman, D.W.F. Study on Transport Phenomena and Intrinsic Kinetics for CO2 Adsorption in Solid Amine Sorbent. Chem. Eng. J. 2019, 377, 120374. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, X.; Chen, Y.; Wang, R.; Ge, T. The Analysis and Evaluation of Direct Air Capture Adsorbents on the Material Characterization Level. Chem. Eng. J. 2022, 450, 137958. [Google Scholar] [CrossRef]
- Driessen, R.T.; Knaken, B.; Buzink, T.; Jacobs, D.A.F.; Hrstka, J.; Brilman, D.W.F. Design and Proof of Concept of a Continuous Pressurized Multi-Stage Fluidized Bed Setup for Deep Sour Gas Removal Using Adsorption. Powder Technol. 2020, 366, 859–872. [Google Scholar] [CrossRef]
- Low, M.-Y.; Danaci, D.; Sturman, C.; Petit, C. Quantification of Temperature-Dependent CO2 Adsorption Kinetics in Lewatit VP OC 1065, Purolite A110, and TIFSIX-3-Ni for Direct Air Capture. Chem. Eng. Res. Des. 2025, 215, 443–452. [Google Scholar] [CrossRef]
- Gebald, C.; Wurzbacher, J.A.; Borgschulte, A.; Zimmermann, T.; Steinfeld, A. Single-Component and Binary CO2 and H2O Adsorption of Amine-Functionalized Cellulose. Environ. Sci. Technol. 2014, 48, 2497–2504. [Google Scholar] [CrossRef]
- Deschamps, T.; Kanniche, M.; Grandjean, L.; Authier, O. Modeling of Vacuum Temperature Swing Adsorption for Direct Air Capture Using Aspen Adsorption. Clean Technol. 2022, 4, 258–275. [Google Scholar] [CrossRef]
- de Joannis, P.; Castel, C.; Kanniche, M.; Favre, E.; Authier, O. Techno-Economic Analysis of Packed Bed and Structured Adsorbent for Direct Air Capture. Carbon Capture Sci. Technol. 2025; 100518, in press. [Google Scholar] [CrossRef]
- Lanxess. Product Information Lewatit® VP OC 1065, edition 2023-11-06, Lanxess, Germany 2023. Available online: https://lanxess.com/en-us/products-and-brands/products/l/lewatit--vp-oc-1065 (accessed on 1 January 2024).
- Soukri, M.; Sitaula, P.; Izenson, M.G.; Phillips, S.D. Development of Advanced Solid Sorbents for Direct Air Capture; RTI International: Durham, NC, USA, 2024. [Google Scholar]
- Aspentech. Aspen ADSIM 10.1 Casebook; Aspentech: Bedford, MA, USA, 1999. [Google Scholar]
- Membrane. Technology and Research Large Pilot Testing of the MTR Membrane Post-Combustion CO2 Capture Process; Membrane: Vista, CA, USA, 2018. [Google Scholar]
- Kaur, C.; Sayari, A. Enhancing Oxidation Stability of Amine-Containing CO2 Adsorbents Using Hydroxyethyl Starch. Chem. Eng. J. 2024, 496, 153756. [Google Scholar] [CrossRef]
- Favre, E. Membrane Separation Processes and Post-Combustion Carbon Capture: State of the Art and Prospects. Membranes 2022, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Favre, E.; Merkel, T.C. Carbon Capture, Membrane Processes and Energy Requirement. Chem. Eng. J. 2024, 482, 148934. [Google Scholar] [CrossRef]
- Bozorg, M.; Ramírez-Santos, Á.A.; Addis, B.; Piccialli, V.; Castel, C.; Favre, E. Optimal Process Design of Biogas Upgrading Membrane Systems: Polymeric vs High Performance Inorganic Membrane Materials. Chem. Eng. Sci. 2020, 225, 115769. [Google Scholar] [CrossRef]
- Breen, A.; Baker, R.; Behm, P.; Freeman, B.; Hao, P.; Hofmann, T.; Kniep, J.; Merkel, T.; Salim, W.; McKaskle, R.; et al. Large Pilot Testing of MTR’s Membrane-Based Post-Combustion CO2 Capture Process; Social Science Research Network: Rochester, NY, USA, 2024. [Google Scholar]
- Robeson, L.M. Correlation of Separation Factor versus Permeability for Polymeric Membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Bounaceur, R.; Berger, E.; Pfister, M.; Ramirez Santos, A.A.; Favre, E. Rigorous Variable Permeability Modelling and Process Simulation for the Design of Polymeric Membrane Gas Separation Units: MEMSIC Simulation Tool. J. Membr. Sci. 2017, 523, 77–91. [Google Scholar] [CrossRef]
- Brinkmann, T.; Lillepärg, J.; Notzke, H.; Pohlmann, J.; Shishatskiy, S.; Wind, J.; Wolff, T. Development of CO2 Selective Poly(Ethylene Oxide)-Based Membranes: From Laboratory to Pilot Plant Scale. Engineering 2017, 3, 485–493. [Google Scholar] [CrossRef]
- Brinkmann, T.; Naderipour, C.; Pohlmann, J.; Wind, J.; Wolff, T.; Esche, E.; Müller, D.; Wozny, G.; Hoting, B. Pilot Scale Investigations of the Removal of Carbon Dioxide from Hydrocarbon Gas Streams Using Poly (Ethylene Oxide)–Poly (Butylene Terephthalate) PolyActiveTM) Thin Film Composite Membranes. J. Membr. Sci. 2015, 489, 237–247. [Google Scholar] [CrossRef]
- Brinkmann, T.; Notzke, H.; Wolff, T.; Zhao, L.; Luhr, S.; Stolten, D. Characterization of a New Flat Sheet Membrane Module Type for Gas Permeation. Chem. Ing. Tech. 2019, 91, 30–37. [Google Scholar] [CrossRef]
- Brinkmann, T.; Pohlmann, J.; Withalm, U.; Wind, J.; Wolff, T. Theoretical and Experimental Investigations of Flat Sheet Membrane Module Types for High Capacity Gas Separation Applications. Chem. Ing. Tech. 2013, 85, 1210–1220. [Google Scholar] [CrossRef]
- Ministère de la Transition Ecologique et de la Cohésion des Territoires. Réglementation Environnementale RE2020; Ministère de la Transition Ecologique et de la Cohésion des Territoires: Paris, France, 2024.
- Maruyama, R.T.; Pai, K.N.; Subraveti, S.G.; Rajendran, A. Improving the Performance of Vacuum Swing Adsorption Based CO2 Capture under Reduced Recovery Requirements. Int. J. Greenh. Gas Control 2020, 93, 102902. [Google Scholar] [CrossRef]
- Patton, A.; Crittenden, B.D.; Perera, S.P. Use of the Linear Driving Force Approximation to Guide the Design of Monolithic Adsorbents. Chem. Eng. Res. Des. 2004, 82, 999–1009. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-0-470-11539-8. [Google Scholar]
- Franco, F.; Bolland, O.; Booth, N.; van Dorst, E.; Ekstrom, C.; Fernandes, E.S.; Anantharaman, R.; Macchi, E.; Manzolini, G.; Nikolic, D.; et al. European Best Practice Guidelines for Assessment of CO2 Capture Technologies; CAESAR project; EU: Brussels, Belgium, 2011. [Google Scholar]
- Aspentech. Aspen Icarus Reference Guide V14; Aspentech: Bedford, MA, USA, 2022. [Google Scholar]
- Fasihi, M.; Efimova, O.; Breyer, C. Techno-Economic Assessment of CO2 Direct Air Capture Plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- Caldera, U.; Breyer, C. Learning Curve for Seawater Reverse Osmosis Desalination Plants: Capital Cost Trend of the Past, Present, and Future. Water Resour. Res. 2017, 53, 10523–10538. [Google Scholar] [CrossRef]
- Nemet, G.F.; Brandt, A.R. Willingness to Pay for a Climate Backstop: Liquid Fuel Producers and Direct CO2 Air Capture. Energy J. 2011, 33, 53–82. [Google Scholar] [CrossRef]
- Hart, P.W.; Sommerfeld, J.T. Cost Estimation of Specialty Chemicals from Laboratory-Scale Prices. Cost Eng. 1997, 39, 31–35. [Google Scholar]
- Grimm, A.; Kramer, G.J.; Gazzani, M. How Would Ideal Sorbents Improve the Technical and Economic Performance of Adsorption-Based Direct Air Capture? Energy Fuels 2024, 38, 18781–18799. [Google Scholar] [CrossRef]
- ISO 27913:2024; Carbon Dioxide Capture, Transportation and Geological Storage—Pipeline Transportation Systems. ISO: Geneva, Switzerland, 2024.
- Shirley, P.; Myles, P. Quality Guidelines for Energy System Studies: CO2 Impurity Design Parameters; NETL: Pittsburgh, PA, USA, 2019.
- Young, J.; McQueen, N.; Charalambous, C.; Foteinis, S.; Hawrot, O.; Ojeda, M.; Pilorgé, H.; Andresen, J.; Psarras, P.; Renforth, P.; et al. The Cost of Direct Air Capture and Storage Can Be Reduced via Strategic Deployment but Is Unlikely to Fall below Stated Cost Targets. One Earth 2023, 6, 899–917. [Google Scholar] [CrossRef]
- Stampi-Bombelli, V.; Mazzotti, M. Exploring Geometric Properties and Cycle Design in Packed Bed and Monolith Contactors Using Temperature-Vacuum Swing Adsorption Modeling for Direct Air Capture. Ind. Eng. Chem. Res. 2024, 63, 19728–19743. [Google Scholar] [CrossRef]
- Sabatino, F.; Grimm, A.; Gallucci, F.; Van Sint Annaland, M.; Kramer, G.J.; Gazzani, M. A Comparative Energy and Costs Assessment and Optimization for Direct Air Capture Technologies. Joule 2021, 5, 2047–2076. [Google Scholar] [CrossRef]
- Wurzbacher, J.A.; Gebald, C.; Brunner, S.; Steinfeld, A. Heat and Mass Transfer of Temperature–Vacuum Swing Desorption for CO2 Capture from Air. Chem. Eng. J. 2016, 283, 1329–1338. [Google Scholar] [CrossRef]
- Schmitt, T.; Leptinsky, S.; Turner, M.; Zoelle, A.; White, C.W.; Hughes, S.; Homsy, S.; Woods, M.; Hoffman, H.; Shultz, T.; et al. Cost and Performance Baseline for Fossil Energy Plants Volume 1: Bituminous Coal and Natural Gas to Electricity; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA; Albany, OR, USA, 2022.
- GRT. Gaz Open Season Pour la Réalisation d’une D’infrastructure de Transport de CO2 Dans la Zone Portuaire de Dunkerque—Proposition de Spécifications Dioxyde de Carbone; GRT: London, UK, 2023. [Google Scholar]
- Kemper, J.; Sutherland, L.; Watt, J.; Santos, S. Evaluation and Analysis of the Performance of Dehydration Units for CO2 Capture. Energy Procedia 2014, 63, 7568–7584. [Google Scholar] [CrossRef]
- Sircar, S.; Myers, A. Gas Separation by Zeolites; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar] [CrossRef]


| Isotherm Model | Parameter | Value |
|---|---|---|
| Toth isotherm (Pure CO2) | (molCO2/kg) | 3.7604 |
| (1/Pa) | 0.001382 | |
| (kJ/molCO2) | 103.05 | |
| (K) | 353.15 | |
| (-) | 0.30894 | |
| (-) | 0.34148 | |
| Co-adsorption isotherm CO2 | (-) | 3.5803 |
| (kg/molCO2) | −1.2153 | |
| GAB isotherm (H2O) | (molH2O/kg) | 2.15 |
| (J/molH2O) | 48,459 | |
| (1/K) | 0.02342 | |
| (J/molH2O) | 57,197 | |
| (J/(molH2O.K)) | −44.931 |
| Parameter | Symbol | Value | Unit | Source |
|---|---|---|---|---|
| Intra-sorbent voidage | 0.34 | - | [34] | |
| Pore radius | 28.5 | nm | [43] | |
| CO2 heat of adsorption | 73 | kJ/mol | [42] | |
| H2O heat of adsorption | 44 | kJ/mol | [42] |
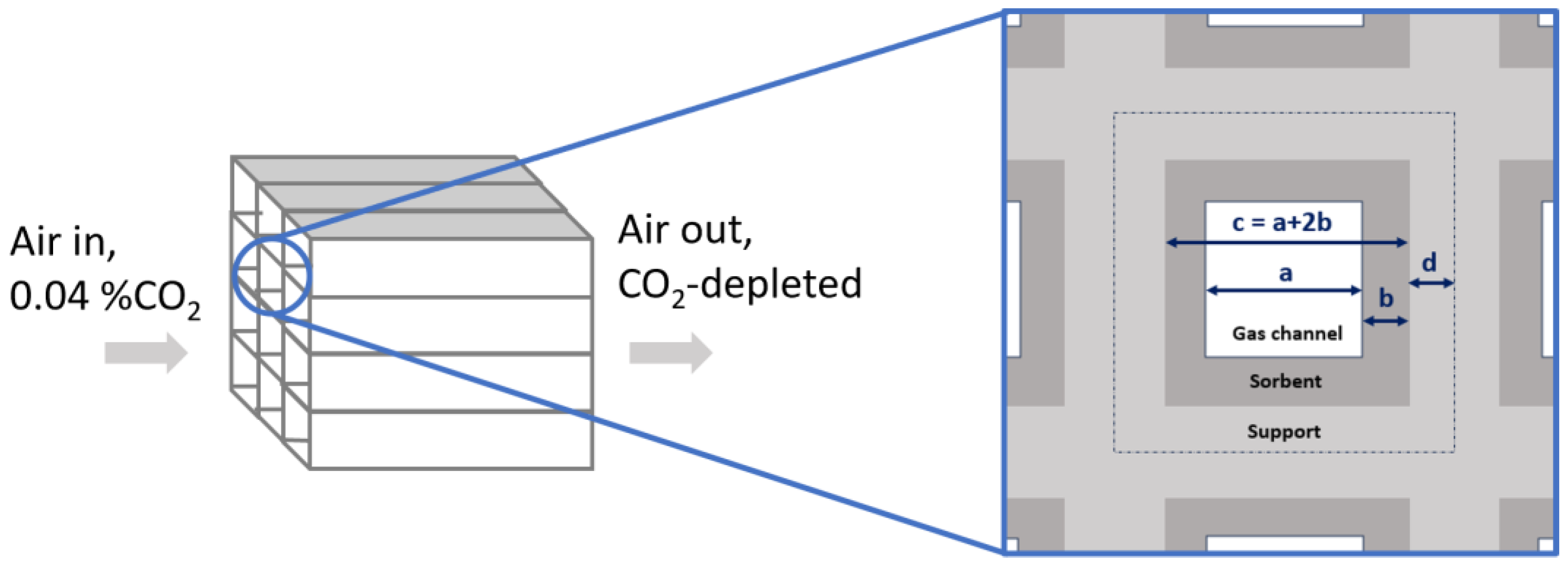
| Parameter | Unit | Value |
|---|---|---|
| Coating thickness | mm | 0.3 |
| mm | 1 | |
| mm | 0.25 | |
| m3/m3 | 0.39 | |
| m3/m3 | 0.23 | |
| Sorbent volume fraction | m3sorbent/m3 | 0.35 |
| Support volume fraction | m3support/m3 | 0.42 |
| Bed density (i.e., sorbent) | kg/m3 | 281 |
| Support density | kgsupport/m3 | 3301 |
| Apparent sorbent interfacial area | m2/m3 | 907 |
| Parameter | Unit | Value |
|---|---|---|
| Bed height | m | 0.5 |
| Bed diameter | m | 2 |
| Superficial air velocity | m/s | 2 |
| Air temperature | °C | 15 |
| Relative humidity (RH) | - | 0.8 |
| Regeneration vacuum pressure | bar | 0.1 |
| Regeneration temperature | °C | 70 or 100 |
| Parameter | Unit | Value |
|---|---|---|
| CO2 permeance | GPU | 1000 |
| CO2/N2 selectivity | - | 45.9 |
| CO2/O2 selectivity | - | 16.8 |
| CO2/Ar selectivity | - | 16.8 |
| CO2/H2O selectivity | - | 0.1 |
| Parameter | Range |
|---|---|
| ) | 0.05–0.95 |
| ) | 0.05–0.5 |
| Capture rate constraint | >80% |
| Purity constraint | >0.9–0.95 |
| Objective |
| Case | Regeneration | Pressure, bar | Temperature, °C |
|---|---|---|---|
| VTSA-100 °C | VTSA | 0.1 | 100 |
| VTSA-70 °C | VTSA | 0.1 | 70 |
| TSA-100 °C | TSA | 1.013 | 100 |
| TSA-70 °C | TSA | 1.013 | 70 |
| Parameter | Unit | Description |
|---|---|---|
| Outlet CO2 purity | molCO2/mol | % CO2 purity in the outlet |
| Separation ratio | - | Ratio of inlet % CO2 purity over outlet % CO2 purity |
| Capture rate | % | CO2 capture rate |
| Ads. CO2 working capacity | molCO2/kgsorbent | CO2 captured by sorbent per mass of sorbent |
| m2 | membrane stages | |
| CO2 productivity | kgCO2/(h.m3) | Ratio of the flux of CO2 captured per bed volume, adjusted with an annual load factor of 0.9 |
| H2O production | kgH2O/kgCO2 | Ratio of the mass of H2O captured per mass of CO2 captured |
| Indirect heat | GJ/tCO2 | Indirect heating requirement for ads. regeneration |
| Direct heat | GJ/tCO2 | Regeneration air heating requirement for ads. regeneration |
| Fan work | MWh/tCO2 | Fan work |
| Vacuum work | MWh/tCO2 | Vacuum pump work |
| Heat. energy | GJ/tCO2 | Sum of direct and indirect |
| Elec. energy | MWh/tCO2 | Sum of fan work and/or vacuum work and/or CPU compressors work |
| Primary energy | GJ/tCO2 | Energy requirements using a primary energy factor of 2.3 for electricity (=thermal energy + electrical energy × 2.3) [59] |
| Cost of capture | €/tCO2 | Cost of capture per tonne of CO2 captured |
| Parameter | Unit | VTSA-100 °C | VTSA-70 °C | TSA-100 °C | TSA-70 °C |
|---|---|---|---|---|---|
| Desorption criterion | molCO2/kg | 0.4 | 0.6 | 0.3 | 0.5 |
| Hot air flowrate | mol/s | 0.005 | 0.005 | 0.01 | 0.1 |
| Ads. outlet CO2 purity | molCO2/mol | 0.321 | 0.15 | 0.093 | 0.01 |
| Ads. separation ratio | - | 803 | 374 | 233 | 25.5 |
| Ads. capture rate | % | 63 | 59 | 61 | 62 |
| CO2 working capacity | molCO2/kgsorbent | 1.05 | 0.83 | 1.16 | 0.98 |
| Ads. CO2 productivity | kgCO2/(h.m3) | 2.2 | 1.6 | 1.7 | 1.6 |
| H2O production | kgH2O/kgCO2 | 3.3 | 4.1 | 3.1 | 4.4 |
| Indirect heat | GJ/tCO2 | 27.7 | 27.2 | 27.1 | 22.7 |
| Direct heat | GJ/tCO2 | 0.06 | 0.09 | 0.2 | 1.5 |
| Ads. fan work | MWh/tCO2 | 1.3 | 1.4 | 1.4 | 1.3 |
| Ads. vacuum work | MWh/tCO2 | 1.4 | 1.9 | 0 | 0 |
| Ads. heat. energy | GJ/tCO2 | 27.7 | 27.3 | 27.3 | 24.2 |
| Ads. elec. energy | MWh/tCO2 | 2.7 | 3.3 | 1.4 | 1.3 |
| Ads. primary energy | GJ/tCO2 | 50.1 | 54.9 | 38.5 | 35.5 |
| Ads. cost of capture | €/tCO2 | 1630 | 1991 | 1651 | 1688 |
| Case | CO2 | N2 | O2 | H2O | Ar |
|---|---|---|---|---|---|
| VTSA-100 °C | 32.1 | 49.8 | 13.3 | 4.2 | 0.6 |
| VTSA-70 °C | 15 | 62.9 | 17.2 | 4.2 | 0.7 |
| TSA-100 °C | 9.3 | 67.6 | 18.1 | 4.2 | 0.8 |
| TSA-70 °C | 1 | 74 | 19.9 | 4.2 | 0.9 |
| Parameter | Unit | VTSA-100 °C-0.95 | VTSA-70 °C-0.945 | VTSA-70 °C-0.9 | TSA-100 °C-0.9 |
|---|---|---|---|---|---|
| Memb. inlet CO2 purity | molCO2/mol | 0.321 | 0.15 | 0.15 | 0.093 |
| Memb. outlet CO2 purity | molCO2/mol | 0.95 | 0.945 | 0.9 | 0.9 |
| Memb. separation ratio | - | 3 | 6.3 | 6 | 9.6 |
| Memb. capture rate | % | 80 | 80 | 80 | 80 |
| m2 | 30,371 | 131,896 | 3915 | 7808 | |
| m2 | 3536 | 3892 | 7808 | 7060 | |
| Memb. CO2 productivity | kgCO2/(h.m3) | 336 | 84 | 95 | 58 |
| Memb. fan work | MWh/tCO2 | 0.001 | 0.004 | 0.002 | 0.003 |
| Memb. vacuum work | MWh/tCO2 | 0.48 | 1.22 | 0.35 | 0.68 |
| Memb. elec. energy | MWh/tCO2 | 0.48 | 1.22 | 0.35 | 0.68 |
| Memb. cost of capture | €/tCO2 | 101 | 226 | 82 | 140 |
| Parameter | Unit | VTSA-100 °C-0.95 | VTSA-70 °C-0.945 | VTSA-70 °C-0.9 | TSA-100 °C-0.9 |
|---|---|---|---|---|---|
| Ads. outlet CO2 purity | molCO2/mol | 0.321 | 0.15 | 0.15 | 0.093 |
| Ads. separation ratio | - | 803 | 374 | 374 | 233 |
| Ads. heat. energy | GJ/tCO2 | 27.7 | 27.3 | 27.3 | 27.3 |
| Ads. elec. energy | MWh/tCO2 | 2.7 | 3.3 | 3.3 | 1.4 |
| Ads. cost of capture | €/tCO2 | 1630 | 1991 | 1991 | 1651 |
| Memb. outlet CO2 purity | molCO2/mol | 0.95 | 0.945 | 0.9 | 0.9 |
| Memb. separation ratio | - | 3 | 6.3 | 6 | 9.6 |
| Memb. elec. energy | MWh/tCO2 | 0.48 | 1.22 | 0.35 | 0.68 |
| Memb. cost of capture | €/tCO2 | 101 | 226 | 82 | 140 |
| CPU outlet CO2 purity | molCO2/mol | 0.995 | 0.99 | 0.94 | 0.94 |
| CPU elec. energy | MWh/tCO2 | 0.1 | 0.1 | 0.1 | 0.1 |
| CPU cost of capture | €/tCO2 | 62 | 62 | 63 | 63 |
| DAC heat. energy | GJ/tCO2 | 27.7 | 27.3 | 27.3 | 27.3 |
| DAC elec. energy | MWh/tCO2 | 3.3 | 4.6 | 3.8 | 2.2 |
| DAC primary energy | GJ/tCO2 | 54.9 | 65.6 | 58.4 | 45.4 |
| DAC cost of capture | €/tCO2 | 1793 | 2279 | 2136 | 1854 |
| Parameter | Unit | VTSA-100 °C-0.95 | VTSA-70 °C-0.945 | VTSA-70 °C-0.9 | TSA-100 °C-0.9 |
|---|---|---|---|---|---|
| O2 concentration after CPU | molO2/mol | 0.0014 | 0.0027 | 0.035 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Joannis, P.; Castel, C.; Kanniche, M.; Favre, E.; Authier, O. Techno-Economic Analysis of Hybrid Adsorption–Membrane Separation Processes for Direct Air Capture. ChemEngineering 2025, 9, 102. https://doi.org/10.3390/chemengineering9050102
de Joannis P, Castel C, Kanniche M, Favre E, Authier O. Techno-Economic Analysis of Hybrid Adsorption–Membrane Separation Processes for Direct Air Capture. ChemEngineering. 2025; 9(5):102. https://doi.org/10.3390/chemengineering9050102
Chicago/Turabian Stylede Joannis, Paul, Christophe Castel, Mohamed Kanniche, Eric Favre, and Olivier Authier. 2025. "Techno-Economic Analysis of Hybrid Adsorption–Membrane Separation Processes for Direct Air Capture" ChemEngineering 9, no. 5: 102. https://doi.org/10.3390/chemengineering9050102
APA Stylede Joannis, P., Castel, C., Kanniche, M., Favre, E., & Authier, O. (2025). Techno-Economic Analysis of Hybrid Adsorption–Membrane Separation Processes for Direct Air Capture. ChemEngineering, 9(5), 102. https://doi.org/10.3390/chemengineering9050102








