1. Introduction
A prevailing trend observed across most copper deposits in Kazakhstan is the depletion of high-grade ore reserves and the accumulation of large volumes of subeconomic waste dumps, with copper contents ranging between 0.3 and 0.5%. Such low-grade raw materials render pyrometallurgical processing economically unfeasible. Currently, approximately 19.5% of global copper production from low-grade mineral and anthropogenic sources—primarily consisting of oxidized copper minerals—is conducted using solvent extraction–electrowinning (SX-EW) technology. However, conventional hydrometallurgical approaches for copper recovery are generally restricted to oxidized ores of simple composition and low concentrations of divalent iron compounds. In contrast, many copper deposits are characterized by a complex mineralogical composition and elevated levels of ferrous iron, typically in the form of pyrite, pyrrhotite, and other iron-bearing minerals. These features complicate the leaching process and result in significantly increased consumption of sulfuric acid, the primary leaching agent. Consequently, preliminary treatment of such raw materials using oxidizing agents is required to convert ferrous iron to its ferric form. Although chemical oxidants such as sodium peroxide and potassium hypochlorite are effectively applied in the leaching of precious metals, their use in copper ore processing is economically inefficient. This highlights the need for developing alternative, cost-effective methods for the oxidative pretreatment of complex copper-bearing materials [
1,
2,
3,
4,
5].
Bacterial cultures are increasingly employed as biological oxidizing agents in global metallurgical practice [
6,
7]. The primary advantages of microbial oxidation include the high efficiency of converting ferrous iron to ferric iron and the relatively low operational costs of this technology. Biooxidation presents an attractive alternative to conventional physicochemical ore processing methods, offering lower resource consumption and a significantly reduced environmental footprint [
3,
8,
9,
10].
The foundation of biogeotechnology lies in the utilization of specific microorganisms capable of selectively extracting metals from ores and mine wastes. The taxonomic composition and physiological stability of these microbial communities directly influence the efficiency of metal recovery. Among various applications, bioleaching is most extensively implemented for the processing of gold- and copper-bearing raw materials. Through microbial oxidation of metal sulfides—such as chalcopyrite or chalcocite—metal sulfates are formed, which are readily soluble in acidic media. Copper ions can then be recovered from these solutions using conventional hydrometallurgical techniques.
Currently, bioleaching technologies are applied on an industrial scale in over 20 countries at approximately 40 facilities, primarily through heap and in situ leaching methods. These operations target low-grade and subeconomic ores, as well as processing wastes from beneficiation plants and mining operations.
Industrial-scale application of bioleaching for copper recovery began in 1982 at the Lo Aguirre copper mine operated by Sociedad Minera Pudahuel in Chile [
11]. The success of this approach led to its subsequent adoption at 17 facilities, including 10 copper mines in Chile, as well as sites in the United States, Peru, and Australia.
Ongoing global research efforts aim to further expand the application of biotechnological approaches in mineral processing. The growing demand for copper, combined with the progressive depletion of high-grade ore reserves, continues to drive innovation and the development of novel microbial technologies in this field.
The bioleaching of copper from dust generated by reverberatory copper smelting furnaces and converters at the Sarcheshmeh plant in Iran was investigated in [
12]. The application of microbiological methods was necessitated by the insufficient efficiency of conventional extraction technologies. The copper-containing dust, predominantly composed of chalcocite, proved to be a favorable substrate for bioleaching, as mesophilic bacteria readily decompose this mineral. Using a consortium of
Thiobacillus ferrooxidans and
Thiobacillus thiooxidans in a standard 9K nutrient medium, copper recovery reached 87%.
In a separate study by Iranian researchers [
13], the catalytic role of pyrite in the bioleaching of copper sulfides was highlighted. In many porphyry copper sulfide ores, pyrite occurs as a vein phase associated with copper minerals, which hinders pyrometallurgical extraction. However, in the context of bioleaching, pyrite demonstrates a positive catalytic effect, particularly in enhancing the dissolution of chalcopyrite. Under optimized bioleaching conditions, copper recoveries exceeding 90% were achieved, emphasizing the importance of controlling the redox potential of the leaching system.
Further advancements were reported by Chinese researchers [
14], who studied the activity of thermoacidophilic archaea capable of oxidizing sulfur, pyrite, chalcopyrite, and other sulfide minerals in extreme environments. Their experiments demonstrated that thermophilic bacteria achieved copper recoveries of up to 97.0%, in contrast to only 32.43% using mesophilic strains. Specifically, chalcopyrite dissolution reached 97.05% with thermophilic microbes, compared to just 15.43% with mesophilic cultures.
A mechanistic investigation by Japanese scientists [
15] into the bioleaching of copper from enargite using thermophilic, iron-oxidizing
Acidianus brierleyi revealed process optimization pathways that enabled copper extraction efficiencies of 90.93%. These findings reinforce the viability of bacterial oxidation not only for the treatment of mineral ores but also for the valorization of man-made, technogenic waste materials.
For example, the primary objective of the study described in [
16] was to evaluate the bioleaching of copper from tailings using alkali-tolerant bacteria isolated from the tailings pulp. The bacteria were identified as a previously unclassified strain belonging to the
Thiobacillus genus. The experiments were conducted at 26 °C, with the culture medium maintained at a pH of 8–10 and a cultivation period of 8–9 days. Under these conditions, copper recovery during the 50-day bioleaching process reached only 25%.
In another study, Serbian researchers [
17] investigated the leaching of copper smelter slag—characterized by a fayalite structure—using both sulfuric acid and microbiological methods. The slag composition included 4.2% Cu, 34.5% Fe(II), and 30.9% SiO
2, with more than 75% of the copper present in sulfide form. A mesophilic microbial consortium was employed for bioleaching. The results indicated that copper extraction from amorphous smelting slags was highly efficient: recovery rates reached 82% using sulfuric acid and 99% with microbial leaching.
In recent years, alternative bioleaching methods utilizing natural organic compounds—bioreagents—have emerged [
18,
19]. Notably, a number of studies have explored copper leaching from low-grade ores in the presence of amino acids. In [
20], the leaching behavior of chalcopyrite concentrate from the Sarcheshmeh copper mine (Kerman, Iran) was investigated in a glycine (aminoacetic acid) solution with oxygen as the oxidizing agent. Optimal leaching conditions were identified, including temperature, pH, oxygen flow rate, and pulp density. The authors of [
21] further demonstrated that copper recovery from low-grade ores could be enhanced using sodium chloride solutions supplemented with amino acids or amino acid-containing compounds, such as protein hydrolysates and baker’s yeast. Under optimal conditions, copper recovery reached up to 92%.
Although direct studies on the influence of alcohols on copper bioleaching are lacking in the available literature, fusel oils—mixtures of aliphatic alcohols—have been reported to enhance the flotation performance of polymetallic ores. This suggests that fusel oils may also affect the biooxidation behavior of copper-bearing materials, warranting further investigation.
Overall, the literature demonstrates a growing application of bioleaching technologies. However, the declining quality and changing phase composition of raw materials necessitate continued research in this field. The accumulation of new knowledge will provide a foundation for developing technical solutions aimed at improving the recovery of valuable components from complex raw materials.
The aim of the present research was to study the effect of bioadditives—specifically amino acids and aliphatic alcohols—on the biooxidation of low-grade copper-containing ores.
2. Materials and Methods
Research Methods
Low-grade copper ore from a deposit in Kazakhstan was used in this study. A previously developed and adapted strain of Acidithiobacillus ferrooxidans (A. ferrooxidans), tailored to the specific chemical and mineralogical characteristics of the feed material, was employed to prepare a bacterial culture solution for the biochemical oxidation of the ore.
During the adaptation phase, the bacterial culture was incubated with the ore material, and after 10 days, the viability of the microbial cells was assessed. Simultaneously, the concentrations of ferrous (Fe2+) and ferric (Fe3+) iron ions were analyzed. Changes in iron concentrations were attributed to both bacterial oxidative metabolism and concurrent abiotic reactions. A marked increase in Fe3+ levels, accompanied by a decrease in Fe2+ concentration, was indicative of active bacterial growth and metabolic activity.
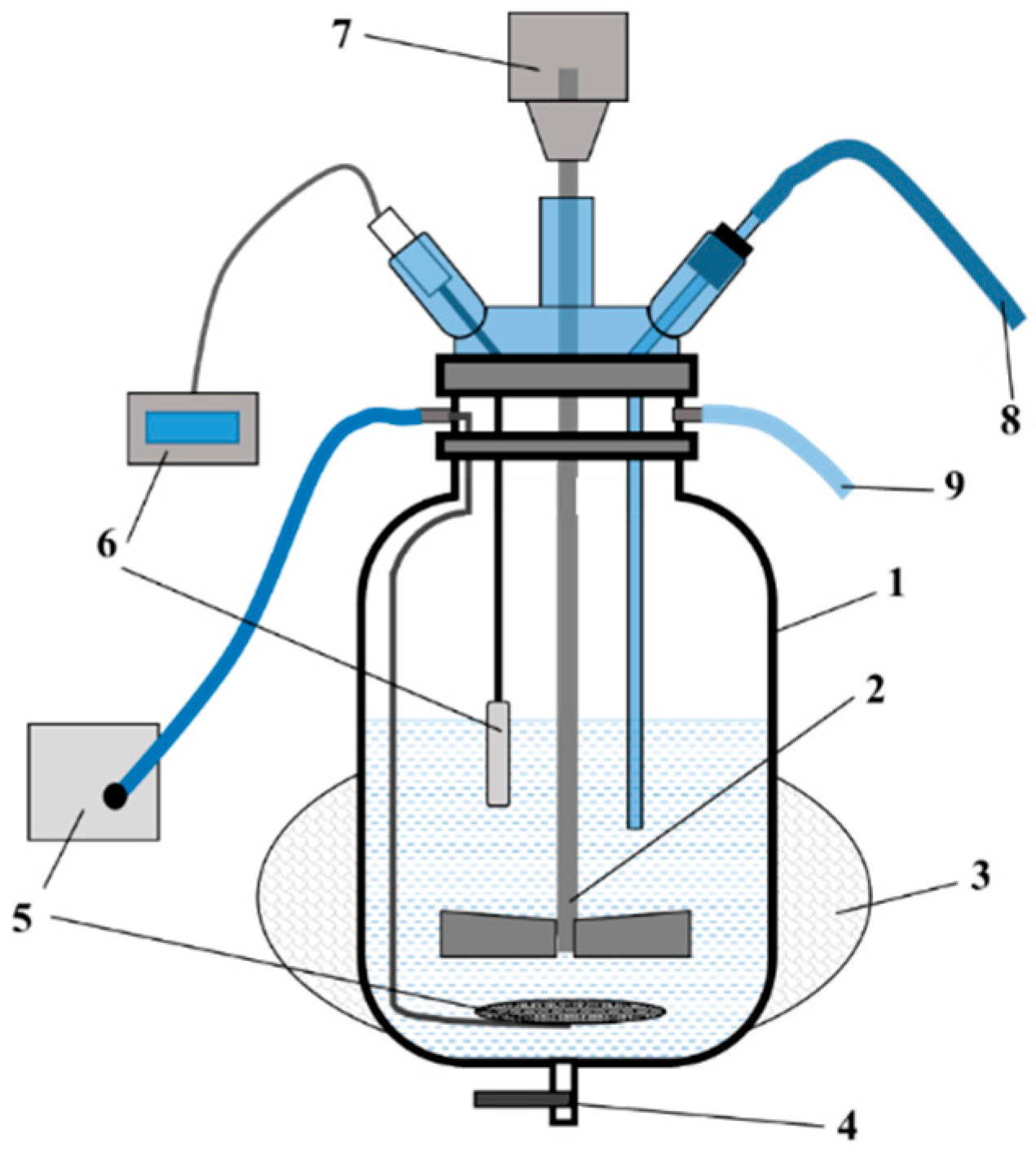
The microbial biomass production system consisted of a bioreactor vessel equipped with air and nutrient medium supply systems, pH and temperature monitoring sensors, an agitation mechanism, gas exhaust ports, and an outlet for the final product—the culture liquid of the A. ferrooxidans strain. The bioreactor vessel was constructed from stainless steel and enclosed in a sealed thermal jacket designed to maintain optimal cultivation temperature via circulating water.
A schematic diagram of the bioreactor system is presented in
Figure 1, and the external view of the unit is shown in
Figure 2.
The bacterial biomass was cultivated using a batch (periodic) cultivation method. In this approach, the sterilized fermenter was pre-filled with a nutrient medium containing the required microorganisms and amino acids. Biochemical processes proceeded for 24 h, until the concentration of viable cells reached at least 4.0 × 106 cells/cm3. Upon completion of the cultivation cycle, the resulting bacterial culture was transferred to a storage tank, where key parameters of the medium—such as pH and temperature—were maintained within optimal ranges.
During storage, a reduction in temperature was permissible. According to recent studies [
1,
2],
A. ferrooxidans exhibits the ability to maintain cell viability and stable population density even under extreme conditions, including temperatures as low as 4 °C.
The adapted A. ferrooxidans strain was subsequently employed in the agitation leaching of copper-bearing mineral pulp using the prepared bacterial culture.
The influence of pH variation on bacterial growth and activity was experimentally investigated. The results are summarized in
Table 1.
The data presented in
Table 1 indicate that the growth of
A. ferrooxidans bacterial culture on copper-bearing mineral pulp occurs only at pH values above 1.2. At pH = 1.5, the concentration of bacterial cells in the initial solution increased from 0.1 to 0.4 × 10
6 cells/cm
3. Simultaneously, the concentration of ferrous ions (Fe
2+) decreased by 50%, from 3.1 g/dm
3 to 1.55 g/dm
3. This reduction in Fe
2+ was accompanied by a corresponding increase in ferric ions (Fe
3+), resulting from both the microbial oxidation of Fe
2+ and additional iron leaching from the mineral matrix.
A further increase in pH to 1.8 led to a ninefold acceleration in bacterial growth, while at pH = 2.0, the cell concentration increased 14-fold compared to the initial value. The maximum bacterial growth was observed at pH = 2.3, where the cell count reached 2.8 × 106 cells/cm3—an increase of 28 times. At this pH level, the concentration of Fe2+ decreased by 92.9%, reaching 0.22 g/L, while Fe3+ concentration increased to 4.3 g/L.
The results also demonstrate that a decrease in pH below 1.2 has a detrimental effect on bacterial viability. At pH = 1.0, the number of viable cells dropped by 85%, and at pH = 0.8, by 90%. In these highly acidic conditions, only a slight decrease in Fe2+ concentration and a minor increase in Fe3+ were observed, which can be primarily attributed to abiotic (chemical) oxidation rather than microbial activity.
These findings confirm that A. ferrooxidans is unable to proliferate in extremely acidic environments. A gradual increase in pH above 1.2 creates favorable conditions for bacterial survival and activity. The pH range of 1.2–1.4 corresponds to limited viability and weak growth, while the range of 1.5–1.8 supports stable growth and active metabolic functioning of the bacterial culture.
The stirring speed of the solution in the fermenter was maintained at 300 rpm.
At the initial stage of the experiment, the ore sample was incubated in the bacterial solution at a solid-to-liquid (S:L) ratio of 1:1 for 72 h. A biologically active additive—either an amino acid or an aliphatic alcohol—was introduced at a concentration of 0.5 M. The average inoculum concentration in the biooxidation solution was 1.0 × 106 cells/cm3. Based on the experimental setup (50 g of ore and 50 mL of bacterial solution), the total inoculum count per experiment was approximately 5.0 × 107 cells.
Following this biooxidation stage, leaching of the pretreated ore was conducted using a sulfuric acid solution with a concentration of 20 g/dm3 at an S:L ratio of 1:3. The concentration of copper in solution was determined both after biooxidation and subsequent acid leaching.
Reagents and Materials:
Amino acids used in the experiments included glycine, leucine, asparagine, histidine, and cysteine. All were supplied in powder form with a purity of ≥99% (TLC grade), supplied by Sigma-Aldrich, St. Louis, MO, USA.
Aliphatic alcohols included the following:
- ○
Butyl alcohol (analytical grade), supplied by Technocrat LLP, Almaty, Kazakhstan.
- ○
Amyl alcohol (ACS grade, 99.0%), supplied by Sigma-Aldrich, St. Louis, MO, USA.
Sulfuric acid, chemically pure, was provided by Technocrat LLP, Almaty, Kazakhstan.
Instrumental Analysis:
The quantitative determination of copper content in the initial ore sample and leachates was performed using an Optima 8000 DV inductively coupled plasma atomic emission spectrometer (ICP-AES) (PerkinElmer, State of Connecticut, Norwalk, CA, USA).
X-ray diffraction (XRD) analysis was conducted using a D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) equipped with a cobalt anode and employing Cu Kα radiation. X-ray fluorescence (XRF) data were acquired using a Venus 200 wavelength-dispersive spectrometer (PANalytical, Almelo, The Netherlands).
Diffraction patterns were interpreted, and interplanar spacings were calculated using EVA software (HVAC version, LBS, Bhandup West, Mumbai, India). Phase identification and sample characterization were carried out using the Search/Match program with reference to the ASTM Powder Diffraction File (PDF) database.
Infrared (IR) spectroscopy was performed using an Avatar 370 FTIR spectrometer (Nicolet Instrument Corporation, Madison, WI, USA) equipped with a CsI beam splitter, operating in the spectral range of 4000–300 cm−1.
3. Results
At the initial stage, a comprehensive characterization of low-grade copper-bearing ore from a deposit in Kazakhstan was performed. The investigation involved X-ray fluorescence (XRF), chemical analysis, X-ray diffraction (XRD), and mineralogical analysis, as well as scanning electron microscopy (SEM) and X-ray microanalysis (XMSA).
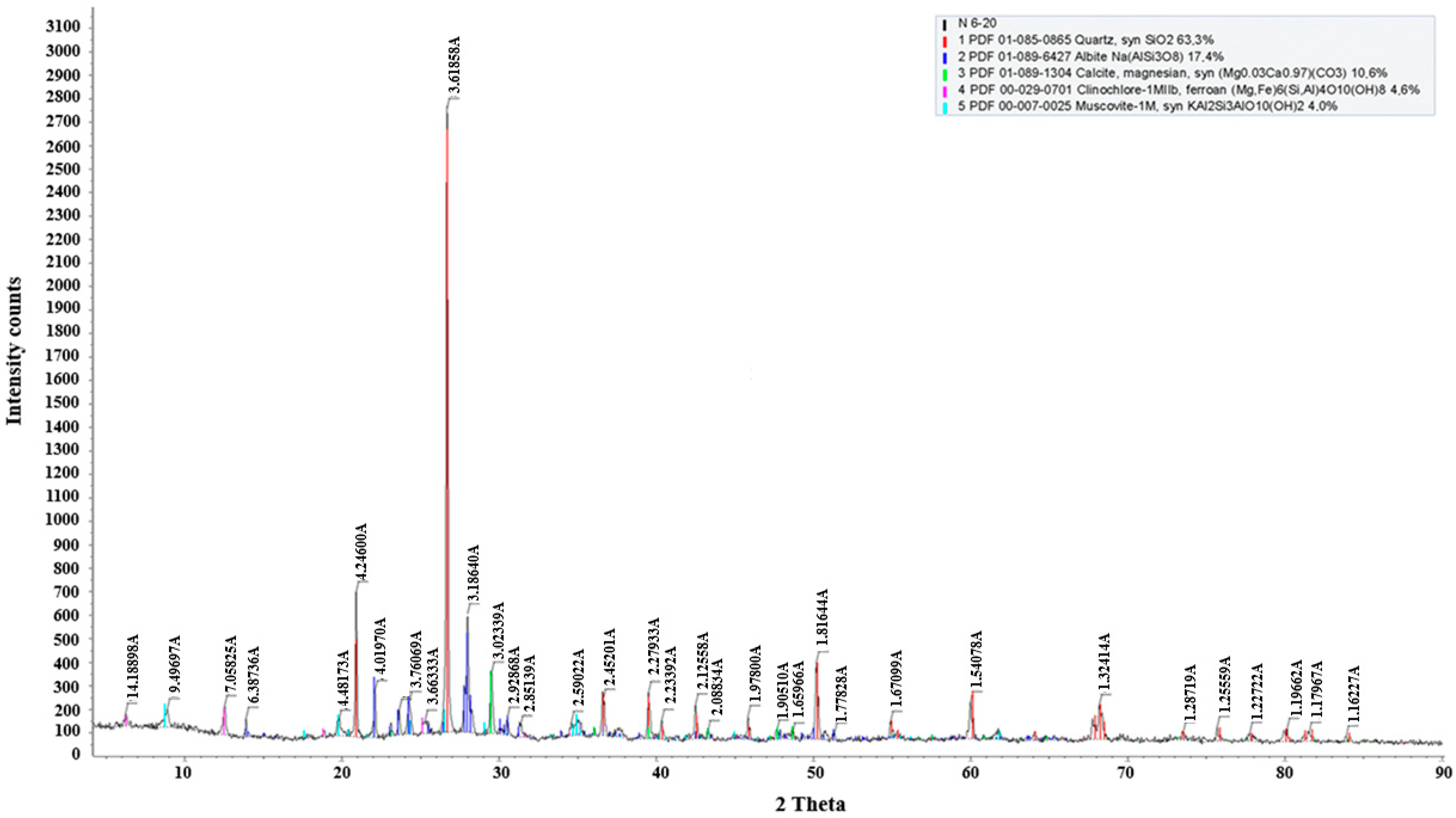
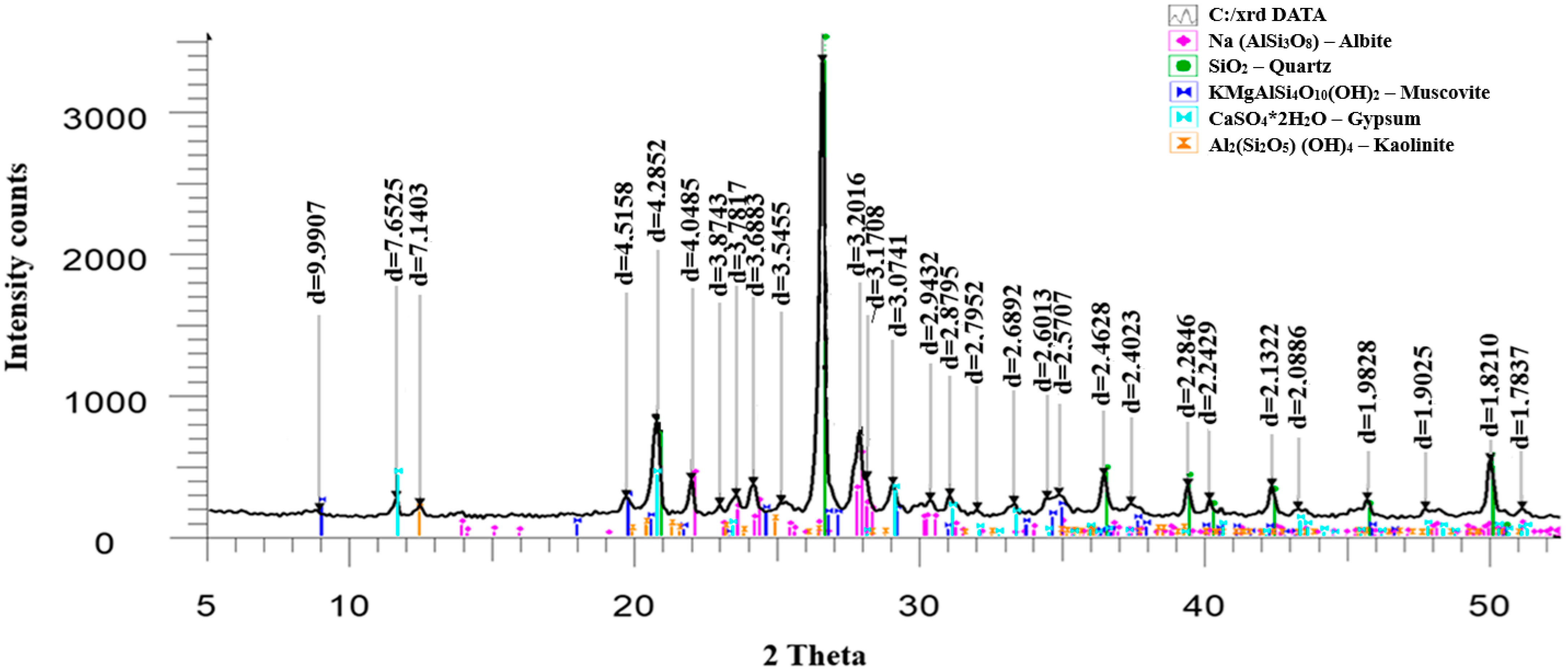
According to the phase analysis results (
Table 2,
Figure 3), the primary mineral component of the ore is quartz, indicating a predominance of silicate gangue. In addition to quartz, the ore also contains albite, clinochlore, and muscovite, along with calcite and magnesite in smaller amounts.
Table 2.
Phase composition of ore.
Table 2.
Phase composition of ore.
| Name | Formula | S-Q, % |
|---|
| Quartz, syn | SiO2 | 63.3% |
| Albite | Na(AlSi3O8) | 17.4% |
| Calcite, magnesian, syn | (Mg0.03Ca0.97)(CO3) | 10.6% |
| Clinochlore-1MIIb, ferroan | (Mg,Fe)6(Si,Al)4O10(OH)8 | 4.6% |
| Muscovite-1M, syn | KAl2Si3AlO10(OH)2 | 4.0% |
Table 3.
Results of X-ray fluorescence analysis of the original sample.
Table 3.
Results of X-ray fluorescence analysis of the original sample.
| Name of Element | Content in Samples, % |
|---|
| O | 51.04 |
| Na | 1.455 |
| Mg | 1.17 |
| Al | 6.097 |
| Si | 24.165 |
| P | 0.059 |
| S | 0.026 |
| Cl | 0.009 |
| K | 1.112 |
| Ca | 3.135 |
| Ti | 0.529 |
| V | 0.007 |
| Mn | 0.089 |
| Fe | 2.648 |
| Cu | 0.374 |
| Zn | 0.01 |
| Rb | 0.008 |
| Sr | 0.009 |
| Zr | 0.013 |
Mineralogical analysis of the initial ore sample revealed the presence of pyrite, chalcopyrite, and chalcocite. Chalcopyrite (CuFeS
2) occurs as irregularly shaped grains and is commonly observed in intergrowths with both chalcocite and pyrite. Chalcocite (Cu
2S) is present as anhedral grains with undulatory boundaries; it appears both as isolated fine grains and in intergrowths with chalcopyrite. Pyrite (FeS
2), a mineral of the cubic crystal system, is found as irregularly shaped grains, often intergrown with chalcopyrite (
Figure 4).
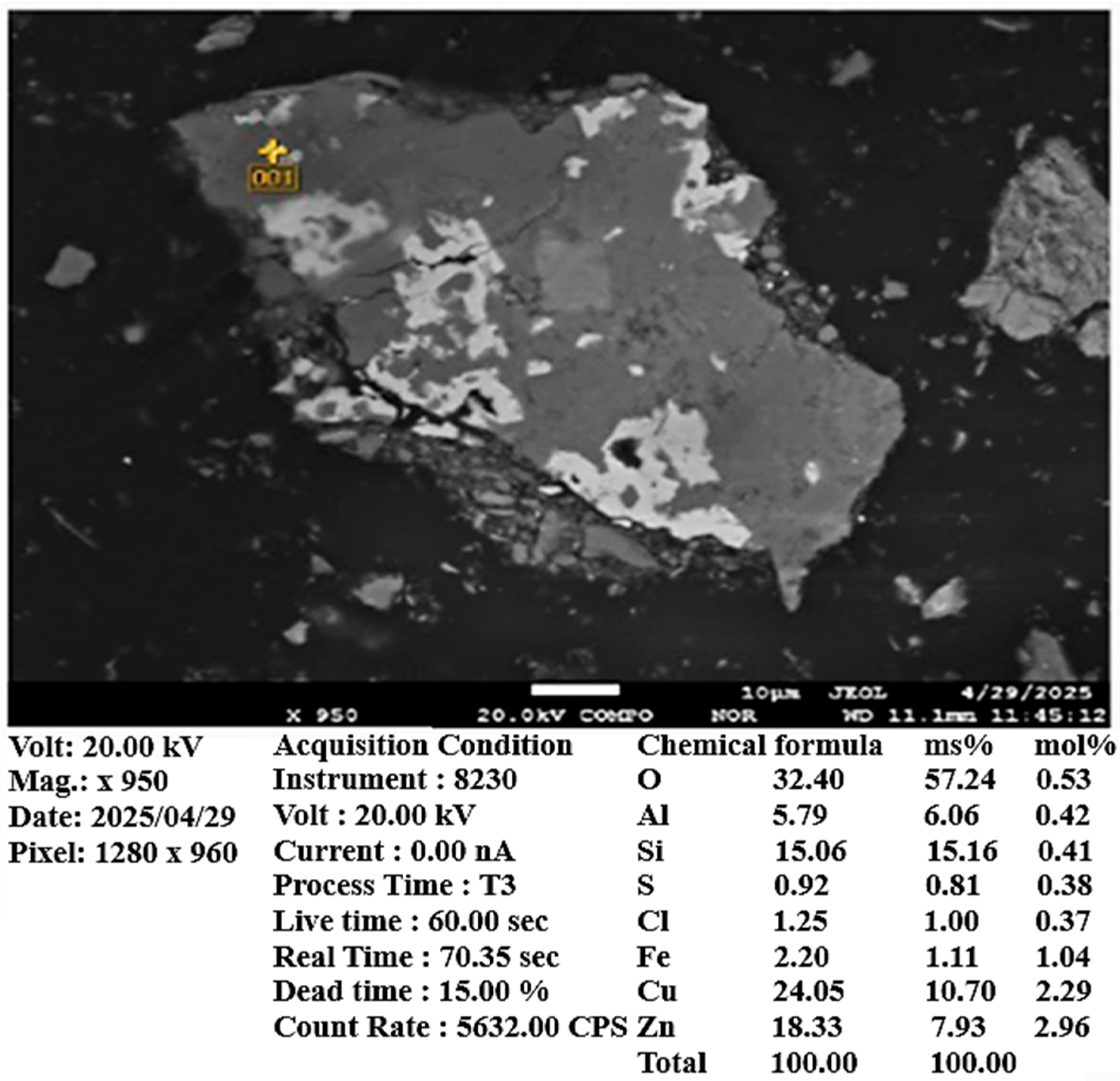
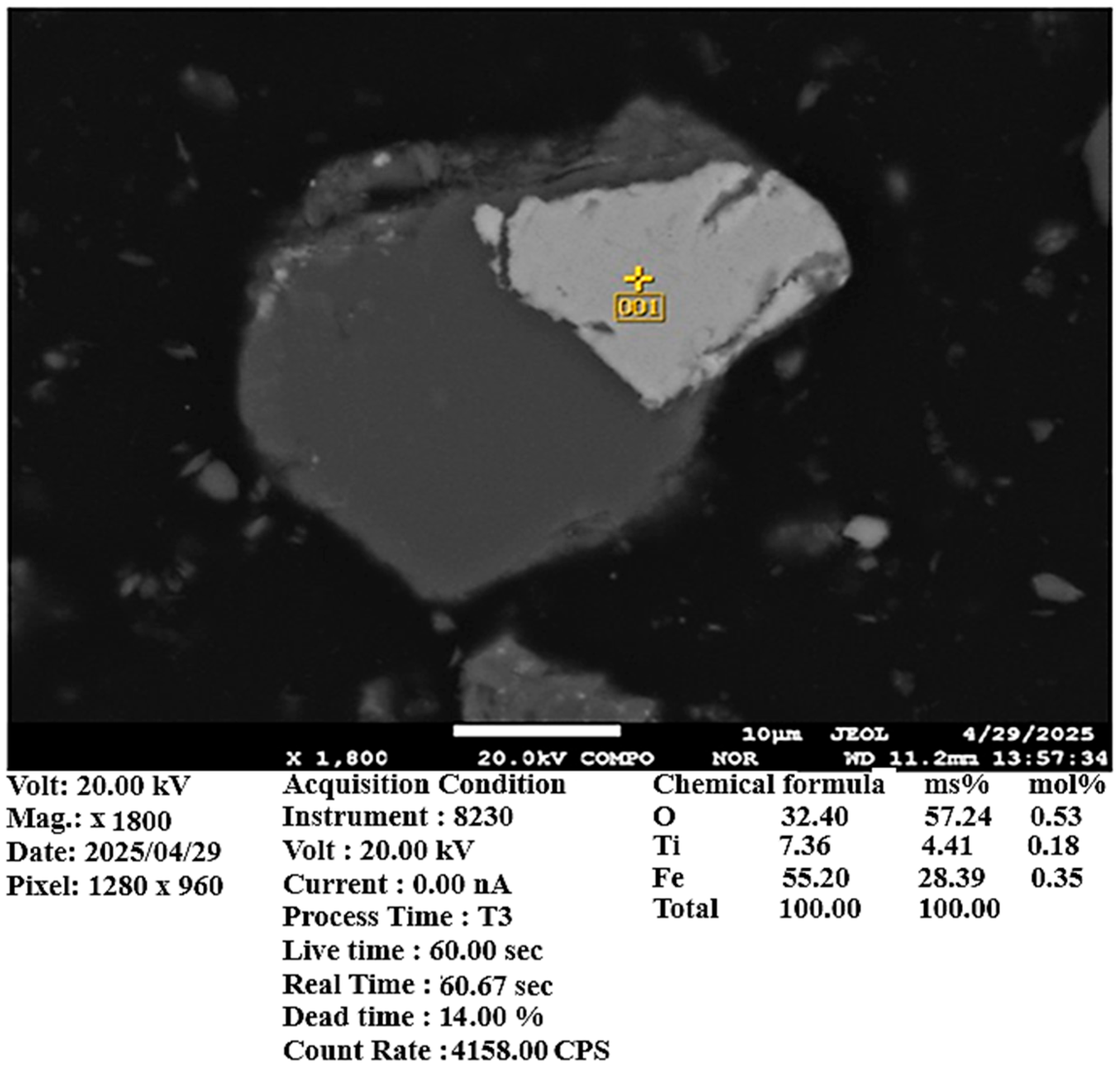
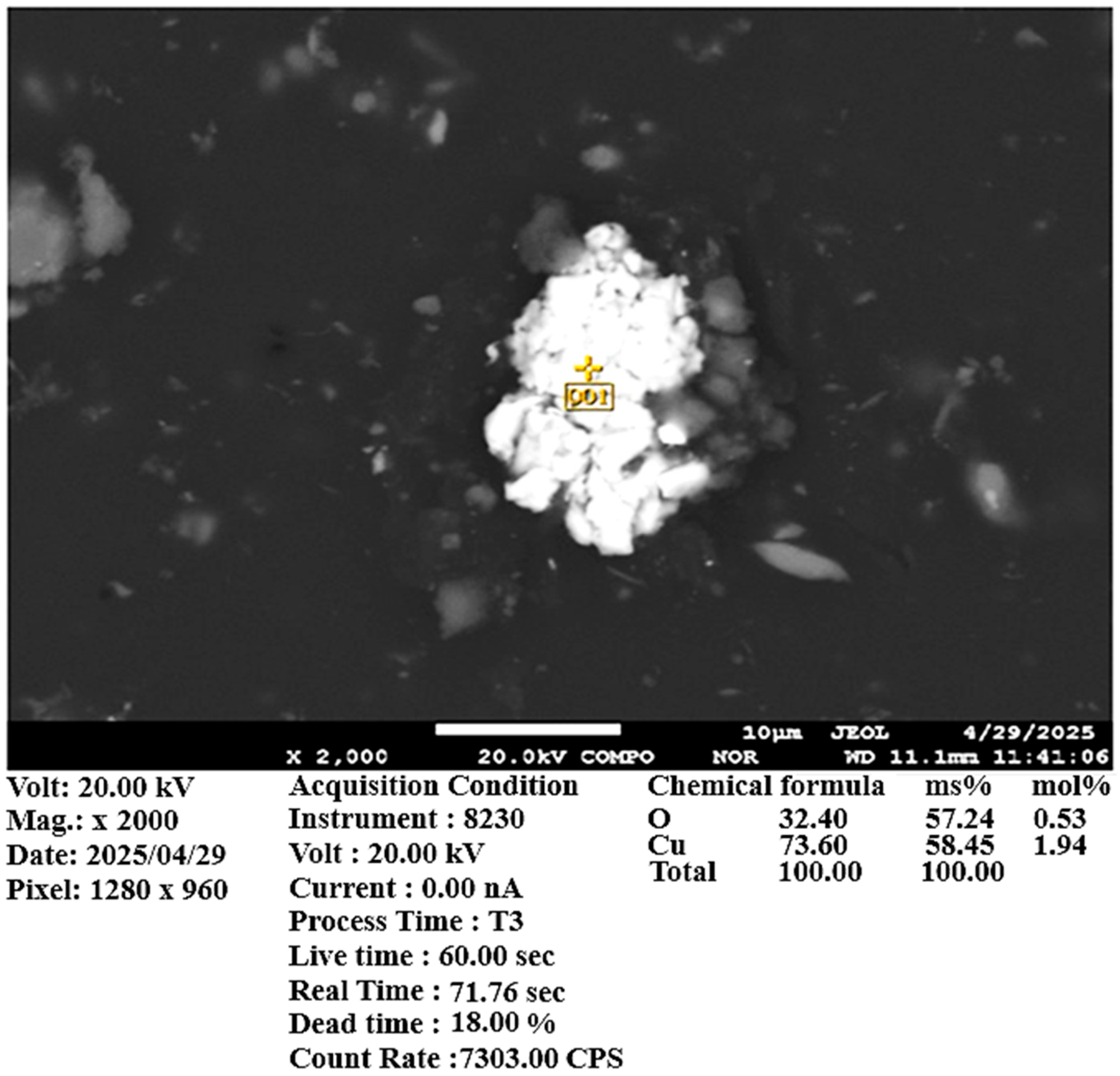
Scanning electron microscopy (SEM) and X-ray spectral microanalysis (XMSA) confirmed a significant presence of copper sulfide phases. Additionally, the sample contains titanomagnetite and various silicon oxide minerals (
Figure 5,
Figure 6 and
Figure 7). According to chemical analysis, the copper content in the ore sample is 0.374 wt.%.
In the present bioleaching experiments, chemolithotrophic bacteria Acidithiobacillus ferrooxidans were employed, as previously described.
The role of these microorganisms in the leaching of sulfide ores is well established. During biogeotechnological processing, metals are transformed from water-insoluble sulfides into water-soluble sulfates. A. ferrooxidans are capable of oxidizing a wide range of metal sulfides and derive the carbon required for cellular growth from carbon dioxide.
In this study, the influence of various amino acids differing in structure and composition on the biooxidation of copper was investigated. The amino acids used can be categorized as follows. Glycine and leucine are aliphatic amino acids and possess the simplest molecular structures among the group. Asparagine and histidine exhibit more complex side chains, with asparagine containing an amide functional group and histidine featuring an imidazole ring. Cysteine is structurally distinct from the others due to the presence of a sulfur atom in its side chain.
Biooxidation and the subsequent leaching of copper were carried out according to the methodology described earlier. The optimal concentration of the amino acids was determined experimentally, based on previously reported studies [
20,
21,
22]. The experimental results are summarized in
Table 4 and
Table 5.
The results presented in the tables indicate that during the biooxidation process, the presence of glycine and leucine leads to more than 30% of copper being transferred into the solution, whereas with other amino acids, this value does not exceed 12.5%. Under these conditions, the total copper recovery after both stages (biooxidation and subsequent sulfuric acid leaching) is significantly higher when glycine and leucine are used compared to other amino acids.
Moreover, a greater amount of sulfur and iron is leached into the solution, indicating more intensive oxidation, which is further confirmed by the formation of sulfate ions (S6+ as SO42−). In contrast, in the presence of asparagine, histidine, and cysteine, sulfur is only partially oxidized to the hexavalent state; the remainder exists in lower oxidation states. These results suggest that aliphatic amino acids exert a stronger effect on the biooxidation process. This behavior can be attributed to the structure of the side chain: the more complex the side chain, the less pronounced its positive influence on biooxidation efficiency.
According to Butlerov’s theory [
23] (Frolov V.V. Chemistry. Moscow, Russia: Higher School, 1996–543 p.), the reactivity of atoms within a molecule is influenced by the nature of the atoms they are bonded to. The chemical activity is determined by the mutual electronic interactions of the directly bonded atoms. In amino acids with aliphatic side chains, the electron density within the carboxyl group is redistributed toward the oxygen atoms, which promotes acid dissociation into hydrogen ions and the conjugate base. However, as the side chain becomes more complex, this redistribution becomes less pronounced due to internal electron delocalization within the side chain itself. As a result, the carboxyl group becomes less acidic, which in turn reduces the reaction rate and oxidation efficiency of the ore.
During biooxidation, the phase composition of the ore changes significantly (
Table 6,
Figure 8). The content of quartz decreases, while albite and muscovite increase. New mineral phases, such as gypsum (CaSO
4·2H
2O) and kaolinite [Al
2(Si
2O
5)(OH)
4], are formed at the expense of calcite, magnesite [(Mg
0.
03Ca
0.
97)(CO
3)], and clinochlore-1MIIb, ferroan [(Mg,Fe)
6(Si,Al)
4O
10(OH)
8]. Notably, the phase transformations do not depend on the type of amino acid used during biooxidation.
In summary, the results demonstrate that the addition of amino acids to the bacterial culture during biooxidation has a significant effect on the copper extraction as well as on the leaching of iron and sulfur. The effectiveness of amino acids in enhancing the process follows the order
Glycine > Leucine > Cysteine > Histidine > Asparagine.
Literary sources indicate a positive effect of fusel oils on the flotation process of polymetallic, copper-molybdenum, and gold-bearing ores [
24,
25]. Fusel oils (FOs) are waste products of ethyl alcohol production, a mixture of amphiatic alcohols of different structures, and they are used as raw materials for their production. At this stage, we have studied the effect of butyl and isoamyl alcohols on the degree of copper bioleaching from low-grade ores, as well as industrial waste based on aliphatic alcohols—fusel oils. The studies were conducted according to the methodology described above. The experimental results are presented in
Table 7.
The data in the table indicate that the addition of aliphatic alcohols to the pulp during the bioleaching process contributes to an increased degree of copper extraction into the solution. This effect becomes even more pronounced when fusel oils are introduced instead of individual aliphatic alcohols. The composition of the fusel oil used in the experiments is presented in
Table 8.As shown in the table, fusel oil contains a mixture of aliphatic alcohols, which explains the synergistic effect observed in copper extraction, as compared to the use of individual alcohols.
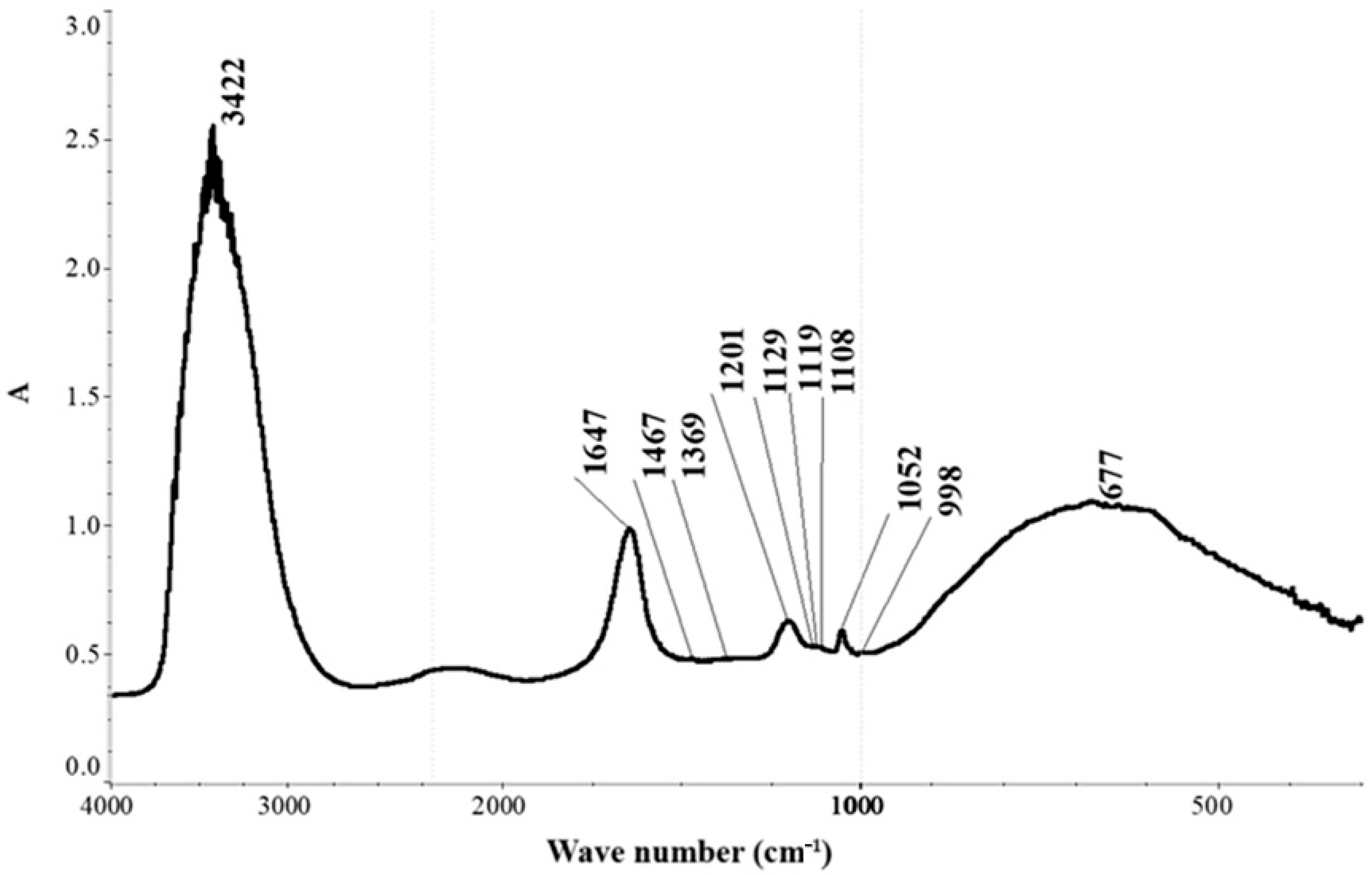
Figure 9 and
Figure 10 present the IR spectra of the solutions obtained after bioleaching copper-bearing ore in the presence of fusel oils.
Figure 9 corresponds to the aqueous phase, while
Figure 10 represents the organic phase.
The IR spectrum of the aqueous phase (
Figure 9) exhibits characteristic absorption bands corresponding to molecular water: the valence vibration ν(OH) at 3422 cm
−1, deformation vibration δ(HOH) at 1647 cm
−1, and the libration mode ν_L(H
2O) with a maximum at 677 cm
−1 [
26]. Superimposed on the water absorption profile, weak bands are observed corresponding to the deformation vibrations of –CH
2 and –CH
3 groups at 1467 and 1369 cm
−1 [
26,
27], respectively.
Additional bands are attributed to hydrosulfate ions (HSO
4−) at 1201 and 1052 cm
−1 [
28] and the sulfate group (SO
42−) at 1129, 1119, and 1108 cm
−1 [
25,
29]. The simultaneous presence of three bands corresponding to ν
3(F
2) of SO
42− at 1129, 1119, and 1108 cm
−1, along with a ν
1(A
1) SO
42− vibration at 998 cm
−1, suggests a lowering of the sulfate ion symmetry and the possible formation of sulfate complexes [
25]. Furthermore, bands at 1119 and 997 cm
−1 may also indicate the presence of thiosulfate ions (S
2O
32−) [
25].
The IR spectrum of the organic phase (
Figure 10) shows distinct absorption bands corresponding to 3-methyl-1-butanol (C
5H
12O), with characteristic peaks at 2958, 2931, 2873, 1466, 1385, 1367, 1265, 1213, 1170, 1125, 1058, 1011, 967, 941, 920, 905, 836, 765, 529, and 475 cm
−1 [
25].
Additionally, in the region associated with O–H and N–H stretching vibrations [
26,
27], a broad absorption band centered at 3354 cm
−1 is observed. The spectrum also contains bands corresponding to carbonyl (C=O) stretching vibrations, including the following:
Aldehydes, esters, and α-amino acid hydrochlorides at 1739 cm
−1 [
27];
Hydrochlorides of other amino acids, saturated ketones, and carboxylic acids at 1719 and 1713 cm
−1 [
30];
Amides at 1647 cm
−1 [
27].
Furthermore, the band at 1647 cm
−1 also coincides with the deformation vibration of water molecules (δHOH) [
25,
26,
27,
28,
29,
30,
31]. Additional signals include the following:
Deformation vibrations of N–H bonds in secondary amides and amines at 1564 cm
−1 [
26,
27];
C–N stretching vibrations in amides and amines at 1253 cm
−1 [
32,
33].
These spectral features suggest the possible formation of sulfo-complexes during copper bioleaching in the presence of aliphatic alcohols.
Given the complex and diverse composition of fusel oil as an organic component during the bioleaching process, it is reasonable to assume the formation of mixed complexes involving sulfo groups and organic ligands, which likely contribute to the enhanced mobilization of copper from low-grade ores into the solution.
4. Conclusions
The decline in the quality of copper-bearing feedstock necessitates the development of innovative, efficient, and cost-effective processing technologies. In this context, biogeotechnological approaches, particularly bioleaching, have gained significant attention. Recent research has increasingly focused on the use of natural organic compounds—bioreagents—as environmentally friendly enhancers of microbial metal extraction.
This study presents the results of physicochemical investigations of substandard copper ore from a deposit in Kazakhstan. The ore was found to have a complex composition, predominantly containing pyrite, chalcopyrite, and chalcocite, with minor amounts of titanomagnetite.
The effects of various additives—amino acids with different side-chain structures, aliphatic alcohols, and industrial waste containing alcohols (fusel oils)—on the biooxidation process were investigated. A pre-adapted strain of Acidithiobacillus ferrooxidans, tolerant to the chemical and mineralogical characteristics of the ore, was used to initiate biochemical oxidation.
The results demonstrate a correlation between amino acid side-chain complexity and its impact on copper biooxidation; the simpler the structure, the more pronounced the effect. This trend aligns with Butlerov’s theory of chemical structure, and the amino acid impact was observed to decrease in the following order: glycine > leucine > cysteine > histidine > asparagine.
A notable enhancement in copper biooxidation was observed upon the addition of aliphatic alcohols and fusel oils. According to IR spectroscopic data, this improvement may be attributed to the formation of copper sulfo-complexes with organic ligands, facilitating more efficient copper mobilization into the solution.