Production of 2,2,3,3,4,4,4-Heptafluorobutyl Acetate from Acetic Acid and 2,2,3,3,4,4,4-Heptafluorobutan-1-ol by Batch Reactive Distillation
Abstract
1. Introduction
2. Materials and Methods
3. Experimental Results and Discussion
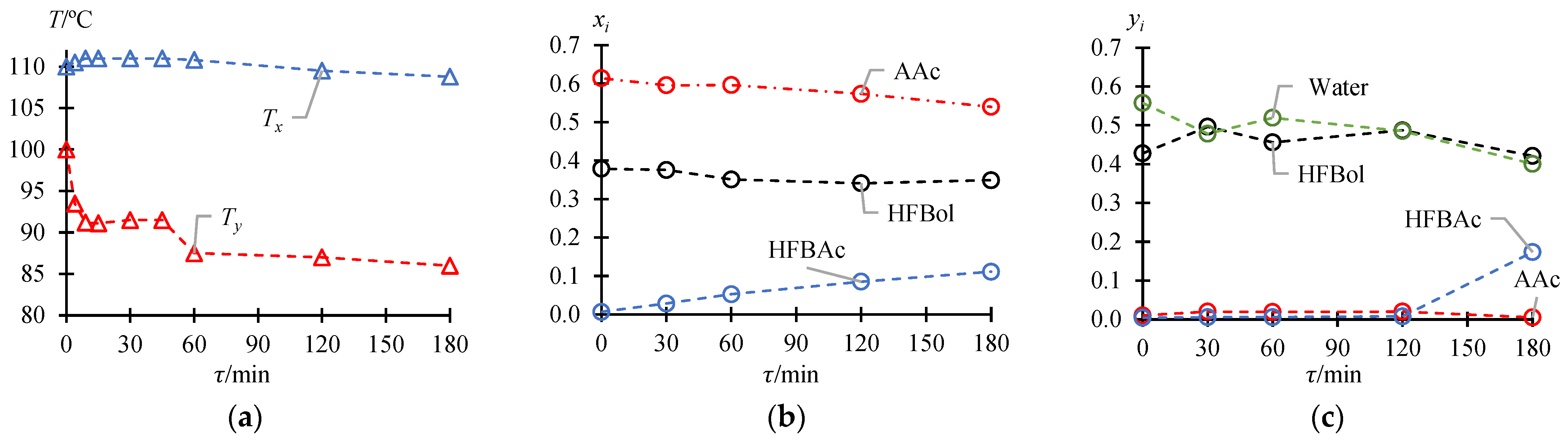
3.1. Total Reflux Mode
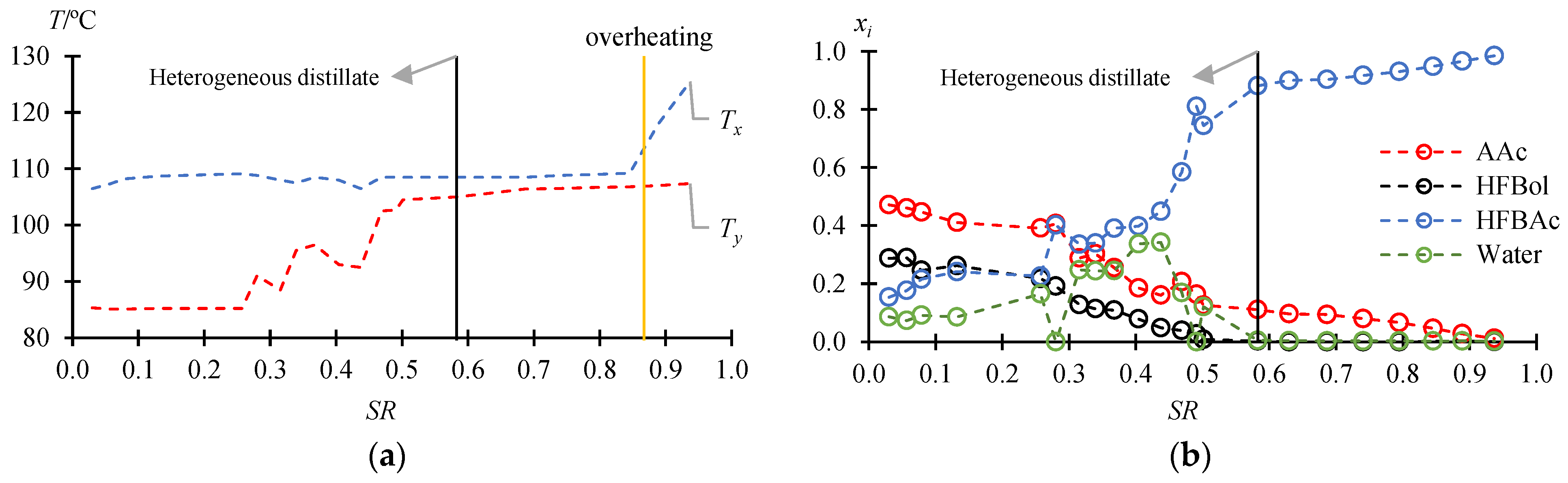
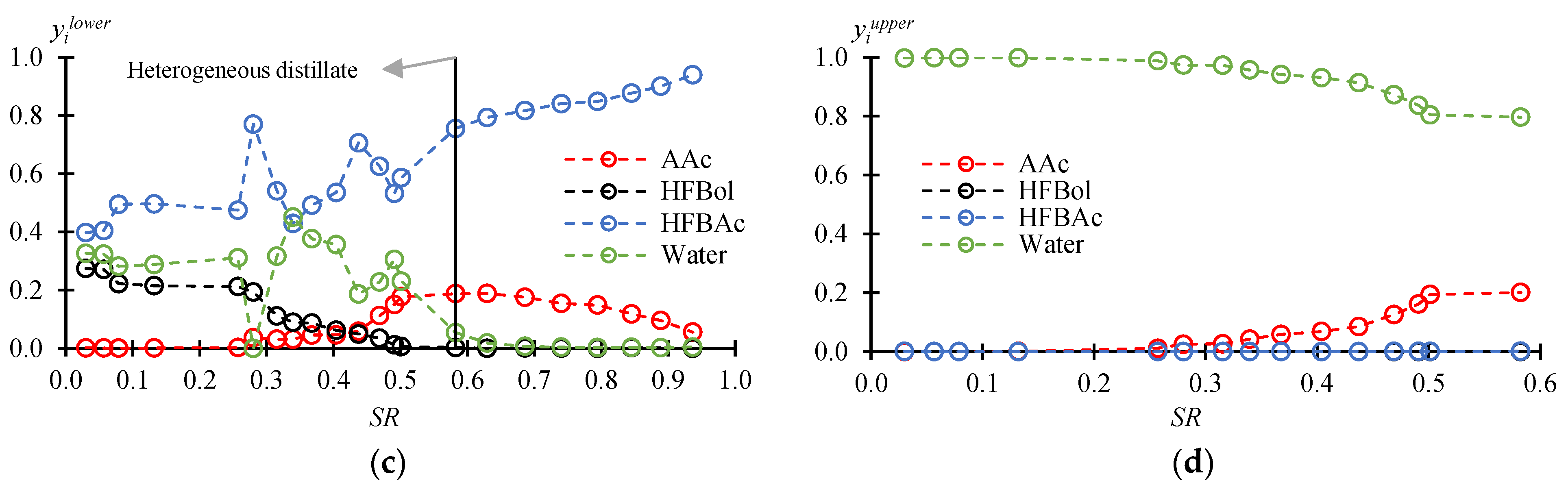
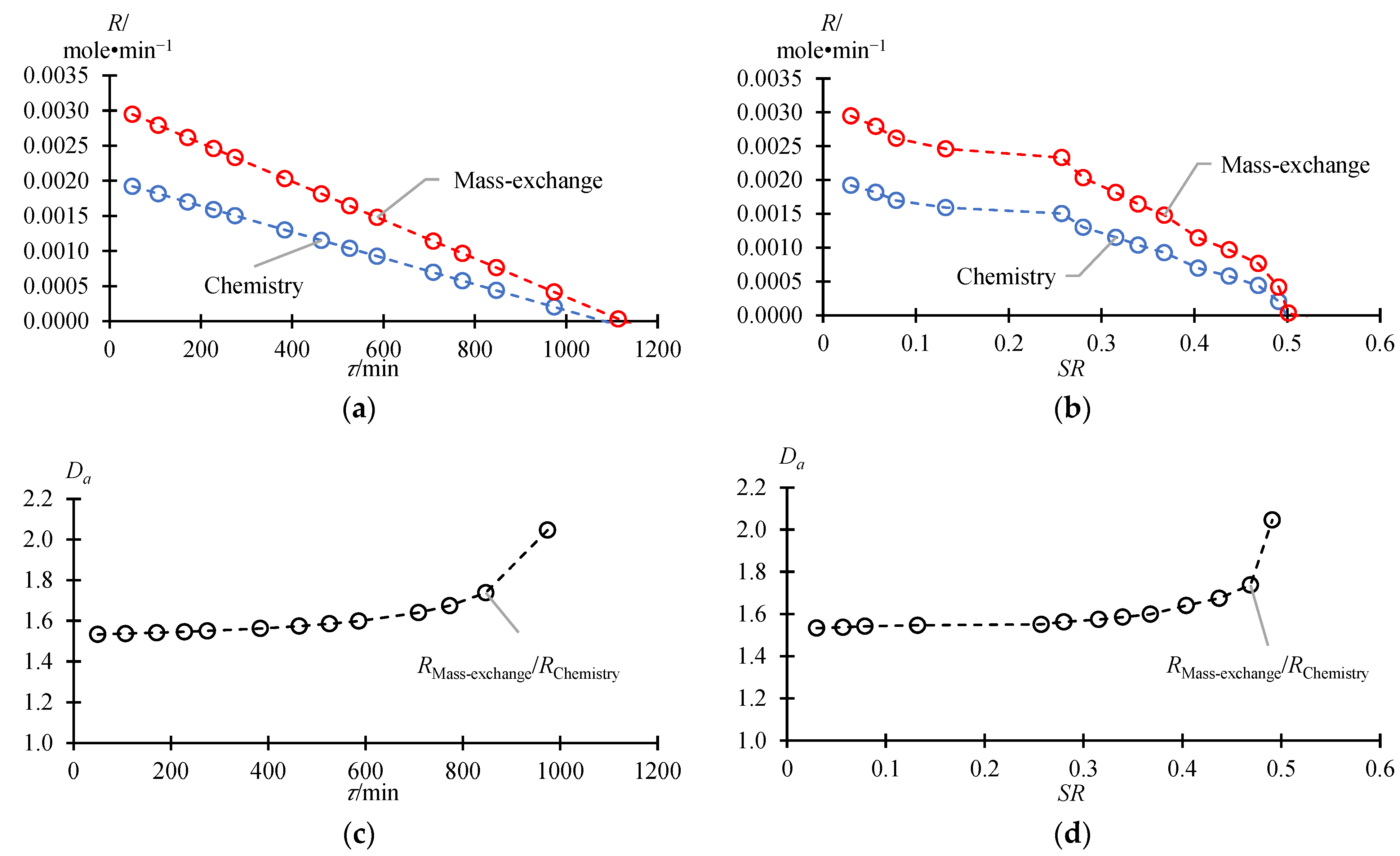
3.2. Fractionation Mode
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAc | acetic acid |
| BRD | batch reactive distillation |
| DMSO | dimethyl sulfoxide |
| GC | gas chromatography |
| HFBAc | 2,2,3,3,4,4,4-heptafluorobutyl acetate |
| HFBol | 2,2,3,3,4,4,4-heptafluorobutan-1-ol |
| NMR | nuclear magnetic resonance |
| RD | reactive distillation |
| Symbols | |
| in the bottom of the column, mole fr. | |
| in the distillate phase, mole fr. | |
| P | pressure, kPa |
| T | temperature, °C |
| u | standard uncertainty |
| SR | sampling ratio |
| N | quantity of substance loaded, mole |
| n | amount of substance sampled, mole |
| m | mass, g |
| , g·mole−1 | |
| Damköhler number | |
| Δτ | accumulation time of the fraction, min |
| rate of mass exchange, mole·min−1 | |
| rate of water formation, mole·min−1 | |
| change in the amount of HFBol in the system during the accumulation time of the fraction, mole | |
| τ | time, min |
| Indexes | |
| bottom | |
| distillate | |
| component number | |
References
- Polkovnichenko, A.V.; Voshkin, A.A.; Kovaleva, E.I.; Selivanov, N.A.; Kvashnin, S.Y.; Lupachev, E.V. Heptafluorobutyl acetate: Heptafluorobutanol and isopropyl acetate reaction in the presence of an acidic catalyst—Chemistry, phase behavior, batch reactive distillation process. Chem. Eng. Res. Des. 2025, 218, 95–116. [Google Scholar] [CrossRef]
- Rogueda, P. Novel Aerosol Formulation Containing Polar Fluorinated Molecules. JP2004502719A, 29 January 2004. [Google Scholar]
- Iwasawa, H.; Hayashi, A.; Shimokawa, T.; Yamamoto, M. Polysiloxane, Process for Production Thereof and Radiation-Sensitive Resin Composition. WO2002090423A1, 14 November 2002. [Google Scholar]
- Kekicheff, P.; Clauzel, M. Covering a Substrate with a Polymer Film That Is Stable in a Liquid Medium. EP2271438A1, 8 November 2011. [Google Scholar]
- Noguchi, T.; Uehara, M. Nonaqueous Electrolyte with Fluorine Containing Ether Compound for Lithium Secondary Battery. US10003100B2, 19 June 2018. [Google Scholar]
- Polymeric Materials Using Controlled Free Radical Initiators and Methods of Making. CN109476774B, 12 January 2021.
- Film Exterior Battery and Battery Module Including the Same. JP6597631B2, 30 October 2019.
- Olson, D.B.; Savu, P.M.; Hebrink, T.J. Copolymers Including Ultraviolet Absorbing Groups and Fluoropolymer Compositions Including Them. US20150353662A1, 10 December 2015. [Google Scholar]
- Olson, D.B.; Savu, P.M.; North, D. Copolymers Including a Triazine Group and Compositions Including Them. WO2015200657A1, 30 December 2015. [Google Scholar]
- Olson, D.B.; Savu, P.M.; North, D. Fluoropolymer Composition Including at Least One Oligomer. US20170198129A1, 13 July 2017. [Google Scholar]
- Yamazaki, J.; Kinoshita, M.; Kuwajima, Y.; Takahashi, K. Manufacturing Method of Ethyllithium Sulfate. JP2021141069A, 16 September 2021. [Google Scholar]
- Nakano, T.; Okano, K.; Shirota, N.; Kashiwagi, K.; Morizawa, Y.; Hamatani, Y. Process for Producing Charge Retention Medium. US9427777B2, 30 August 2016. [Google Scholar]
- Chicheva, D.S.; Krasnykh, E.L.; Shakun, V.A. Kinetic regularities of neopentyl glycol esterification with acetic and 2-ethylhexanoic acids. Fine Chem. Technol. 2024, 19, 28–38. [Google Scholar] [CrossRef]
- Brace, N.O. Some approaches to the synthesis of fluorinated alcohols and esters. I. Completely fluorinated esters from the hunsdiecker reaction of silver F-alkanoates with iodine. J. Fluor. Chem. 1981, 18, 515–524. [Google Scholar] [CrossRef]
- Brace, N.O. Some approaches to the synthesis of fluorinated alcohols and esters. II. Use of F-alkyl iodides for the synthesis of F-alkyl alkanols. J. Fluor. Chem. 1982, 20, 313–327. [Google Scholar] [CrossRef]
- Brace, N.O. Syntheses with perfluoroalkyl iodides. A review. J. Fluor. Chem. 2001, 108, 147–175. [Google Scholar] [CrossRef]
- Dou, Z.; Zhai, W.; Xia, K.; Chen, D.; Yu, H. Continuous Synthesis Method and Synthesis Device of Ethyl Trifluoroacetate. CN118307407A, 6 August 2024. [Google Scholar]
- Desbois, M.; Amiet, L. Process for Preparing Trifluoroacetic or Trichloroacetic acid Esters. U.S. Patent 4701551A, 20 October 1987. [Google Scholar]
- Mahajan, Y.S.; Shah, A.K.; Kamath, R.S.; Salve, N.B.; Mahajani, S.M. Recovery of trifluoroacetic acid from dilute aqueous solutions by reactive distillation. Sep. Purif. Technol. 2008, 59, 58–66. [Google Scholar] [CrossRef]
- Talnikar, V.D.; Deorukhkar, O.A.; Katariya, A.; Mahajan, Y.S. Value-Added Esterification for the Recovery of Trifluoroacetic Acid: Batch Kinetics and Reactive Distillation Studies. Chem. Eng. Commun. 2017, 204, 356–364. [Google Scholar] [CrossRef]
- Ji, Y.; Blankenship, A.N.; van Bokhoven, J.A. Heterogeneous Mn-Based Catalysts for the Aerobic Conversion of Methane-to-Methyl Trifluoroacetate. ACS Catal. 2023, 13, 3896–3901. [Google Scholar] [CrossRef]
- Ravi, M.; van Bokhoven, J.A. Homogeneous Copper-Catalyzed Conversion of Methane to Methyl Trifluoroacetate in High Yield at Low Pressure. ChemCatChem 2018, 10, 2383–2386. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Han, Z.; Huang, S.; Yuan, D.; Su, W. Atmosphere-Pressure Methane Oxidation to Methyl Trifluoroacetate Enabled by a Porous Organic Polymer-Supported Single-Site Palladium Catalyst. ACS Catal. 2021, 11, 1008–1013. [Google Scholar] [CrossRef]
- Dang, H.T.; Cheong, S.; Kim, J.; Tran, N.T.; Kim, H.; Lee, H. Tetrachlorocobaltate-Catalyzed Methane Oxidation to Methyl Trifluoroacetate. Catalysts 2022, 12, 1419. [Google Scholar] [CrossRef]
- Cheong, S.-H.; Kim, D.; Dang, H.T.; Kim, D.; Seo, B.; Cheong, M.; Hong, S.H.; Lee, H. Methane oxidation to methyl trifluoroacetate by simple anionic palladium catalyst: Comprehensive understanding of K2S2O8-based methane oxidation in CF3CO2H. J. Catal. 2022, 413, 803–811. [Google Scholar] [CrossRef]
- Hylland, K.T.; Schmidtke, I.L.; Wragg, D.S.; Nova, A.; Tilset, M. Synthesis of substituted (N,C) and (N,C,C) Au(iii) complexes: The influence of sterics and electronics on cyclometalation reactions. Dalton Trans. 2022, 51, 5082–5097. [Google Scholar] [CrossRef]
- Devale, R.R.; Katariya, A.; Mahajan, Y.S. Ethyl trifluoroacetate formation as a means to recover trifluoroacetic acid from dilute aqueous mixture: Reaction, separation and purification. J. Chin. Inst. Eng. 2023, 46, 781–794. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, X.; Zhou, L.; Huang, Z. Investigation of Ethyl Lactate Reactive Distillation Process. Chem. Eng. Res. Des. 2007, 85, 525–529. [Google Scholar] [CrossRef]
- Kiss, A.A. Novel applications of dividing-wall column technology to biofuel production processes. J. Chem. Technol. Biotechnol. 2013, 88, 1387–1404. [Google Scholar] [CrossRef]
- Chen, H.; Huang, K.; Zhang, L.; Wang, S. Reactive Distillation Columns with a Top-Bottom External Recycle. Ind. Eng. Chem. Res. 2012, 51, 14473–14488. [Google Scholar] [CrossRef]
- Bhatia, S.; Mohamed, A.; Ahmad, A.; Chin, S. Production of isopropyl palmitate in a catalytic distillation column: Comparison between experimental and simulation studies. Comput. Chem. Eng. 2007, 31, 1187–1198. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hong, W.H.; Wozny, G. Effect of recycle and feeding method on batch reactive recovery system of lactic acid. Korean J. Chem. Eng. 2002, 19, 808–814. [Google Scholar] [CrossRef]
- Kumar, R.; Mahajani, S.M.; Nanavati, H.; Noronha, S.B. Recovery of lactic acid by batch reactive distillation. J. Chem. Technol. Biotechnol. 2006, 81, 1141–1150. [Google Scholar] [CrossRef]
- Kumar, R.; Nanavati, H.; Noronha, S.B.; Mahajani, S.M. A continuous process for the recovery of lactic acid by reactive distillation. J. Chem. Technol. Biotechnol. 2006, 81, 1767–1777. [Google Scholar] [CrossRef]
- Kamble, S.P.; Barve, P.P.; Joshi, J.B.; Rahman, I.; Kulkarni, B.D. Purification of Lactic Acid via Esterification of Lactic Acid Using a Packed Column, Followed by Hydrolysis of Methyl Lactate Using Three Continuously Stirred Tank Reactors (CSTRs) in Series: A Continuous Pilot Plant Study. Ind. Eng. Chem. Res. 2012, 51, 1506–1514. [Google Scholar] [CrossRef]
- Kumar, R.; Mahajani, S.M. Esterification of Lactic Acid with n-Butanol by Reactive Distillation. Ind. Eng. Chem. Res. 2007, 46, 6873–6882. [Google Scholar] [CrossRef]
- Steinigeweg, S.; Gmehling, J. Esterification of a Fatty Acid by Reactive Distillation. Ind. Eng. Chem. Res. 2003, 42, 3612–3619. [Google Scholar] [CrossRef]
- Zhou, D.; Guo, J.; Zhu, Y.; Zhuo, Y.; Sha, Y. Synthesis and Analysis of Reactive Dividing-Wall Distillation for Methylal Production. Chem. Eng. Technol. 2020, 43, 1350–1360. [Google Scholar] [CrossRef]
- Polkovnichenko, A.V.; Kovaleva, E.I.; Selivanov, N.A.; Ksenofontova, T.D.; Kvashnin, S.Y.; Lupachev, E.V. 2,2,3,3,4,4,4-Heptafluorobutyl Acetate: Transesterification Reaction of 2,2,3,3,4,4,4-Heptafluoro-1-Butanol and Isopropyl Acetate—Side-Product Composition. International Electronic Conference on Processes. Eng. Proc. 2024, 67, 40. [Google Scholar]
- Polkovnichenko, A.V.; Kovaleva, E.I.; Privalov, V.I.; Selivanov, N.A.; Kvashnin, S.Y.; Lupachev, E.V. 2,2,3,3,4,4,4-Heptafluorobutyl Acetate—Chemical Equilibrium and Kinetics of the Esterification Reaction of 2,2,3,3,4,4,4-Heptafluorobutan-1-ol and Acetic Acid in the Presence of an Acidic Catalyst. Molecules 2025, 30, 1744. [Google Scholar] [CrossRef]
- Polkovnichenko, A.V.; Lupachev, E.V.; Kisel’, A.V.; Kvashnin, S.Y.; Kulov, N.N. Perfluoro(7-Methylbicyclo [4.3.0]Nonane) and Perfluoro(Butylcyclohexane): Physicochemical, Thermophysical, and Spectral Data. J. Chem. Eng. Data 2023, 68, 499–517. [Google Scholar] [CrossRef]
- Serafimov, L.A. Thermodynamic and topological analysis of heterogeneous equilibrium diagrams of multicomponent mixtures. Russ. J. Phys. Chem. A 2002, 76, 1211–1224. [Google Scholar]
- Serafimov, L.A. Thermodynamic and topological analysis of liquid–vapor phase equilibrium diagrams and problems of rectification of multicomponent mixtures. In Mathematical Method in Contemporary Chemistry; Kuchanov, S.I., Ed.; Gordon and Breach Publishers: Amsterdam, The Netherlands, 1996; Chapter 10; pp. 557–605. [Google Scholar]
- Chelyuskina, T.V.; Bedretdinov, F.N.; Pronina, D.S. Studying the structure of the vapor–liquid equilibrium diagram of the butyl propionate–propionic acid–butyl butyrate–butyric acid system. Theor. Found. Chem. Eng. 2016, 50, 1043–1048. [Google Scholar] [CrossRef]

| Chemical Name | CAS-No | Molar Mass M/g·Mole−1 | Supplier | Mass Fraction Purity | Purification in Laboratory | Mass Fraction After Purification (GC 1) |
|---|---|---|---|---|---|---|
| HFBol | 375-01-9 | 200.05 | P&M Invest (Moscow, Russia) | 0.60–0.90 | Heteroazeotropic distillation; distillation | ≥0.998 |
| AAc | 64-19-7 | 60.05 | EKOS-1 (Moscow, Russia) | 0.99 | none | - |
| DMSO-d6 | 2206-27-1 | 84.17 | Solvex-D (Moscow, Russia) | 0.998 atom % D | none | - |
| Amberlyst 35 WET by Rohm and Haas France S.A.S.; ionic form supplied: H+; weight TOT capacity (H+): >5.2 eq·kg−1 | ||||||
| Component | /g | /Mole | /Mole fr. |
|---|---|---|---|
| AAc | 113.0040 | 1.8818 | 0.5498 |
| HFBol | 308.3300 | 1.5412 | 0.4502 |
| Amberlist 35 WET | 6.6685 | 0.0347 * | 0.0101 * |
| τ/min | /°C | /°C | /Mole fr. | /Mole fr. (Lower/Organic Phase of Distillate) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AAc | HFBol | HFBAc | H2O | AAc | HFBol | HFBAc | H2O | |||
| Feed | 0.5290 | 0.4710 | 0 | 0 | ||||||
| 0 | 110.0 | 100.0 | 0.6147 | 0.3788 | 0.0065 | 0 | 0.0100 | 0.4279 | 0.0042 | 0.5579 |
| 30 | 111.0 | 91.5 | 0.5960 | 0.3757 | 0.0283 | 0 | 0.0200 | 0.4964 | 0.0054 | 0.4782 |
| 60 | 110.8 | 87.5 | 0.5965 | 0.3510 | 0.0525 | 0 | 0.0188 | 0.4560 | 0.0058 | 0.5194 |
| 120 | 109.5 | 87.0 | 0.5735 | 0.3412 | 0.0853 | 0 | 0.0198 | 0.4871 | 0.0077 | 0.4854 |
| 180 | 108.8 | 86.0 | 0.5398 | 0.3490 | 0.1112 | 0 | 0.0050 | 0.4213 | 0.1733 | 0.4004 |
| τ/min | /°C | /°C | /Mole fr. | /Mole fr. (Lower/Organic Phase) | /Mole fr. (Upper/Aqueous Phase) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aac | HFBol | HFBAc | H2O | Aac | HFBol | HFBAc | H2O | Aac | HFBol | HFBAc | H2O | ||||
| Heterogeneous distillate | |||||||||||||||
| 50 | 0.0300 | 106.5 | 85.3 | 0.4721 | 0.2880 | 0.1536 | 0.0863 | 0.0012 | 0.2748 | 0.3975 | 0.3265 | 0.0005 | 0.0010 | 0.0010 | 0.9975 |
| 107 | 0.0567 | 107.3 | 85.1 | 0.4610 | 0.2891 | 0.1766 | 0.0733 | 0.0010 | 0.2710 | 0.4048 | 0.3232 | 0.0004 | 0.0003 | 0.0006 | 0.9987 |
| 171 | 0.0789 | 108.2 | 85.1 | 0.4470 | 0.2460 | 0.2155 | 0.0915 | 0.0007 | 0.2221 | 0.4950 | 0.2822 | 0.0006 | 0.0002 | 0.0005 | 0.9987 |
| 228 | 0.1320 | 108.7 | 85.2 | 0.4108 | 0.2624 | 0.2412 | 0.0856 | 0.0010 | 0.2149 | 0.4962 | 0.2879 | 0.0008 | 0 | 0.0003 | 0.9989 |
| 275 | 0.2572 | 109.1 | 85.2 | 0.3912 | 0.2178 | 0.2260 | 0.1650 | 0.0024 | 0.2120 | 0.4748 | 0.3108 | 0.0116 | 0.0001 | 0.0001 | 0.9882 |
| 384 | 0.2802 | 108.8 | 91.0 | 0.4066 | 0.1915 | 0.4019 | 0 | 0.0362 | 0.1934 | 0.7704 | 0.0000 | 0.0255 | 0.0003 | 0.0006 | 0.9736 |
| 464 | 0.3154 | 108.0 | 88.5 | 0.2884 | 0.1282 | 0.3358 | 0.2476 | 0.0312 | 0.1112 | 0.5410 | 0.3166 | 0.0266 | 0.0001 | 0.0003 | 0.9730 |
| 526 | 0.3395 | 107.5 | 95.5 | 0.3027 | 0.1137 | 0.3399 | 0.2437 | 0.0313 | 0.0893 | 0.4287 | 0.4507 | 0.0427 | 0.0001 | 0.0001 | 0.9571 |
| 586 | 0.3676 | 108.5 | 96.5 | 0.2551 | 0.1089 | 0.3910 | 0.2450 | 0.0453 | 0.0862 | 0.4919 | 0.3766 | 0.0584 | 0.0001 | 0.0001 | 0.9414 |
| 709 | 0.4040 | 108.0 | 93.0 | 0.1856 | 0.0789 | 0.3983 | 0.3372 | 0.0460 | 0.0620 | 0.5354 | 0.3566 | 0.0685 | 0.0002 | 0.0003 | 0.9310 |
| 773 | 0.4374 | 106.5 | 92.5 | 0.1607 | 0.0485 | 0.4485 | 0.3423 | 0.0588 | 0.0488 | 0.7061 | 0.1863 | 0.0856 | 0.0005 | 0.0004 | 0.9135 |
| 847 | 0.4689 | 108.5 | 102.5 | 0.2071 | 0.0391 | 0.5846 | 0.1692 | 0.1123 | 0.0345 | 0.6253 | 0.2279 | 0.1262 | 0.0007 | 0.0004 | 0.8727 |
| 974 | 0.4909 | 108.5 | 102.7 | 0.1633 | 0.0265 | 0.8102 | 0 | 0.1505 | 0.0118 | 0.5322 | 0.3055 | 0.1619 | 0.0007 | 0.0004 | 0.8370 |
| 1114 | 0.5014 | 108.5 | 104.5 | 0.1262 | 0.0087 | 0.7443 | 0.1208 | 0.1771 | 0.0060 | 0.5865 | 0.2304 | 0.1944 | 0.0009 | 0 | 0.8047 |
| 1228 | 0.5824 | 108.5 | 105.0 | 0.1110 | 0.0025 | 0.8812 | 0.0053 | 0.1878 | 0.0029 | 0.7555 | 0.0538 | 0.2013 | 0.0016 | 0 | 0.7971 |
| Homogeneous distillate | |||||||||||||||
| 1351 | 0.6296 | 108.5 | 105.6 | 0.0964 | 0 | 0.8999 | 0.0037 | 0.1885 | 0.0000 | 0.7935 | 0.0180 | - | - | - | - |
| 1411 | 0.6862 | 108.5 | 106.4 | 0.0939 | 0 | 0.9030 | 0.0031 | 0.1753 | 0.0011 | 0.8168 | 0.0068 | - | - | - | - |
| 1481 | 0.7404 | 108.8 | 106.5 | 0.0801 | 0 | 0.9170 | 0.0029 | 0.1542 | 0.0008 | 0.8410 | 0.0040 | - | - | - | - |
| 1556 | 0.7945 | 109.0 | 106.7 | 0.0667 | 0 | 0.9304 | 0.0029 | 0.1487 | 0.0000 | 0.8483 | 0.0030 | - | - | - | - |
| 1626 | 0.8453 | 109.2 | 106.8 | 0.0464 | 0.0027 | 0.9481 | 0.0028 | 0.1181 | 0.0018 | 0.8770 | 0.0031 | - | - | - | - |
| 1702 | 0.8890 | 118.0 | 107.0 | 0.0279 | 0.0031 | 0.9659 | 0.0031 | 0.0953 | 0.0018 | 0.9008 | 0.0021 | - | - | - | - |
| 1762 | 0.9369 | 125.5 | 107.4 | 0.0121 | 0.0006 | 0.9851 | 0.0022 | 0.0557 | 0 | 0.9400 | 0.0043 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polkovnichenko, A.V.; Lupachev, E.V.; Kovaleva, E.I.; Kvashnin, S.Y.; Chelyuskina, T.V.; Voshkin, A.A. Production of 2,2,3,3,4,4,4-Heptafluorobutyl Acetate from Acetic Acid and 2,2,3,3,4,4,4-Heptafluorobutan-1-ol by Batch Reactive Distillation. ChemEngineering 2025, 9, 72. https://doi.org/10.3390/chemengineering9040072
Polkovnichenko AV, Lupachev EV, Kovaleva EI, Kvashnin SY, Chelyuskina TV, Voshkin AA. Production of 2,2,3,3,4,4,4-Heptafluorobutyl Acetate from Acetic Acid and 2,2,3,3,4,4,4-Heptafluorobutan-1-ol by Batch Reactive Distillation. ChemEngineering. 2025; 9(4):72. https://doi.org/10.3390/chemengineering9040072
Chicago/Turabian StylePolkovnichenko, Andrei V., Egor V. Lupachev, Evgenia I. Kovaleva, Sergey Ya. Kvashnin, Tatiana V. Chelyuskina, and Andrey A. Voshkin. 2025. "Production of 2,2,3,3,4,4,4-Heptafluorobutyl Acetate from Acetic Acid and 2,2,3,3,4,4,4-Heptafluorobutan-1-ol by Batch Reactive Distillation" ChemEngineering 9, no. 4: 72. https://doi.org/10.3390/chemengineering9040072
APA StylePolkovnichenko, A. V., Lupachev, E. V., Kovaleva, E. I., Kvashnin, S. Y., Chelyuskina, T. V., & Voshkin, A. A. (2025). Production of 2,2,3,3,4,4,4-Heptafluorobutyl Acetate from Acetic Acid and 2,2,3,3,4,4,4-Heptafluorobutan-1-ol by Batch Reactive Distillation. ChemEngineering, 9(4), 72. https://doi.org/10.3390/chemengineering9040072








