Abstract
Oily wastewater is a major environmental issue resulting from different industrial and manufacturing activities. Contaminated water with oil represents a significant environmental hazard that can harm numerous life forms. Several methodologies have been tested for the removal of oily wastewater from aqueous solutions, and adsorption in a flow-through reactor is an effective mechanism to reduce these effluents. This study focuses on evaluating the ability of Fe3O4/Bent material to adsorb gasoline emulsion from a solution using a fixed-bed column, and it involves analyzing the resulting breakthrough curves. The FT-IR, SEM, EDX, and XRD techniques were used to characterize Fe3O4/Bent. Various ranges of variables were examined, including bed height (2–4 cm), flow rate (3–3.8 mL/min), and initial concentration (200–1000 mg/L), to determine their impacts on the mass transfer zone (MTZ) length and the adsorption capacity (qe). It was shown that a higher bed height and a lower flow rate contributed to a longer time of breakthrough and exhaustion. At the same time, it was noted that under high initial gasoline concentrations, the fixed-bed system rapidly reached breakthrough and exhaustion. Models like the Yoon–Nelson and Thomas kinetic column models were employed to predict the breakthrough curves. Thomas and Yoon–Nelson’s breakthrough models provided a good fit for the breakthrough curves with a correlation coefficient of R2 > 0.95. Furthermore, with a fixed-bed system, the Thomas and Yoon–Nelson models best describe the breakthrough curves for gasoline removal.
1. Introduction
There has been a steady evolution of oil spill cleaning methods in recent years in response to the increasing frequency of accidents and leaks, intending to address these issues and reduce their catastrophic consequences swiftly [1,2]. The fact that oil spill pollution is a prominent illustration of how human activities are destroying water quality and impacting aquatic ecosystems all across the world makes this need even more pressing [3,4]. The “Prestige” shipwreck in the North Atlantic in 2002 [3], the “Sea Diamond” in Santorini in 2007 [3], the Deep Horizon in the Gulf of Mexico in 2010 [2], and the “Agia Zoni II” in the Saronic Gulf in 2017 [4,5] are all notable instances.
Oil quickly spreads into water, creates a slick on the surface, and then gradually sinks into and builds up in the sediments. The degradation and eventual extinction of large swaths of marine life and habitats can be hastened by winds and currents [4]. In addition to the factors mentioned earlier, the rate at which oil spills spread in natural bodies of water can be affected by several physicochemical parameters, including salinity and temperature [5,6].
There are numerous methods to remove oily wastewater, including biological processes [7], ultrafiltration and nanofiltration [8], microfiltration [9], adsorption [10,11,12], reverse osmosis [13,14], electroflotation [15,16], advanced oxidation processes [17], de-emulsification [18], chemical coagulation, electrocoagulation, and flocculation [19]. With the exception of adsorption, most of these approaches have drawbacks, including higher operational costs than adsorption techniques, complicated processes, and high sludge formation.
Adsorption is a widely used method for treating wastewater due to its simplicity in operation, regenerable adsorbents, cost-effectiveness, and having a high-efficiency rate [20,21,22]. Moreover, it can handle many effluents without making any nasty sludge, which is a major plus [23]. As an alternative to commercial adsorbents, eco-friendly and inexpensive adsorbents like chitosan [24], clay materials [25,26,27], rice husk ash [28], wasted coffee powder [29], crab shells [30], and tea leaves [31] have started to attract attention. The main physical forces that govern adsorption are polarity, hydrophobicity, hydrogen bonding, steric interactions, π-π interactions, dipole-induced dipole interactions, and Van der Waals forces [32]. Noteworthy, adsorption has become the method of choice for treating oily water because it is inexpensive and easy to implement.
Many studies have utilized adsorption methods to remove oil spills from water using batch experiments with diverse low-cost adsorbents [33,34,35]. Nevertheless, treating oily wastewater from large volumes of water using batch adsorption proved challenging at an industrial scale. Instead, continuous-flow fixed-bed column adsorption has proven effective for handling large quantities of water and using low-cost adsorbents [36].
One kind of adsorbent is chitosan, which is well known for its low cost, non-toxicity, and excellent adsorption capability [37]. However, it can be dried and distorted, has poor mechanical strength, and has lower solubility in acidic solutions [38]. Although zeolites and biomass have been considered potential adsorbents, their poor adsorption rates severely restrict their potential uses [38,39]. On the other hand, clay minerals have great adsorption capacities, but they are not very useful because they are so dispersed in water; hence, they are hard to recycle or reuse [40]. It is worth mentioning that the transformation of volcanic ash and turf produces bentonite, a clay mineral composed mainly of aluminum phyllosilicate [41]. It has many intriguing properties, such as a large surface area, the ability to swell, the capability to exchange cations, and strong bond formations [42,43]. An enhanced adsorption capacity and substantially increasing the specific surface area are the anticipated outcomes of shrinking bentonite to nanoscale size [44]. Furthermore, bentonite can be used as a base for various metals and metal oxides, opening up new possibilities for composites in different industries [42]. Activated carbon and ferric oxide nanoparticles were proposed as high-efficiency adsorbents for the removal of emulsified oil (gasoline, diesel, and kerosene) from polluted water [45,46].
Ferric oxide nanoparticles (Fe3O4 NPs) are renowned for their biological adaptation, magnetic properties, large surface-to-volume ratio, eco-friendliness, and high capacity to remove organic contaminants from wastewater [47,48]. Because of the magnetic property of Fe3O4 NPs, encasing them in an inorganic matrix (like bentonite) makes it easier to separate the magnetic composite from an aqueous solution using an external magnetic field. A wider variety of active sites, increased chemical stability of Fe3O4 NPs, and a large specific surface area are only a few of the outstanding physicochemical qualities that the produced magnetic composite may exhibit [49]. However, magnetite nanoparticles have two main issues: rapid agglomeration and oxidation by ambient oxygen [50].
For the purpose of removing inorganic water contaminants, a few studies have described the synthesis of an iron oxide/bentonite nanocomposite, which exhibited outstanding adsorption performance [35,36,37,38,51,52,53,54]. Due to its easiness of use, low operating temperature, low cost, and scalability, the co-precipitation approach is often selected over alternative synthesis procedures [55]. Iron oxide nanoparticles have a high surface energy and, thus, a tendency to aggregate. By lowering the particle size, ensuring a uniform distribution of pore sizes, and preventing the aggregation of Fe3O4 NPs, sonochemical-assisted co-precipitation can improve the composite’s adsorption performance [55]. This study used an ultrasound-assisted co-precipitation technique to create a nanocomposite of iron oxide and bentonite called Fe3O4/Bent NPs. This composite was employed to remove oil from an oil–water emulsion because, to our knowledge, no other study has reported on how the material adsorbs organic pollutants. It is important to note that batch studies are commonly utilized to assess the adsorption efficiency in removing particular adsorbates and to ascertain the highest possible adsorption capacity. Most of the time, the results of batch studies are only applicable to small-scale laboratory systems and cannot be used in large-scale industrial systems. Therefore, basic data for the design of continuous-flow sorption must be obtained through a fixed-bed column study.
This investigation explores the removal of gasoline emulsion using Fe3O4/Bent material in a fixed-bed column. Previous research has validated Fe3O4/Bent NCs as adsorbents for the removal of oil content from oil–water emulsions through batch experiments [33]. However, batch adsorption is less suitable for large-scale applications due to the overestimation of sorption capacities [56]. In contrast, fixed-bed column adsorption is more effective and practical for water treatment, allowing for continuous operation and scalability. Furthermore, no studies used continuous-flow fixed-bed column methods to demonstrate the gasoline emulsion adsorption capability of Fe3O4/Bent. Hence, this study aims to (i) evaluate the usage of fixed-bed columns with Fe3O4/Bent for gasoline removal, (ii) analyze the effects of operating parameters through breakthrough curve analysis, and (iii) compare experimental data with theoretical models to predict the adsorption behavior. In general, the practical application of the findings of this study is the treatment of pollution sources from gas stations, including fuel leaks and the discharge of wastewater from cleaning tanks.
2. Materials and Methods
2.1. Chemicals
Samples of natural bentonite clays were gathered from “Traifawi”, a region near the Syrian border, and were provided by the General Company for Geological Survey and Mining in Baghdad, Iraq. Iron oxide Nanopowder/nanoparticles was obtained from SkySpring Nanomaterials, Inc. (Houston, TX, USA) (Fe3O4, 98% purity, 20–30 nm), HCL (37%), and NaOH (99%) were supplied from HiMedia (Thane, India). The liquid gasoline was obtained from a local Iraqi gas station.
2.2. Synthesis of Fe3O4/Bent NC
In the composite synthesis, 4 g of bentonite was introduced into 100 milliliters of a solution containing 2 g of Fe3O4 nanoparticles (Fe3O4 NPs were purchased). This mixture was continuously stirred for 6 h and then allowed to settle for 24 h. Subsequently, the mixture underwent washing and centrifugation to isolate the Fe3O4/Bent composite from the solution. The sample was dried in an oven set to 60 °C for 12 h as the last stage in the synthesis process [57,58].
2.3. Synthesis of Oil-Produced Water Emulsion
Deionized (DI) water and gasoline obtained from a local Iraqi gas station were utilized to produce the emulsified gasoline solution. As illustrated in Figure 1 and Figure 2, the emulsion was generated by adding gasoline oil droplets to DI water, followed by the homogenization of the mixture at 23 °C for 30 min. The range of gasoline concentrations in the oil–water emulsion was between 200 and 1000 mg/L, which is comparable to the oil concentrations found in actual produced effluent. The gasoline emulsion concentration was determined using a chemical oxygen demand (COD) device, namely a Lovibond ET 108 model manufactured in Dortmund, Germany.

Figure 1.
Emulsion stock solution.

Figure 2.
Chemical oxygen demand device.
2.4. Characterization
Instrumental methods of the physicochemical properties of the raw bentonite before and after being modified with the Fe3O4 NC were examined using SEM, EDX, XRD, and FTIR. For SEM analysis and EDX data, experiments were conducted using a Bruker INSPECT550. X-ray diffraction (XRD) experiments were performed at the Cu Kα wavelength with 2θ angles ranging from 10° to 90° using the Rigaku MiniFlex XRD. The FTIR analysis was carried out utilizing a German Bruker-Tensor 27. The point of zero charge (pHpzc) analysis was used to estimate the surface charge of the bentonite clay. Using the standard salt addition method, the pHzc for Fe3O4/Bent was recorded as 7.3. In order to accomplish this, 10 individual Erlenmeyer flasks were used to collect 30 mL of 0.02 M NaCl. Using 0.1 M HCl and 0.1 M NaOH solutions, the pH was adjusted to a range of 2 to 12. For 24 h at room temperature, each flask was shaken in an orbital shaker at 150 rpm with 0.1 g of absorbent added. The samples were separated using Whatman filter paper No. 41 while the system was in equilibrium. After measuring the filtrate’s pH, a graph was generated to illustrate the correlation between the initial and final pH values (Figure 3). According to Bassim et al. [59], the pHzc can be found when the pH difference is zero.
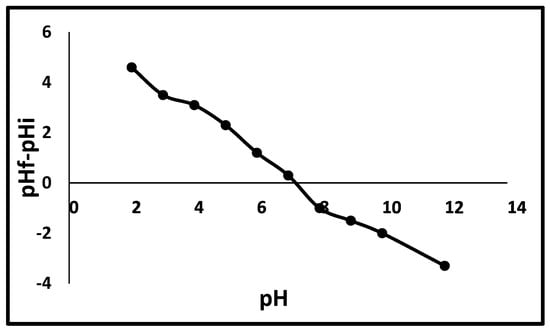
Figure 3.
pH point of zero charge of Fe3O4/Bent.
2.5. Fixed-Bed Experiments
Glass columns of 32 cm in length and 1.7 cm in inner diameter were used for fixed-bed research (Figure 4). Glass beads were added to the top of the Fe3O4/Bent bed to guarantee that the effluent flow was dispersed uniformly when it entered the fixed bed. Glass wool and plastic mesh were added to the bottom of the column to stop the adsorbent from being lost throughout the process. A peristaltic pump was used to feed the influent at the top of the column and run it in a downflow mode. On the shape of the breakthrough curve and breakthrough capacity, the effects of beginning concentration (200–1000 mg/L), flow rate (3–3.8 mL/min), and bed height (2–4 cm) were investigated. At prearranged intervals, samples were taken at the bottom of the column. Before being analyzed, the collected effluent was preserved by acidification with 1 N HNO3, filtered through a 0.45 µm Whatman filter, and stored at 8 °C. A chemical oxygen demand device (COD) (type Lovibond ET 108/Germany) was used to measure the remaining concentration of gasoline emulsion.
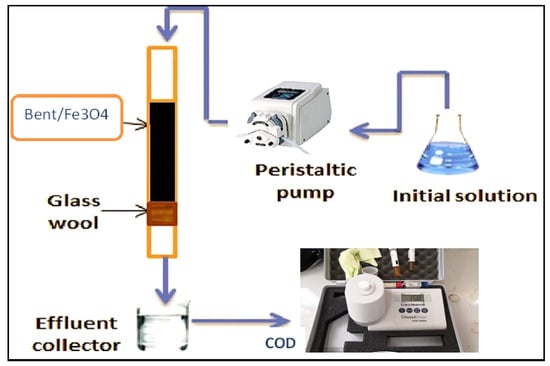
Figure 4.
Fixed-bed adsorption.
2.6. Fixed-Bed Data Analysis
The shape of the breakthrough curve indicates the performance of the fixed-bed experiment. Plotting Ct/C0 against time represents the breakthrough curve. The initial gasoline emulsion concentration (C0) and the effluent concentration at any given time (Ct) are denoted by the relevant variables. The treated effluent concentration at 90% and 10% of the original concentration, respectively, was used to define the exhaustion and breakthrough time [60,61].
By applying the given concentration (Ct) and flow rate (Q) to the fixed-bed column, the total amount of adsorbed gasoline (qtotal, mg) was determined using Equation (1) [62,63].
where Q is the volumetric flow rate (mL/min), and C0 (mg/L) and Ct (mg/L) refer to the initial gasoline emulsion concentration and effluent concentration at any time t, respectively.
By applying Equation (2), we were able to calculate the equilibrium adsorption capacity (qeq, mg/g) of the gasoline emulsion at the point of exhaustion for each unit dry weight of Fe3O4/Bent added to the bed column [64].
where m (g) is the total mass of Fe3O4/Bent loaded to the glass column.
The adsorption zone length, which represents the adsorbent efficiency in the fixed bed, was determined using Equation (3), which is also known as the mass transfer zone (LMTZ) length [65].
where L is the bed height (cm), tb is the breakthrough time (min), and te is the exhaustion time (min).
2.7. Fixed-Bed Adsorption Model
Kinetic models are used to represent the experimental data of gasoline emulsion adsorption under fixed-bed circumstances. The Yoon–Nelson and Thomas models are used to predict the breakthrough curves. The model parameters were estimated in the nonlinear identification procedure using the experimental data and the least-square method to fit the breakthrough curves [66,67]. The Thomas model is the most general and widely used theoretical model for evaluating column performance and forecasting the concentration–time profile of the complete breakthrough curve. It is assumed that adsorption is governed by mass transfer at the interface rather than chemical interactions between molecules. The model is based on experimental data obtained using the Langmuir adsorption isotherm model with no axial dispersion and the second-order reversible kinetic model [68,69]. Equation (4) provides the Thomas model’s nonlinear form.
where KTh is the constant of the kinetic sorption rate for the Thomas model (mL/mg. min). qTh is the maximum sorption capacity that is estimated via the Thomas model (mg/g), t is the time (min), and m is the mass of the adsorbent (g). Thomas parameters (KTh and qTh) are determined employing the nonlinear regression via drawing (Ct/C0) vs. time.
On the other hand, the Yoon–Nelson kinetic model is employed due to its simplicity, flexibility, and practicality, making it an appropriate choice for predicting the breakthrough curves in fixed-bed adsorption systems. Furthermore, it assumes that the decline in the adsorption rate for each adsorbate molecule is proportional to the probability of adsorption and breakthrough adsorption on the adsorbent. Moreover, it does not require any detailed data related to the characteristics of the adsorbent or the adsorbate’s characteristics, such as the adsorption isotherm or the mass transfer properties, and also about the physical properties of the adsorption bed [70]. In addition, the Yoon–Nelson model simplifies the prediction process of the breakthrough curves by requiring only two critical parameters: the time necessary for a 50% breakthrough (t50) and the rate constant (KYN). This simplification would allow for easy curve prediction when the adsorbent becomes saturated, and the containment adsorbate would begin to emerge in the effluent [71]. Equation (5) depicts the Yoon–Nelson model’s nonlinear form.
In this case, τ is the amount of time in minutes required for a 50% adsorbate breakthrough, t is the sampling time in minutes, and KYN is the rate constant (min−1). The Yoon model constants (KYN, τ) can be acquired by plotting Ct/C0 versus time (t).
3. Results and Discussion
3.1. Characterization of Fe3O4/Bent
Bentonite’s surface morphology (Figure 5a) was coarse and comprised a variety of irregularly shaped aggregates, the majority of which were spherical and had several tiny pores and voids [72]. This demonstrates that bentonite is an excellent support material for coating nanometric particles because of these properties, which raise the likelihood of foreign molecules adhering to its surface. As illustrated in Figure 5b, bentonite’s surface underwent notable alterations following the application of Fe3O4 magnetite nanoparticles. It was remarked that more big channels and pores formed on the brighter Fe3O4/Bent surface. In the SEM of Fe3O4/Bent nanocomposites, it was noticed that minuscule particles were as small as a few nm in diameter. This suggests that the Fe3O4 magnetite nanoparticle coating on bentonite was successful.
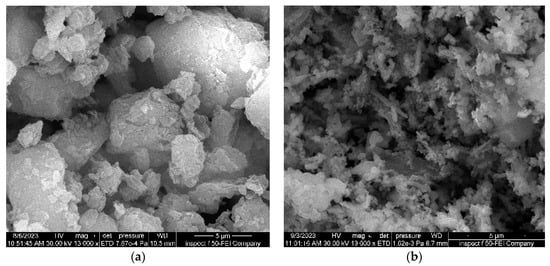
Figure 5.
SEM micrographs: (a) raw bentonite, (b) Fe3O4/Bent.
Table 1 presents the results which indicate a significant increase in specific surface area (from 17.57 to 81.75 m2·g−1) and total pore volume (from 0.029 to 0.057 cm3·g−1) resulting from modified bentonite with magnetic iron nanoparticles. Fe3O4 nanoparticles can overlap between the layers of bentonite, causing an increase in the interlayer spacing. This expands the structure and increases the overall surface area. Fe3O4 nanoparticles can also coat the external surfaces of bentonite, contributing to an increased surface area by providing additional surface sites for interaction. Incorporating Fe3O4 nanoparticles creates new pores and voids within the bentonite structure. These additional pores enhance the overall porosity, increasing the available surface area for adsorption. The nanoparticles create a rougher and more complex surface topology on bentonite. This roughness provides more active sites for adsorption and interaction, thereby increasing the BET surface area.

Table 1.
Structure properties of raw Bent and Fe3O4/Bent.
An essential characterization parameter, FTIR, reveals the structural changes in bentonite concerning important functional groups. According to Al-Essa [73], the bands observed at 3417 and 1651 cm−1 in Figure 6 of the RB spectra resulted from the vibrations generated by the hydration molecules’ (OH groups’) stretching and bending on the bentonite’s surface. The stretching vibration of H2O is accompanied by a wide band at 3533.59 cm−1 when water is adsorbed. According to Anirudhan and Ramachandran [74], the wide band at 3618.46 cm−1 is caused by the stretching vibration of the hydroxyl (O-H) bond on the surface of the Si-OH (silanol) group of RB. The strong absorption of the band at 1033 cm−1 for stretching vibration Si-O bands provides strong evidence of a silicate structure. The Al-O-Si and Si-O-Si bending vibrations can be seen as two distinct bands at 524 and 470 cm−1. According to Luangklang and Rangsriwatananon [75], the band at 524 cm−1 is the most sensitive in octahedral sheets because any remaining Al+3 species may cause it. The spectra include lines at 690 and 798 cm−1, which identify quartz and cristobalite, respectively [76,77]. In addition, Eisazadeh et al. [78] reported the detection and reporting of bands at 918 cm−1, which are almost 913 cm−1, linked to the bending vibrations of Al-Al-OH. The detection of bending vibrations in Al-Mg-OH was achieved at 840 cm−1, which is close to the wavelength of 830 cm−1 [79].
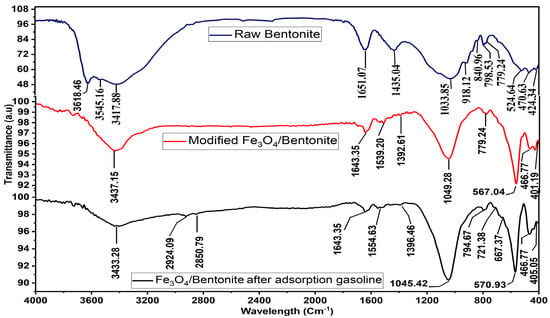
Figure 6.
FTIR for raw bentonite, modified Fe3O4/Bent, and Fe3O4/Bent after adsorption gasoline.
The FTIR spectrum presented in Figure 6 also shows the functional groups on the surface of Fe3O4/Bent. The peaks observed between 500 cm−1 and 600 cm−1 are related to the presence of the Fe-O bond [80]. The absorption peak at approximately 567 cm−1 can be ascribed to the Fe-O vibrational mode of Fe3O4 nanoparticles [54]. The absorption peaks at 1643.35 cm−1 approximately correspond to the stretching vibration of -FeOO- [81]. Also, Fe3O4/Bent peaks show a slight shift and increase the intensity in some absorption peak positions. For instance, there is an absorption peak at 1033.85 cm−1 in the case of raw bentonite corresponding to the Si-O group, but this peak is shifted to 1049.28 cm−1 in the case of Fe3O4/Bent. This change could be due to the interaction of the magnetite nanoparticles with the clay sheets [82].
According to the results of the Energy-Dispersive X-ray (EDX) examination (Table 2), the primary constituents of the bentonite sample are aluminum (Al), silicon (Si), and oxygen (O), all of which are basic elements of the smectite group. Following the Fe3O4 alteration, Fe3O4/Bent NC production is validated using EDS. The EDS findings for the Fe3O4/Bent NC confirm the presence of iron (Fe), oxygen (O), carbon (C), silicon (Si), and aluminum (Al) components.

Table 2.
Elemental compositions of raw bentonite and Fe3O4/Bent.
The structural alterations and layer spacing of natural bentonite before and after Fe3O4 NC modification were investigated using XRD analysis. The expansion of bentonite is principally caused by its hydrated structure, in which cations sit within the interlayer gap, surrounded by absorbed water molecules. Figure 7 shows the diffractograms for both RB and Fe3O4/Bent. Montmorillonite is the major component of raw bentonite, with quartz, palygorskite, and calcite also present. Different diffraction peaks were seen at different angles, showing the following composition: montmorillonite (19.8, 25.23, 35.17, and 62 degrees), quartz (20.8 and 26.5 degrees), and calcite (29.32 and 50 degrees) [83].
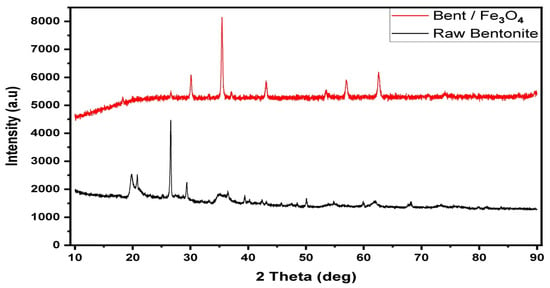
Figure 7.
XRD analysis of raw bentonite and Fe3O4/Bent.
After loading bentonite with Fe3O4 NPs, the XRD pattern shows obvious peaks at 2θ = 18.26, which are for bentonite, while the absorption peaks at 2θ = 30.07°, 35.43°, 43.07°, 53.44°, 56.96°, and 62.54° are for Fe3O4NPs [84]. Furthermore, the decrease in peak intensity suggests that the crystallinity decreased in the montmorillonite layers, which could be related to the intercalation of Fe3O4 [85]. This result indicated that the iron molecules were successfully intercalated into bentonite.
3.2. Effect of Bed Height
The gasoline emulsion solutions were allowed to pass through the adsorption column at varying bed heights (2, 3, and 4 cm) with a constant flow rate of 3.4 mL/min and an initial influent concentration of 200 mg/L to investigate the impact of bed height. The time consumed to start the breakthrough and the components emerging from the bed’s outlet would rise with a higher bed height, as illustrated in Figure 8. The fixed bed quickly becomes exhausted at lower bed heights due to the breakthrough curve’s sharpening. A typical S-shaped profile appears in the curves, indicating an optimal adsorption system [86]. Table 3 indicates that increasing the bed heights would lead to elevated qe values. A longer bed of 4 cm denotes a higher amount of adsorbent loaded in the fixed bed, indicating a greater number of binding sites accessible for adsorption in the process of removing gasoline emulsion. Furthermore, longer influent solution residence time in the Fe3O4/Bent bed would result from a longer bed of 4 cm. In other words, increasing the bed height results in increasing the contact time between the emulsion and the adsorbent particles. This allows gasoline ions to permeate into the adsorbent bed, facilitating the overall adsorption. It was noticed that the length of the MTZ was increased as the bed’s height was raised from 2 to 4 cm.
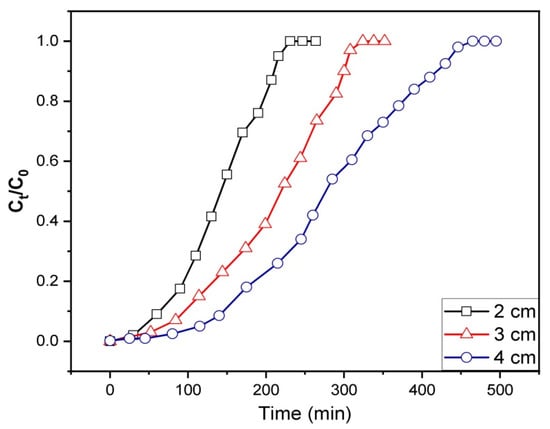
Figure 8.
The impact of bed height on the breakthrough capability of a fixed-bed containing Fe3O4/Bent for the elimination of gasoline.

Table 3.
The breakthrough analysis parameters for Fe3O4/Bent in the fixed bed.
3.3. Effect of Flow Rate
One of the most important factors in determining how long the solute remains in contact with the adsorbent surface is the flow rate. Figure 9 shows the gasoline breakthrough curves on Fe3O4/Bent at a range of flow rates from 3 to 3.8 mL/min, with a fixed starting concentration of 200 mg/L and a bed height of 3 cm. Increasing the flow rate from 3 mL/min to 3.8 mL/min accelerated the starting of breakthrough and exhaustion, according to the data. A decrease in the flow rate was found to cause a rightward shift of the breakthrough curves. However, the breakthrough curves grew steeper as the flow rates increased. Data such as the column’s adsorption capacity at exhaustion (qe) and MTZ length are presented in Table 3. The value of qe increased as the flow rate was reduced from 3.8 to 3 mL/min, as shown in Table 3, due to increasing the contact time between the adsorptive materials (adsorbate/adsorbent), leading to increasing the adsorption capacity. At the same time, when the flow rate was low, the MTZ values were low as well, which meant that the MTZ was becoming shorter. Because the MTZ travels more slowly over the fixed bed at a low flow rate, adsorption takes longer to occur, and the fixed bed takes longer to reach breakthrough and exhaustion [19]. Conversely, low qe values would be the consequence of a short duration of time for the gasoline emulsion to diffuse from the solute onto the binding sites on the surface and pores of the Fe3O4/Bent due to rapid flow rates.
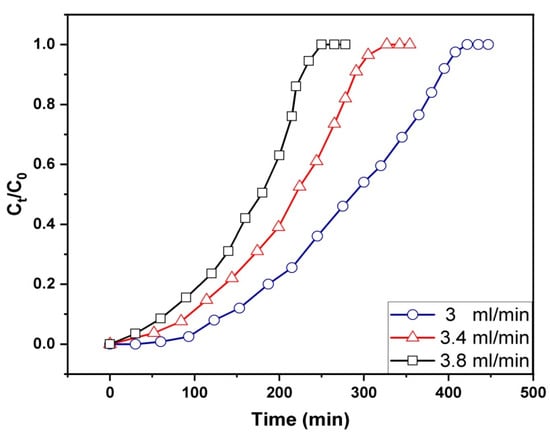
Figure 9.
Effect of flow rate on breakthrough capacity of fixed-bed Fe3O4/Bent for removal of gasoline.
3.4. Effect of Initial Concentration
The gasoline emulsion breakthrough curves under various starting concentrations are shown in Figure 10. The curves show a constant flow rate of 3.4 mL/min and a bed height of 4 cm. The initial concentration was increased from 200 to 1000 mg/L, and a decrease in the time to breakthrough and exhaustion was noted. The breakthrough curves became more scattered, and the time to breakthrough was longer at low initial concentrations. The breakthrough curves steepened as the starting concentration was raised to 1000 mg/L, indicating the rapid saturation of the fixed bed. Table 3 shows that when the initial gasoline concentration was raised, qe values were seen to rise due to introducing more adsorbate molecules to the bed that can be adsorbed on the adsorbent’s surface, giving an increase in the adsorbed amount. As the starting concentration was lowered from 1000 mg/L to 200 mg/L, the value of the MTZ also decreased. The concentration gradient, or the difference in emulsion concentration on the Fe3O4/Bent surface and gasoline concentration in the solution, is the primary driving factor behind adsorption [70]. As a rule of thumb, increasing the concentration difference leads to higher adsorption rates. It also improves the gasoline uptake onto Fe3O4/Bent, as it provides a stronger driving force to overcome the mass transfer resistance. This explains that the higher the starting concentrations, the higher the qe values that can be obtained.
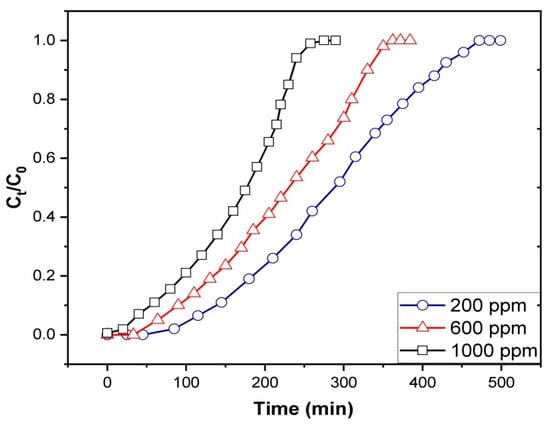
Figure 10.
Effect of initial concentration on breakthrough capacity of fixed-bed Fe3O4/Bent for gasoline removal.
3.5. Breakthrough Curve Modeling
The Thomas and Yoon–Nelson models are two examples of the various kinetic column models that must be employed to assess and predict the breakthrough curves in order to build a fixed-bed adsorption system, particularly if it would be used for an industrial scale. The Thomas model is frequently used to determine the adsorption capacity of the adsorbent. The rate constant and adsorption capacity were obtained from the nonlinear regression plot of Equation (4), and numerical values are presented in Table 4. The breakthrough curves may be predicted by the Thomas model, as evidenced by the high values of the coefficient of determination (R2). High R2 values also suggest a good match between the theoretical nonlinear data produced by the Thomas model and the experimental data. Figure 11a–c, which show a strong agreement between the experimental data and the theoretical nonlinear plot from the Thomas model with manipulating flow rate, bed height, and beginning concentration, validate this. All things considered, the Thomas model can adequately describe the gasoline emulsion adsorption on Fe3O4/Bent in a fixed-bed system. The findings indicate that while mass transfer resistance is reduced at greater flow rates, KTh values rise. In the meantime, a high flow rate was seen in the values of qTh, which is explained by the insufficient amount of time that gasoline molecules spend in contact with binding sites on the surface of Fe3O4/Bent. The driving force of adsorption, a better concentration gradient, was seen when the starting concentration was raised from 200 to 1000 mg/L, which resulted in an increase in qTh values [87]. Meanwhile, because of the impact of steric crowding, the KTh values were lower at high starting concentrations. On the other hand, using nonlinear regression Equation (5), the Yoon–Nelson model constants R2, qYN, and KYN were determined. As the flow rate increased, KYN increased from 0.015 to 0.025, as seen in Table 4. The shorter solute residence time in the column was the reason for this disparity. The time required to obtain a 50% breakthrough increased from 142.16 to 279.05 min when the bed length was raised, whereas KYN reduced from 0.029 to 0.016 min. There were more adsorption sites accessible at deeper bed depths. The time needed for a 50% breakthrough time (τ) dropped from 279.05 to 172.75 when the initial concentration of gasoline emulsion was increased. In contrast, KYN increased from 0.016 to 0.022 as a result of the greater gasoline concentration acting as a driving force for adsorption. The Thomas and Yoon–Nelson models were found to be a good fit for describing the breakthrough behavior of the gasoline emulsion, based on the R2 value (R2 > 0.95) [88].

Table 4.
Data from fixed beds with “Thomas and Yoon–Nelson models” characteristics for the adsorption of gasoline under various conditions.
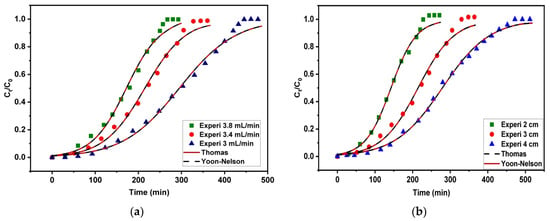
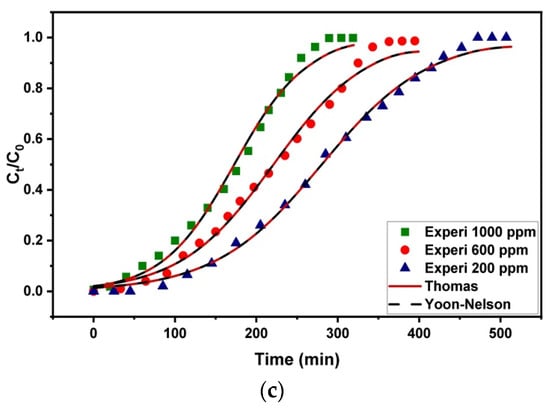
Figure 11.
Thomas and Yoon–Nelson modeling results based on experimental and theoretical breakthrough curves of gasoline adsorption on Fe3O4/Bent at various (a) flow rates, (b) bed heights, and (c) initial concentrations.
3.6. Adsorption Mechanism
The FTIR spectra verified the chemisorption nature of the adsorption process, as illustrated in Figure 6 by the appearance of new peaks at (2924.09 cm−1 and 2850.79 cm−1). The presence of these peaks denotes a C-H bond, which indicates that the adsorbents and oil molecules are interacting chemically. The sharpening of the Si-OH, O-H, and Si-O-Si peak intensities, which increased, further suggests that the nanocomposite and gasoline oil interact strongly. Furthermore, because bentonite and iron oxide are hydrophobic, the nanocomposite may interact hydrophobically with oil and absorb oil molecules [89,90]. Most of the oil that is adsorbed on the Fe3O4/Bent NC does so via interactions that are hydrophobic and chemically bound. The results show that the composite material is an effective adsorbent for cleaning greasy water, which is a promising development. Moreover, changes in pH have a considerable impact on surface charge, adsorbent stability, and pollutant structure [91]. The effect of emulsified gasoline pH on the adsorption capacity of Fe3O4/Bent was examined using batch process testing with varying the pH from 3.0 to 9.0 at a specified adsorbent dose, initial gasoline concentration, and contact time. The removal of gasoline emulsion increased as pH increased, reaching a peak at pH = 6, which corresponds to the neutral pH of the gasoline emulsion. As a result, removal was reduced, reaching its lowest proportion at pH 9. In general, removal percentages were lower in acidic or alkaline conditions compared to neutral ones. This pattern may be explained by the electrostatic attraction between gasoline droplets and Fe3O4/Bent, which occurs when surface functional groups partially dissociate in acidic or alkaline emulsified gasoline solutions.
4. Conclusions
In this work, gasoline was adsorbed out of a water solution using a fixed-bed method. The effects of several column factors on the fixed-bed performance were verified, including initial concentration, bed height, and flow rate. It was found that low beginning concentrations, declining flow rate, and rising bed height all contributed to longer breakthrough and exhaustion times. Under conditions of reduced bed height, elevated start concentrations, and accelerated flow velocity, breakthrough curves became more pointed. The breakthrough curves showed that gasoline adsorption on Fe3O4/Bent was the most efficient at a flow rate of 3.4 mL/min, an influent gasoline concentration of 1000 mg/L, and a 4 cm bed height. The highest amount of gasoline adsorbed onto the Fe3O4/Bent material’s surface was 269.46 mg/g of adsorbent. For the purpose of defining breakthrough curve models, kinetic models like the Thomas and Yoon–Nelson models were employed. The Thomas and Yoon–Nelson models provided an adequate fit for the breakthrough curves. The nonlinear plots produced by the Thomas and Yoon–Nelson models from the experimental data showed a good degree of agreement. Overall, the findings demonstrate the ability of Fe3O4/Bent to extract the emulsion from aqueous solution in a laboratory setting when used in fixed-bed settings. Prior to being implemented in the industry, more research on the fixed-bed system’s design parameters on a pilot scale is advised. The current work presents a straightforward synthetic method for creating a Fe3O4/Bent composite material that may be used to extract gasoline from industrial wastewater.
Author Contributions
Conceptualization, M.A.S. (Mohammed A. Sarran), A.A.A., M.F.A., K.T.R. and M.A.S. (Mohammed Ahmed Shehab); Methodology, M.A.S. (Mohammed A. Sarran), A.A.A. and M.F.A.; Validation, A.D.J.A.-B. and K.T.R.; Formal analysis, A.A.A., M.F.A., A.D.J.A.-B., K.T.R., M.A.S. (Mohammed Ahmed Shehab), H.H.M., S.A., M.A. (Mahmood Alhafadhi) and M.A. (Mohammed Alktranee); Investigation, M.A.S. (Mohammed A. Sarran), A.A.A., M.A.S. (Mohammed Ahmed Shehab), H.H.M. and M.A. (Mahmood Alhafadhi); Writing—original draft, M.A.S. (Mohammed A. Sarran), A.A.A. and M.F.A.; Writing—review & editing, M.A.S. (Mohammed A. Sarran); Visualization, A.A.A., K.T.R., M.A.S. (Mohammed Ahmed Shehab), H.H.M., S.A. and M.A. (Mohammed Alktranee); Supervision, A.A.A. and M.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
The authors are grateful to the Chemical Engineering Department, University of Technology, Baghdad, Iraq, and Al-Mustaqbal University, Hilla, Iraq, for providing space and facilities to conduct this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chang, S.E.; Stone, J.; Demes, K.; Piscitelli, M. Consequences of oil spills: A review and framework for informing planning. Ecol. Soc. 2014, 19, 25. [Google Scholar] [CrossRef]
- Brody, T.M.; Di Bianca, P.; Krysa, J. Analysis of inland crude oil spill threats, vulnerabilities, and emergency response in the midwest United States. Risk Anal. Int. J. 2012, 32, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Kolokoussis, P.; Karathanassi, V. Oil spill detection and mapping using sentinel 2 imagery. J. Mar. Sci. Eng. 2018, 6, 4. [Google Scholar] [CrossRef]
- Ghadhban, M.Y.; Rashid, K.T.; AbdulRazak, A.A.; Alsalhy, Q.F. Recent progress and future directions of membranes green polymers for oily wastewater treatment. Water Sci. Technol. 2023, 87, 57–82. [Google Scholar] [CrossRef]
- Fingas, M. Oil spill dispersants: A technical summary. In Oil Spill Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 435–582. [Google Scholar] [CrossRef]
- Zekri, A.Y.; Chaalal, O. Effect of temperature on biodegradation of crude oil. Energy Sources 2005, 27, 233–244. [Google Scholar] [CrossRef]
- Cisterna-Osorio, P.; Arancibia-Avila, P. Comparison of biodegradation of fats and oils by activated sludge on experimental and real scales. Water 2019, 11, 1286. [Google Scholar] [CrossRef]
- Sherhan, B.Y.; Abbas, A.D.; Alsalhy, Q.F.; Rashad, A.A.; Rashad, Z.W.; Shawkat, A.A.; Abbas, T.K.; Kareem, N.A.A. Produced water treatment using ultrafiltration and nanofiltration membranes. Al-Khwarizmi Eng. J. 2016, 12, 10–18. [Google Scholar] [CrossRef]
- Samal, K.; Geed, S.R.; Mohanty, K. Hybrid biological processes for the treatment of oily wastewater. In Advances in Oil-Water Separation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 423–435. [Google Scholar] [CrossRef]
- Alsulaili, A.D.; Fahim, A.M. Oil removal from produced water by agriculture waste adsorbents. Int. J. Environ. Waste Manag. 2020, 25, 12–31. [Google Scholar] [CrossRef]
- Azeez, R.A. Removal oil from produced water by using adsorption method with adsorbent a Papyrus reeds. Eng. Technol. J. 2019, 37, 157–165. [Google Scholar] [CrossRef]
- El-Din, G.A.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the use of banana peels for oil spill removal. Alexandria Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Piemonte, V.; Prisciandaro, M.; Mascis, L.; Di Paola, L.; Barba, D. Reverse osmosis membranes for treatment of produced water: A process analysis. Desalination Water Treat. 2015, 55, 565–574. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Abdul Karim, Z.; Ismail, A.F. Thin film composite membrane for oily waste water treatment: Recent advances and challenges. Membranes 2018, 8, 86. [Google Scholar] [CrossRef]
- Sellami, M.; Loudiyi, K.; Bellemharbet, K.; Djabbour, N. Electro-Coagulation Treatment and De-oiling of Wastewaters Arising from Petroleum Industries. J. Pet. Environ. Biotechnol. 2016, 7, 1000290. [Google Scholar] [CrossRef]
- Xie, A.; Ladner, D.A.; Popat, S.C. Electrocoagulation-electroflotation for primary treatment of animal rendering wastewater to enable recovery of fats. Chem. Eng. J. 2022, 431, 133910. [Google Scholar] [CrossRef]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Álvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef]
- Maddah, Z.H.; Naife, T.M. Demulsification of water in Iraqi crude oil emulsion. J. Eng. 2019, 25, 37–46. [Google Scholar] [CrossRef]
- Ahmed, F.S.; Alsaffar, M.A.; AbdulRazak, A.A. One-step synthesis of magnetic fly ash composites for methylene blue removal: Batch and column study. Environ. Sci. Pollut. Res. 2022, 30, 124748–124766. [Google Scholar] [CrossRef]
- Ghasemi, A.; Ghasemi, Z. Application of SD/MNP/PEI Nanocomposite for Heavy Metals Sorption. Chem. Chem. Technol. 2023, 17, 878–886. [Google Scholar] [CrossRef]
- Khalaf, I.H.; Al-Sudani, F.T.; AbdulRazak, A.A.; Aldahri, T.; Rohani, S. Optimization of Congo red dye adsorption from wastewater by a modified commercial zeolite catalyst using response surface modeling approach. Water Sci. Technol. 2021, 83, 1369–1383. [Google Scholar] [CrossRef]
- Sarran, M.A.; AbdulRazak, A.A.; Abid, M.F.; Jawad, A.D. Oily wastewater treatment using low-cost and highly efficient natural and activated Iraqi bentonite. Desalin. Water Treat. 2024, 319, 100412. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mondal, S.; De, S. Design and scaling up of fixed bed adsorption columns for lead removal by treated laterite. J. Clean. Prod. 2018, 177, 760–774. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wilson, L.D. Phosphate uptake studies of cross-linked chitosan bead materials. J. Colloid Interface Sci. 2017, 485, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, V.; Bosco, G.E.; Fadini, P.S.; Mozeto, A.A.; Cestari, A.R.; Carvalho, W.A. Use of a La (III)-modified bentonite for effective phosphate removal from aqueous media. J. Hazard. Mater. 2014, 274, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, P.; Ning, P.; Su, Y. Enhanced adsorption removal of phosphate from water by mixed lanthanum/aluminum pillared montmorillonite. Chem. Eng. J. 2009, 151, 141–148. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, Y.; Wu, X.; Fan, P.; Song, R. La (III)-bentonite/chitosan composite: A new type adsorbent for rapid removal of phosphate from water bodies. Appl. Clay Sci. 2020, 190, 105547. [Google Scholar] [CrossRef]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+ & Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Nguyen, T.-B.; Dat, N.D.; Huu, B.T.; Nguyen, X.-C.; Tran, T.; Bui, M.-H.; Dong, C.-D.; Bui, X.-T. Adsorption of norfloxacin from aqueous solution on biochar derived from spent coffee ground: Master variables and response surface method optimized adsorption process. Chemosphere 2022, 288, 132577. [Google Scholar] [CrossRef]
- Jeon, C. Removal of Cr (VI) from aqueous solution using amine-impregnated crab shells in the batch process. J. Ind. Eng. Chem. 2019, 77, 111–117. [Google Scholar] [CrossRef]
- Yang, A.; Yang, S.; Zhu, Y. Magnetic modification of used tea leaves for uranium adsorption. New Carbon Mater. 2021, 36, 821–826. [Google Scholar] [CrossRef]
- Sabir, S. Approach of cost-effective adsorbents for oil removal from oily water. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1916–1945. [Google Scholar] [CrossRef]
- Ewis, D.; Benamor, A.; Ba-Abbad, M.M.; Nasser, M.; El-Naas, M.; Qiblawey, H. Removal of oil content from oil-water emulsions using iron oxide/bentonite nano adsorbents. J. Water Process Eng. 2020, 38, 101583. [Google Scholar] [CrossRef]
- Ewis, D.; Mahmud, N.; Benamor, A.; Ba-Abbad, M.M.; Nasser, M.; El-Naas, M. Enhanced Removal of Diesel Oil Using New Magnetic Bentonite-Based Adsorbents Combined with Different Carbon Sources. Water Air Soil Pollut. 2022, 233, 195. [Google Scholar] [CrossRef]
- Rodrigues, S.C.G.; Rodrigues, M.G.F.; Pereira, K.R.O.; Valezuela-Diaz, F.R. Performance of organophilic clay as adsorbent in the oil/water separation process. J. Pet. Gas 2010, 4. (In Brazilian) [Google Scholar] [CrossRef]
- de, M.; Ferreira, R.; Ribeiro, B.D.; Stapelfeldt, D.M.A.; de FR Moreira, M. Treatment of Water Contaminated by Ship Oil: Study of Adsorption in a Fixed-Bed Column. Analytica 2024, 5, 203–218. [Google Scholar]
- Shahnaz, T.; Patra, C.; Sharma, V.; Selvaraju, N. A comparative study of raw, acid-modified and EDTA-complexed Acacia auriculiformis biomass for the removal of hexavalent chromium. Chem. Ecol. 2020, 36, 360–381. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Majid, Z.; AbdulRazak, A.A.; Noori, W.A.H. Modification of zeolite by magnetic nanoparticles for organic dye removal. Arab. J. Sci. Eng. 2019, 44, 5457–5474. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Taubert, A. Clay–polymer nanocomposites (CPNs): Adsorbents of the future for water treatment. Appl. Clay Sci. 2014, 99, 83–92. [Google Scholar] [CrossRef]
- Mo, W.; He, Q.; Su, X.; Ma, S.; Feng, J.; He, Z. Preparation and characterization of a granular bentonite composite adsorbent and its application for Pb2+ adsorption. Appl. Clay Sci. 2018, 159, 68–73. [Google Scholar] [CrossRef]
- Pandey, S. A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment. J. Mol. Liq. 2017, 241, 1091–1113. [Google Scholar] [CrossRef]
- Sharma, V.; Shahnaz, T.; Subbiah, S.; Narayanasamy, S. New insights into the remediation of water pollutants using nanobentonite incorporated nanocellulose chitosan based aerogel. J. Polym. Environ. 2020, 28, 2008–2019. [Google Scholar] [CrossRef]
- Shahnaz, T.; Sharma, V.; Subbiah, S.; Narayanasamy, S. Multivariate optimisation of Cr (VI), Co (III) and Cu (II) adsorption onto nanobentonite incorporated nanocellulose/chitosan aerogel using response surface methodology. J. Water Process Eng. 2020, 36, 101283. [Google Scholar] [CrossRef]
- Asif, M.; Boumaza, M.M.; Kumar, N.S.; Al-Ghurabi, E.H.; Shahabuddin, M. Adsorptive Removal of Emulsified Automobile Fuel from Aqueous Solution. Separations 2023, 10, 493. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Liu, H. Oil adsorption and reuse performance of multi-walled carbon nanotubes. Procedia Eng. 2015, 102, 1896–1902. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Damasceno, B.S.; da Silva, A.F.V.; de Araújo, A.C.V. Dye adsorption onto magnetic and superparamagnetic Fe3O4 nanoparticles: A detailed comparative study. J. Environ. Chem. Eng. 2020, 8, 103994. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.; Hu, L.; Li, Y.; Luo, D.; Mei, S. Recent applications of magnetic composites as extraction adsorbents for determination of environmental pollutants. TrAC Trends Anal. Chem. 2019, 119, 115611. [Google Scholar] [CrossRef]
- Bassim, S.; Mageed, A.K.; AbdulRazak, A.A.; Majdi, H.S. Green synthesis of Fe3O4 nanoparticles and its applications in wastewater treatment. Inorganics 2022, 10, 260. [Google Scholar] [CrossRef]
- Hashemian, S.; Saffari, H.; Ragabion, S. Adsorption of cobalt (II) from aqueous solutions by Fe3O4/bentonite nanocomposite. Water Air Soil Pollut. 2015, 226, 2212. [Google Scholar] [CrossRef]
- Yan, L.; Li, S.; Yu, H.; Shan, R.; Du, B.; Liu, T. Facile solvothermal synthesis of Fe3O4/bentonite for efficient removal of heavy metals from aqueous solution. Powder Technol. 2016, 301, 632–640. [Google Scholar] [CrossRef]
- Křišťan, P.; Chlan, V.; Štěpánková, H.; Řezníček, R.; Kouřil, K.; Štěpánek, J.; Poláková, K.; Procházka, V.; Čuda, J.; Medřík, I. Bentonite/iron oxide composites: Preparation and characterization by hyperfine methods. J. Nanomater. 2013, 2013, 179794. [Google Scholar] [CrossRef]
- Lou, Z.; Zhou, Z.; Zhang, W.; Zhang, X.; Hu, X.; Liu, P.; Zhang, H. Magnetized bentonite by Fe3O4 nanoparticles treated as adsorbent for methylene blue removal from aqueous solution: Synthesis, characterization, mechanism, kinetics and regeneration. J. Taiwan Inst. Chem. Eng. 2015, 49, 199–205. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. Part B Eng. 2019, 162, 538–568. [Google Scholar] [CrossRef]
- Negrea, A.; Mihailescu, M.; Mosoarca, G.; Ciopec, M.; Duteanu, N.; Negrea, P.; Minzatu, V. Estimation on fixed-bed column parameters of breakthrough behaviors for gold recovery by adsorption onto modified/functionalized amberlite XAD7. Int. J. Environ. Res. Public Health 2020, 17, 6868. [Google Scholar] [CrossRef] [PubMed]
- Nyankson, E.; Adjasoo, J.; Efavi, J.K.; Amedalor, R.; Yaya, A.; Manu, G.P.; Asare, K.; Amartey, N.A. Characterization and evaluation of zeolite A/Fe3O4 nanocomposite as a potential adsorbent for removal of organic molecules from wastewater. J. Chem. 2019, 2019, 8090756. [Google Scholar] [CrossRef]
- Mohammadifard, A.; Allouss, D.; Vosoughi, M.; Dargahi, A.; Moharrami, A. Synthesis of magnetic Fe3O4/activated carbon prepared from banana peel (BPAC@ Fe3O4) and salvia seed (SSAC@ Fe3O4) and applications in the adsorption of Basic Blue 41 textile dye from aqueous solutions. Appl. Water Sci. 2022, 12, 88. [Google Scholar] [CrossRef]
- Bassim, S.; Mageed, A.K.; AbdulRazak, A.A.; Al-Sheikh, F. Photodegradation of methylene blue with aid of green synthesis of CuO/TiO2 nanoparticles from extract of citrus aurantium juice. Bull. Chem. React. Eng. Catal. 2023, 18, 1–16. [Google Scholar] [CrossRef]
- Mekonnen, D.T.; Alemayehu, E.; Lennartz, B. Fixed-bed column technique for the removal of phosphate from water using leftover coal. Materials 2021, 14, 5466. [Google Scholar] [CrossRef]
- Futalan, C.M.; Wan, M.-W. Fixed-bed adsorption of lead from aqueous solution using chitosan-coated bentonite. Int. J. Environ. Res. Public Health 2022, 19, 2597. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S. Highly efficient as-synthesized and oxidized multi-walled carbon nanotubes for copper (II) and zinc (II) ion adsorption in a batch and fixed-bed process. J. Mater. Res. Technol. 2021, 15, 2848–2872. [Google Scholar] [CrossRef]
- Lai, K.C.; Hiew, B.Y.Z.; Tee, W.T.; Thangalazhy-Gopakumar, S.; Gan, S.; Lee, L.Y. Usage of a new macro-hierarchical graphene sponge in batch adsorption and packed column configuration for efficient decontamination of cadmium in aqueous environment. J. Environ. Chem. Eng. 2021, 9, 106057. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Fagundes, J.L.S.; Georgin, J.; Salau, N.P.G.; Dotto, G.L. A mass transfer study considering intraparticle diffusion and axial dispersion for fixed-bed adsorption of crystal violet on pecan pericarp (Carya illinoensis). Chem. Eng. J. 2020, 397, 125423. [Google Scholar] [CrossRef]
- Patel, P.; Gupta, S.; Mondal, P. Modeling of continuous adsorption of greywater pollutants onto sawdust activated carbon bed integrated with sand column. J. Environ. Chem. Eng. 2022, 10, 107155. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Zhao, X.; Wang, Y.; Xie, F.; Cheng, J.; Tang, M. Adsorption of methylene blue by phoenix tree leaf powder in a fixed-bed column: Experiments and prediction of breakthrough curves. Desalination 2009, 245, 284–297. [Google Scholar] [CrossRef]
- Ghorbanian, S.; Davoudinejad, M.; Khakpay, A.; Radpour, S. Modeling breakthrough curves of citric acid adsorption onto anionic resins in an aqueous solution. J. Eng. 2015, 2015, 139041. [Google Scholar] [CrossRef]
- Ramirez, A.; Giraldo, S.; García-Nunez, J.; Flórez, E.; Acelas, N. Phosphate removal from water using a hybrid material in a fixed-bed column. J. Water Process Eng. 2018, 26, 131–137. [Google Scholar] [CrossRef]
- Jung, K.-W.; Jeong, T.-U.; Choi, J.-W.; Ahn, K.-H.; Lee, S.-H. Adsorption of phosphate from aqueous solution using electrochemically modified biochar calcium-alginate beads: Batch and fixed-bed column performance. Bioresour. Technol. 2017, 244, 23–32. [Google Scholar] [CrossRef]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Ye, Y.; Jiao, J.; Kang, D.; Jiang, W.; Kang, J.; Ngo, H.H.; Guo, W.; Liu, Y. The adsorption of phosphate using a magnesia–pullulan composite: Kinetics, equilibrium, and column tests. Environ. Sci. Pollut. Res. 2019, 26, 13299–13310. [Google Scholar] [CrossRef]
- Shabani, E.; Salimi, F.; Jahangiri, A. Removal of arsenic and copper from water solution using magnetic iron/bentonite nanoparticles (Fe3O4/bentonite). Silicon 2019, 11, 961–971. [Google Scholar] [CrossRef]
- Al-Essa, K. Activation of Jordanian bentonite by hydrochloric acid and its potential for olive mill wastewater enhanced treatment. J. Chem. 2018, 2018, 8385692. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process Saf. Environ. Prot. 2015, 95, 215–225. [Google Scholar] [CrossRef]
- Pluangklang, C.; Rangsriwatananon, K. Facile method by bentonite treated with heat and acid to enhance pesticide adsorption. Appl. Sci. 2021, 11, 5147. [Google Scholar] [CrossRef]
- Mekhamer, W.K. Energy storage through adsorption and desorption of water vapour in raw Saudi bentonite. Arab. J. Chem. 2016, 9, S264–S268. [Google Scholar] [CrossRef]
- Zhirong, L.; Uddin, M.A.; Zhanxue, S. FT-IR and XRD analysis of natural Na-bentonite and Cu (II)-loaded Na-bentonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1013–1016. [Google Scholar] [CrossRef]
- Eisazadeh, A.; Kassim, K.A.; Nur, H. Physicochemical characteristics of phosphoric acid stabilized bentonite. Electron. J. Geotech. Eng. 2010, 15, 327–336. [Google Scholar]
- AKÇAY, G.; Yurdakoc, M.K. Nonyl-and dodecylamines intercalated bentonite and illite from Turkey. J. Chem. 1999, 23, 105–114. (In Turkish) [Google Scholar]
- Khatamian, M.; Divband, B.; Shahi, R. Ultrasound assisted co-precipitation synthesis of Fe3O4/bentonite nanocomposite: Performance for nitrate, BOD and COD water treatment. J. Water Process Eng. 2019, 31, 100870. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses; Wiley-vch Weinheim: Weinheim, Germany, 2003; Volume 664. [Google Scholar] [CrossRef]
- Adisu, N.; Balakrishnan, S.; Tibebe, H. Synthesis and Characterization of Fe3O4-Bentonite Nanocomposite Adsorbent for Cr (VI) Removal from Water Solution. Int. J. Chem. Eng. 2022, 2022, 4441718. [Google Scholar] [CrossRef]
- Tasew, A.T. Physicochemical Characterization and Activation of Local Bentonite to Adsorb Dye and Chemical Oxygen Demand from the Effluent of a Textile Factory; Addis Ababa Science and Technology University: Addis Ababa, Ethiopia, 2020. [Google Scholar]
- Mahmud, N.; Nasser, M.S.; El-Naas, M.H.; Ba-Abbad, M.M.; Mohammad, A.W.; Mansour, S.; Benamor, A. Synthesis and characterization of Fe3O4 nanoparticles using different experimental methods. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kuala Lumpur, Malaysia, 30–31 October 2020; IOP Publishing: Bristol, UK, 2020; Volume 778, p. 12028. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Balkanloo, P.G.; Nozad, E. A facile method for dye and heavy metal elimination by pH sensitive acid activated montmorillonite/polyethersulfone nanocomposite membrane. J. Polym. Sci. 2018, 36, 49–57. (In Chinese) [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Ho, Y.-S. Removal of lead (II) ions from synthetic and real effluents using immobilized Pinus sylvestris sawdust: Adsorption on a fixed-bed column. J. Hazard. Mater. 2005, 123, 135–144. [Google Scholar] [CrossRef]
- Kumari, U.; Mishra, A.; Siddiqi, H.; Meikap, B.C. Effective defluoridation of industrial wastewater by using acid modified alumina in fixed-bed adsorption column: Experimental and breakthrough curves analysis. J. Clean. Prod. 2021, 279, 123645. [Google Scholar] [CrossRef]
- Hussein, F.B.; Mayer, B.K. Fixed-bed column study of phosphate adsorption using immobilized phosphate-binding protein. Chemosphere 2022, 295, 133908. [Google Scholar] [CrossRef]
- Yaghoubi, Z.; Basiri-Parsa, J. Modification of ultrafiltration membrane by thermo-responsive Bentonite-poly (N-isopropylacrylamide) nanocomposite to improve its antifouling properties. J. Water Process Eng. 2020, 34, 101067. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Deliv. Rev. 2020, 163, 65–83. [Google Scholar] [CrossRef]
- Xia, T.; Yan, N.; Li, S.; Lin, Y.; Su, T. Adsorption of tylosin and sulfamethazine by carbon nanotubes and titanium dioxide nanoparticles: pH-dependent mechanisms. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 581, 123851. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).