Abstract
The chemical industry, a cornerstone of the global economy essential for modern life, has raised significant concerns due to its unique nature. Chemical technologies often require high energy inputs, involving ecotoxic reagents thus assessing risks from an economic standpoint becomes complex. While the economic aspects of chemical technologies have been discussed and economic tools have been used to inform investment decisions in this field, many fundamental issues remain unexplored, such as the clear definition of chemical technology economics and the reasons for its importance. The primary contribution of this article is to synthesize insights into these fundamental issues and propose pathways for future research in chemical technology economics. This review is divided into two sections: the first provides an overview of the significance of economic factors in chemical technologies, and the second explores the fundamentals of economics and their application to chemical technology considerations. Our research underscores that economic theories significantly influence the profile of chemical technologies, viewing the chemical sector as a dual asset. First, the sector has a unique opportunity to lead the way in promoting sustainable economic development, and second, it can adopt economic behaviors that align with environmental and societal needs.
1. Introduction
The science of chemistry was envisioned as a solution to many of society’s needs, becoming a central science [1]. Consequently, chemistry has a long history of inventing essential and beneficial products and provides the chemical knowledge necessary for technological development, leading to unexpected discoveries that drive innovation. These innovations directly impact the chemical economy [2]. As a result, chemical industries are synonymous with economic growth. Since the first industrial revolution, particularly in Europe, the chemical industry has held a leading role in financial and social development [3]. The chemical industry contributes an astounding USD 5.7 trillion to the global economic output and supports 120 million jobs worldwide. The global chemical market doubled from 2000 to 2017 and is projected to double again by 2030 [4].
However, this technological progress has often been achieved with a narrow focus on function, overlooking adverse consequences [5]. The modern chemical sector faces significant threats to the ecological environment due to the large number of toxic and harmful substances it produces. Consequently, chemical industries can also be seen as the opposite of environmental and health solutions [6]. Many chemical products are designed for their intended use but rely on circumstantial controls to limit exposure to unassessed hazards, often due to a historical lack of tools and models [5]. While local issues caused by chemicals in the 1970s and 1980s have been addressed, several have evolved into global phenomena. These include greenhouse gas emissions, climate change, and micro- and nanoplastic pollution [1].
It is crucial to urgently transition the chemical industry and its entire value chain to safer and more sustainable chemistry and manufacturing practices. This necessity has driven the development of technologies aimed at mitigating environmental impact. Typical wastewater treatments generally involve a combination of physicochemical and biological processes [7]. Solids are separated from wastewater via physical processes utilizing screens and filters, while microorganisms in biological processes break down and remove harmful waste. Chemical methods are often integrated with physical techniques to tackle more complex contaminants effectively [8]. The final choice of treatment depends on whether high performance (quality and purity) or lower costs and high production levels are prioritized. Sustainable wastewater management must be considered from a multidisciplinary perspective, highlighting the need for economic analysis [9,10].
Green chemistry and chemical technologies offer both incremental and innovative solutions to technological challenges, providing demonstrable environmental and economic benefits. The concept of green chemistry aims to reinvent existing chemical processes to minimize environmental impact [11]. Sustainable technologies have been developed in various ways, including the application of the 12 principles of green chemistry [12], which have remained relevant for 25 years [13]. Although only principle 2 explicitly mentions the economy, all principles are influenced by cost implications. Cost can be a proxy for environmental friendliness under process design conditions (e.g., worker safety, energy usage, waste production, material usage). Evaluating the performance of wastewater treatment involves engineering, environmental, and economic (3E) factors [14], and the economic value of wastewater is often underexplored [15].
The assessment of chemical technologies revealed two principal factors: viewing chemical technologies as opportunities and viewing them as risks. Despite missteps along the way, we are now entering a decade of debate. Given the essential functions provided by chemical technologies, future considerations must include two goals: (i) maintaining and expanding advances in performance while (ii) demonstrating the economic returns of chemical technologies to support both scientific and economic aspirations. Addressing this is an urgent scientific challenge.
This study aims to investigate the core issues of chemical technologies economics and suggest directions for future research. The paper is organized as follows: Section 1 defines the field of chemical technologies economics, Section 2 explores its motivations, Section 3 outlines the main approaches used, Section 4 identifies open issues for future research, and Section 5 presents the conclusions drawn from the study.
1.1. The Concept of Chemical Technologies Economics (CTE)
Figure 1A depicts a scenario in which chemical technologies and economics operate entirely independently, with no consideration of their interrelationships in decision making. Figure 1B depicts the initial stage of the economics of chemical technologies. At this stage, the relationship between a chemical technology and its economic implications is roughly considered. For example, during research, development, and demonstration, the current approach provides factors that serve as a foundation for forecasting the economic prospects of the planned mature technology [16].
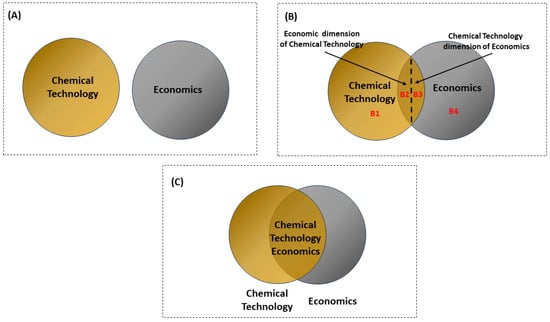
Figure 1.
The connections between chemical technology, economics, and the economics of chemical technology. (A) depicts a scenario in which chemical technologies and economics operate entirely independently. (B) depicts the initial stage of the economics of chemical technologies, when the relationship between a chemical technology and its economic implications is roughly considered. (C) illustrates the ideal scenario of integrating chemical technology and economics to establish a cohesive decision-making framework.
Figure 1B diagram is split into four sections (B1, B2, B3, and B4). Part B4 represents economics without considering chemical technologies, where chemical technology is not a factor. Part B1 denotes chemical technologies without considering economic aspects. The overlap of the two circles (Parts B2 and B3) signifies the interdependencies between chemical technologies and economics. At this stage, the chemical technology economy can be understood in two dimensions: the chemical technology dimension of economics (CTE) (e.g., technology parameters serving as a basis for forecasting economic prospects [8]) and the economic dimension of chemical technologies (ECT) (e.g., considering the chemical technology budget in decision-making).
Part B2 represents the CTE, where chemical technology issues (such as treatment facilities using technology based on purity goals, pollutant characteristics, and concentrations) are analyzed to achieve long-term profits and increased sustainability. Part B3 represents the ECT, where economic factors are integrated into the analysis of chemical technology to enhance the profitability of technology-adjacent decision-making.
Figure 1C illustrates the ideal scenario of integrating chemical technology and economics (B2) to establish a cohesive decision-making framework. This interdisciplinary approach influences stakeholders and economic entities involved in chemical technology, facilitating investment decisions aimed at enhancing profitability. It requires balancing costs and economic benefits from both the perspectives of CTE and ECT. A critical focus lies in modeling the interrelationships between economics and chemical technologies, highlighting their interconnected nature.
1.2. Chemical Industries and Economic Development
The growing chemical production in both developed and developing countries is expected to present significant growth opportunities for the chemical technology market. Chemical technology is utilized within the chemical industry to improve the production processes of various chemicals [17]. The global chemicals market is projected to experience strong growth in the coming years [18]. According to a report by Allied Market Research titled “Chemical Technology Market”, the market was valued at USD 1.4 billion in 2021 and is forecasted to reach USD 2.5 billion by 2031, growing at a compound annual growth rate (CAGR) of 6.2% from 2022 to 2031 [17]. Key economies like the U.S., Japan, China, and India rely heavily on a strong chemical industry, which spans sectors such as agrochemicals, pharmaceuticals, industrial solvents, food colorants, and specialty chemicals, among others. In the UK, the chemical and pharmaceutical industry contributes over £3 billion to the economy and employs more than 10,000 people. This underscores the global significance of the chemical industry as a crucial driver of economic growth [19]. According to Das and Icart [20], the chemical industry is essential to economic development and wealth creation. In the U.S., the chemical industry supports 788,000 jobs, contributes 25% to the GDP, and is a USD 760 billion enterprise (ACC, 2021) [21].
2. Chemical Technologies
The chemical process industries cover chemicals and related products, utilizing chemical production, processing, and equipment [22]. This encompasses sectors like pharmaceuticals, detergents, cosmetics, fertilizers, catalysts, chemicals, and synthetic materials [22]. These industries employ diverse chemical processes, including reactions and refining methods, to manufacture a broad array of materials [23].
Wastewater from the chemical industry significantly impacts the environment due to its content of hazardous, toxic organic compounds and salts, depending on the chemicals produced. Various technologies are employed to remediate emerging contaminants in water. Among these, nanomaterials-dominated environmental chemical technology stands out for its efficiency, low energy consumption, and ease of recovery [24], making it highly effective in pollution control and environmental remediation. The concept of “wastewater as a resource” reflects a paradigm shift, recognizing wastewater as a valuable resource for addressing challenges in water supply and sanitation [15].
Wastewater treatment technology generally consists of three main systems: pretreatment, biochemical, and advanced treatment [25]. Advanced approaches are classified into physical separation, chemical, and biochemical methods [26]. Physical treatments use physical effects to isolate contaminants from water without altering the wastewater’s composition. Examples include sedimentation, membranes, electro-dialysis, and ion exchange. Chemical treatments have the capability to produce insoluble solids and gasses, transform non-biodegradable compounds into biodegradable ones, and deactivate chelating agents to eliminate substances from wastewater [27]. Biological treatments are effective for removing carbonaceous organic materials, Biological Oxygen Demand (BOD), phosphorus, and nitrification and denitrification, with aerobic processes generally achieving better results. Anaerobic processes focus on resource recovery and pollution limitation [27].
The cost of wastewater management is a significant concern, given the increasing demand for this essential resource [28]. Handling wastewater incurs significant costs and prompts considerations regarding funding and cost minimization [29]. Examining and researching the costs linked to various treatment methods is essential for enhancing efficiency, cutting costs, and promoting widespread adoption [30]. Although many papers discuss various aspects of treatment costs (investment or operational), few focus specifically on this topic. Comparisons between technologies from different studies are challenging due to differing assumptions, leading to varied cost analyses [31]. Table 1 compares different chemical technologies in terms of technological and economic aspects.

Table 1.
Comparison of chemical technologies.
3. Approaches in Chemical Technology Economics
In economics, microeconomics and macroeconomics represent distinct fields. Macroeconomics examines aggregate economic measures such as national output, unemployment, and inflation, whereas microeconomics analyzes the behavior of individual economic agents such as consumers, workers, and firms. Microeconomics specifically investigates and explains the decision-making processes of these agents from an economic and rational perspective [52].
Economics within the chemical industry encompasses various parameters including research and development, raw materials, labor, productivity, pricing, and classical techno-economic evaluation models. Economic indicators play a crucial role in assessing chemical technologies [53,54,55].
This review adopts a microeconomic perspective, aiming to integrate economic knowledge into the decision-making processes of chemical technologies. The objective is to improve decision making using economic methods, models, and information. While economic considerations of chemical technologies constitute just one facet of their overall economic management, as illustrated in Figure 2, it remains a pivotal component interconnected with all other aspects of chemical technology management.
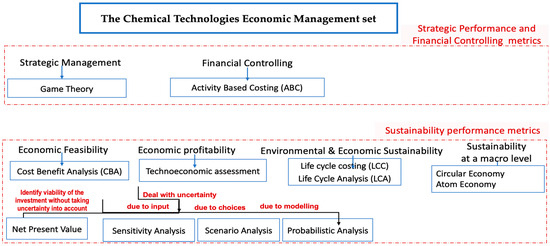
Figure 2.
The chemical technologies economic management set.
Economics encompasses various approaches (such as concepts, models, and theories) tied to financial considerations in any form they manifest. Clearly, economic factors play a crucial role in dealing with chemical technologies. Managing technologies always involves making decisions and allocating available budgets optimally. Economic analysis can aid in decision-making regarding investments, enhancing profitability, and contributing to a business strategy aimed at long-term sustainability and robust organizational health.
3.1. Economic Concepts
3.1.1. Strategic Performance and Financial Controlling
Application of Game Theory in Chemical Technologies
Game theory offers a powerful framework for addressing multi-objective problems by studying strategic decision-making among multiple interacting agents. It provides insights into player behavior in competitive and cooperative settings, aiding the analysis and prediction of outcomes. Understanding its principles, types, applications, and benefits helps develop effective strategies for various scenarios. Game theory has proven useful in solving complex engineering challenges like production design and planning, enhancing computational efficiency. One notable advantage is its ability to achieve faster solutions for distributed algorithms compared to heuristic methods [56]. Various studies (outlined in Table 2) have examined the game theory concept applied to chemical technologies for strategic decision making.
Recently, evolutionary game theory (EGT) has been increasingly applied to waste management and environmental governance. EGT is a promising method for analyzing interactions among multiple agents and their strategic behaviors. The core concept of EGT posits that interactions among individuals within a group are dynamic processes involving moves and countermoves, shaped by a constantly changing gaming environment. This tool is commonly utilized to examine decision-making issues in waste management. EGT has been applied in waste management to explore recycling practices for construction, electronic, and food waste. Researchers have developed dynamic evolutionary game models to analyze the impact of government incentive policies on the evolution of China’s construction waste recycling processes [57].
Another study proposes a methodological framework that integrates game theory and multi-criteria decision-making (MCDM) to address decision-making complexities in sustainable sludge management. The research suggests that Strategy S3 could benefit both the sludge treatment facility and the government, especially under conditions where the facility pays a tipping fee ranging from USD 0.19 to 7.59 per kWh of net electricity generation [58].

Table 2.
Game theory concept applied in chemical technologies for strategic decision making.
Table 2.
Game theory concept applied in chemical technologies for strategic decision making.
| Economic Theory | Chemical Technology | Uncertainty Analysis | Main Findings | Ref. |
|---|---|---|---|---|
| Game Theory | Several sludge valorization technologies were evaluated as alternative strategies, including incineration for electricity generation followed by landfill (S1), incineration for electricity generation followed by cement production (S2), biogas production from sludge digestion for electricity generation using fuel cells (S3), and biogas production from sludge digestion for electricity generation via combustion (S4). | Sensitivity Analysis | The results of the framework can enhance the sustainable decision-making process for the parties involved and facilitate more efficient agreements. | [58] |
| Nash non-cooperative game theory | Wastewater discharge reduction technology | - | Developing a WPDP system requires effective management of total wastewater discharge and improved supervision of water pollution sources. Allocating wastewater discharge permits involves negotiating with stakeholders to align their interests. These negotiations among provinces can be viewed as examples of non-cooperative game behavior driven by self-interest. | [59] |
In a prior study, researchers developed a DEA-based fixed cost allocation model using Nash’s non-cooperative game theory. This model aimed to allocate and compare wastewater discharge permits among provinces in 2017. Correlation analysis between allocation ratios and environmental efficiency showed that the proposed scheme incorporated environmental considerations. The allocation approach, based on non-cooperative game behavior among Decision-Making Units (DMUs), prioritizes equity and environmental compatibility. It represents a more holistic and acceptable method compared to previous schemes focused solely on efficiency. Policy recommendations were derived from a comparative analysis of water pollution permit utilization rates across 31 provinces [59].
Application of Activity-Based Costing in Chemical Technologies
Simple preparation methods and short synthesis processes are inherent to low-cost graphene production, which may help offset the use of any toxic materials. However, only a comprehensive economic model can fully identify these critical issues. Utilizing systematic synthesis methodologies can create topological pathways focused on minimizing costs [60]. While it is widely acknowledged that Life Cycle Assessment (LCA) can be used to evaluate any product or process, economic impact often provides a more significant indication of solvent performance, at least from an LCA standpoint. LCA does not account for fundamental factors like worker safety, which is a key component of Principle 12 of green chemistry [61]. The synthesis process involves a series of activities requiring step-by-step decisions, generally divided into two main categories: (i) planning and (ii) design. Additionally, the synthesis process can be organized based on the specific tasks and methods used. Understanding the dynamics of managing the synthesis process requires detailed assessments such as Activity-Based Costing (ABC) [62].
ABC is a strategic cost management method that measures the cost and efficiency of activities, products, and services based on the resources used in production [62]. It assumes that resources are consumed by the activities necessary to produce a product or service. ABC is especially useful in considering environmental costs when calculating product costs. It is a valuable performance evaluation tool for companies because it identifies unprofitable products or services, allowing them to be discontinued or for their prices to be adjusted if costs are incorrectly applied. Additionally, it helps improve a company’s strategic position by identifying areas for cost reduction (Table 3) and providing accurate information for managerial decision making [63].

Table 3.
Activity-Based Costing.
3.1.2. Sustainability Performance Metrics
Application of Techno-Economic Assessment, Life Cycle Assessment, and Life Cycle Cost and Cost–Benefit Analysis in Chemical Technologies
Assessing the economic competitiveness of emerging green chemical technologies is essential for their adoption over conventional methods. Evaluating these technologies from environmental life cycle and techno-economic perspectives is crucial. One prominent method for assessing economic viability is techno-economic assessment (TEA), which integrates technological and economic evaluations. TEA allows for the direct influence of technological parameters on economic indicators, facilitating informed decisions on the adoption of new green chemistry technologies [65].
TEA is a crucial tool for evaluating costs, benefits, and risks in innovative ventures, particularly in nanotechnology-based wastewater treatment. It supports effective resource management, fosters clear stakeholder communication, and helps decision makers develop effective strategies. TEA aids in proactive risk management by identifying and assessing risks, ensuring economic sustainability [66]. Furthermore, TEA is essential for strategic long-term planning, facilitating continual process refinement, technology comparisons, and ongoing enhancement initiatives. It aids in securing financing by presenting persuasive investment propositions to stakeholders, highlighting anticipated returns. Ultimately, TEA serves to connect the imperative for sustainable wastewater treatment with the economic feasibility and sustainability of nanotechnology ventures [67].
The increasing emphasis on sustainability has led to the development of assessment tools such as Life Cycle Analysis (LCA), Life Cycle Costing (LCC), and Cost–Benefit Analysis (CBA) [68]. TEA and LCC are systematic methodologies used to evaluate economic feasibility. TEA typically examines feasibility from an investor’s viewpoint, focusing on cradle-to-gate system boundaries, while LCC assesses costs across all stages of a product’s life cycle [69].
The goal of LCA and LCC is to evaluate both the environmental impacts and costs associated with various methods of producing nZVI (nanoscale zero-valent iron). These evaluations strive to pinpoint manufacturing methods that reduce both costs and environmental impacts across the lifecycle of nZVI. Figure 3 delineates all phases of nZVI production, from extracting raw materials to producing nZVI particles, using a “cradle to gate” methodology. In LCC, the analysis encompasses processes (Figure 3) involving economic expenses, especially direct costs, and incorporates external environmental costs like carbon emissions. The assessment unit is 1.00 kg of nZVI manufactured [70].
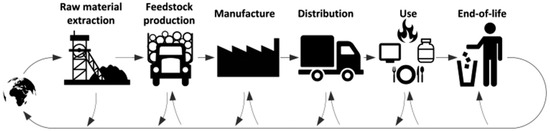
Figure 3.
General life cycle phases of a product or process including recycling streams [65].
LCA is a thorough methodology used to analyze the environmental impacts that may arise from a product throughout its complete life cycle. LCA enables the evaluation of the environmental sustainability of alternatives for recovering resources from wastewater, and it can be integrated with other specific assessment techniques such as life cycle costing and risk assessments [71]. CBA is widely recognized as the primary tool for assessing the economic efficiency of projects. It is utilized not only to quantify potential impacts on human health and the environment but also to identify the most effective strategies for mitigating these impacts. CBA involves financial analysis to forecast future financial flows of the project, determining its feasibility and return on investment. Additionally, it includes economic analysis to evaluate the project’s overall contribution to regional or state economic prosperity [72].
Forecasting methods used to predict the future potential of emerging technologies inherently involve uncertainty. To ensure accurate interpretation of prospective technology assessment results, it is crucial to evaluate the impact of this uncertainty. There are three primary types of uncertainty that affect outcomes: uncertainty in input data, uncertainty arising from decision-making choices, and uncertainty inherent in the modeling process. Various methods exist to address these uncertainties, enabling a balanced interpretation of the assessment indicators. Uncertainty in input data can be managed via uncertainty and sensitivity analyses, along with data quality assessments. The impact of choices can be evaluated using what-if or scenario analyses. Lastly, modeling uncertainty can be addressed by employing a standardized assessment approach [65].
Several studies (outlined in Table 4 have examined the techno-economic viability of introducing advanced technologies into wastewater treatment facilities. For example, despite the various benefits of adsorption—such as its straightforwardness, effectiveness, and selectivity over traditional treatment methods—its economic feasibility remains a notable challenge. The expense of adsorbents is notably impacted by their origin, manufacturing process, and surface characteristics [73]. Advanced evaluation techniques, such as LCA and CBA, have improved the assessment of adsorbents from both economic and ethical standpoints. These methodologies offer a structured method to evaluate the sustainability and economic viability of adsorbent technologies across their entire lifecycle, from acquiring raw materials to disposal or regeneration. They quantify the environmental impacts and economic costs linked to various adsorbent systems, facilitating informed decision-making in choosing and refining water remediation strategies [74].
Mahmoud et al. [75] examined the application of Fe/Cu nanoparticles in coagulation and adsorption processes for treating textile wastewater in plants (WWTPs). They estimated annual capital expenditures at approximately USD 0.021/m3, energy costs at USD 0.001/m3, and material costs for coagulant and adsorbent at USD 0.15/m3 and USD 6.1/m3, respectively. Labor and maintenance costs were assessed at USD 0.1/m3 and USD 0.0037/m3, leading to projected total operational expenses of USD 6.35/m3. This operational cost exceeds the reported operational costs of USD 5.8/m3 by Yin et al. who studied electrocoagulation and ozonation processes in textile water treatment. Mahmoud et al. suggested potential cost reductions via the use of nanoparticles synthesized via green methods rather than chemical synthesis. Additionally, they explored the use of proanthocyanidin-functionalized Fe3O4 nanoparticles, synthesized using proanthocyanidins from cereals, legume seeds, and fruits, for heavy metal extraction [76].
Caroline Visentin et al. conducted a study to assess the ecological and financial effect of various production approaches of nano zero-valent iron (nZVI) used in remediating polluted sites. The researchers assessed three synthesis methods: milling, liquid chemical reduction using sodium borohydride, and chemical reduction using hydrogen gas. They utilized LCA to evaluate environmental factors, employing the Simapro tool with the ecoinvent database and Impact 2002+ method. This analysis encompassed impact categories such as climate change, ecosystem quality, resource consumption, and human health. The study focused on raw material extraction and manufacturing stages, excluding nZVI’s post-production use phase. In terms of economic analysis, LCC was conducted using Simapro to evaluate internal and external costs. Results indicated that the sodium borohydride reduction method had the least environmental impact, while the hydrogen gas reduction method had a higher impact. In economic terms, the milling approach showed decreased costs as opposed to hydrogen gas reduction. Therefore, the study concluded that methods involving sodium borohydride reduction and milling are most advantageous due to their reduced environmental impacts and economic feasibility [70].
In a recent study, a TEA was conducted on the production of MgO nanoparticles using the sol–gel method. The findings revealed that the project, which involves sourcing MgO raw materials and manufacturing MgO nanoparticles, is highly profitable. Annual sales significantly exceed operational costs, resulting in a substantial profit of USD 337k. To maintain viability, initial investment costs, including equipment purchases, must remain below USD 192k. Over a span of 10 years, the project anticipates achieving a remarkable cumulative net present value/total investment cost ratio of 4977.18%, demonstrating substantial financial returns. Additionally, the payback period, where the initial investment is recovered, is projected to be within the third year, underscoring the project’s robust financial viability [77].

Table 4.
Techno-economic Analysis, LCA/LCC, and Cost–Benefit Assessment.
Table 4.
Techno-economic Analysis, LCA/LCC, and Cost–Benefit Assessment.
| Nanomaterial Used | Chemical Technology | Economic Theory | Goal of the Study | Main Findings (Environmental and Economic Benefits) | Ref. |
|---|---|---|---|---|---|
| Aluminum hydroxide/oxide nanoparticles (AHNP) | Adsorption, electrocoagulation | LCA | Eco-environmental assessment Economically, the combined material and energy costs of the adsorption process were found to be nearly seven times higher than those of the electrocoagulation process. | The environmental impacts of both methods were analyzed. The results revealed that the dissolution of the aluminum electrode and electricity usage in the electrocoagulation process are major contributors to environmental impacts. The adsorption process (GWP 35.2 kg CO2 eq.) exhibits nearly eight times greater environmental impact compared to electrocoagulation (GWP 4.5 kg CO2 eq.), due to the higher adsorption capacity of in situ generated coagulant compared to pre-precipitated adsorbents. | [78] |
| ZnCl2 | Adsorption, electrocoagulation | LCA | Environmental and economic impacts. The cost analysis concludes that electrocoagulation is more economically efficient than adsorption | This study conducts a life cycle assessment of both processes. The results reveal that the adsorbent production stage in the adsorption system significantly affects the environment. Recycling coproducts and recovering energy during adsorbent production helps reduce the overall energy demand. In contrast, the electrocoagulation process requires considerably less energy and raw materials, resulting in eight times lower global warming potential. Scenario analysis shows that using electricity from natural gas minimizes environmental impacts, compared to other energy sources. Sensitivity analysis, with a ±20% variation in process parameters, was also conducted. | [79] |
| Nanoscale zero-valent iron (nZVI) | Iron milling, liquid chemical reduction with sodium borohydride, and chemical reduction with hydrogen gas. | LCA, LCC | The impacts of synthesizing nano-iron for remediation of contaminated sites | The LCC analysis compared the costs of different methods for synthesizing nano-iron. It found that the milling method required less funds compared to the hydrogen gas reduction method. This cost difference is due to the higher energy consumption linked with hydrogen gas reduction. | [70] |
| NH2-functionalized magnetic graphene oxide | Hydrothermal method. Modified by 1st and 2nd grafting PAMAM type dendrimer. | LCA- LCC analysis, NPV, IRR | Eco-environmental assessment. | The price of mGO-NH2 was approximately USD 143.7 per kilogram, achieving an NPV of USD 21,064.8 per kilogram of mercury removed over a twenty-year lifespan. The project proved economically viable with a 64% internal rate of return (IRR) and required a capital investment of USD 742.31 per kilogram of mercury removed. This study highlights the environmental implications of mGO-NH2, paving the way for potential industrial-scale process modifications. | [80] |
| MgO Nanoparticles | Sol–Gel | Gross Profit Margin, Internal Rate Return, Break Even Point, Payback Period, and Cumulative Net Present Value | Economic Feasibility of magnesium oxide (MgO) nanoparticles using the sol–gel method | The Payback Period analysis reveals that profitability is achieved after a period exceeding three years. From an economic standpoint, it is evident that this project holds significant potential, despite potential losses resulting from deviations from optimal conditions. These study findings are anticipated to provide a framework for the development of economically efficient and commercially viable MgO nanoparticle production projects. | [77] |
| Fe-Cu nanoparticle | Coagulation and adsorption | Capital expenditures (CAPEX) and operating expenses (OPEX) | To evaluate the viability and effectiveness of the pilot prototype system using Fe-Cu nanoparticles | The overall cost of treatment (incorporating annual capital expenditures and operational expenditures) per cubic meter in the suggested system was USD 4.5, aimed at reusing treated textile wastewater for irrigating forest trees. This research demonstrates that the innovative multistage treatment approach (beginning with coagulation followed by adsorption using Fe/Cu nanoparticles) effectively addresses real textile wastewater at a competitive cost. | [75] |
| MoS2 nanoparticles | solvothermal method | The life cycle assessment (LCA) was conducted using a combination of Aspen Plus and LCIA IMPACT 2002+ from cradle-to-gate, with a functional unit defined as 1 kg of MoS2 ENM. | To evaluate the environmental and health impacts of scaling up the production of MoS2 NPs | The study proposes replacing LiOH with NaOH (sodium hydroxide) to alleviate resource scarcity and reduce associated environmental impacts during processing. This substitution has the potential to decrease estimated impacts by up to 56%. The findings provide valuable guidance for scientific committees and stakeholders, aiding informed decisions in selecting and developing nanomaterial production processes, specifically for MoS2. | [81] |
| - | Coagulants derived from chemicals (alum) and bio-sources (neem leaves) | CBA | To assess the efficacy of chemical-based and bio-based coagulants in treating real aquaculture wastewater. | Neither scenario proves economically viable for generating profit, whereas employing neem coagulant provides benefits for water reuse and sludge utilization. | [82] |
| - | Membrane bioreactor (MBR) technology | CBA | The study evaluated the cost-effectiveness, environmental benefits, net earnings, and effectiveness of MBRs using economic methodologies such as cost analysis, CBA, and data envelopment analysis (DEA) with non-radial directional distance function (NDDF). Data from 35 large-scale MBR installations were analyzed to assess investment and operational aspects. | The economic feasibility of 35 large-scale MBR plants, each with a capacity of more than 10 k m3/d, was assessed using techno-economic methods. CBA, which included shadow pricing of environmental benefits, indicated that the MBRs yielded positive net profits, averaging approximately USD 4.9/m3. This analysis affirmed the feasibility of Membrane bioreactor (MBR) technology from a techno-economic standpoint. | [83] |
| - | Moving bed biofilm reactor (MBBR) | CBA | An evaluation of the feasibility of improving wastewater treatment systems in an area of North Macedonia. | According to their economic analysis, they concluded that it is justified to invest in the WWTP project. | [10] |
The study conducted a comparison between chemical-based (alum) and bio-based (neem leaves) coagulants in treating aquaculture wastewater, assessing their economic viability. Alum demonstrated higher efficiency in removing total suspended solids (99.7%), turbidity (98.8%), and color (97.3%), whereas neem coagulant achieved similar performance with lower dosages. Total costs, including capital and operational expenses, were evaluated alongside potential benefits from water reuse and valuable product recovery from sludge. Net profit analysis indicated negative values for both scenarios, with cost–benefit ratios of 0 for alum and 0.06 for neem coagulants. The study suggests further exploration using the Social Return on Investment (SROI) method to integrate non-traditional factors like social and environmental impacts into the cost–benefit analysis of these scenarios [82].
Another study conducted a comprehensive evaluation of MBR technology, focusing on its techno-economic aspects. It analyzed 35 large-scale plants, each with a capacity of more than 10 k m3/d, using CBA and DEA. By examining investment and operational data, the study assessed marginal costs and environmental benefits, incorporating shadow prices of pollutants, resulting in an average net profit of approximately USD 4.9/m3 [83].
Using NDDF in DEA, the study found the average cost efficiency and energy efficiency of the MBRs to be 0.77 and 0.66, respectively. The study found significant variations in net profits and efficiency metrics among MBR installations, influenced by factors such as geographical location, effluent standards, and operational duration, as verified via statistical tests. It compared costs across MBRs treating different wastewater types, with varying capacities, conforming to diverse effluent standards, situated in different construction locations, and located in various geographical regions. This research group presented findings from a financial analysis of a WWTP in Dojran, North Macedonia. Variant 3 emerged as the most cost-effective option, with the lowest overall and discounted costs, resulting in the lowest cost per cubic meter of treated water and the highest financial benefit. The economic assessment indicated that investing in the WWTP project is justified, supported by an economic internal rate of return of 16.38%, a positive economic NPV, and an economic benefit–cost ratio of 2.11. Environmental benefits were predominantly from nitrogen at approximately 49% of the total, followed by phosphorus at approximately 46% [10].
3.1.3. Sustainability at a Macro Level
Sustainable, Green, Circular, and Low Carbon Chemistry
- Green Chemistry and Atom Economy
Screening methods offer an initial glimpse into the potential of emerging green technologies. These methods are often qualitative or semi-quantitative and serve as a rough, preliminary assessment. Simple metrics can be employed to evaluate the performance of new technologies [65]. However, the chemistry community often overlooks the importance of linking molecular-scale chemical phenomena to broader macroeconomic impacts [84]. In response to growing environmental concerns, green chemistry emerged as a pivotal concept starting in the 1980s and gained prominence by the late 1990s. Initially, it emphasized pollution prevention rather than waste cleanup. However, recognizing that not all aspects of green chemistry may be sustainable, there is a need to define and apply sustainable chemistry principles to assess each chemical, reaction, or process. A recent perspective emphasizes the need to explicitly assess sustainability using Green Chemistry metrics [85]. Green chemistry addresses the issue of chemical pollution by promoting the design of chemicals and production processes that minimize or eliminate the use and generation of hazardous substances [86]. Over the past decade, the field has gained significant attention for its ability to leverage chemical innovation to simultaneously achieve environmental and economic goals [87]. Green chemistry is based on twelve principles (Figure 4) as proposed by Anastas and Warner [88] that focus on reducing the environmental and health impacts of hazardous chemicals, materials, and practices
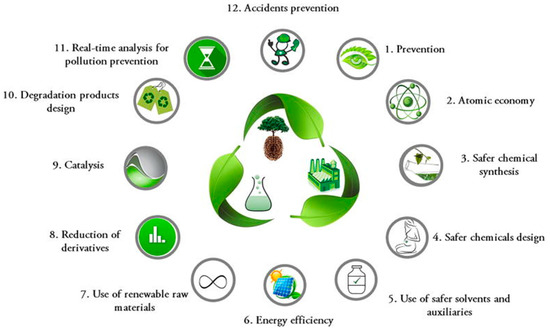
Figure 4.
The 12 Principles of Green Chemistry proposed by Anastas and Warner [88].
These principles have remained unchanged for the past 25 years, highlighting their enduring relevance [13]. The Twelve Principles of Green Chemistry serve as “design rules” that guide chemists in their pursuit of sustainability. The discipline is characterized by the careful planning of chemical synthesis and molecular design to minimize negative consequences [87].
The majority of the principles of green chemistry focus on strategies for chemical production, such as atom and energy efficiency, the use of safer solvents, and catalysts. Others address the environmental impact of chemical processes, including waste prevention, product degradability at the end of life, and the prevention of pollution and accidents. While green chemistry is a valuable tool and guiding philosophy for reducing pollution and protecting the environment, it still operates within the framework of a linear economy (i.e., take-make-use-dispose) [86].
- Atom Economy
Principle 2, the concept of atom economy, introduced new criteria for the efficient and sustainable production of synthetic compounds. Atom economy, first proposed by Barry Trost [89,90], is defined as the percentage of atoms from the reactants that are incorporated into the final product. An ideal reaction would convert all atoms from the starting materials into the desired product, achieving 100% atom economy (Figure 5). This metric defines the efficiency of a chemical reaction and is widely used to evaluate the “greenness” of a synthesis. The advancement of this concept was primarily driven by synthetic chemists seeking to develop reactions with higher atom economy (Table 5). However, this principle can and should be applied more broadly to measure the efficiency of all types of synthesis, including the production of functional materials [91].
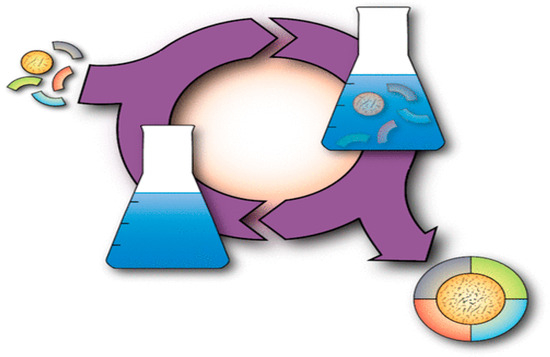
Figure 5.
A visualization of the principle of atom economy illustrating the complete conversion of starting materials into the desired product without generating any waste (100% atom economy) [91].
Although only Principle 2 explicitly mentions the word “economy”, all twelve principles are influenced by cost considerations. Cost can serve as a proxy for greenness in process design, factoring in aspects such as worker safety, reduced energy consumption, minimized waste, and lower material use. While the cost of synthesis is a significant concern, economic considerations are often an afterthought during the design phase of chemical synthesis. A number of studies focus on the use of less harmful reagents, catalysts, or routes (e.g., [92,93]).

Table 5.
Atom Economy.
Table 5.
Atom Economy.
| Economic Theory | Chemical Technology | Main Findings | References |
|---|---|---|---|
| Atom economy | Synthesis | AQ reagent-free, 100% atom-economic, scalable, and sustainable synthetic method for the highly regio- and stereoselective iodosulfenylation of alkynes. This method utilizes only iodine and disulfides to produce important classes of stereodefined alkenes, such as (E)-β-iodoalkenyl sulfides, with moderate to excellent yields of up to 96%. | [94] |
| Atom economy | Synthesis | Dichloromeldrum’s acid is introduced as a bench-stable, non-volatile reagent for the dichloroacetylation of anilines and alkyl amines, yielding α,α-dichloroacetamides, which are key motifs in medicinal chemistry. The products are obtained in good to excellent yields using reagent-grade solvents, and since the only byproducts are acetone and CO2, no column chromatography is necessary. This reagent offers a practical, efficient, and environmentally friendly approach for the dichloroacetylation of primary amines. | [92] |
Introducing green modifications into established processes is often challenging [20]. Not all chemicals, reactions, or processes that are sustainable are necessarily green. Therefore, the selection of chemicals, reactions, and processes that are both sustainable and green should be prioritized in design and innovation efforts [1]. Addressing these challenges is supported by significant discoveries from various research groups [95,96,97,98].
- Sustainable Chemistry
Sustainable chemistry aims to utilize resources, including energy, at a rate that aligns with natural replenishment rates, and it ensures that waste generation does not exceed the capacity for remediation. Importantly, sustainable chemistry does not necessarily equate to green chemistry in all cases. Therefore, prioritizing processes that are both green and sustainable should be the focus of design and innovation efforts [1]. The three pillars of sustainability are often denoted as the 3Ps—people, planet, and profit. Unlike green chemistry, sustainability encompasses economic considerations as well [99].
- Circular Chemistry and Circular Economy
The chemical industry has identified a third significant trend: the increasing prominence of the circular economy. This trend has gained traction alongside rising public apprehension about plastic waste polluting oceans, rivers, and extensive landfill sites. In response, the industry is taking proactive steps by establishing global partnerships focused on reducing plastic waste. These initiatives involve collaboration among stakeholders throughout the value chain, including producers, brand owners, and waste management organizations [100]. Chemistry has been closely intertwined with the socioeconomic system since the Industrial Revolution. Implementing circular economy practices in the chemical industry is considered a practical approach to achieving sustainability goals. However, the degree of circularity within a process ultimately determines its overall sustainability [101].
Circular chemistry incorporates the principles of circular economy (CE), utilizing life cycle approaches and systems thinking to address and understand sustainability issues related to chemical processes and products [102]. It particularly emphasizes resource efficiency throughout chemical value chains and underscores the importance of developing innovative chemical reactions for the reuse and recycling of chemicals. This approach aims to advance towards a closed-loop, waste-free chemical industry [103]. Consequently, the field of chemistry stands on the verge of a unique opportunity to fundamentally rethink and reinvent its practices via new technologies and innovations that align with circularity and sustainability [102].
The circular economy promises to provide a pathway to decouple environmental impacts from economic growth, aiming to achieve higher profits with a reduced environmental footprint. When examining the interplay between chemicals and circularity, green chemistry establishes a fundamental basis for establishing robust and sustainable circular economy practices. By integrating green chemistry principles and circular economy strategies, nations can optimize resource utilization, reduce raw material procurement inputs and waste management costs, and mitigate the depletion rate of non-renewable resources [104].
In this light, wastewater treatment is crucial within the framework of the circular economy, where wastewater is seen as a valuable asset rather than a liability. This approach emphasizes the reuse and regeneration of materials and products to reduce dependence on natural resources and enhance environmental sustainability. Wastewater treatment facilities have transformed into vital sources of energy, clean water, fertilizers, and nutrients. For example, biogas produced from wastewater can serve both industrial and domestic needs, reducing reliance on fossil fuels and alleviating pressure on natural resources. Governments worldwide have recognized the potential of wastewater as a valuable resource rather than merely disposing of it into nearby water bodies. This multifaceted utility has attracted considerable interest from policymakers seeking alternative avenues for economic growth [15]. Table 6 highlights the application of circular economy theory in various chemical technologies.

Table 6.
Circular Economy: the interplay between chemical technology and circularity.
Gherghel et al. evaluated treatment technologies and highlighted those significant challenges persist at lower Technology Readiness Levels (TRLs), necessitating further studies on technology, expenses, and impact on the environment. Among the technologies showing great promise in line with circular economy principles are those dedicated to recovering phosphorus via struvite precipitation, as well as generating energy via anaerobic digestion, thermal hydrolysis, and co-digestion with organic wastes. These technologies exhibit higher TRL values, indicating their readiness for market penetration and potential to substantially transform current wastewater treatment plant paradigms [111].
- Low-Carbon Chemistry
In the context of sustainability, low-carbon chemistry is gaining significance, and integrating the concept of circular economy (CE) will be crucial for more comprehensively applying green and sustainable chemistry principles to the resource-dependent chemical industry [101]. A key driver for sustainability is achieving products with low-carbon footprints, measured as ‘embodied carbon’, which reflects reduced carbon emissions throughout the product’s life cycle. This includes emissions from raw material procurement, production, transportation, and end-of-life disposal [112]. To achieve low-carbon products, raw materials should have minimal carbon footprints via careful sourcing, optimized logistics, and reusability. The use of renewable or alternative chemicals (such as bioethanol, lactic acid, sugars, lipids, and gasses like H2, CO2, CH4, CO, etc.) in production processes contributes to a low-carbon approach [29]. Additionally, implementing energy-efficient strategies and incorporating renewable energy into processes can help lower the embedded carbon [112].
Various assessment tools have been developed and implemented in the field of economic sustainability. Green, circular, and low-carbon chemistry are related and interconnected concepts but are not interchangeable. While both green and circular chemistry are significant, they address different economic models—linear and circular economies, respectively. Applying the principles of green or circular chemistry to the same chemical process can lead to distinct outcomes for the triple bottom line: social, environmental, and economic sustainability, often referred to as “People, Planet, Profit” [86]. Low-carbon chemistry is becoming increasingly important in the context of sustainability, and linking it to the circular economy will be essential for more fully integrating green and sustainable chemistry principles into the resource-dependent chemical industry [102].
4. Challenges and Future Perspectives
Economic analysis is crucial for guiding investment decisions across various sectors. In the context of wastewater treatment projects, the viability of investment hinges on anticipated returns. However, the benefits from such projects often manifest indirectly, adding complexity to decision-making. Therefore, conducting a thorough economic study is essential for management to explore feasible options, especially in wastewater management.
Several principles outlined in chemical processes for water treatment are applicable here. For instance, when considering coagulant recovery within these principles, technologies for coagulant recovery align well with objectives such as waste reduction, enhancing atom economy, and promoting the use of coagulants as reusable catalysts rather than consumables [11]. Technical specifications, such as treatment process kinetics, are considered key indicators for assessing required initial investments. Economic factors, including initial investments and operational and maintenance costs, also play a significant role in selecting the optimal industrial wastewater treatment option. However, there remains a scarcity of studies focusing on the economic viability of diverse treatment technologies, particularly those that are newly emerging [113].
Chemical systems and processes are integral to achieving a balance between resource management and remediation of wastes [1]. However, each treatment method encounters distinct challenges: physical separation methods face issues such as pollutant accumulation and slow diffusion; chemical treatments are limited by economic feasibility and scalability; and biochemical methods, while cutting-edge in industrial wastewater treatment research [26], are hindered by slow microbial activity and stringent habitat requirements. Adsorption emerges as a viable solution—effective, cost-efficient, and non-destructive—for removing diverse pollutants from wastewater. Its efficacy spans minerals, metal ions, dyes, and other contaminants, as evidenced by various wastewater treatment studies [36].
Although economic analysis plays a crucial role in decision-making processes for WWTPs, it has been relatively neglected compared to environmental and technological assessments, as emphasized by Rashidi et al. [114]. Selecting the best treatment option is a complicated undertaking, influenced by unique strengths and weaknesses inherent in each approach. Considerations extend beyond costs to encompass effectiveness, practicality, environmental impacts, sludge generation, operational complexities, pre-treatment requirements, and potential risks associated with by-products. Among the various methods available for wastewater treatment, only a few have gained widespread acceptance in the industrial sector, influenced by advancements in technology and economic factors.
5. Conclusions
Approaches in chemical economics encompass a range of methodologies, including TEA, CBA, LCA, LCC, ABC, atom economy, game theory, and circular economy principles. These methods are summarized and analyzed to enhance understanding and application in evaluating chemical technologies from an economic perspective. The economic aspect is crucial in decision-making processes, especially when selecting chemical technologies, as it can significantly impact treatment efficiency and costs. The complexity of choosing chemical technologies highlights the need for thorough studies on the criteria and variables involved in decision-making processes. Various researchers emphasize the importance of economic analysis in this context. This study characterizes the economics of chemical technologies as a field that spans across disciplines, investigating the mutual influences and development of economics and chemical technologies to enhance decision-making processes. The primary focus is on understanding how chemical technologies and economics interact, with chemical technology costs serving as a critical link between the two.
Author Contributions
Methodology, D.A.G., A.C.M. and G.Z.K.; writing—original draft preparation, D.A.G.; writing—review and editing, D.A.G., A.C.M. and G.Z.K.; supervision, G.Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Horváth, I.T. Introduction: Sustainable Chemistry. Chem. Rev. 2018, 118, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Committee on Enhancing the U.S. Chemical Economy Through Investments in Fundamental Research in the Chemical Sciences; Board on Chemical Sciences and Technology; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. The Importance of Chemical Research to the U.S. Economy; National Academies Press: Washington, DC, USA, 2022; p. 26568. ISBN 978-0-309-68863-5. [Google Scholar]
- Biniecka, M.; Campana, P.; Iannilli, I. The Technological and Economic Management of the Environmental Variable in the Pharmaceutical–Chemical Industry. Microchem. J. 2005, 79, 325–329. [Google Scholar] [CrossRef]
- Royle, M.; Chachuat, B.; Xu, B.; Gibson, E.A. The Pathway to Net Zero: A Chemicals Perspective. RSC Sustain. 2024, 2, 1337–1349. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a Green Chemistry Future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Discussion on the Development of Green Chemistry and Chemical Engineering. IOP Conf. Ser. Earth Environ. Sci. 2017, 94, 012136. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Yenkie, K.M. Integrating the Three E’s in Wastewater Treatment: Efficient Design, Economic Viability, and Environmental Sustainability. Curr. Opin. Chem. Eng. 2019, 26, 131–138. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Hernández-Sancho, F.; Sala-Garrido, R. Economic Feasibility Study for Wastewater Treatment: A Cost–Benefit Analysis. Sci. Total Environ. 2010, 408, 4396–4402. [Google Scholar] [CrossRef]
- Ćetković, J.; Knežević, M.; Lakić, S.; Žarković, M.; Vujadinović, R.; Živković, A.; Cvijović, J. Financial and Economic Investment Evaluation of Wastewater Treatment Plant. Water 2022, 14, 122. [Google Scholar] [CrossRef]
- Keeley, J.; Jarvis, P.; Judd, S.J. Coagulant Recovery from Water Treatment Residuals: A Review of Applicable Technologies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2675–2719. [Google Scholar] [CrossRef]
- Dubé, M.A.; Salehpour, S. Applying the Principles of Green Chemistry to Polymer Production Technology. Macromol. React. Eng. 2014, 8, 7–28. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK; New York, NY, USA, 1998; ISBN 978-0-19-850234-0. [Google Scholar]
- Su, X.; Chiang, P.; Pan, S.; Chen, G.; Tao, Y.; Wu, G.; Wang, F.; Cao, W. Systematic Approach to Evaluating Environmental and Ecological Technologies for Wastewater Treatment. Chemosphere 2019, 218, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Buchner, G.A.; Zimmermann, A.W.; Hohgräve, A.E.; Schomäcker, R. Techno-Economic Assessment Framework for the Chemical Industry—Based on Technology Readiness Levels. Ind. Eng. Chem. Res. 2018, 57, 8502–8517. [Google Scholar] [CrossRef]
- Allied Market Research Chemical Technology Market. Available online: https://www.alliedmarketresearch.com/press-release/chemical-technology-market.html (accessed on 9 April 2024).
- Research and Markets Chemicals Global Market Report. 2024. Available online: https://www.researchandmarkets.com/reports/5781388/chemicals-global-market-report?srsltid=AfmBOoo2W-cq4I_cpUdN4j5YuB6avuZ5heZWtg6mi86CSGq378aDrtTA (accessed on 9 April 2024).
- Nyirenda, J.; Malabo, H. Mineral and Bioresource Exploitation for Transformation and Sustainability of the Chemical Industry in Zambia. Humanit. Soc. Sci. Commun. 2024, 11, 19. [Google Scholar] [CrossRef]
- Das, S.; Brunet Icart, I. Innovation Policy of European Chemical Companies with Special Focus on Large Companies. Rev. Int. Organ. 2015, 123–157. [Google Scholar] [CrossRef]
- American Chemistry Council. Available online: https://www.americanchemistry.com/about-acc (accessed on 9 April 2024).
- Garrett, D.E. Economy of the Chemical Industry. In Chemical Engineering Economics; Springer: Dordrecht, The Netherlands, 1989; pp. 107–153. ISBN 978-94-011-6546-4. [Google Scholar]
- Wojcik-Jurkiewicz, M.; Masztalerz, M.; Lew, G.; Lulek, A.; Sadowska, B. The Impact of Chemical Companies on the Environment and Local Communities in the Aspect of Business Model. Eur. Res. Stud. J. 2021, XXIV, 548–563. [Google Scholar] [CrossRef]
- Xu, X.; Liu, H.; Wang, J.; Chen, T.; Ding, X.; Chen, H. Insight into Surface Hydroxyl Groups for Environmental Purification: Characterizations, Applications and Advances. Surf. Interfaces 2021, 25, 101272. [Google Scholar] [CrossRef]
- Gopal, R.; Chinnapan, M.M.; Bojarajan, A.K.; Rotte, N.K.; Ponraj, J.S.; Ganesan, R.; Atanas, I.; Nadarajah, M.; Manavalan, R.K.; Gaspar, J. Facile Synthesis and Defect Optimization of 2D-Layered MoS2 on TiO2 Heterostructure for Industrial Effluent, Wastewater Treatments. Sci. Rep. 2020, 10, 21625. [Google Scholar] [CrossRef]
- Mu, X.; Lu, S.; Li, Q. How to Promote the Development of Industrial Wastewater Treatment Technological Innovation in China: A Tripartite Evolutionary Game Analysis. Sustainability 2023, 15, 15359. [Google Scholar] [CrossRef]
- Li, Y. Technology Review and Selection Guide for Industry Wastewater Treatment. Comput. Water Energy Environ. Eng. 2020, 9, 22–35. [Google Scholar] [CrossRef]
- Gallego Valero, L.; Moral Pajares, E.; Román Sánchez, I.; Sánchez Pérez, J. Analysis of Environmental Taxes to Finance Wastewater Treatment in Spain: An Opportunity for Regeneration? Water 2018, 10, 226. [Google Scholar] [CrossRef]
- Moral Pajares, E.; Gallego Valero, L.; Román Sánchez, I. Cost of Urban Wastewater Treatment and Ecotaxes: Evidence from Municipalities in Southern Europe. Water 2019, 11, 423. [Google Scholar] [CrossRef]
- San Juan, J.L.; Caligan, C.J.; Garcia, M.M.; Mitra, J.; Mayol, A.P.; Sy, C.; Ubando, A.; Culaba, A. Multi-Objective Optimization of an Integrated Algal and Sludge-Based Bioenergy Park and Wastewater Treatment System. Sustainability 2020, 12, 7793. [Google Scholar] [CrossRef]
- Cañizares, P.; Paz, R.; Sáez, C.; Rodrigo, M.A. Costs of the Electrochemical Oxidation of Wastewaters: A Comparison with Ozonation and Fenton Oxidation Processes. J. Environ. Manag. 2009, 90, 410–420. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Joshiba, G.J.; Femina, C.C.; Varshini, P.; Priyadharshini, S.; Arun Karthick, M.S.; Jothirani, R. A Critical Review on Recent Developments in the Low-Cost Adsorption of Dyes from Wastewater. Desalin. Water Treat. 2019, 172, 395–416. [Google Scholar] [CrossRef]
- Ngueagni, P.T.; Woumfo, E.D.; Kumar, P.S.; Siéwé, M.; Vieillard, J.; Brun, N.; Nkuigue, P.F. Adsorption of Cu(II) Ions by Modified Horn Core: Effect of Temperature on Adsorbent Preparation and Extended Application in River Water. J. Mol. Liq. 2020, 298, 112023. [Google Scholar] [CrossRef]
- Wan, S.; Bi, H.; Sun, L. Graphene and Carbon-Based Nanomaterials as Highly Efficient Adsorbents for Oils and Organic Solvents. Nanotechnol. Rev. 2016, 5, 3–22. [Google Scholar] [CrossRef]
- Yang, A.; Zhu, Y.; Huang, C.P. Facile Preparation and Adsorption Performance of Graphene Oxide-Manganese Oxide Composite for Uranium. Sci. Rep. 2018, 8, 9058. [Google Scholar] [CrossRef]
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Ijaz Khan, M.; Ben Moussa, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater Treatment: A Short Assessment on Available Techniques. Alex. Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Bello, L.A.; Omoboye, A.J.; Abiola, T.; Oyetade, J.; Akinropo; Udorah, D.; Racheal, A.E. Treatment Technologies for Wastewater from Cosmetic Industry—A Review. Int. J. Chem. Biomol. Sci. 2018, 4, 69–80. [Google Scholar]
- Ray, S.S.; Gusain, R.; Kumar, N. Regeneration and Recyclability of Carbon Nanomaterials after Adsorption. In Carbon Nanomaterial-Based Adsorbents for Water Purification; Elsevier: Amsterdam, The Netherlands, 2020; pp. 349–363. ISBN 978-0-12-821959-1. [Google Scholar]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Wu, J.; Niu, J. Insights into the Electrochemical Degradation of Sulfamethoxazole and Its Metabolite by Ti/SnO2-Sb/Er-PbO2 Anode. Chin. Chem. Lett. 2020, 31, 2673–2677. [Google Scholar] [CrossRef]
- Aziz, H.A.; Abu Amr, S.S. (Eds.) Advanced Oxidation Processes (AOPs) in Water and Wastewater Treatment; Advances in Environmental Engineering and Green Technologies; IGI Global: Hershey, PA, USA, 2019; ISBN 978-1-5225-5766-1. [Google Scholar]
- Samer, M. Biological and Chemical Wastewater Treatment Processes. In Wastewater Treatment Engineering; Samer, M., Ed.; InTech: London, UK, 2015; ISBN 978-953-51-2179-4. [Google Scholar]
- Vo, D.-T.; Phan, H.-V.-T.; Hoang, L.-T.-T.-T.; Nguyen, V.-K.; Tran, T.-N.; Dao, M.-T. Application of an Anaerobic–Anoxic–Oxic–Oxic (AAO/O) Model to the Treatment of Real Domestic Wastewater. J. Chem. 2022, 2022, 9456026. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J.; Chang, Y.; Lee, Y.-J. Sludge Treatment: Current Research Trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad, S. Cost Estimation and Economical Evaluation of Three Configurations of Activated Sludge Process for a Wastewater Treatment Plant (WWTP) Using Simulation. Appl. Water Sci. 2017, 7, 2513–2521. [Google Scholar] [CrossRef]
- Arif, A.U.A.; Sorour, M.T.; Aly, S.A. Cost Analysis of Activated Sludge and Membrane Bioreactor WWTPs Using CapdetWorks Simulation Program: Case Study of Tikrit WWTP (Middle Iraq). Alex. Eng. J. 2020, 59, 4659–4667. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Bampos, G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes 2022, 10, 2304. [Google Scholar] [CrossRef]
- Oscar Omondi, D.; Caren Navalia, A. Constructed Wetlands in Wastewater Treatment and Challenges of Emerging Resistant Genes Filtration and Reloading. In Inland Waters—Dynamics and Ecology; Devlin, A., Pan, J., Manjur Shah, M., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83968-294-0. [Google Scholar]
- Wu, Y.; Han, R.; Yang, X.; Zhang, Y.; Zhang, R. Long-Term Performance of an Integrated Constructed Wetland for Advanced Treatment of Mixed Wastewater. Ecol. Eng. 2017, 99, 91–98. [Google Scholar] [CrossRef]
- Stefanakis, A. (Ed.) Constructed Wetlands for Industrial Wastewater Treatment; Challenges in Water Management Series; John Wiley & Sons/Blackwell: Hoboken, NJ, USA, 2018; ISBN 978-1-119-26841-3. [Google Scholar]
- Reniers, G.L.L.; Van Erp, N. Operational Safety Economics: A Practical Approach Focused on the Chemical and Process Industries; Wiley: Chichester, UK, 2016; ISBN 978-1-118-87112-6. [Google Scholar]
- Wang, Y.; Li, G.; Liu, Z.; Cui, P.; Zhu, Z.; Yang, S. Techno-Economic Analysis of Biomass-to-Hydrogen Process in Comparison with Coal-to-Hydrogen Process. Energy 2019, 185, 1063–1075. [Google Scholar] [CrossRef]
- Cormos, A.-M.; Cormos, C.-C. Techno-Economic Assessment of Combined Hydrogen & Power Co-Generation with Carbon Capture: The Case of Coal Gasification. Appl. Therm. Eng. 2019, 147, 29–39. [Google Scholar] [CrossRef]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carriers (LOHCs)—Techno-Economic Analysis of LOHCs in a Defined Process Chain. Energy Environ. Sci. 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Renna, P. A Review of Game Theory Models to Support Production Planning, Scheduling, Cloud Manufacturing and Sustainable Production Systems. Designs 2024, 8, 26. [Google Scholar] [CrossRef]
- Ding, M.; Zeng, H. Multi-Agent Evolutionary Game in the Recycling Utilization of Sulfate-Rich Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 8770. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, J. Developing a Sustainability-Oriented Multi-Criteria Game Theoretical Decision Analysis Framework: A Case Study of Sludge Management. J. Clean. Prod. 2022, 354, 131807. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, Q.; Rao, K.; Dai, Q. Water Pollutant Discharge Permit Allocation Based on DEA and Non-Cooperative Game Theory. J. Environ. Manag. 2022, 302, 113962. [Google Scholar] [CrossRef]
- Kuhlmann, H.; Skiborowski, M. Optimization-Based Approach To Process Synthesis for Process Intensification: General Approach and Application to Ethanol Dehydration. Ind. Eng. Chem. Res. 2017, 56, 13461–13481. [Google Scholar] [CrossRef]
- García-Quintero, A.; Palencia, M. A Critical Analysis of Environmental Sustainability Metrics Applied to Green Synthesis of Nanomaterials and the Assessment of Environmental Risks Associated with the Nanotechnology. Sci. Total Environ. 2021, 793, 148524. [Google Scholar] [CrossRef]
- Johnson, H.T.; Kaplan, R.S. Relevance Lost: The Rise and Fall of Management Accounting; Harvard Business School Press: Boston, MA, USA, 1991; ISBN 978-0-87584-254-7. [Google Scholar]
- Akgün, M.; Katanalp, B.; Can, A.V.; Kıymaz Kıvraklar, M. Adapting the Activity-Based Costing Method for Water Footprint Accounting. J. Clean. Prod. 2023, 400, 136691. [Google Scholar] [CrossRef]
- Gkika, D.A.; Filiz, V.; Rangou, S.; Kyzas, G.Z.; Mitrοpoulos, A.C. Cost Profile of Membranes That Use Polymers of Intrinsic Microporosity (PIMs). Membranes 2022, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, G.; Van Dael, M.; Van Passel, S.; You, F. How to Assess the Potential of Emerging Green Technologies? Towards a Prospective Environmental and Techno-Economic Assessment Framework. Green Chem. 2019, 21, 4868–4886. [Google Scholar] [CrossRef]
- Mpongwana, N.; Rathilal, S. A Review of the Techno-Economic Feasibility of Nanoparticle Application for Wastewater Treatment. Water 2022, 14, 1550. [Google Scholar] [CrossRef]
- Kuppens, T.; Van Dael, M.; Vanreppelen, K.; Thewys, T.; Yperman, J.; Carleer, R.; Schreurs, S.; Van Passel, S. Techno-Economic Assessment of Fast Pyrolysis for the Valorization of Short Rotation Coppice Cultivated for Phytoextraction. J. Clean. Prod. 2015, 88, 336–344. [Google Scholar] [CrossRef]
- Hoogmartens, R.; Van Passel, S.; Van Acker, K.; Dubois, M. Bridging the Gap between LCA, LCC and CBA as Sustainability Assessment Tools. Environ. Impact Assess. Rev. 2014, 48, 27–33. [Google Scholar] [CrossRef]
- Miah, J.H.; Koh, S.C.L.; Stone, D. A Hybridised Framework Combining Integrated Methods for Environmental Life Cycle Assessment and Life Cycle Costing. J. Clean. Prod. 2017, 168, 846–866. [Google Scholar] [CrossRef]
- Visentin, C.; Trentin, A.W.D.S.; Braun, A.B.; Thomé, A. Lifecycle Assessment of Environmental and Economic Impacts of Nano-Iron Synthesis Process for Application in Contaminated Site Remediation. J. Clean. Prod. 2019, 231, 307–319. [Google Scholar] [CrossRef]
- Corominas, L.; Byrne, D.M.; Guest, J.S.; Hospido, A.; Roux, P.; Shaw, A.; Short, M.D. The Application of Life Cycle Assessment (LCA) to Wastewater Treatment: A Best Practice Guide and Critical Review. Water Res. 2020, 184, 116058. [Google Scholar] [CrossRef]
- Turková, J.; Korytárová, J. Methods for Evaluation of WWTPs Environmental Impacts: A Review. IOP Conf. Ser. Earth Environ. Sci. 2019, 222, 012004. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and Challenges in Adsorption-Based Wastewater Remediation: A Comprehensive Review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Mudhoo, A.; Mohan, D.; Pittman, C.U.; Sharma, G.; Sillanpää, M. Adsorbents for Real-Scale Water Remediation: Gaps and the Road Forward. J. Environ. Chem. Eng. 2021, 9, 105380. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mostafa, M.K.; Peters, R.W. A Prototype of Textile Wastewater Treatment Using Coagulation and Adsorption by Fe/Cu Nanoparticles: Techno-Economic and Scaling-up Studies. Nanomater. Nanotechnol. 2021, 11, 184798042110411. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, Y.; Deng, S.; Zhao, B.; Fu, Y.; Liu, Z. Synthesis of Proanthocyanidins-Functionalized Fe3O4 Magnetic Nanoparticles with High Solubility for Removal of Heavy-Metal Ions. Chem. Phys. Lett. 2020, 753, 137600. [Google Scholar] [CrossRef]
- Noorlela, A.; Nandiyanto, A.B.D.; Ragadhita, R.; Fiandini, M.; Kurniawan, T. Techno-Economic Analysis of the Production of Magnesium Oxide Nanoparticles Using Sol-Gel Method. J. Mech. Eng. Sci. Innov. 2023, 3, 1–13. [Google Scholar] [CrossRef]
- Goyal, H.; Mondal, P. Life Cycle Assessment (LCA) of the Arsenic and Fluoride Removal from Groundwater through Adsorption and Electrocoagulation: A Comparative Study. Chemosphere 2022, 304, 135243. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Gupta, S.; Mondal, P. Life Cycle Assessment (LCA) of Greywater Treatment Using ZnCl2 Impregnated Activated Carbon and Electrocoagulation Processes: A Comparative Study. Ind. Eng. Chem. Res. 2023, 62, 3259–3270. [Google Scholar] [CrossRef]
- Pakzad Toochaei, S.; Abyar, H.; Einollahipeer, F. Comprehensive Life Cycle Assessment of NH2-Functionalized Magnetic Graphene Oxide for Mercury Removal: Carbon Emissions and Economic Evaluation. Environ. Pollut. 2024, 347, 123737. [Google Scholar] [CrossRef]
- Hachhach, M.; Akram, H.; Kasmi, A.E.; Hanafi, M.; Achak, O.; Chafik, T. Life Cycle Assessment of Large-Scale Production of MoS2 Nanomaterials through the Solvothermal Method. J. Nanopart. Res. 2022, 24, 181. [Google Scholar] [CrossRef]
- Kurniawan, S.; Ahmad, A.; Imron, M.; Abdullah, S.R.S.; Abu Hasan, H.; Othman, A.R.; Kuncoro, E.P. Performance of Chemical-Based vs Bio-BasedCoagulants in Treating Aquaculture Wastewater and Cost-Benefit Analysis. Pol. J. Environ. Stud. 2023, 32, 1177–1187. [Google Scholar] [CrossRef]
- Gao, T.; Xiao, K.; Zhang, J.; Zhang, X.; Wang, X.; Liang, S.; Sun, J.; Meng, F.; Huang, X. Cost-Benefit Analysis and Technical Efficiency Evaluation of Full-Scale Membrane Bioreactors for Wastewater Treatment Using Economic Approaches. J. Clean. Prod. 2021, 301, 126984. [Google Scholar] [CrossRef]
- Constable, D.J.C. Green and Sustainable Chemistry—The Case for a Systems-Based, Interdisciplinary Approach. iScience 2021, 24, 103489. [Google Scholar] [CrossRef] [PubMed]
- De Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of Green Chemistry and Its Multidimensional Impacts: A Review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.N.; Zhang, D.; Miller, S.J.; Rossen, K.; Chirik, P.J.; Kozlowski, M.C.; Zimmerman, J.B.; Brooks, B.W.; Savage, P.E.; Allen, D.T.; et al. Green Chemistry: A Framework for a Sustainable Future. Org. Process Res. Dev. 2021, 25, 1455–1459. [Google Scholar] [CrossRef]
- Mutlu, H.; Barner, L. Getting the Terms Right: Green, Sustainable, or Circular Chemistry? Macromol. Chem. Phys. 2022, 223, 2200111. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Trost, B.M. The Atom Economy—A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Trost, B.M. Atom Economy—A Challenge for Organic Synthesis: Homogeneous Catalysis Leads the Way. Angew. Chem. Int. Ed. Engl. 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Freund, R.; Lächelt, U.; Gruber, T.; Rühle, B.; Wuttke, S. Multifunctional Efficiency: Extending the Concept of Atom Economy to Functional Nanomaterials. ACS Nano 2018, 12, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Heard, D.M.; Lennox, A.J.J. Dichloromeldrum’s Acid (DiCMA): A Practical and Green Amine Dichloroacetylation Reagent. Org. Lett. 2021, 23, 3368–3372. [Google Scholar] [CrossRef]
- Nitti, A.; Osw, P.; Calcagno, G.; Botta, C.; Etkind, S.I.; Bianchi, G.; Po, R.; Swager, T.M.; Pasini, D. One-Pot Regiodirected Annulations for the Rapid Synthesis of π-Extended Oligomers. Org. Lett. 2020, 22, 3263–3267. [Google Scholar] [CrossRef]
- Satyanarayana, A.N.V.; Mukherjee, N.; Chatterjee, T. 100% Atom-Economical and Highly Regio- and Stereoselective Iodosulfenylation of Alkynes: A Reagentless and Sustainable Approach to Access (E)-β-Iodoalkenyl Sulfides and (Z)-Tamoxifen. Green Chem. 2023, 25, 779–788. [Google Scholar] [CrossRef]
- Kindalkar, V.S.; Kumara, K.; Bhat, S.; Dharmaprakash, S.M. An Eco-Friendly Approach for the Reduction of Graphene Oxide Using Syzygium Samarangense Fruit Extract. Mater. Chem. Phys. 2021, 261, 124224. [Google Scholar] [CrossRef]
- Vatandost, E.; Saraei, A.G.; Chekin, F.; Raeisi, S.N.; Shahidi, S. Antioxidant, Antibacterial and Anticancer Performance of Reduced Graphene Oxide Prepared via Green Tea Extract Assisted Biosynthesis. ChemistrySelect 2020, 5, 10401–10406. [Google Scholar] [CrossRef]
- Mahendran, G.B.; Ramalingam, S.J.; Rayappan, J.B.B.; Kesavan, S.; Periathambi, T.; Nesakumar, N. Green Preparation of Reduced Graphene Oxide by Bougainvillea Glabra Flower Extract and Sensing Application. J. Mater. Sci. Mater. Electron. 2020, 31, 14345–14356. [Google Scholar] [CrossRef]
- Thiyagarajulu, N.; Arumugam, S. Green Synthesis of Reduced Graphene Oxide Nanosheets Using Leaf Extract of Lantana Camara and Its In-Vitro Biological Activities. J. Clust. Sci. 2021, 32, 559–568. [Google Scholar] [CrossRef]
- Omran, B.A.; Baek, K.-H. Valorization of Agro-Industrial Biowaste to Green Nanomaterials for Wastewater Treatment: Approaching Green Chemistry and Circular Economy Principles. J. Environ. Manag. 2022, 311, 114806. [Google Scholar] [CrossRef]
- Lange, J.-P. Towards Circular Carbo-Chemicals—The Metamorphosis of Petrochemicals. Energy Environ. Sci. 2021, 14, 4358–4376. [Google Scholar] [CrossRef]
- Mohan, S.V.; Katakojwala, R. The Circular Chemistry Conceptual Framework: A Way Forward to Sustainability in Industry 4.0. Curr. Opin. Green Sustain. Chem. 2021, 28, 100434. [Google Scholar] [CrossRef]
- Guarieiro, L.; Rezende, M.; Barbosa, W.; Da Rocha, G.; Pereira, P.A.; Fernandes, D.; Lopes, W.; Mota, C.; De Andrade, J. Reaching Circular Economy through Circular Chemistry: The Basis for Sustainable Development. J. Braz. Chem. Soc. 2022, 33, 1353–1374. [Google Scholar] [CrossRef]
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular Chemistry to Enable a Circular Economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef]
- Ncube, A.; Mtetwa, S.; Bukhari, M.; Fiorentino, G.; Passaro, R. Circular Economy and Green Chemistry: The Need for Radical Innovative Approaches in the Design for New Products. Energies 2023, 16, 1752. [Google Scholar] [CrossRef]
- Arias, B.G.; Merayo, N.; Millán, A.; Negro, C. Sustainable Recovery of Wastewater to Be Reused in Cooling Towers: Towards Circular Economy Approach. J. Water Process Eng. 2021, 41, 102064. [Google Scholar] [CrossRef]
- Acevedo-García, V.; Rosales, E.; Puga, A.; Pazos, M.; Sanromán, M.A. Synthesis and Use of Efficient Adsorbents under the Principles of Circular Economy: Waste Valorisation and Electroadvanced Oxidation Process Regeneration. Sep. Purif. Technol. 2020, 242, 116796. [Google Scholar] [CrossRef]
- Vallini, J.; Willson, V.; Fernández Luco, L.; Saralegui, A.B.; Boeykens, S.P.; Piol, M.N. Demolition Waste for Adsorption of Metals: A Step towards the Circular Economy. J. Environ. Manag. 2023, 342, 118200. [Google Scholar] [CrossRef] [PubMed]
- Mladenovic, N.; Petkovska, J.; Dimova, V.; Dimitrovski, D.; Jordanov, I. Circular Economy Approach for Rice Husk Modification: Equilibrium, Kinetic, Thermodynamic Aspects and Mechanism of Congo Red Adsorption. Cellulose 2022, 29, 503–525. [Google Scholar] [CrossRef]
- Madeła, M.; Skuza, M. Towards a Circular Economy: Analysis of the Use of Biowaste as Biosorbent for the Removal of Heavy Metals. Energies 2021, 14, 5427. [Google Scholar] [CrossRef]
- Poddar, K.; Sarkar, D.; Sarkar, A. Norfloxacin Adsorption by Torrefied Coco Peat Biochar as a Novel Adsorbent in a Circular Economy Framework. Environ. Res. 2024, 251, 118711. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A Review on Wastewater Sludge Valorisation and Its Challenges in the Context of Circular Economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Dahiya, S.; Katakojwala, R.; Ramakrishna, S.; Mohan, S.V. Biobased Products and Life Cycle Assessment in the Context of Circular Economy and Sustainability. Mater. Circ. Econ. 2020, 2, 7. [Google Scholar] [CrossRef]
- Kamali, M.; Persson, K.M.; Costa, M.E.; Capela, I. Sustainability Criteria for Assessing Nanotechnology Applicability in Industrial Wastewater Treatment: Current Status and Future Outlook. Environ. Int. 2019, 125, 261–276. [Google Scholar] [CrossRef]
- Rashidi, J.; Rhee, G.; Kim, M.; Nam, K.; Heo, S.; Yoo, C.; Karbassi, A. Life Cycle and Economic Assessments of Key Emerging Energy Efficient Wastewater Treatment Processes for Climate Change Adaptation. Int. J. Environ. Res. 2018, 12, 815–827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).