Study of Microstructure, Texture, and Cooking Qualities of Reformulated Whole Wheat Flour Pasta by Substituting Water with Stearic Acid–Candelilla Wax–Groundnut Oil Oleogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oleogel Samples
2.3. Pasta Preparation
2.4. Thickness and Width of the Pasta
2.5. Colorimetry
2.6. Microscopy
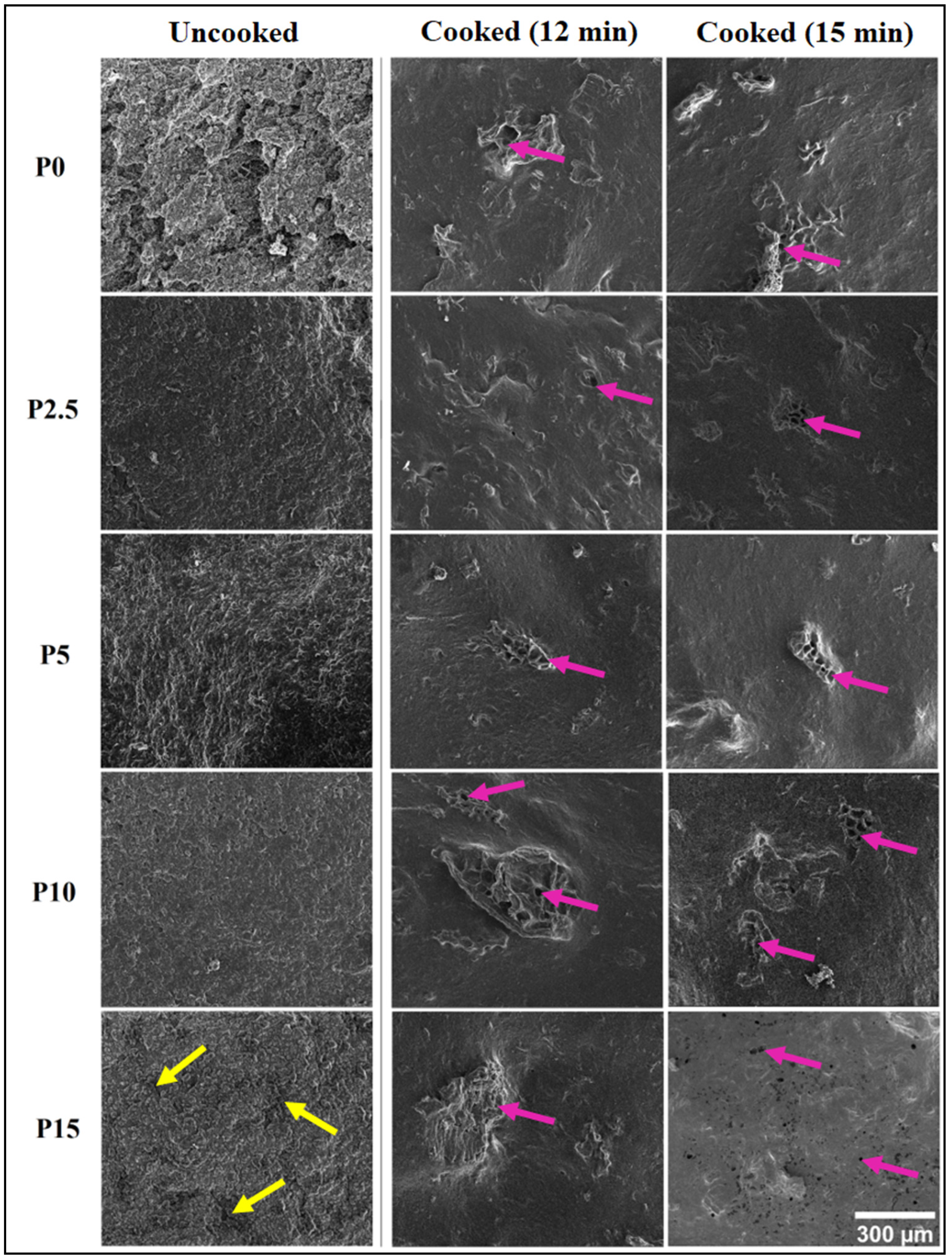
2.6.1. Surface Topology
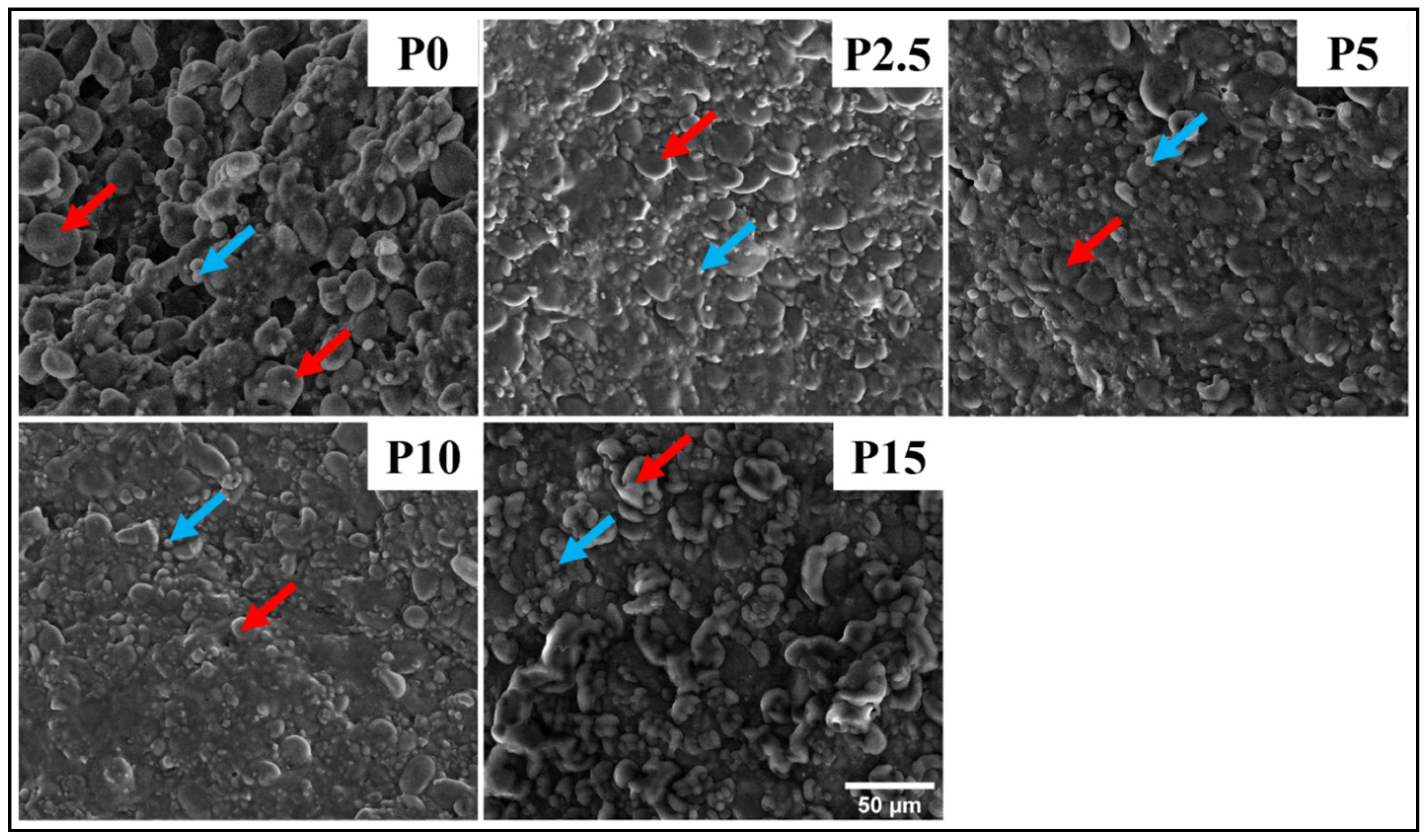
2.6.2. FESEM
2.7. Cooking Qualities of Pasta
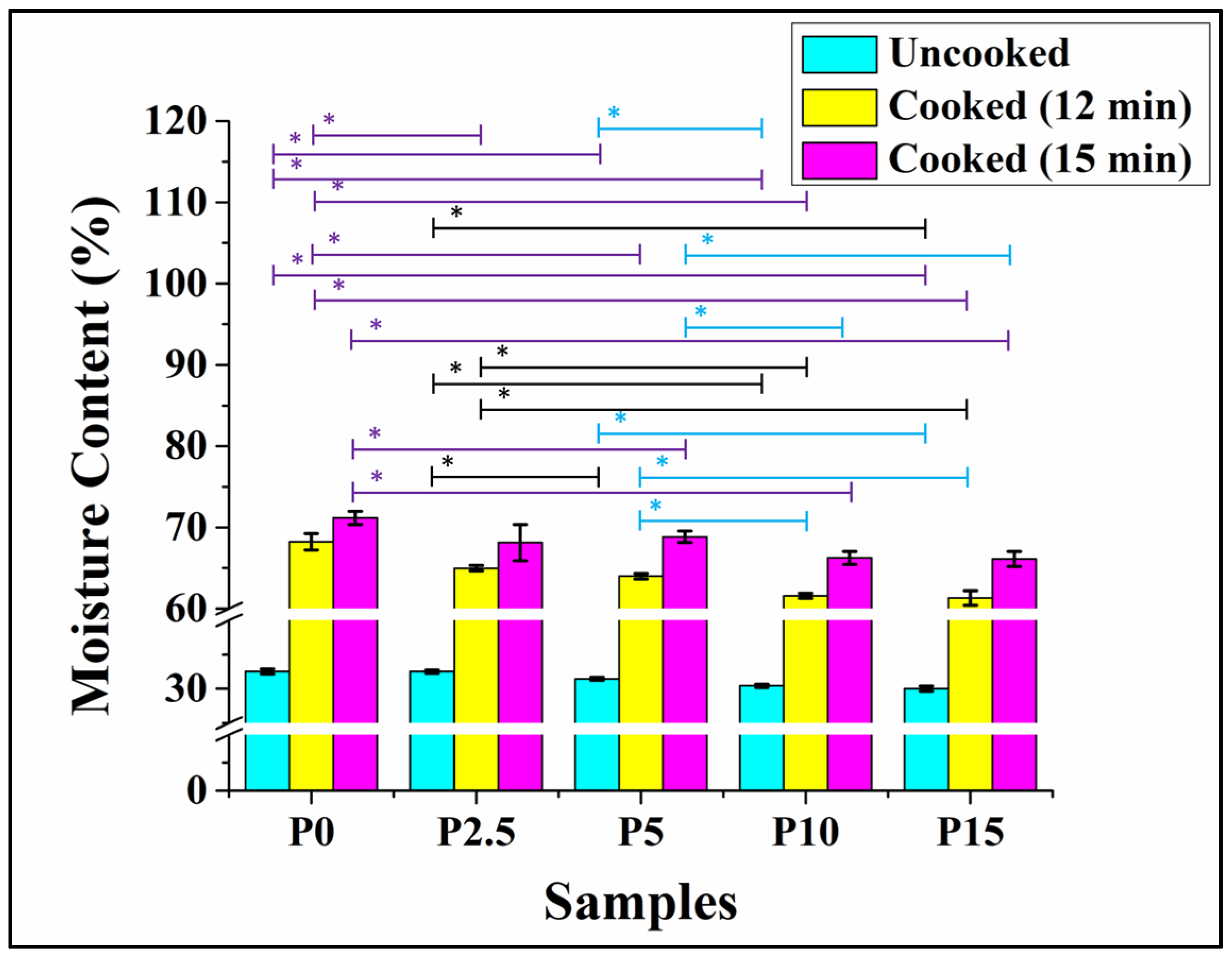
2.8. Moisture Analysis
2.9. Texture Analysis
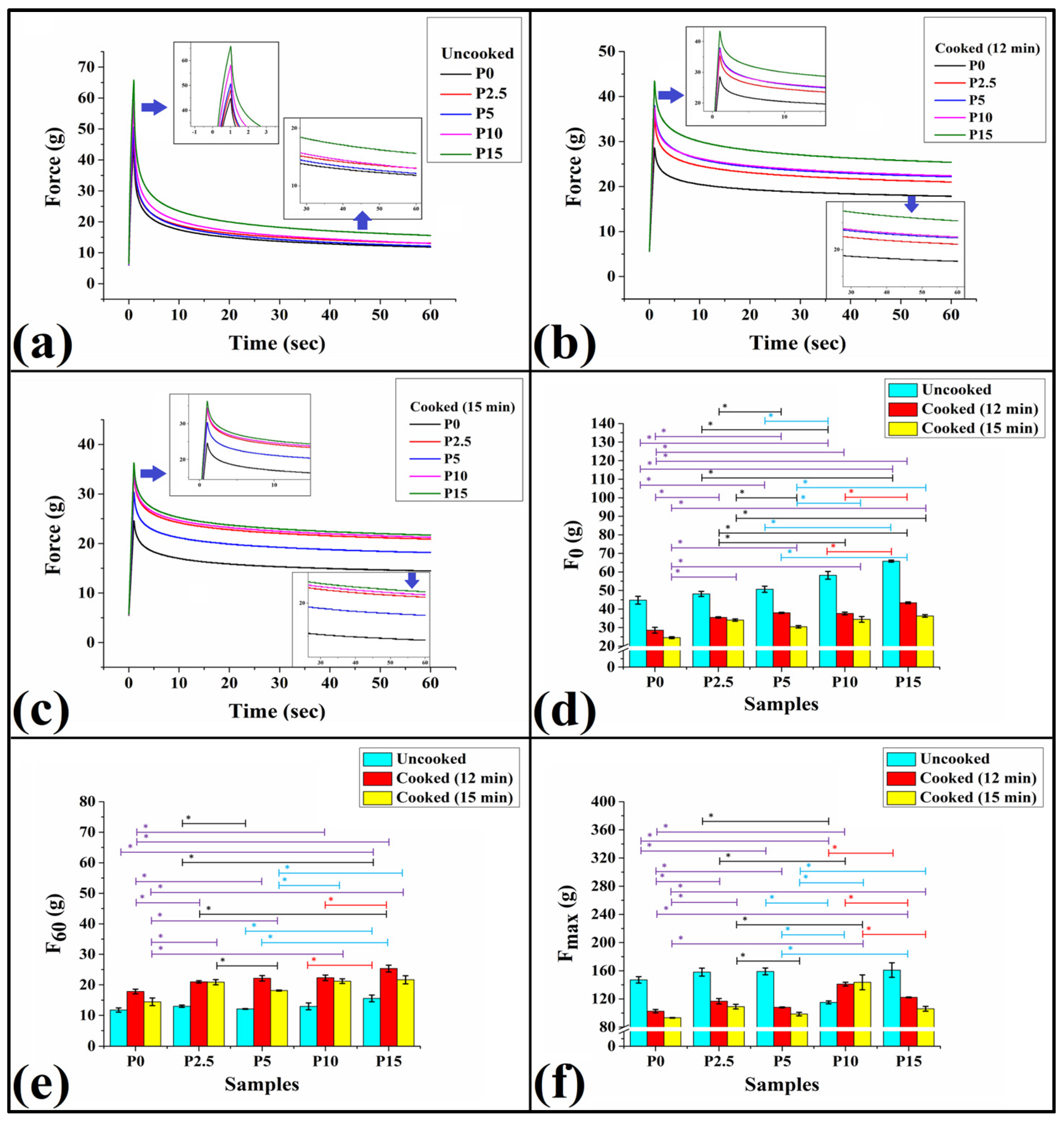
2.9.1. Stress Relaxation
2.9.2. Puncture Test
2.10. FTIR
2.11. Statistical Analysis
3. Results
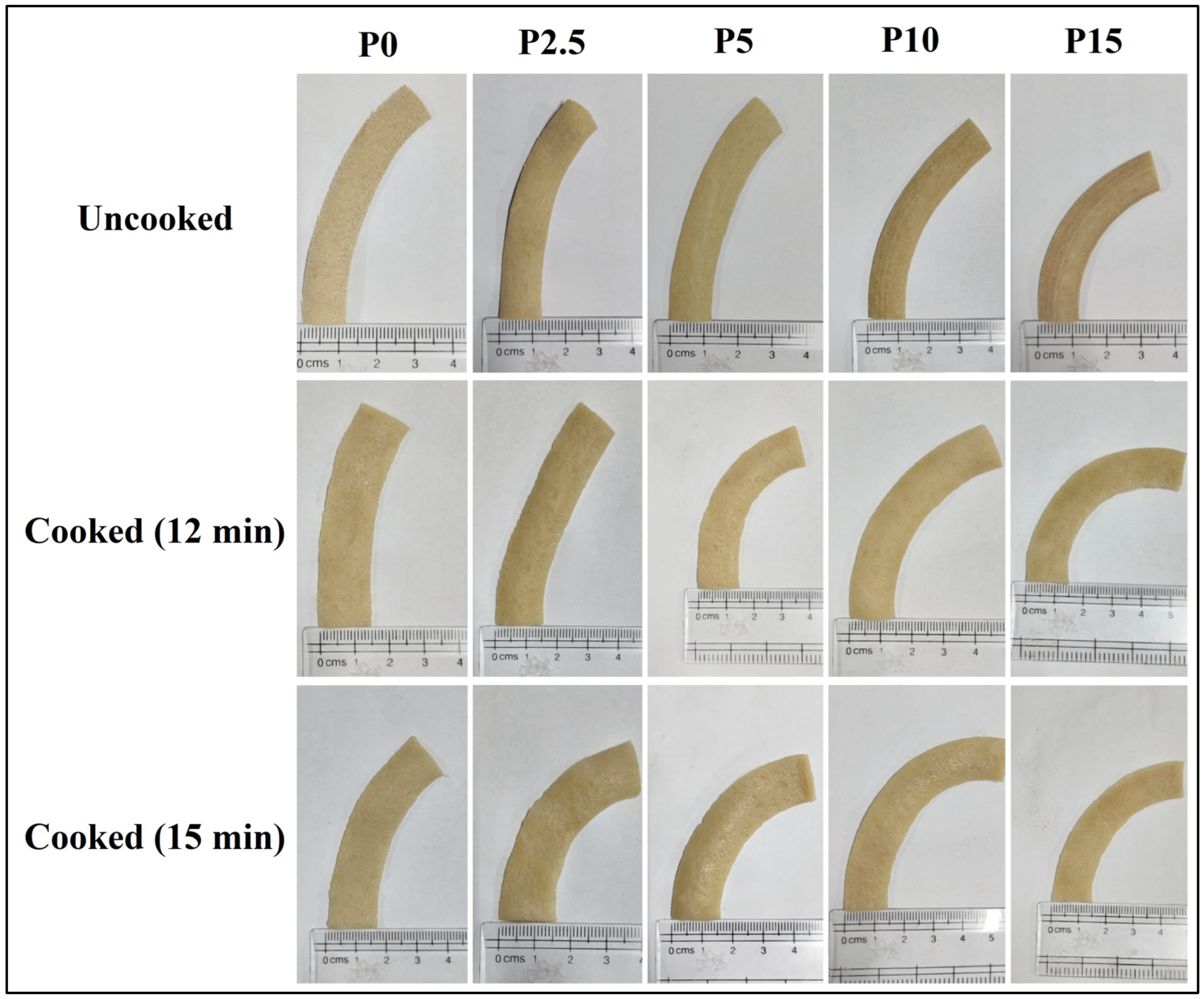
3.1. Visual Appearance of Pasta Samples
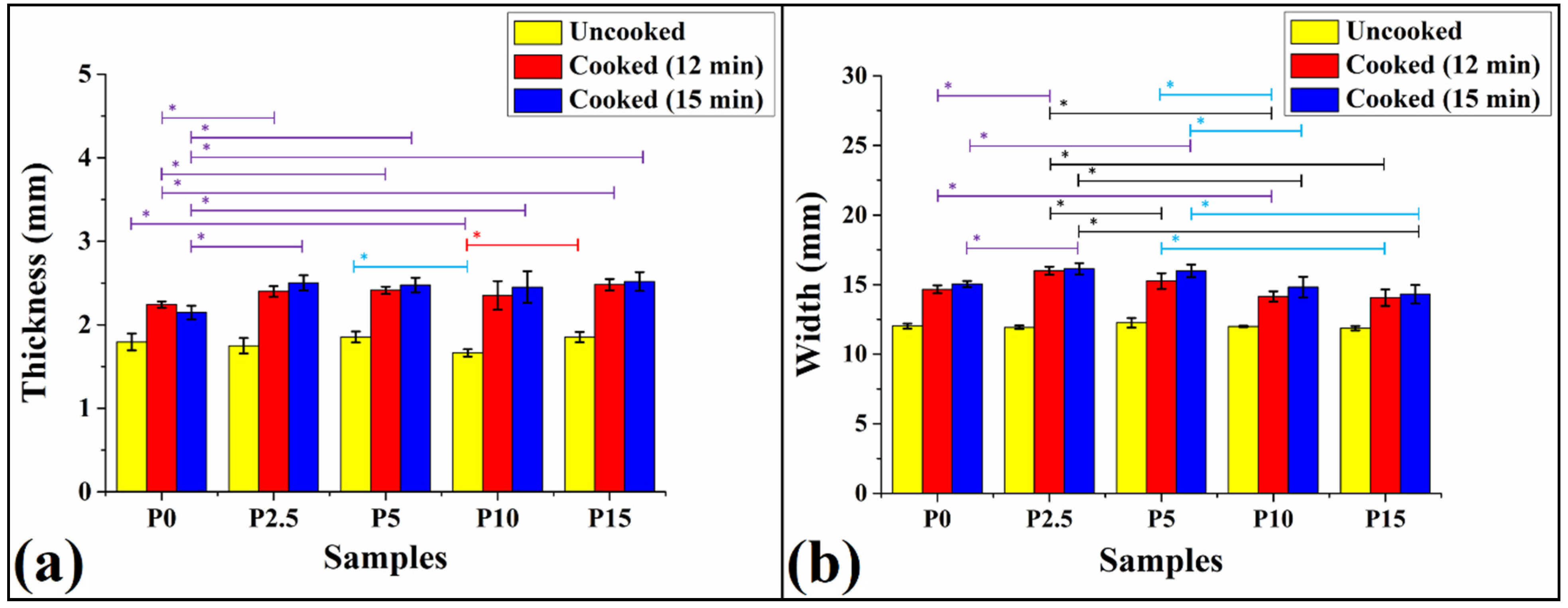
3.2. Thickness and Width of Pasta Samples
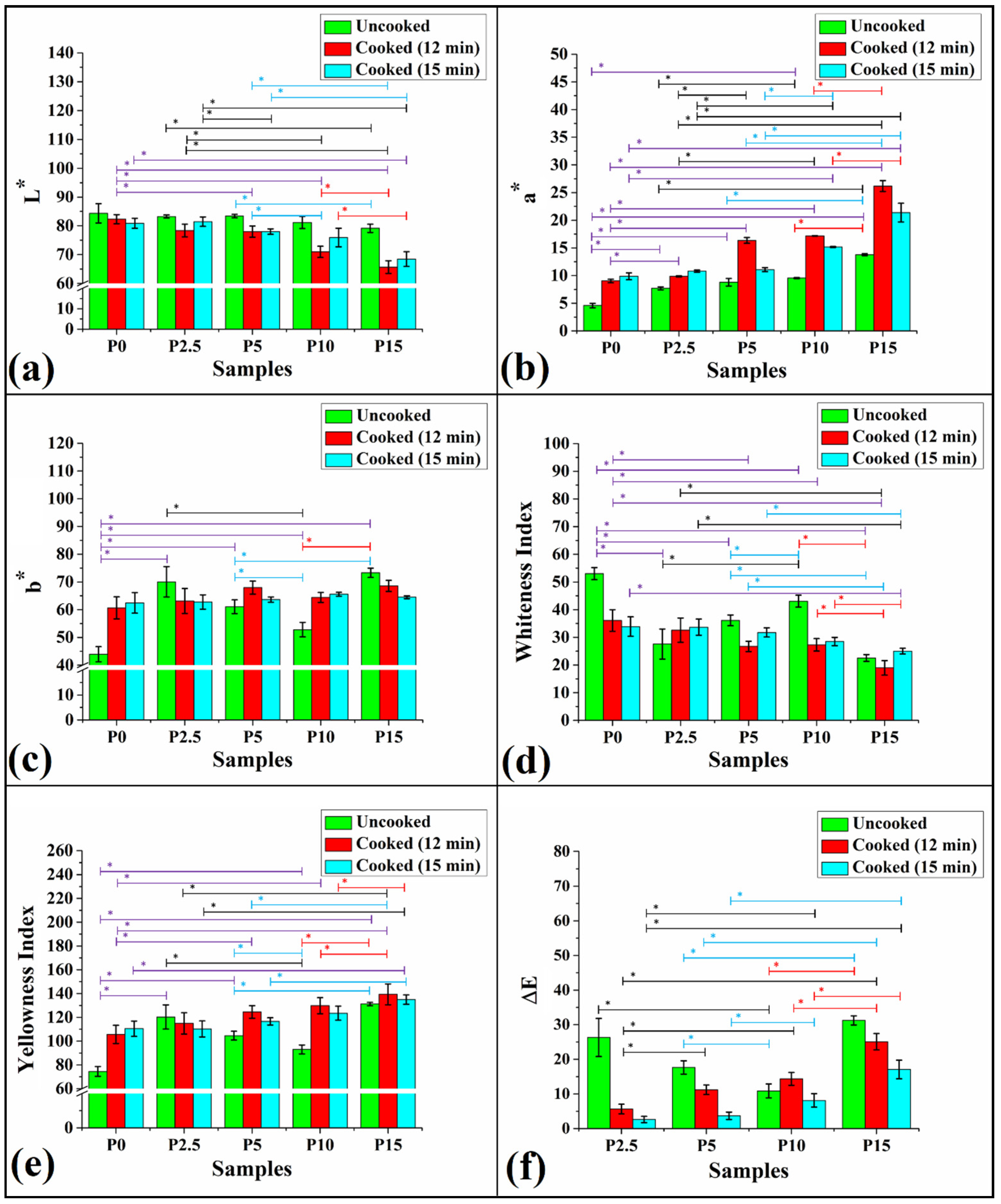
3.3. Colorimetry
3.4. Microscopy
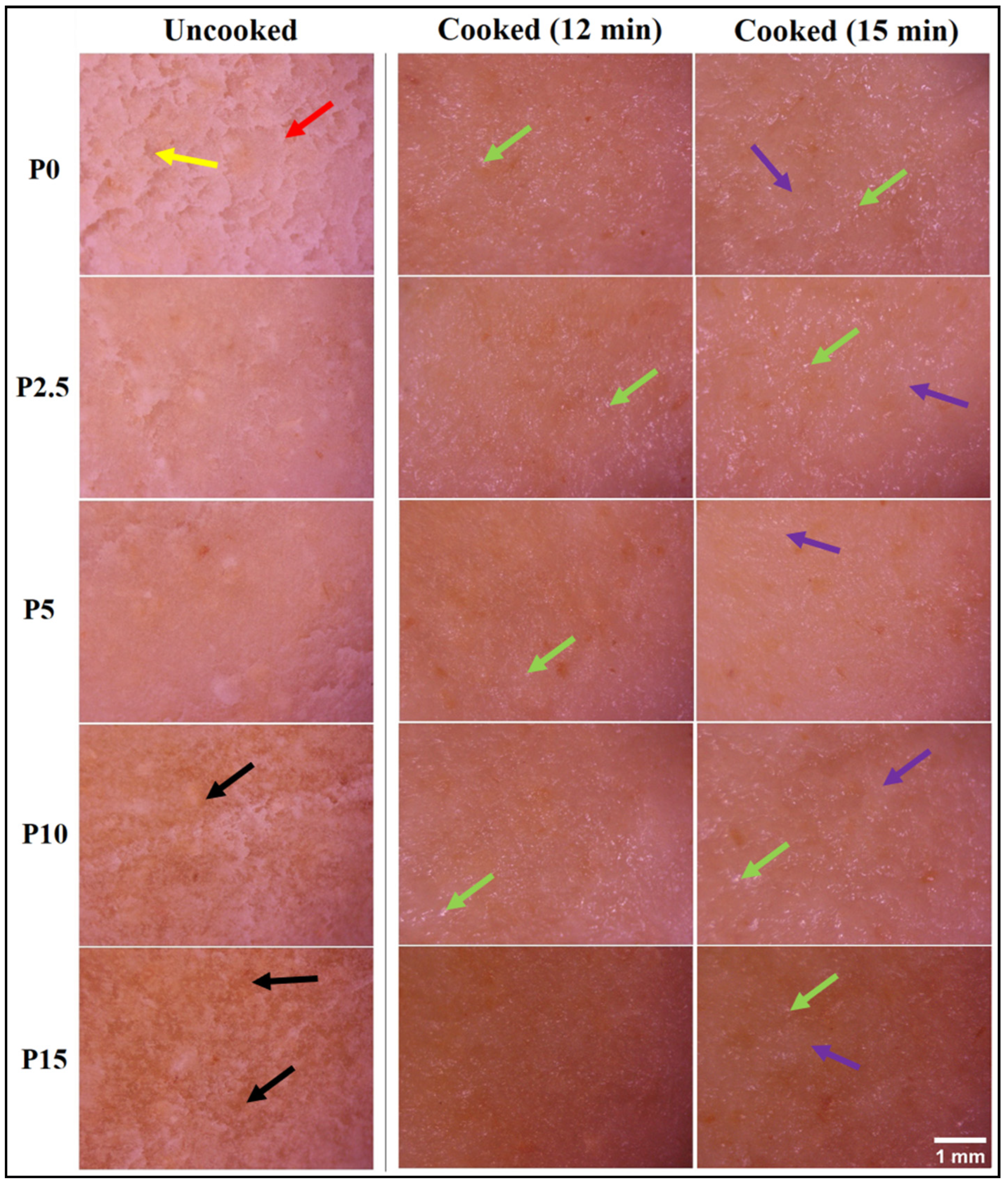
3.4.1. Surface Topology
3.4.2. FESEM
3.5. Cooking Qualities of Pasta
Water Absorption Capacity (WAC), Swelling Index (SI), and Dry Matter
3.6. Moisture Analysis
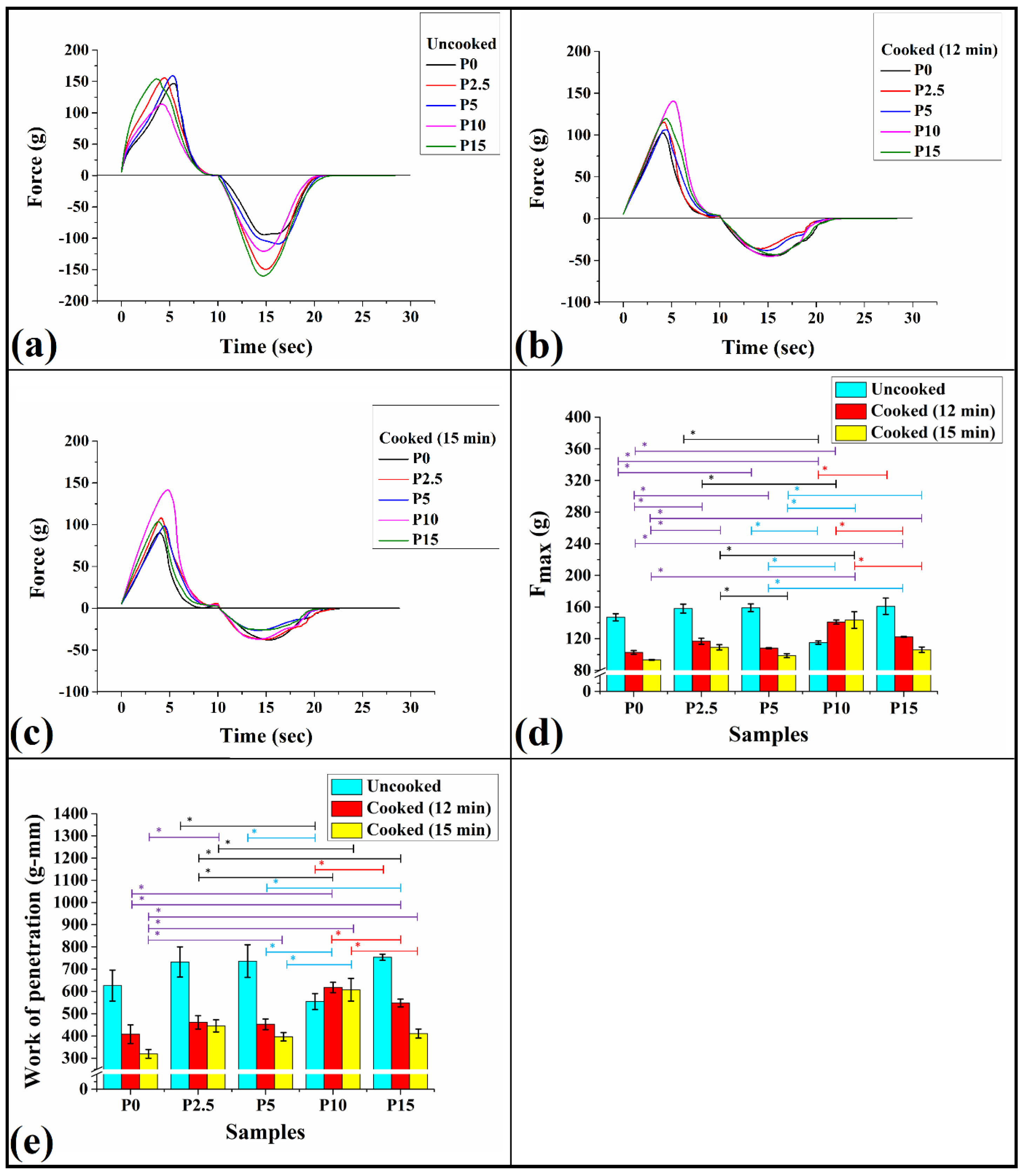
3.7. Texture Analysis
3.7.1. Stress Relaxation Test
3.7.2. Puncture Test
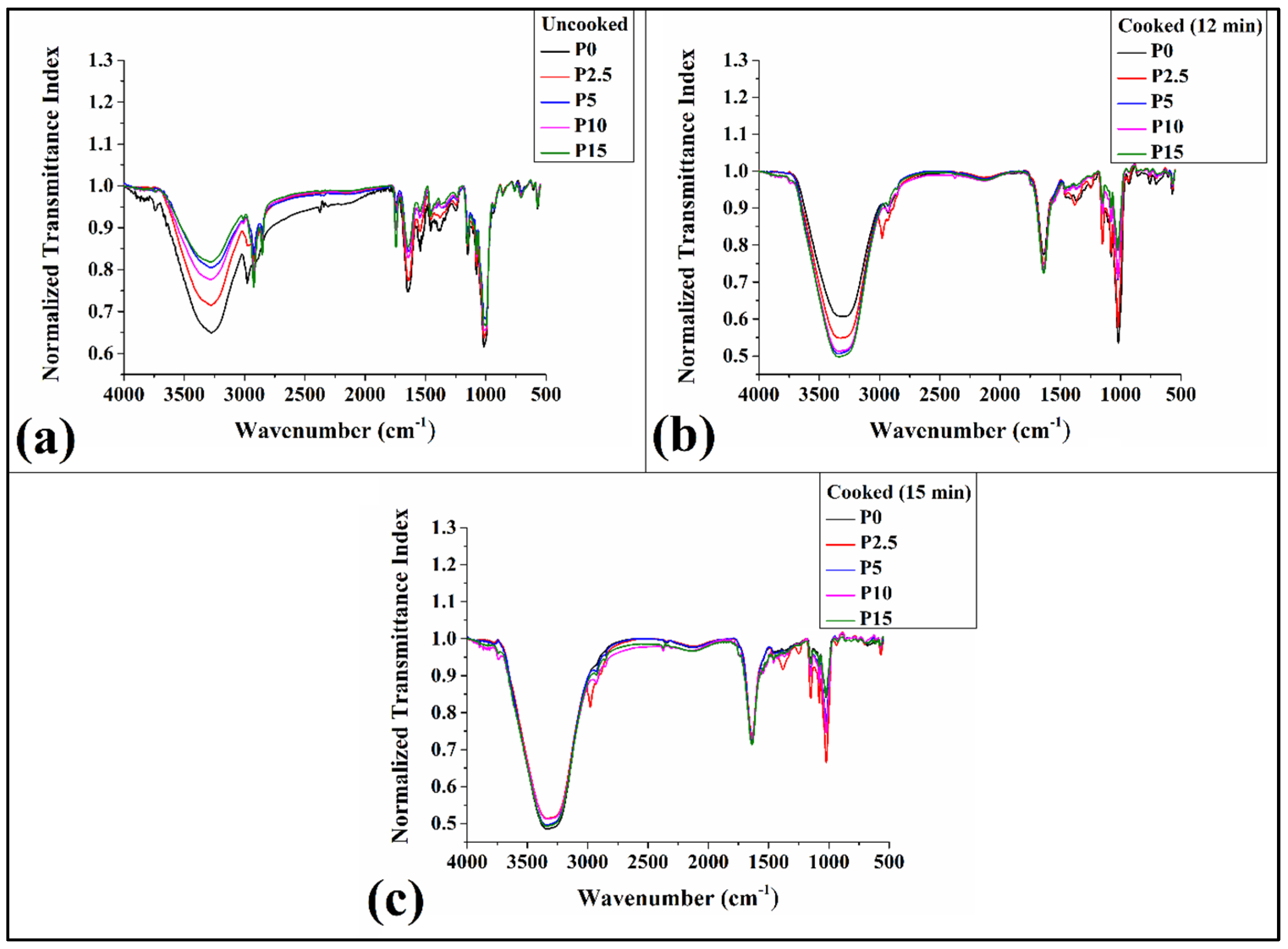
3.8. FTIR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Palla, C.A.; Wasinger, M.F.; Carrín, M.E. Monoglyceride oleogels as fat replacers in filling creams for sandwich cookies. J. Sci. Food Agric. 2021, 101, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Giacomozzi, A.S.; Carrín, M.E.; Palla, C.A. Muffins made with monoglyceride oleogels: Impact of fat replacement on sensory properties and fatty acid profile. J. Am. Oil Chem. Soc. 2023, 100, 343–349. [Google Scholar] [CrossRef]
- Martins, A.J.; Lorenzo, J.M.; Franco, D.; Vicente, A.A.; Cunha, R.L.; Pastrana, L.M.; Quiñones, J.; Cerqueira, M.A. Omega-3 and polyunsaturated fatty acids-enriched hamburgers using sterol-based oleogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1900111. [Google Scholar] [CrossRef]
- Oh, I.K.; Amoah, C.; Lim, J.; Jeong, S.; Lee, S. Assessing the effectiveness of wax-based sunflower oil oleogels in cakes as a shortening replacer. LWT 2017, 86, 430–437. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Żbikowska, A. Effect of high-oleic rapeseed oil oleogels on the quality of short-dough biscuits and fat migration. J. Food Sci. Technol. 2020, 57, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, E.; Öğütcü, M. The texture, sensory properties and stability of cookies prepared with wax oleogels. Food Funct. 2015, 6, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Bascuas, S.; Espert, M.; Llorca, E.; Quiles, A.; Salvador, A.; Hernando, I. Structural and sensory studies on chocolate spreads with hydrocolloid-based oleogels as a fat alternative. LWT 2021, 135, 110228. [Google Scholar] [CrossRef]
- Zulim Botega, D.C.; Marangoni, A.G.; Smith, A.K.; Goff, H.D. The potential application of rice bran wax oleogel to replace solid fat and enhance unsaturated fat content in ice cream. J. Food Sci. 2013, 78, C1334–C1339. [Google Scholar] [CrossRef] [PubMed]
- Espert, M.; Hernández, M.; Sanz, T.; Salvador, A. Reduction of saturated fat in chocolate by using sunflower oil-hydroxypropyl methylcellulose based oleogels. Food Hydrocoll. 2021, 120, 106917. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in microstructural and physicochemical properties of candelilla wax/rice bran oil–derived oleogels using sunflower lecithin and soya lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Cerqueira, M.A.; Mohanty, B.; Habibullah, S.; Banerjee, I.; Pal, K. Effect of biodegradable hydrophilic and hydrophobic emulsifiers on the oleogels containing sunflower wax and sunflower oil. Gels 2021, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Bharti, D.; Kim, D.; Banerjee, I.; Rousseau, D.; Pal, K. Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels. Gels 2022, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Brochard, M.; Correia, P.; Barroca, M.J.; Guiné, R.P. Development of a new pasta product by the incorporation of chestnut flour and bee pollen. Appl. Sci. 2021, 11, 6617. [Google Scholar] [CrossRef]
- Sissons, M.; Sestili, F.; Botticella, E.; Masci, S.; Lafiandra, D. Can manipulation of durum wheat amylose content reduce the glycaemic index of spaghetti? Foods 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Fuad, T.; Prabhasankar, P. Role of ingredients in pasta product quality: A review on recent developments. Crit. Rev. Food Sci. Nutr. 2010, 50, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Brennan, M.A.; Brennan, C.S. The effect of semolina replacement with protein powder from fish (Pseudophycis bachus) on the physicochemical characteristics of pasta. LWT 2018, 89, 52–57. [Google Scholar] [CrossRef]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. LWT 2019, 114, 108368. [Google Scholar] [CrossRef]
- De Pasquale, I.; Verni, M.; Verardo, V.; Gómez-Caravaca, A.M.; Rizzello, C.G. Nutritional and functional advantages of the use of fermented black chickpea flour for semolina-pasta fortification. Foods 2021, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Lomuscio, E.; Bianchi, F.; Cervini, M.; Giuberti, G.; Simonato, B.; Rizzi, C. Durum Wheat Fresh Pasta Fortification with Trub, a Beer Industry By-Product. Foods 2022, 11, 2496. [Google Scholar] [CrossRef] [PubMed]
- Vernon-Carter, E.J.; Alvarez-Ramirez, J.; Meraz, M.; Bello-Perez, L.A.; Garcia-Diaz, S. Canola oil/candelilla wax oleogel improves texture, retards staling and reduces in vitro starch digestibility of maize tortillas. J. Sci. Food Agric. 2020, 100, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, D.; Bharti, D.; Dhal, S.; Sahu, D.; Behera, H.; Sahoo, M.; Kim, D.; Jarzębski, M.; Anis, A.; Mohanty, B. Role of stearic acid as the crystal habit modifier in candelilla wax-groundnut oil oleogels. ChemEngineering 2023, 7, 96. [Google Scholar] [CrossRef]
- Patil, S.; Sonawane, S.K.; Arya, S. Chemometric approach-based characterization and screening of gluten free flours for development of Indian unleavened flatbread. J. Food Sci. Technol. 2021, 58, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Prerana, S.; Anupama, D. Influence of carrot puree incorporation on quality characteristics of instant noodles. J. Food Process Eng. 2020, 43, e13270. [Google Scholar] [CrossRef]
- Kadiri, O.; Gbadamosi, S.O.; Akanbi, C.T. Texture profile analysis and stress relaxation characteristics of protein fortified sweetpotato noodles. J. Texture Stud. 2020, 51, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Pradhan, B.K.; Mahapatra, P.; Ray, S.S.; Chakravarty, S.; Pal, K. Development of a low-cost food color monitoring system. Color Res. Appl. 2021, 46, 430–445. [Google Scholar] [CrossRef]
- Sena, B.; Dhal, S.; Sahu, D.; Sarkar, P.; Mohanty, B.; Jarzębski, M.; Wieruszewski, M.; Behera, H.; Pal, K. Variations in Microstructural and Physicochemical Properties of Soy Wax/Soybean Oil-Derived Oleogels Using Soy Lecithin. Polymers 2022, 14, 3928. [Google Scholar] [CrossRef] [PubMed]
- International, A.; Latimer, G.W. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Tudorica, C.; Kuri, V.; Brennan, C. Nutritional and physicochemical characteristics of dietary fiber enriched pasta. J. Agric. Food Chem. 2002, 50, 347–356. [Google Scholar] [CrossRef]
- Noonim, P.; Rajasekaran, B.; Venkatachalam, K. Structural Characterization and Peroxidation Stability of Palm Oil-Based Oleogel Made with Different Concentrations of Carnauba Wax and Processed with Ultrasonication. Gels 2022, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Alzuwaid, N.T.; Fellows, C.M.; Laddomada, B.; Sissons, M. Impact of wheat bran particle size on the technological and phytochemical properties of durum wheat pasta. J. Cereal Sci. 2020, 95, 103033. [Google Scholar] [CrossRef]
- Lehnert, S.; Dubinina, A.; Deynichenko, H.; Khomenko, O.; Gapontseva, O.; Antoniuk, I.; Medvedeva, A.; Demichkovska, M.; Vasylieva, O. The study of influence of natural antioxidants on quality of peanut and linseed oil blends during their storage. Вoстoчнo-Еврoпейский Журнал Передoвых Технoлoгий 2018, 3, 44–50. [Google Scholar] [CrossRef]
- Nurhasanah, S.; Setyadi, A.; Munarso, S.; Subroto, E.; Filianty, F. Shelf-Life Prediction of Peanut Oil (Arachis hypogaea L.) Using an Accelerated Shelf-Life Testing (ASLT) Method in the Polypropylene Packaging. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 3rd International Conference on Agricultural Postharvest Handling an Processing, Bogor, Indonesia, 12–14 October 2021; IOP Publishing Ltd.: Bristol, UK, 2022; p. 012056. [Google Scholar]
- Kuntzler, S.G.; Costa, J.A.V.; Brizio, A.P.D.R.; de Morais, M.G. Development of a colorimetric pH indicator using nanofibers containing Spirulina sp. LEB 18. Food Chem. 2020, 328, 126768. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, X.; Zhu, Y.; Zeng, Y.; Fang, C.; Liu, Y.; Hu, S.; Ge, Y.; Jiang, W. Preparation and application of a colorimetric film based on sodium alginate/sodium carboxymethyl cellulose incorporated with rose anthocyanins. Food Chem. 2022, 393, 133342. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.S.; Bharti, D.; Pradhan, B.K.; Sahu, D.; Dhal, S.; Kim, N.M.; Jarzębski, M.; Pal, K. Analysis of the Physical and Structure Characteristics of Reformulated Pizza Bread. Foods 2022, 11, 1979. [Google Scholar] [CrossRef]
- Demirkesen, I.; Mert, B. Recent developments of oleogel utilizations in bakery products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2460–2479. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.J.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Edible oleogels: An opportunity for fat replacement in foods. Food Funct. 2018, 9, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ramirez, J.; Vernon-Carter, E.; Carrera-Tarela, Y.; Garcia, A.; Roldan-Cruz, C. Effects of candelilla wax/canola oil oleogel on the rheology, texture, thermal properties and in vitro starch digestibility of wheat sponge cake bread. LWT 2020, 130, 109701. [Google Scholar] [CrossRef]
- Jung, D.; Oh, I.; Lee, J.; Lee, S. Utilization of butter and oleogel blends in sweet pan bread for saturated fat reduction: Dough rheology and baking performance. LWT 2020, 125, 109194. [Google Scholar] [CrossRef]
- Turgut, Y.; Turgut, S.S.; Karacabey, E. Use of ohmic heating as an alternative method for cooking pasta. J. Sci. Food Agric. 2021, 101, 5529–5540. [Google Scholar] [CrossRef]
- Espinosa-Solis, V.; Zamudio-Flores, P.B.; Tirado-Gallegos, J.M.; Ramírez-Mancinas, S.; Olivas-Orozco, G.I.; Espino-Díaz, M.; Hernández-González, M.; García-Cano, V.G.; Sánchez-Ortíz, O.; Buenrostro-Figueroa, J.J. Evaluation of cooking quality, nutritional and texture characteristics of pasta added with oat bran and apple flour. Foods 2019, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, A.; Surasani, V.K.R.; Sharma, S. Influence of supplementation with pangas protein isolates on textural attributes and sensory acceptability of semolina pasta. J. Food Meas. Charact. 2021, 15, 1317–1326. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Q.; Zhou, X.; Li, X.; Wang, F.; Liu, Y. Identification of characteristic starch properties of wheat varieties used to commercially produce dried noodles. Int. J. Food Sci. Technol. 2021, 56, 794–803. [Google Scholar] [CrossRef]
- Ikegwu, T.M.; Nkama, I.; Okafor, I.G. Comparative studies of the proximate, microscopic and thermal properties of processed maize, wheat, millet, cassava and Bambara nut flours. Acta Sci. Nutr. Health 2023, 7, 38–47. [Google Scholar] [CrossRef]
- Zhao, M.; Rao, J.; Chen, B. Effect of high oleic soybean oil oleogels on the properties of doughs and corresponding bakery products. J. Am. Oil Chem. Soc. 2022, 99, 1071–1083. [Google Scholar] [CrossRef]
- González, A.; Bordón, M.G.; Bustos, M.C.; Cordova Salazar, K.L.; Ribotta, P.D.; Martínez, M.L. Study of the incorporation of native and microencapsulated chia seed oil on pasta properties. Int. J. Food Sci. Technol. 2021, 56, 233–241. [Google Scholar] [CrossRef]
- Witek, M.; Maciejaszek, I.; Surówka, K. Impact of enrichment with egg constituents on water status in gluten-free rice pasta–nuclear magnetic resonance and thermogravimetric approach. Food Chem. 2020, 304, 125417. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Oliva, N.; Cini, E. A systematic review of gluten-free dough and bread: Dough rheology, bread characteristics, and improvement strategies. Appl. Sci. 2020, 10, 6559. [Google Scholar] [CrossRef]
- Martin, C.; Morel, M.-H.; Reau, A.; Cuq, B. Kinetics of gluten protein-insolubilisation during pasta processing: Decoupling between time-and temperature-dependent effects. J. Cereal Sci. 2019, 88, 103–109. [Google Scholar] [CrossRef]
- Purwadi, R.; Teguh, C.; Mazaya, D. Fermented cassava as an alternative flour for pasta noodle. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Seminar on Chemical Engineering Soehadi Reksowardojo (STKSR 2020), Bandung, Indonesia, 16–17 November 2020; IOP Publishing Ltd.: Bristol, UK, 2021; p. 012042. [Google Scholar]
- Chen, H.; Guo, X.-N.; Zhu, K.-X. The effect of chitosan oligosaccharides on the shelf-life and quality of fresh wet noodles. Carbohydr. Polym. 2023, 309, 120704. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, J. Mechanical properties of maize kernel horny endosperm, floury endosperm and germ. Int. J. Food Prop. 2019, 22, 863–877. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Cao, M.-J.; Liu, G.-M. Texture analyzers for food quality evaluation. In Evaluation Technologies for Food Quality; Elsevier: Amsterdam, The Netherlands, 2019; pp. 441–463. [Google Scholar]
- Garcia-Valle, D.E.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Structural characteristics and in vitro starch digestibility of pasta made with durum wheat semolina and chickpea flour. LWT 2021, 145, 111347. [Google Scholar] [CrossRef]
- Garcia-Valle, D.E.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effects of mixing, sheeting, and cooking on the starch, protein, and water structures of durum wheat semolina and chickpea flour pasta. Food Chem. 2021, 360, 129993. [Google Scholar] [CrossRef] [PubMed]
- Kamble, D.B.; Singh, R.; Rani, S.; Pratap, D. Physicochemical properties, in vitro digestibility and structural attributes of okara-enriched functional pasta. J. Food Process. Preserv. 2019, 43, e14232. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Dong, F.; Tian, A.; Li, Z.; Dai, Y. Physical, mechanical and antimicrobial properties of starch films incorporated with ε-poly-l-lysine. Food Chem. 2015, 166, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yue, Q.; Liu, C.; Zheng, X.; Hong, J.; Li, L.; Bian, K. Effect of gliadin/glutenin ratio on pasting, thermal, and structural properties of wheat starch. J. Cereal Sci. 2020, 93, 102973. [Google Scholar] [CrossRef]

| Samples | Wheat Flour (g) | Water (g) | Oleogel Amount (g) | Total Amount (g) |
|---|---|---|---|---|
| P0 | 250 | 95 | 0 | 345 |
| P2.5 | 250 | 92.625 | 2.375 | 345 |
| P5 | 250 | 90.25 | 4.75 | 345 |
| P10 | 250 | 85.5 | 9.5 | 345 |
| P15 | 250 | 80.75 | 14.25 | 345 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaturvedi, D.; Dhal, S.; Sahu, D.; Jarzębski, M.; Anis, A.; Kim, D.; Pal, K. Study of Microstructure, Texture, and Cooking Qualities of Reformulated Whole Wheat Flour Pasta by Substituting Water with Stearic Acid–Candelilla Wax–Groundnut Oil Oleogel. ChemEngineering 2024, 8, 51. https://doi.org/10.3390/chemengineering8030051
Chaturvedi D, Dhal S, Sahu D, Jarzębski M, Anis A, Kim D, Pal K. Study of Microstructure, Texture, and Cooking Qualities of Reformulated Whole Wheat Flour Pasta by Substituting Water with Stearic Acid–Candelilla Wax–Groundnut Oil Oleogel. ChemEngineering. 2024; 8(3):51. https://doi.org/10.3390/chemengineering8030051
Chicago/Turabian StyleChaturvedi, Diksha, Somali Dhal, Deblu Sahu, Maciej Jarzębski, Arfat Anis, Doman Kim, and Kunal Pal. 2024. "Study of Microstructure, Texture, and Cooking Qualities of Reformulated Whole Wheat Flour Pasta by Substituting Water with Stearic Acid–Candelilla Wax–Groundnut Oil Oleogel" ChemEngineering 8, no. 3: 51. https://doi.org/10.3390/chemengineering8030051
APA StyleChaturvedi, D., Dhal, S., Sahu, D., Jarzębski, M., Anis, A., Kim, D., & Pal, K. (2024). Study of Microstructure, Texture, and Cooking Qualities of Reformulated Whole Wheat Flour Pasta by Substituting Water with Stearic Acid–Candelilla Wax–Groundnut Oil Oleogel. ChemEngineering, 8(3), 51. https://doi.org/10.3390/chemengineering8030051









