Microwave-Assisted Synthesis of Titanosilicates Using a Precursor Produced from Titanium Ore Concentrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Abbreviations
2.2. Methods for Analysis
2.2.1. Synthesis of a Titanium-Containing Precursor
2.2.2. Microwave Synthesis of IONSIVE-911 Phase
2.2.3. TGA and DSC Analysis
2.2.4. IR Analysis
2.2.5. XRD Analysis
2.2.6. BET Surface Properties
2.2.7. Sorption of Cs+ Cations from CsCl Model Solution
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, N.; Singh, A.; Ahmad, W. Microwave Assisted Green Synthesis of Silver Nanoparticles and Its Application: A Review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 663–672. [Google Scholar] [CrossRef]
- Kubrakova, I.V.; Koshcheeva, I.Y.; Pryazhnikov, D.V.; Martynov, L.Y.; Kiseleva, M.S.; Tyutyunnik, O.A. Microwave Synthesis, Properties and Analytical Capabilities of Nanoscale Magnetite-Based Sorption Materials. J. Anal. Chem. 2014, 69, 378–389. [Google Scholar] [CrossRef]
- Gabano, E.; Ravera, M. Microwave-Assisted Synthesis: Can Transition Metal Complexes Take Advantage of This “Green” Method?: A Review. Molecules 2022, 27, 4249. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Dwyer, F.G.; Vartuli, J.C. Crystallization Method Employing Microwave Radiation. U.S. Patent 4778666, 18 October 1988. [Google Scholar]
- Li, Y.; Yang, W. Microwave Synthesis of Zeolite Membranes: A Review. J. Memb. Sci. 2008, 316, 3–17. [Google Scholar] [CrossRef]
- Cundy, C.S. Microwave Techniques in the Synthesis and Modification of Zeolite Catalysts. A Review. Collect. Czechoslov. Chem. Commun. 1998, 63, 1699–1723. [Google Scholar] [CrossRef]
- Chan, C.; Manap, N.I.; Din, N.S.M.N.M.; Hazmi, A.S.A.H.; Kow, K.W.; Ho, Y.K. Strategy to scale up Microwave Synthesis with insight into the thermal and non-thermal effects from energy-based perspective. Chem. Eng. Process.-Process Intensif. 2021, 168, 108594. [Google Scholar] [CrossRef]
- Coutinho, D.; Losilla, J.A.; Balkus, K.J. Microwave Synthesis of ETS-4 and ETS-4 Thin Films. Microporous Mesoporous Mater. 2006, 90, 229–236. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, Y. Porous Materials for Catalysis: Toward Sustainable Synthesis and Applications of Zeolites. In Sustainable Nanoscale Engineering; Szekely, G., Livingston, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 5; pp. 115–137. [Google Scholar] [CrossRef]
- Losilla, J.A.; Balkus, K.J. Microwave Assisted Synthesis of ETS-10. J. Porous Mater. 2009, 16, 487. [Google Scholar] [CrossRef]
- Kuznicki, S.M.; Bell, V.A.; Nair, S.; Hillhouse, H.W.; Jacubinas, R.M.; Braunbarth, C.M.; Toby, B.H.; Tsapatsis, M. A Titanosilicate Molecular Sieve with Adjustable Pores for Size-Selective Adsorption of Molecules. Nature 2001, 412, 720–724. [Google Scholar] [CrossRef]
- Shariaty, P.; Jahandar Lashaki, M.; Hashisho, Z.; Sawada, J.; Kuznicki, S.; Hutcheon, R. Effect of ETS-10 Ion Exchange on Its Dielectric Properties and Adsorption/Microwave Regeneration. Sep. Purif. Technol. 2017, 179, 420–427. [Google Scholar] [CrossRef]
- De Raffele, G.; Aloise, A.; De Luca, P.; Vuono, D.; Tagarelli, A.; Nagy, J.B. Kinetic and Thermodynamic Effects during the Adsorption of Heavy Metals on ETS-4 and ETS-10 Microporous Materials. J. Porous Mater. 2016, 23, 389–400. [Google Scholar] [CrossRef]
- Celestian, A.J.; Powers, M.; Rader, S. In Situ Raman Spectroscopic Study of Transient Polyhedral Distortions during Cesium Ion Exchange into Sitinakite. Am. Mineral. 2013, 98, 1153–1161. [Google Scholar] [CrossRef]
- Figueiredo, B.R.; Cardoso, S.P.; Portugal, I.; Rocha, J.; Silva, C.M. Inorganic Ion Exchangers for Cesium Removal from Radioactive Wastewater. Sep. Purif. Rev. 2018, 47, 306–336. [Google Scholar] [CrossRef]
- Koo, T.H.; Kim, J.W.; Park, K.R. Formation of Pharmacosiderite (KFe4(AsO4)3(OH)4∙6-7H2O) in the Acid-Sulfate-Chloride (ASC) Geothermal Spring, Norris Geyser Basin, Yellowstone National Park, USA: Implication of Fe and As Redox Reaction Associated with Microbe and Clay Minerals. Appl. Clay Sci. 2022, 216, 106343. [Google Scholar] [CrossRef]
- Majzlan, J.; Haase, P.; Plášil, J.; Dachs, E. Synthesis and Stability of Some Members of the Pharmacosiderite Group, AFe4(OH)4(AsO4)3∙nH2O (A = K, Na, 0.5Ba, 0.5Sr). Can. Mineral. 2019, 57, 663–675. [Google Scholar] [CrossRef]
- Lin, Z.; Ferdov, S. Temperature and Time Controlled Crystallization in Na2O–SiO2–TiO2–H2O System. Microporous Mesoporous Mater. 2022, 335, 111835. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Kalashnikova, G.O.; Nikolaev, A.I.; Perovskiy, I.A.; Bazai, A.V.; Yakovenchuk, V.N.; Bocharov, V.N.; Kabanova, N.A.; Krivovichev, S.V. Ion-Exchange-Induced Transformation and Mechanism of Cooperative Crystal Chemical Adaptation in Sitinakite: Theoretical and Experimental Study. Minerals 2022, 12, 248. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Nikolaev, A.P.; Selivanova, E.A.; Pakhomovsky, Y.A.; Korchak, J.A.; Spiridonova, D.V.; Zalkind, O.A.; Krivovichev, S.V. Ivanyukite-Na-T, Ivanyukite-Na-C, Ivanyukite-K, and Ivanyukite-Cu: New Microporous Titanosilicates from the Khibiny Massif (Kola Peninsula, Russia) and Crystal Structure of Ivanyukite-Na-T. Am. Mineral. 2009, 94, 1450–1458. [Google Scholar] [CrossRef]
- Santos-Vieira, I.C.M.S.; Lin, Z.; Rocha, J. Towards the Sustainable Synthesis of Microporous and Layered Titanosilicates: Mechanochemical Pre-Treatment Reduces the Water Amount. Green Chem. 2022, 24, 5088–5096. [Google Scholar] [CrossRef]
- Milyutin, V.V.; Nekrasova, N.A.; Yanicheva, N.Y.; Kalashnikova, G.O.; Ganicheva, Y.Y. Sorption of Cesium and Strontium Radionuclides onto Crystalline Alkali Metal Titanosilicates. Radiochemistry 2017, 59, 65–69. [Google Scholar] [CrossRef]
- Samburov, G.O.; Kalashnikova, G.O.; Panikorovskii, T.L.; Bocharov, V.N.; Kasikov, A.; Selivanova, E.; Bazai, A.V.; Bernadskaya, D.; Yakovenchuk, V.N.; Krivovichev, S.V. A Synthetic Analog of the Mineral Ivanyukite: Sorption Behavior to Lead Cations. Crystals 2022, 12, 311. [Google Scholar] [CrossRef]
- Nikolaev, A.I.; Gerasimova, L.G.; Maslova, M.V.; Shchukina, E.S. Sorption of Cesium and Strontium Radionuclides by Synthetic Ivanyukite from Model and Process Solutions. Theor. Found. Chem. Eng. 2021, 55, 1078–1085. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L.; Cui, H. Functional Ag-Doped Coralloid Titanosilicate Zeolite (CTS-Ag) for Efficiently Catalytic and Photodegradative Removal of Free Cyanides and Copper/Zinc-Cyanide Complexes in Real Wastewater. J. Alloys Compd. 2022, 926, 166848. [Google Scholar] [CrossRef]
- Kosa, S.A.; El Maksod, I.H.; Hegazy, E.Z.; Baamer, D.F. Photo Catalytic Behavior of Some Pharmacosiderite Titanium Analogs. Silicon 2020, 12, 813–820. [Google Scholar] [CrossRef]
- Tratnjek, T.; Deschanels, X.; Hertz, A.; Rey, C.; Causse, J. Ti/Si Ratio as a Tool to Tailor the Microstructure of Titanate-Based Crystalline Phases Able to Selectively Trap Strontium over Calcium. J. Hazard. Mater. 2022, 440, 129755. [Google Scholar] [CrossRef]
- Oleksiienko, O.; Sillanpää, M. Sol–Gel Synthesized Titanosilicates for the Uptake of Radionuclides. In Advanced Water Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 361–444. ISBN 9780128192160. [Google Scholar]
- Milcent, T.; Hertz, A.; Barré, Y.; Grandjean, A. Influence of the Nb Content and Microstructure of Sitinakite-Type Crystalline Silicotitanates (CST) on Their Sr2+ and Cs+ Sorption Properties. Chem. Eng. J. 2021, 426, 131425. [Google Scholar] [CrossRef]
- Perovskiy, I.A.; Khramenkova, E.V.; Pidko, E.A.; Krivoshapkin, P.V.; Vinogradov, A.V.; Krivoshapkina, E.F. Efficient Extraction of Multivalent Cations from Aqueous Solutions into Sitinakite-Based Sorbents. Chem. Eng. J. 2018, 354, 727–739. [Google Scholar] [CrossRef]
- Perovskiy, I.A.; Yanicheva, N.Y.; Stalyugin, V.V.; Panikorovskii, T.L.; Golov, A.A. Sorption of Multivalent Cations on Titanosilicate Obtained from Natural Raw Materials. The Mechanism and Thermodynamics of Sorption. Microporous Mesoporous Mater. 2021, 311, 110716. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Men’shikov, Y.P. Khibiny; F. Wall. Laplandia Minerals: Apatity, Russia, 2005; ISBN 5-900395-48-0. p. 467. [Google Scholar]
- Oleksiienko, O.; Wolkersdorfer, C.; Sillanpää, M. Titanosilicates in Cation Adsorption and Cation Exchange—A Review. Chem. Eng. J. 2017, 317, 570–585. [Google Scholar] [CrossRef]
- Li, G.; Li, M.; Zhang, X.; Cao, P.; Jiang, H.; Luo, J.; Jiang, T. Hydrothermal Synthesis of Zeolites-Calcium Silicate Hydrate Composite from Coal Fly Ash with Co-Activation of Ca(OH)2-NaOH for Aqueous Heavy Metals Removal. Int. J. Min. Sci. Technol. 2022, 32, 563–573. [Google Scholar] [CrossRef]
- Gerasimova, L.; Nikolaev, A.; Maslova, M.E.S. Method for Producing Titanium-Silicon Sodium-Containing Product. RU Patent 2680493 C1.Bull №6, 21 February 2019. [Google Scholar]
- Gerasimova, L.; Shukina, E.; Maslova, M.; Nikolaev, A.; Toshio, O.O.H. Method of Preparation of Sodium-Containing Titanosilicate Sorbent. RU Patent 2644614 C1. Bull №25, 21 February 2019. [Google Scholar]
- Maslova, M.; Ivanenko, V.; Gerasimova, L.; Larsson, A.-C.; Antzutkin, O.N. Synthesis of Titanium Phosphates from Unconventional Solid Precursor and Their Ion-Exchange and Electrochemical Properties. J. Mater. Sci. 2021, 56, 9929–9950. [Google Scholar] [CrossRef]
- Maslova, M.; Ivanenko, V.; Yanicheva, N.; Gerasimova, L. The Effect of Heavy Metal Ions Hydration on Their Sorption by a Mesoporous Titanium Phosphate Ion-Exchanger. J. Water Process Eng. 2020, 35, 101233. [Google Scholar] [CrossRef]
- Gerasimova, L.G.; Nikolaev, A.I.; Shchukina, E.S.; Maslova, M.V. Hydrothermal Synthesis of Framed Titanosilicates with a Structure of Ivanyukite Mineral. Rep. Acad. Sci. 2019, 487, 289–292. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases (accessed on 15 September 2023).
- Schulman, E.; Wu, W.; Liu, D. Two-Dimensional Zeolite Materials: Structural and Acidity Properties. Materials 2020, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Feliczak-Guzik, A. Hierarchical Zeolites: Synthesis and Catalytic Properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Cazula, B.; Oliveira, L.; Machado, B.; Alves, H. Optimization of experimental conditions for the synthesis of Si-MCM-41 molecular sieves using different methods and silica sources. Mater. Chem. Phys. 2021, 266, 124553. [Google Scholar] [CrossRef]
- Chen, T.; Maddrell, E.; Gandy, A.; Stennett, M. Hriljac, Transformation of Cs-IONSIV into a ceramic wasteform by hot isostatic pressing. J. Nucl. Mater. 2018, 498, 33–43. [Google Scholar] [CrossRef]
- Gerasimova, L.G.; Maslova, M.V.; Shchukina, E.S. The Technology of Sphene Concentrate Treatment to Obtain Titanium Salts. Theor. Found. Chem. Eng. 2009, 43, 464–467. [Google Scholar] [CrossRef]
- Votyakov, S.; Kiseleva, D.; Grokhovsky, V.; Shchapova, Y. Minerals: Structure, Properties, Methods of Investigation; Votyakov, S., Kiseleva, D., Grokhovsky, V., Shchapova, Y., Eds.; Springer Proceedings in Earth and Environmental Sciences: Cham, Switzerland; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-00924-3. [Google Scholar]
- Chukanov, N.V. eBook. Infrared Spectra of Mineral Species: Extended Library; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2014; Volume 1, ISBN 978-94-007-7127-7. [Google Scholar]
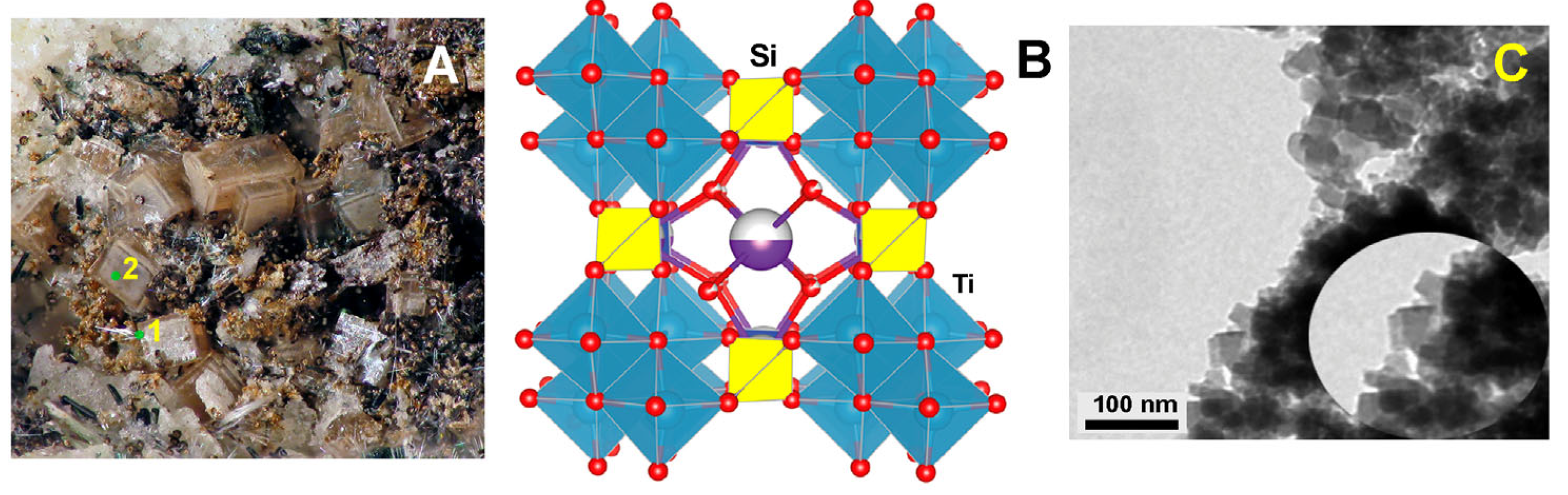

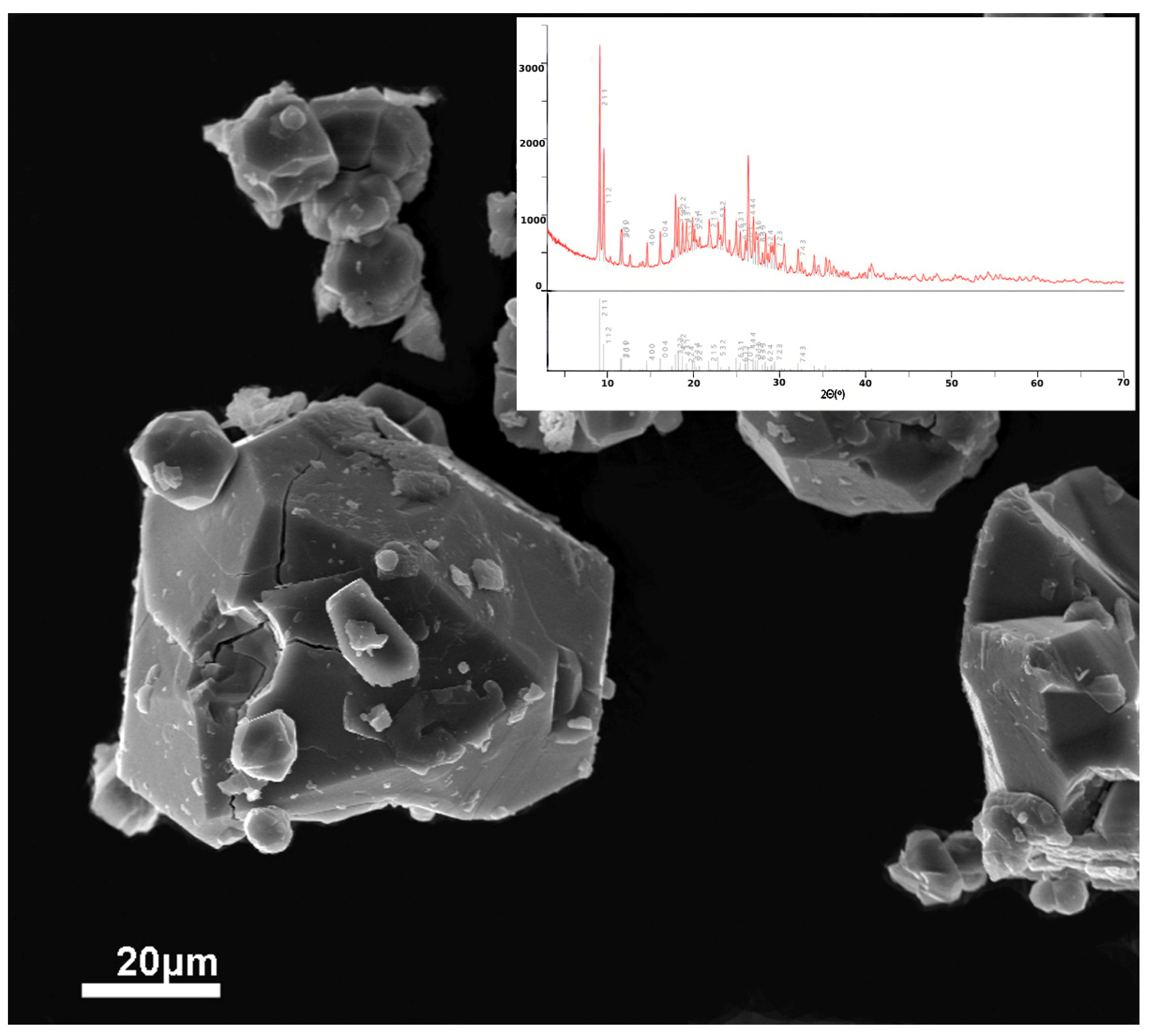
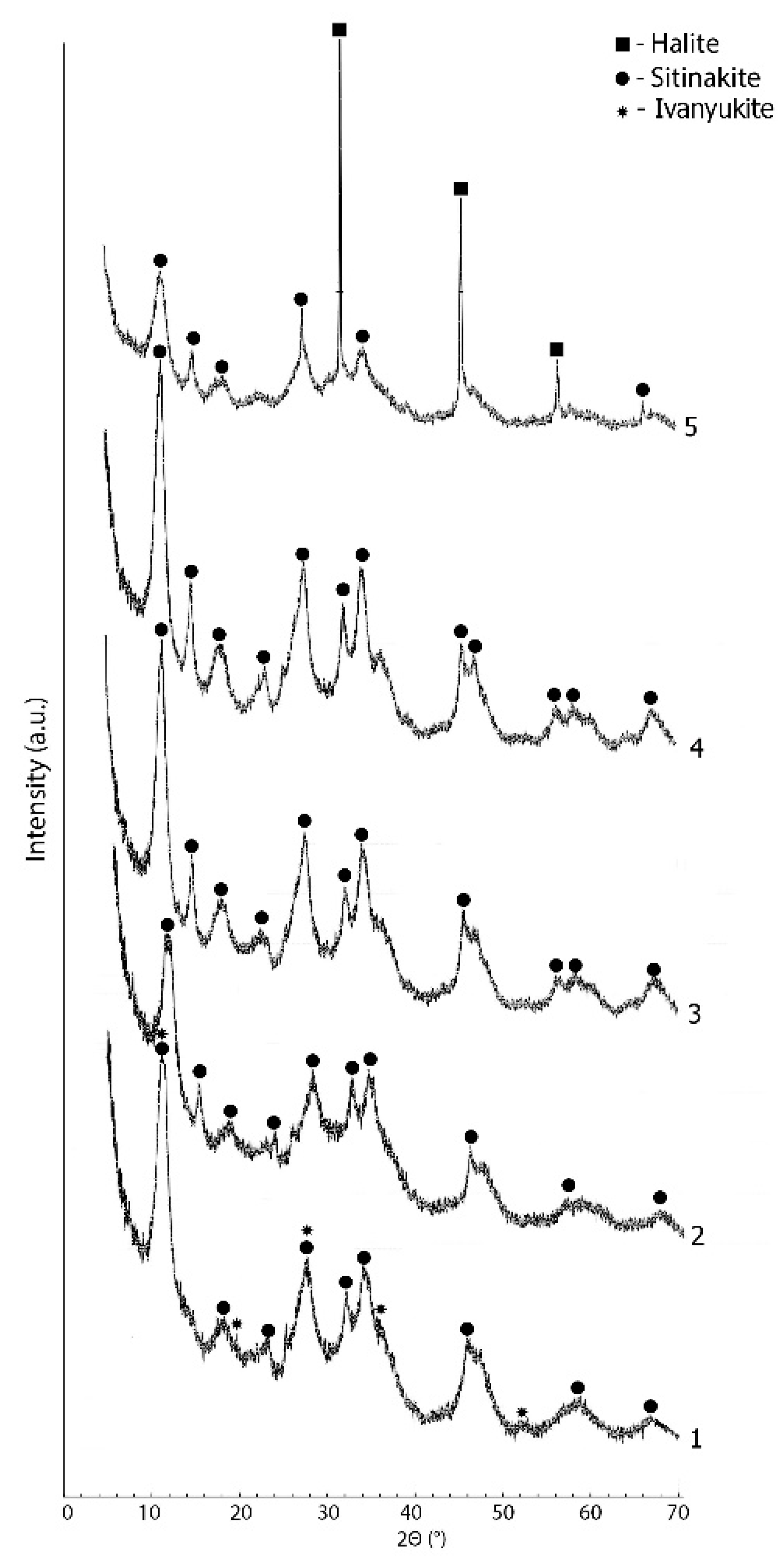

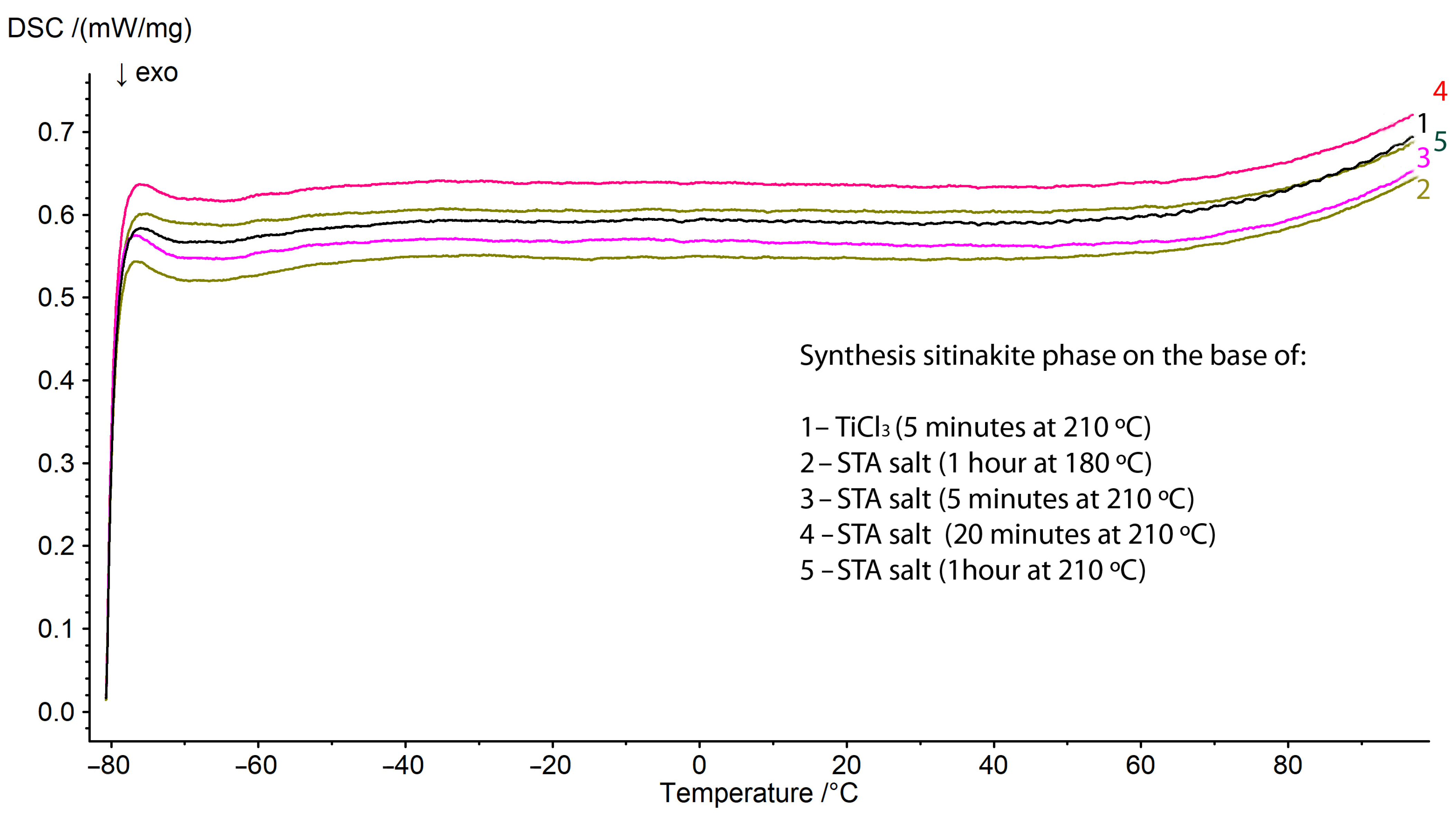
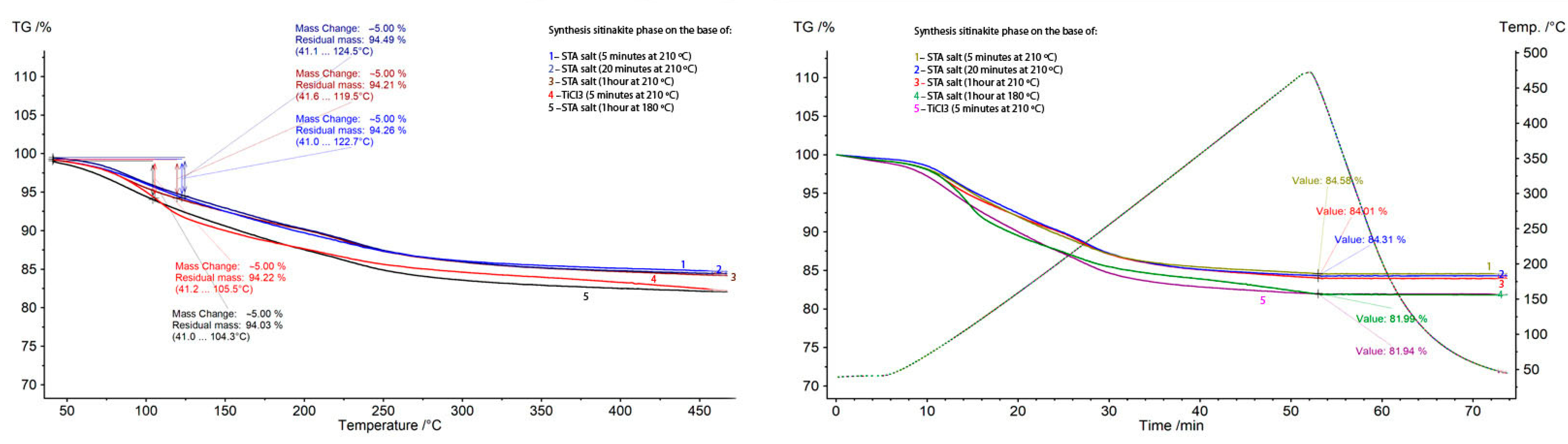
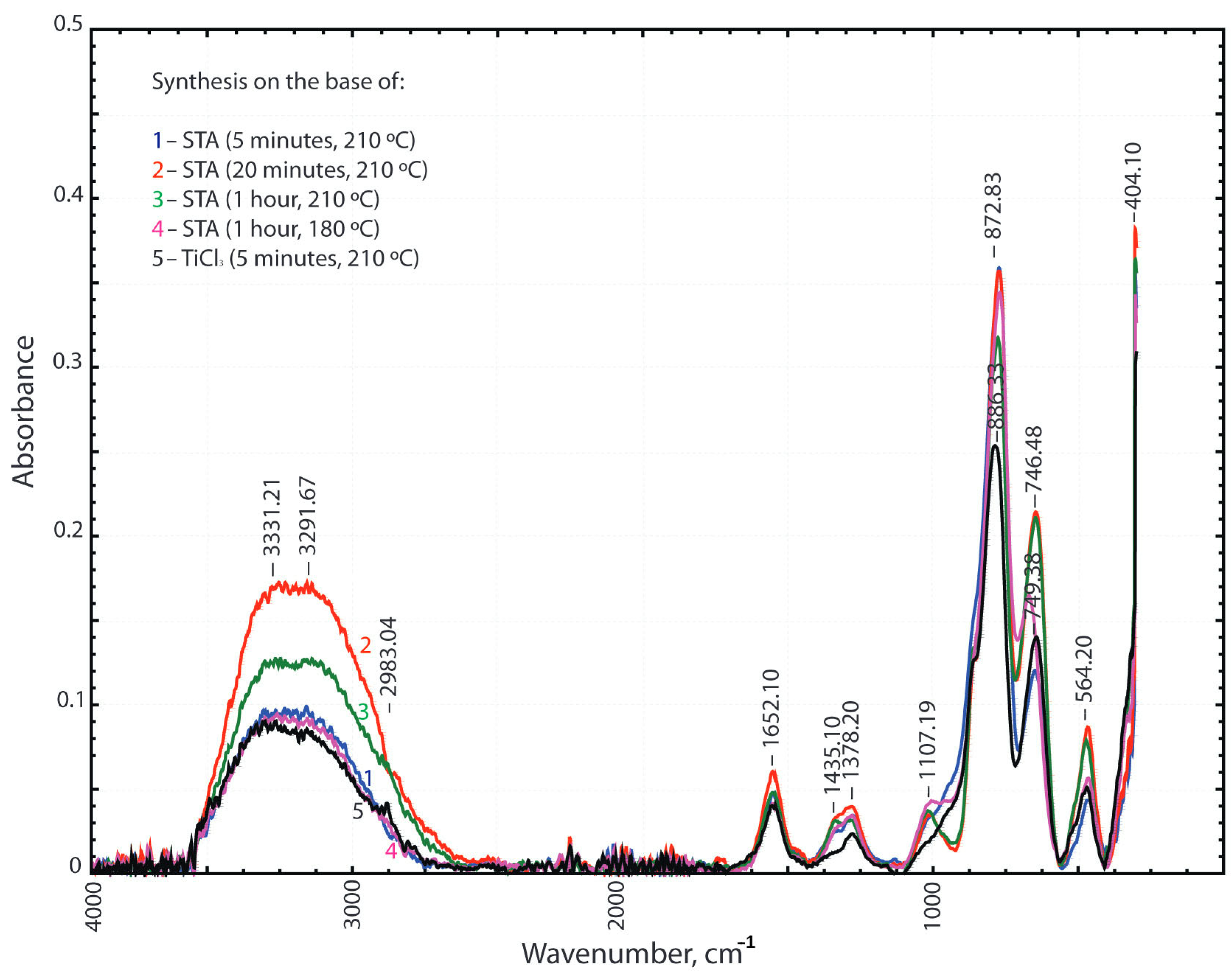
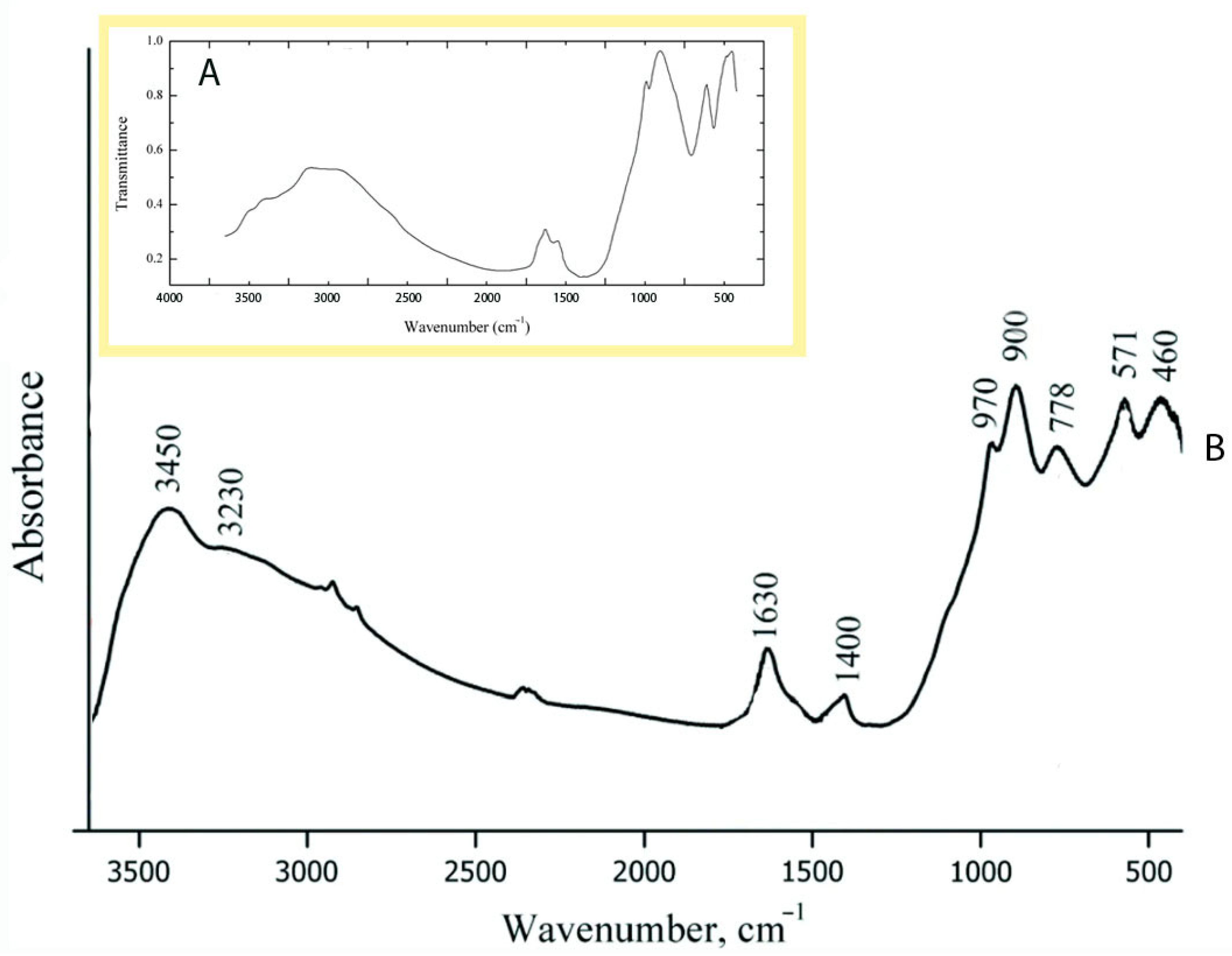
| Initial Components for Synthesis with STA Salt | Treatment Time, Min | Initial Components for Synthesis with TiCl3, g | Treatment Time, Min | ||||
|---|---|---|---|---|---|---|---|
| 5 | 20 | 60 | 5 | 20 | 60 | ||
| Temperature 150 °C | |||||||
| Weight of Components, g | |||||||
| STA-Based Product | TiCl3-Based Product | ||||||
| Na2SiO3∙5H2O | 7.802 | 7.798 | 7.796 | - | - | - | - |
| NaOH | 2.520 | 2.528 | 2.530 | - | - | - | - |
| STA salt | 4.636 | 4.635 | 4.637 | - | - | - | - |
| H2Odistilled | 50 | 50 | 50 | - | - | - | - |
| Temperature 180 °C | |||||||
| Weight of Components, g | |||||||
| STA-Based Product | TiCl3-Based Product | ||||||
| Na2SiO3∙5H2O | 7.797 | 7.799 | 7.796 | Na2SiO3∙5H2O | 7.797 | 7.797 | 7.799 |
| NaOH | 2.520 | 2.519 | 2.521 | NaOH | 2.527 | 2.519 | 2.527 |
| STA salt | 4.634 | 4.635 | 4.635 | TiCl3 | 16.800 | 16.800 | 16.800 |
| H2Odistilled | 50 | 50 | 50 | H2Odistilled | 25 | 25 | 25 |
| Temperature 210 °C | |||||||
| Weight of Components, g | |||||||
| STA-Based Product | TiCl3-Based Product | ||||||
| Na2SiO3∙5H2O | 7.796 | 7.797 | 7.798 | Na2SiO3∙5H2O | 7.798 | 7.786 | 7.799 |
| NaOH | 2.560 | 2.522 | 2.600 | NaOH | 5.262 | 2.564 | 2.561 |
| STA salt | 4.637 | 4.636 | 4.637 | TiCl3 | 16.800 | 16.800 | 16.800 |
| H2Odistilled | 50 | 50 | 50 | H2Odistilled | 25 | 25 | 25 |
| Number of Synthesis | Ti Source | T, °C | Time, min | XRD Result | Chemical Composition of the Mother Solutions, g/L | |||
|---|---|---|---|---|---|---|---|---|
| Na | S | Si | Ti | |||||
| 1 | STA | 150 | 5 | - | 37.2 | 17.4 | 9.73 | 29.5 |
| 2 | STA | 150 | 20 | - | 38.1 | 18.1 | 9.97 | 26.0 |
| 3 | STA | 150 | 60 | - | 37.6 | 18.0 | 9.96 | 23.0 |
| 4 | STA | 180 | 5 | 4 reflects, no identification | 38.1 | 18.4 | 9.82 | 24.0 |
| 5 | STA | 180 | 20 | - | 40.7 | 18.5 | 10.2 | 18.5 |
| 6 | STA | 180 | 60 | IONSIVE + SIV | 40.1 | 18.6 | 11.7 | 3.50 |
| 7 | STA | 210 | 5 | IONSIVE | 39.7 | 17.7 | 10.8 | 21.5 |
| 8 | STA | 210 | 20 | IONSIVE | 40.5 | 18.5 | 12.4 | <2 |
| 9 | STA | 210 | 60 | IONSIVE | 40.4 | 17.9 | 12.2 | <2 |
| 10 | TiCl3 | 180 | 5 | halite | 47.8 | <0.001 | 0.48 | <2 |
| 11 | TiCl3 | 180 | 20 | halite | 49.7 | <0.001 | 0.07 | <2 |
| 12 | TiCl3 | 180 | 60 | halite | 51.8 | <0.001 | 0.07 | <2 |
| 13 | TiCl3 | 210 | 5 | halite + IONSIVE | 66.0 | <0.001 | 21.5 | 18.0 |
| 14 | TiCl3 | 210 | 20 | halite | 47.9 | <0.001 | 0.68 | <2 |
| 15 | TiCl3 | 210 | 60 | halite | 50.8 | <0.001 | 0.04 | <2 |
| Conditions of the Synthesis of Sitinakite Phases | BET Surface Area, m2/g | BJH Adsorption Average Pore Diameter, nm |
|---|---|---|
| STA, 1 h, 180 °C | 180.15 | 9.62 |
| STA, 5 min, 210 °C | 104.70 | 11.73 |
| STA, 20 min, 210 °C | 179.28 | 8.75 |
| STA, 1 h, 210 °C | 158.03 | 10.40 |
| TiCl3, 5 min, 210 °C | 131.12 | 13.00 |
| Components | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Na2O | 17.31 | 16.45 | 14.17 | 10.79 |
| SiO2 | 21.56 | 24.06 | 19.20 | 17.80 |
| TiO2 | 36.67 | 38.66 | 38.45 | 43.22 |
| Al2O3 | 0.97 | 0.49 | 0.44 | 0.08 |
| Sample | Cs+ Concentration, g/dm3 | Sorption IONSIVE Capacity Synthesized from Titanite Concentrate, mg/g [Current Paper] | Sorption IONSIVE Capacity Synthesized from Leucoxene Concentrate, mg/g [30] | Sorption IONSIVE Capacity o Synthesized from Loparite Concentrate, mg/g [30] |
|---|---|---|---|---|
| Method of synthesis | microvawe-assisted synthesis | hydrothermal synthesis | hydrothermal synthesis | |
| Initial solution | 1.10 | - | - | - |
| After treatment with Sorbent 2 (Figure 4) | 0.85 | 25 | 170 | 297 |
| After treatment with Sorbent 3 (Figure 4) | 0.53 | 57 | ||
| After treatment with Sorbent 4 (Figure 4) | 0.63 | 47 | ||
| After treatment with Sorbent 5 (Figure 4) | 0.69 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalashnikova, G.O.; Gryaznova, D.V.; Baranchikov, A.E.; Britvin, S.N.; Yakovenchuk, V.N.; Samburov, G.O.; Veselova, V.O.; Pulyalina, A.Y.; Pakhomovsky, Y.A.; Bazai, A.V.; et al. Microwave-Assisted Synthesis of Titanosilicates Using a Precursor Produced from Titanium Ore Concentrate. ChemEngineering 2023, 7, 118. https://doi.org/10.3390/chemengineering7060118
Kalashnikova GO, Gryaznova DV, Baranchikov AE, Britvin SN, Yakovenchuk VN, Samburov GO, Veselova VO, Pulyalina AY, Pakhomovsky YA, Bazai AV, et al. Microwave-Assisted Synthesis of Titanosilicates Using a Precursor Produced from Titanium Ore Concentrate. ChemEngineering. 2023; 7(6):118. https://doi.org/10.3390/chemengineering7060118
Chicago/Turabian StyleKalashnikova, Galina O., Darya V. Gryaznova, Alexander E. Baranchikov, Sergey N. Britvin, Victor N. Yakovenchuk, Gleb O. Samburov, Varvara O. Veselova, Aleksandra Y. Pulyalina, Yakov A. Pakhomovsky, Ayya V. Bazai, and et al. 2023. "Microwave-Assisted Synthesis of Titanosilicates Using a Precursor Produced from Titanium Ore Concentrate" ChemEngineering 7, no. 6: 118. https://doi.org/10.3390/chemengineering7060118
APA StyleKalashnikova, G. O., Gryaznova, D. V., Baranchikov, A. E., Britvin, S. N., Yakovenchuk, V. N., Samburov, G. O., Veselova, V. O., Pulyalina, A. Y., Pakhomovsky, Y. A., Bazai, A. V., Glazunova, M. Y., Shirokaya, A. A., Kozerozhets, I. V., Nikolaev, A. I., & Ivanov, V. K. (2023). Microwave-Assisted Synthesis of Titanosilicates Using a Precursor Produced from Titanium Ore Concentrate. ChemEngineering, 7(6), 118. https://doi.org/10.3390/chemengineering7060118








