Abstract
This article proposes a better alternative method to prepare CNT antifreeze nanofluid in EG/water by modifying the conventional method that requires long hours of sonication. Sonicating a sample for long hours is time and energy consuming and may deform the structure of CNT. In the modified method, the nanofluid preparation was carried out by dispersion of CNT in EG via sonication followed by adding water and again sonication. The study shows that nanofluid could be prepared in less sonication time of 1.5 h compared to the 5 h required in the conventional method. FTIR spectroscopy revealed that interaction of EG with CNT occurs via trans conformation resulting in greater stabilization and better interaction of nanofluid prepared by this method (85 days) as compared to nanofluid prepared by the conventional method (50 days). The nanofluid prepared by this method has better physical–chemical properties compared to nanofluid prepared by the conventional method. The nanofluid prepared by this method showed higher stability and better physical–chemical properties at a lower sonication time. Hence it is a more effective and cost efficient technique for preparing CNT (EG/water) nanofluid.
1. Introduction
Numerous thermal applications utilize water as a heat transfer fluid. Sometimes, ethylene glycol (EG) is added to water as the mixture of EG/water acquires anti-freeze and anti-boil properties. Generally, this mixture is used for heat transfer applications in car radiators, solar cells, etc. in areas where the surrounding is too cold, which freezes water [,]. This mixture provides us with a large range of temperatures for heat transfer applications; however, this mixture has low thermal conductivity and, hence, a low rate of heat transfer. The concept of nanofluid (adding nanoparticles to conventional heat transfer fluid) is helpful in improving the thermal conductivity of EG/water systems.
High thermal conductivity and thermal stability of CNT make it a viable option for the preparation of CNT based EG/water nanofluid [,,,,]. A major issue with the use of CNT nanofluid is the aggregation of CNT in the base fluid due to its high aspect ratio and strong van der Waals interaction [,,]. Various researchers prepared CNT (EG/water) nanofluid by using surfactants. Kumarsen et al., 2012 [] prepared 0.45 vol% CNT (EG/water) nanofluid by 30 min stirring and 90 min sonication. They utilized SDBS as a surfactant for proper dispersion and reported an enhancement of 19.73% in thermal conductivity. Sandhu et al., 2016 [] prepared 0.1 vol% CNT nanofluid by 80 min sonication. They utilized GA as a dispersant and reported a stability of 39 days and an enhancement of 28% in thermal conductivity. Similarly, Ganeshkumar et al., 2017 [] prepared 0.9 wt% of CNT (EG/water) nanofluid by using SDBS as a surfactant. The sample was stirred for 20 min and sonicated for 180 min for homogenous dispersion of CNT. They reported an enhancement of 11% in thermal conductivity. Although using surfactants is an easier and more cost-effective way of preparing nanofluids, using surfactants at higher temperatures is not feasible as they degrade at higher temperatures. Additionally, excessive foaming is also a problem while using surfactants as a dispersing agent. Furthermore, surfactant particles increase thermal resistance between CNT and the base fluid, which may limit the enhancement of thermal conductivity. Therefore, various researchers prepared nanofluid without surfactants.
Izadi et al., 2018 [] worked in a range of 0.05 to 1 vol% of CNT without surfactants by using 5–6 h sonication in an EG/water mixture and reported non-Newtonian behavior of nanofluid. Soltanimehr and Afrand, 2016 [] prepared nanofluid by dispersing (0.025–1 vol%) FCNT in EG/water (40:60) with 2.5 h magnetic stirring followed by 6 h sonication. They reported an enhancement of 34.7% in thermal conductivity as compared to the base fluid. Mirabagheri et al., 2018 [] prepared nanofluid by using (0.25–0.8 vol%) FCNT in EG/water (20:80) by using 2.5 h stirring and 6 h sonication. They reported 27.3% enhancement in thermal conductivity. They reported that the amount of CNT and percentage of EG and water affect the thermal conductivity noticeably. Yan et al., 2020 [] prepared nanofluid by using (0.075–1.2 vol%) ZnO-MWCNT hybrid in EG/water (20:80) by 120 min magnetic stirring and 300–360 min sonication and reported an enhancement in viscosity with volume fraction and decrement in viscosity with temperature increment. Esfe et al., 2023 [] prepared nanofluid by using MWCNT-TiO2, EG/water (50:50) by 3 h stirring and 5 h sonication and reported 36.30% enhancement in thermal conductivity and 2 weeks stability. Shahsavani et al., 2018 [] prepared COOH-MWCNT nanofluid by 2 h mixing and 5–6 h sonication for studying the rheological behavior of nanofluid. They reported that viscosity increases with an increase in solid volume fraction while a decrease was observed with an increase in temperature. Dekhogi et al., 2017 [] prepared COOH-SWCNT nanofluid by 2.30 h stirring and 6 h sonication and reported 10 days of stability. According to this study, the thermal conductivity increases with volume fraction and temperature. Bagheri et al., 2018 [] prepared ZnO-MWCNT nanofluid by 2 h stirring and 5 h sonication and reported an enhancement of 30% at 1.2 vol% and a stability of 1 week. Moradi et al., 2019 [] prepared MWCNT-TiO2 nanofluid by 2 h stirring and 5.5 h sonication. They reported an enhancement of 34% at 1 vol% CNT. Eshgarf et al., 2016 [] synthesized nanofluid of MWCNT-SiO2 via 2 h stirring and 5–6 h sonication and studied the viscosity at different shear rates.
The literature showed that researchers supply sonication energy for a longer duration for preparing CNT nanofluid. Preparation of nanofluid via long hour sonication is a time- and energy-consuming process; additionally, it may deform the structure of CNT [].
Apart from this, none of these research articles has discussed the role of the preparation method and the nature of interactions responsible for stabilization of nanofluid. Thus, it is imperative to understand the nature of EG/water and CNT for preparation of a stable nanofluid. Dispersing CNT in EG is easier in comparison to its dispersion in water owing to π–π interactions between CNT and EG. Therefore, CNT dispersion in EG first and then in the subsequent addition of water appears as a superior approach for preparing CNT antifreeze nanofluid in EG/water. A similar approach was used by Yadav et al., 2023 [] for synthesizing CNT nanofluid in (60:40) EG/water. EG/water (50:50) has lower viscosity and cost in comparison to EG/water (60:40) ratio. Therefore, this article prepared CNT antifreeze nanofluid with (50:50) EG/water. No research article in the literature discusses and compares the results of nanofluid prepared by conventional method (Method 1) and modified method (Method 2) at this ratio. Therefore, preparing nanofluids by using this ratio will provide new information to researchers working in the field of heat transfer. The nanofluid prepared by Method 2 showed better stabilization and physical–chemical properties at lower sonication time; therefore, this can be seen as a viable option of preparing nanofluid from a commercial perspective.
2. Material and Method
2.1. Materials
Ad Nano technology Pvt. Ltd. Karnataka, India provided COOH functionalized MWCNT (OD = 50–80 nm, ID = 5–15 nm, length = 10–20 m, specific surface area = 200 g/mL, density = 2.1 g/mL) with a purity of 95%. Thermo Fisher Scientific India Pvt. Ltd. provided EG 98% pure (Molecular weight = 62.07 (g/mol), density = 1113.2 (kg/m3)). Easy-Still Mark 2000 DDQ XL horizontal distillation unit) was used to double-distill water. Labman LMUC-3 ultrasonicator bath-sonicator with power = 100 W, frequency = 40 kHz, was utilized to disperse CNT in the base fluid. Shimadzu ATX224, (Shimadzu Philippines manufacturing. Inc) digital weighting balance with accuracy up to 1 mg was utilized to weigh CNT. As a base fluid, 50:50 (EG/water) mixture was utilized.
2.2. Methodology
Nanofluid was synthesized using two techniques as described below:
2.2.1. Preparation of Nanofluid by Conventional Method (Method 1)
CNT nanofluids with different concentrations of CNT (0.001, 0.005, 0.01, 0.025, 0.05 and 0.075 w/v%) were formulated by this method. The concentration range was selected based on their stability. Initially, 50:50 EG/water was mixed in six bottles by adding 50 mL water and 50 mL EG and stirring for about 1 min by a glass rod. Next, the required amount of CNT was added to each bottle and ultrasonication was carried out in the temperature range of 30–40 °C for varied durations (2, 3, 4, 5 and 6 h). The absorbance of samples was recorded and plotted by originPro 8.5.0 SR1b161 software. These nanofluids were used for further studies.
2.2.2. Preparation of Nanofluid by Modified Method (Method 2)
CNT nanofluids with different concentrations of CNT (0.001, 0.005, 0.01, 0.025, 0.05 and 0.075 w/v%) were again made with Method 2 by adding the requisite quantity of CNT to 50 mL EG and conducting sonication for S0 time period. Thereafter, 50 mL water was added and it was again subjected to a second slot of sonication for Sf time period. Various combinations of S0 (40, 50, 60 and 70 min) and Sf (30, 60 and 90 min) were tried to optimize the nanofluid. The absorbance was recorded at each combination and plotted by origin software. Ultrasonicator temperature was fixed in 30–40 °C range. These nanofluids were used for further studies.
2.3. Characterization
Absorbance of the prepared nanofluids was recorded by UV-vis spectrophotometer (HITACHI U-2190) (Hitachi High Technology Tokyo, Japan) for estimating the stability of the nanofluids. Zetasizer Nano ZS (Malvern RDET 48125) (Malvern Instrument limited, United Kingdom) was employed to record the particle size of the prepared nanofluids. Thermal conductivity was recorded by Thermal Property Analyzer (Decagon Inc., Pullman, WA, USA) using a KS-1 single-needle (60 mm long and 1.3 mm diameter) sensor. For error estimation, the instrument was calibrated using glycerin. The measurements were within 98% accuracy. Rotational rheometer (Anton Paar MCR-102) (Anton Paar Austria GmbH) was used to measure the dynamic viscosity at a variable shear rate of 10–100/s. Calibration was performed by BW 20 oil at 30 °C as per national standards and uncertainty was 0.25%. The Pak and Cho correlation [], based on mixing theory (Equation (1)), was used to estimate theoretical density of the nanofluids:
ρ(nf) = Φ ρ(p) + (1 − Φ)ρ(bf)
Experimentally, density was measured by a pycnometer. Uncertainty was ±0.005%. Three concordant readings for every sample ensured accuracy and repeatability. The average values of these experimental data were used in Equation (2) to calculate density:
ρ(nf) = (Mnf − Mbf)/Vnf
Advance neo 500, Bruker NMR spectrophotometer (Bruker, Germany) and Tensor 37, Bruker FTIR spectrophotometer (Bruker, Germany) were used for NMR and FTIR studies, respectively.
3. Result and Discussion
3.1. Effect of Sonication Time
Ultrasonication is a common way to break up agglomerates and promote the dispersion of nanoparticles in base fluid to obtain more stable nanofluid [].
3.1.1. Effect of Sonication Time on Nanofluid Prepared by Method 1
For nanofluids prepared by Method 1, CNT mixed with 50:50 EG/water was sonicated for 2, 3, 4, 5 and 6 h and their absorbance is recorded at each interval and plotted in Figure 1. From Figure 1a–f, it was noted that on enhancing sonication up to 5 h, the absorbance rises for all concentrations. The absorbance decreases as the sonication time increases beyond 5 h.
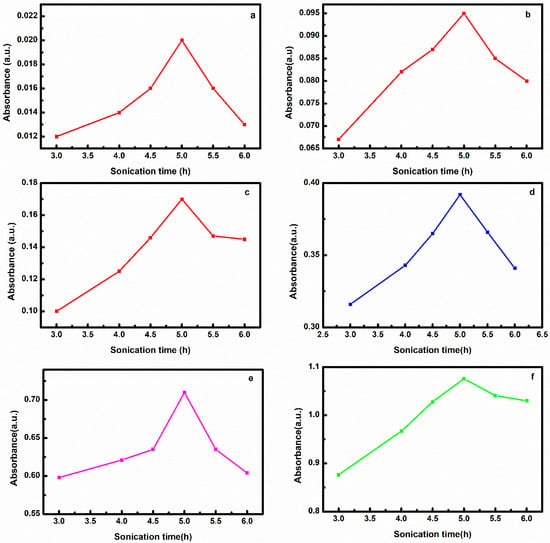
Figure 1.
Effect of sonication time on nanofluid prepared by Method 1 at (a) 0.001, (b) 0.005, (c) 0.01, (d) 0.025, (e) 0.05, (f) 0.075 w/v% CNT.
Sonication lower than 5 h is not enough for effective dispersion of CNT in a mixture of EG/water. Thus, absorbance rises up to 5 h. More than 5 h of sonication can create active areas on the CNT surface increasing the possibility of particle collision and formation of new aggregates resulting in decrement of absorbance. This type of trend was seen by other researchers also [,,].
3.1.2. Effect of Sonication Time on Nanofluid Prepared by Method 2
Figure 2a–f represent the absorbance data of nanofluid prepared by Method 2 at various combinations of sonication time intervals, S0 (40, 50, 60 and 70 min) and Sf (30, 60 and 90 min). Absorbance was maximum for S0 = 60 min and thereafter decreased at S0 = 70 mins. This clearly indicated S0 = 60 min was the optimum dispersion time, as maximum dispersion is seen at S0 = 60 min and all Sf (30, 60 and 90 min).
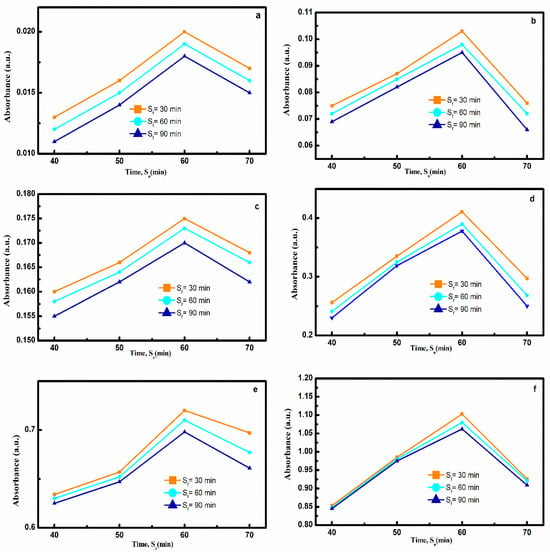
Figure 2.
Effect of sonication time on dispersion of CNT in nanofluid prepared by Method 2 at (a) 0.001, (b) 0.005, (c) 0.01, (d) 0.025, (e) 0.05, (f) 0.075 w/v% CNT.
The effect of Sf (30, 60 and 90 min) was observed at the optimized S0 (60 min). As Sf increased from 30 to 90 mins, absorbance decreased. Therefore, S0 = 60 min and Sf = 30 min was obtained as optimized time for sonication.
Applying sonication for S0 > 60 min and Sf > 30 min enhances the defects on surface of CNT leading to the formation of new aggregates resulting in a decrement in absorbance [].
3.2. Stability Analysis
Pantzali et al., 2009 [] reported that nanofluids have sufficient capability to design efficient heat transfer systems but their long-term stability could be a practical issue in their commercialization. Thus, in this study, evaluation of nanofluid stability was performed with Dynamic light scattering and UV-vis spectroscopy.
3.2.1. UV-Vis Spectroscopy
Stability of prepared nanofluid was analyzed under static conditions by measuring their absorbance using UV-vis spectroscopy. It was found that stability varied with the concentration of nanofluids as plotted in Figure 3 and Figure 4. As CNT concentration increased, absorbance also increased as represented in Figure 3 and Figure 4.
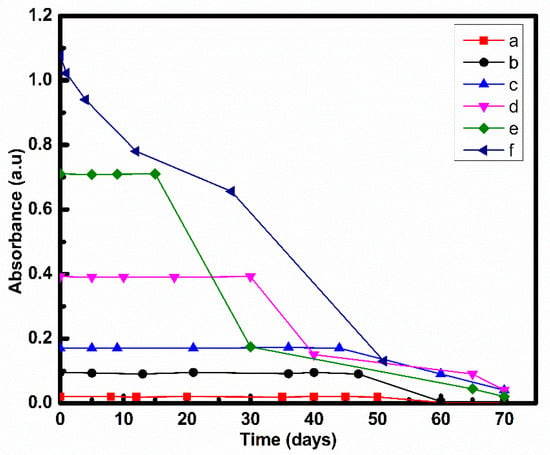
Figure 3.
Stability of nanofluids prepared by Method 1 at (a) 0.001, (b) 0.005, (c) 0.01, (d) 0.025 (e) 0.05, (f) 0.075 w/v% CNT.
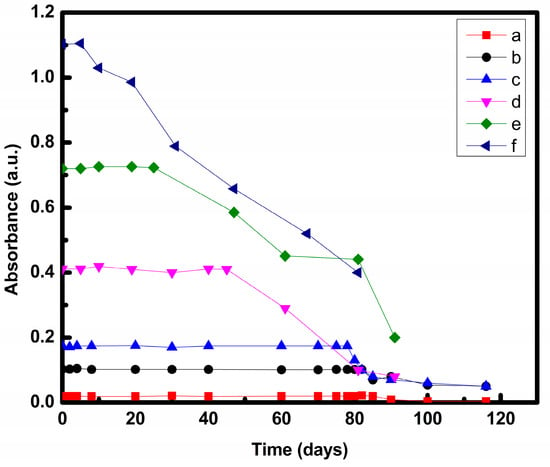
Figure 4.
Stability of nanofluid prepared by Method 2 at (a) 0.001, (b) 0.005, (c) 0.01, (d) 0.025 (e) 0.05, (f) 0.075 w/v% CNT.
In case of nanofluids prepared by Method 1, nanofluids at 0.001, 0.005, 0.01, 0.025 and 0.05 w/v% of CNT showed no change in absorbance up to 50, 47,44, 30 and 15 days, respectively, and a decrease was seen thereafter. CNT nanofluid with 0.075 w/v% CNT showed a constant decrement in absorbance.
In case of nanofluids prepared by Method 2, nanofluids containing 0.001, 0.005, 0.01, 0.025, and 0.05 w/v% CNT showed no change in absorbance up to 85, 82, 78, 45 and 25 days, respectively, and a decrease was seen thereafter. Nanofluid with 0.075 w/v% CNT showed a constant decrement in absorbance after 5 days.
For nanofluids prepared by both Method 1 and Method 2, stability decreased as concentration increases. A similar trend was also reported by other researchers [,]. Average particle separation reduces with concentration. A lower separation means higher Vander Waals attractive potential. A higher Vander Waals attractive potential than electrostatic repulsive potential leads to particle agglomeration, which settle at the bottom [].
It was found that nanofluids prepared by Method 2 wa more stable as compared to nanofluid prepared by Method 1 at all concentrations as shown in Table 1. This may be because of the change in preparation procedures. In Method 2, CNT was allowed to interact with EG first thereafter water was added to the system. CNT interacts with EG more strongly as compared to water [], which blocks CNT and water contact. Hence, it prevents agglomeration of CNT []. Therefore, proper dispersion was obtained by Method 2.

Table 1.
Stability of nanofluid prepared by Method 1 and Method 2.
Since nanofluids with 0.05 and 0.075 w/v% of CNT showed stability less than 1 month, these were not considered for further analysis owing to the lack of practical applicability for heat transfer.
3.2.2. Dynamic Light Scattering
Figure 5 showed that hydrodynamic size increases with the increment in concentration of CNT in both nanofluids prepared by Method 1 and Method 2. Larger concentrations showed enhanced particle sizes as agglomeration enhances with increased loading [].
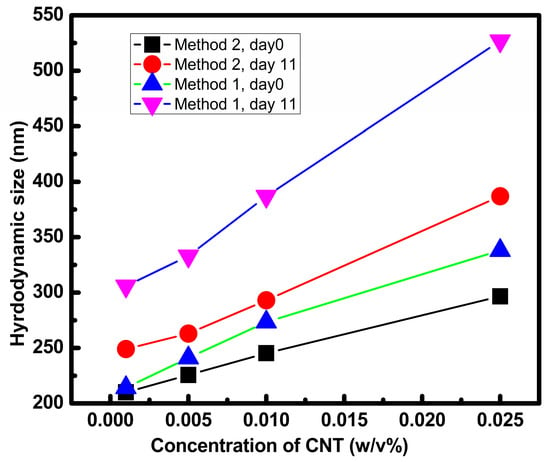
Figure 5.
Hydrodynamic size of nanofluid prepared by Method 1 and Method 2 at day 0 and day 11.
Comparing the hydrodynamic size at day 0 for nanofluids prepared by Method 1 and Method 2 at each concentration, it was found that nanoparticles prepared by Method 2 were smaller than those prepared by Method 2 at all concentrations.
The hydrodynamic size of both nanofluids prepared by Method 1 and Method 2 showed an increment on keeping with time as shown by Figure 5 (comparing day 0 and day 11). It was also found that nanofluid prepared by Method 1 showed a larger increment in size or agglomerated faster with time as compared to nanofluid prepared by Method 2. This could be due to the arrangement of water and EG around CNT. The presence of more EG molecules around CNT helps in stabilizing its dispersion, while the presence of water leads to agglomeration of CNT. In Method 2, the CNT was first dispersed or wrapped by EG before being mixed with water. The strong propensity of EG to form hydrogen bonding with water stabilizes the (CNT-EG) system in water.
On the other hand, in Method 1, CNT was added to the water and EG mixture and the water molecules around CNT caused faster agglomeration of CNT and destabilization of nanofluid at a faster rate.
Larger enhancement in size with time was also observed at a high concentration in comparison to lower concentration of CNT.
An increase in hydrodynamic size or aggregation shows lack of stability of nanofluid []. The enhancement in size observed by DLS matches well to the decrease in stability as indicated by UV-vis spectroscopy.
3.3. Study of Interactions between CNT and Base Fluid
3.3.1. FTIR Spectroscopy
FTIR data of EG/water mixture, nanofluid prepared by Method 1 and nanofluid prepared by Method 2 are shown in Figure 6. The peak at 3276 cm−1 in a mixture of EG/water indicate hydrogen bonding. A shift was observed in this peak in the case of both nanofluids (3292 and 3297 cm−1) because of the formation of new hydrogen bonds between CNT-EG-water system.
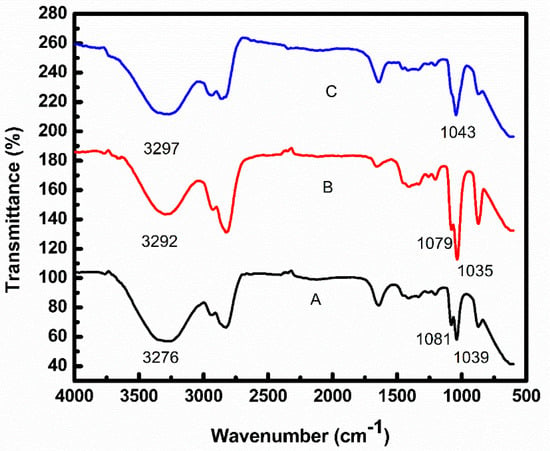
Figure 6.
FTIR spectra of (A) EG/water mixture, (B) nanofluid prepared by Method 1, (C) nanofluid prepared by Method 2.
Two bands at 1039 and 1081 cm−1 were due to O-C-C-O stretching vibration modes in trans and gauche conformation of EG in EG/water mixture, respectively []. This indicates that in a mixture of EG/water, EG exists in both trans and gauche conformations. A minute shift was seen for the trans peak in nanofluid prepared by Method 1, indicating interaction of both water and EG with CNT in nanofluid prepared by Method 1. In the case of nanofluid prepared by Method 2, the intensity of gauche conformation (1081 cm−1 band) diminishes and only trans-conformation exists (1043 cm−1 band), indicating that gauche form changes to trans form in EG. The reason is that EG interacts by trans form, having no dipole moment with the nonpolar surface of CNT, eliminating the gauche form having a dipole moment [,].
As no new peak appeared in nanofluids prepared by Method 1 and Method 2 as compared to the base fluid, it indicated that nanofluids were stabilized via noncovalent interactions.
3.3.2. NMR Spectroscopy
Figure 7 depicts the NMR spectra (500 MHz, DMSOd6, TMS) of EG/water mixture, nanofluid prepared by Method 1 and nanofluid prepared by Method 2. In the case of nanofluid prepared by Method 1 (Figure 7B), all peaks become broader as compared with a mixture of EG/water (Figure 7A). It showed enhanced hydrogen bonding in nanofluid. The peak at 4.47 ppm (due to water) and 5.11 ppm (due to OH of EG) [] in nanofluid prepared by Method 1 were broader than nanofluid prepared by Method 2. It indicated that OH of EG and water showed more interaction among themselves and with CNT in case of nanofluid prepared by Method 1. In the case of nanofluid prepared by Method 2 (Figure 7C), peak at 3.45 ppm (due to CH2-EG) was broader in comparison with nanofluid prepared by Method 1, indicating stronger stacking interaction of CH2 (EG) with CNT.
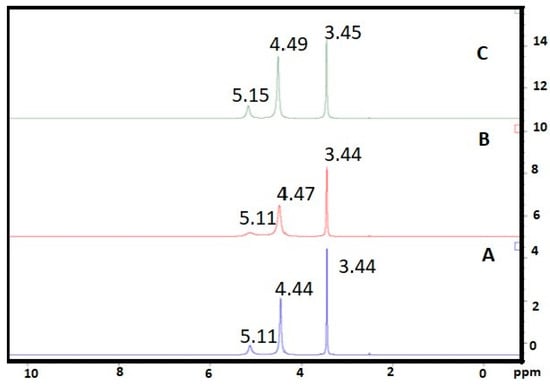
Figure 7.
1H NMR spectra of (A) EG/water mixture, (B) nanofluid prepared by Method 1, (C) nanofluid prepared by Method 2.
These results were in line with preparation protocols. No new peak appears in nanofluid prepared by Method 1 and Method 2 in comparison to a mixture of EG/water, indicating the presence of noncovalent interactions such as hydrogen bonding, Vander walls interactions, and hydrophilic–hydrophobic interactions for stabilizing CNT in EG/water mixture.
3.4. Physical–Chemical Properties
3.4.1. Viscosity
An increase in viscosity leads to an increment in the pumping power required to pump the nanofluid, so a study of viscosity is important for its role in heat transfer application. The rheological behaviour of the base fluid and both nanofluids prepared by Method 1 and Method 2 at different concentrations is shown in Figure 8. It was observed that both nanofluids have higher viscosity as compared to the base fluid. This will increase the pumping power required to pump the nanofluid.
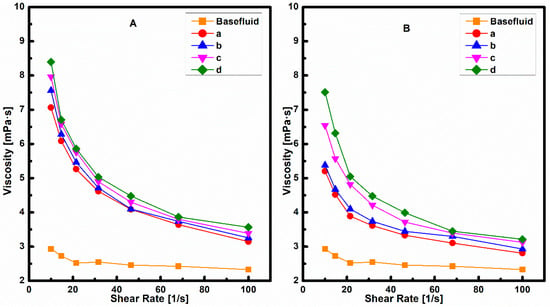
Figure 8.
Viscosity of nanofluid prepared by (A) Method 1 (B) Method 2.
Viscosity increases with an increase in particle concentration. Similar trend was also observed by other researchers and it was due to the formation of agglomerates of CNT [].
The dynamic viscosity of nanofluid was observed to decrease as the shear rate increased as shown in Table 2 and Table 3. It clearly indicates the shear thinning behaviour of the nanofluid. This may be because of breaking of agglomerates of CNT-CNT and particle–base fluid interactions at a higher shear rate [,].

Table 2.
Viscosity of nanofluid prepared by Method 1.

Table 3.
Viscosity of nanofluid prepared by Method 2.
It was observed that nanofluids prepared by Method 1 had higher viscosity as compared to nanofluids prepared by Method 2 at all shear rates as shown in Figure 8A,B and in Table 2 and Table 3. Higher viscosity means more energy required for pumping the fluid, which eventually leads to an increase in operation costs. Therefore, nanofluid prepared by Method 2 is a viable option for heat removal in industrial application in comparison to nanofluid prepared by Method 1. This may be because of larger particle size and low stability of CNT nanoparticles in nanofluid prepared by Method 1 as indicated by DLS and UV vis data.
3.4.2. Density
The density of the nanofluid prepared by both Method 1 and Method 2 are given in Table 4. The density of all the prepared nanofluids was slightly higher than the base fluid due to the presence of CNT nanoparticles. A negligible enhancement in density of nanofluid prepared by Method 2 as compared to nanofluid prepared by Method 1 was observed at all concentrations. This means that the prepared nanofluids are suitable for heat transfer applications.

Table 4.
Density of nanofluid.
3.4.3. Thermal Conductivity
Figure 9 shows an enhancement in thermal conductivity with concentration of CNT for both nanofluids prepared by Method 1 and Method 2. The enhancement in thermal conductivity is the result of homogenous dispersion in the base fluid and formation of a nanolayer [,,,]. The liquid nanolayer at the interface has a higher thermal conductivity than the bulk liquid []. Nanofluid prepared by Method 2 has higher thermal conductivity as compared to nanofluid prepared by Method 1. Higher thermal conductivity means higher heat removal tendency. Therefore, nanofluid prepared by Method 2 is a better nanofluid. This is because in Method 2, CNT is first dispersed in EG. EG form interactions with CNT via trans form (as described by FTIR data). This means higher interaction between CNT and EG and more ordered liquid layer is formed around CNT in Method 2 that results in higher thermal conductivity [].
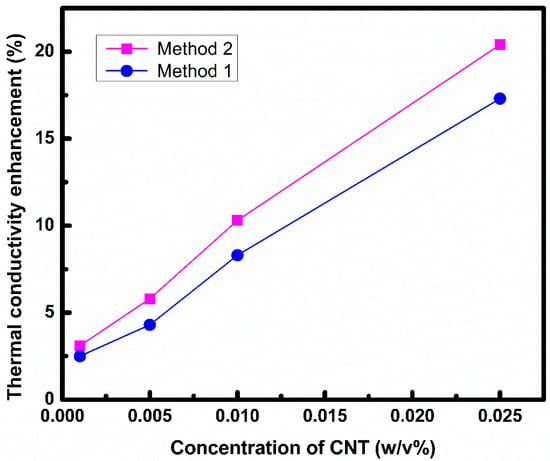
Figure 9.
Thermal conductivity of nanofluids prepared by Method 1 and Method 2.
4. Comparison of Present Work with Work Reported in Literature
As shown in Table 5, work has been carried out in literature to prepare surfactant-free nanofluid using EG/water (50:50). However, when compared to previous literature, the current study showed that Method 2 requires less sonication time and shows better stability.

Table 5.
Comparison of present study with reported work.
5. Conclusions
In this study, stable CNT antifreeze nanofluid was prepared in 50:50 EG/water without surfactants and their physical–chemical properties were investigated. Nanofluid was prepared by two methods, first by the conventional method (Method 1) and second by a modified method (Method 2) in a concentration range of 0.001 to 0.075 w/v%. Both Method 1 and Method 2 are successful in preparing stable nanofluids with enhanced thermal conductivity. These nanofluids remain stable without the addition of any surfactant, thus solving the problem of foaming. These nanofluids could be useful in a large range of temperatures including cold (subzero) areas for heat transfer applications. Thermal conductivity of both nanofluids increases linearly with concentration and their stability decreases with concentration. The conclusions of the aforementioned study are listed below:
- ▪
- Method 2 requires less time (1.5 h) for preparation of CNT antifreeze nanofluid in EG/water as compared to Method 1 (5 h).
The nanofluid prepared by Method 2 was more stable, having smaller particle sizes as shown by UV-vis and DLS data, respectively. This means better dispersion was obtained by this method.
- ▪
- The preparation method plays an important role in stabilization of nanofluid. It was found that noncovalent interactions are responsible for stabilization of CNT nanofluid.
- ▪
- A higher enhancement in thermal conductivity was observed in the case of Method 2 (20%) in comparison to Method 1 (17%).
- ▪
- A 7.4% greater increase in viscosity was seen in the case of nanofluid prepared by Method 1 in comparison to Method 2 at 100/s shear rate.
- ▪
- In both nanofluids, a marginal difference is seen in density as compared with the base fluid.
- ▪
- This study provides an alternate way of producing CNT antifreeze nanofluid in EG/water system, without the use of surfactant or the need of long term sonication. The better way of preparing such type of nanofluids is to first disperse CNT in EG, which has a higher capability of dispersing CNT, followed by the addition of water.
Nanofluid prepared by Method 2 showed greater stability, enhanced thermal conductivity and lower viscosity in less sonication time. Therefore, Method 2 is a time-saving and better approach for the preparation of CNT nanofluid in EG/water mixture. Thus, the outcome of this study added new information to the scientific literature and will be very helpful for the forthcoming studies on heat transfer and material science.
Author Contributions
Conceptualization: P.Y., S.M.G. and S.K.S.; methodology: P.Y.; software: P.Y.; validation: S.M.G. and S.K.S.; formal analysis: S.M.G. and S.K.S.; investigation: S.M.G. and S.K.S.; resources: S.M.G. and S.K.S.; data curation: P.Y., S.M.G. and S.K.S.; writing—original draft preparation: P.Y.; writing—review and editing, S.M.G. and S.K.S.; visualization: P.Y.; supervision, S.M.G. and S.K.S.; project administration, S.M.G.; funding acquisition: S.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by GGSIP University, New Delhi, India, through Faculty Research Grant Scheme: GGSIPU/DRC/FRGS/2022/1223/4 and the APC was funded Priyanka Yadav.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
| CNT | Carbon Nanotube |
| EG | Ethylene Glycol |
| Φ | CNT amount (V%) |
| ρ(bf) | Base fluid density (Kg/m3) |
| ρ(nf) | Nanofluid Density (Kg/m3) |
| M(nf) | Nanofluid mass (Kg) |
| M(bf) | Base fluid mass (Kg) |
| V(nf) | Nanofluid volume (m3) |
| S0 | Sonication time prior to addition of water (min) |
| Sf | Sonication time after addition of water (min) |
References
- American Society of Heating, Refrigerating and Air-Conditioning Engineers. Ashrae Handbook: Fundamentals; ASHRAE: Atlanta, GA, USA, 2009. [Google Scholar]
- Roohani, E.; Toghraie, D. Heat transfer improvement of antifreeze by changing it to hybrid nanofluid: Effects of hybrid magnesium oxide–graphene oxide nanopowders. J. Therm. Anal. Calorim. 2022, 147, 6777–6791. [Google Scholar] [CrossRef]
- Afrand, M. Experimental study on thermal conductivity of ethylene glycol containing hybrid nano-additives and development of a new correlation. Appl. Therm. Eng. 2017, 110, 1111–1119. [Google Scholar] [CrossRef]
- Izadi, F.; Ranjbarzadeh, R.; Kalbasi, R.; Afrand, M. A new experimental correlation for non-Newtonian behavior of COOH-DWCNTs/antifreeze nanofluid. Phys. E Low Dimens. Syst. Nanostruct. 2018, 98, 83–89. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; de Castro, C.A.N. Conduction and convection heat transfer characteristics of ethylene glycol based nanofluids—A review. Appl. Energy 2016, 184, 681–695. [Google Scholar] [CrossRef]
- Azmi, W.H.; Hamid, K.A.; Usri, N.A.; Mamat, R.; Sharma, K.V. Heat transfer augmentation of ethylene glycol: Water nanofluids and applications—A review. Int. Commun. Heat Mass Transf. 2016, 75, 13–23. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirlohi, A.; Nazari, M.A.; Ghasempour, R. A review of thermal conductivity of various nanofluids. J. Mol. Liq. 2018, 265, 181–188. [Google Scholar] [CrossRef]
- Kumar, L.H.; Kazi, S.N.; Masjuki, H.H.; Zubir, M.N.M. A review of recent advances in green nanofluids and their application in thermal systems. Chem. Eng. J. 2022, 429, 132321. [Google Scholar] [CrossRef]
- Said, Z.; Sundar, L.S.; Tiwari, A.K.; Ali, H.M.; Sheikholeslami, M.; Bellos, E.; Babar, H. Recent advances on the fundamental physical phenomena behind stability, dynamic motion, thermophysical properties, heat transport, applications, and challenges of nanofluids. Phys. Rep. 2022, 946, 1–94. [Google Scholar] [CrossRef]
- Rudyak, V.Y.; Tret’yakov, D.S. Rheological Properties of Water- and Ethylene-Glycol-Based Nanofluids with Single-Walled Carbon Nanotubes. J. Eng. Phys. Thermophys. 2021, 94, 1208–1216. [Google Scholar] [CrossRef]
- Kumaresan, V.; Velraj, R. Experimental investigation of the physical-chemical properties of water-ethylene glycol mixture based CNT nanofluids. Thermochim Acta 2012, 545, 180–186. [Google Scholar] [CrossRef]
- Sandhu, H.; Gangacharyulu, D. An experimental study on stability and some thermophysical properties of multiwalled carbon nanotubes with water–ethylene glycol mixtures. Part. Sci. Technol. 2017, 35, 547–554. [Google Scholar] [CrossRef]
- Ganeshkumar, J.; Kathirkaman, D.; Raja, K.; Kumaresan, V.; Velraj, R. Experimental study on density, thermal conductivity, specific heat, and viscosity of water-ethylene glycol mixture dispersed with carbon nanotubes. Therm. Sci. 2017, 21 Pt A, 255–265. [Google Scholar] [CrossRef]
- Soltanimehr, M.; Afrand, M. Thermal conductivity enhancement of COOH-functionalized MWCNTs/ethylene glycol–water nanofluid for application in heating and cooling systems. Appl. Therm. Eng. 2016, 105, 716–723. [Google Scholar] [CrossRef]
- Mirbagheri, M.H.; Akbari, M.; Mehmandoust, B. Proposing a new experimental correlation for thermal conductivity of nanofluids containing of functionalized multiwalled carbon nanotubes suspended in a binary base fluid. Int. Commun. Heat Mass Transf. 2018, 98, 216–222. [Google Scholar] [CrossRef]
- Yan, S.R.; Toghraie, D.; Abdulkareem, L.A.; Alizadeh, A.; Barnoon, P.; Afrand, M. The rheological behavior of MWCNTs–ZnO/Water–Ethylene glycol hybrid non-Newtonian nanofluid by using of an experimental investigation. J. Mater. Res. Technol. 2020, 9, 8401–8406. [Google Scholar] [CrossRef]
- Esfe, M.H.; Alidoust, S.; Tamrabad, S.N.H.; Toghraie, D.; Hatami, H. Thermal conductivity of MWCNT-TiO2/Water-EG hybrid nanofluids: Calculating the price performance factor (PPF) using statistical and experimental methods (RSM). Case Stud. Therm. Eng. 2023, 48, 103094. [Google Scholar] [CrossRef]
- Shahsavani, E.; Afrand, M.; Kalbasi, R. Experimental study on rheological behavior of water–ethylene glycol mixture in the presence of functionalized multi-walled carbon nanotubes: A novel correlation for the non-Newtonian nanofluid. J. Therm. Anal. Calorim. 2018, 131, 1177–1185. [Google Scholar] [CrossRef]
- Dehkordi, R.A.; Esfe, M.H.; Afrand, M. Effects of functionalized single walled carbon nanotubes on thermal performance of antifreeze: An experimental study on thermal conductivity. Appl. Therm. Eng. 2017, 120, 358–366. [Google Scholar] [CrossRef]
- Bagheri, H.; Nadooshan, A.A. The effects of hybrid nano-powder of zinc oxide and multi walled carbon nanotubes on the thermal conductivity of an antifreeze. Phys. E Low Dimens. Syst. Nanostruct. 2018, 103, 361–366. [Google Scholar] [CrossRef]
- Moradi, A.; Zareh, M.; Afrand, M.; Khayat, M. Effects of temperature and volume concentration on thermal conductivity of TiO2-MWCNTs (70-30)/EG-water hybrid nano-fluid. Powder Technol. 2020, 362, 578–585. [Google Scholar] [CrossRef]
- Eshgarf, H.; Sina, N.; Esfe, M.H.; Izadi, F.; Afrand, M. Prediction of rheological behavior of MWCNTs–SiO2/EG–water non-Newtonian hybrid nanofluid by designing new correlations and optimal artificial neural networks. J. Therm. Anal. Calorim. 2018, 132, 1029–1038. [Google Scholar] [CrossRef]
- Yadav, P.; Gupta, S.M.; Sharma, S.K. A review on stabilization of carbon nanotube nanofluid. J. Therm. Anal. Calorim. 2022, 147, 6537–6561. [Google Scholar] [CrossRef]
- Yadav, P.; Gupta, S.M.; Sharma, S.K. Preparation and characterization of surfactant-free CNT based nanofluid in EG/water (60:40 ratio) basefluid for refrigerant application. J. Therm. Anal. Calorim. 2023, 148, 10037–10050. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Islam, M.M.; Chong, T.H.; Shah, S.K.; Shahrul, I.M.; Rahman, S.; Bui, L.D.; Ma, A. Effect of ultrasonication duration on colloidal structure and viscosity of alumina-water nanofluid. Ind. Eng. Chem. Res. 2014, 53, 6677–6684. [Google Scholar] [CrossRef]
- Javadian, S.; Motaee, A.; Sharifi, M.; Aghdastinat, H.; Taghavi, F. Dispersion stability of multi-walled carbon nanotubes in catanionic surfactant mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 141–149. [Google Scholar] [CrossRef]
- Pantzali, M.N.; Mouza, A.A.; Paras, S.V. Investigating the efficacy of nanofluids as coolants in plate heat exchangers (PHE). Chem. Eng. Sci. 2009, 64, 3290–3300. [Google Scholar] [CrossRef]
- Zhao, M.; Lv, W.; Li, Y.; Dai, C.; Zhou, H.; Song, X.; Wu, Y. A study on preparation and stabilizing mechanism of hydrophobic silica nanofluids. Materials 2018, 11, 1385. [Google Scholar] [CrossRef]
- Haghighi, E.B.; Nikkam, N.; Saleemi, M.; Behi, M.; Mirmohammadi, S.A.; Poth, H.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M.; Palm, B. Shelf stability of nanofluids and its effect on thermal conductivity and viscosity. Meas. Sci. Technol. 2013, 24, 105301. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Witharana, S.; Palabiyik, I.; Musina, Z.; Ding, Y. Stability of glycol nanofluids—The theory and experiment. Powder Technol. 2013, 239, 72–77. [Google Scholar] [CrossRef]
- Suganthi, K.S.; Rajan, K.S. A formulation strategy for preparation of ZnO-Propylene glycol-water nanofluids with improved transport properties. Int. J. Heat Mass Transf. 2014, 71, 653–663. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Marneni, N. Stability of Nanofluids. In Topics in Mining, Metallurgy and Materials Engineering; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2017; pp. 1–31. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Synthesis, characterization and dispersion stability of water-based Cu–CNT hybrid nanofluid without surfactant. Microfluid. Nanofluidics 2021, 25, 14. [Google Scholar] [CrossRef]
- Guo, Y.C.; Cai, C.; Zhang, Y.H. Observation of conformational changes in ethylene glycol-water complexes by FTIR-ATR spectroscopy and computational studies. AIP Adv. 2018, 8, 055308. [Google Scholar] [CrossRef]
- Krishnan, B.K.; Krishnan, D.R.S. Raman and infrared spectra of ethylene glycol. Proc. Indian Acad. Sci. Sect. A 1966, 64, 111–122. [Google Scholar] [CrossRef]
- Balamurugan, K.; Baskar, P.; Kumar, R.M.; Das, S.; Subramanian, V. Interaction of carbon nanotube with ethylene glycol-water binary mixture: A molecular dynamics and density functional theory investigation. J. Phys. Chem. C 2012, 116, 4365–4373. [Google Scholar] [CrossRef]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; De Amicis, C.V.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry. Org. Process Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Viscosity measurements of multi-walled carbon nanotubes-based high temperature nanofluids. Mater. Lett. 2014, 122, 212–215. [Google Scholar] [CrossRef]
- Amiri, A.; Sadri, R.; Ahmadi, G.; Chew, B.T.; Kazi, S.N.; Shanbedi, M.; Alehashem, M.S. Synthesis of polyethylene glycol-functionalized multi-walled carbon nanotubes with a microwave-assisted approach for improved heat dissipation. RSC Adv. 2015, 5, 35425–35434. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Baik, Y.J.; Koo, J. Quantitative analyses of factors affecting thermal conductivity of nanofluids using an improved transient hot-wire method apparatus. Int. J. Heat Mass Transf. 2015, 89, 116–123. [Google Scholar] [CrossRef]
- Hays, A.; Marsh, C.P.; Alvarado, J.; Franks, R. The Effect of Nanoparticle Agglomeration on Enhanced Nanofluidic Thermal Conductivity. 2006. Available online: http://docs.lib.purdue.edu/iracc/829 (accessed on 8 November 2022).
- Kumar, R.M.; Baskar, P.; Balamurugan, K.; Das, S.; Subramanian, V. Interaction of ethylene glycol-water clusters with aromatic surfaces. RSC Adv. 2013, 3, 7798–7807. [Google Scholar] [CrossRef]
- Kakavandi, A.; Akbari, M. Experimental investigation of thermal conductivity of nanofluids containing of hybrid nanoparticles suspended in binary base fluids and propose a new correlation. Int. J. Heat Mass Transf. 2018, 124, 742–751. [Google Scholar] [CrossRef]
- Irani, M.; Afrand, M.; Mehmandoust, B. Curve fitting on experimental data of a new hybrid nano-antifreeze viscosity: Presenting new correlations for non-Newtonian nanofluid. Phys. A Stat. Mech. Its Appl. 2019, 531, 120837. [Google Scholar] [CrossRef]
- Eshgarf, H.; Afrand, M. An experimental study on rheological behavior of non-Newtonian hybrid nano-coolant for application in cooling and heating systems. Exp. Therm. Fluid Sci. 2016, 76, 221–227. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).