Fabrication and Characterization of Poly(lactic acid)-Based Biopolymer for Surgical Sutures
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Methodology for Manufacturing Absorbable Medical Sutures
3. Characterization and Tests
3.1. Elongation and Thickness Measurement
3.2. DSC Testing
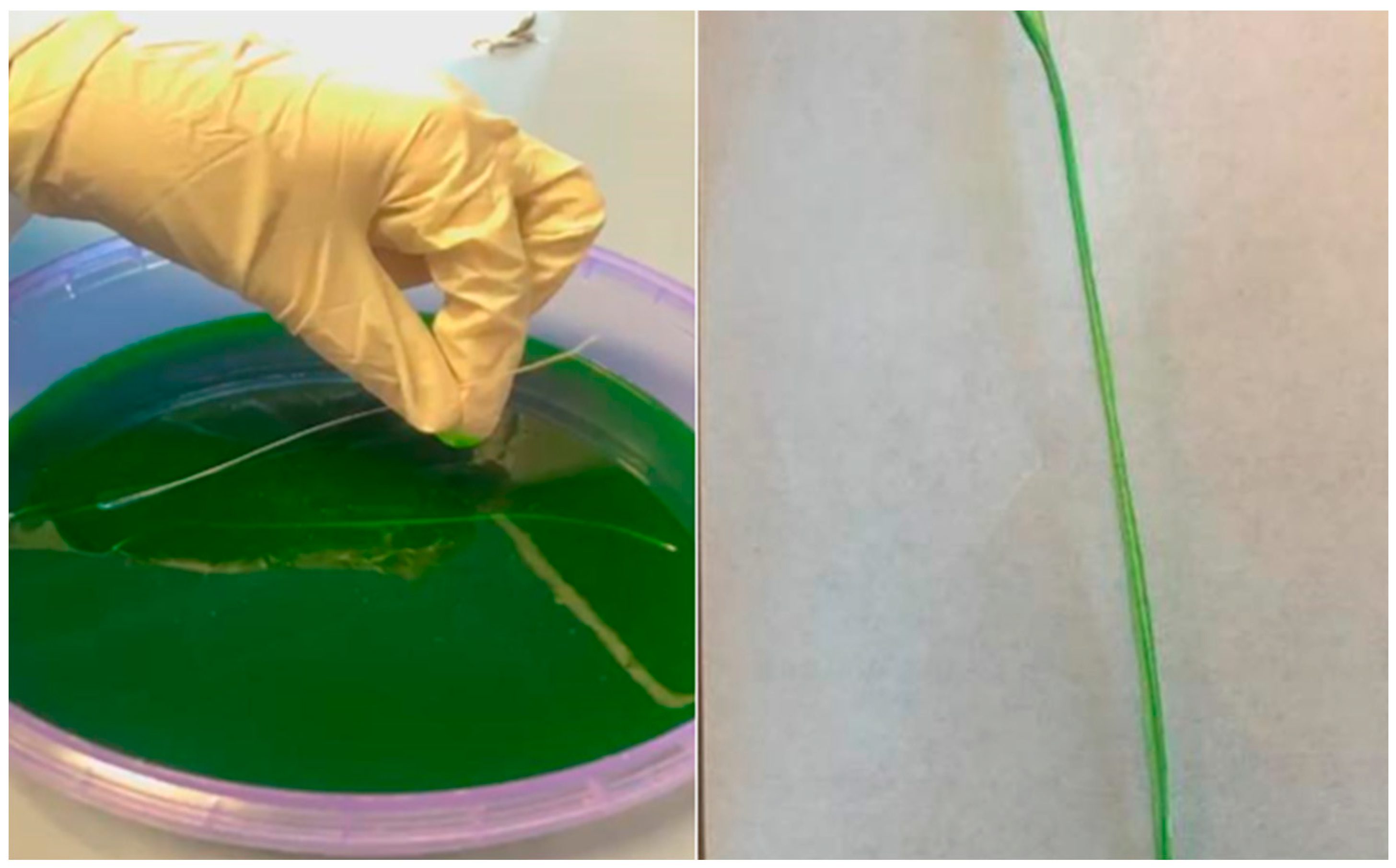
3.3. Human Skin Suture Practice Simulator Testing
3.4. Mouse Implantation Testing
3.5. Degradation Study
4. Results and Discussion
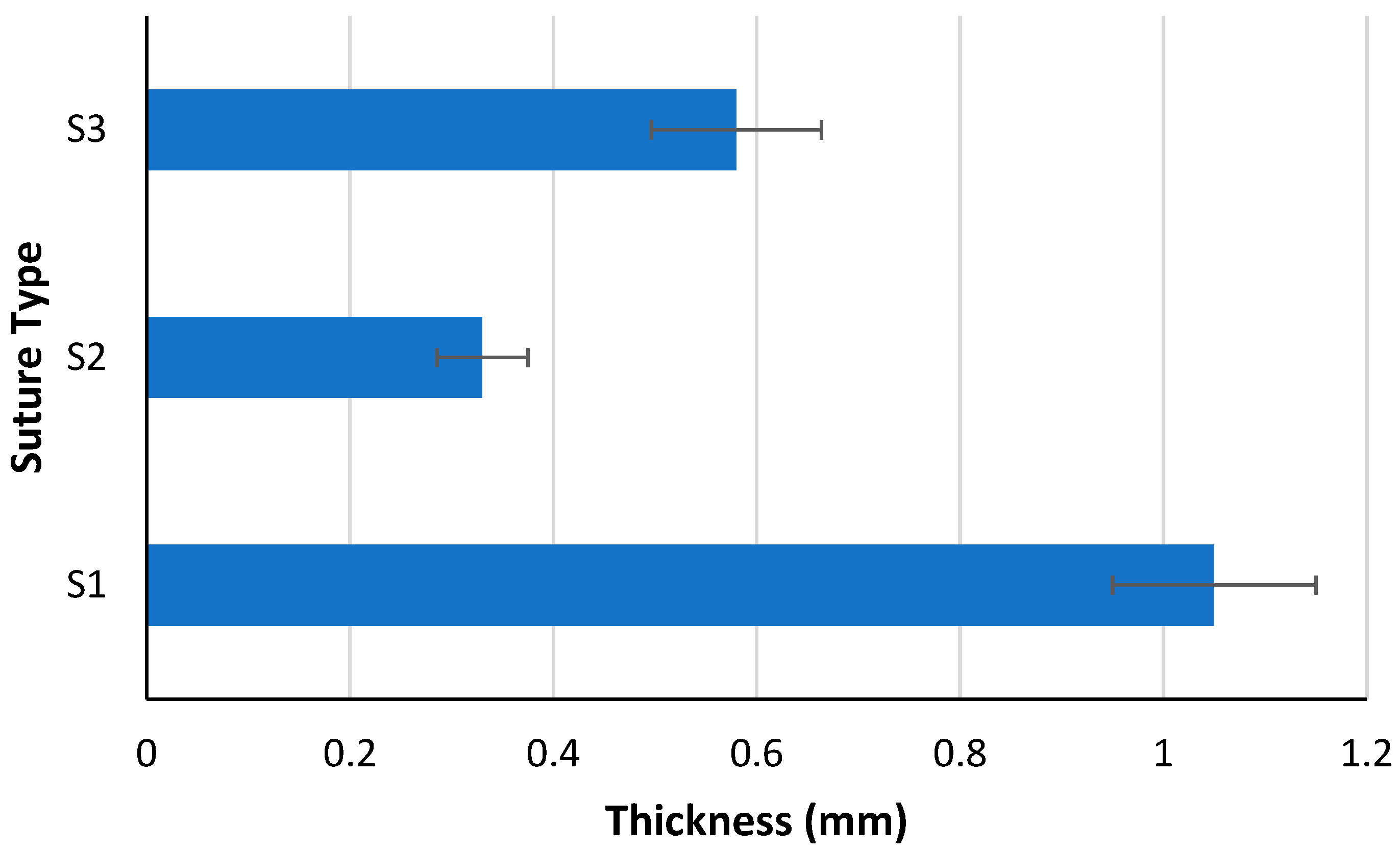
4.1. Elongation and Thickness Analysis
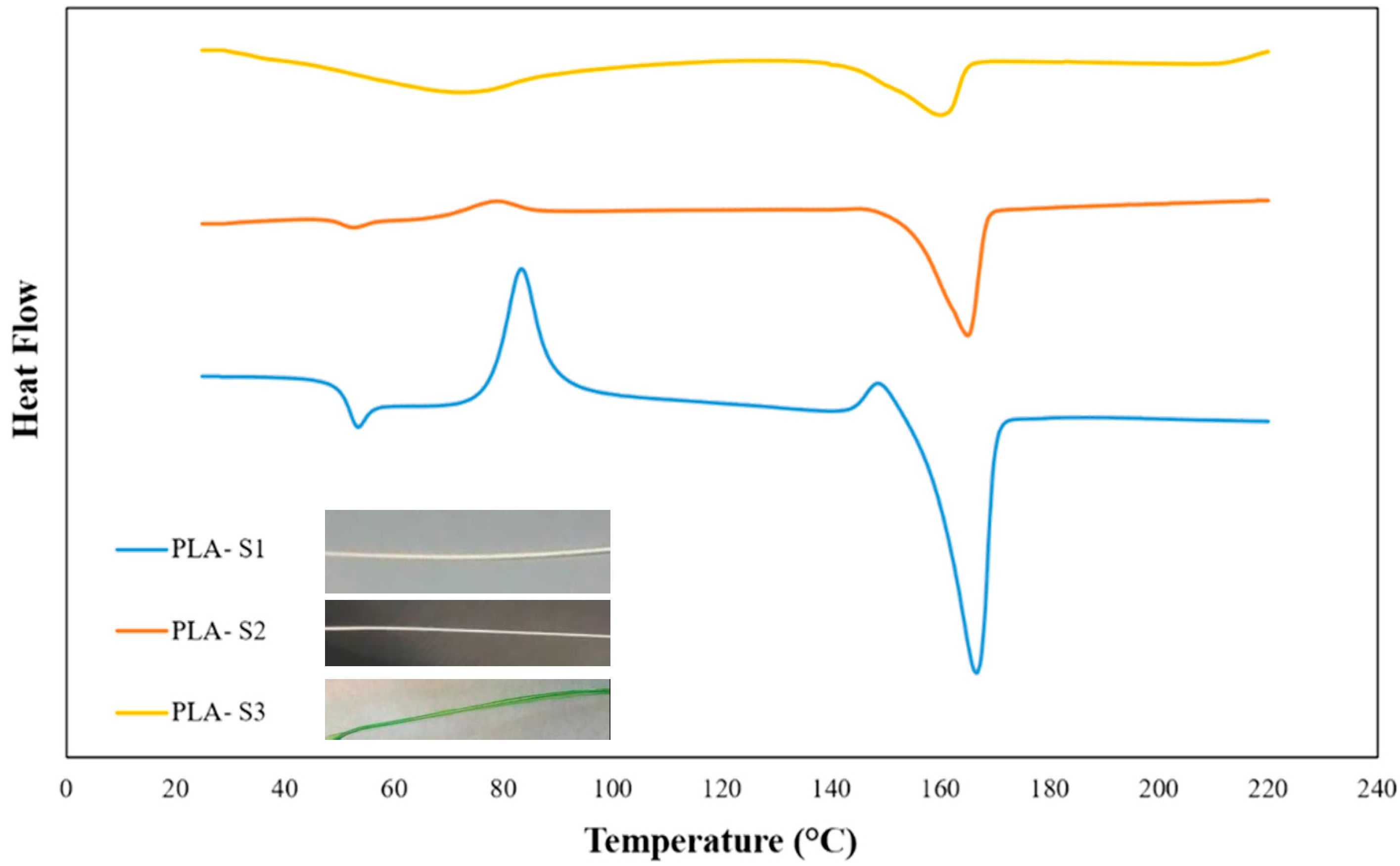
4.2. DSC Analysis
4.3. Suture Implantation in a Human Skin Simulator
4.4. Suture Implantation in a Lab Rat
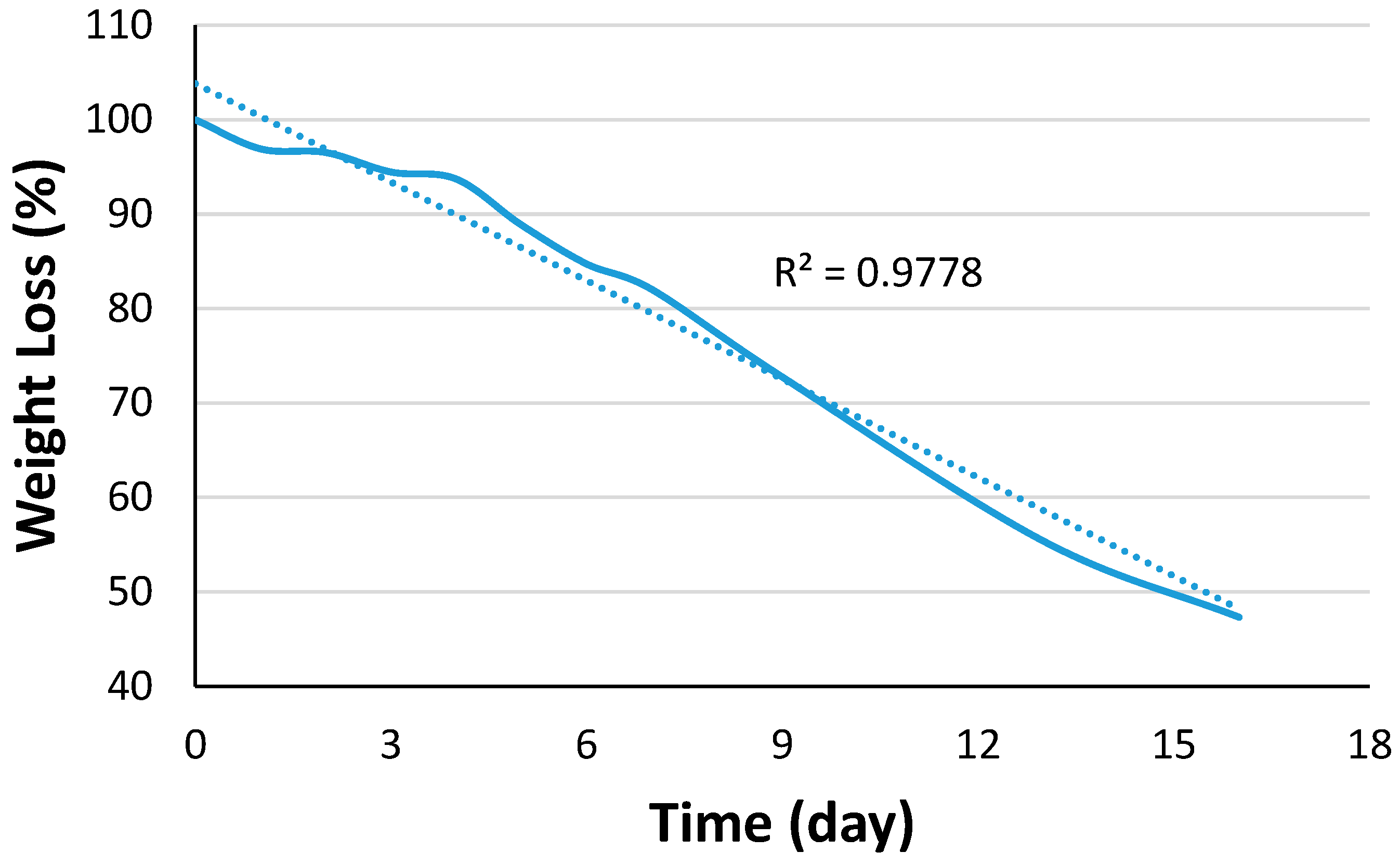
4.5. Suture Degradation
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Susin, C.; Fiorini, T.; Lee, J.; De Stefano, J.A.; Dickinson, D.P.; Wikesjö, U.M. Wound healing following surgical and regenerative periodontal therapy. Periodontol. 2000 2015, 68, 83–98. [Google Scholar] [PubMed]
- You, H.J.; Han, S.K. Cell therapy for wound healing. J. Korean Med. Sci. 2014, 29, 311–319. [Google Scholar] [PubMed]
- Matarasso, A.; Paul, M.D. Barbed sutures in aesthetic plastic surgery: Evolution of thought and process. Aesthetic Surg. J. 2013, 33, 17S–31S. [Google Scholar]
- Nicks, B.A.; Ayello, E.A.; Woo, K.; Nitzki-George, D.; Sibbald, R.G. Acute wound management: Revisiting the approach to assessment, irrigation, and closure considerations. Int. J. Emerg. Med. 2010, 3, 399–407. [Google Scholar]
- Niamtu, J., III. Expanding Hematoma in Face-lift Surgery: Literature Review, Case Presentations, and Caveats. Dermatol. Surg. 2005, 31, 1134–1144. [Google Scholar]
- Sathaiah, C. A Prospective Study to Compare Continous Versus Interrupted X Suture in Prevention of Burst Abdomen. Ph.D. Thesis, Madurai Medical College, Madurai, India, 2019. [Google Scholar]
- Theopold, C.; Potter, S.; Dempsey, M.; O’Shaughnessy, M. A randomised controlled trial of absorbable versus non-absorbable sutures for skin closure after open carpal tunnel release. J. Hand Surg. 2012, 37, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Breuing, K.; Butler, C.E.; Ferzoco, S.; Franz, M.; Hultman, C.S.; Kilbridge, J.F.; Ventral Hernia Working Group. Incisional ventral hernias: Review of the literature and recommendations regarding the grading and technique of repair. Surgery 2010, 148, 544–558. [Google Scholar]
- Zhao, W.; Liu, L.; Zhang, F.; Leng, J.; Liu, Y. Shape memory polymers and their composites in biomedical applications. Mater. Sci. Eng. C 2019, 97, 864–883. [Google Scholar]
- Chellamani, K.P.; Veerasubramanian, D.; Balaji, R.V. Surgical sutures: An overview. J. Acad. Indus. Res. 2013, 1, 778–781. [Google Scholar]
- Dennis, C.; Sethu, S.; Nayak, S.; Mohan, L.; Morsi, Y.; Manivasagam, G. Suture materials—Current and emerging trends. J. Biomed. Mater. Res. Part A 2016, 104, 1544–1559. [Google Scholar]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Paoletti, S. Adhesive and sealant interfaces for general surgery applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 626–639. [Google Scholar]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun medical sutures for wound healing: A review. Polymers 2022, 14, 1637. [Google Scholar]
- Narasimhan, A.K.; Rahul, T.S.; Krishnan, S. Revisiting the properties of suture materials: An overview. In Advanced Technologies and Polymer Materials for Surgical Sutures; Woodhead Publishing: Cambridge, UK, 2023; pp. 199–235. [Google Scholar]
- Al-Abdullah, T.; Plint, A.C.; Fergusson, D. Absorbable versus nonabsorbable sutures in the management of traumatic lacerations and surgical wounds: A meta-analysis. Pediatr. Emerg. Care 2007, 23, 339–344. [Google Scholar] [CrossRef]
- Köhler, G.; Luketina, R.R.; Emmanuel, K. Sutured repair of primary small umbilical and epigastric hernias: Concomitant rectus diastasis is a significant risk factor for recurrence. World J. Surg. 2015, 39, 121–126. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar]
- Ozdil, D.; Aydin, H.M. Polymers for medical and tissue engineering applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810. [Google Scholar]
- Joseph, B.; George, A.; Gopi, S.; Kalarikkal, N.; Thomas, S. Polymer sutures for simultaneous wound healing and drug delivery–a review. Int. J. Pharm. 2017, 524, 454–466. [Google Scholar]
- Zheng, K.; Gu, Q.; Zhou, D.; Zhou, M.; Zhang, L. Recent progress in surgical adhesives for biomedical applications. Smart Mater. Med. 2022, 3, 41–65. [Google Scholar]
- Schlich, T. The Origins of Organ Transplantation: Surgery and Laboratory Science; University Rochester Press: Rochester, NY, USA, 2010; pp. 1880–1930. [Google Scholar]
- Byrne, M.; Aly, A. The surgical suture. Aesthetic Surg. J. 2019, 39, S67–S72. [Google Scholar]
- Bennett, R.G. Selection of wound closure materials. J. Am. Acad. Dermatol. 1988, 18, 619–637. [Google Scholar]
- Laurén, P.; Somersalo, P.; Pitkänen, I.; Lou, Y.R.; Urtti, A.; Partanen, J.; Yliperttula, M. Nanofibrillar cellulose-alginate hydrogel coated surgical sutures as cell-carrier systems. PLoS ONE 2017, 12, e0183487. [Google Scholar]
- Akombaetwa, N.; Bwanga, A.; Makoni, P.A.; Witika, B.A. Applications of electrospun drug-eluting nanofibers in wound healing: Current and future perspectives. Polymers 2022, 14, 2931. [Google Scholar]
- Rakhmatullayeva, D.; Ospanova, A.; Bekissanova, Z.; Jumagaziyeva, A.; Savdenbekova, B.; Seidulayeva, A.; Sailau, A. Development and characterization of antibacterial coatings on surgical sutures based on sodium carboxymethyl cellulose/chitosan/chlorhexidine. Int. J. Biol. Macromol. 2023, 236, 124024. [Google Scholar]
- Yang, J.; Pan, H.; Li, X.; Sun, S.; Zhang, H.; Dong, L. A study on the mechanical, thermal properties and crystallization behavior of poly (lactic acid)/thermoplastic poly (propylene carbonate) polyurethane blends. R. Soc. Chem. Adv. 2017, 7, 46183–46194. [Google Scholar]
- Oksiuta, Z.; Jalbrzykowski, M.; Mystkowska, J.; Romanczuk, E.; Osiecki, T. Mechanical and thermal properties of polylactide (PLA) composites modified with Mg, Fe, and polyethylene (PE) additives. Polymers 2020, 12, 2939. [Google Scholar] [PubMed]
- Felfel, R.M.; Gupta, D.; Zabidi, A.Z.; Prosser, A.; Scotchford, C.A.; Sottile, V.; Grant, D.M. Performance of multiphase scaffolds for bone repair based on two-photon polymerized poly (D, L-lactide-co-ɛ-caprolactone), recombinamers hydrogel and nano-HA. Mater. Des. 2018, 160, 455–467. [Google Scholar]
- Chopra, S.; Deshmukh, K.A.; Deshmukh, A.D.; Gogte, C.L.; Peshwe, D. Prediction, evaluation and mechanism governing interphase strength in tensile fractured PA-6/MWCNT nanocomposites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 255–262. [Google Scholar]
- Barden, J.M., III. Influence of Molecular Architecture on the Structure and Properties of Co-Polyaramid Fibers. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2015. [Google Scholar]
- Wang, W.; Ping, P.; Chen, X.; Jing, X. Polylactide-based polyurethane and its shape-memory behavior. Eur. Polym. J. 2006, 42, 1240–1249. [Google Scholar]
- El-Bakary, M.A.; El-Farahaty, K.A.; El-Sayed, N.M. Investigating the mechanical behavior of PGA/PCL copolymer surgical suture material using multiple-beam interference microscopy. Fibers Polym. 2019, 20, 1116–1124. [Google Scholar]
- Yatigala, N.S.; Bajwa, D.S.; Bajwa, S.G. Compatibilization improves physico-mechanical properties of biodegradable biobased polymer composites. Compos. Part A Appl. Sci. Manuf. 2018, 107, 315–325. [Google Scholar]
- Radhakumary, C.; Nair, P.D.; Mathew, S.; Nair, C.R. Biopolymer composite of chitosan and methyl methacrylate for medical applications. Trends Biomater. Artif. Organs 2005, 18, 117–124. [Google Scholar]
- Budnyak, T.M.; Yanovska, E.S.; Kichkiruk, O.Y.; Sternik, D.; Tertykh, V.A. Natural minerals coated by biopolymer chitosan: Synthesis, physicochemical, and adsorption properties. Nanoscale Res. Lett. 2016, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Issaadi, K.; Habi, A.; Grohens, Y.; Pillin, I. Maleic anhydride-grafted poly (lactic acid) as a compatibilizer in poly (lactic acid)/graphene oxide nanocomposites. Polym. Bull. 2016, 73, 2057–2071. [Google Scholar]
- Khasraghi, S.S.; Shojaei, A.; Sundararaj, U. Bio-based UV curable polyurethane acrylate: Morphology and shape memory behaviors. Eur. Polym. J. 2019, 118, 514–527. [Google Scholar]
- Singh, P.; Pandey, P.; Arya, D.K.; Anjum, M.M.; Poonguzhali, S.; Kumar, A.; Rajinikanth, P.S. Biomimicking dual drug eluting twisted electrospun nanofiber yarns for post-operative wound healing. Biomed. Mater. 2023, 18, 035006. [Google Scholar]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67–103. [Google Scholar]
- Polimeni, G.; Koo, K.T.; Pringle, G.A.; Agelan, A.; Safadi, F.F.; Wikesjö, U.M. Histopathological observations of a polylactic acid-based device intended for guided bone/tissue regeneration. Clin. Implant. Dent. Relat. Res. 2008, 10, 99–105. [Google Scholar]
- Felfel, R. Manufacture and Characterisation of Bioresorbable Fibre Reinforced Composite Rods and Screws for Bone Fracture Fixation Applications. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2013. [Google Scholar]
- Yannie, H.M.; Norman, T.L. Accelerated Degradation Tests of Polylactic Acid (PLA) Scaffolds for Tissue Engineering Applications. In The Research and Scholarship Symposium; Cedarville University: Cedarville, OH, USA, 2021; Volume 14. [Google Scholar]
- Castañeda-Rodríguez, S.; González-Torres, M.; Ribas-Aparicio, R.M.; Del Prado-Audelo, M.L.; Leyva-Gómez, G.; Gürer, E.S.; Sharifi-Rad, J. Recent advances in modified poly (lactic acid) as tissue engineering materials. J. Biol. Eng. 2023, 17, 21. [Google Scholar]


| USP Size | Suture Diameter Size (mm) |
|---|---|
| 3 | 0.600–0.699 |
| 2 | 0.500–0.599 |
| 1 | 0.400–0.499 |
| 0 | 0.350–0.399 |
| 2-0 | 0.300–0.339 |
| 3-0 | 0.200–0.249 |
| 4-0 | 0.150–0.199 |
| 5-0 | 0.100–0.149 |
| 6-0 | 0.070–0.099 |
| 7-0 | 0.050–0.069 |
| 8-0 | 0.040–0.049 |
| 9-0 | 0.030–0.039 |
| Properties | Value | Unit |
|---|---|---|
| Density | 1.30~1.4 | |
| Vicat Softening Point Temperature | ≥85 | °C |
| Tensile Strength | ≥50 | MPa |
| Impact Strength | ≥2 | |
| Melt flow rate | 8–15 | |
| Mold shrinkage | 0.003 | % |
| S1 Length (mm) | S2 Length (mm) | Change in Length (mm) | Elongation Percentage (%) | |
|---|---|---|---|---|
| Average Value | 15.25 | 37.25 | 22.00 | 143.48 |
| Sample | Tg (°C) | Tc (°C) | Tm (°C) | Crystallinity (%) |
|---|---|---|---|---|
| PLA-Pellet | 60.17 | 96.04 | 156.23 | 41.38 |
| PLA-S1 | 50.63 | 75.35 | 153.43 | 4.54 |
| PLA-S2 | 48.91 | 69.84 | 184.19 | 27.27 |
| PLA-S3 | 60.47 | - | 164.01 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhulaybi, Z.A. Fabrication and Characterization of Poly(lactic acid)-Based Biopolymer for Surgical Sutures. ChemEngineering 2023, 7, 98. https://doi.org/10.3390/chemengineering7050098
Alhulaybi ZA. Fabrication and Characterization of Poly(lactic acid)-Based Biopolymer for Surgical Sutures. ChemEngineering. 2023; 7(5):98. https://doi.org/10.3390/chemengineering7050098
Chicago/Turabian StyleAlhulaybi, Zaid Abdulhamid. 2023. "Fabrication and Characterization of Poly(lactic acid)-Based Biopolymer for Surgical Sutures" ChemEngineering 7, no. 5: 98. https://doi.org/10.3390/chemengineering7050098
APA StyleAlhulaybi, Z. A. (2023). Fabrication and Characterization of Poly(lactic acid)-Based Biopolymer for Surgical Sutures. ChemEngineering, 7(5), 98. https://doi.org/10.3390/chemengineering7050098





