Abstract
A bicarbonate-peroxide (BAP) system was evaluated to improve the quality of industrial tannery wastewater using an I-optimal experimental design with four variables (temperature, initial pH, bicarbonate, and H2O2 concentration). The response variables were COD removal, ammonia nitrogen removal, and nitrate concentration. The most critical variables were optimized using a The process was carried out in 500 mL reactors, the operational volume of 250 mL, and the agitation was at 550 rpm. A new I-optimal reaction surface design at two levels (bicarbonate concentration 0.01–0.3 mol/L and H2O2 0.05–0.35 mol/L) was used to obtain the optimal data of the experimental design. Optimal conditions were validated by one-way ANOVA statistical analysis using Prism software. Temperatures above 50 °C promote the efficiency of the BAP system, and slightly acidic initial pHs allow stabilization of the system upon inclusion of bicarbonate and peroxide in the concentration of bicarbonate, which is critical for the reaction with peroxide and formation of reactive oxygen species. With the validated optimal data, removal percentages above 78% were achieved for nitrites, ammonia nitrogen, chromium, TSS, BOD, conductivity, chromium, and chlorides; for COD and TOC, removal percentages were above 45%, these results being equal and even higher than other AOPs implemented for this type of water.
Keywords:
BAP system; bicarbonate; AOPs; hydrogen peroxide; COD; ammonium oxidation; tannery wastewater 1. Introduction
The tanning industry generates highly polluting waste, especially in its effluents. The operational processes of this industry require large amounts of water; it has been reported that an average of 10–25 m3 of water is consumed in the various stages of the process, and an average of 8 to 20 m3 of wastewater may be generated; this amount may vary depending on the technological development of each industry [1]. These effluents are characterized by high levels of pollutants from organic matter such as meat particles, hair, blood, soluble proteins, and fertilizer; in addition, there is the inorganic load of dyes, chromium salts as tanning agents, sulfur and lime salts, and other recalcitrant compounds [2]. These effluents also contain heavy metals such as lead (Pb2+), cadmium (Cd2+), chromium (Cr6+), zinc (Zn2+), copper (Cu2+), and iron (Fe3+) [3], as well as solvent–bone pollutants [4] that alter the natural characteristics of the ecosystems into which these effluents are discharged if they are not treated beforehand, and which can have harmful effects on those who consume them; liver and kidney damage, skin irritation, chronic bronchitis, nasal irritation, cancer, and DNA (deoxyribonucleic acid) abnormalities leading to mutations and malformations, as well as other complications in the digestive system, are possible harmful effects [5,6]. It is estimated that the waste generated by this industry in the form of water accounts for just over 300 million tons of toxic wastewater and approximately 64,320 tons of sludge [7], making the treatment of wastewater from the leather industry a challenge.
Advanced oxidation processes (AOPs) have proven to be a favorable alternative for the treatment of tannery effluents; these processes generate highly reactive species such as hydroxyl radicals (-OH) and other oxidizing species that can degrade organic compounds, emerging contaminants, dyes, heavy metals and different recalcitrant compounds present in wastewater, such as tannery wastewater [8,9]. These techniques were developed as an alternative to conventional treatment methods such as coagulation–flocculation, sedimentation, and biological treatment [10]. AOPs can remove a wide range of organic and inorganic contaminants, including those that are not biodegradable [11,12]. Studies show that AOPs are effective in treating tannery effluents, including ozone, Fenton, and H2O2/UV [13,14]. Fenton is one of the most widely used processes in the treatment of tannery effluents; the generation of hydroxyl radicals (-OH) occurs by the reaction of H2O2 with ferrous ions (Fe2+); it has been reported that this process can achieve 93% COD (Chemical Oxygen Demand), 98% BOD (Biochemical Oxygen Demand), and 62% chromium removal in combined processes with biological processes [15]; however, the Fenton process has limitations, such as the high cost of chemicals, sludge generation, and the need to carefully control the pH. Ozonation is another process used in tannery effluent treatment, involving ozone (O3) as a potent oxidizing agent to degrade pollutants in wastewater. It has been found that this system can effectively remove color (80–90%), odor, and organic compounds from tannery wastewater (60–80% COD), partly due to rapid reaction kinetics. This process does not generate chemical residues and can eliminate by-products generated during oxidation. However, ozone generation and exhaust gas management costs can be significant challenges [16]. Photocatalysis is another widely studied process in tannery wastewater treatment, using H2O2 activated by ultraviolet (UV) light to generate reactive species (-OH and superoxide radicals) that degrade organic pollutants. It has shown promising results in the treatment of tannery wastewater, especially in the degradation of dyes and aromatic compounds, achieving 70% COD, 76% chromium, and 60% color removal. However, photocatalytic processes require UV light sources, and their effectiveness can be affected by the presence of suspended solids or high concentrations of organic matter, characteristics present in tannery effluents [17].
The use of H2O2 in combination with ultraviolet light has been shown to reveal trace organic pollutants in tannery effluents [13]. Although these systems are efficient at the time of treatment, the complexity of the process and the high cost of expansion have hampered their adoption in the tanning industry, especially in developing countries. The H2O2 bicarbonate system (BAP) is an advanced oxidation process that has proven to be an alternative for treating complex wastewater, especially in the presence of dyes. In this system, the use of H2O2 and bicarbonate (HCO3−) produces hydroxyl radicals and other species, such as peroxymonocarbonate (HCO4−) and superoxide ions (O2−), which are highly reactive and can break down organic and recalcitrant contaminants in wastewater [18]. Recent studies have shown that the BAP system can be used to degrade a wide range of organic pollutants, dyes, heavy metals, nitrogenous compounds such as N-NH3 (ammoniacal nitrogen) and N-NO2− (nitrite), and other pollutants contained in wastewater containing dyes and organic compounds similar to those found in tannery wastewater, such as dyeing and other wastewater [19]. One of the aspects that draws attention to the BAP system concerns the low cost of implementation compared to other AOPs or other treatment systems, such as biological or physicochemical systems, due to the low use of sophisticated equipment, a low-complexity infrastructure, short reaction times [14], and low consumption of reagents, since the bicarbonate, in addition to activating H2O2, can also contribute to the neutralization of acidic effluents often produced in tanneries [20].
The BAP system is subject to the influence of several variables that can significantly affect its efficiency and performance and must be considered [21]. Some of the most important factors to be considered are the hydrogen peroxide concentration, which can affect the formation of hydroxyl radicals and, consequently, the efficiency of oxidation of organic compounds [22]. On the other hand, the bicarbonate concentration directly affects the pH of the wastewater and, consequently, the degradation of the acidic pollutants it contains [23]. Moreover, the pH of the system plays a fundamental role in the formation and stability of hydroxyl radicals, which are critical factors in the oxidation process [24]. Finally, it is essential to mention that temperature also plays a fundamental role in this process, as it acts as a catalyst for the reactions; as the temperature increases, the rate of the reactions involved also increases [25].
Currently, there are no reports on the use of a BAP system in the treatment of tannery wastewater, so this work aims to contribute to the knowledge of the potential applications of the BAP system in the treatment of tannery wastewater, including the optimization of process parameters such as pH, temperature, H2O2 concentration, and bicarbonate concentration to maximize the removal of contaminants. The response surface analysis method was used to determine the effects of these variables on the BAP system. In this analysis, experiments were conducted in which the variables of interest were systematically varied, and the response of the process was measured using the data collected. Based on this information, it was possible to determine the optimal conditions, which were later statistically validated to maximize the efficiency of the BAP system.
2. Materials and Methods
2.1. Tannery Wastewater
The tannery wastewater was obtained from a cattle skin tannery in Cúcuta (Norte de Santander, Colombia). A composite sample was obtained in duplicate and collected for 30 min during the working day, with a volume of 300 mL per sample. The sample was kept cold at 4 °C and with H2SO4 (sulfuric acid) for COD analysis. Characterization and analysis were performed at the water laboratory and the INNOVALGAE laboratory of the Universidad Francisco de Paula Santander (UFPS).
2.2. Physicochemical Characterization of the Tannery Effluents
The Tannery wastewater was physiochemically characterized at the UFPS Water Laboratory, according to the 23rd edition of Standard Methods for the Examination of Water and Wastewater (Table 1). Measurements were performed in triplicate.

Table 1.
Physicochemical characterization.
2.3. Experimental Analysis
An experimental design with three levels of I-optimal reaction area, as in Table 2, was used and analyzed with Design Expert software. The variables evaluated were temperature, initial pH, bicarbonate concentration, and hydrogen peroxide concentration, and the response variables were COD removal percentage, ammonia nitrogen removal percentage, and nitrate concentration. The experimental design resulted in 28 experiments; each experiment was performed in triplicate.

Table 2.
Experimental design.
The wastewater was passed through a grease trap for 5 min and then sedimented for 30 min in the pilot plant of the UFPS Operations Laboratory. A catalytic reactor of 600 mL and an operating volume of 250 mL was used, with temperature and pH control (Figure 1). Stirring was performed at 550 rpm. HCl (hydrochloric acid) and NaOH (sodium hydroxide) at a concentration of 0.1 mol/L were used for pH control. The H2O2 concentration was 35%, from which the concentrations used in the experimental design were calculated. Finally, 90% commercial purity grade sodium bicarbonate was used to determine the design concentrations.
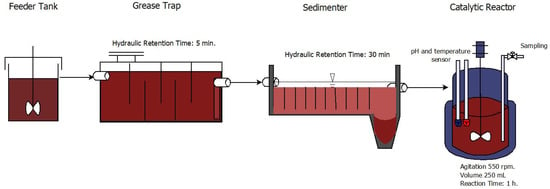
Figure 1.
Experimental setup for the bicarbonate/hydrogen peroxide process.
2.4. Analytical Methods
2.4.1. TOC Determination
TOC was quantified using a TORCH TOC analyzer from TELEDYNE (Cincinnati, OH, USA). The operating conditions were sample volume of 0.5 mL, water chase volume of 1.0 mL, injection line rinse on, injection line rinse volume of 0.5 mL, acid volume of 0.5 mL, ICS parge flow 200 mL min/L, carrier gas delay time of 0.40 min, ICS parge time of 50 min, detector sweep flow of 500 mL/min, furnace sweep time of 1.0 min, and system flow of 200 mL/min.
2.4.2. COD Determination
The quantification of the COD was determined by the method 5220 C-Closed reflux of the 23rd edition of Standard Methods [26], using potassium dichromate as an oxidant agent in an acid medium. For this, its digestion was carried out by adding 1.5 mL of digester solution and 3.5 mL of catalyst solution to 2.5 mL of sample; it was stirred in the vortex, heated for 2 h at 150 °C, and let cool to room temperature (25 °C). The sample was then transferred to a larger vessel, and 0.05 to 0.10 mL (1 to 2 drops) of ferroin indicator was added and stirred rapidly on a magnetic stirrer while titrating with standardized 0.10 mol/L FAS. The endpoint was an abrupt color change from blue-green to reddish brown. The calculation was performed using the formula (1). The COD calibration curve was generated from 5 patterns of potassium acid phthalate, made up of 5 concentrations 0, 50, 100, 250, and 500 mg/L. Each experiment was carried out by duplicate.
where
- B = mL FAS used for sample;
- A = mL FAS used for blank;
- M = molarity of FAS;
- 8000 = milliequivalent weight of oxygen ∗ 1000 mL/L.
2.4.3. Nitrate Quantification
For nitrates, the ultraviolet spectrophotometric detection method was used (SM-4500-NO3−B), scanning initially at a wavelength of 250 to 200 nm. Subsequently, the second derivative was calculated on the spectrophotometer in the range of 230 to 220 nm, and this record was used to determine the nitrate concentration.
2.4.4. Hydrogen Peroxide Determination
The methodology proposed by [27] was used to determine the concentration of hydrogen peroxide. A 1 L colorimetric solution was prepared using ammonium metavanadate (7.0188 g) and sulfuric acid (19.989 mL) in deionized water in a 1 L volumetric flask. A total of 1.033 mL of colorimetric solution was used, and 1 mL of sample was added and made up to 10 mL in a volumetric balance. The reading was made at a wavelength of 450 nm using a TERMO GENESYS UV-VIS spectrophotometer.
2.5. Data Analysis
The results from the experimental design were analyzed using the custom analysis in Design Expert 13. Prisma 8.0 software was used to analyze the data. Likewise, to determine the validation of the results between the expected value yielded by the experimental design and those obtained experimentally, ten replicates were used, and the statistical significance was determined using the Holm–Sidak method, with alpha = 0.05. Each row was analyzed individually without assuming a consistent SD number of t-tests equal to 10.
3. Results and Discussion
3.1. Physicochemical Characterization of Tannery Wastewater
Table 3 shows the physicochemical characterization of the tannery effluents evaluated in this study. The tannery effluent used in this study had a high COD concentration (6535.66 ± 15.33 mg/L), indicating low biodegradability given the BOD concentration (1245.52 ± 7.45 mg/L), resulting in a BOD/COD ratio of 0.19, similar to other studies [18,28,29,30,31]. The BOD/COD ratio allows determination of the degree of biodegradability of wastewater; values < 0.4 indicate low biodegradability of the effluent and the presence of recalcitrant compounds; and typical values in the range of 0.1–0.4 BOD/COD [32] have been reported for tannery effluents. The low biodegradability may be due to phenolic compounds, red and black dyes, and other recalcitrant compounds used in leather washing and tanning [33]. A dark brown color characterizes tannery wastewater due to the different dyes used in the process; a strong odor is due to volatile organic compounds, organic and inorganic carbon, nitrogen and phosphorus compounds, fats, and other highly polluting compounds such as total dissolved solids (TDS), chlorides, sulfates, and heavy metals like zinc (Zn) and chromium (Cr), among others [1,4,34,35,36]. These characteristics were similar to those found in this study, although the concentration of chromium was lower compared to other studies. Regarding pH, acidic values between 3.4 and 5.5 and basic values between 8 and 11 have been reported [3,37]. In this study, the pH remained within slightly acid values (5.45); this parameter can affect the biodegradability of tannery effluents. Values above 12 or below 4.5 can cause inhibition in the different microorganisms; likewise, the high turbidity of the effluents affects the photosynthetic activity [29].

Table 3.
Physicochemical characterization of tannery wastewater.
3.2. Experimental Design
3.2.1. COD
The results of the experimental design for COD are shown in Table 4. The model F-value of 22.57 implies that the model is significant (p < 0.0001); there is only a 0.01% chance that such a high F-value is due to noise. Similarly, p-values less than 0.0500 indicate that the model terms are significant. In this analysis, it is found that H2O2, bicarbonate, pH, and temperature affect the process and the H2O2 and pH interaction, and finally, the quadratic adjustment of pH and temperature is significant in the process. The lack of fit F-value of 0.43 implies that the lack of fit is not significant relative to the pure error. There is an 89.38% chance that such a large F-value of lack of fit is due to noise. The non-significant lack of fit is good.

Table 4.
Analysis ANOVA for COD.
Figure 2 shows the response surface for COD, where a lower concentration of bicarbonate and peroxide enhances COD removal.
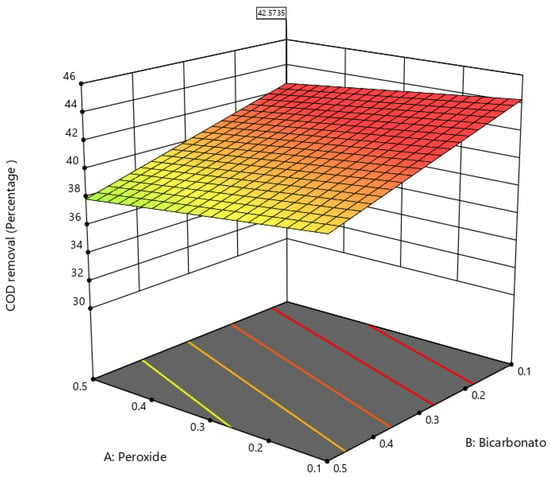
Figure 2.
Response surface for COD.
COD removal in a BAP system depends on several factors that play an important role in the efficiency of the COD degradation process. Some reports on effluents containing dyes similar to those used in the tanning industry have indicated that the initial COD concentration is an important factor [18,38,39]. Very high COD concentrations may require longer reaction times and high doses of H2O2 and bicarbonate to achieve significant COD removal due to the complexity of the tannery wastewater matrix [40]. Although there are no reports on applying the BAP system in tannery wastewater, the effects of bicarbonate and H2O2 dosing have been reported in wastewater from dyeing plants comparable to tanneries. Regarding bicarbonate, it was found that it can affect the total pH and alkalinity of wastewater, so determining the optimal dose of bicarbonate is essential to keep the pH in the range where oxidation reactions occur and the generation of hydroxyl radicals (-OH) is efficient [41,42]. As for H2O2, the dose supplied to the system is crucial for generating a significant number of hydroxyl radicals to ensure the oxidation process of organic compounds and the reduction in COD. Excess H2O2 can increase the COD concentration of the wastewater. Moreover, residual H2O2 acts as a scavenger of (-OH), negatively affecting the removal of various organic pollutants [2]. As for pH and temperature, these parameters can affect the rate of oxidation reactions. It has been pointed out that at temperatures above 50 °C in effluents containing dyes and high COD concentrations, such as those found in tannery effluents, the reactions proceed easily because the reactants can favorably overcome the reaction energy barrier, which enables the degradation of COD [25]. In terms of pH, under alkaline conditions, bicarbonate may decompose into CO2 or CO32−, which may reduce the activation of H2O2; under low pH conditions, the interaction between HCO3− and H2O2 is beneficial for the formation of HCO4−, but may not lead to the formation of (-OH); a high pH was beneficial for the formation of % HO) and HCO4−. However, when the pH is very high, it promotes the formation of singlet oxygen (1O2), the least oxidative species in the BAH system, thus affecting the pollutant oxidation process [24,43]. Finally, it is essential to highlight that bicarbonate can increase the total pH of tannery wastewater so that the initial pH under weakly acidic conditions allows the increase to a low alkaline pH that guarantees the optimal range for the BAP system, which was demonstrated in the results of the experimental analysis and obtained the optimal conditions, which were subsequently validated in this work.
3.2.2. Nitrification
Table 5 shows the experimental design results for ammonia nitrogen removal. The results for ammonia nitrogen removal showed that the design was significant (F = 34.37); there is only a 0.01% chance that such a significant F value is due to noise. p-values less than 0.0500 for this case indicate that the model terms are significant. It is evident that the variables H2O2 concentration, bicarbonate, pH, temperature, and H2O2 temperature interaction presented a linear behavior and are significant in the model. Likewise, the model’s quadratic trend of pH and temperature is significant. The F-value for lack of fit of 1.13 implies that the lack of fit is not significant relative to the pure error. There is a 48.14% chance that such a large F-value of lack of fit is due to noise.

Table 5.
Analysis ANOVA for ammonia nitrogen removal and nitrate generated.
For the case of nitrate generation (Table 5), the model F-value of 22.05 implies that the model is significant; there is only a 0.01% chance that such a large F-value is due to noise. p-values less than 0.0500, in this case, indicate that the model terms are significant. The variables of bicarbonate, temperature, and the H2O2-pH interaction were found to be significant when they have linear behavior; while pH and temperature with the quadratic trend are significant in the model. The F-value for lack of fit of 1.10 implies that the lack of fit is not significant relative to the pure error. There is a 49.25% chance that such a large F-value for lack of fit is due to noise. Figure 3 shows the response surface for ammonium nitrogen removal and nitrate generation.
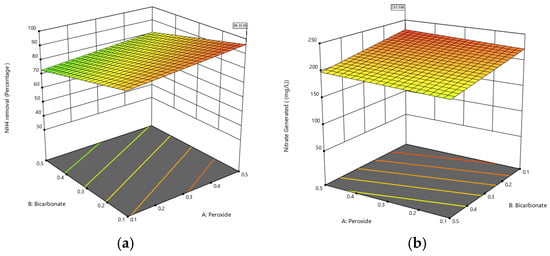
Figure 3.
Response surface for ammonia nitrogen removal (a) and nitrate produced (b).
One of the essential aspects of implementing a BAP system for tannery wastewater is the oxidation of ammonium nitrogen to nitrate. This less toxic compound can have added value for cultivating microorganisms such as microalgae and cyanobacteria that use nitrate as a growth source [44]. There are no reports in the literature on the oxidation of ammonium nitrogen to nitrate by a BAP system in tannery wastewater. However, recent studies with tannery-like dyeing waters have indicated that the oxidation of ammonium nitrogen (NH3-N) to nitrate (NO3−) depends on several factors. The pH plays a fundamental role in the oxidation of ammonium nitrogen to nitrite and subsequently to nitrate in various oxidation processes; very high pH values favor the conversion of N-NH3 to N-NO2, while slightly acidic values favor the oxidation of N-NO2 to N-NO3 and the addition of bicarbonate can maintain the pH in the optimal range (5–8) for the complete oxidation of N-NH3 [45,46]. The dosage of bicarbonate and H2O2 is crucial for the N-NH3 oxidation process; the formation of reactive oxygen species (ROS) depends on the reaction of bicarbonate and H2O2 and the pH of the medium to allow the formation of reactive species such as peroxymonocarbonate (HCO4−), superoxide ions (O2−), or OH—radicals [31], as shown in this work, where the interaction between peroxide and hydrogen is important for the BAP process. Another important factor is temperature, which can accelerate the oxidation process. Research with various AOPs has found that higher temperatures accelerate the reaction rate and the degradation of nitrogen compounds produced when dyes are used during the tanning process [47].
3.3. Process Optimization
According to the previous design, the concentration of both peroxide and bicarbonate significantly affects both the removal of COD and the generation of NO3; therefore, in order to improve both responses, a Central Composite Design was employed (2 factors, 3 levels, and 13 experiments). Table 6 shows the levels of both factors. For each experiment, pH was kept constant at 5.1, the temperature was maintained at 57.5 °C, and the mixing of the samples was performed at 550 rpm.

Table 6.
Central Composite Design for process optimization.
The results of the CCD design can be found in Table 7. The results of the experimental design for the COD case show that the model F-value of 13.54 implies that the model is significant; there is only a 0.12% chance that such a large F-value is due to noise. p-values less than 0.0500 indicate that the model terms are significant. In this case, the trend of the variables is quadratic, with H2O2 and bicarbonate being significant in the model. Values greater than 0.1000 indicate that the model terms are not significant. The lack of fit F-value of 0.52 implies that the lack of fit is not significant relative to the pure error; there is a 72.70% chance that such a significant lack of fit F-value is due to noise. For the case of nitrate generated from ammonium oxidation, the design results showed that the model F-value of 61.12 implies that the model is significant. There is only a 0.01% chance that such a large F-value is due to noise. p-values less than 0.0500 indicate that the model terms are significant. H2O2 concentration is significant in the model when it follows a quadratic trend, while for bicarbonate concentration, this is significant when it takes both a linear and quadratic trend. Values greater than 0.1000 indicate that the model terms are not significant. The lack of fit F-value of 3.88 implies that the lack of fit is not significant relative to the pure error. There is a 10.88% chance that such a large F-value of lack of fit is due to noise.

Table 7.
Analysis ANOVA for the removal of COD and nitrate formation.
The surface response of the interaction between H2O2 and bicarbonate concentration for COD removal and nitrate generation (Figure 4) shows that the optimal concentration of bicarbonate was 0.175 mol/L, while that of H2O2 was 0.2 mol/L. The highest COD removal and nitrate generation were maximized by the software.
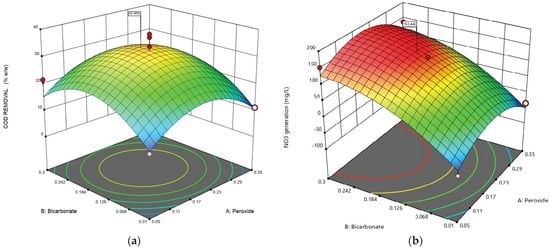
Figure 4.
Response surface validation of optimal conditions for COD (a) and nitrate removal (b).
Figure 4a shows that the response surface analysis between bicarbonate concentration and H2O2 dosage had a statistically significant effect on the COD removal rate at the central level of initial pH and temperature. As shown in Figure 4a, the COD removal rate was favored by bicarbonate and peroxide concentration up to the central level and then decreased rapidly with ozone concentration. Therefore, the COD removal rate was affected from the concentration of 0.06 mol/L to 0.3 mol/L, where the COD removal rate was favored. For the case of peroxide in this study, it was found that values above 0.23 mol/L affect the COD degradation process, decreasing the removal rate; studies of AOPs using H2O2 have indicated the importance of determining the optimal dosage of peroxide since high dosages can affect the process and increase the concentration of COD in the wastewater [48], as could be evidenced in this study. On the other hand, Figure 4b shows that the response surface analysis between the bicarbonate concentration and the H2O2 dose had a statistically significant effect on the rate of oxidation of ammonium to nitrate at the central level of initial pH and temperature. The oxidation rate was favored by both the bicarbonate and peroxide concentration; dosages between 0.01 mol/L and 0.4 mol/L in the case of bicarbonate facilitated the oxidation process of ammonium to nitrate, as did peroxide dosages between 0.05 mol/L and 0.23 mol/L. To the best of the authors’ knowledge, there is no evidence in the literature regarding the influence of bicarbonate concentration on ammonium removal. However, Ref. [46] reported that the removal of ammonium through the ozonation process could be affected by high concentrations of bicarbonate since it can make the medium too alkaline and therefore interferes with the generation of nitrates, hence the importance of determining the optimal dose. Regarding the influence of H2O2 on the removal of ammonium, it has been reported that the oxidation of ammonium to nitrate occurs due to the release of oxygen radicals and the degradation of azo dyes present in these types of effluents, such as tannery effluents, which leads to the search for ways to optimize dosages that do not exceed the optimum and interfere with the oxidation rate [49].
The results of the experimental design and response surface in this work allowed the determination of the variables to confirm the following process conditions: a temperature of 57 ± 0.2 °C, a pH of 5.1 ± 0.1, a bicarbonate concentration of 0.175 mol/L, and an H2O2 concentration of 0.2 mol/L. Ten experiments (one original plus nine replicates) were performed to confirm these conditions, and a t-test was performed using Prism (GraphPad, Version 10.0.0). Figure 5 shows the results of the verification of the optimal conditions.
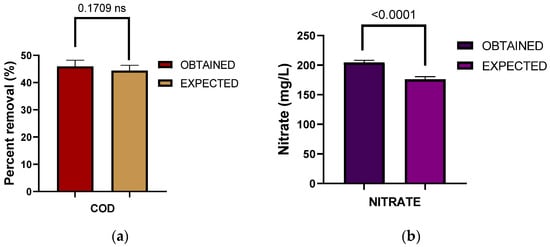
Figure 5.
Validation results between the expected value resulting from the experimental design and the experimentally determined value for the percentage of COD removal (a) and nitrate formation (b).
It can be observed that in the case of COD, there are no significant differences between the observed and expected values, while in the case of nitrates, there are significant differences between the experimentally determined value and the expected value. However, the experimentally determined value is higher, indicating an efficient nitrification process. Figure 6 shows the percentage removal of the different pollutants using the optimization variables.
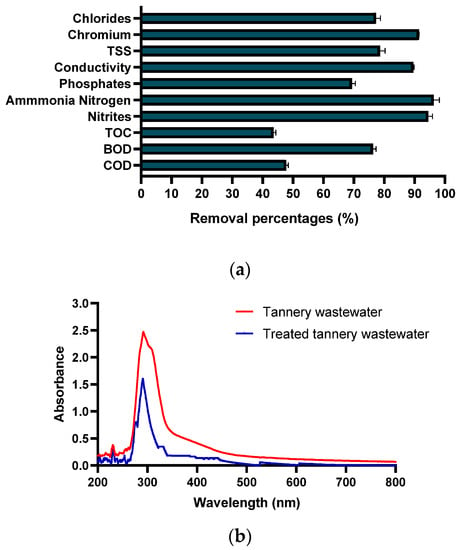
Figure 6.
Removal of contaminants (a) and color removal (b).
It can be demonstrated that the BAP process for the treatment of tannery wastewater achieves removal of over 78% for nitrites, ammonium nitrogen, chromium, TSS, BOD, conductivity, chromium, and chlorides (Table 8); based on Colombian regulations for tannery wastewater discharges, it can be seen that the BOD, TSS, chromium, and chloride parameters are below the maximum permissible values. Regarding nitrogen compounds (nitrites and ammoniacal nitrogen) and phosphates, the regulations do not indicate an allowable value, but only indicate analysis and reporting; in this sense, the toxicity of ammoniacal nitrogen in these effluents may vary depending on the species exposed, the duration of exposure, and the specific characteristics of the wastewater. However, authors have indicated that high concentrations of ammonia nitrogen and phosphates can generate eutrophication in the water bodies where they are discharged and be toxic to aquatic organisms, hence the importance of eliminating these compounds [50]. Concerning COD, removal of 49.5% was demonstrated, and the ratio of BOD/COD was 0.11 after the BAP process; several authors have reported COD removals between 40 and 50%, which are similar to those reported in this study [20,40,51]. The achieved removals for chromium, TOC, and BOD are, in some cases, superior to other AOPs for tannery wastewater [30,47,52]; the removal rates depend on the physicochemical properties of the wastewater and the concentration of the initial pollutant load [53], as well as on the factors evaluated in this work. The optimal values obtained during the process allowed the treatment of tannery wastewater due to the reactive species generated by the interaction of bicarbonate with peroxide, producing an effluent that can be used by microorganisms such as microalgae or cyanobacteria, which can improve the physicochemical conditions of the effluent and also produce biomass with potential industrial interest [1,19].

Table 8.
Final concentration of contaminant parameters using BAP system.
3.4. Treatment Costs Evaluation
To determine the cost feasibility for the application of the BAP system treatments in tannery wastewater, the costs of each of the variables analyzed in the experimental system were determined. The treatment costs were calculated taking into account the experimental conditions and the data obtained from the optimization process, namely a temperature of 57 ± 0.2 °C, a pH of 5.1 ± 0.1, a bicarbonate concentration of 0.175 mol/L, an H2O2 concentration of 0.2 mol/L, and a reaction time of V = 1 L. The energy prices and reagents used were as follows: 1.66 EUR/Kg H2O2 50% (w/v), 0.96 EUR/Kg sodium bicarbonate, 0.19 EUR/kWh energy value in Cúcuta (Colombia) for industrial applications; and 0.8 Kwh heating equipment. Table 9 shows the costs of different AOPs used to treat tannery wastewater.

Table 9.
System costs and BAP and other AOPs used in tannery wastewater treatment.
In the available literature, few studies present the costs of different AOPs in tannery wastewater; analyzing the different costs of the implementation of AOPs for the treatment of tannery wastewater, the BAP and O3 systems presented the lowest costs. It is essential to indicate that the BAP system implemented in this research used raw wastewater without pretreatment. In contrast, the ozone system reported by [57] worked with an effluent with a lower organic load than the one used in this study. The pollutant load in tannery effluents is a variable that influences the cost of treatment, affecting the amount of energy required, the reaction time, and the reagents to be consumed. Some studies have implemented various combinations of AOPs, such as ozone, UV, and H2O2, and found that energy consumption and treatment cost was in the order of UV > UV/H2O2 > UV/O3 > UV/O3/H2O2 > O3/H2O2 > O3 > O3 [55]. In contrast to the BAP system, there is still no report of the costs of this process, but it can be indicated that the system is more economical than the photocatalytic processes, and in comparison with ozone, the system is more favorable to implement and operate, which makes it a promising strategy not only for the treatment of tannery effluents but of their possible reuse in the process, allowing sustainability of the water resource.
The future use of AOPs and the BAP system is mainly in its coupling to biological systems, such as the use of microalgae and cyanobacteria that can improve effluents and generate biomass and metabolites of interest, such as lipids, carotenoids, and phycocyanins, as well as in evaluating the photoperiod conditions and dilutions of the wastewater that allow for the establishment of the effectiveness of coupling.
4. Conclusions
In this work, the effect of bicarbonate concentration, hydrogen peroxide, Ph, and temperature on implementing a BAP system for treating tannery wastewater was analyzed using response surface analysis. The BAP system buffers the pH and maintains it throughout the tannery wastewater treatment process; the generation of ROS gives it a particular advantage as an activating agent for hydrogen peroxide. The HCO3−-H2O2 system can degrade the organic pollutants in the tannery wastewater and oxidize the nitrogen species present, resulting in an effluent with a lower pollutant load, which proves the promoting effect of bicarbonate in the degradation of the analyzed pollutants based on H2O2. The analysis of the reaction surface showed that temperatures above 50 °C promote the efficiency of the BAP system and that slightly acidic initial pH values allow the stabilization of the system at the time of the inclusion of bicarbonate and peroxide in the treatment process. It is important to further investigate the efficiency of the BAP system in the treatment of tannery wastewater and to couple the system with biotechnological processes, such as the use of microalgae and cyanobacteria, as well as with other AOPs, such as UV or ozone, which allow the integration of a process leading to the reintegration of treated wastewater into tannery production systems in terms of the circular economy.
Author Contributions
Conceptualization, F.M.-M.; data curation, A.F.B.-S., J.B.G.-M., N.A.U.-S. and G.L.L.-B.; funding acquisition, F.M.-M.; investigation, N.A.U.-S. and C.J.S.-P.; resources, A.F.B.-S. and F.M.-M.; software, N.A.U.-S., J.B.G.-M. and A.F.B.-S.; supervision, A.F.B.-S. and F.M.-M.; writing—original draft, N.A.U.-S. and C.J.S.-P.; writing—review and editing, A.F.B.-S., G.L.L.-B. and F.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Universidad del Valle (Colombia) with the project “Evaluation of a coupled system for advanced oxidation of industrial effluents and production of microalgae to obtain high value-added metabolites. ID CI 21144” and Newton Fund Institutional Links, ID 527624805.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our sincere gratitude to Universidad del Valle and Universidad Francisco de Paula Santander for the equipment for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Urbina-suarez, N.A.; Machuca-martínez, F.; Barajas-solano, A.F. Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater. Molecules 2021, 26, 3222. [Google Scholar] [CrossRef]
- Sivagami, K.; Sakthivel, K.P.; Nambi, I.M. Advanced Oxidation Processes for the Treatment of Tannery Wastewater. J. Environ. Chem. Eng. 2018, 6, 3656–3663. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Mishra, S. Hexavalent Chromium Reduction Potential of Cellulosimicrobium Sp. Isolated from Common Effluent Treatment Plant of Tannery Industries. Ecotoxicol. Environ. Saf. 2018, 147, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hansen, É.; Monteiro de Aquim, P.; Hansen, A.W.; Cardoso, J.K.; Ziulkoski, A.L.; Gutterres, M. Impact of Post-Tanning Chemicals on the Pollution Load of Tannery Wastewater. J. Environ. Manag. 2020, 269, 110787. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, S.; Hooda, A.; Rana, L.; Dhankhar, R. Chromium (VI) Biosorption by Immobilized Aspergillus Niger in Continuous Flow System with Special Reference to FTIR Analysis. J. Environ. Biol. 2010, 31, 561–566. [Google Scholar] [PubMed]
- Daneshvar, E.; Zarrinmehr, M.J.; Kousha, M.; Hashtjin, A.M.; Saratale, G.D.; Maiti, A.; Vithanage, M.; Bhatnagar, A. Hexavalent Chromium Removal from Water by Microalgal-Based Materials: Adsorption, Desorption and Recovery Studies. Bioresour. Technol. 2019, 293, 122064. [Google Scholar] [CrossRef]
- Rezgui, S.; Ghazouani, M.; Bousselmi, L.; Akrout, H. Efficient Treatment for Tannery Wastewater through Sequential Electro-Fenton and Electrocoagulation Processes. J. Environ. Chem. Eng. 2022, 10, 107424. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Heal. 2019, 17, 170. [Google Scholar] [CrossRef]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen Reduction Reaction in the Field of Water Environment for Application of Nanomaterials. Nanomater 2020, 10, 1719. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Q.; Tang, Y.; Zhou, J.; Guo, H. Tannery Wastewater Treatment: Conventional and Promising Processes, an Updated 20-Year Review. J. Leather Sci. Eng. 2022, 4, 10. [Google Scholar] [CrossRef]
- Muruganandham, M.; Suri, R.P.S.; Jafari, S.; Sillanpää, M.; Lee, G.J.; Wu, J.J.; Swaminathan, M. Recent Developments in Homogeneous Advanced Oxidation Processes for Water and Wastewater Treatment. Int. J. Photoenergy 2014, 2014. [Google Scholar] [CrossRef]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the Degradation of Molecular and Ionic Ibuprofen in a UV/H2O2 System. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Schrank, S.G.; José, H.J.; Moreira, R.F.P.M.; Schröder, H.F. Comparison of Different Advanced Oxidation Process to Reduce Toxicity and Mineralisation of Tannery Wastewater. Water Sci. Technol. 2004, 50, 329–334. [Google Scholar] [CrossRef]
- Shokri, A.; Fard, M.S. A Critical Review in Fenton-like Approach for the Removal of Pollutants in the Aqueous Environment. Environ. Chall. 2022, 7, 100534. [Google Scholar] [CrossRef]
- Jessieleena, A.A.; Priyanka, M.; Saravanakumar, M.P. Comparative Study of Fenton, Fe2+/NaOCl and Fe2+/(NH4)2S2O8 on Tannery Sludge Dewaterability, Degradability of Organics and Leachability of Chromium. J. Hazard. Mater. 2021, 402, 123495. [Google Scholar] [CrossRef]
- Houshyar, Z.; Khoshfetrat, A.B.; Fatehifar, E. Influence of Ozonation Process on Characteristics of Pre-Alkalized Tannery Effluents. Chem. Eng. J. 2012, 191, 59–65. [Google Scholar] [CrossRef]
- Korpe, S.; Rao, P.V. Application of Advanced Oxidation Processes and Cavitation Techniques for Treatment of Tannery Wastewater—A Review. J. Environ. Chem. Eng. 2021, 9, 105234. [Google Scholar] [CrossRef]
- Urbina-Suarez, N.A.; Rivera-Caicedo, C.; González-Delgado, Á.D.; Barajas-Solano, A.F.; Machuca-Martínez, F. Bicarbonate-Hydrogen Peroxide System for Treating Dyeing Wastewater: Degradation of Organic Pollutants and Color Removal. Toxics 2023, 11, 366. [Google Scholar] [CrossRef]
- Pan, H.; Gao, Y.; Li, N.; Zhou, Y.; Lin, Q.; Jiang, J. Recent Advances in Bicarbonate-Activated Hydrogen Peroxide System for Water Treatment. Chem. Eng. J. 2021, 408, 127332. [Google Scholar] [CrossRef]
- Kan, H.; Soklun, H.; Yang, Z.; Wu, R.; Shen, J.; Qu, G.; Wang, T. Purification of Dye Wastewater Using Bicarbonate Activated Hydrogen Peroxide: Reaction Process and Mechanisms. Sep. Purif. Technol. 2020, 232, 115974. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Yang, Y.; Yao, B.; Tang, Y.; Luo, L.; Zhi, D.; Wan, Z.; Wang, L.; Zhou, Y. A Review on Percarbonate-Based Advanced Oxidation Processes for Remediation of Organic Compounds in Water. Environ. Res. 2021, 200, 111371. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Choi, W. Bicarbonate-Induced Activation of H2O2 for Metal-Free Oxidative Desulfurization. J. Hazard. Mater. 2016, 304, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.; Lu, X.; Chen, Z.; Yin, G. Degradation of Chlorophenols by Supported Co-Mg-Al Layered Double Hydrotalcite with Bicarbonate Activated Hydrogen Peroxide. J. Phys. Chem. A 2014, 118, 10028–10035. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, H.; Zhao, S. Fast Degradation of Acid Orange II by Bicarbonate-Activated Hydrogen Peroxide with a Magnetic S-Modified CoFe2O4 Catalyst. J. Taiwan Inst. Chem. Eng. 2015, 55, 90–100. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Soklun, H.; Qu, G.; Xia, T.; Guo, X.; Jia, H.; Zhu, L. A Green Strategy for Simultaneous Cu(II)-EDTA Decomplexation and Cu Precipitation from Water by Bicarbonate-Activated Hydrogen Peroxide/Chemical Precipitation. Chem. Eng. J. 2019, 370, 1298–1309. [Google Scholar] [CrossRef]
- Lipps, W.C.; Braun-Howland, E.B.; Baxter, T.E. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 0875532993. [Google Scholar]
- Nogueira, R.F.P.; Oliveira, M.C.; Paterlini, W.C. Simple and Fast Spectrophotometric Determination of H2O2 in Photo-Fenton Reactions Using Metavanadate. Talanta 2005, 66, 86–91. [Google Scholar] [CrossRef]
- Appiah-Brempong, M.; Essandoh, H.M.K.; Asiedu, N.Y.; Dadzie, S.K.; Momade, F.W.Y. Artisanal Tannery Wastewater: Quantity and Characteristics. Heliyon 2022, 8, e08680. [Google Scholar] [CrossRef]
- Genawi, N.M.; Ibrahim, M.H.; El-Naas, M.H.; Alshaik, A.E. Chromium Removal from Tannery Wastewater by Electrocoagulation: Optimization and Sludge Characterization. Water 2020, 12, 1374. [Google Scholar] [CrossRef]
- Tamil Selvan, S.; Velramar, B.; Ramamurthy, D.; Balasundaram, S.; Sivamani, K. Pilot Scale Wastewater Treatment, CO2 Sequestration and Lipid Production Using Microalga, Neochloris Aquatica RDS02. Int. J. Phytoremediation 2020, 22, 1462–1479. [Google Scholar] [CrossRef]
- Monira, U.; Sattar, G.S.; Mostafa, M.G. Characterization of Tannery Effluent and Efficiency Assessment of Central Effluent Treatment Plant (CETP) at Savar in Bangladesh. Asian J. Sci. Appl. Technol. 2023, 12, 48–53. [Google Scholar] [CrossRef]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and Biological Treatment Technologies for Leather Tannery Chemicals and Wastewaters: A Review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef]
- Akan, J.C.; Abdulrahman, F.I.; Ayodele, J.T.; Ogugbuaja, V.O. Impact of Tannery and Textile Effluent on the Chemical Characteristics of Challawa River, Kano State, Nigeria. Aust. J. Basic Appl. Sci. 2009, 3, 1933–1947. [Google Scholar]
- Khanh Tran, T.; Jyh Leu, H.; Quyet Vu, T.; Tam Nguyen, M.; Anh Pham, T.; Kiefer, R. Hydrogen Production from the Tannery Wastewater Treatment by Using Agriculture Supports Membrane/Adsorbents Electrochemical System. Int. J. Hydrogen Energy 2020, 45, 3699–3711. [Google Scholar] [CrossRef]
- Reyes-Serrano, A.; López-Alejo, J.E.; Hernández-Cortázar, M.A.; Elizalde, I. Removing Contaminants from Tannery Wastewater by Chemical Precipitation Using CaO and Ca(OH)2. Chin. J. Chem. Eng. 2020, 28, 1107–1111. [Google Scholar] [CrossRef]
- Saranya, D.; Shanthakumar, S. An Integrated Approach for Tannery Effluent Treatment with Ozonation and Phycoremediation: A Feasibility Study. Environ. Res. 2020, 183, 109163. [Google Scholar] [CrossRef]
- Meenachi, S.; Kandasamy, S. Pre-Treatment of Tannery Chrome Wastewater by Green Synthesised Iron Oxide Nanocatalyst. Int. J. Environ. Anal. Chem. 2019, 101, 2087–2099. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Chen, Z.X.; Zhang, J.; Gong, L.; Wang, Y.X.; Zhao, H.Q.; Mu, Y. Carbonate-Activated Hydrogen Peroxide Oxidation Process for Azo Dye Decolorization: Process, Kinetics, and Mechanisms. Chemosphere 2018, 192, 372–378. [Google Scholar] [CrossRef]
- Urbina-Suarez, N.A.; Barajas-Solano, A.F.; Zuorro, A.; Machuca, F. Advanced Oxidation Processes with Uv-H2o2 for Nitrification and Decolorization of Dyehouse Wastewater. Chem. Eng. Trans. 2022, 95, 235–240. [Google Scholar] [CrossRef]
- Jawad, A.; Chen, Z.; Yin, G. Bicarbonate Activation of Hydrogen Peroxide: A New Emerging Technology for Wastewater Treatment. Chin. J. Catal. 2016, 37, 810–825. [Google Scholar] [CrossRef]
- Mergeay, M. Heavy Metal Resistances in Microbial Ecosystems. In Molecular Microbial Ecology Manual; Springer: Dordrecht, The Netherlands, 1995; pp. 439–455. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Z.; Ruan, X.; Xia, D.; Zeng, Q.; Xu, A. Kinetics and Mechanism of Organic Pollutants Degradation with Cobalt–Bicarbonate–Hydrogen Peroxide System: Investigation of the Role of Substrates. Appl. Catal. A Gen. 2012, 411–412, 24–30. [Google Scholar] [CrossRef]
- Balagam, B.; Richardson, D.E. The Mechanism of Carbon Dioxide Catalysis in the Hydrogen Peroxide N-Oxidation of Amines. Inorg. Chem. 2008, 47, 1173–1178. [Google Scholar] [CrossRef]
- Sforza, E.; Kumkum, P.; Barbera, E.; Kumar, S. Bioremediation of Industrial Effluents: How a Biochar Pretreatment May Increase the Microalgal Growth in Tannery Wastewater. J. Water Process Eng. 2020, 37, 101431. [Google Scholar] [CrossRef]
- Wang, J.; Song, M.; Chen, B.; Wang, L.; Zhu, R. Effects of PH and H2O2 on Ammonia, Nitrite, and Nitrate Transformations during UV254nm Irradiation: Implications to Nitrogen Removal and Analysis. Chemosphere 2017, 184, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, B.; Zanetti, M. Orthophosphate vs. Bicarbonate Used as a Buffering Substance for Optimizing the Bromide-Enhanced Ozonation Process for Ammonia Nitrogen Removal. Sci. Total Environ. 2019, 692, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Korpe, S.A.; Landge, V.; Hakke, V.S.; Rao, P.V.; Sonawane, S.H.; Sonawane, S.S. Advanced Oxidation Processes for Tannery Industry Wastewater Treatment. In Novel Approaches towards Wastewater Treatment and Resource Recovery Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 253–276. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An Overview on the Advanced Oxidation Processes Applied for the Treatment of Water Pollutants Defined in the Recently Launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Jóźwiakowski, K.; Marzec, M.; Fiedurek, J.; Kamińska, A.; Gajewska, M.; Wojciechowska, E.; Wu, S.; Dach, J.; Marczuk, A.; Kowlaczyk-Juśko, A. Application of H2O2 to Optimize Ammonium Removal from Domestic Wastewater. Sep. Purif. Technol. 2017, 173, 357–363. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, W. A Review for Tannery Wastewater Treatment: Some Thoughts under Stricter Discharge Requirements. Environ. Sci. Pollut. Res. 2019, 26, 26102–26111. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Veli, S.; Bingöl, D. Use of Response Surface Methodology for Pretreatment of Hospital Wastewater by O3/UV and O3/UV/H2O2 Processes. Sep. Purif. Technol. 2014, 132, 561–567. [Google Scholar] [CrossRef]
- Pire-Sierra, M.C.; Cegarra-Badell, D.D.; Carrasquero-Ferrer, S.J.; Angulo-Cubillan, N.E.; Díaz-Montiel, A.R. Nitrogen and COD Removal from Tannery Wastewater Using Biological and Physicochemical Treatments. Rev. Fac. Ing. Univ. Antioquia 2016, 2016, 63–73. [Google Scholar] [CrossRef]
- Saravanathamizhan, R.; Perarasu, V.R.; Dhandapani, B. Advanced Oxidation Process for Effluent Treatment in Textile, Pharmaceutical, and Tannery Industries. In Photocatalytic Degradation of Dyes, Current Trends and Future Perspectives; Elsevier: Amsterdam, The Netherlands, 2021; pp. 719–745. [Google Scholar] [CrossRef]
- Módenes, A.N.; Espinoza-Quiñones, F.R.; Borba, F.H.; Manenti, D.R. Performance Evaluation of an Integrated Photo-Fenton—Electrocoagulation Process Applied to Pollutant Removal from Tannery Effluent in Batch System. Chem. Eng. J. 2012, 197, 1–9. [Google Scholar] [CrossRef]
- Wardenier, N.; Liu, Z.; Nikiforov, A.; Van Hulle, S.W.H.; Leys, C. Micropollutant Elimination by O3, UV and Plasma-Based AOPs: An Evaluation of Treatment and Energy Costs. Chemosphere 2019, 234, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Cañizares, P.; Paz, R.; Sáez, C.; Rodrigo, M.A. Costs of the Electrochemical Oxidation of Wastewaters: A Comparison with Ozonation and Fenton Oxidation Processes. J. Environ. Manag. 2009, 90, 410–420. [Google Scholar] [CrossRef]
- Gomes, A.I.; Soares, T.F.; Silva, T.F.C.V.; Boaventura, R.A.R.; Vilar, V.J.P. Ozone-Driven Processes for Mature Urban Landfill Leachate Treatment: Organic Matter Degradation, Biodegradability Enhancement and Treatment Costs for Different Reactors Configuration. Sci. Total Environ. 2020, 724, 138083. [Google Scholar] [CrossRef]
- Isarain-Chávez, E.; De La Rosa, C.; Godínez, L.A.; Brillas, E.; Peralta-Hernández, J.M. Comparative Study of Electrochemical Water Treatment Processes for a Tannery Wastewater Effluent. J. Electroanal. Chem. 2014, 713, 62–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).